Introduction
A glioma is a tumour produced by glial cells and is
the most common primary malignant tumour in the central nervous
system, accounting for ~30% of the tumours in this region (1,2).
Astrocytoma is one of the most aggressive gliomas, with a poor
prognosis (3,4). Astrocytomas can be found in various
parts of the central nervous system and are a common
neuroepithelial tumour that can occur in individuals of all ages
(5). The average survival time
ranges from 17 weeks to 3 years (6).
The tumours develop slowly and progressively, and epilepsy is often
the first symptom. Approximately 50% of patients experience the
onset of epilepsy and the majority of patients experience
headaches, psychomotor muscle weakness, vomiting and an obvious
disturbance of consciousness (7).
According to the 2016 World Health Organization
(WHO) classification, astrocytoma can be divided into four grades
(I–IV) based on its histological and morphological characteristics
(8): Low-grade astrocytomas are
classified as grades I and II, and high-grade astrocytomas are
classified as grades III and IV. The prognosis of high-grade
astrocytoma is very poor and the average survival time is only
0.6–0.7 years (9).
The growth pattern of astrocytomas in the brain
consists of invasive or local invasive growth, and as the
invasiveness increases, the tumour grade increases and the survival
rate decreases (10). At present,
the conventional treatment is primarily surgical resection
supplemented by radiotherapy and chemotherapy (11). There are a number of surgical methods
for astrocytoma, including excisional biopsy (EB), subtotal
resection (STR), gross resection (GR), partial resection (PR) and
gross total resection (GTR). Although the efficacy of conventional
treatment has made some progress in recent years, the prognosis for
astrocytoma patients is still poor, which is primarily associated
with incomplete resection, recurrence and radiotherapy and
chemotherapy resistance after surgical resection (12–14).
Therefore, it is important to find an optimal treatment for
patients with astrocytoma.
The purpose of the present study was to use the
Surveillance, Epidemiology and End Results (SEER) database to
characterize the different therapies for patients with astrocytoma
at a population level and the recommendations for treatment
use.
Patients and methods
Data source and patients
The SEER database includes ~28% of the US population
and collects demographic information, primary tumour location,
tumour grade, tumour stage, treatment method and survival time data
for patients with cancer (15). The
National Cancer Institute SEER*Stat software [version 8.3.5; SEER
18 Regs Custom Data (with additional treatment field), Nov 2017 Sub
(1973–2015 varying) database] was used to identify 46,717 patients
diagnosed with astrocytoma between January 2004 and December 2015.
Histological ICD-O-3 codes (The 3rd edition of The International
Classification of Diseases for Oncology) (16) were used to select the following
subtypes: Subependymal giant cell astrocytoma, malignant (ICD-O-3
code 9384/3); astrocytoma, NOS (ICD-O-3 code 9400/3); astrocytoma,
anaplastic (ICD-O-3 code 9401/3); protoplasmic astrocytoma (ICD-O-3
code 9410/3); gemistocytic astrocytoma (ICD-O-3 code 9411/3);
fibrillary astrocytoma (ICD-O-3 code 9420/3); pilocytic
astrocytoma, malignant (ICD-O-3 code 9421/3); pleomorphic
xanthoastrocytoma (ICD-O-3 code 9424/3); glioblastoma, NOS (ICD-O-3
code 9440/3); giant cell glioblastoma (ICD-O-3 code 9441/3); and
gliosarcoma (ICD-O-3 code 9442/3).
The exclusion criteria in the present study were: i)
Unknown survival time (n=206); ii) unknown household income (n=2);
iii) age <18 years (n=3,597); and surgical code not 00, 20, 21,
30, 40 or 55 (n=688; http://seer.cancer.gov/manuals/2018/AppendixC/Surgery_Codes_Brain_2018.pdf).
Ultimately, a total of 42,224 eligible patients diagnosed with
astrocytoma.
There were several methods used to confirm the
diagnosis of patients in the SEER database, such as histological
diagnosis and radiography. Overall, 90.7% of patients were
confirmed by positive histological diagnosis and 7.8% by
radiography (Fig. S1).
Study variables
The study variables in the present study included
age at diagnosis, year of diagnosis, sex, ethnicity, marital
status, urban-rural residence, household income, summary stage, and
surgical, radiotherapy and chemotherapy information. According to
the surgical code, patients were divided into four groups: No
surgery (code 00), EB (code 20), STR (code 21), GR (code 30), PR
(code 40) and GTR (code 55) (17).
Astrocytomas were divided into four groups according to the 2016
WHO classification: Grade I, grade II, grade III and grade IV
(8). Demographic and
clinicopathological characteristics included age at diagnosis
(18–40, 41–60, 61–80 and >80 years), sex (male and female),
ethnicity (white, black and other), marital status (married,
unmarried and unknown), urban-rural residence (metropolitan and
non-metropolitan), summary stage (localized, regional, distant and
unstaged/unknown), radiotherapy (yes or no) and chemotherapy (yes
or no). Household income was divided into three groups: Low-income
group (<4,219), middle-income group (4,219–5,191) and
high-income group (>5,191). Overall survival (OS) and
cancer-specific survival (CSS) were the primary endpoints of the
present study.
Statistical analysis
The OS time corresponded to the length of time from
the date of diagnosis to the death from any cause or the date on
which data were censored. When analyzing CSS, mortality cases
associated with other causes were excluded. SPSS version 20.0 (IBM
Corp.) was used for all statistical analyses. χ2 tests
were used to analyze factors associated with the surgical methods.
Kaplan-Meier curve analyses and the log-rank test were used to
analyze the OS and CSS times of patients with regard to different
surgical methods and other variables. Multivariate Cox regression
was used to analyze factors associated with OS and CSS. P<0.05
was considered to indicate a statistically significant
difference
Results
Demographic and clinicopathological
characteristics of the astrocytoma
A total of 42,224 eligible astrocytoma patients from
between January 2004 and 2015 December were included in the present
study cohort. Among them, 11,427 (27.1%) patients did not receive
surgery, 7,661 (18.1%) received EB, 5,520 (13.1%) received STR,
6,037 (14.3%) received GR, 5,314 (12.6%) received PR and 6,265
(14.8%) received GTR (Fig. 1A).
Table I shows the demographic and
clinicopathological characteristics of patients with astrocytoma
and the association between surgical method and each variable as
analyzed by the χ2 test. χ2 tests showed that
age of diagnosis, year of diagnosis, sex, ethnicity, marital
status, urban-rural residence, household income, summary stage,
radiotherapy and chemotherapy information were all associated
factors (all P<0.001). Among all 42,224 patients, over time, the
number of patients diagnosed with astrocytoma increased. The
majority of patients were white [37,462 (88.7%); Table I], between 41–80 years old [33,344
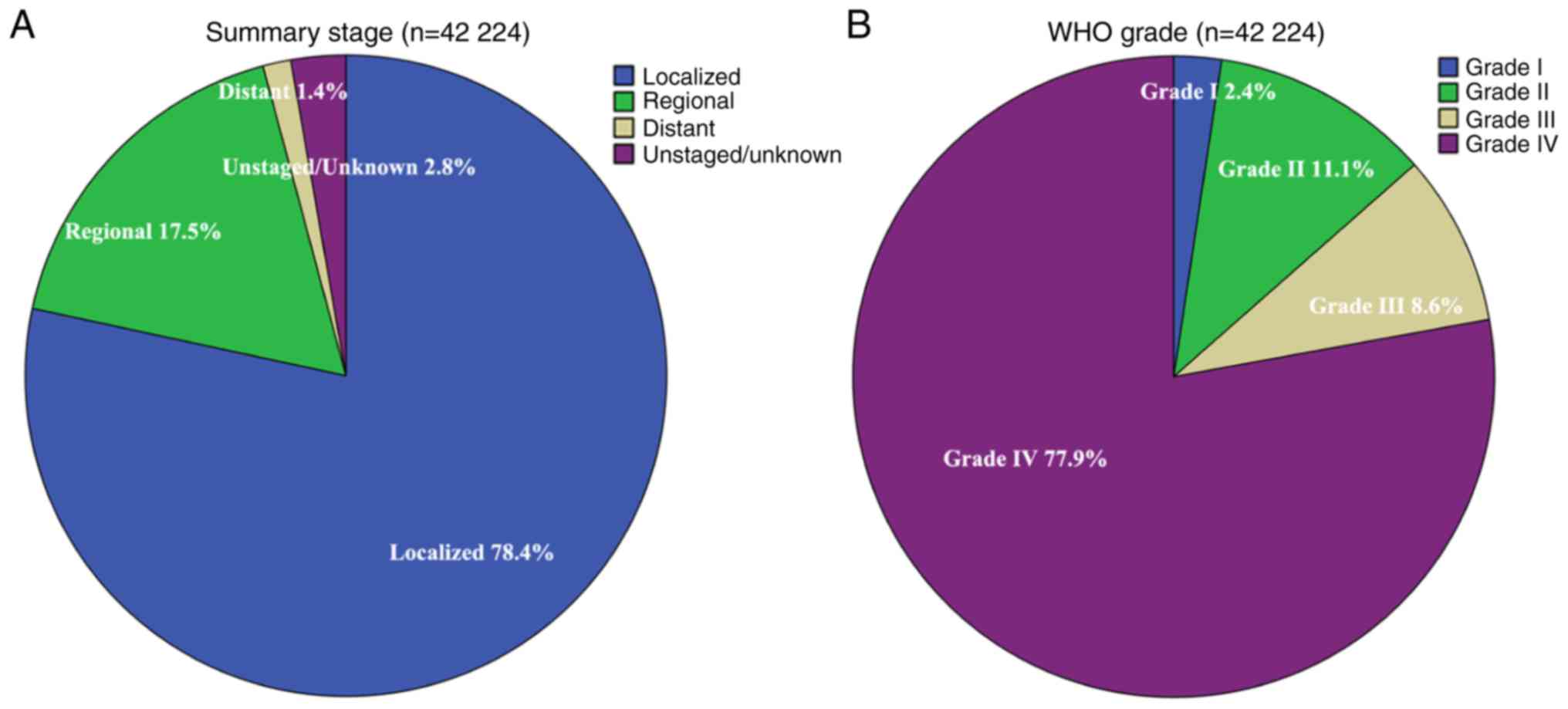
(79.0%)], had localized disease [33,090 (78.4%); Fig. 2A] and were WHO grade IV [32,876
(77.9%); Fig. 2B].
