Introduction
Superselective intra-arterial infusion of cisplatin
with concomitant radiotherapy has been introduced to increase the
treatment efficacy of superselective intra-arterial
chemoradiotherapy (SSIACRT) for advanced head and neck cancer
(1). It has an 80% complete response
(CR) rate in advanced head and neck cancer (2). However, despite the significant CR
rate, the survival rate of SSIACRT remains unsatisfactory (3). Previous studies have shown that T and N
classification are prognostic factors that are correlated with
overall survival (OS) and disease-free survival (DFS), and the risk
of nodal disease is directly associated with more advanced T
classification (4). Furthermore,
most treatment failures occur at the primary tumor site, followed
by regional nodal failure (5,6).
However, classic parameters, such as TNM classification, are not
useful for prediction of responses, and the establishment of
useful, effective, pretreatment risk stratification is vital
(7,8).
18F-Fluorodeoxyglucose positron emission tomography
with computed tomography (FDG-PET-CT) is a medical imaging
technique that is based on the study of glucose metabolism in tumor
cells. FDG-PET-CT in oral cancer has been evaluated by numerous
studies. The maximum standardized uptake value (SUVmax), a
semiquantitative measure of tumor uptake, has shown varied results
in its role as a prognostic factor for head-and-neck squamous cell
carcinoma (HNSCC) that is treated with definitive chemoradiotherapy
(9–11).
The present study aimed to evaluate the treatment
results and compare the pre-treatment SUV (pre-SUV) and
post-treatment SUV (post-SUV) with OS and local control (LC) rates
in patients with advanced oral cancer treated with SSIACRT.
Materials and methods
Patients
Between May 2003 and February 2018, 74 patients with
advanced oral cancer were treated with SSIACRT at Hirosaki
University Hospital, Hirosaki Japan. Of these, 55 patients
underwent PET-CT, one prior to and several times after treatment at
Hirosaki University Hospital. In this study, we restricted our
population to patients who had both pre-SUV and post-SUV
measurements. We excluded 5 patients who were diagnosed with neck
recurrence only after they had been treated with SSIACRT because
these patients did not have a primary tumor. We also excluded 2
patients with metallic artifacts of dental crowns because they
hinder in SUV measurement.
Finally, 37 patients were analyzed in
this study (Table I)
The inclusion criteria were as follows: oral
squamous cell carcinoma (SCC) of the tongue and lower gum, floor of
mouth, buccal mucosa, upper jaw and fauces and, Union for
International Cancer Control TNM classification stage III to IV. A
total of 34 patients were fresh newly diagnosed and 3 patients had
local recurrence (LR) cases. Among the 3 patients, one had not only
local LR but also neck recurrence.
This study was approved by the ethics committee of
Hirosaki University Hospital, Hirosaki Japan, and informed consent
was obtained from each participant. Patients provided oral and
written consent to the collection of their data and consented to
publication of the PET scan data as well as participation. We have
read the Helsinki Declaration and have followed the guidelines in
this study.
Treatment procedure
The extent of tumor invasion was assessed by CT,
magnetic resonance imaging (MRI), and PET-CT. Primary tumors and
all nodal areas were irradiated with 50 Gy in 25 fractions, 5
fractions a week, in a period of 5 weeks. An additional dose of 16
Gy by a boost irradiation in 8 fractions was applied on the primary
tumors, with a total dose of 66 Gy. All patients received
concurrent intra-arterial DOC (40 mg/mm2) and CDGP (80
mg/mm2) infusion thrice every 4 weeks in the following
manner (12).
Anticancer drugs were partially delivered to the
regional neck area in patients with bulky nodal diseases confirmed
to have multiple feeding arteries. The dose of drug for each feeder
of bulky nodal diseases was determined by CT angiography. When the
number of feeding arteries was more than 4 or the feeding artery
was not identified using a microcatheter, an arterial
redistribution technique was used. Unnecessary branches of the
external carotid artery (ECA) were embolized with microcoils
(Trufill Pushable Ceoil, Codman, Neurovascular, and Tornado
Embolization Microcoil, Cook) via a microcatheter. The procedure
was performed within the extent of the ECA. Drug infusion was
performed in the radiology suite by interventional radiologists
(12). This treatment has been
approved by the appropriate ethical committees of Hirosaki
University Hospital (Hirosaki, Japan).
Evaluation of response to therapy
Responses to therapy were assessed by clinical
examination, CT, and MRI 4 weeks after the completion of SSIACRT.
FDG-PET was performed 8 weeks after the completion of radiotherapy
to avoid false positive results caused by inflammation (12).
The final treatment effect for a primary tumor and
cervical lymph nodes was determined, considering inspection,
palpation, and various diagnostic imaging modalities.
PET-CT imaging
FDG-PET-CT scans were performed using Discovery ST
Elite 16 (GE Medical Systems). Up take time was 50–60 min after
injection. FDG was injected intravenously at a dose of 100–300
MBq.
