Introduction
Breast cancer is the most commonly diagnosed cancer
in women, with 1,671,149 newly cases in 2012, accounting for 25.1%
of all types of cancer, as well as its significant
cancer-associated mortality in developed and developing countries
(1,2). Estimating the condition of patients,
including the size and stage of the tumor, and establishing
scientific therapy are key to obtaining satisfactory therapeutic
effects (3). A careful and complete
examination prior to surgery is important for selection of the
suitable operation method and for surgical planning (4). One of the most important pieces of
information required from preoperative examination is the size and
area of extension of the tumor, which is important for surgical
treatment and chemo-radiotherapy (5). Thus, assessing tumor size and
estimating the tumor stage is imperative in clinical practice.
Although a variety of imaging modalities have been used to diagnose
breast cancer, mammography remains the traditional standard method
for diagnosing breast cancer (6),
with a reported sensitivity of 85%, decreasing to 68% in women with
dense breasts (7). However, this
method does not estimate tumor size well, and it exhibits certain
limitations regarding the qualitative diagnosis of breast tumors
(8). It is best suited for breast
examination and cancer screening (9). Additionally, the question of whether
mammography should be recommended or not to women between the ages
of 40 and 50 years depends upon the publication of relevant
clinical trial data due to test sensitivity, disease prevalence,
cost of screening and consequences of a false-positive result
(10,11).
Although magnetic resonance imaging (MRI) has been
widely used in clinical practice for years, the appropriate use of
MRI in elderly patients with breast cancer remains unclear. It has
been reported that MRI has the greatest benefit in women presenting
with an occult primary cancer and minimal additional benefit in
elderly patients with breast cancer undergoing MRI for extent of
disease evaluation or in post-treatment surveillance (12). MRI exhibits a high resolution and has
been reported to accurately estimate tumor size (13,14).
However, MRI has also been reported to overestimate tumor size,
particularly in patients with tumor (T)2 or T3 stage (15,16).
Although, MRI displays high sensitivity and specificity in breast
cancer detection, the above limitation cannot be ignored.
Sonography is more widely used in clinical practice, albeit as a
method of cancer screening rather than tumor size estimation
(17). Positron emission tomography
(PET)/computed tomography (CT) using 2-deoxy-2
fluoro-18-fluoro-D-glucose (18F-FDG) is a useful modality in the
diagnosis of patients with breast cancer (18). By evaluating glucose metabolism, it
can also be used in staging, restaging and post-therapeutic
response evaluation (16,19). In a previous study,
18F-FDG PET/CT was considered inappropriate for
evaluation of tumor size and not recommended as a priority option
in clinical practice due to its poor spatial resolution, which
limits clear delineation of the tumor boundary (20). However, other studies have
demonstrated the advantage of 18F-FDG PET/CT in tumor
size estimation (21). To the best
of our knowledge, few studies have compared the sensitivity and
accuracy of 18F-FDG PET/CT with other traditional
imaging modalities such as sonography and MRI. Besides, Jun et
al (22) found a distinct layer
between the tumor and background activity using a 10-step color
scale with specific window level settings and named the distinct
layer the peritumoral halo layer (PHL). Based on 18F-FDG, Jun et
al, proposed a volume measurement method using PHL (22). However, although the newly developed
PHL method appears promising for accurate estimation of tumor size,
it has not yet been fully validated.
This study aimed to evaluate the concordance between
the 18F-FDG PET/CT based peri-tumoral halo uptake layer
(PHL) method and the pathologic size of breast cancer. By comparing
the reliability and correlation of 18F-FDG PET/CT,
sonography and MRI in pathological tumor size assessment,
experimental evidences were provided for the selection of imaging
modalities in breast cancer diagnosis. In order to examine the
factors associated with discordance size, the accuracy of
18F-FDG PET/CT, sonography and MRI was also compared in
different tumor sizes (T1-T3), histologic subgroups and intrinsic
subtypes.
Materials and methods
Patients
A total of 79 female patients with breast cancer
selected from the database of Changxing People's Hospital (Huzhou,
China) were included in this retrospective study. The staging of
breast cancer was performed according to the 6th Edition of the
American Joint Committee on Cancer Staging Manual (23). The inclusion criteria included: i)
Patients who had undergone 18F-FDG PET/CT, preoperative
sonography and MRI examinations prior to initial treatment between
May 2015 and June 2018; ii) patients who underwent the
aforementioned mentioned imaging modalities within 10 days of
diagnosis prior to surgery, and iii) patients with complete medical
history and stored paraffin sample of the tumor obtained from
surgery. The average time point when patients finished imaging
using different modalities was 3 days before surgery. The exclusion
criteria included: i) Patients with a history of neo-adjuvant
treatment; ii) patients who had undergone preoperative mammotomy;
iii) patients with non-avid tumor and clustered tumors, and iv)
patients with incomplete data or insufficient results (such as MRI
without contrast enhancement and pathology results without exact
tumor size). The present study was supported by the Ethics
Committee of Changxing People's Hospital (Huzhou, China; approval
no. CPH 201505). All the patients signed informed consent
forms.
Diagnostic imaging examination
A high-resolution 5–12 MHz linear array transducer,
VOLUSON 530 and 730D (Kretztechnik AG) was used with a width of 50
mm to perform sonography imaging and the results were recorded
according to the estimation of two independent examiners based on a
widely accepted standard described in a previous study (24). The echo-poor center of the lesion and
the echogenic halo were considered for the measurement of tumor
size. Sonography was carried out by 2 independent experienced,
board-certified breast radiologists.
