Introduction
Lung cancer is a common malignancy and is the
leading cause of cancer-associated mortality worldwide (1,2).
Non-small cell lung cancer (NSCLC) accounts for ~85% of all lung
cancers (3). Lung adenocarcinoma
(LUAD) is the most common pathologic subtype of NSCLC in
non-smoking males, and in all females (both smokers and
non-smokers) (4,5). Although numerous resources have been
directed towards the development of novel LUAD treatments, the
prognosis of patients with advanced LUAD remains unsatisfactory,
with a 5 year survival rate <10% in 2018 (6). LUAD is relatively sensitive to primary
chemotherapy, but tumors rapidly acquire chemoresistance, leading
to death for most patients (7,8).
Cisplatin is one of the most effective
chemotherapeutic drugs and is used to treat various tumors,
including testicular cancer, ovarian cancer, cervix carcinoma,
breast cancer, prostate carcinoma, bladder cancer, lung cancer,
melanoma and head-and-neck cancer (9,10).
Cisplatin has a broad-spectrum anticancer activity, but its use is
limited due to it causing severe side effects and due to a number
of tumors acquiring cisplatin resistance (9). Although the side effects caused by
cisplatin have been mildly alleviated by newly-developed
antagonists (11), cisplatin
resistance, which commonly originates from multiple cellular
self-defense adaptations, often results in disease recurrence
(12). Thus, the development of
cisplatin resistance remains a substantial challenge for
chemotherapeutics.
A major impediment to a comprehensive understanding
of the molecular mechanisms underlying cisplatin-induced drug
resistance is that most currently available results were generated
using isolated cell lines. These studies can be misleading when
extended to in vivo experiments and clinical trials
(12,13). However, the integration of cell line
data with clinical information, especially overall survival (OS)
time, may improve this issue. For example, Zhao et al
(14) used The Cancer Genome Atlas
(TCGA) database to demonstrate that patients expressing high levels
of the long non-coding RNA (lncRNA) HOMEOBOX A11 antisense RNA
(HOXA11-AS) have shorter survival rates compared to the low
expression level group; mechanistic experiments subsequently showed
that the microRNA (miRNA/miR) targeted by HOXA11-AS affects
cisplatin resistance in LUAD cells. The aforementioned study thus
provides a framework for the identification of additional miRNAs
associated with cisplatin resistance in LUAD cells.
In the present study, the framework of Zhao et
al (14) was used to identify
miRNA targets that may be useful for the mitigation of cisplatin
resistance. The present study aimed to: i) Identify differentially
expressed (DE) mRNAs (DEmRNAs), DEmiRNAs and DElncRNAs between two
LUAD cell lines, namely A549 (cisplatin-sensitive) and A549-DDP
(cisplatin-resistant), using data from the Gene Expression Omnibus
(GEO) database (15); ii) quantify
the expression levels of these DEmRNAs in samples of patients with
LUAD using data downloaded from the TCGA database; iii) construct a
competing endogenous RNA (ceRNA) network based on the
aforementioned data; and iv) assess the associations between the
elements of the ceRNA network and patient OS time to identify
potential research targets.
Materials and methods
A549/A549-DDP data retrieval
Two miRNA and mRNA expression datasets were
downloaded from the GEO database (16): GSE43249 (17), which was derived from the GPL14613
(miRNA-2) Affymetrix Multispecies miRNA-2 Array, and GSE43493
(18), which was derived from the
GPL15314 Arraystar Human LncRNA microarray V2.0 (Agilent_033010
Probe Name version). Each dataset contained six samples, three that
were cisplatin-sensitive and three that were
cisplatin-resistant.
A549/A549-DDP data pre-processing
The raw microarray data were read using the package
affy v1.52.0 (19) in R v3.4.3
(http://www.bioconductor.org/packages/release/bioc/html/affy.html),
and was standardized using the robust multi-array average (20,21)
method, with background adjustment, quantile normalization and
summarization on a log2 scale. Using the platform
annotation file, the probe was annotated and the unmatched probe
was removed. To map different probes to the same mRNA or miRNA
data, the mean value of each different probe was used as the final
expression, and the genes were divided into mRNAs and lncRNAs
following the guidelines of the HUGO Gene Nomenclature Committee
(22).
Identification of DEmRNAs, DEmiRNAs
and DElncRNAs
The DEmRNAs, DElncRNAs and DEmiRNAs were identified
in the GEO datasets using the R package limma v3.34.9 (23). The classical Bayesian test was used
to calculate P-values. mRNAs, lncRNAs and miRNAs were considered
significantly differentially expressed if |log2 (fold
change)|≥1 and P<0.05. To visualize the DEmRNAs, DElncRNAs and
DEmiRNAs, heat maps and volcano maps were generated using the R
packages ggplot2 (24) and heatmap2
(25), respectively.
TCGA patient data retrieval
RNA sequence data and clinical information
(specifically, cisplatin treatment status and OS time) for 576
patients with LUAD were retrieved from the TCGA database
(https://www.cancer.gov/tcga; accessed on
August 29, 2017). The use of TCGA data in the present study is in
accordance with TCGA publication guidelines (https://cancergenome.nih.gov/publications/publicationguidelines).
Since the patient data used originated from the TCGA database, no
further ethical approval was required.
Identification of DEmRNAs associated
with patient survival
The expression levels of each of the identified
DEmRNAs were quantified in each patient with LUAD. For each DEmRNA,
patients were divided into a low- and a high-expression group based
on mean gene expression. Kaplan-Meier survival curves were
generated, and the DEmRNAs that were significantly associated with
OS were identified using a log-rank test. P<0.05 was considered
to indicate a statistically significant difference.
Functions and interactions of the
survival-associated DEmRNAs
The Database for Annotation, Visualization and
Integrated Discovery v.6.8 (26) was
used to identify the Gene Ontology (GO) (27) terms and the Kyoto Encyclopedia of
Genes and Genomes (KEGG) (28)
pathways significantly enriched in the survival-associated DEmRNAs
(i.e. those with P<0.05). The STRING database v10.5 (29) was used to predict protein-protein
interactions (PPIs) of the survival-associated DEmRNAs. PPI scores
≤0.15 were considered of low confidence. Cytoscape v3.6.1 (30) was used to visualize the PPI network
and to calculate node degrees.
Co-expression of DElncRNAs and
survival-associated DEmRNAs
The Pearson correlation coefficient between each
DElncRNA and each survival-associated DEmRNA was calculated.
P-values were adjusted using the false discovery rate (FDR) to
control for the effects of multiple comparisons. DElncRNAs and
survival-associated DEmRNAs were considered to be co-expressed when
|r|>0.95 and P<0.05 (FDR-adjusted). The functions of the
co-expressed DElncRNAs were predicted based on the lncRNA-mRNA
regulatory network; the R package clusterProfiler (31) was used to identify the pathways
significantly enriched in the target genes of the co-expressed
DElncRNAs. Pathways with Benjamini-Hochberg-adjusted P-values
<0.05 were considered significantly enriched.
DEmiRNA regulatory networks and KEGG
pathway enrichment
The target gene prediction module of miRWalk v2.0
(http://zmf.umm.uni-heidelberg.de/apps/zmf/mirwalk2/miRretsys-self.html)
(32) was used to identify possible
target genes of the DEmiRNAs in eight databases miRWalk (http://mirwalk.umm.uni-heidelberg.de),
Microt4 (http://mirtarbase.mbc.nctu.edu.tw/php/index.php),
MiRanda (http://www.microrna.org/microrna/home.do), miRDB
(http://www.mirdb.org/miRDB/policy.html), miRMap
(https://mirmap.ezlab.org), PITA (http://genie.weizmann.ac.il/pubs/mir07/mir07_dyn_data.html),
RNA22 (https://cm.jefferson.edu/rna22) and
Targetscan (http://www.targetscan.org/vert_71/). To increase the
reliability of the search results, only genes identified in ≥5
databases were used to construct the miRNA control network, which
was visualized with Cytoscape v3.6.1 (30). The KEGG pathway enrichment of the
predicted DEmiRNA target genes was investigated using
clusterProfiler (31).
Construction of a ceRNA regulatory
network
lncRNAs associated with the DEmiRNAs were identified
using the prediction module of DIANA-LncBase v2 (33); only lncRNAs with scores >0.75 were
included. Subsequently, a ceRNA network based on several data
sources was constructed: The lncRNA-miRNA regulatory network; the
miRNA-target mRNA regulatory network; and the DElncRNAs that were
positively co-expressed with survival-associated DEmRNAs.
Association between OS time and the
expression levels of selected lncRNA targets
Preliminary results demonstrated that the lncRNAs
HOXD-AS2, LNC01123 and FIRRE appeared in one or more ceRNA axes.
Therefore, the expression levels of these lncRNAs were quantified,
as well as those of the co-expressed lncRNAs and
survival-associated DEmRNAs, in non-LUAD tumors using the Gene
Expression Profiling Interactive Analysis (GEPIA) server (34); GEPIA analyses RNA expression in 9,736
tumors and 8,587 normal samples from the TCGA and the
Genotype-Tissue Expression projects.
Statistical analysis
The classical Bayesian test was used to test
differentially expressed mRNAs, lncRNAs and miRNAs. DEmRNAs that
were significantly associated with OS time were identified using
the log-rank test. Fisher's exact test was applied for the GO
enrichment of DEmRNAs associated with OS time. All comparisons were
between cisplatin-resistant A549-DDP cells and cisplatin-sensitive
A549 cells. P<0.05 was considered to indicate a statistically
significant difference, unless otherwise specified. The statistical
analysis was performed with R v.3.4.3 (35).