Data analysis of PET results
For the semiquantitative evaluation of FDG uptake in
the primary tumor, region of interest (ROI) was placed on the area
of highest FDG uptake on the PET images and the SUVmax of the
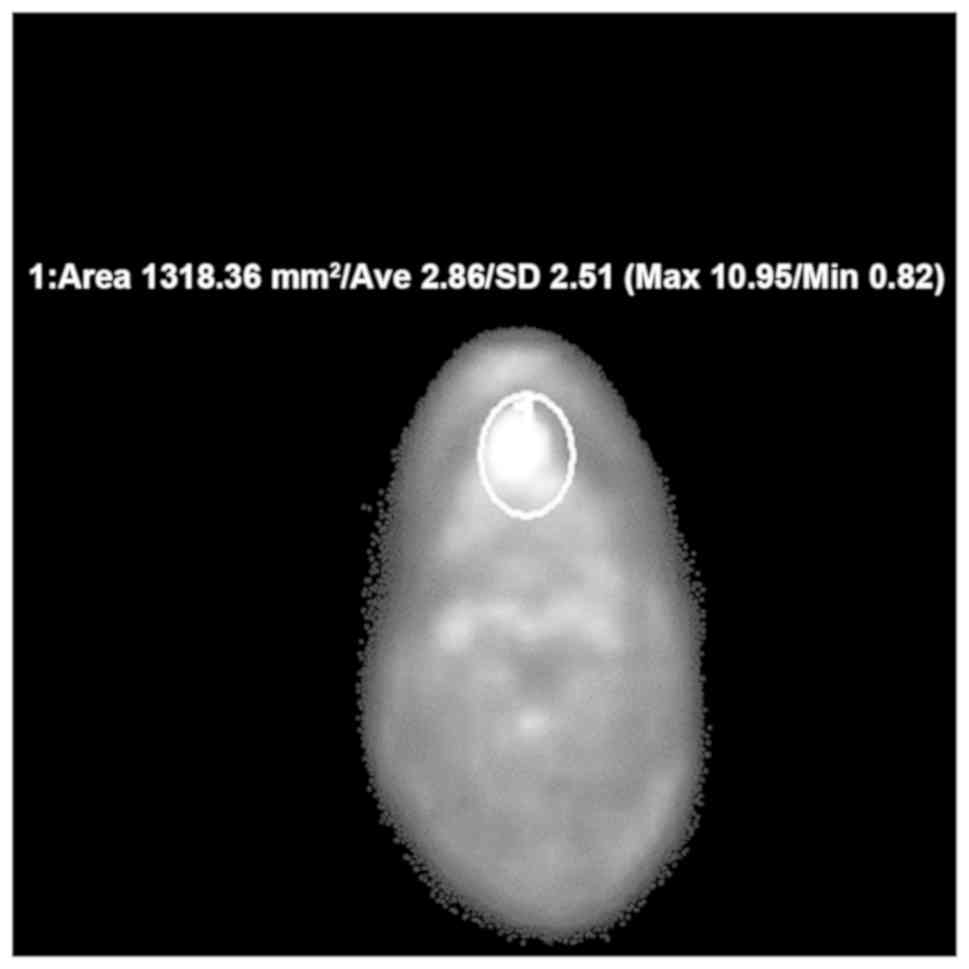
primary tumor was automatically calculated (Fig. 1). Measurement of SUVmax was performed
using Shade Quest View R software (Yokogawa Electric Corporation).
In the evaluation, we preferred to use SUVmax as the evaluation
index in order to minimize partial volume effects in the relatively
small ROI (13). We measured the
pre-treatment SUV (pre-SUV) and post-treatment SUV (post-SUV).
Finally, we compared the pre-SUV and post-SUV with OS and LC
rates.
Statistical analysis
Survival curves were obtained using the Kaplan-Meier
method. The significance differences of the OS, disease free (DF),
and LC rates were analyzed by two-sided log rank test. SUV cut-off
points were set based on the result of receiver operating
characteristic (ROC) curve analysis. Paired t-test was used for the
comparison of the averages between two groups of patients.
Statistical significance was a defined as P-value
<0.05. Statistical analyses were performed with EZR (Saitama
Medical Center, Jichi Medical University, Saitama, Japan), which is
a graphical user interface for R (The R Foundation for Statistical
Computing). More precisely, it is a modified version of R commander
designed to add statistical functions frequently used in
biostatistics (14).