A MAGNETOM Verio 3.0T (Siemens AG) was used to
perform MRI. All patients were imaged in the prone position with
both breasts placed into a dedicated 16-channel breast coil. The
MRI protocols included the following: i) Bilateral axial
turbo-spin-echo fat-suppressed T2-weighted image (Time of
repetition (TR) / Time of echo (TE), 4,630/70 msec; field of view,
320 mm; slice thickness, 3 mm and number of excitations, 1); ii)
axial turbo-spin-echo T1-weighted image (TR/TE, 736/9.1 msec; field
of view, 320 mm; slice thickness, 3 mm and number of excitations,
1); iii) diffusion-weighted images (TR/TE, 5,800/82 msec; field of
view, 360 mm; slice thickness, 3 mm and b values 0, 400 and 800
sec/mm2) and iv) measurement of the apparent
diffusion-coefficient value.
Two PET/CT scanners [DSTe 8, (GE Medical Systems)
and Gemini 64, (Philips Medical Systems)] were used to perform
18F-FDG PET/CT examinations. All the patients fasted for
6 h before scanning and serum glucose levels were measured prior to
18F-FDG injection (DSTe 8, 0.2 mCi/kg and Gemini 64, 0.1 mCi/kg). A
CT scan was performed in all the patients 1 h after 18F-FDG
injection and images were collected. Each patient was scanned from
the base of the skull to the mid-thigh level. Following low-dose CT
scanning to correct for attenuation, PET acquisition began
immediately in the same anatomical position (3-dimensional mode,
1.5–2.5 min per bed position). The acquired images were
reconstructed using an iterative ordered subsets expectation
maximization algorithm and then transferred to Advantage
Workstation 4.5 (GE Healthcare). According to the methods described
in a previous study, the PHL results of the tumors were determined
(22). Sonography, MRI and
18F-FDG PET/CT were performed by 3 independent groups.
Each group was blinded to the results of the other groups. The
quality control of machines was also monitored by radiologists
according to widely accepted standards in China (25–27).
Quality control consisted of daily, weekly, monthly and seasonally
quality examination. Daily and weekly examinations included
self-examination of hardware equipment and fast calibration to
maintain the accuracy and uniformity of PET/CT. Monthly and
seasonally examinations included normalized correction of PET
scanner's sensitivity and image correction using a Well counter.
The measurement of tumor size by sonography, MRI and
18F-FDG PET/CT was performed by two independent
radiologists in each examination and the average tumor size from
two independent radiologists were calculated as final result.
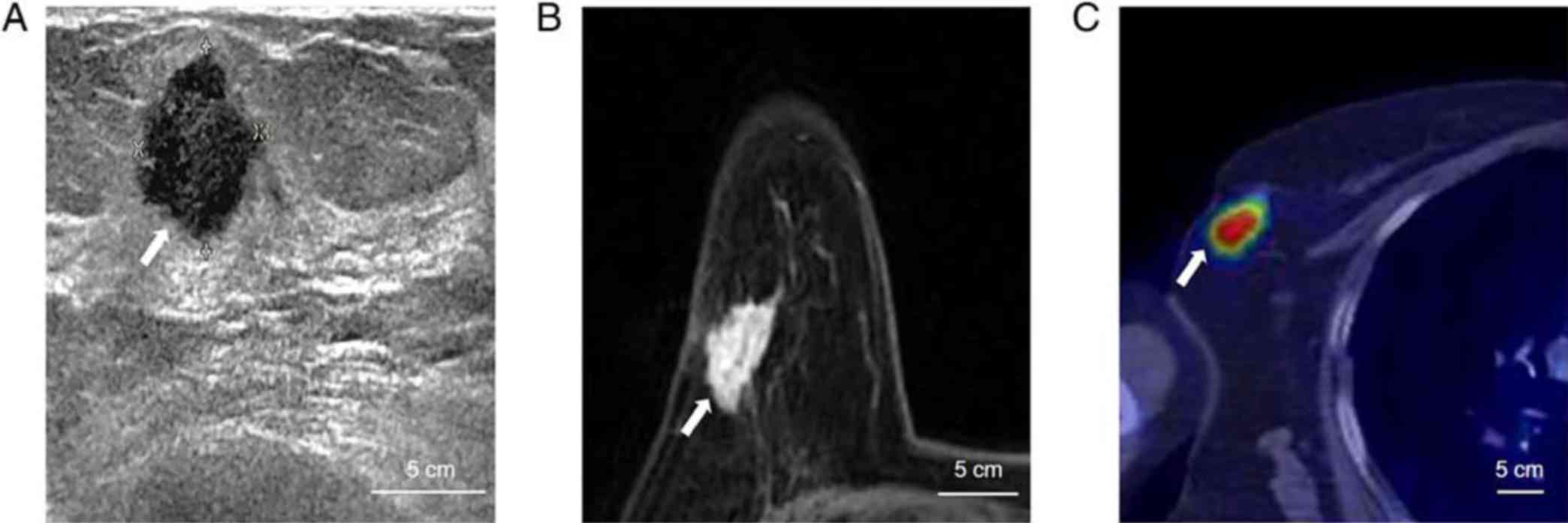
Fig. 1 shows an example of
sonography, MRI and 18F-FDG PET/CT examination results
of the representative case from a 54-year-old patient with right
breast cancer.
Concordance between images and
pathological results
Tumors were excised according to the direction of
largest diameter indicated by previous imaging examination. The
largest diameter from 2 edges of the paraffin sample were
calculated as the tumor size. Pathological reports were completed
by 2 independent pathologists from Department of Pathology,
Changxing People's Hospital (Huzhou, China). The average diameter
of tumors, recorded by two independent pathologists were defined as
the standard of objective tumor size. The most widely accepted
cut-off point of 0.5 cm described in previous studies was used in
the current study to estimate the results of the imaging modalities
(9,28–30). The
results were calculated using the image-derived tumor size minus
the histopathologically determined tumor size and were considered
to be concordant within ± 0.5 cm. Values <0.5 cm were graded
underestimated, while those >0.5 cm were graded overestimated
regarding tumor size estimation.
Comparison of the results of the
different imaging modalities among different subgroups of
patients
The reliabilities of 18F-FDG PET/CT,
sonography and MRI in terms of tumor size prediction were compared
in different subgroups. Tumor size was classified into different
subgroups as T1, T2 and T3. The imaging results for different
histological subtypes were then compared with histopathologically
derived tumor size.