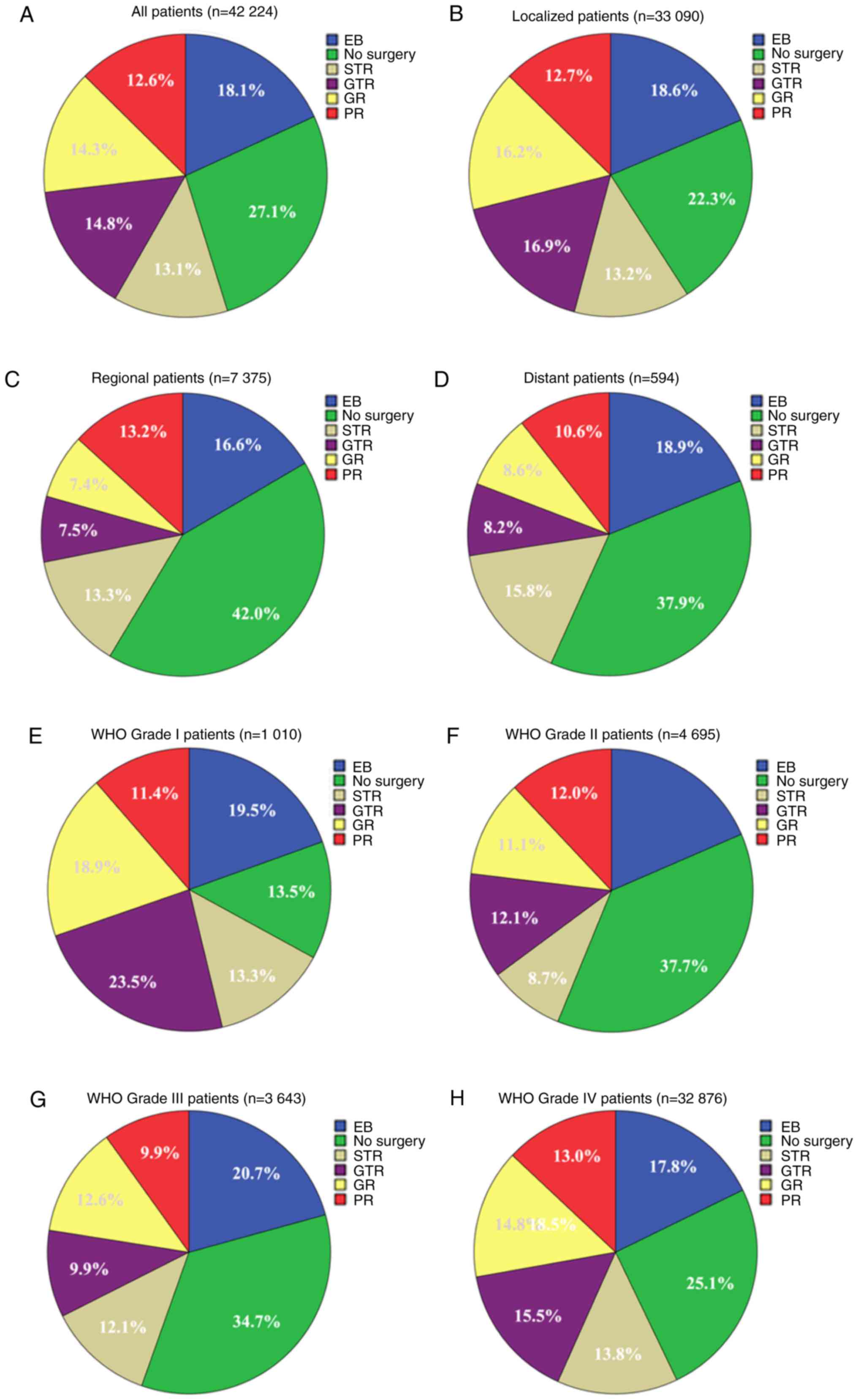 | Figure 1.Number and proportion of surgical
methods performed on patients with astrocytoma with different
summary statuses between 2004 and 2015. (A) All patients, n=42,224.
(B) Localized patients, n=33,090. (C) Regional patients, n=7,375.
(D) Distant patients, n=594. (E) WHO grade I (7) patients, n=1,010. (F) WHO grade II
patients, n=4,695. (G) WHO grade III patients, n=3,643. (H) WHO
grade IV patients, n=32,876. WHO, World Health Organization; EB,
excision biopsy; STR, subtotal resection; GR, gross resection; PR,
partial resection; GTR, gross total resection. |
 | Table I.Characteristics of patients with
astrocytoma stratified by surgical method. |
Table I.
Characteristics of patients with
astrocytoma stratified by surgical method.