Results
DEmRNAs, DEmiRNAs and DEIncRNAs in the
A549 and A549-DDP cell lines
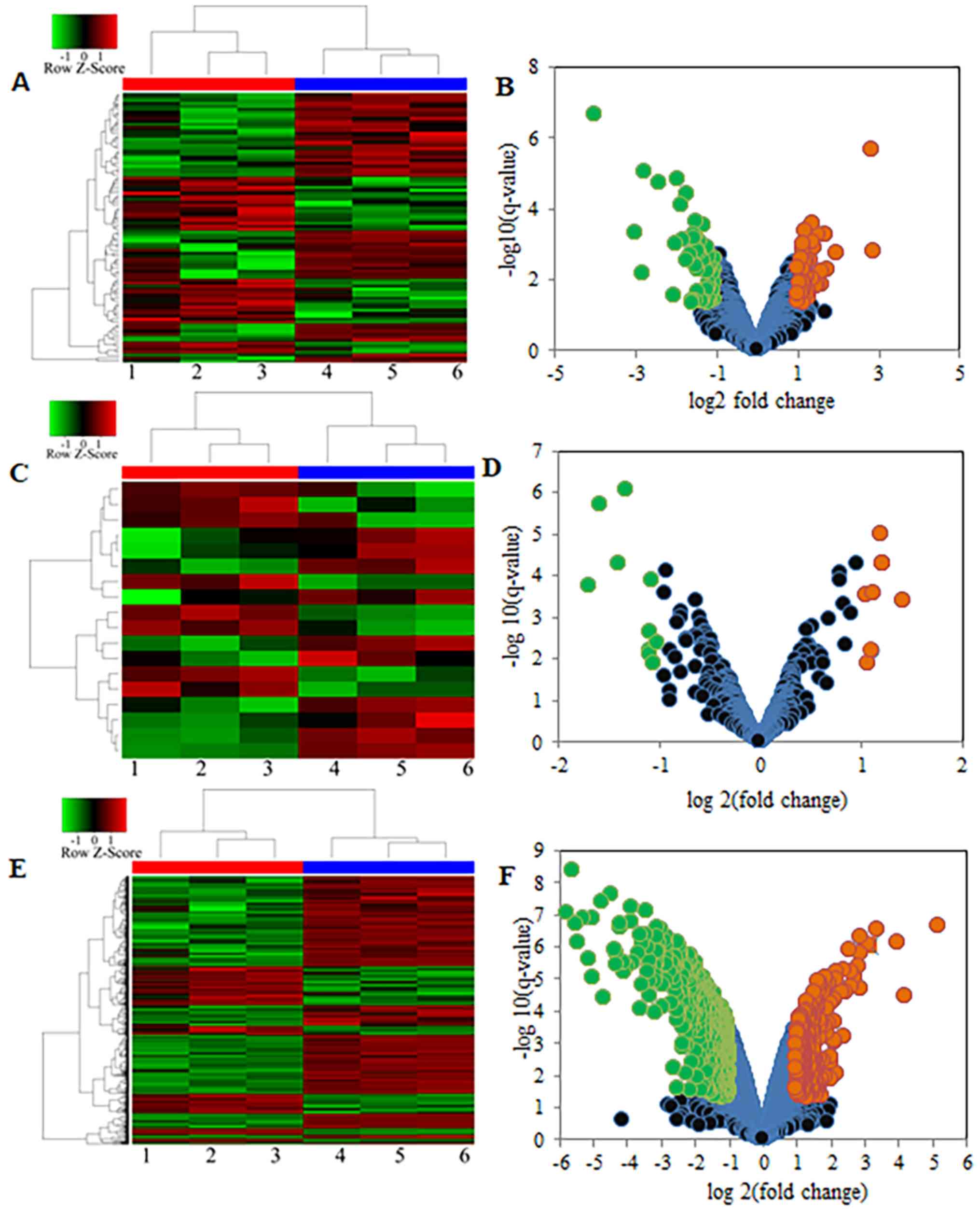
A total of 842 mRNAs were identified to be
differentially expressed between the A549 and A549-DDP cell lines.
Among these DEmRNA, 245 (29.10%) were upregulated in the A549-DDP
cell line compared with the A549 cell line, while 597 (70.90%) were
downregulated (Fig. 1). In addition,
90 DElncRNAs and 18 DEmiRNAs were identified. Among these DEmiRNAs
and DElncRNAs, 37 DElncRNAs (41.11%) and 8 DEmiRNAs (44.44%) were
upregulated in the A549-DDP cell line compared with the A549 cell
line, while 53 lncRNAs (58.89%) and 10 miRNAs (55.56%) were
downregulated (Fig. 1; Table SI).
Survival-associated DEmRNAs
In the TCGA patient dataset, 86 patients treated
with cisplatin were identified. These patients expressed 786 of the
identified DEmRNAs. Among these, 33 DEmRNAs were significantly
associated with OS time (Table I).
Five upregulated DEmRNAs were associated with low OS time: Rh
family B glycoprotein (RHBG), phosphatase orphan 2 (PHOSPHO2),
activity regulated cytoskeleton associated protein (ARC),
thioredoxin (TXN) and kinesin family member 26A (KIF26A; Fig. 2A-E; Table
SII). Four other upregulated DEmRNAs were associated with high
OS time: Zinc finger protein 417, neural cell adhesion molecule 1
(NCAM1), mediator complex subunit 12 (MED12) and ADP ribosylation
factor 4 (ARF4; Fig. 2F-G; Table SII). These nine DEmRNAs comparing
with the other 24 DEmRNAs, were more related to the prognosis of
patients.
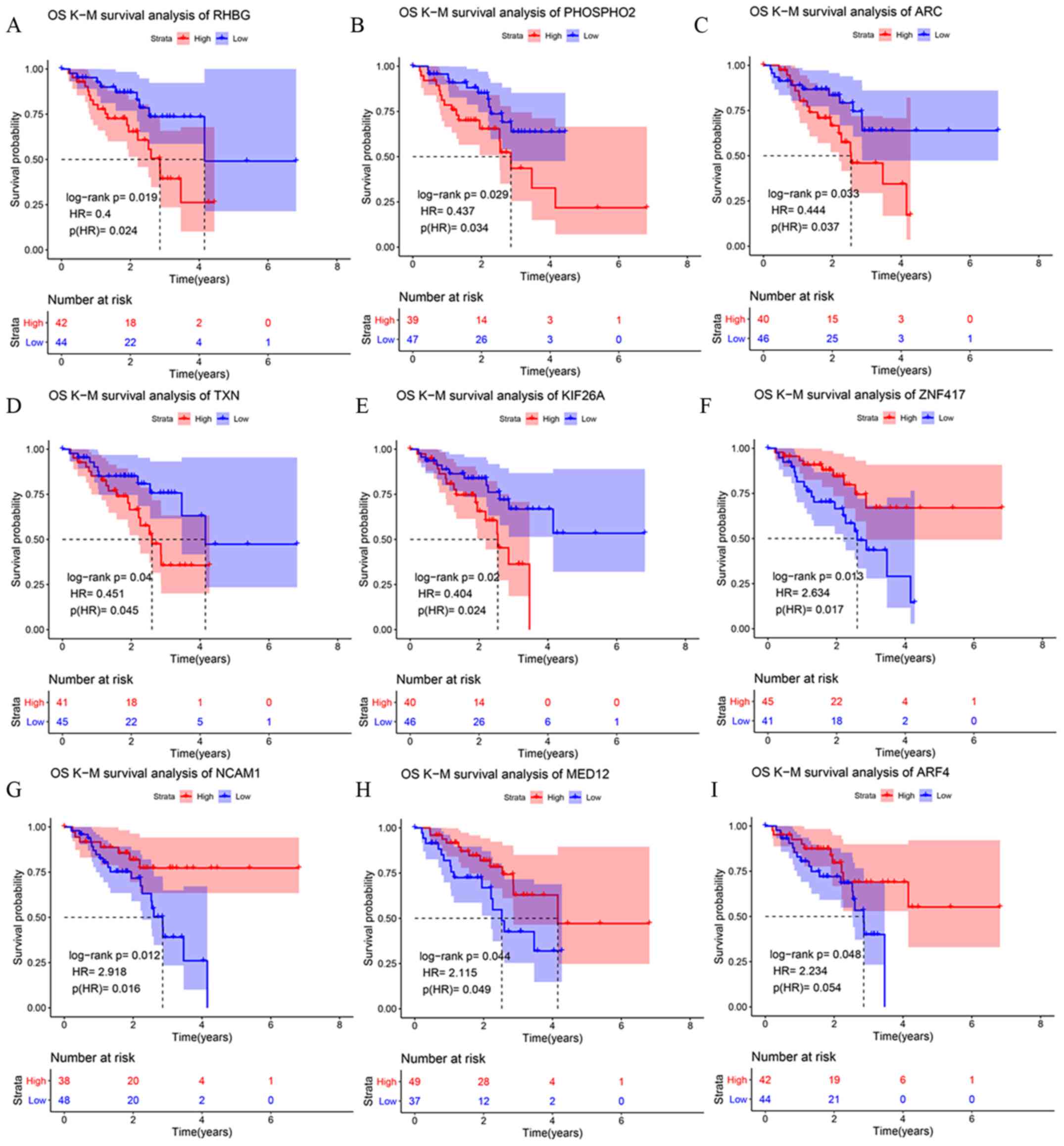 | Figure 2.Kaplan-Meier survival curves for the
six most upregulated mRNAs in the cisplatin-resistant A549-DDP cell
line compared with the cisplatin-sensitive A549 cell line. (A)
RHBG. (B) PHOSPHO2. (C) ARC. (D) TXN. (E) KIF26A. (F) ZNF417. (G)
NCAM1. (H) MED12. (I) ARF4. Red lines represent the overall
survival of the patients expressing low levels of each mRNA; blue
lines represent the overall survival of the patients expressing
high levels of each mRNA. The cut-off point for high/low levels was
the mean of gene expression. The shaded areas are the 95% CI of the
corresponding groups. RHBG, Rh family B glycoprotein; PHOSPHO2,
phosphatase, orphan 2; ARC, activity regulated
cytoskeleton-associated protein; TXN, thioredoxin; KIF26A, kinesin
family member 26A; ZNF417, zinc finger protein 417; NCAM1, neural
cell adhesion molecule 1; MED12, mediator complex subunit 12; ARF4,
ADP ribosylation factor 4. |
 | Table I.Survival-associated mRNAs
differentially expressed between cisplatin-resistant and
cisplatin-sensitive cell lines. |
Table I.
Survival-associated mRNAs
differentially expressed between cisplatin-resistant and
cisplatin-sensitive cell lines.