Statistical analysis
All the collected data were described as mean ±
standard deviation. One-way ANOVA followed by Tukey's post-hoc test
were performed for the evaluation of differences among 3 groups.
Categorical variables were normally tested by the r2
test when appropriate. Kappa statistics were used to evaluate the
inter-observer agreement for determination of PHL surrounding each
tumor. Associations between pathology and MRI-determined tumor
sizes, or between pathology and 18F-FDG
PET/CT-determined tumor sizes, were evaluated using linear
regression analysis. Intraclass correlation coefficient (ICC) and
Bland-Altman analyses were used to examine the concordance and
reliability of tumor sizes obtained using MRI and
18F-FDG PET/CT. Two-sided P<0.05 were considered to
indicate a statistically significant difference. Statistical
analyses were conducted using SPSS v.19.0 software (IBM Corp.).
Results
Patient characteristics
A total of 79 Chinese female patients with breast
cancer from Changxing People's Hospital were included in the
current study. The clinicopathological characteristics and the
results of tumor size estimation based on different imaging
modalities are summarized in Table
I. The age range of the patients was 42–76 years and the mean
age was 53 years. Among all the patients, those with ductal
adenocarcinoma were more numerous compared with non-ductal
adenocarcinoma, and T2 was the most frequent tumor stage among
patients. During 18F-FDG PET/CT examination, the mean
standardized uptake value (SUVmax) of the primary tumor was 4.80
(range, 1.12–19.61). The tumor sizes according to the results of
sonography, MRI and 18F-FDG PET/CT were 2.65, 3.82 and
2.98 cm, respectively. The mean pathological size of the tumor was
3.05 cm, ranging from 1.28–8.65 cm, which was considered as the
final standard size of each tumor.
 | Table I.Clinical characteristics of patients
with breast cancer. |
Table I.
Clinical characteristics of patients
with breast cancer.
|
Characteristics | No. of
patients/mean (range) |
|---|
| Number of
patients | 79 |
| Sex | Female |
| Age, years | 53 (42–76) |
| Pathological
type |
|
|
Ductal | 68 |
|
Non-ductal | 11 |
| Surgery | 48 |
|
Breast-conservation
therapy |
|
|
Mastectomy | 31 |
| T stage |
|
| T1 | 23 |
| T2 | 37 |
| T3 | 19 |
| Tumor size, cm |
|
|
Pathology | 3.05
(1.28–8.65) |
|
Sonography | 2.65
(0.95–7.58) |
|
MRI | 3.82
(2.87–10.34) |
|
PET/CT | 2.98
(1.20–8.72) |
| SUVmax
of primary tumor | 4.80
(1.12–19.61) |
PHL determination of tumors in
patients with breast cancer
PHL, which was determined by 2 radiologist reviewers
based on the results of 18F-FDG PET/CT, exhibited good
consistency with a contingency coefficient of 0.79 (P<0.01). The
PHL of the majority of tumors detected was below the 40–50% band,
while the area between 20 and 30% (olive-green color) of the band
of SUVmax is the area where the PHL of each tumor was most commonly
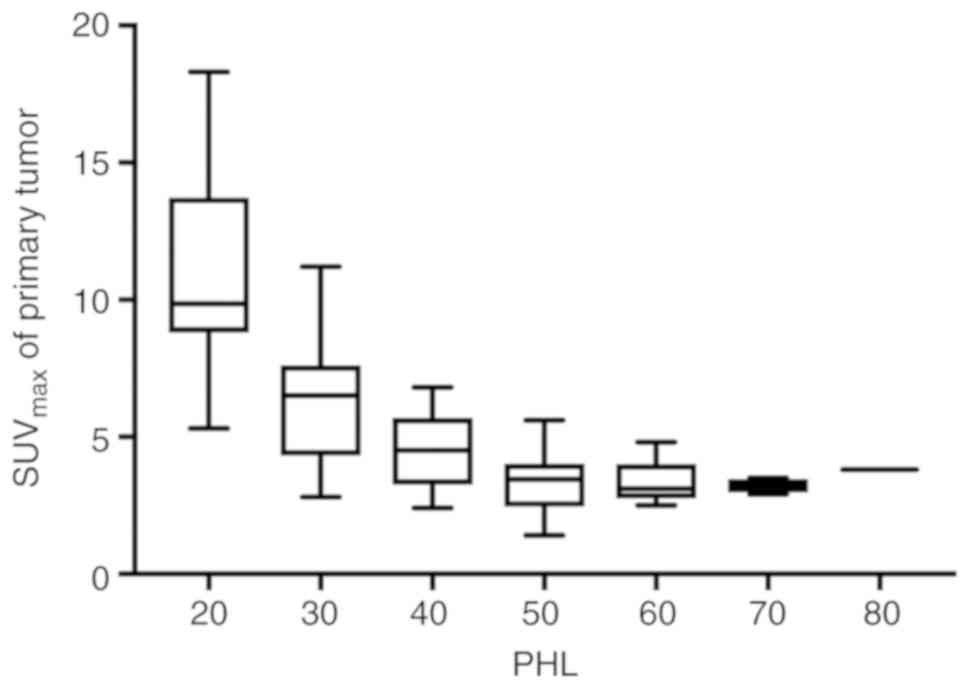
recorded (24%; data not shown). The PHL exhibited a negative
association with the SUVmax of separate tumors (Fig. 2), with the exception of 5 cases, as
presented in Table II.
 | Table II.Discordant cases (n=5) of PHL
determined by 2 independent radiologists. |
Table II.