| Characteristic | n (%) | No surgery, n
(%) | EB, n (%) | STR, n (%) | GR, n (%) | PR, n (%) | GTR, n (%) | P-valuea |
|---|
| Total patients | 42,224 | 11,427 (27.1) | 7,661 (18.1) | 5,520 (13.1) | 6,037 (14.3) | 5,314 (12.6) | 6,265 (14.8) |
|
| Age at diagnosis |
|
|
|
|
|
|
| <0.001 |
|
18–40 | 4,991 (11.8) | 924 (18.5) | 947 (19.0) | 701 (14.0) | 886 (17.8) | 623 (12.5) | 910 (18.2) |
|
|
41–60 | 14,445 (34.2) | 3,028 (21.0) | 2,736 (18.9) | 2,035 (14.1) | 2,198 (15.2) | 2,043 (14.1) | 2,405 (16.6) |
|
|
61–80 | 18,899 (44.8) | 5,269 (27.9) | 3,454 (18.3) | 2,497 (13.2) | 2,691 (14.2) | 2,358 (12.5) | 2,630 (13.9) |
|
|
>80 | 3,889 (9.2) | 2,206 (56.7) | 524 (13.5) | 287 (7.4) | 162 (6.7) | 290 (7.5) | 320 (8.2) |
|
| Year of
diagnosis |
|
|
|
|
|
|
| <0.001 |
|
2004 | 3,281 (7.8) | 1,000 (30.5) | 390 (11.9) | 43 (1.3) | 50 (1.5) | 778 (23.7) | 1,020 (31.1) |
|
|
2005 | 3,182 (7.5) | 1,003 (31.5) | 321 (10.1) | 39 (1.2) | 54 (1.7) | 788 (24.8) | 977 (30.7) |
|
|
2006 | 3,180 (7.5) | 961 (30.2) | 388 (12.2) | 26 (0.8) | 50 (1.6) | 724 (22.8) | 1,031 (32.4) |
|
|
2007 | 3,432 (8.1) | 997 (29.1) | 544 (15.9) | 30 (0.9) | 54 (1.6) | 846 (24.7) | 961 (28.0) |
|
|
2008 | 3,408 (8.1) | 1,039 (30.5) | 919 (27.0) | 16 (0.5) | 46 (1.3) | 751 (22.0) | 637 (18.7) |
|
|
2009 | 3,462 (8.2) | 1,008 (29.1) | 1,055 (30.5) | 99 (2.9) | 97 (2.8) | 625 (18.1) | 578 (16.7) |
|
|
2010 | 3,504 (8.3) | 890 (25.4) | 876 (25.0) | 570 (16.3) | 473 (13.5) | 363 (10.4) | 332 (9.5) |
|
|
2011 | 3,581 (8.5) | 949 (26.5) | 678 (18.9) | 840 (23.5) | 805 (22.5) | 114 (3.2) | 195 (5.4) |
|
|
2012 | 3,764 (8.9) | 894 (23.8) | 627 (16.7) | 913 (24.3) | 1,065 (28.3) | 105 (2.8) | 160 (4.3) |
|
|
2013 | 3,783 (9.0) | 912 (24.1) | 652 (17.2) | 980 (25.9) | 1,027 (27.1) | 74 (2.0) | 138 (3.6) |
|
|
2014 | 3,776 (8.9) | 842 (22.3) | 604 (16.0) | 1,007 (26.7) | 1,102 (29.2) | 89 (2.4) | 132 (3.5) |
|
|
2015 | 3,871 (9.2) | 932 (24.1) | 607 (15.7) | 957 (24.7) | 1,214 (31.4) | 57 (1.5) | 104 (2.7) |
|
| Sex |
|
|
|
|
|
|
| <0.001 |
|
Male | 2,4209 (57.3) | 6,254 (25.8) | 4,431 (18.3) | 3,264 (13.95) | 3,493 (14.4) | 3,125 (12.9) | 3,642 (15.0) |
|
|
Female | 18,015 (42.7) | 5,173 (28.7) | 3,230 (17.9) | 2,256 (12.5) | 2,544 (14.1) | 2,189 (12.2) | 2,623 (14.6) |
|
| Ethnicity |
|
|
|
|
|
|
| <0.001 |
|
White | 3,7462 (88.7) | 10,080 (26.9) | 6,766 (18.1) | 4,859 (13.0) | 5,391 (14.4) | 4,733 (17.4) | 5,633 (15.0) |
|
|
Black | 2,467 (5.8) | 656 (26.6) | 510 (20.7) | 332 (13.5) | 355 (14.4) | 292 (16.4) | 322 (13.1) |
|
|
Other | 2,295 (5.4) | 691 (30.1) | 385 (16.8) | 329 (14.3) | 291 (12.7) | 289 (17.3) | 310 (13.5) |
|
| Marital status |
|
|
|
|
|
|
| <0.001 |
|
Married | 2,5825 (61.2) | 6,536 (25.3) | 4,627 (17.9) | 3,418 (13.2) | 3,790 (14.7) | 3,395 (13.1) | 4,059 (15.7) |
|
|
Unmarried | 14,824 (35.1) | 4,454 (30.0) | 2,690 (18.1) | 1,854 (12.5) | 1,978 (13.3) | 1,797 (12.1) | 2,051 (13.8) |
|
|
Unknown | 1,575 (3.7) | 437 (27.7) | 344 (21.8) | 248 (15.7) | 269 (17.1) | 122 (7.7) | 155 (9.8) |
|
| Urban-rural
residence |
|
|
|
|
|
|
| 0.002 |
|
Metropolitan | 37,086 (87.8) | 9,963 (26.9) | 6,662 (18.0) | 4,863 (13.1) | 5,362 (14.5) | 4,690 (12.6) | 5,546 (15.0) |
|
|
Non-metropolitan | 5,138 (12.2) | 1,464 (28.5) | 999 (19.4) | 657 (12.8) | 675 (13.1) | 624 (12.1) | 719 (14.0) |
|
| Income |
|
|
|
|
|
|
| <0.001 |
|
Lower | 13,219 (31.3) | 3,767 (28.5) | 2,479 (18.8) | 1,696 (12.8) | 1,942 (14.7) | 1,571 (11.9) | 1,764 (13.3) |
|
|
Middle | 14,937 (35.4) | 4,142 (27.1) | 2,648 (17.0) | 1,931 (12.9) | 2,170 (14.5) | 2,007 (13.1) | 2,341 (15.4) |
|
|
Upper | 14,068 (33.3) | 3,518 (25.7) | 2,534 (18.8) | 1,893 (13.5) | 1,925 (13.7) | 1,736 (12.7) | 2,160 (15.7) |
|
| Summary stage |
|
|
|
|
|
|
| <0.001 |
|
Localized | 33,090 (78.4) | 7,363 (22.3) | 6,169 (18.6) | 4,379 (13.2) | 5,376 (16.2) | 4,197 (12.7) | 5,606 (16.9) |
|
|
Regional | 7,375 (17.5) | 3,100 (42.0) | 1,222 (16.6) | 979 (13.3) | 547 (7.4) | 974 (13.2) | 553 (7.5) |
|
|
Distant | 594 (1.4) | 225 (37.9) | 112 (18.9) | 94 (15.8) | 51 (8.6) | 63 (10.6) | 49 (8.2) |
|
|
Unstaged/unknown | 1,165 (2.8) | 739 (63.4) | 158 (13.6) | 68 (5.8) | 63 (5.4) | 80 (6.9) | 57 (4.9) |
|
| WHO
gradeb |
|
|
|
|
|
|
| <0.001 |
| I | 1,010 (2.4) | 136 (13.5) | 197 (19.5) | 134 (13.3) | 191 (18.9) | 115 (11.4) | 237 (23.5) |
|
| II | 4,695 (11.1) | 1,770 (37.7) | 868 (18.5) | 407 (8.7) | 520 (11.1) | 564 (12.0) | 566 (12.1) |
|
|
III | 3,643 (8.6) | 1,264 (34.7) | 755 (20.7) | 442 (12.1) | 459 (12.6) | 361 (9.9) | 362 (9.9) |
|
| IV | 3,2876 (77.9) | 8,257 (25.1) | 5,841 (17.8) | 4,537 (13.8) | 4,867 (14.8) | 4,274 (13.0) | 5,100 (15.5) |
|
| Radiotherapy |
|
|
|
|
|
|
| <0.001 |
|
Yes | 28,429 (67.3) | 5,844 (20.6) | 5,296 (18.6) | 4,177 (14.7) | 4,629 (16.3) | 3,858 (13.6) | 46,25 (16.3) |
|
| No | 13,795 (32.7) | 5,583 (40.5) | 2,365 (17.1) | 1,343 (9.7) | 1,408 (10.2) | 1,456 (10.6) | 1,640 (11.9) |
|
| Chemotherapy |
|
|
|
|
|
|
| <0.001 |
|
Yes | 24,910 (59.0) | 4,601 (18.5) | 4,708 (18.9) | 3,869 (15.5) | 4,311 (17.3) | 3,280 (13.2) | 4,141 (16.6) |
|
| No | 17,314 (41.0) | 6,826 (39.4) | 2,953 (17.1) | 1,651 (9.5) | 1,726 (10.0) | 2,034 (11.7) | 2,124 (12.3) |
|
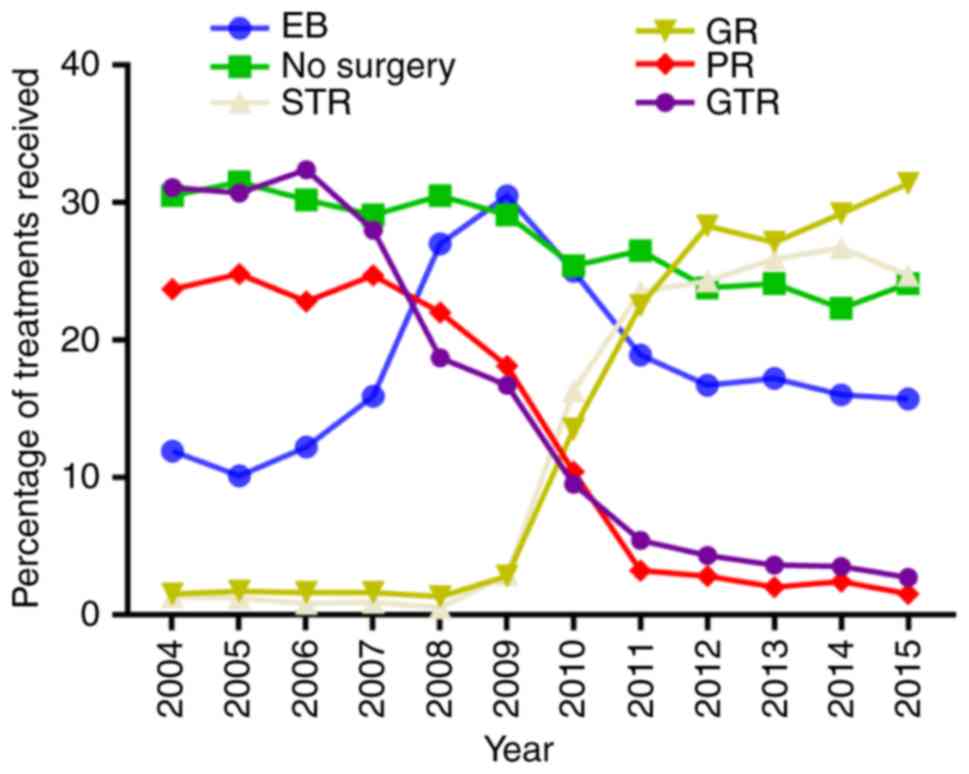
Among all 42,224 patients, the proportion of
patients who underwent STR or GR increased between 2004 and 2015
[43/3,281 patients (1.3%) vs. 957/3,871 (24.7%), P<0.001; and
50/3,281 patients (1.5%) vs. 1,214/3,871 (31.4%), P<0.001,
respectively]. However, the proportion of patients who did not
undergo surgery, PR or GTR decreased between 2004 and 2015
[1,000/3,281 patients (30.5%) vs. 932/3,871 (24.1%), P<0.001;
778/3,281 patients (23.7%) vs. 57/3,871 (1.5%), P<0.001; and
1,020/3,281 patients (31.1%) vs. 104/3,871 (2.7%), P<0.001]
(Fig. 3).