| Symbol | Log2
FC | Low median | High median | Description |
|---|
| MT1A | −2.63 |
| 2.55 | Metallothionein
1A |
| VGF | −2.50 | 4.15 | 2.55 | VGF nerve growth
factor inducible |
| SARM1 | −2.22 | 2.86 | 4.15 | Sterile alpha and
TIR motif containing 1 |
| DPP4 | −2.21 | 2.86 | 4.15 |
Dipeptidyl-peptidase 4 |
| SIRT4 | −1.85 | 2.87 |
| Sirtuin 4 |
| SH2B2 | −1.65 |
| 2.86 | SH2B adaptor
protein 2 |
| PER1 | −1.65 |
| 2.86 | PER1 |
| FKBP1B | −1.60 | 4.15 | 2.60 | FK506 binding
protein 1B |
| FAM117A | −1.55 | 2.86 |
| Family with
sequence similarity 117 member A |
| DIRAS3 | −1.50 |
| 2.60 | DIRAS family GTPase
3 |
| STAC3 | −1.47 |
| 2.60 | SH3 and cysteine
rich domain 3 |
| MAGEH1 | −1.42 | 2.60 |
| MAGE family member
H1 |
| RAB9B | −1.40 | 2.87 |
| RAB9B, member RAS
oncogene family |
| SLC17A9 | −1.28 | 2.86 |
| Solute carrier
family 17 member 9 |
| ADRA1D | −1.21 | 4.15 | 2.55 | Adrenoceptor alpha
1D |
| ELOVL2 | −1.19 |
| 2.87 | ELOVL fatty acid
elongase 2 |
| DBP | −1.19 | 2.87 |
| D-box binding PAR
bZIP transcription factor |
| NR1D1 | −1.10 | 2.87 |
| Nuclear receptor
subfamily 1 group D member 1 |
| HSPA2 | −1.07 | 4.15 | 2.55 | Heat shock protein
family A (Hsp70) member 2 |
| GJA1 | −1.04 | 4.15 | 2.21 | Gap junction
protein alpha 1 |
| CEACAM6 | −1.03 | 4.15 | 2.55 | Carcinoembryonic
antigen related cell adhesion molecule 6 |
| ID4 | −1.01 | 4.15 | 2.60 | Inhibitor of DNA
binding 4, HLH protein |
| NDST3 | 1.01 | 2.53 | 2.53 | N-deacetylase and
N-sulfotransferase 3 |
| ZNF417 | 1.06 | 2.60 |
| Zinc finger protein
417 |
|
PHOSPHO2 | 1.09 |
| 2.86 | Phosphatase, orphan
2 |
| ARC | 1.11 |
| 2.55 | Activity regulated
cytoskeleton associated protein |
| TXN | 1.13 | 4.15 | 2.60 | Thioredoxin |
| ARF4 | 1.15 | 2.86 |
| ADP ribosylation
factor 4 |
| RHBG | 1.27 | 4.15 | 2.86 | Rh family B
glycoprotein (gene/pseudogene) |
| EDEM1 | 1.28 | 2.86 |
| ER degradation
enhancing alpha-mannosidase like protein 1 |
| KIF26A | 1.48 |
| 2.55 | Kinesin family
member 26A |
| NCAM1 | 2.20 | 2.86 |
| Neural cell
adhesion molecule 1 |
| MED12 | 2.46 | 2.53 | 4.15 | Mediator complex
subunit 12 |
Functional enrichment and PPIs of the
survival-associated DEmRNAs
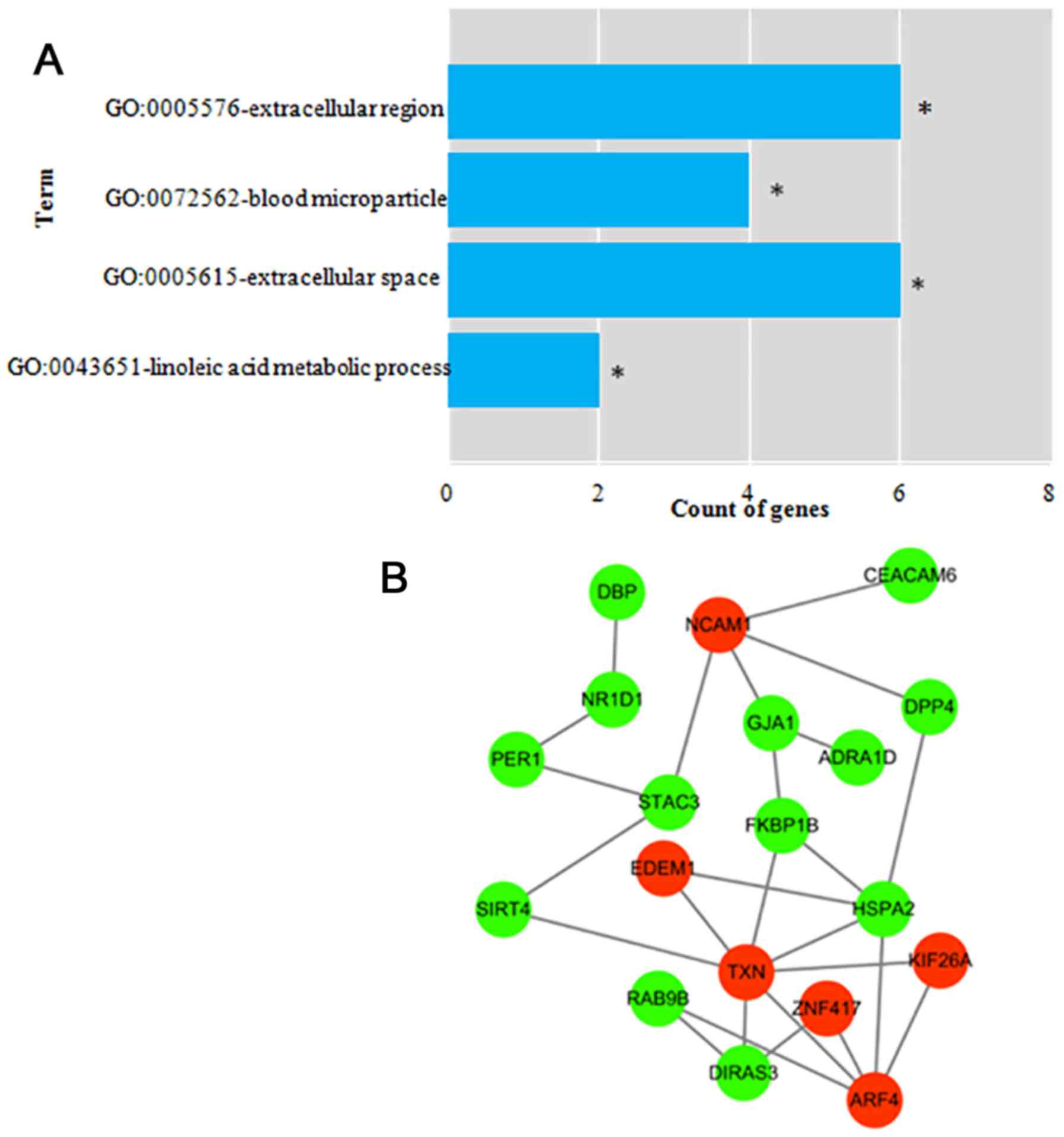
The GO terms most over-represented in the DEmRNAs
annotations were ‘extracellular region’, ‘blood microparticle’,
‘extracellular space’ and ‘linoleic acid metabolic process’
(Fig. 3A; Table SIII). No KEGG pathways enriched in
the DEmRNAs were identified (data not shown). The PPI network of
the survival-associated DEmRNAs (Fig.
3B) contained 19 nodes and 26 interaction pairs, including 6
upregulated and 13 downregulated DEmRNAs.
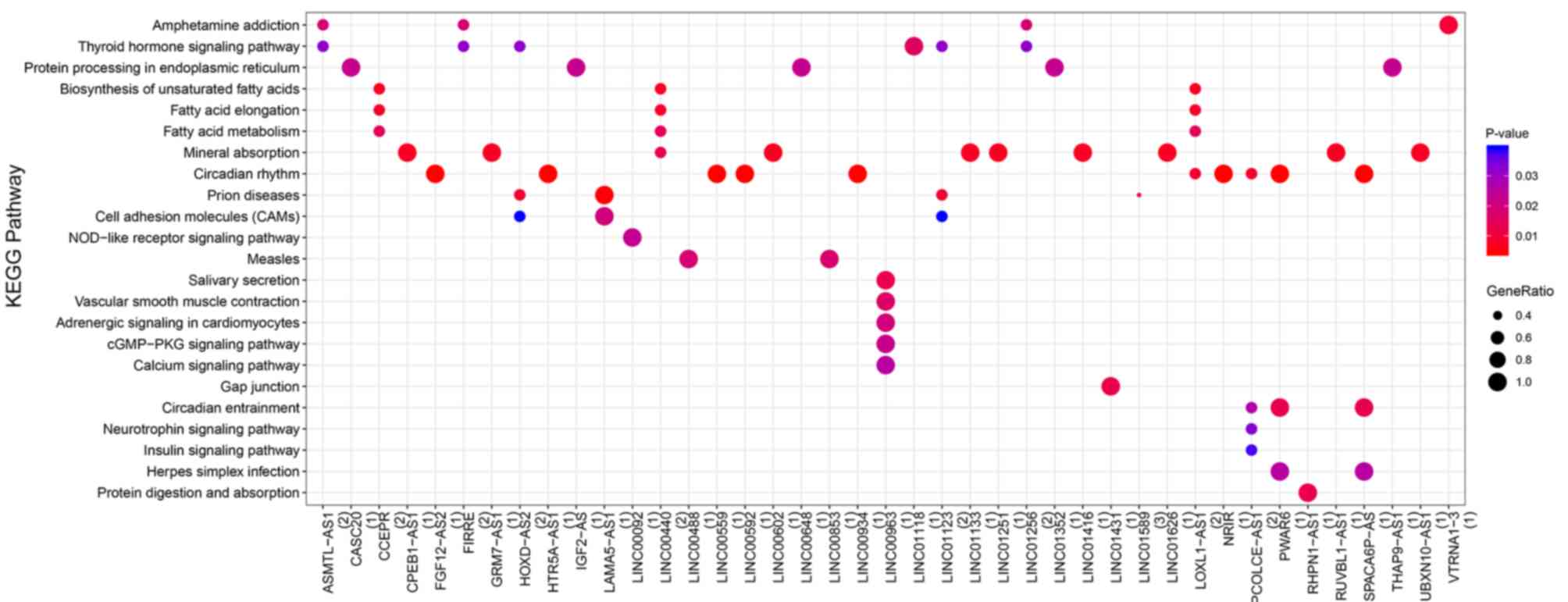
Co-expression of DElncRNAs and
survival-associated DEmRNAs
A total of 168 positively co-expressed pairs of
DElncRNAs and survival-associated DEmRNAs were identified (74
DElncRNAs and 32 DEmRNAs). According to the DElncRNA-DEmRNA
network, the target genes of the co-expressed DElncRNAs were
over-represented in three KEGG pathways: ‘Protein processing in
endoplasmic reticulum’, ‘mineral absorption’ and ‘circadian rhythm’
(Fig. 4; Table SIV).