Discordant cases (n=5) of PHL
determined by 2 independent radiologists.
|
|
|
|
| PHL (%) | Tumor size
(mm) |
|---|
|
|
|
|
|
|
|
|---|
| No. | Age | Histologic
type | SUVmax of
tumor | Radiologist1 | Radiologist2 | Sonography | MRI | PET/CT | Pathology |
|---|
| 1 | 53 | Ductal
adenocarcinoma | 8.2 | 30 | 20 | 7.8 | 8.0 | 7.5 | 7.2 |
| 2 | 48 | Ductal
adenocarcinoma | 3.2 | 70 | 60 | 2.4 | 3.6 | 2.7 | 2.9 |
| 3 | 45 | Ductal
adenocarcinoma | 3.8 | 50 | 60 | 3.1 | 2.9 | 3.8 | 3.5 |
| 4 | 62 | Non-ductal
adenocarcinoma | 2.1 | 70 | 80 | 2.2 | 2.8 | 2.3 | 1.8 |
| 5 | 71 | Ductal
adenocarcinoma | 4.6 | 60 | 70 | 4.2 | 4.3 | 5.1 | 4.9 |
Concordance of tumor sizes measured
different imaging modalities and pathology
All sonography, MRI and PET/CT examination results
had statistically significant associations with pathological
measurements regarding tumor size. Using the pathological size of
the tumor as the final standard size, there were small differences
observed between the imaging and pathology tumor size, namely
sonography, −0.78 ± 1.10 cm; MRI, 1.28 ± 1.25 cm and
18F-FDG PET/CT, 0.13 ± 0.90 cm, respectively (data not
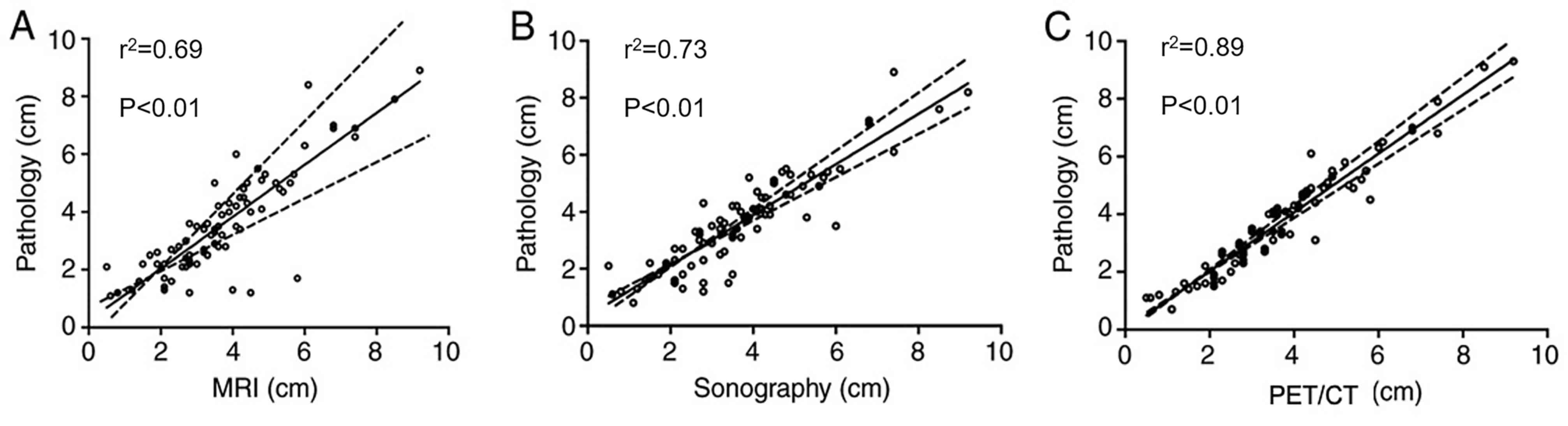
shown). The linear regression between 18F-FDG PET/CT and
pathology measurements (r2=0.89; P<0.01) was more
significant compared with the linear correlation between sonography
(r2=0.73; P<0.01) and MRI (r2=0.69;
P<0.01; Fig. 3).
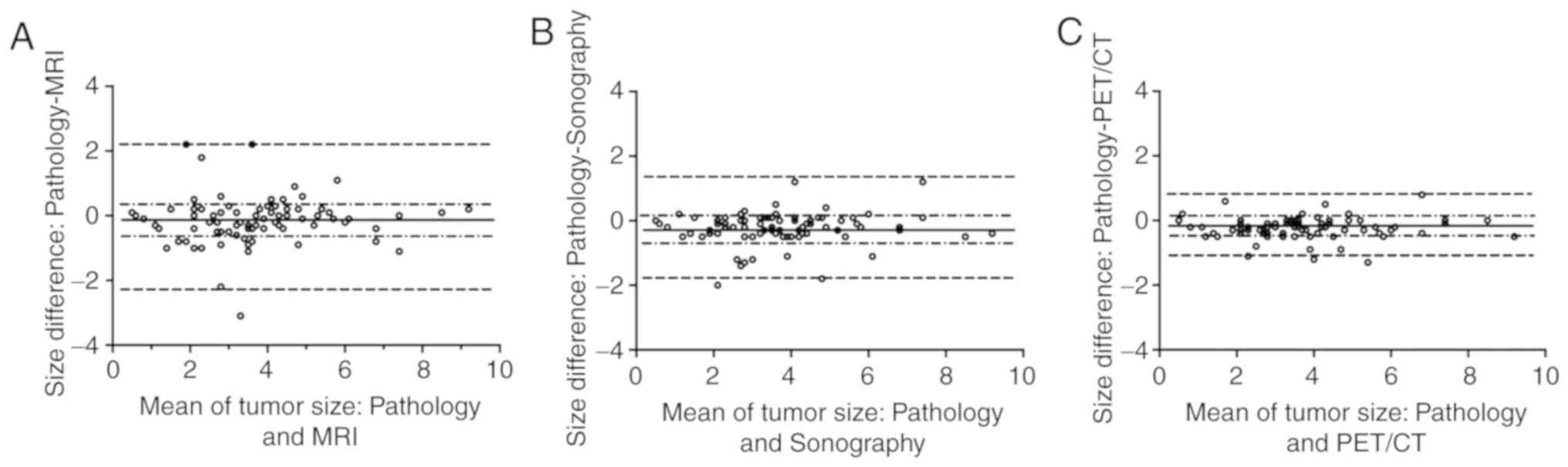
In addition, 18F-FDG PET/CT demonstrated
a smaller bias in tumor size estimation compared with sonography
and MRI in Bland-Altman analysis. 18F-FDG PET/CT-derived
tumor size exhibited a high concordance with pathological tumor
size [0.95; 95% confidence interval (CI), 0.92–0.97] using the ICC
test, which was significantly superior to sonography (0.83; 95% CI,
0.81–0.89) and MRI (0.77; 95% CI, 0.65–0.84; Fig. 4).