Results
After SSIACRT, treatment results of
primary were as follows
Of the 37 patients, 32 (86.5%) had CR, and 5 (13.5%)
patients had partial response (PR). Five of 37 patients (13.5%)
showed LR during follow-up. Of the 9 patients who were evaluated as
PR or stable disease (SD), 5 patients received palliative treatment
and 3 patients were treated surgically but unsuccessfully. One
patient with residual neck disease was treated successfully by
radical neck dissection. Distant metastasis (DM) was noted in 6
patients (16.2%). Twelve patients (32.4%) died: 5 of DM, 4 of LR, 2
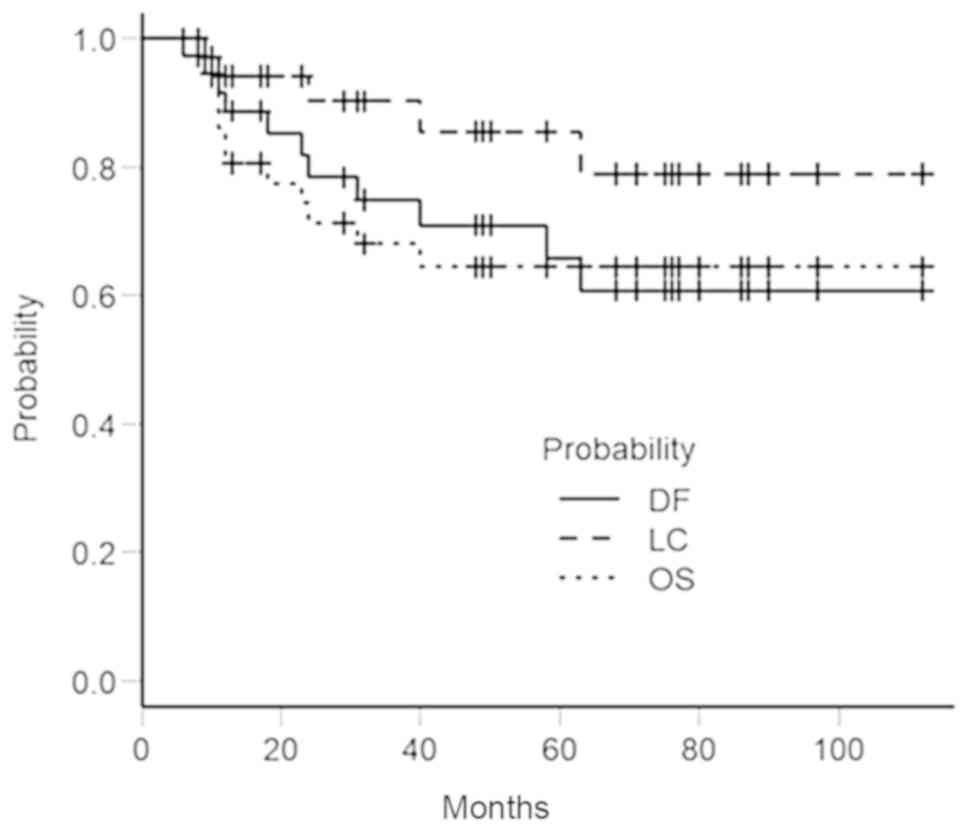
of other diseases and 1 of unknown cause. The 5-year OS, DF and LC
rates were 64.5% [95% confidence interval (CI), 45.7–78.2%], 59.9%
(95% CI; 40.4–74.8%), and 85.5% (95% CI; 65.0–94.5%), respectively
(Fig. 2).
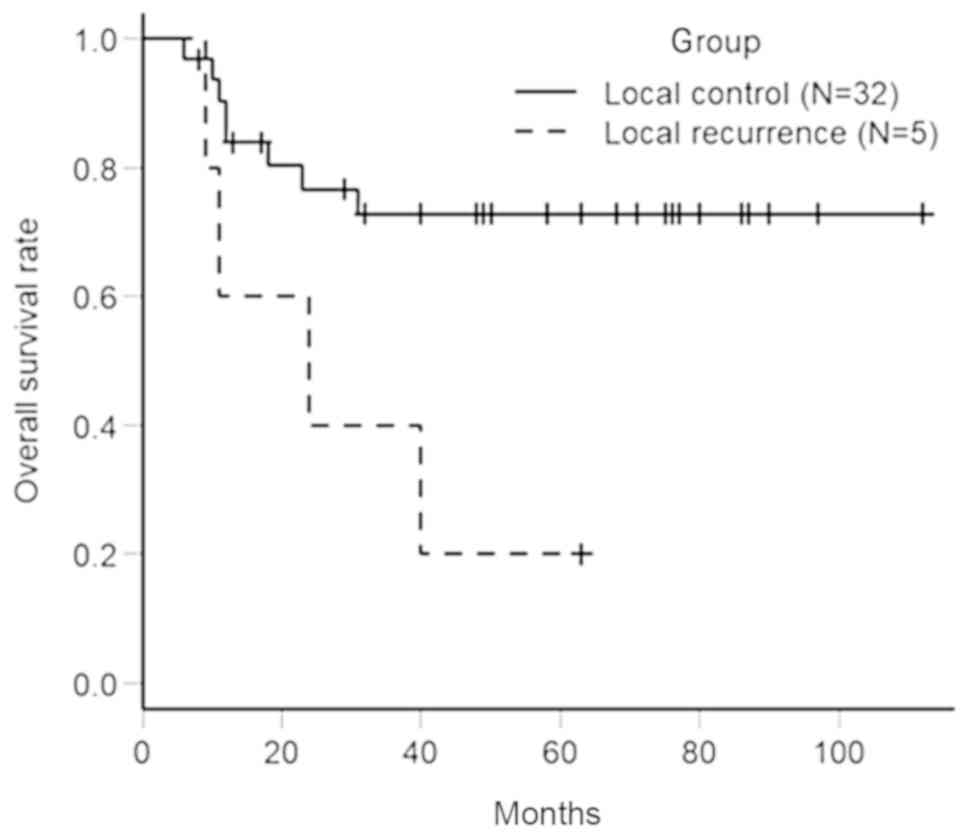
Fig. 3 shows the
difference in OS rates between the LC and LR groups. Of 37
patients, 5 patients (13.5%) were included in LR group and 32
patients (86.5%) were included in LC group. Based on the
Kaplan-Meier method, the 5-year OS rate in the LR group was 20.0%
(95% CI; 0.8–58.2%) and the 5-year OS rate in LC group was 72.8%
(95% CI: 52.7–85.5%). There was a significant difference between
the 2 groups by log-rank test (P=0.0177).