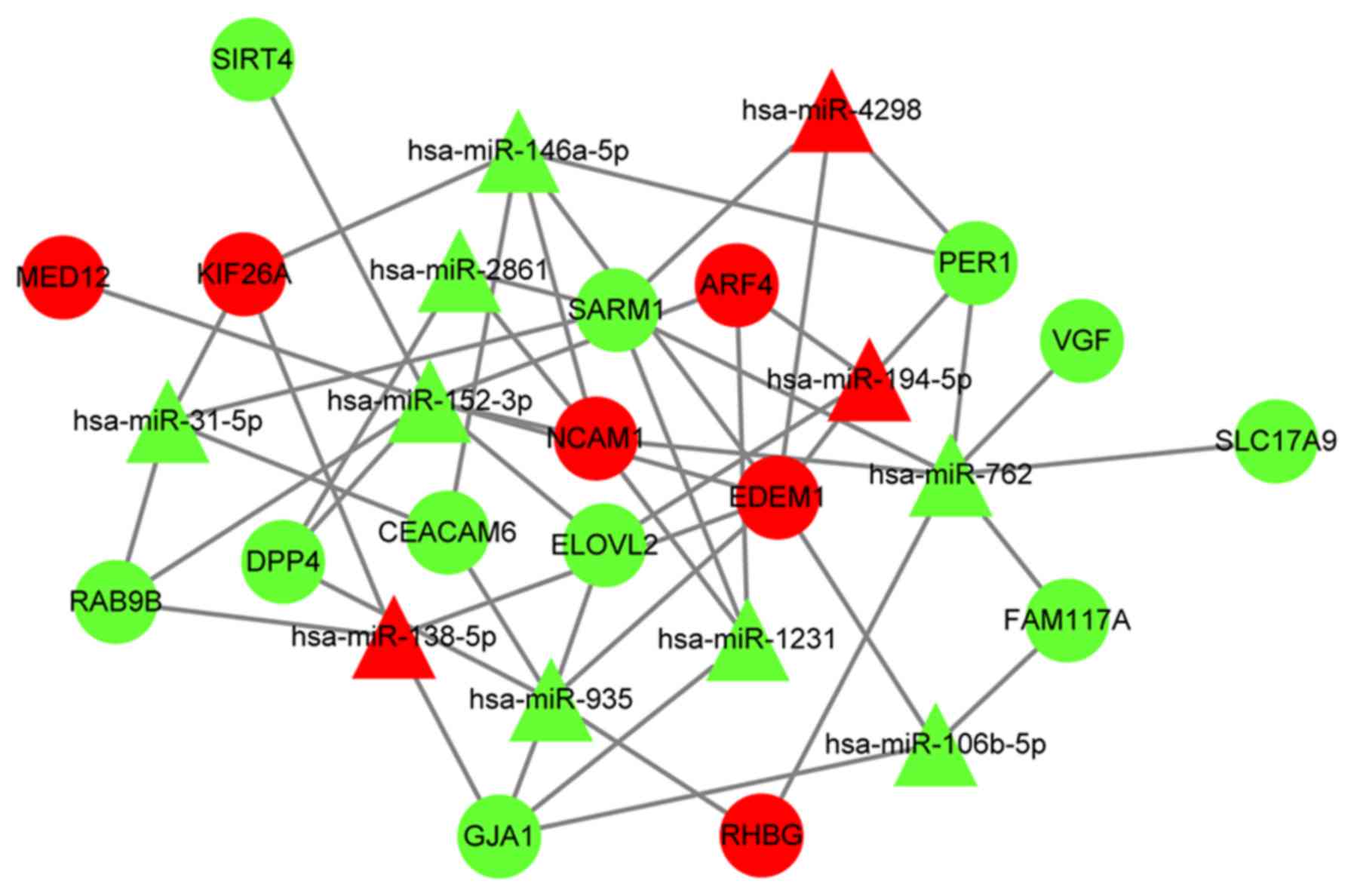
DEmiRNA target gene prediction and
functional enrichment analysis
Using miRWalk v2.0 (32), 11 DEmiRNAs targets, 17
survival-associated DEmRNA targets and 52
DEmiRNA/survival-associated DEmRNA pairs were identified in the
DEmiRNA regulatory network (Fig. 5;
Table SV). Several KEGG pathways,
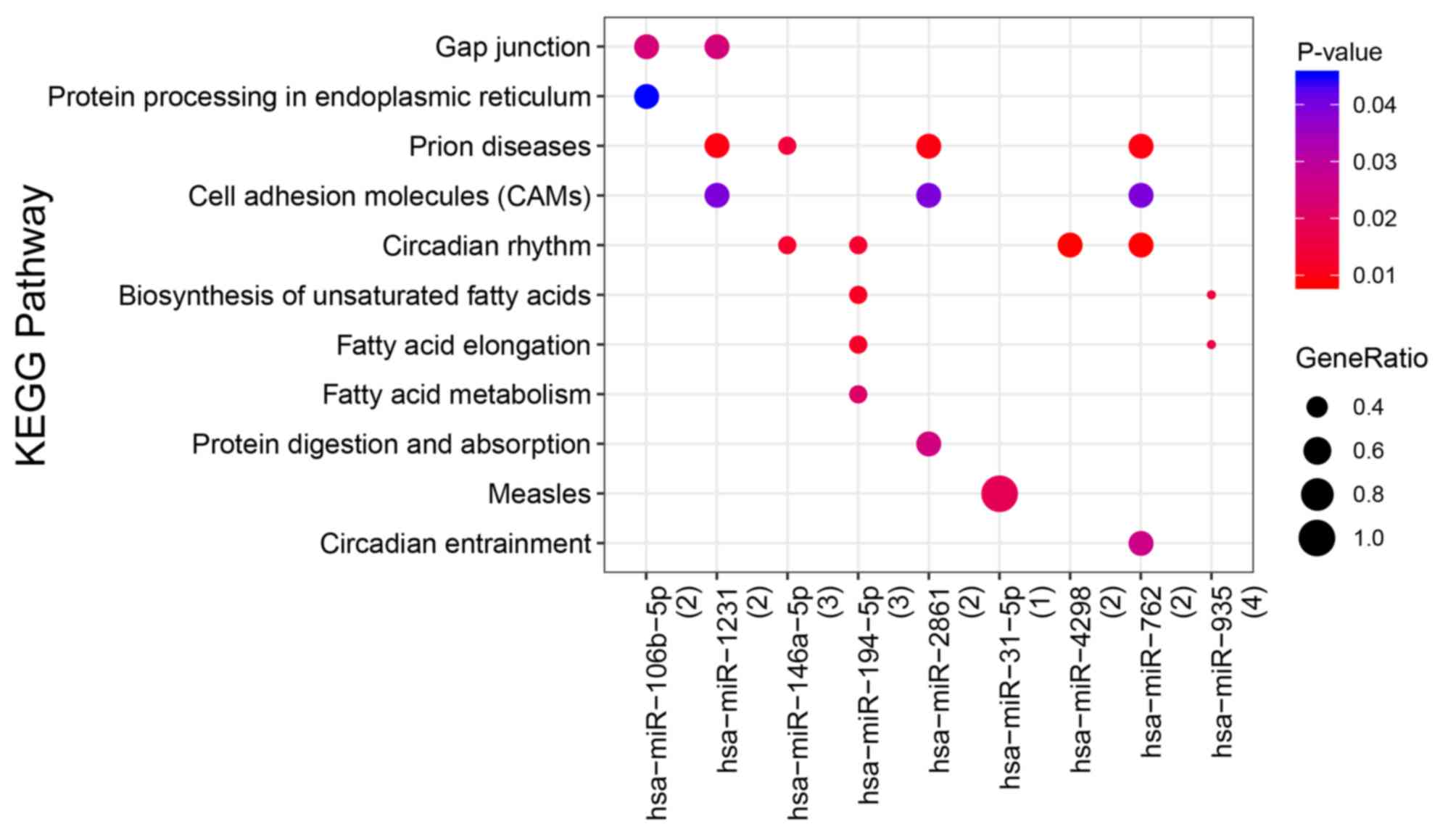
including ‘cell adhesion molecules (CAMs)’ and ‘circadian rhythm’,
were enriched in the target genes (Fig.
6; Table SVI).
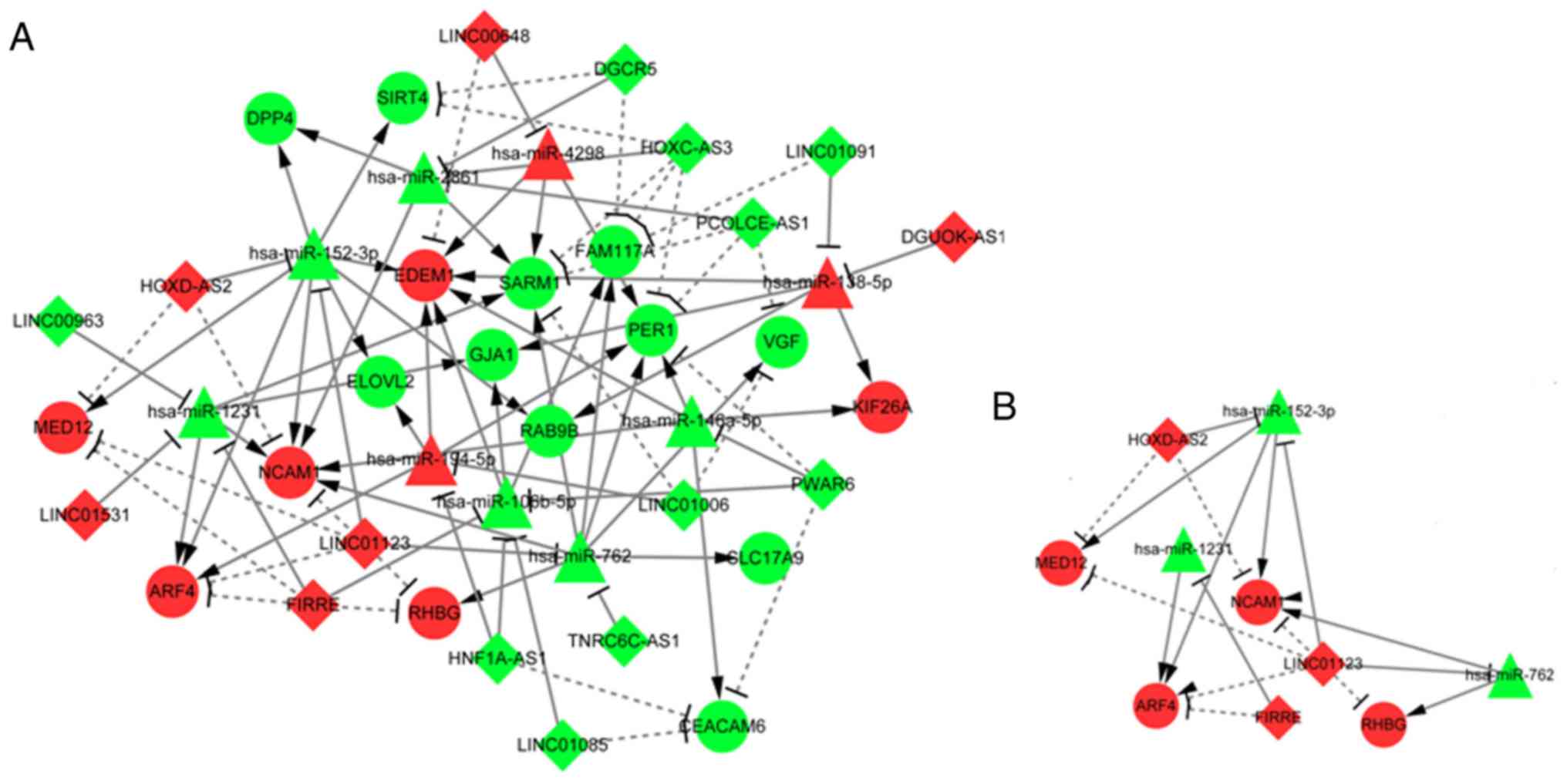
ceRNA regulatory network
Using Cytoscape (30), the DElncRNA-DEmiRNA regulatory
network was combined with the DEmiRNA-DEmRNA network to obtain a
DElncRNA-DEmiRNA-DEmRNA ceRNA network (Fig. 7A; Table
SVII). The ceRNA network included 9 DEmiRNAs, 16 DElncRNAs, 17
target DEmRNAs and 87 pairs with a regulatory association. The
present putative ceRNA network (Fig.
7B) included eight axes: HOXD-AS2/hsa-miR-152-3p/MED12,
HOXD-AS2/hsa-miR-152-3P/NCAM1, LINC01123/hsa-miR-152-3p/MED12,
LINC01123/hsa-miR-152-3P/NCAM1, LINC01123/hsa-miR-152-3P/ARF4,
LINC01123/hsa-miR-762/NCAM1, LINC01123/hsa-miR-762/RHBG and
FIRRE/hsa-miR-1231/ARF4. HOXD-AS2/has-miR-152-3p/ARF4 was not
included in the axes list as HOXD-AS2/hsa-miR-152-3p/ARF4 didn't
form a triangle and there was no line to connect HOXD-AS2 and ARF4
(Fig. 7B). The putative ceRNA axes
included three lncRNAs (HOXD-AS2, LINC01123 and FIRRE), three
miRNAs (hsa-miR-152-3p, hsa-miR-762 and hsa-miR-1231) and four
genes (MED12, RHBG, NCAM1 and ARF4).