Association of sonography, MRI and
18F-FDG PET/CT according to pathology T stage in
patients with breast cancer
In the analysis of subgroups classified by tumor T
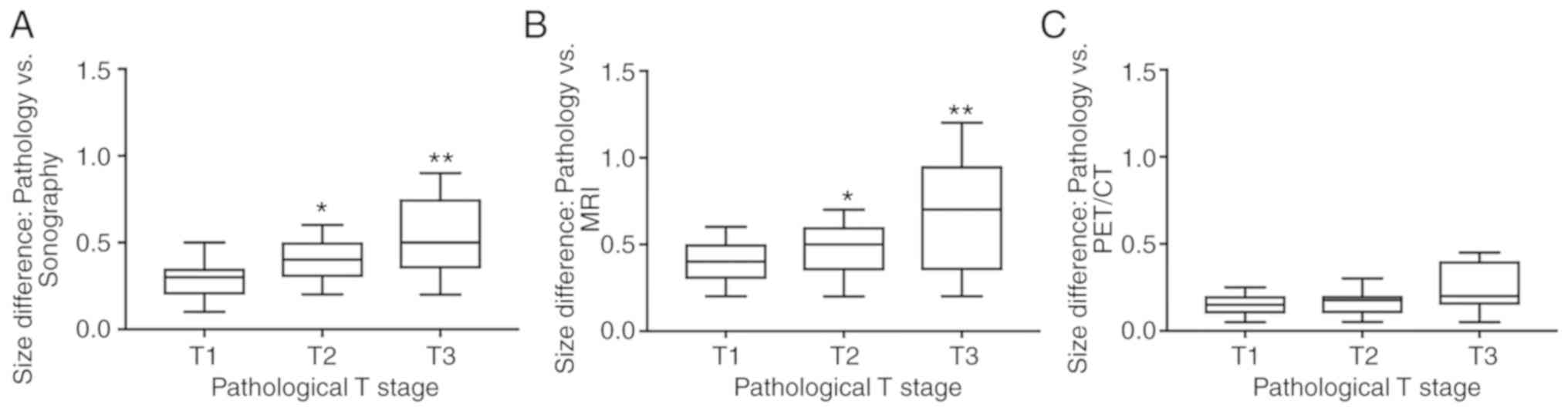
stage, 18F-FDG PET/CT compared with sonography or MRI
demonstrated significantly lower size differences (vs. pathology)
in T2 and T3 stage tumors (P<0.05), but not in T1 stage tumors
(Fig. 5). However, there were cases
where T stage was wrongly estimated using imaging. Table III presents the T stages and tumor
sizes of 12 patients whose T stages were incorrect on sonography,
MRI or 18F-FDG PET/CT. For sonography, there were 5
patients whose tumor T stages were overestimated (3 cases were
considered T2 instead of T1 and 1 was considered T3 instead of T2;
Table III). MRI-assessed T stages
were incorrect for 7 patients, of which 5 cases were upstaged (3
cases from T1 to T2; 1 case from T1 to T3 and 1 case from T2 to T3;
Table III) while 2 cases were
down-staged (1 case from T3 to T2 and 1 case T2 to T1; Table III). A total of 2 patients were
upstaged in 18F-FDG PET/CT assessment (1 case was
upstaged from T1 to T2 and 1 case from T2 to T3; Table III). Among the mismatched 12 cases,
1 case was incorrectly upstaged from T2 to T3 in sonography, MRI
and 18F-FDG PET/CT.
 | Table III.Evaluation of 12 discordant cases of
pathological T stage (22). |
Table III.
Evaluation of 12 discordant cases of
pathological T stage (22).
|
| T stage | Size differences
(vs. PET/CT, mm) |
|---|
|
|
|
|
|---|
| Serial no. | Pathology | Sonography | MRI | PET/CT | Sonography | MRI | PET/CT |
|---|
| 1 | T1 | T2 | T1 | T1 | 0.6 | 0.3 | 0.2 |
| 2 | T1 | T2 | T1 | T1 | 0.8 | 0.2 | 0.2 |
| 3 | T1 | T2 | T2 | T1 | 0.4 | 0.5 | 0.1 |
| 4 | T1 | T3 | T1 | T1 | 1.1 | 0.3 | 0.1 |
| 5 | T2 | T3 | T3 | T3 | 0.3 | 0.5 | 0.3 |
| 6 | T1 | T1 | T3 | T1 | 0.2 | 1.2 | 0.1 |
| 7 | T1 | T1 | T2 | T1 | 0.5 | 0.8 | 0.4 |
| 8 | T3 | T1 | T2 | T3 | 0.7 | 0.4 | 0.2 |
| 9 | T2 | T2 | T1 | T2 | 0.6 | 0.2 | 0.5 |
| 10 | T1 | T1 | T2 | T1 | 0.1 | 0.5 | 0.6 |
| 11 | T2 | T1 | T2 | T2 | 0.7 | 0.3 | 0.2 |
| 12 | T1 | T1 | T1 | T2 | 0.3 | 0.2 | 0.5 |
Additionally, 18F-FDG PET/CT also
demonstrated a significant advantage in accuracy of predicting
tumor size compared with sonography and MRI in different
pathological subgroups of tumors. The concordance rates for of
sonography, MRI and 18F-FDG PET/CT were 45.4, 31.8 and
69.2%, respectively, in ductal adenocarcinoma, while for non-ductal
adenocarcinoma, the concordance rates for sonography, MRI and
18F-FDG PET/CT were 41.2, 29.6 and 72.5%, respectively
(data not shown).