Subgroup analysis for evaluating the
proportion of different surgical methods based on SEER stage and
grade
The proportion of different surgical methods based
on summary stage and grade were evaluated. As shown in Table I and Fig.
1B-D, compared with patients with regional and distant
astrocytoma, patients with localized astrocytoma were more likely
to undergo GR (16.2 vs. 7.4 or 8.6%) and GTR (16.9 vs. 7.5 or
8.2%). In addition, patients with WHO grade I and IV were more
likely to undergo GR (18.9 and 14.8 vs. 11.1 and 12.6%; P<0.001)
and GTR (23.5 and 15.5 vs. 12.1 and 9.9%; P<0.001) compared with
WHO grade II and III (Table I;
Fig. 1E-H).
Effects of different variables on OS
and CSS in patients with astrocytoma
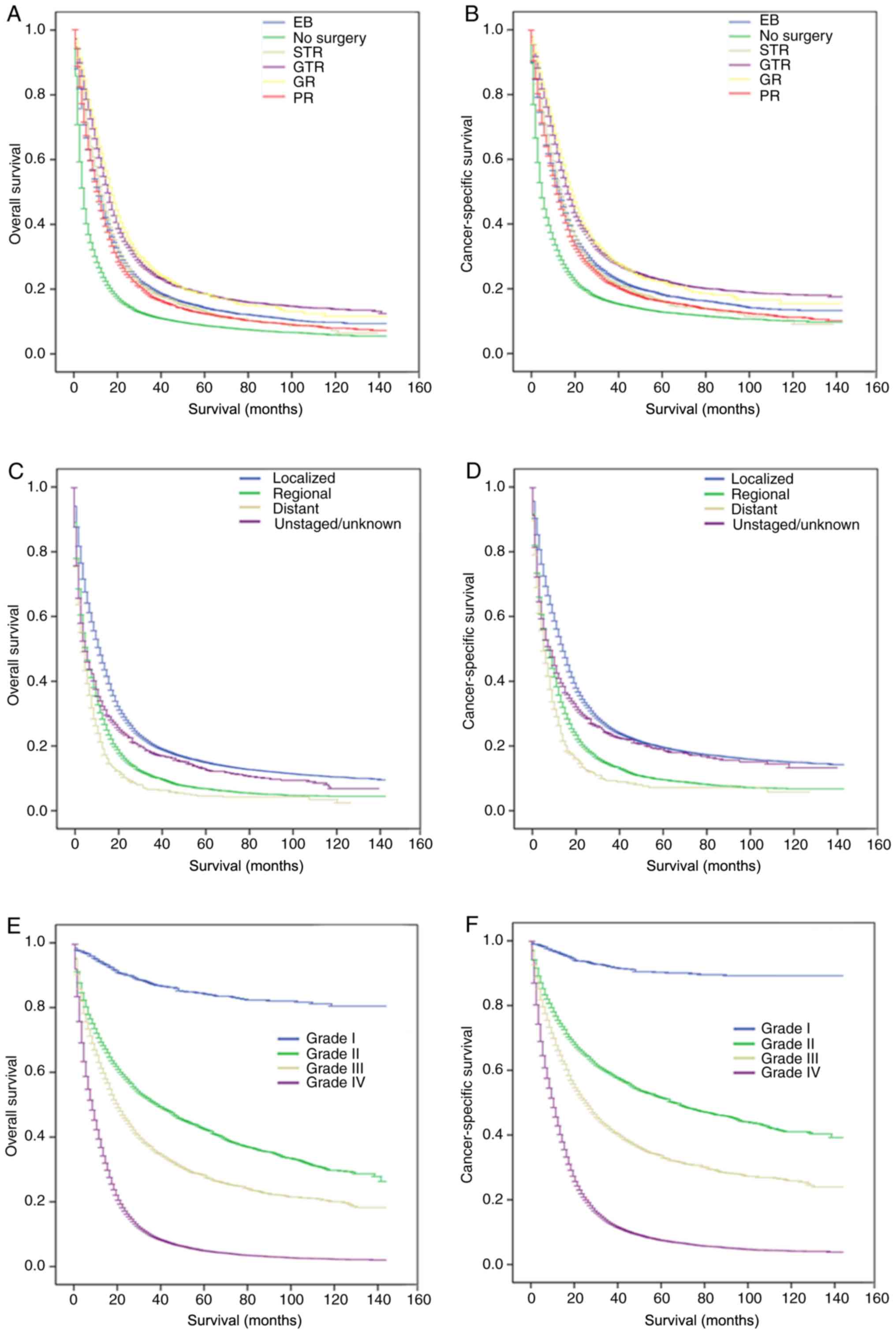
Kaplan-Meier curves were constructed to analyze the
influence of clinical factors on the OS and CSS of patients with
astrocytoma (Table II).
Kaplan-Meier analysis showed that age at diagnosis, ethnicity,
marital status, urban-rural residence, household income, summary
stage, WHO grade, and surgical, radiotherapy and chemotherapy
information were significantly associated with OS and CSS (all
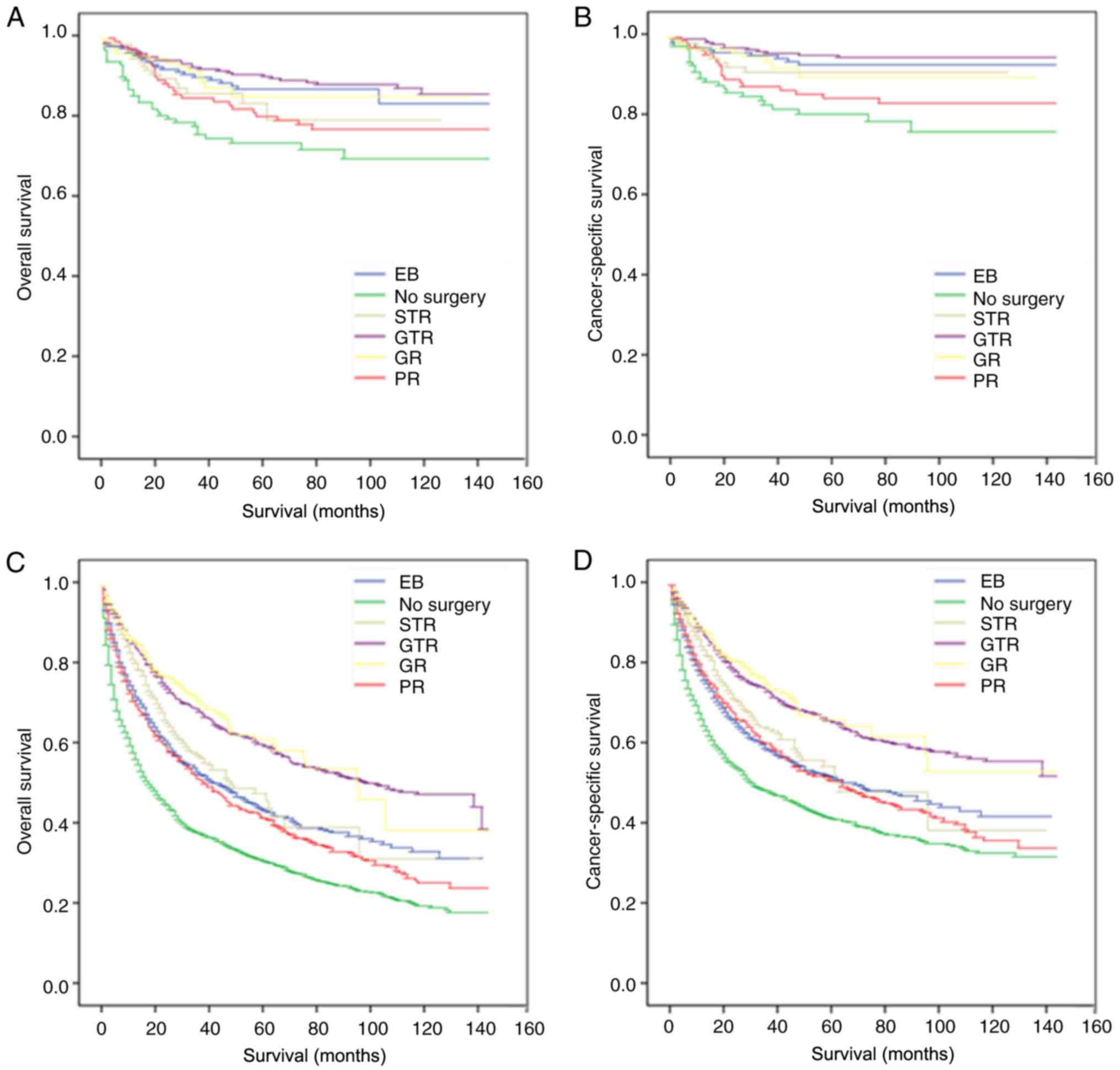
P<0.05). Patients who underwent GR or GTR had longer OS median
survival times (MSTs) (17.00 and 15.00 months) and higher CSS MST
(19.00 and 17.00 months) compared with those in the other surgical
groups (Fig. 4A and B).