Association between OS time and the
expression of selected lncRNA targets
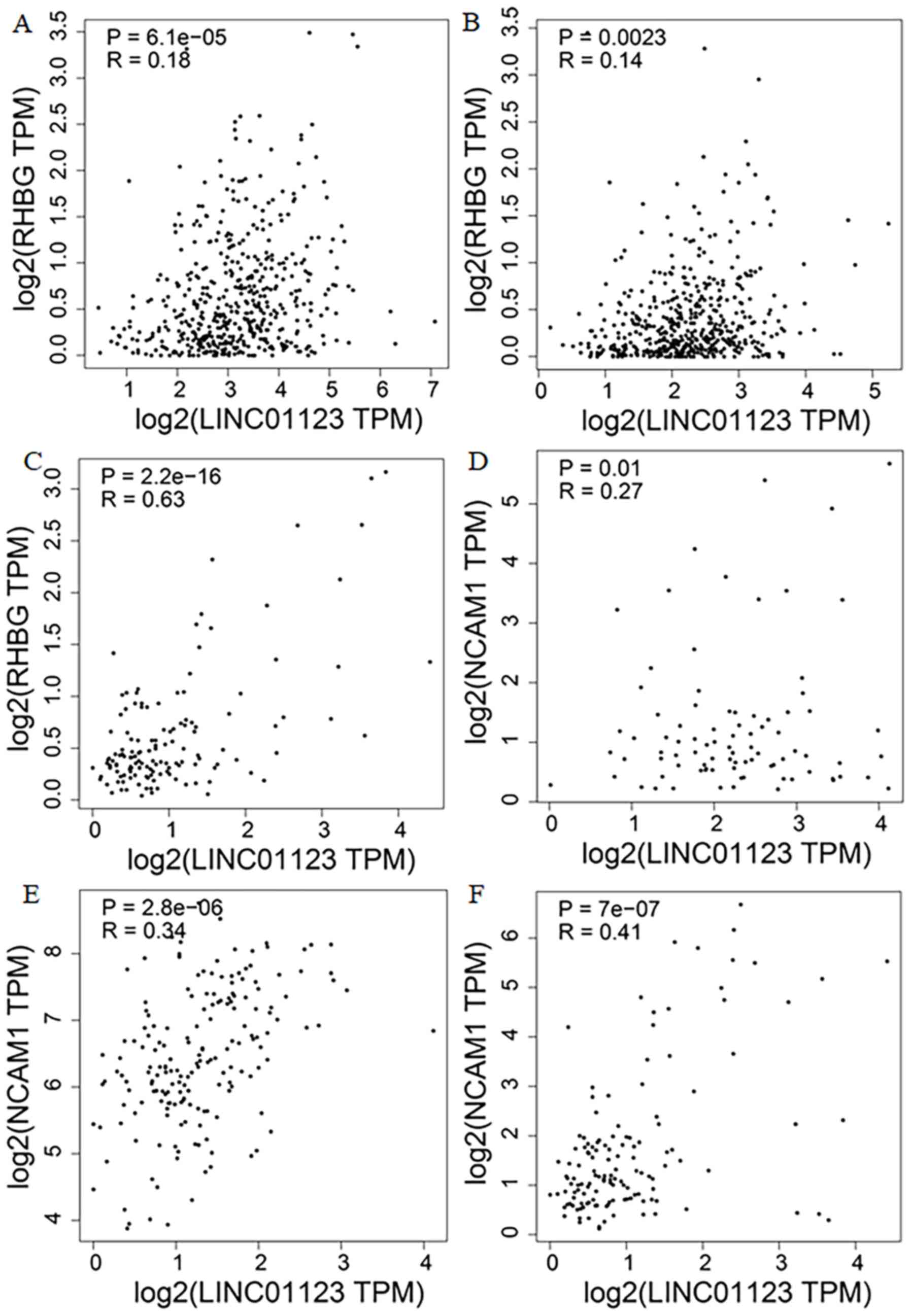
The GEPIA analysis identified RHBG and NCAM1 as
co-expressed genes of HOXD-AS2 and LNC01123, respectively. LNC1123
was positively co-expressed with RHBG in LUAD (R=0.18; Fig. 8A), lung squamous cell carcinoma
(LUSC; R=0.14; Fig. 8B) and
testicular germ cell tumors (TGCT; R=0.63; Fig. 8C). LNC1123 was positively
co-expressed with NCAM1 in mesothelioma (MESO; R=0.27; Fig. 8D), pheochromocytoma and paraganglioma
(PCPG; R=0.34; Fig. 8E), and TGCT
(R=0.41; Fig. 8F). In addition,
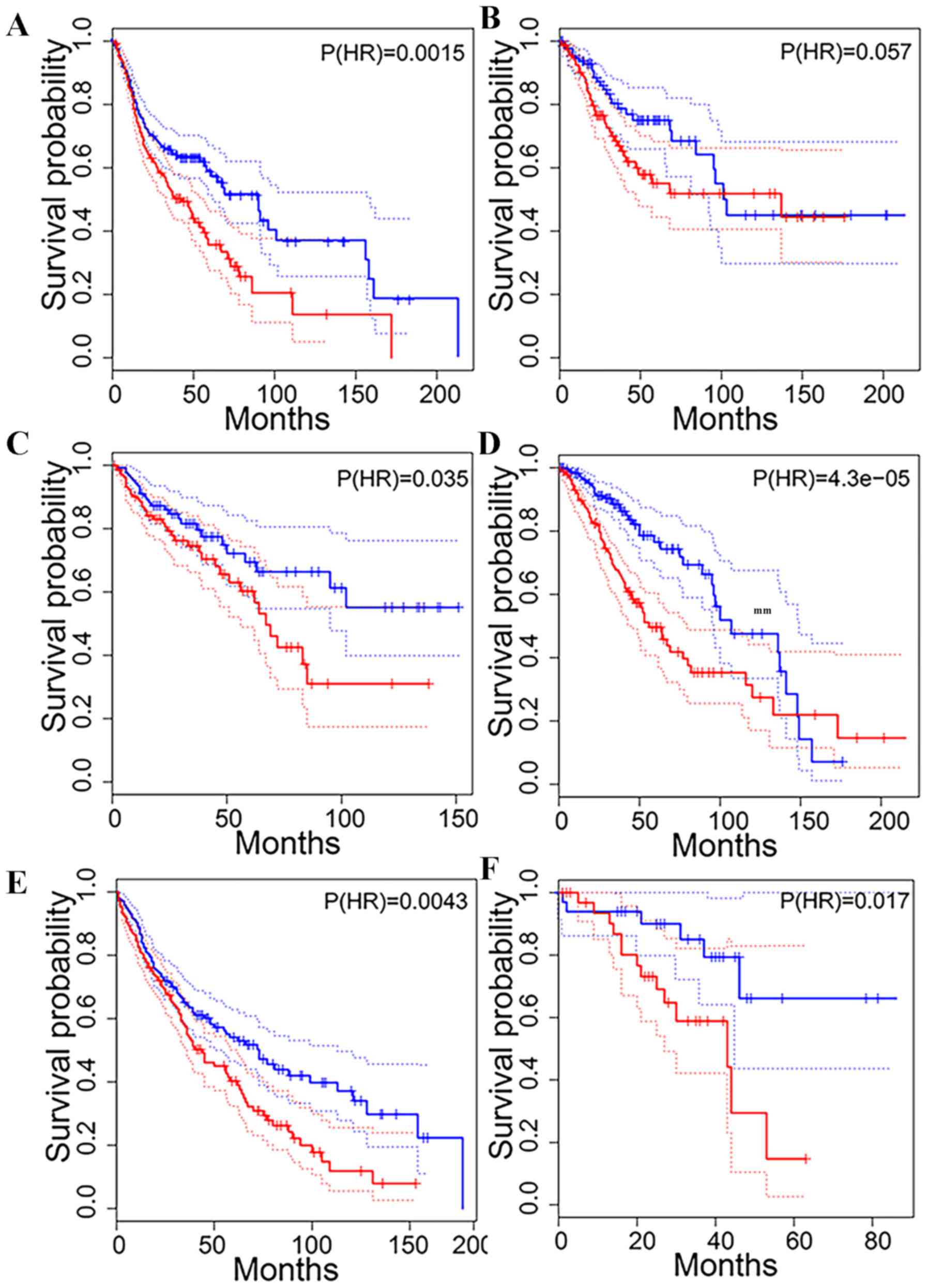
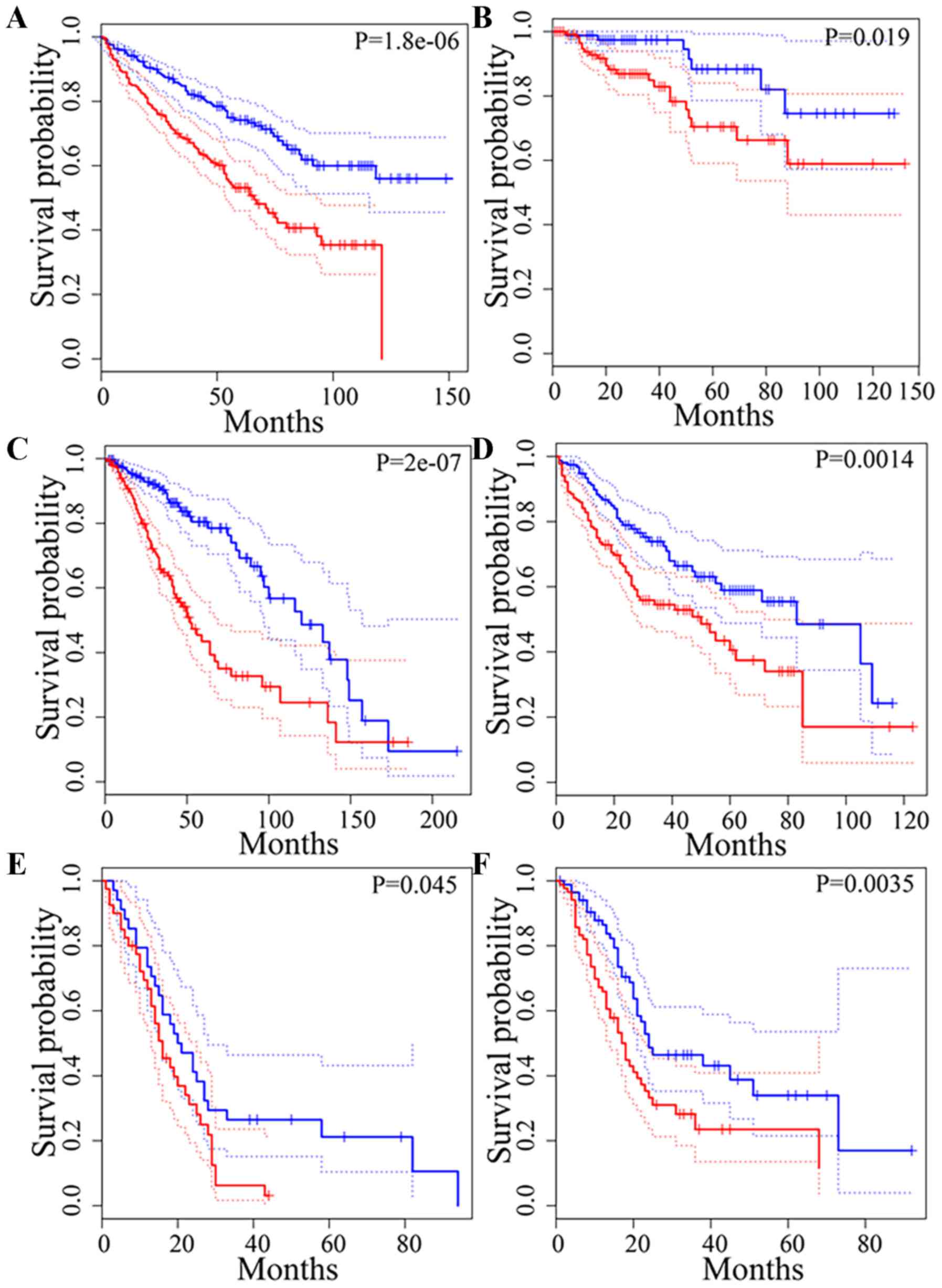
LNC1123 upregulation was associated with shorter patient OS time in
head and neck squamous cell carcinoma (HNSCC; Fig. 9A), and in cervical squamous cell
carcinoma and endocervical adenocarcinoma (P=0.057; Fig. 9B).