Discussion
Previous studies have demonstrated that in early
breast cancer, there is no association between long-term survival
and mastectomy with tumors of relatively small size (5–9). In
addition, complete removal of the tumor reduces the probability of
recurrence (1). Thus, estimating the
size and area of extension of the tumor accurately is important for
chemoradiotherapy decision and surgical treatment. As a
non-invasive method, 18F-FDG PET/CT is applied in
clinical practice. However, FDG PET/CT is not recommended as a
priority option in clinical practice due to poor spatial
resolution, which limits clear delineation of the tumor boundary.
Studies comparing the reliability of 18F-FDG PET/CT with
MRI or sonography in terms of tumor size assessment of breast
cancer are limited (20). The
present study demonstrated that using PHL, the results from the
18F-FDG PET/CT method had a higher accuracy and
association with pathological tumor size compared with those
obtained by MRI and sonography.
It has been previously reported that tumor size is
often underestimated by sonography (31), which was also observed in the current
study. Several hypotheses have been proposed to explain these
discrepancies. Hieken et al (32), demonstrated that these discrepancies
are derived from extensive intraductal in situ components caused by
unclear margins of sonography. In the study by Gruber et al
(31), a novel technique called
panoramic mode, which allows a complete image to be built from
individual sectional sonographic images, was used in order to
obtain more accurate results of the tumor with the diameters
exceeding the width of the transducer. This may solve the issue of
tumor size underestimation with the use of sonography to a certain
degree. Regarding MRI, a previously published study revealed that
pre-operative MRI was associated with lower re-operation rates for
close/positive margins (P<0.05) (3), which affirmed the capacity of MRI for
breast tumor assessment. However, a previous study reported an
overestimation of tumor size using MRI (33), which is consistent with results
obtained in the present study. To the best of our knowledge, the
present study is so far the first to compare the reliability and
association among 18F-FDG PET/CT, MRI and sonography in
breast cancer tumor size assessment. The present study, provides
further guidance for choosing a more effective diagnostic method
for estimating the tumor size in patients with breast cancer.
Since 18F-FDG PET/CT was first
recommended as a method to estimate tumor size, there are several
studies describing the advantages of 18F-FDG PET/CT for
staging breast cancer (34–37). However, only N staging (lymph node
detection) and M staging (distant metastasis) were took into
consideration in these studies, whereas the present study added T
stage for tumor size measurement as well. Furthermore, several
studies reported 18F-FDG PET/CT to have low concordance
with pathology in terms of T stage (38–40),
while the present study detected a good consistency with pathology
and even a lower bias of tumor size. An unclear tumor margin on
18F-FDG PET/CT, arising from its inherently low
resolution and from the confounding factor of surrounding
physiological breast uptake, is likely the main reason why PET/CT
is not used for tumor size evaluation (20). Recently, a method using PHL has been
reported as reliable for measuring tumor volume in cases of thyroid
cancer (41). A recent report
suggested that PHL can enhance tumor margin detection on
18F-FDG PET/CT (41).
In the present study, the longest tumor diameters
obtained with 18F-FDG PET/CT scan compared with MRI and
sonography, exhibited statistically significant smaller differences
and more linear associations with pathological tumor size. This
demonstrated that tumor size estimation by 18F-FDG
PET/CT scan can be more accurate than by sonography and MRI and can
provide a reliable reference for the decision of an appropriate
surgical strategy and prognostic prediction. In T stage assessment,
MRI and sonography displayed higher sizes differences (vs.
pathology) compared with 18F-FDG PET/CT in T2 and T3
stage tumors, but not in T1 stage tumors. Furthermore, MRI
assessments resulted in 2 patients being incorrectly upstaged as
T3, while this error was avoided in 18F-FDG PET/CT
assessments. Size differences by 18F-FDG PET/CT were
also smaller compared to MRI and sonography in 12 incorrectly
staged patients. Overestimation of tumor size can be a serious
mistake, as it may deprive patients of the opportunity of
undergoing breast-conservation therapy, which is a simpler and more
superficial type of therapy compared with mastectomy (4). Thus, tumor size estimates using
18F-FDG PET/CT may be more reliable in guiding surgical
strategy for large-sized tumors and 18F-FDG PET/CT could
be recommended as a better imaging modality in breast cancer tumor
estimation compared with other imaging methods.
The present study has several limitations. First, as
all the patients are enrolled from a single institution, this may
lead to a lack of appropriate representation and selection bias.
However, this may not affect the results severely, since the
purpose of the present study was simply to evaluate and compare the
reliability of a tumor size measurement method using PHL and the
included cases exhibited various tumor sizes. Second, investigator
influence during malignancy assessment of the results due to
previous knowledge of the results derived from other imaging
techniques cannot be excluded. Third, the pathological size that
was used as a reference standard was solely based on previous
pathological reports, while there may be changes in pathological
measurement during preservation and preparation of each tissue
specimen. Additionally, the bias during sonography should be taken
into consideration as it is more likely to be operator-dependent.
Last, because of the flat character of imaging analysis and the
non-planar growth form of the tumors, only the larger diameter
could be measured with the image. Despite the aforementioned
limitations, the results of the present study suggest that the
18F-FDG PET/CT-based PHL method was superior to breast
ultrasound and MRI.