Table II shows the
date of pre-SUV and post-SUV. The range of pre-SUV and post-SUV
were 4.9–43.0 and 1.8–13.9, respectively. The mean ± standard
deviation (SD) of pre-SUV and post-SUV was 15.9 ± 7.1 and 5.1 ±
2.7, respectively. The median of pre-SUV and post-SUV was 15.4 and
4.1, respectively.
 | Table II.Pre-SUV and post-SUV (n=37). |
Table II.
Pre-SUV and post-SUV (n=37).
| Variable | Pre-SUV | Post-SUV |
|---|
| Range |
4.9–43.0 |
1.8–13.9 |
| Mean ± SD | 15.9a±7.1 | 5.1a±2.7 |
| Median | 15.4 | 4.1 |
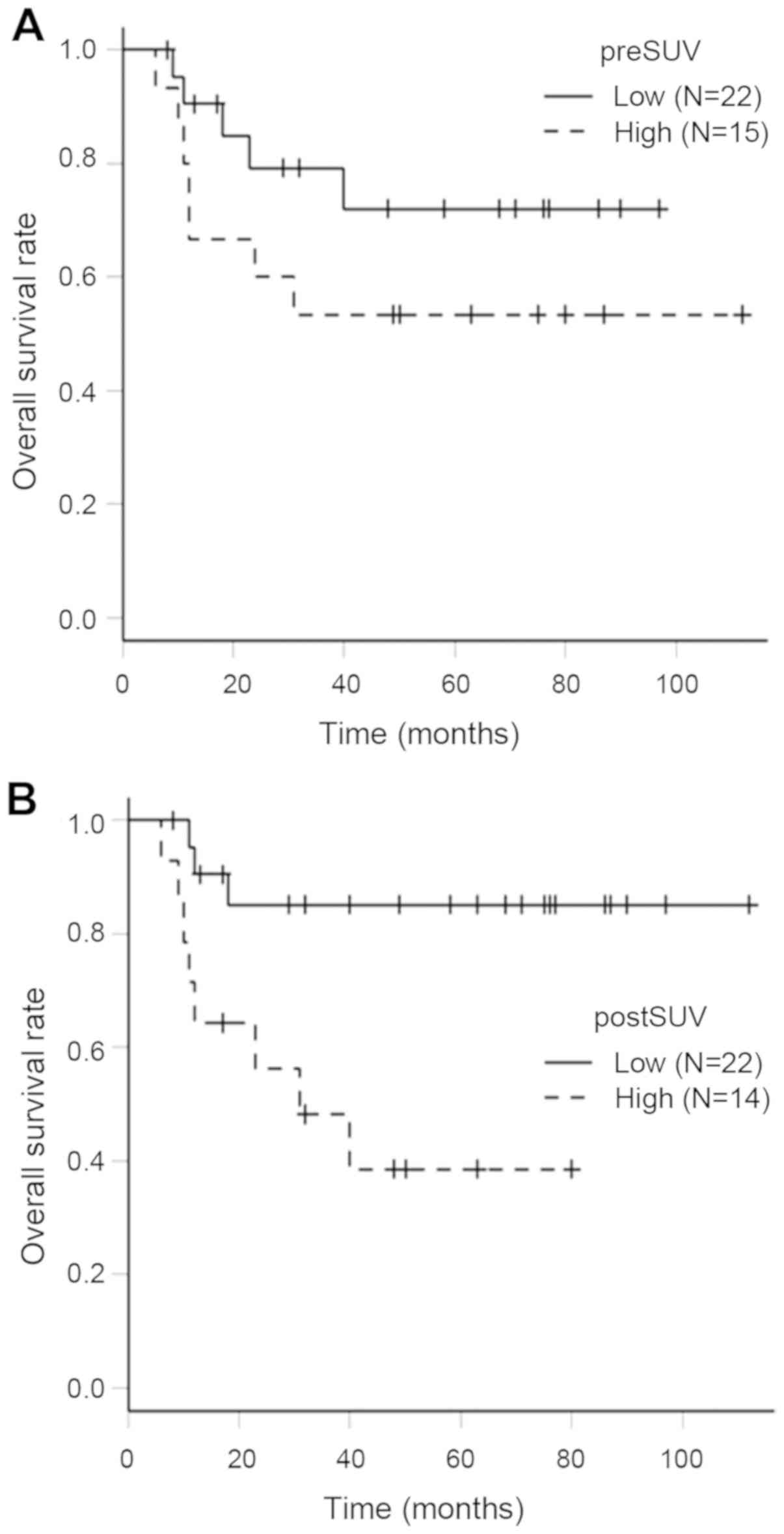
Table III shows the
pre-SUV and post-SUV in the LC and LR groups. A pre-SUV of 16.6 and
post-SUV of 4.4 were identified as the appropriate cut-off points
for ROC curve analysis. Kaplan-Meier curves of OS rates in the
pre-SUV and post-SUV groups are shown in Fig. 4. A 5-year OS rate in the low pre-SUV
(<16.6) group and high pre-SUV (>16.6) group were 75.6% (95%
CI: 46.5–90.3%) and 50.0% (95% CI: 24.5–71.0%), respectively. The
OS rate in the high pre-SUV group was lower than that of low
pre-SUV group. However, there was no significant difference between
the 2 groups by log-rank test (P=0.0726). The 5-year OS rate in the
low post-SUV (<4.4) group and high post-SUV (>4.4) group were
85.2% (95% CI: 60.6–95.0%) and 38.6% (95% CI: 13.4–63.6%),
respectively. There was a significant difference between the 2
groups by log-rank test (P=0.006).