HOXD-AS2 upregulation was associated with shorter
patient OS time in colon adenocarcinoma (COAD; Fig. 9C), brain lower-grade glioma (LGG;
Fig. 9D), LUSC (Fig. 9E) and uveal melanoma (Fig. 9F). HOXD-AS2 was positively
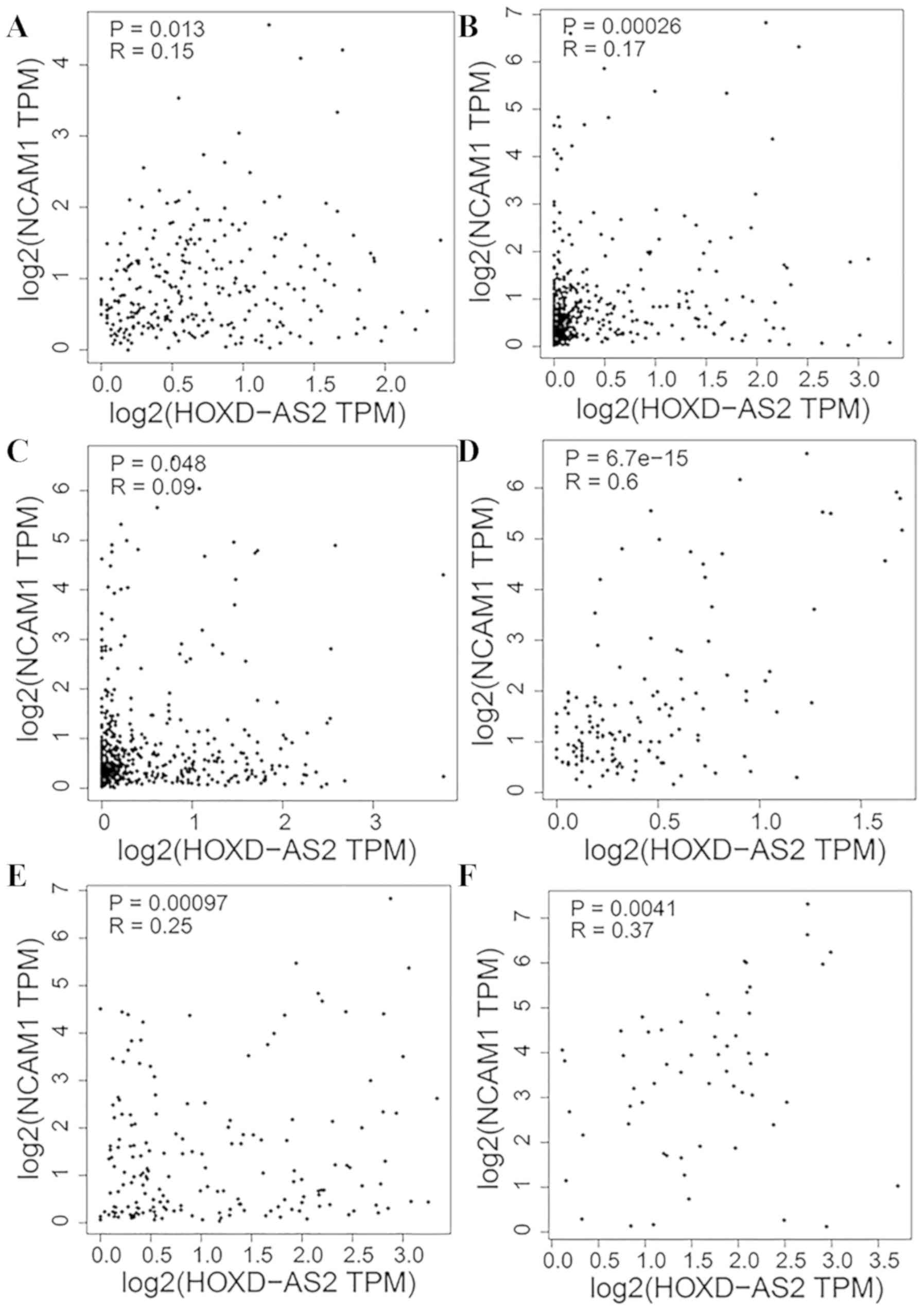
co-expressed with NCAM1 in COAD (R=0.15; Fig. 10A), LUAD (R=0.17; Fig. 10B), LUSC (R=0.09; Fig. 10C), TGCT (R=0.6; Fig. 10D), uterine corpus endometrial
carcinoma (R=0.25; Fig. 10E) and
uterine carcinosarcoma (R=0.37; Fig.
10F). No positive associations were identified between HOXD-AS2
and RHBG (data not shown).
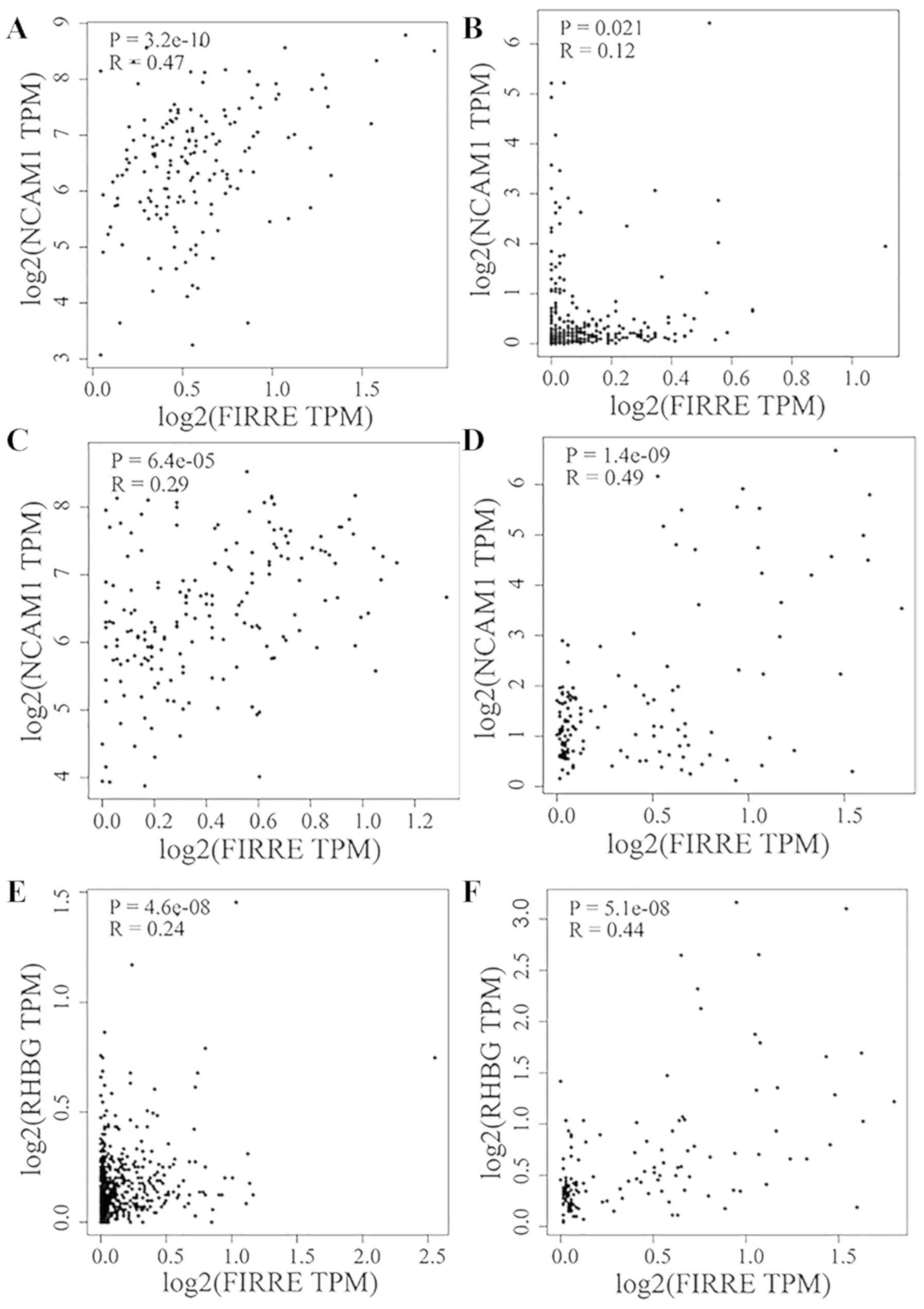
FIRRE was positively co-expressed with NCAM1 in
glioblastoma multiforme (R=0.47; Fig.
11A), liver hepatocellular carcinoma (LIHC; R=0.12; Fig. 11B), PCPG (R=0.29; Fig. 11C) and TGCT (R=0.49; Fig. 11D). FIRRE was positively
co-expressed with RHBG in prostate adenocarcinoma (R=0.24; Fig. 11E) and TGCT (R=0.44; Fig. 11F). In addition, FIRRE upregulation
was associated with shorter patient OS time in kidney renal clear
cell carcinoma (Fig. 12A), kidney
renal papillary cell carcinoma (Fig.
12B), LGG (Fig. 12C), LIHC
(Fig. 12D), MESO (Fig. 12E) and pancreatic adenocarcinoma
(Fig. 12F).
Discussion
The present analysis of gene expression patterns
(based on the GEO datasets) identified 33 genes differentially
expressed in cisplatin-resistant A549-DDP cells compared with in
cisplatin-sensitive A549 cells. Among these, nine were upregulated
in the cisplatin-resistant cells and 24 were downregulated. By
cross-referencing these results with patient data from the TCGA
dataset, five of these upregulated genes (PHOSPHO2, ARC, TXN,
RHBG and KIF26A) were identified to be associated with
poor OS time outcomes. These five genes may be useful potential
targets for the reversal of cisplatin resistance in LUAD.
RHBG was identified as being of particular
interest, as this gene also appeared in one of the axes of the
putative ceRNA network generated in the present study. RHBG is a
non-erythroid membrane glycoprotein of the Rh antigen family, and
the mechanisms regulating RHBG expression remain poorly studied.
Consistent with the results of the present study, RHBG has been
demonstrated to be expressed in LIHC and COAD cell lines (36). Additionally, RHBG has been implicated
in the growth of brain tumors in mice (37). RHBG deserves further study both as a
possible maker of poor LUAD outcomes and as a potential target for
cisplatin-resistance reversal therapy.
Furthermore, NCAM1, MED12 and ARF4
appeared in one or more ceRNA network axes, but increased
expression levels of these genes were associated with improved OS
time. NCAM1 encodes a cellular adhesion protein and is a
well-known potential target of antibody-based cancer
immunotherapies (38). In addition,
NCAM1 has been identified as an immunohistochemical marker for lung
neuroendocrine tumors (39), and it
was recently proposed that the NCAM1-180 splice variant might be a
useful marker for NSCLC (40).
