Introduction
Despite progress in the treatment of cancer through
surgery, radiotherapy, and chemotherapy, the incidence of cancer
remains high, which is largely due to the aging and growth of the
world population. This high incidence of cancer is also associated
with an increase in cancer-causing behaviours, particularly
smoking, in economically developing countries (1), which is concomitant to moderate
improvements in the overall five-year survival rate for oral cancer
(2,3). Therefore, new treatments are needed to
improve the care and outcome of oral cancer patients. Oral squamous
cell carcinoma (OSCC) is the most common type of oral cancer.
Notably, OSCC cells are highly invasive, which frequently leads to
local recurrence and distant lymphatic metastasis (4,5). Hence,
a better understanding of tumour cell invasion mechanisms is
essential to develop more potent treatments for OSCC.
Initially identified during embryogenesis,
epithelial-mesenchymal transition (EMT) has been described as an
essential process related to cell differentiation and morphogenesis
(6). Importantly, EMT induction has
also been associated with tumour progression, tumour cell invasion,
and metastasis (7,8), including in OSCC (9). When EMT occurs, cancer cells lose
epithelial characteristics and acquire mesenchymal properties,
including fibroblast-like morphology, changes in gene expression
patterns, and increased motility. Moreover, the outgrowth of
metastases is generally associated with self-renewal, a defining
trait of cancer stem cells (CSCs). Notably, EMT induction has also
been shown to confer CSC-like characteristics to tumour cells
(10), and such changes have been
associated with increased cell invasion, metastasis, and resistance
to chemotherapy (11–13).
Hepatocyte growth factor (HGF) and its receptor
c-Met have been implicated in EMT in numerous types of cancer
(14). Notably, activation of the
HGF/c-Met signalling pathway has been shown to promote cancer cell
scattering and invasion. Curcumin has been extensively studied for
its potential chemopreventive and anti-tumour activities in
colorectal cancer (15,16). Furthermore, curcumin has been shown
to reduce the expression of EMT markers and act as a potent
inhibitor of EMT in various cancers (17–20).
However, the potential effects of curcumin on EMT in oral cancer
cells remain to be clarified. In this study, we aimed to examine
the potential effects of curcumin on HGF-induced EMT in an OSCC
cell line. In addition, we analysed invasion by an invasion assay
and gelatin zymography, and metastasis by a scratch wound healing
cell migration assay. This study may serve as a stepping stone
towards the development of novel treatments for oral cancer.
Materials and methods
Cell culture and reagents
The human tongue-derived OSCC cell line HSC-4 and
Ca9-22 was purchased from the RIKEN BioResource Center (Ibaraki,
Japan). HSC-4 and Ca9-22 cells were cultured in Dulbecco's modified
Eagle medium (DMEM) supplemented with 10% (v/v) foetal bovine serum
(FBS) in a humidified incubator at 37°C and 5% CO2. DMEM
and FBS were purchased from Gibco (Life Technologies, Tokyo,
Japan). HGF and curcumin were purchased from Sigma-Aldrich. All
antibodies used in this study were commercially available and
included antibodies against c-Met and phosphorylated-cMet
(phospho-c-Met, Tyr1234/1235) (Cell Signaling Technology),
α-tubulin (Sigma-Aldrich), E-cadherin and vimentin (Merck
Millipore), and ERK and phospho-Tyr204-ERK (Tyr204) (both from
Santa Cruz Biotechnology).
Scratch wound healing cell migration
assay
Cell migration was determined using a scratch wound
healing assay, as described previously (21,22),
except for the following modifications. Briefly, semi-confluent
cells in 12-well plates were treated for 4 h with 10 µg/ml
mitomycin C to block cell proliferation, and an artificial wound
was made by scraping the bottom of the dish. The cells were
subsequently wounded with a sterile 200-µl pipette tip to generate
a cell-free gap, ~0.3 mm in width. Cells were then washed with
phosphate-buffered saline (PBS) and photographed to record the
wound width at 0 h (~0.3 mm). Finally, one group of cells was
cultured for 48 h in DMEM supplemented with 10% (v/v) FBS as a
control, while the other groups were treated with 20 ng/ml HGF
(23). For curcumin pre-treatment,
cell monolayers were scratched and incubated for 2 h with DMEM
supplemented with 0.5% (v/v) FBS and 15 µM curcumin before HGF
treatment. The concentration of curcumin was selected based on the
study by Tong et al, who reported that the effective
concentration of curcumin is 10–20 µM (24). At the end of the incubation,
photographs were taken, and the wound width was measured to
evaluate cell migration.
Matrigel cell invasion assay
In vitro cell invasion assays were performed
following the manufacturer's recommendations. HSC-4 cells
(3×105 cells/ml) incubated in the presence or absence of
HGF were seeded in a 6-well plate fitted with a BioCoat Matrigel
Invasion Chamber (Corning; Becton Dickinson). Cells were then
cultured for 48 h in DMEM supplemented with 10% (v/v) FBS in the
presence or absence of 20 ng/ml HGF and 15 µM curcumin. After 48 h,
non-invading cells were removed from the surface of the membrane by
scrubbing, and the invading cells were fixed with 100% methanol and
then stained using the Diff-Quick staining kit after fixation with
methanol. Finally, the number of invading cells in six random
fields was counted using a microscope equipped with a ×200
objective and the Image Pro Express software (Meyer Instruments) to
evaluate the invasion index.