 | Table II.Kaplan-Meier analysis overall
survival and cancer-specific survival for patients with
astrocytoma. |
Table II.
Kaplan-Meier analysis overall
survival and cancer-specific survival for patients with
astrocytoma.
|
|
| Kaplan-Meier |
| Kaplan-Meier |
|---|
|
|
|
|
|
|
|---|
| Characteristic | OS MST, months | Log-rank test | P-value | CSS MST,
months | Log-rank test | P-value |
|---|
| Age at diagnosis,
years |
| 12,929.451 | <0.001 |
| 10,171.401 | <0.001 |
|
18–40 | 77.00 |
|
| 112.00 |
|
|
|
41–60 | 15.00 |
|
| 17.00 |
|
|
|
61–80 | 6.00 |
|
| 8.00 |
|
|
|
>60 | 2.00 |
|
| 3.00 |
|
|
| Sex |
| 2.209 | 0.137 |
| 4.125 | 0.042 |
|
Male | 10.00 |
|
| 13.00 |
|
|
|
Female | 10.00 |
|
| 12.00 |
|
|
| Ethnicity |
| 116.719 | <0.001 |
| 143.124 | <0.001 |
|
White | 10.00 |
|
| 12.00 |
|
|
|
Black | 11.00 |
|
| 15.00 |
|
|
|
Other | 14.00 |
|
| 18.00 |
|
|
| Marital status |
| 7.893 | 0.019 |
| 6.171 | 0.046 |
|
Married | 11.00 |
|
| 13.00 |
|
|
|
Unmarried | 8.00 |
|
| 11.00 |
|
|
|
Unknown | 10.00 |
|
| 13.00 |
|
|
| Urban-rural
residence |
| 87.138 | <0.001 |
| 72.102 | <0.001 |
|
Metropolitan | 11.00 |
|
| 8.00 |
|
|
|
Non-metropolitan | 8.00 |
|
| 6.00 |
|
|
| Income |
| 122.487 | <0.001 |
| 82.768 | <0.001 |
|
Lower | 9.00 |
|
| 11.00 |
|
|
|
Middle | 10.00 |
|
| 12.00 |
|
|
|
Upper | 11.00 |
|
| 14.00 |
|
|
| Summary stage |
| 1,208.088 | <0.001 |
| 1,097.821 | <0.001 |
|
Localized | 12.00 |
|
| 14.00 |
|
|
|
Regional | 6.00 |
|
| 7.00 |
|
|
|
Distant | 4.00 |
|
| 5.00 |
|
|
|
Unstaged/unknown | 5.00 |
|
| 8.00 |
|
|
| WHO grade |
| 6,264.594 | <0.001 |
| 5,846.311 | <0.001 |
| I | – |
|
| – |
|
|
| II | 39.00 |
|
| 66.00 |
|
|
|
III | 20.00 |
|
| 25.00 |
|
|
| IV | 8.00 |
|
| 10.00 |
|
|
| Surgery |
| 3212.688 | <0.001 |
| 2,281.789 | <0.001 |
| No
surgery | 4.00 |
|
| 5.00 |
|
|
| EB | 11.00 |
|
| 13.00 |
|
|
|
STR | 12.00 |
|
| 14.00 |
|
|
| GR | 17.00 |
|
| 19.00 |
|
|
| PR | 10.00 |
|
| 12.00 |
|
|
|
GTR | 15.00 |
|
| 17.00 |
|
|
| Radiotherapy |
| 1,377.534 | <0.001 |
| 712.575 | <0.001 |
|
Yes | 13.00 |
|
| 15.00 |
|
|
| No | 3.00 |
|
| 4.00 |
|
|
| Chemotherapy |
| 1,392.837 | <0.001 |
| 697.593 | <0.001 |
|
Yes | 14.00 |
|
| 16.00 |
|
|
| No | 3.00 |
|
| 5.00 |
|
|
Identification of prognostic factors
for patients with astrocytoma
Multivariate Cox regression was used to analyze the
factors associated with OS and CSS in patients with astrocytoma
(Table III). After adjusting for
age at diagnosis, ethnicity, marital status, urban-rural residence,
household income, summary stage, WHO grade, and surgical,
radiotherapy and chemotherapy information, Cox regression indicated
that compared with EB patients, the non-surgical patients [hazard
ratio (HR), 1.45; 95% confidence interval (CI), 1.41–1.50;
P<0.001) and patients with PR (HR, 1.04; 95% CI, 1.00–1.08;
P=0.038) had less favorable OS, whereas patients with GR (HR, 0.72;
95% CI, 0.69–0.75; P<0.001) and GTR (HR, 0.80; 95% CI,
0.77–0.83; P<0.001) had more favorable OS. In terms of CSS,
compared with other patients, the non-surgical patients (vs. EB;
HR, 1.43; 95% CI, 1.38–1.49; P<0.001) had significantly lower
odds of CSS, whereas GR patients (vs. EB; HR, 0.72; 95% CI,
0.69–0.76; P<0.001) and GTR patients (vs. EB; HR, 0.80; 95% CI,
0.77–0.83; P<0.001) had significantly greater odds of CSS.
Surgical method was an independent prognostic factor for OS and CSS
in patients with astrocytoma.
 | Table III.Risk factors for overall survival and
cancer-specific survival for patients with astrocytoma. |
Table III.
Risk factors for overall survival and
cancer-specific survival for patients with astrocytoma.