In conclusion, the present study has demonstrated
that 18F-FDG PET/CT-based PHL can accurately estimate
tumor size in patients with breast cancer. Despite the fact that
this method may overestimate small size tumors, it is superior to
sonography and MRI, with greater association and reliability for
pathological tumor size assessment in breast cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YW performed patient enrollment according to the
exclusion and inclusion criteria of the study, performed
experiments and analyzed the data. YL and CX performed experiments,
analyzed the data and drafted the manuscript. BL designed and
conceived this study. All authors have read and approved the
manuscript.
Ethics approval and consent to
participate
The retrospective study was approved by The Ethics
Committee of Changxing People's Hospital (Huzhou, China; approval
no. CPH 201505). All patients provided signed informed consent.
Patient consent for publication
All patients enrolled in this study have provided
consent for the publication of their examination information.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
18F-FDG PET/CT
|
positron emission tomography/computed
tomography using 2-deoxy-2-fluoro-18-fluoro-D-glucose
|
|
PHL
|
peri-tumoral halo uptake layer
|
|
MRI
|
magnetic resonance imaging
|
|
ICC
|
intraclass correlation coefficient
|
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ghoncheh M, Pournamdar Z and Salehiniya H:
Incidence and mortality and epidemiology of breast cancer in the
world. Asian Pac J Cancer Prev. 17((S3)): 43–46. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
McGhan LJ, Wasif N, Gray RJ, Giurescu ME,
Pizzitola VJ, Lorans R, Ocal IT, Stucky CC and Pockaj BA: Use of
preoperative magnetic resonance imaging for invasive lobular
cancer: Good, better, but maybe not the best? Ann Surg Oncol. 17
(Suppl 3):255–262. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pop CF, Stanciu-Pop C, Drisis S,
Radermeker M, Vandemerckt C, Noterman D, Moreau M, Larsimont D,
Nogaret JM and Veys I: The impact of breast MRI workup on tumor
size assessment and surgical planning in patients with early breast
cancer. Breast J. 24:927–933. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Miller AB, Baines CJ, To T and Wall C:
Canadian National Breast Screening Study: 1. Breast cancer
detection and death rates among women aged 40 to 49 years. CMAJ.
147:1459–1476. 1992.PubMed/NCBI
|
|
6
|
Don S, Choi E and Min D: Breast mass
segmentation in digital mammography using graph cuts. Convergence
and Hybrid Information Technology. Lee G, Howard D and Ślęzak D:
Vol. 206:Springer. (Berlin, Heidelberg). 88–96. 2011. View Article : Google Scholar
|
|
7
|
Brem RF, Ioffe M, Rapelyea JA, Yost KG,
Weigert JM, Bertrand ML and Stern LH: Invasive lobular carcinoma:
Detection with mammography, sonography, MRI, and breast-specific
gamma imaging. AJR Am J Roentgenol. 192:379–383. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sarno A, Mettivier G and Russo P:
Dedicated breast computed tomography: Basic aspects. Med Phys.
42:2786–2804. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Onesti JK, Mangus BE, Helmer SD and Osland
JS: Breast cancer tumor size: Correlation between magnetic
resonance imaging and pathology measurements. Am J Surg.
196:844–848; discussion 849-850. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Narod SA: Age of diagnosis, tumor size,
and survival after breast cancer: Implications for mammographic
screening. Breast Cancer Res Treat. 128:259–266. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nyström L, Rutqvist LE, Wall S, Lindgren
A, Lindqvist M, Rydén S, Andersson I, Bjurstam N, Fagerberg G,
Frisell J, et al: Breast cancer screening with mammography:
Overview of Swedish randomised trials. Lancet. 341:973–978. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pilewskie M, Hirsch A, Eaton A, Stempel M
and Gemignani ML: Breast cancer in the elderly: Is MRI helpful?
Breast J. 21:651–657. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hatada I, Hayashizaki Y, Hirotsune S,
Komatsubara H and Mukai T: A genomic scanning method for higher
organisms using restriction sites as landmarks. Proc Natl Acad Sci
USA. 88:9523–9527. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Boetes C, Veltman J, van Die L, Bult P,
Wobbes T and Barentsz JO: The role of MRI in invasive lobular
carcinoma. Breast Cancer Res Treat. 86:31–37. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lo GG, Ai V, Chan JKF, Li KW, Cheung PSY,
Wong TT, Ma M, Lee R and Chien D: Diffusion-weighted magnetic
resonance imaging of breast lesions: First experiences at 3 T. J
Comput Assist Tomogr. 33:63–69. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kuhl CK, Schrading S, Leutner CC,
Morakkabati-Spitz N, Wardelmann E, Fimmers R, Kuhn W and Schild HH:
Mammography, breast ultrasound, and magnetic resonance imaging for
surveillance of women at high familial risk for breast cancer. J
Clin Oncol. 23:8469–8476. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Burkett BJ and Hanemann CW: A review of
supplemental screening ultrasound for breast cancer: Certain
populations of women with dense breast tissue may benefit. Acad
Radiol. 23:1604–1609. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Paydary K, Seraj SM, Zadeh MZ,
Emamzadehfard S, Shamchi SP, Gholami S, Werner TJ and Alavi A: The
evolving role of FDG-PET/CT in the diagnosis, staging, and
treatment of breast cancer. Mol Imaging Biol. 21:1–10. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Uematsu T, Kasami M and Yuen S: Comparison
of FDG PET and MRI for evaluating the tumor extent of breast cancer
and the impact of FDG PET on the systemic staging and prognosis of
patients who are candidates for breast-conserving therapy. Breast
Cancer. 16:97–104. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nogami Y, Iida M, Banno K, Kisu I, Adachi
M, Nakamura K, Umene K, Masuda K, Tominaga E, Tanaka K, et al:
Application of FDG-PET in cervical cancer and endometrial cancer:
Utility and future prospects. Anticancer Res. 34:585–592.