 | Table III.Pre-SUV and post-SUV between LC and
LR. |
Table III.
Pre-SUV and post-SUV between LC and
LR.
|
| LC group | LR group |
|---|
|
|
|
|
|---|
| Parameter | Mean ± SD (N) |
|---|
| Pre-SUV | 15.8±7.6
(N=32) | 15.9±3.8 (N=5) |
| Post-SUV | 4.3±1.4 (N=32) | 6.0±2.0 (N=4) |
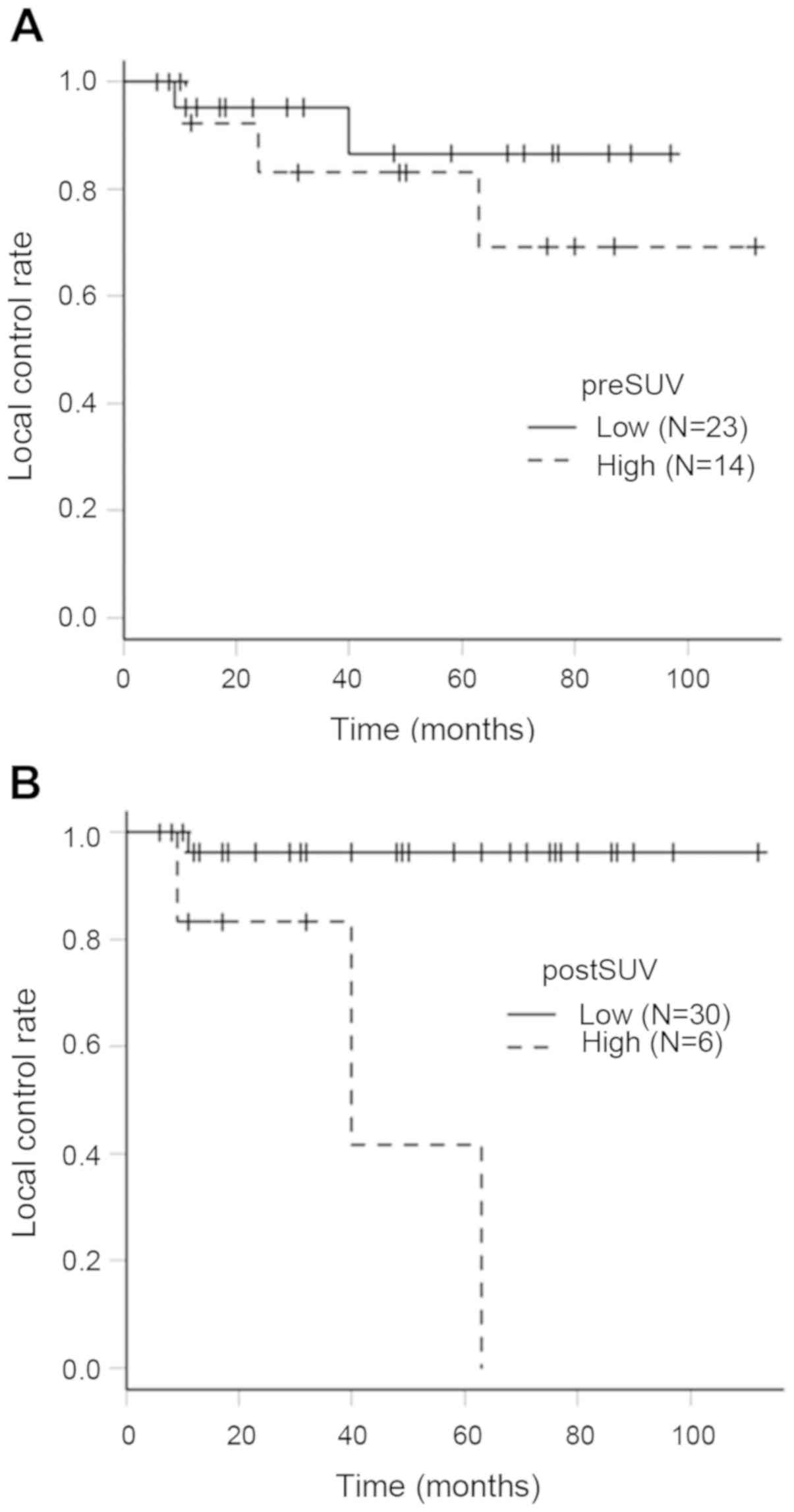
LC rates in the pre-SUV and post-SUV groups
presented in Fig. 5. The 5-year LC
rate in the low pre-SUV (<16.7) and high pre-SUV (>16.7)
groups were 86.6% (95% CI: 53.9–96.7%) and 83.1% (95% CI:
47.2–95.5%), respectively. There was no significant difference
between the 2 groups by log-rank test (P=0.411). The 5-year LC
rates in the low post-SUV (<6.3) group and high post-SUV
(>6.3) group were 96.3% (95% CI: 76.5–99.5%) and 41.7% (95% CI:
1.1–84.3%), respectively. There was a significant difference
between 2 groups by log-rank test (P=0.000187).