|
| OS | CSS |
|---|
|
|
|
|
|---|
| Characteristic | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age at diagnosis,
years |
|
18–40 | Reference |
| Reference |
|
|
41–60 | 2.29
(2.18–2.41) | <0.001 | 2.24
(2.12–2.36) | <0.001 |
|
61–80 | 3.85
(3.66–4.05) | <0.001 | 3.66
(3.47–3.86) | <0.001 |
|
>60 | 5.85
(5.51–6.20) | <0.001 | 5.47
(5.13–5.84) | <0.001 |
| Ethnicity |
|
White | Reference |
| Reference |
|
|
Black | 0.92
(0.88–0.97) | 0.001 | 0.87
(0.82–0.91) | <0.001 |
|
Other | 0.84
(0.80–0.88) | <0.001 | 0.82
(0.77–0.86) | <0.001 |
| Marital status |
|
Married | Reference |
| Reference |
|
|
Unmarried | 1.10
(1.07–1.12) | <0.001 | 1.08
(1.05–1.10) | <0.001 |
|
Unknown | 0.96
(0.91–1.02) | 0.179 | 0.92
(0.86–0.98) | 0.009 |
| Urban-rural
residence |
|
Metropolitan | Reference |
| Reference |
|
|
Non-metropolitan | 1.05
(1.01–1.09) | 0.015 | 1.06
(1.02–1.11) | 0.003 |
| Income |
|
Lower | Reference |
| Reference |
|
|
Middle | 0.94
(0.92–0.97) | <0.001 | 0.97
(0.94–1.00) | 0.055 |
|
Upper | 0.88
(0.85–0.91) | <0.001 | 0.89
(0.86–0.92) | <0.001 |
| Summary stage |
|
Localized | Reference |
| Reference |
|
|
Regional | 1.43
(1.39–1.47) | <0.001 | 1.46
(1.41–1.50) | <0.001 |
|
Distant | 1.49
(1.36–1.62) | <0.001 | 1.53
(1.40–1.69) | <0.001 |
|
Unstaged/unknown | 0.75
(0.70–0.80) | <0.001 | 0.75
(0.70–0.81) | <0.001 |
| WHO grade |
| I | Reference |
| Reference |
|
| II | 5.01
(4.23–5.95) | <0.001 | 6.49
(5.21–8.09) | <0.001 |
|
III | 10.09
(8.50–11.98) | <0.001 | 14.23
(11.40–17.75) | <0.001 |
| IV | 18.34
(15.50–21.70) | <0.001 | 26.39
(21.22–32.82) | <0.001 |
| Surgery |
| No
surgery | 1.45
(1.41–1.50) | <0.001 | 1.43
(1.38–1.49) | <0.001 |
| EB | Reference |
| Reference |
|
|
STR | 0.95
(0.92–0.99) | 0.022 | 0.96
(0.92–1.00) | 0.068 |
| GR | 0.72
(0.69–0.75) | <0.001 | 0.72
(0.69–0.76) | <0.001 |
| PR | 1.04
(1.00–1.08) | 0.038 | 1.04
(1.00–1.08) | 0.069 |
|
GTR | 0.80
(0.77–0.83) | <0.001 | 0.80
(0.77–0.83) | <0.001 |
| Radiotherapy |
|
Yes | Reference |
| Reference |
|
| No | 1.61
(1.56–1.67) | <0.001 | 1.58
(1.52–1.64) | <0.001 |
| Chemotherapy |
|
Yes | Reference |
| Reference |
|
| No | 1.55
(1.50–1.60) | <0.001 | 1.49
(1.45–1.55) | <0.001 |
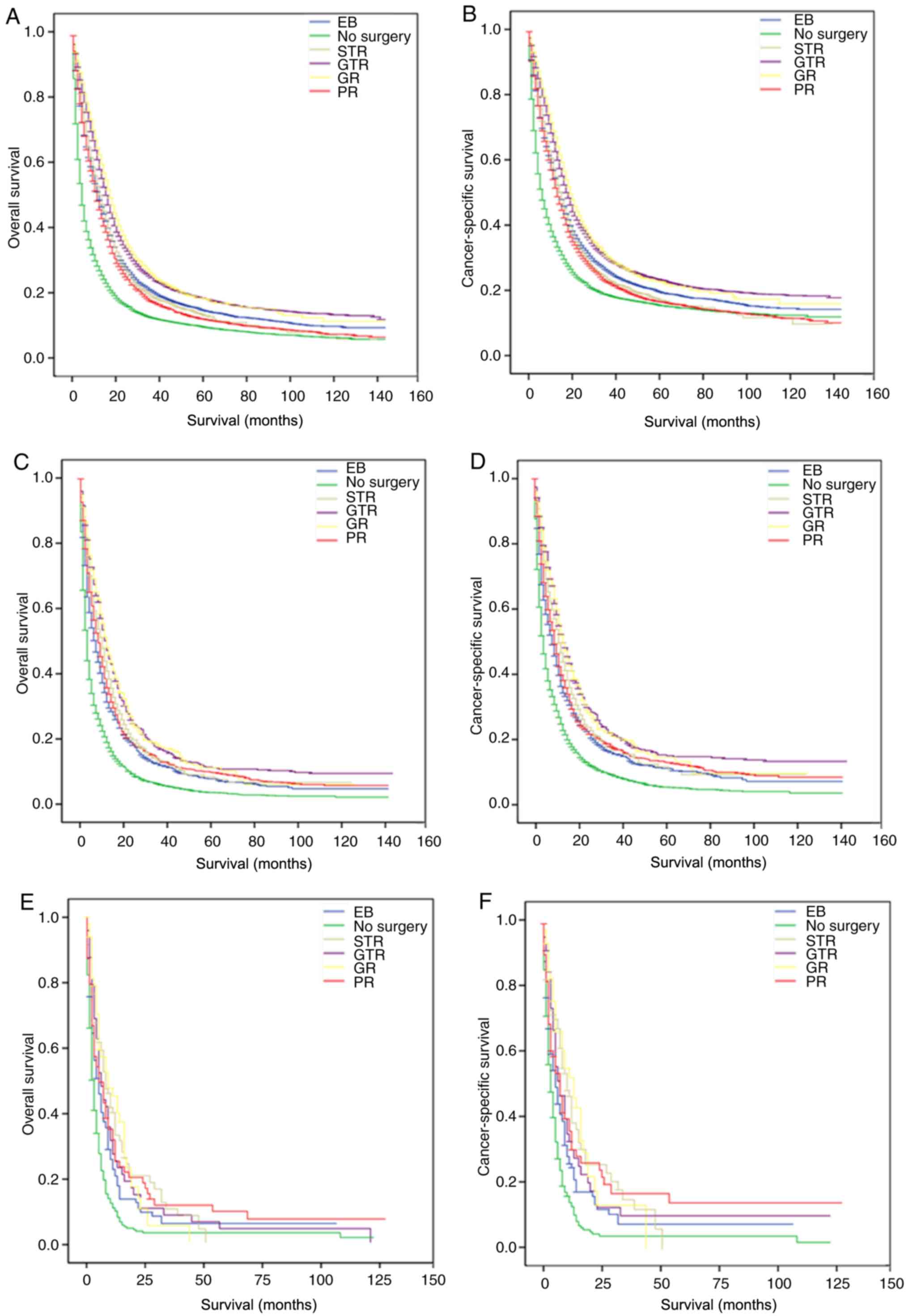
Subgroup analysis for evaluating the
effect of surgical method on OS and CSS based on summary stage and
WHO grade
Based on summary stage and WHO grade, the difference
between surgical method and prognosis among the subgroups of
astrocytoma patients was further examined (Table IV). It was found that for OS and
CSS, surgical method was still an independent prognostic factor for
patients with localized, regional, distant, grade II, grade III and
grade IV. Compared with patients with EB, patients with GTR in the
localized group (OS: HR, 0.82; 95% CI, 0.79–0.86; P<0.001; CSS:
HR, 0.82, 95% CI, 0.79–0.86; P<0.001), regional group (OS: HR,
0.68; 95% CI, 0.61–0.76; P<0.001; CSS: HR, 0.67; 95% CI,
0.59–0.75; P<0.001), distant group (OS: HR, 0.75; 95% CI,
0.53–1.06; P<0.001; CSS: H, R0.69; 95% CI, 0.47–1.00; P=0.051),
grade II group (OS: HR, 0.77; 95% CI, 0.66–0.90; P<0.001; CSS:
HR, 0.78; 95% CI, 0.66–0.92; P<0.001), grade III group (OS: HR,
0.57; 95% CI, 0.48–0.68; P<0.001; CSS: HR, 0.57; 95% CI,
0.48–0.69; P<0.001) and grade IV group (OS: HR, 0.82; 95% CI,
0.79–0.87; P<0.001; CSS: HR, 0.83; 95% CI, 0.79–0.86;
P<0.001) had higher relative survival rates. Moreover, in the
grade II and III subgroups, GTR was associated with the highest OS
and CSS MST for patients. However, for patients in the grade I
subgroup, the multivariate Cox regression showed that the surgical
method had no significant effect on OS or CSS (P>0.05). The
subtype stratification based on summary stage and WHO grade is
graphically displayed in Figs. 5 and
6, respectively.
 | Table IV.Subgroup analyses stratified by
summary stage and grade for overall survival and cancer-specific
survival for patients with. |
Table IV.