2014.PubMed/NCBI
|
|
21
|
Park SH, Seo M, Choi HJ, Bae K, Bang M and
Jun S: More accurate than MRI measurement of tumor size in breast
cancer by using the peri-tumoral halo uptake layer method of the
18F-FDG PET/CT scan. Hell J Nucl Med. 21:108–114. 2018.PubMed/NCBI
|
|
22
|
Jun S, Kim H and Nam HY: A new method for
segmentation of FDG PET metabolic tumour volume using the
peritumoural halo layer and a 10-step colour scale. A study in
patients with papillary thyroid carcinoma. Nucl Med (Stuttg).
54:272–285. 2015.
|
|
23
|
Singletary SE, Allred C, Ashley P, Bassett
LW, Berry D, Bland KI, Borgen PI, Clark GM, Edge SB, Hayes DF, et
al: Staging system for breast cancer: revisions for the 6th edition
of the AJCC Cancer Staging Manual. Surg Clin North Am. 83:803–819.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mendelson E, Baum J, Berg W, Merritt C and
Rubin E: Breast imaging reporting and data system. BI-RADS.
2003.
|
|
25
|
Zhengfeng L: Diagnostic ultrasound system
quality control testing methods. Chin Med Dev. 07:27–32. 2011.(In
Chinese).
|
|
26
|
Hengdi W: Image quality control standards
and procedures of MRI devices. POSTRUM. 28:19–23. 2013.(In
Chinese).
|
|
27
|
Jun Y, Jianwei W, Shuyue A and Wei H:
Quality control and administration of PET/CT. Chin Med Euipment J.
27:71–73. 2006.(In Chinese).
|
|
28
|
Grimsby GM, Gray R, Dueck A, Carpenter S,
Stucky CC, Aspey H, Giurescu ME and Pockaj B: Is there concordance
of invasive breast cancer pathologic tumor size with magnetic
resonance imaging? Am J Surg. 198:500–504. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Luparia A, Mariscotti G, Durando M, Ciatto
S, Bosco D, Campanino PP, Castellano I, Sapino A and Gandini G:
Accuracy of tumour size assessment in the preoperative staging of
breast cancer: Comparison of digital mammography, tomosynthesis,
ultrasound and MRI. Radiol Med (Torino). 118:1119–1136. 2013.
View Article : Google Scholar
|
|
30
|
Lai HW, Chen DR, Wu YC, Chen CJ, Lee CW,
Kuo SJ, Chen ST and Wu HK: Comparison of the diagnostic accuracy of
magnetic resonance imaging with sonography in the prediction of
breast cancer tumor size: A concordance analysis with
histopathologically determined tumor size. Ann Surg Oncol.
22:3816–3823. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gruber I, Rueckert M, Kagan K, Staebler A,
Siegmann KC, Hartkopf A, Wallwiener D and Hahn M: Measurement of
tumour size with mammography, sonography and magnetic resonance
imaging as compared to histological tumour size in primary breast
cancer. BMC Cancer. 13:3282013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hieken TJ, Harrison J, Herreros J and
Velasco JM: Correlating sonography, mammography, and pathology in
the assessment of breast cancer size. Am J Surg. 182:351–354. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jethava A, Ali S, Wakefield D, Crowell R,
Sporn J and Vrendenburgh J: Diagnostic accuracy of MRI in
predicting breast tumor size: Comparative analysis of MRI vs
histopathological assessed breast tumor size. Conn Med. 79:261–267.
2015.PubMed/NCBI
|
|
34
|
Caresia Aroztegui AP, García Vicente AM,
Alvarez Ruiz S, Delgado Bolton RC, Orcajo Rincon J, Garcia Garzon
JR, de Arcocha Torres M and Garcia-Velloso MJ: 18F-FDG PET/CT in
breast cancer: Evidence-based recommendations in initial staging.
Tumour Biol. 39:10104283177282852017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pritchard KI, Julian JA, Holloway CM,
McCready D, Gulenchyn KY, George R, Hodgson N, Lovrics P, Perera F,
Elavathil L, et al: Prospective study of
2-[18F]fluorodeoxyglucose positron emission tomography
in the assessment of regional nodal spread of disease in patients
with breast cancer: An Ontario clinical oncology group study. J
Clin Oncol. 30:1274–1279. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Riegger C, Herrmann J, Nagarajah J,
Hecktor J, Kuemmel S, Otterbach F, Hahn S, Bockisch A, Lauenstein
T, Antoch G, et al: Whole-body FDG PET/CT is more accurate than
conventional imaging for staging primary breast cancer patients.
Eur J Nucl Med Mol Imaging. 39:852–863. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Avril N, Rosé CA, Schelling M, Dose J,
Kuhn W, Bense S, Weber W, Ziegler S, Graeff H and Schwaiger M:
Breast imaging with positron emission tomography and fluorine-18
fluorodeoxyglucose: Use and limitations. J Clin Oncol.
18:3495–3502. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hyun SH, Ahn HK, Park YH, Im YH, Kil WH,
Lee JE, Nam SJ, Cho EY and Choi JY: Volume-based metabolic tumor
response to neoadjuvant chemotherapy is associated with an
increased risk of recurrence in breast cancer. Radiology.
275:235–244. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kim J, Yoo SW, Kang SR, Cho SG, Oh JR,
Chong A, Min JJ, Bom HS, Yoon JH and Song HC: Prognostic
significance of metabolic tumor volume measured by (18)F-FDG PET/CT
in operable primary breast cancer. Nucl Med Mol Imaging.
46:278–285. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Oh JR, Seo JH, Chong A, Min JJ, Song HC,
Kim YC and Bom HS: Whole-body metabolic tumour volume of 18F-FDG
PET/CT improves the prediction of prognosis in small cell lung
cancer. Eur J Nucl Med Mol Imaging. 39:925–935. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jun S, Park JG and Seo Y: Accurate FDG PET
tumor segmentation using the peritumoral halo layer method: A study
in patients with esophageal squamous cell carcinoma. Cancer
Imaging. 18:352018. View Article : Google Scholar : PubMed/NCBI
|