Discussion
This study showed treatment results and relationship
between FDG-PET SUV and prognosis of patients with advanced oral
cancer treated with SSIACRT. Kobayashi et al reported that
the survival rate of patients with advanced oral cancer treated
with SSIACRT was significantly superior to that of the surgical
group (3). Furthermore, SSIACRT not
only has a higher survival rate but also resulted in a better QOL
compared with surgical procedures (15). Our result showed a relatively high
5-year OS rate (64.5%) and high LC rate (85.5%) of even advanced
oral cancers. From the results of previous studies and the present
study, SSIACRT is a reliable treatment modality with respect to
survival in advanced oral cancer. However, DF rate (59.9%) was low
compared to the LC rate and 16.2% of patients had distant
metastasis, so this problem should be considered in the future.
Several studies concluded that the LC rate correlated with the OS
rate (3). Our result in the
comparison of OS rates between the LC and LR groups showed that the
LR group had a poorer survival rate than the LC group.
Previous studies have shown that cut-off points of
SUVmax of the primary tumor were set as median of SUVmax or
calculated factors of DFS (16–25). In
this study, SUV cut-off points for OS and LC were set based on the
results of the ROC curve analysis. ROC curve analysis is used to
evaluate the strength of relationships and examine the usefulness
of diagnostics results (26).
In our analysis, there was a significant difference
in the 5-year OS and LC rates between the low post-SUV group and
high post-SUV groups. Our results suggest that higher post-SUV is
predictive of worse clinical outcomes. Minn et al reported
that high FDG uptake in HNSCC is associated with poor survival
(16). A similar association has
also been demonstrated for other malignancies such as brain, lung,
malignant lymphomas, and anus (27–30).
Morikawa et al reported that pret-SUV-max was correlated
with OS rate in patients with oral SCC treated with mainly surgery
(26). In contrast, Suzuki et
al reported that pretreatment SUV-max failed to predict the OS
rate, and metabolic tumor volume using an SUV threshold of 5.0 was
a significant prognostic factor for OS rate in patients with oral
SCC who were treated with SSIA-CRT (31). Compared to those in their study, our
result did not show that there was a significant difference in OS
and LC rates between the low pre-SUV and high pre-SUV groups.
We suggest that the residual tumor cells expressed
as post-SUV were critical factors in metastasis and poor prognosis,
something no previous studies have shown clearly with SSLACRT. It
is well known that cancer stem cells are highly resistant to
radiotherapy and chemotherapy, and can contribute to recurrence and
disease progression (32,33). This may suggest that SSIACRT is a
powerful treatment for advanced oral cancer even if the pre-SUV is
high. From a point of functional organ preservation, SSIACRT should
be recommended as an alternative to surgical procedures (15). However, this study also shows that
although SSIACRT is a powerful treatment method, further treatments
need to be developed to completely eradicate cancer stem cells. If
such a treatment is developed, even patients with high SUV will
have better prognosis for advanced oral cancer.
This study has several limitations. First, our study
had a retrospective design and relatively small sample size of 37.
Pre-SUV might also be selected as a statistically important factor
if the analyzed sample size was larger. Second, to measure SUV of a
tumor in the oral cavity, inflammation after SSIACRT and metallic
artifacts of dental crowns cause inaccurate mismeasurement of
SUV.
Therefore, SSIACRT achieved a high LC rate even in
advanced oral cancer and a low post-SUV is statistically associated
with increased OS and LC rates after SSIACRT independent of T and N
classification. Our results suggest that post-SUV is an accurate
predictor of prognosis and may be useful and potentially more
specific in predicting LR in patients with advanced oral cancer
treated with SSIACRT.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
TO and KA performed statistical analysis and drafted
the article. KH and RF produced figures and tables, and critically
reviewed the articles. KW and MA performed patient treatment,
provided patient data and provided valuable insight from their
fields. YH designed the study and revised the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the ethics committee of
Hirosaki University Hospital, Hirosaki, Japan. Patients agreed to
the participation in this study and signed an informed consent
form. Patients provided oral and written consent to the collection
of their data and participation in the current study.