Subgroup analyses stratified by
summary stage and grade for overall survival and cancer-specific
survival for patients with.
|
|
| OS |
| CSS |
|---|
|
|
|
|
|
|
|---|
| Characteristic | OS MST, months | HR (95% CI) | P-value | CSS MST,
months | HR (95% CI) | P-value |
|---|
| Localized |
| No
surgery | 4.00 | 1.54
(1.48–1.60) | <0.001 | 6.00 | 1.51
(1.44–1.57) | <0.001 |
| EB | 12.00 | Reference | – | 15.00 | Reference | – |
|
STR | 13.00 | 0.99
(0.94–1.04) | 0.611 | 15.00 | 1.00
(0.96–1.05) | 0.879 |
| GR | 17.00 | 0.73
(0.70–0.77) | <0.001 | 19.00 | 0.74
(0.70–0.77) | <0.001 |
| PR | 11.00 | 1.07
(1.02–1.12) | 0.002 | 13.00 | 1.07
(1.02–1.12) | 0.007 |
|
GTR | 16.00 | 0.82
(0.79–0.86) | <0.001 | 14.00 | 0.82
(0.79–0.86) | <0.001 |
| Regional |
| No
surgery | 3.00 | 1.31
(1.22–1.41) | <0.001 | 4.00 | 1.30
(1.20–1.40) | <0.001 |
| EB | 7.00 | Reference | – | 8.00 | Reference | – |
|
STR | 10.00 | 0.85
(0.78–0.94) | 0.001 | 11.00 | 0.83
(0.75–0.92) | <0.001 |
| GR | 12.00 | 0.70
(0.63–0.79) | <0.001 | 13.00 | 0.71
(0.62–0.80) | <0.001 |
| PR | 8.00 | 0.94
(0.86–1.03) | 0.175 | 9.00 | 0.95
(0.86–1.04) | 0.277 |
|
GTR | 11.00 | 0.68
(0.61–0.76) | <0.001 | 13.00 | 0.67
(0.59–0.75) | <0.001 |
| Distant |
| No
surgery | 2.00 | 1.21
(0.94–1.55) | 0.133 | 2300 | 1.20
(0.92–1.56) | 0.177 |
| EB | 5.00 | Reference | – | 5.00 | Reference | – |
|
STR | 8.00 | 0.79
(0.58–1.07) | 0.131 | 10.00 | 0.72
(0.52–1.01) | 0.056 |
| GR | 9.00 | 0.65
(0.45–0.94) | 0.023 | 13.00 | 0.58
(0.39–0.88) | 0.010 |
| PR | 5.00 | 0.68
(0.49–0.94) | 0.021 | 7.00 | 0.63
(0.44–0.90) | 0.012 |
|
GTR | 6.00 | 0.75
(0.53–1.06) | 0.099 | 7.00 | 0.69
(0.47–1.00) | 0.051 |
| Grade I |
| No
surgery |
| NA | 0.112 |
| NA | 0.169 |
| EB | – | Reference | – |
| Reference | – |
|
STR | – | NA | 0.564 |
| NA | 0.388 |
| GR | – | NA | 0.823 |
| NA | 0.706 |
| PR | – | NA | 0.724 |
| NA | 0.356 |
|
GTR | – | NA | 0.885 |
| NA | 0.310 |
| Grade II |
| No
surgery | 18.00 | 1.26
(1.13–1.40) | <0.001 | 31.00 | 1.17
(1.04–1.32) | 0.012 |
| EB | 44.00 | Reference | – | 69.00 | Reference | – |
|
STR | 49.00 | 0.88
(0.73–1.06) | 0.063 | 63.00 | 0.86
(0.70–1.05) | 0.138 |
| GR | 96.00 | 0.71
(0.58–0.86) | <0.001 | – | 0.71
(0.57–0.88) | 0.002 |
| PR | 39.00 | 1.14
(0.99–1.31) | 0.062 | 64.00 | 1.05
(0.90–1.23) | 0.516 |
|
GTR | 104.00 | 0.77
(0.66–0.90) | 0.001 | – | 0.78
(0.66–0.92) | 0.004 |
| Grade III |
| No
surgery | 8.00 | 1.45
(1.30–1.62) | <0.001 | 10.00 | 1.51
(1.34–1.69) | <0.001 |
| EB | 20.00 | Reference | – | 24.00 | Reference | – |
|
STR | 35.00 | 0.85
(0.72–1.01) | 0.064 | 47.00 | 0.83
(0.69–1.00) | 0.050 |
| GR | 72.00 | 0.55
(0.45–0.67) | <0.001 | 75.00 | 0.55
(0.45–0.68) | <0.001 |
| PR | 29.00 | 1.02
(0.87–1.18) | 0.836 | 33.00 | 1.02
(0.87–1.20) | 0.809 |
|
GTR | 78.00 | 0.57
(0.48–0.68) | <0.001 | 119.00 | 0.57
(0.48–0.69) | <0.001 |
| Grade IV |
| No
surgery | 3.00 | 1.45
(1.40–1.51) | <0.001 | 4.00 | 1.42
(1.37–1.48) | <0.001 |
| EB | 9.00 | Reference | – | 10.00 | Reference | – |
|
STR | 10.00 | 0.98
(0.94–1.02) | 0.339 | 11.00 | 0.99
(0.95–1.04) | 0.703 |
| GR | 14.00 | 0.74
(0.71–0.77) | <0.001 | 16.00 | 0.75
(0.710.78) | <0.001 |
| PR | 9.00 | 1.03
(0.99–1.08) | 0.138 | 10.00 | 1.04
(0.99–1.08) | 0.116 |
|
GTR | 13.00 | 0.82
(0.79–0.87) | <0.001 | 14.00 | 0.83
(0.79–0.86) | <0.001 |
Discussion
The present study used a large, population-based
database to quantitatively compare the impact of four different
surgical methods on survival in patients with astrocytoma. The
effect of surgical method on OS and CSS rate in patients with
astrocytoma was analyzed and it was found that surgical method was
an independent prognostic factor for patients with astrocytoma.
Patients in the GR and GTR groups had higher OS and CSS time MST
compared with those in the non-surgical, EB and PR groups and
similar results were obtained in subgroup analyses based on summary
stage and grade. However, although GR and GTR had higher OS and CSS
time, it was observed that the percentage of patients who underwent
GTR decreased between 2004 and 2015.
Surgical resection serves a key role in the
management of patients with grade I, II and III astrocytoma
(18). For patients with grade I
astrocytoma, surgical resection is usually effective and these
patients rarely receive radiotherapy and chemotherapy (19). Johnson et al (20) retrospectively analyzed 865 adult
patients with pilocytic astrocytoma aged 20 years and older, and
reported that GTR was a significant predictor of survival compared
with STR or biopsy. Several studies have shown that the extent of
resection can affect the OS of patients with grade II and III
astrocytoma (21–23). Fouladi et al (24) found that for patients with
pleomorphic xanthoastrocytoma, GTR without adjuvant therapy
prolonged disease control. Moreover, for patients with glioblastoma
(GBM), there is evidence to support the benefit of GTR with regard
to survival (25,26). A large single-center study based on
1,229 patients with GBM showed that GTR significantly prolonged MST
compared with incomplete resection (27).
In the present study, statistical analysis of all
patients with astrocytoma was performed, demonstrating that GTR and
GR were beneficial for the survival of astrocytoma patients and
could reduce the risk of death. Subsequently, a stratified analysis
based on the summary stage and WHO grade was conducted and showed
that GTR was beneficial for OS and CSS. For patients with
localized, regional and distant astrocytoma, GR was associated with
the longest OS and CSS time MST, whereas GTR was associated with
similar survival times and benefits. In the stratified analysis
according to WHO stage, the benefits of GTR were more prominent
compared with other analyses, and GTR could lead to the longest OS
and CSS time MST in patients with grade II and III.
Maximizing the benefit of resection is a core
principle of neurosurgical oncology and every effort should be made
to achieve GTR during the initial surgery. The present study
observed that the proportion of patients who received STR increased
from 2004 to 2015. This may be since studies have shown that
patients who underwent STR of thalamic and brain stem gliomas had a
relatively good prognosis (28,29).
Minehan et al (30) studied
136 patients with spinal astrocytoma and found that 11 patients
with GTR had the shortest median survival time, which may be the
reason for the decrease in the proportion of patients with GTR.
In the present study of patients with astrocytoma,
the mortality rate of patients treated with PR was higher compared
with that of patients receiving EB, STR, GR or GTR. After further
stratified analysis of the summary stage, it was observed that this
effect gradually weakened with increased stage. For patients with
distant summary stage, PR was more beneficial compared with GTR.
This phenomenon was further analyzed and it was indicated that this
may be associated with the fact that PR produces a smaller residual
tumour volume compared with EB, which can slow the tumour growth
rate. In addition, GTR is more traumatic for the patient compared
with PR (31). Total surgical
resection should be considered with caution by the surgeon, as it
is strictly dependent on the anatomical location of the tumour, as
well as the presence of patient comorbidities (17). Therefore, for different patients with
astrocytoma, different and individual treatments are necessary.
There are limitations to the present study. Firstly,
the SEER database is a retrospective dataset with its own
retrospective study limitations. Secondly, the patients' physical
conditions were unclear and patients with several comorbidities may
pursue more conservative treatment. Thirdly, there may be selection
bias with patients receiving GTR compared with STR. This may be due
to the surgeons who would consider the postoperative complications
of GTR surgery for astrocytoma patients. In addition, for
chemotherapy and radiotherapy, the present study does not
distinguish whether adjuvant or neoadjuvant therapy was used and
there is no information on the specific radiotherapy technique,
including dose, fractionation and beam energy, or chemotherapy
regimen used.