Furthermore, NCAM1 may be a useful biomarker and therapeutic target
for acute myeloid leukemia (41),
the follicular variant of papillary thyroid carcinoma (42) and breast cancer (43). Although NCAM1 has been associated
with cisplatin resistance in ovarian cancer (44,45), the
in vitro expression of NCAM improved the response of
multiple myeloma cells to Bortezomib (Btz) treatment (46). Consistent with this, the present
study revealed that NCAM upregulation was associated with improved
patient OS time outcomes.
MED12 is a component of the CDK8 subcomplex. MED12
mutations are associated with tumorigenesis (47). Indeed, somatic mutations in MED12
exon 2 have been observed in uterine leiomyosarcoma, colorectal
cancer (CRC) (47), uterine
leiomyoma, breast fibroadenoma, phyllodes tumors and prostate
cancer (48). Additionally,
inhibition of MED12 expression has been associated with resistance
to cisplatin and other chemotherapy drugs (49,50).
This is consistent with the results of the present study, in which
patients with high levels of MED12 had improved OS time.
ARF4 is a small guanine-binding protein that serves
a role in vesicular trafficking (51). Although the results of the present
study suggested that ARF4 upregulation was associated with
improved patient outcomes, it has been previously reported that
high expression levels of ARF4 in patients with breast
cancer are significantly associated with increased risk of distant
metastasis and shorter OS time. Conversely, ARF4 silencing
reduces the colonization of the lung by metastatic breast cancer
cells in vivo (51). These
contradictory results suggest that the role of ARF4 in LUAD
deserves further investigation.
The present putative ceRNA network included three
miRNAs (hsa-miR-152-3p, hsa-miR-762 and hsa-miR-1231) across the
eight axes. In several types of cancer (including prostate, ovarian
and breast), miR-152 expression has been shown to reduce tumor cell
viability and proliferation (52–54). In
addition, the suppression of miR-152 biogenesis increases cisplatin
resistance in epithelial ovarian cancer (55). However, the overexpression of miR-152
increases cisplatin resistance and proliferation of nasopharyngeal
carcinoma cells (56), while the
overexpression of miR-762 stimulates the development of various
tumors, including ovarian (57) and
breast cancer (58). Conversely, the
expression of miR-762 (in combination with other miRNAs) leads to
the apoptosis of breast cancer cells (59). In the present study, miR-152 and
miR-762 were downregulated in the cisplatin-resistant LUAD cells.
Overall, these results suggested that the behavior of these miRNAs
may vary in different types of cancer.
By contrast, miR-1231 expression consistently
negatively regulates the progression of various types of cancer,
including glioma (60,61), pancreatic cancer (62) and papillary thyroid cancer (63). Additionally, miR-1231 has been
identified as an independent prognostic factor; low expression of
miR-1231 is associated with worse patient outcomes compared with
high expression of miR-1231 in glioma and pancreatic cancer
(60–62). Consistent with these results, the
present study revealed that miR-1231 upregulation was associated
with improved patient OS time and cisplatin sensitivity.
The present putative ceRNA axes included three
lncRNAs (HOXD-AS2, LINC01123 and FIRRE). Each of these three
lncRNAs has been shown to be upregulated in one or more types of
cancer, and each one is commonly associated with poor patient
prognosis. For example, LINC01123 is upregulated in intrahepatic
cholangiocarcinomas (64) and is
associated with poor prognosis in prostate cancer (65). Similarly, HOXD-AS2 is upregulated in
glioma cells and is associated with poor prognosis (66). Consistent with these previous
studies, the present study revealed that LINC01123 and HOXD-AS2
were upregulated in numerous types of cancer and were associated
with reduced patient OS time. Importantly, the
HOXD-AS2/hsa-miR-152-3P/NCAM1 and LINC01123/hsa-miR-762/RHBG axes
in the present putative ceRNA network were supported by the
co-expression results, which showed that LINC01123 was co-expressed
with RHBG and that HOXD-AS2 was co-expressed with NCAM1.
FIRRE upregulation is associated with poor OS time
in diffuse large B-cell lymphoma, CRC and HNSCC (67). However, FIRRE upregulation is also
associated with significantly improved OS time in CRC (68). The present study revealed that FIRRE
was upregulated in numerous types of cancer, possibly indicating
that this lncRNA behaves differently under different
circumstances.
In combination with the aforementioned results, the
analyses of the current study revealed that the mRNAs, miRNAs and
lncRNAs that form the potential axes in the present putative ceRNA
network serve various important roles in cancer pathogenesis and
progression. Importantly, a number of these molecules may serve
different roles in different types of cancer. Thus, the results of
the present study suggest these molecules as important targets for
future studies focused on cancer diagnosis, prognosis and therapy.
NCAM1 and miR-152 are particularly intriguing targets with respect
to cisplatin resistance, as NCAM1 increases Btz sensitivity and
miR-152 reduces cisplatin-induced effects (46). However, further investigations are
necessary to determine the ceRNA mechanisms underlying cisplatin
resistance in LUAD.
In addition, the present study presents some
limitations, such as that the TCGA dataset included relatively few
patients that met the set criteria and that the available clinical
survival data was restricted to OS time. Future studies should
recruit patients with lung cancer for cisplatin chemotherapy,
collect lung lesion biopsy samples from patients with disease
progression after three cycles of chemotherapy and then quantify
the expression levels of the candidate lncRNAs (HOXD-AS2 and
LINC01123), miRNAs (hsa-miR-152-3p and hsa-miR-762) and mRNAs
(NCAM1, MED12 and ARF4) identified herein in the biopsy samples.
Additionally, patients should be followed-up at 3 months, 6 months,
1 year and 3 years after chemotherapy to determine survival
rates.
Despite these limitations, the results of the
present study suggested that the integration of cell line
experimental data with clinical information may be a valuable
method to identify key cancer genes and potentially useful research
targets.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present work was supported by grants from the
Affiliated Hospital of Youjiang Medical University for
Nationalities Outstanding Scholar Funding (Baise, Guangxi, China;
grant no. R20196313) and Doctorate Awarding Unit Funding of the
Affiliated Hospital of Youjiang Medical University for
Nationalities [grant no. (2019)48]. The funders had no role in the
design of the study, the collection, analyses or interpretation of
data, the writing of the manuscript or the decision to publish the
results.
Availability of data and materials
The data and materials used and/or analyzed during
the current study are available from the corresponding author on
reasonable request.
Authors' contributions
YL was involved in design of the study, analysis and
interpretation of data and drafting the manuscript. BY gave final
approval of the version of the manuscript to be published and was
involved in data analysis. SH revised the manuscript critically for
important intellectual content and was involved in the acquisition
and analysis of data. ZW made substantial contributions to
conception and design. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Molina JR, Yang P, Cassivi SD, Schild SE
and Adjei AA: Non-Small cell lung cancer: Epidemiology, risk
factors, treatment, and survivorship. Mayo Clin Proc. 83:584–594.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu L, Wu S, Yang Y, Cai J, Zhu X, Wu J,
Wu J, Li M and Guan H: SOSTDC1 is down-regulated in non-small cell
lung cancer and contributes to cancer cell proliferation. Cell
Biosci. 6:242016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li X, Shi Y, Yin Z, Xue X and Zhou B: An
eight-miRNA signature as a potential biomarker for predicting
survival in lung adenocarcinoma. J Transl Med. 12:1592014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Riaz SP, Lüchtenborg M, Coupland VH,
Spicer J, Peake MD and Møller H: Trends in incidence of small cell
lung cancer and all lung cancer. Lung Cancer. 75:280–284. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Murray N and Turrisi AT III: A review of
first-line treatment for small-cell lung cancer. J Thorac Oncol.
1:270–278. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sarvi S, Mackinnon AC, Avlonitis N,
Bradley M, Rintoul RC, Rassl DM, Wang W, Forbes SJ, Gregory CD and
Sethi T: CD133+ cancer stem-like cells in small cell lung cancer
are highly tumorigenic and chemoresistant but sensitive to a novel
neuropeptide antagonist. Cancer Res. 74:1554–1565. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Voigt W, Dietrich A and Schmoll HJ:
Overview of development status and clinical action. Cisplatin and
its analogues. Pharm Unserer Zeit. 35:134–143. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Silver DP, Richardson AL, Eklund AC, Wang
ZC, Szallasi Z, Li Q, Juul N, Leong CO, Calogrias D, Buraimoh A, et
al: Efficacy of neoadjuvant cisplatin in triple-negative breast
cancer. J Clin Oncol. 28:1145–1153. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Santabarbara G, Maione P, Rossi A and
Gridelli C: Pharmacotherapeutic options for treating adverse
effects of cisplatin chemotherapy. Expert Opin Pharmacother.
17:561–570. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yimit A, Adebali O, Sancar A and Jiang Y:
Differential damage and repair of DNA-adducts induced by
anti-cancer drug cisplatin across mouse organs. Nat Commun.
10:3092019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Eastman A: Improving anticancer drug
development begins with cell culture: Misinformation perpetrated by
the misuse of cytotoxicity assays. Oncotarget. 8:8854–8866. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao X, Li X, Zhou L, Ni J, Yan W, Ma R,
Wu J, Feng J and Chen P: LncRNA HOXA11-AS drives cisplatin
resistance of human LUAD cells via modulating miR-454-3p/Stat3.
Cancer Sci. 109:3068–3079. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Edgar R, Domrachev M and Lash AE: Gene
expression omnibus: NCBI gene expression and hybridization array
data repository. Nucleic Acids Res. 30:207–210. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Barrett T, Troup DB, Wilhite SE, Ledoux P,
Rudnev D, Evangelista C, Kim IF, Soboleva A, Tomashevsky M and
Edgar R: NCBI GEO: Mining tens of millions of expression
profiles-database and tools update. Nucleic Acids Res.
35:D760–D765. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang Y, Li H, Liu J and Wang J: Noncoding
RNA expression profiling of cisplatin-resistant cells derived from
the A549 lung cell line (miRNA). NCBI. 2013, https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE43249January
2–2013
|
|
18
|
Yang Y, Li H, Liu J and Wang J: Noncoding
RNA expression profiling of cisplatin-resistant cells derived from
the A549 lung cell line (lncRNA and mRNA). NCBI. 2013, https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE43493January
14–2013
|
|
19
|
Gautier L, Cope L, Bolstad BM and Irizarry
RA: Affy-Analysis of affymetrix genechip data at the probe level.
Bioinformatics. 20:307–315. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bolstad BM, Irizarry RA, Astrand M and
Speed TP: A comparison of normalization methods for high density
oligonucleotide array data based on variance and bias.
Bioinformatic. 19:185–193. 2003. View Article : Google Scholar
|
|
21
|
Irizarry RA, Hobbs B, Collin F,
Beazer-Barclay YD, Antonellis KJ, Scherf U and Speed TP:
Exploration, normalization, and summaries of high density
oligonucleotide array probe level data. Biostatistics. 4:249–264.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Guidelines of HUGO Gene Nomenclature
Committee (HGNC). European Bioinformatics Institute (EMBL-EBI).
https://www.genenames.org/about/guidelines/
|
|
23
|
Ritchie ME, Phipson B, Wu D, Hu Y, Law CW,
Shi W and Smyth GK: Limma powers differential expression analyses
for RNA-sequencing and microarray studies. Nucleic Acids Res.