Patient consent for publication
Patients provided oral and written consent for the
publication of PET scan data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Robbins KT, Storniolo AM, Kerber C,
Seagren S, Berson A and Howell SB: Rapid superselective high-dose
cisplatin infusion for advanced head and neck malignancies. Head
Neck. 14:364–371. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Robbins KT, Kumar P, Wong FS, Hartsell WF,
Flick P, Palmer R, Weir AB III, Neill HB, Murry T, Ferguson R, et
al: Targeted chemoradiation for advanced head and neck cancer:
Analysis of 213 patients. Head Neck. 22:687–693. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kobayashi W, Teh BG, Narita N, Ito R,
Saito Y, Furudate K, Kimura H, Kakehata S and Kawaguchi H:
Comparative study of superselective intra-arterial
chemoradiotherapy versus radical surgery on distant metastasis for
advanced oral cancer. J Oral Oncol. 2014:1927342014.
|
|
4
|
Myerson RJ, Outlaw ED, Chang A, Birnbaum
EH, Fleshman JW, Grigsby PW, Kodner IJ, Malayapa RS, Mutch MG,
Parikh P, et al: Radiotherapy for epidermoid carcinoma of the anus:
Thirty year' experience. Int J Radiat Oncol Biol Phys. 75:428–435.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Das P, Bhatia S, Eng C, Ajani JA, Skibber
JM, Rodriguez-Bigas MA, Chang GJ, Bhosale P, Delclos ME, Krishnan
S, et al: Predictors and patterns of recurrence after definitive
chemoradiation for anal cancer. Int J Radiat Oncol Biol Phys.
68:794–800. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wright JL, Patil SM, Temple LK, Minsky BD,
Saltz LB and Goodman KA: Squamous cell carcinoma of the anal canal:
Patterns and predictors of failure and implications for
intensity-modulated radiation treatment planning. Int J Radiat
Oncol Biol Phys. 78:1064–1072. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Salesiotis AN and Cullen KJ: Molecular
markers predictive of response and prognosis in the patient with
advanced squamous cell carcinoma of the head and neck: Evolution of
a model beyond TNM staging. Curr Opin Oncol. 12:29–39. 2000.
View Article : Google Scholar
|
|
8
|
Zhang Y, He W and Zhang S: Seeking for
correlative genes and signaling pathways with bone metastasis from
breast cancer by integrated analysis. Front Oncol. 9:1382019.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
La TH, Filion EJ, Turnbull BB, Chu JN, Lee
P, Nguyen K, Maxim P, Quon A, Graves EE, Loo BW Jr and Le QT:
Metabolic tumor volume predicts for recurrence and death in
head-and-neck cancer. Int J Radiat Oncol Biol Phys. 74:1335–1341.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Murphy JD, La TH, Chu K, Quon A, Fischbein
NJ, Maxim PG, Graves EE, Loo BW Jr and Le QT: Postradiation
metabolic tumor volume predicts outcome in head-and-neck cancer.
Int J Radiat Oncol Biol Phys. 80:514–521. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shimizu M, Mitsudo K, Koike I, Taguri M,
Iwai T, Koizumi T, Oguri S, Kioi M, Hirota M, Inoue T and Tohnai I:
Prognostic value of 2-[(18) F]fluoro-2-deoxy-D-glucose positron
emission tomography for patients with oral squamous cell carcinoma
treated with retrograde superselective intra-arterial chemotherapy
and daily concurrent radiotherapy. Oral Surg Oral Med Oral Pathol
Oral Radiol. 121:239–247. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kobayashi W, Teh BG, Sakaki H, Sato H,
Kimura H, Kakehata S and Nagahata M: Superselective intra-arterial
chemoradiotherapy with docetaxel-nedaplatin for advanced oral
cancer. Oral Oncol. 46:860–863. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Keramida G, Potts J, Bush J, Dizdarevic S
and Peters AM: Hepatic steatosis is associated with increased
hepatic FDG uptake. Eur J Radiol. 83:751–755. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kanda Y: Investigation of the freely
available easy-to-use software ‘EZR’ for medical statistics. Bone
Marrow Transplant. 48:452–458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kobayashi W, Kukobota K, Ito R, Sakaki H,
Nakagawa H and Teh BG: Can superselective intra-arterial
chemoradiotherapy replace surgery followed by radiation for
advanced cancer of the tongue and floor of the mouth? J Oral
Maxillofac Surg. 74:1248–1254. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Minn H, Lapela M, Klemi PJ, Grénman R,
Leskinen S, Lindholm P, Bergman J, Eronen E, Haaparanta M and
Joensuu H: Prediction of survival with
fluorine-18-fluoro-deoxyglucose and PET in head and neck cancer. J
Nucl Med. 38:1907–1911. 1997.PubMed/NCBI
|
|
17
|
Koyasu S, Nakamoto Y, Kikuchi M, Suzuki K,
Hayashida K, Itoh K and Togashi K: Prognostic value of pretreatment
18F-FDG PET/CT parameters including visual evaluation in patients
with head and neck squamous cell carcinoma. AJR Am J Roentgenol.