In the present study, it was demonstrated that the
survival benefit of GTR was higher compared with unsuccessful or
not attempted GTR, therefore more patients need to be encouraged to
undergo GTR to improve OS and CSS times.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and material
The datasets generated and/or analyzed during the
present study are available in the Surveillance, Epidemiology, and
End Results Program repository (seer.cancer.gov/).
Authors' contributions
HM and XL were involved in the study conception and
design. HM collected and assembled data. HM and WM were involved in
data analysis and interpretation. HM and XL wrote the manuscript.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
No applicable.
Patient consent for publication
No applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Teng YD, Abd-El-Barr M, Wang L, Hajiali H,
Wu L and Zafonte RD: Spinal cord astrocytomas: Progresses in
experimental and clinical investigations for developing recovery
neurobiology-based novel therapies. Exp Neurol. 311:135–147. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Iida T, Tomogane Y, Takagi T, Miyaji Y,
Sakamoto D, Yoshida Y, Ishikura R, Ando K, Nakagomi N, Hirota S and
Yoshimura S: Grading of astrocytomas using the PRESTO (principles
of echo-shifting with a train of observations) magnetic resonance
imaging sequence. Clin Neurol Neurosurg. 173:91–95. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xie JC, Yang S, Liu XY and Zhao YX:
Marital status is associated with survival of patients with
astrocytoma. J Clin Neurosci. 56:79–87. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wen PY and Kesari S: Malignant gliomas in
adults. N Engl J Med. 359:492–507. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ostrom QT, Cioffi G, Gittleman H, Patil N,
Waite K, Kruchko C and Barnholtz-Sloan JS: CBTRUS statistical
report: Primary brain and other central nervous system tumors
diagnosed in the United States in 2012–2016. Neuro Oncol. 21 (Suppl
5):v1–v100. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Walker DG and Kaye AH: Diagnosis and
management of astrocytomas, oligodendrogliomas and mixed gliomas: A
review. Australas Radiol. 45:472–482. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Donofrio CA, Gagliardi F, Callea M, da
Passano CF, Terreni MR, Cavalli A, Spina A, Acerno S, Bailo M,
Elbabaa SK and Mortini P: Pediatric cerebellar pilocytic
astrocytoma presenting with spontaneous intratumoral hemorrhage.
Neurosurg Rev. 2018.(Epub ahead of print). PubMed/NCBI
|
|
8
|
Louis DN, Perry A, Reifenberger G, von
Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD,
Kleihues P and Ellison DW: The 2016 world health organization
classification of tumors of the central nervous system: A summary.
Acta Neuropathol. 131:803–820. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Weidmann MJ: Neurosurgery. Med J Aust.
161:392–394. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tavares CB, Gomes-Braga FDCS, Sousa EB,
Borges US, Escórcio-Dourado CS, Silva-Sampaio JPD and Silva BBD:
Evaluation of estrogen receptor expression in low-grade and
high-grade astrocytomas. Rev Assoc Med Bras (1992). 64:1129–1133.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Guastella AR, Michelhaugh SK, Klinger NV,
Fadel HA, Kiousis S, Ali-Fehmi R, Kupsky WJ, Juhász C and Mittal S:
Investigation of the aryl hydrocarbon receptor and the intrinsic
tumoral component of the kynurenine pathway of tryptophan
metabolism in primary brain tumors. J Neurooncol. 139:239–249.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Delgado-Lopez PD, Corrales-Garcia EM,
Martino J, Lastra-Aras E and Duenas-Polo MT: Diffuse low-grade
glioma: A review on the new molecular classification, natural
history and current management strategies. Clin Transl Oncol.
19:931–944. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Patel S, DiBiase S, Meisenberg B, Flannery
T, Patel A, Dhople A, Cheston S and Amin P: Phase I clinical trial
assessing temozolomide and tamoxifen with concomitant radiotherapy
for treatment of high-grade glioma. Int J Radiat Oncol Biol Phys.
82:739–742. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ghotme KA, Barreto GE, Echeverria V,
Gonzalez J, Bustos RH, Sanchez M, Leszek J, Yarla NS, Gomez RM,
Tarasov VV, et al: Gliomas: New perspectives in diagnosis,
treatment and prognosis. Curr Top Med Chem. 17:1438–1447. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mao W, Huang X, Kong M, Fan J and Geng J:
More lymph node dissection improves survival in patients with newly
diagnosed lymph node-positive penile cancer. Int Urol Nephrol.
51:641–654. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mao W, Kong M, Yu H, Wang D, Huang X, Yao
X, Fan J and Geng J: Prognosis and treatment differences between
initial and second primary chondrosarcoma. Oncol Lett. 18:207–218.
2019.PubMed/NCBI
|
|
17
|
Alattar AA, Brandel MG, Hirshman BR, Dong
X, Carroll KT, Ali MA, Carter BS and Chen CC: Oligodendroglioma
resection: A surveillance, epidemiology, and end results (SEER)
analysis. J Neurosurg. 128:1076–1083. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dong X, Noorbakhsh A, Hirshman BR, Zhou T,
Tang JA, Chang DC, Carter BS and Chen CC: Survival trends of grade
I, II, and III astrocytoma patients and associated clinical
practice patterns between 1999 and 2010: A SEER-based analysis.
Neurooncol Pract. 3:29–38. 2016.PubMed/NCBI
|
|
19
|
Karajannis M, Allen JC and Newcomb EW:
Treatment of pediatric brain tumors. J Cell Physiol. 217:584–589.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Johnson DR, Brown PD, Galanis E and
Hammack JE: Pilocytic astrocytoma survival in adults: Analysis of
the surveillance, epidemiology, and end results program of the
national cancer institute. J Neurooncol. 108:187–193. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Keles GE, Chang EF, Lamborn KR, Tihan T,
Chang CJ, Chang SM and Berger MS: Volumetric extent of resection
and residual contrast enhancement on initial surgery as predictors
of outcome in adult patients with hemispheric anaplastic
astrocytoma. J Neurosurg. 105:34–40. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Smith JS, Chang EF, Lamborn KR, Chang SM,
Prados MD, Cha S, Tihan T, Vandenberg S, McDermott MW and Berger
MS: Role of extent of resection in the long-term outcome of
low-grade hemispheric gliomas. J Clin Oncol. 26:1338–1345. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
McGirt MJ, Chaichana KL, Attenello FJ,
Weingart JD, Than K, Burger PC, Olivi A, Brem H and
Quinoñes-Hinojosa A: Extent of surgical resection is independently
associated with survival in patients with hemispheric infiltrating
low-grade gliomas. Neurosurgery. 63:700–707. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fouladi M, Jenkins J, Burger P, Langston
J, Merchant T, Heideman R, Thompson S, Sanford A, Kun L and Gajjar
A: Pleomorphic xanthoastrocytoma: Favorable outcome after complete
surgical resection. Neuro Oncol. 3:184–192. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lacroix M, Abi-Said D, Fourney DR,
Gokaslan ZL, Shi W, DeMonte F, Lang FF, McCutcheon IE, Hassenbusch
SJ, Holland E, et al: A multivariate analysis of 416 patients with
glioblastoma multiforme: Prognosis, extent of resection, and
survival. J Neurosurg. 95:190–198. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sanai N and Berger MS: Glioma extent of
resection and its impact on patient outcome. Neurosurgery.
62:753–764; discussion 264–756. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li YM, Suki D, Hess K and Sawaya R: The
influence of maximum safe resection of glioblastoma on survival in
1229 patients: Can we do better than gross-total resection? J
Neurosurg. 124:977–988. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Freeman CR and Suissa S: Brain stem tumors
in children: Results of a survey of 62 patients treated with
radiotherapy. Int J Radiat Oncol Biol Phys. 12:1823–1828. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Grigsby PW, Thomas PR, Schwartz HG and
Fineberg BB: Multivariate analysis of prognostic factors in
pediatric and adult thalamic and brainstem tumors. Int J Radiat
Oncol Biol Phys. 16:649–655. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Minehan KJ, Brown PD, Scheithauer BW,
Krauss WE and Wright MP: Prognosis and treatment of spinal cord
astrocytoma. Int J Radiat Oncol Biol Phys. 73:727–733. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hongo H, Takai K, Komori T and Taniguchi
M: Intramedullary spinal cord ependymoma and astrocytoma:
Intraoperative frozen-section diagnosis, extent of resection, and
outcomes. J Neurosurg Spine. 30:133–139. 2019. View Article : Google Scholar
|