43:e472015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wickham H: ggplot2: Elegant graphics for
data analysis. New York. Springer–Verlag. 2016.
|
|
25
|
Zhao S, Yin L, Guo Y, Sheng Q and Shyr Y:
heatmap3: An Improved Heatmap Package. R package version 1.1.6.
https://CRAN.R-project.org/package=heatmap3December
1–2018
|
|
26
|
Huang DW, Sherman BT and Lempicki RA:
Systematic and integrative analysis of large gene lists using DAVID
bioinformatics resources. Nat Protoc. 4:44–57. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ashburner M, Ball CA, Blake JA, Botstein
D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS and Eppig
JT: Gene ontology: Tool for the unification of biology. The gene
ontology consortium. Nat Genet. 25:25–29. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kanehisa M: KEGG: Kyoto encyclopedia of
genes and genomes. Nucleic Acids Res. 28:27–30. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Szklarczyk D, Franceschini A, Wyder S,
Forslund K, Heller D, Huerta-Cepas J, Simonovic M, Roth A, Santos
A, Tsafou KP, et al: STRING v10: Protein-protein interaction
networks, integrated over the tree of life. Nucleic Acids Res.
43:D447–D452. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shannon P, Markiel A, Ozier O, Baliga NS,
Wang JT, Ramage D, Amin N, Schwikowski B and Ideker T: Cytoscape: A
software environment for integrated models of biomolecular
interaction networks. Genome Res. 13:2498–2504. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yu G, Wang LG, Han Y and He QY:
ClusterProfiler: An R package for comparing biological themes among
gene clusters. OMICS. 16:284–287. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dweep H and Gretz N: MiRWalk2.0: A
comprehensive atlas of microRNA-target interactions. Nat Methods.
12:6972015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Paraskevopoulou MD, Vlachos IS, Karagkouni
D, Georgakilas G, Kanellos I, Vergoulis T, Zagganas K, Tsanakas P,
Floros E, Dalamagas T, et al: DIANA-LncBase v2: Indexing microRNA
targets on non-coding transcripts. Nucleic Acids Res. 44:D231–D238.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tang Z, Li C, Kang B, Gao G, Li C and
Zhang Z: GEPIA: A web server for cancer and normal gene expression
profiling and interactive analyses. Nucleic Acids Res. 45:W98–W102.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liao Y, Xie B, Zhang H, He Q, Guo L,
Subramaniapillai M, Fan B, Lu C and Mclntyer RS: Efficacy of
omega-3 PUFAs in depression: A meta-analysis. Transl Psychiatr.
9:1902019. View Article : Google Scholar
|
|
36
|
Merhi A, De Mees C, Abdo R, Victoria
Alberola J and Marini AM: Wnt/β-Catenin signaling regulates the
expression of the ammonium permease gene RHBG in human cancer
cells. PLoS One. 10:e01286832015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Johansson FK, Brodd J, Eklof C, Ferletta
M, Hesselager G, Tiger CF, Uhrbom L and Westermark B:
Identification of candidate cancer-causing genes in mouse brain
tumors by retroviral tagging. Proc Natl Acad Sci USA.
101:11334–11337. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jensen M and Berthold F: Targeting the
neural cell adhesion molecule in cancer. Cancer Lett. 258:9–21.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kashiwagi K, Ishii J, Sakaeda M, Arimasu
Y, Shimoyamada H, Sato H, Sato H, Miyata C, Kamma H, Aoki I and
Yazawa T: Differences of molecular expression mechanisms among
neural cell adhesion molecule 1, synaptophysin, and chromogranin A
in lung cancer cells. Pathol Int. 62:232–245. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Vander Borght A, Duysinx M, Broers JLV,
Ummelen M, Falkenberg FW, Hahnel C and van der Zeijst BAM: The 180
splice variant of NCAM-containing exon 18-is specifically expressed
in small cell lung cancer cells. Transl Lung Cancer Res. 7:376–388.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sasca D, Szybinski J, Schüler A, Shah V,
Heidelberger J, Haehnel PS, Dolnik A, Kriege O, Fehr EM, Gebhardt
WH, et al: NCAM1 (CD56) promotes leukemogenesis and confers drug
resistance in AML. Blood. 133:2305–2319. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cho H, Kim JY and Oh YL: Diagnostic value
of HBME-1, CK19, galectin 3, and CD56 in the subtypes of follicular
variant of papillary thyroid carcinoma. Pathol Int. 68:605–613.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ghaderi F, Ahmadvand S, Ramezan A,
Montazer M and Ghaderi A: Production and characterization of
monoclonal antibody against a triple negative breast cancer cell
line. Biochem Biophys Res Commun. 505:181–186. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhao X, Tang DY, Zuo X, Zhang TD and Wang
C: Identification of lncRNA-miRNA-mRNA regulatory network
associated with epithelial ovarian cancer cisplatin-resistant. J
Cell Physiol. 234:19886–19894. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Fang L, Wang H and Li P: Systematic
analysis reveals a lncRNA-mRNA co-expression network associated
with platinum resistance in high-grade serous ovarian cancer.
Invest New Drugs. 36:187–194. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yoshida T, Ri M, Kinoshita S, Narita T,
Totani H, Ashour R, Ito A, Kusumoto S, Ishida T, Komatsu H and Iida
S: Low expression of neural cell adhesion molecule, CD56, is
associated with low efficacy of bortezomib plus dexamethasone
therapy in multiple myeloma. PLoS One. 13:e01967802018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kämpjärvi K, Mäkinen N, Kilpivaara O,
Arola J, Heinonen HR, Böhm J, Abdel-Wahab O, Lehtonen HJ, Pelttari
LM, Mehine M, et al: Somatic MED12 mutations in uterine
leiomyosarcoma and colorectal cancer. Br J Cancer. 107:1761–1765.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kämpjärvi K, Kim NH, Keskitalo S, Clark
AD, von Nandelstadh P, Turunen M, Heikkinen T, Park MJ, Mäkinen N,
Kivinummi K, et al: Somatic MED12 mutations in prostate cancer and
uterine leiomyomas promote tumorigenesis through distinct
mechanisms. Prostate. 76:22–31. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Huang S, Hölzel M, Knijnenburg T,
Schlicker A, Roepman P, McDermott U, Garnett M, Grernrum W, Sun C,
Prahallad A, et al: MED12 controls the response to multiple cancer
drugs through regulation of TGF-β receptor signaling. Cell.
151:937–950. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Feliciano P: MED12 in cancer drug
resistance. Nat Genet. 45:112012. View Article : Google Scholar
|
|
51
|
Howley BV, Link LA, Grelet S, El-Sabban M
and Howe PH: A CREB3-regulated ER-Golgi trafficking signature
promotes metastatic progression in breast cancer. Oncogene.
37:1308–1325. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ramalho-Carvalho J, Gonçalves CS, Graça I,
Bidarra D, Pereira-Silva E, Salta S, Godinho MI, Gomez A, Esteller
M, Costa BM, et al: A multiplatform approach identifies miR-152-3p
as a common epigenetically regulated onco-suppressor in prostate
cancer targeting TMEM97. Clin Epigenetics. 10:402018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ge S, Wang D, Kong Q, Gao W and Sun J:
Function of miR-152 as a tumor suppressor in human breast cancer by
targeting PIK3CA. Oncol Res. 25:1363–1371. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Li LW, Xiao HQ, Ma R, Yang M, Li W and Lou
G: MiR-152 is involved in the proliferation and metastasis of
ovarian cancer through repression of ERBB3. Int J Mol Med.
41:1529–1535. 2018.PubMed/NCBI
|
|
55
|
Wang Y, Bao W, Liu Y, Wang S, Xu S, Li X,
Li Y and Wu S: MiR-98-5p contributes to cisplatin resistance in
epithelial ovarian cancer by suppressing miR-152 biogenesis via
targeting dicer1. Cell Death Dis. 9:4472018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Huang S, Li X and Zhu H: MicroRNA-152
targets phosphatase and tensin homolog to inhibit apoptosis and
promote cell migration of nasopharyngeal carcinoma cells. Med Sci
Monit. 22:4330–4337. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Hou R, Yang Z, Wang S, Chu D, Liu Q, Liu J
and Jiang L: MiR-762 can negatively regulate menin in ovarian
cancer. Onco Targets Ther. 10:2127–2137. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Li Y, Huang R, Wang L, Hao J, Zhang Q,
Ling R and Yun J: MicroRNA-762 promotes breast cancer cell
proliferation and invasion by targeting IRF7 expression. Cell
Prolif. 48:643–649. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Shi Y, Jia Y, Zhao W, Zhou L, Xie X and
Tong Z: Histone deacetylase inhibitors alter the expression of
molecular markers in breast cancer cells via microRNAs. Int J Mol
Med. 42:435–442. 2018.PubMed/NCBI
|
|
60
|
Wang H, Wu J, Luo WJ and Hu JL: Low
expression of miR-1231 in patients with glioma and its prognostic
significance. Eur Rev Med Pharmacol Sci. 22:8399–8405.
2018.PubMed/NCBI
|
|
61
|
Zhang J, Zhang J, Qiu W, Zhang J, Li Y,
Kong E, Lu A, Xu J and Lu X: MicroRNA-1231 exerts a tumor
suppressor role through regulating the EGFR/PI3K/AKT axis in
glioma. J Neurooncol. 139:547–562. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Chen SL, Ma M, Yan L, Xiong SH, Liu Z, Li
S, Liu T, Shang S, Zhang YY, Zeng H, et al: Clinical significance
of exosomal miR-1231 in pancreatic cancer. Zhonghua Zhong Liu Za
Zhi. 41:46–49. 2019.(In Chinese; Abstract available in Chinese from
the publisher). PubMed/NCBI
|
|
63
|
Pan Y, Xu T, Liu Y, Li W and Zhang W:
Upregulated circular RNA circ_0025033 promotes papillary thyroid
cancer cell proliferation and invasion via sponging miR-1231 and
miR-1304. Biochem Biophys Res Commun. 510:334–338. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Yang W, Li Y, Song X, Xu J and Xie J:
Genome-Wide analysis of long noncoding RNA and mRNA co-expression
profile in intrahepatic cholangiocarcinoma tissue by RNA
sequencing. Oncotarget. 8:26591–26599. 2017.PubMed/NCBI
|
|
65
|
Ma W, Chen X, Ding L, Ma J, Jing W, Lan T,
Sattar H, Wei Y, Zhou F and Yuan Y: The prognostic value of long
noncoding RNAs in prostate cancer: A systematic review and
meta-analysis. Oncotarget. 8:57755–57765. 2017.PubMed/NCBI
|
|
66
|
Qi Y, Wang Z, Wu F, Yin B, Jiang T, Qiang
B, Yuan J, Han W and Peng X: Long noncoding RNA HOXD-AS2 regulates
cell cycle to promote glioma progression. J Cell Biochem.
120:281172018.
|
|
67
|
Shi X, Cui Z, Liu X, Wu S, Wu Y, Fang F
and Zhao H: LncRNA FIRRE is activated by MYC and promotes the
development of diffuse large B-cell lymphoma via Wnt/β-catenin
signaling pathway. Biochem Biophys Res Commun. 510:594–600. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Li M, Zhao LM, Li SL, Li J, Gao B, Wang
FF, Wang SP, Hu XH, Cao J and Wang GY: Differentially expressed
lncRNAs and mRNAs identified by NGS analysis in colorectal cancer
patients. Cancer Med. 7:4650–4664. 2018. View Article : Google Scholar : PubMed/NCBI
|