202:851–858. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Brun E, Kjellén E, Tennvall J, Ohlsson T,
Sandell A, Perfekt R, Perfekt R, Wennerberg J and Strand SE: FDG
PET studies during treatment: prediction of therapy outcome in head
and neck squamous cell carcinoma. Head Neck. 24:127–135. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schwartz DL, Rajendran J, Yueh B, Coltrera
MD, Leblanc M, Eary J and Krohn K: FDG-PET prediction of head and
neck squamous cell cancer outcomes. Arch Otolaryngol Head Neck
Surg. 130:1361–1367. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Machtay M, Natwa M, Andrel J, Hyslop T,
Anne PR, Lavarino J, Intenzo CM and Keane W: Pretreatment FDG-PET
standardized uptake value as a prognostic factor for outcome in
head and neck cancer. Head Neck. 31:195–201. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Halfpenny W, Hain SF, Biassoni L, Maisey
MN, Sherman JA and McGurk M: FDG-PET. A possible prognostic factor
in head and neck cancer. Br J Cancer. 86:512–516. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Torizuka T, Kanno T, Futatsubashi M,
Naitou K, Ueda Y and Ouchi Y: Prognostic value of 18F-FDG PET in
patients with head and neck squamous cell cancer. AJR Am J
Roentgenol. 192:156–160. 2009. View Article : Google Scholar
|
|
23
|
Allal AS, Slosman DO, Kebdani T, Allaoua
M, Lehmann W and Dulguerov P: Prediction of outcome in
head-and-neck cancer patients using the standardized uptake value
of 2-[18F]fluoro-2-deoxy-D-glucose. Int J Radiat Oncol Biol Phys.
59:1295–1300. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Allal AS, Dulguerov P, Allaoua M,
Haenggeli CA, El-Ghazi el A, Lehmann W and Slosman DO: Standardized
uptake value of 2-[(18)F] fluoro-2-deoxy-D-glucose in predicting
outcome in head and neck carcinomas treated by radiotherapy with or
without chemotherapy. J Clin Oncol. 20:1398–1404. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Roh JL, Pae KH, Choi SH, Kim JS, Lee S,
Kim SB, Nam SY and Kim SY: 2-[18F]-Fluoro-2-deoxy-D-glucose
positron emission tomography as guidance for primary treatment in
patients with advanced-stage resectable squamous cell carcinoma of
the larynx and hypopharynx. Eur J Surg Oncol. 33:790–795. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Morikawa T, Futoo E, Bessho H, Yakushiji
T, Nomura T, Onodera S, Uchino Y, Takano N, Shibahara T and
Katakura A: 18F-FDG PET/CT parameters as imaging biomarker is
useful in oral squamous cell carcinoma patients. Jpn J Soc Oral
Oncol. 29:23–35. 2017. View Article : Google Scholar
|
|
27
|
Patronas NJ, Di Chiro G, Kufta C,
Bairamian D, Kornblith PL, Simon R and Larson SM: Prediction of
survival in glioma patients by means of positron emission
tomography. J Neurosurg. 62:816–822. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Okada J, Yoshikawa K, Itami M, Imaseki K,
Uno K, Itami J, Kuyama J, Mikata A and Arimizu N: Positron emission
tomography using fluorine-18-fluorodeoxyglucose in malignant
lymphoma: A comparison with proliferative activity. J Nucl Med.
33:325–329. 1992.PubMed/NCBI
|
|
29
|
Ahuja V, Coleman RE, Herndon J and Patz EF
Jr: The prognostic significance of fluorodeoxyglucose positron
emission tomography imaging for patients with nonsmall cell lung
carcinoma. Cancer. 83:918–924. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cardenas ML, Spencer CR, Markovina S,
DeWees TA, Mazur TR, Weiner AA, Parikh PJ and Olsen JR:
Quantitative FDG-PET/CT predicts local recurrence and survival for
squamous cell carcinoma of the anus. Adv Radiat Oncol. 2:281–287.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Suzuki-Shibata S, Yamamoto Y, Yoshida T,
Mizoguchi N, Nonaka T, Kubota A, Narimatsu H, Miyagi Y, Kobayashi
T, Kaneta T and Inoue T: Prognostic value of volumetric FDG PET/CT
parameters in patients with oral tongue squamous cell carcinoma who
were treated by superselective intra-arterial chemoradiotherapy.
Jpn J Radiol. 35:740–747. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chi HC, Tsai CY, Tsai MM, Yeh CT and Lin
LH: Roles of long noncoding RNAs in recurrence and metastasis of
radiotherapy-resistant cancer stem cells. Int J Mol Sci. 18(pii):
E19032017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sen Z, Zhan XK, Jing J, Yi Z and Wanqi Z:
Chemosensitizing activities of cyclotides from Clitoria ternatea in
paclitaxel-resistant lung cancer cells. Oncol Lett. 5:641–644.
2013. View Article : Google Scholar : PubMed/NCBI
|