Gelatin zymography
The gelatinolytic activity of HSC-4 cells was
examined by gelatin zymography. Proteins from serum-free
conditioned medium were diluted in sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer
and separated using a 12.5% (w/v) polyacrylamide gel containing
0.1% (w/v) gelatin. For each sample, the amount of material loaded
onto the gel was corrected for the number of cells in culture and
corresponded to the proteins secreted by ten cells. Electrophoresis
was carried out for 2 h at 60 mA and 4°C, and the gel was incubated
overnight in 50 mM Tris-HCl buffer (pH 7.5) containing 10 mM
CaCl2 and 1X Halt Protease Inhibitor Cocktail (Thermo
Fisher Scientific). Finally, the gel was stained using 0.25%
Coomassie Blue in a methanol:acetic acid:water (50:10:40) solution
and destained using the same solution without dye. Negative
staining (white bands against a dark background) indicated
proteolysis and, therefore, gelatinolytic activity.
Western blotting
Following treatment, HSC-4 and Ca9-22 cells were
collected, washed with PBS, and lysed using RIPA buffer (10 mM
Tris-HCl pH 8.0, 150 mM NaCl, 1% (v/v) Nonidet P-40, 0.5% (w/v)
deoxycholic acid, 0.1% (w/v) SDS, 5 mM ethylenediaminetetraacetic
acid, 1X Halt Protease Inhibitor Cocktail, and 1X Halt Protein
Phosphatase Inhibitor (Thermo Fisher Scientific, Inc.). The protein
concentration of the cell lysates was determined using a BCA
Protein Assay kit (Thermo Fisher Scientific, Inc.), and equal
amounts of protein were subjected to SDS-PAGE. The separated
proteins were transferred onto a PVDF membrane (GE Healthcare),
which was then blocked for 1 h at room temperature with 5% (w/v)
bovine serum albumin in Tris-buffered saline (TBS)/Tween-20 (TBS-T)
to prevent non-specific binding. The membrane was incubated
overnight at 4°C with antibodies diluted in TBS-T, washed, and
incubated with HRP-conjugated secondary antibodies diluted in
TBS-T. Finally, antibody-antigen complexes were detected using the
ECL Plus Western Blotting Detection Reagent (GE Healthcare).
Statistical analysis
All data are presented as the mean ± standard
deviation from three independent experiments unless stated
otherwise. The statistical significance of a difference between two
groups was evaluated using unpaired Student's t-test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Curcumin inhibits HGF-induced invasion
and migration of HSC-4 cells
To assess the effect of curcumin on oral cancer
cells, we first examined HGF-induced cell motility of HSC-4 cells
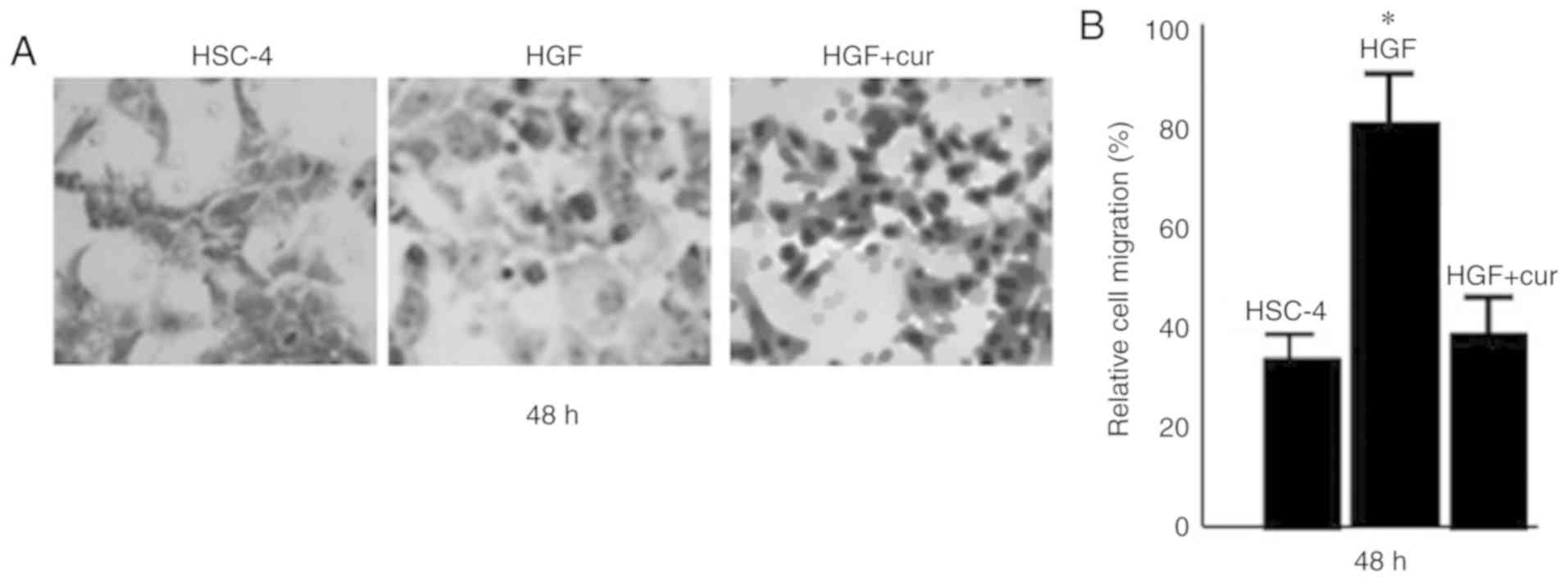
using an invasion assay (Fig. 1).
HGF-induced cells exhibited a 2-fold increase in the number of
invasive cells compared to control cells (P<0.05). In
stark contrast, curcumin pre-treatment significantly inhibited
HGF-induced invasion of HSC-4 cells (P<0.05), with a
number of invasive cells that was comparable to that of the control
cells.
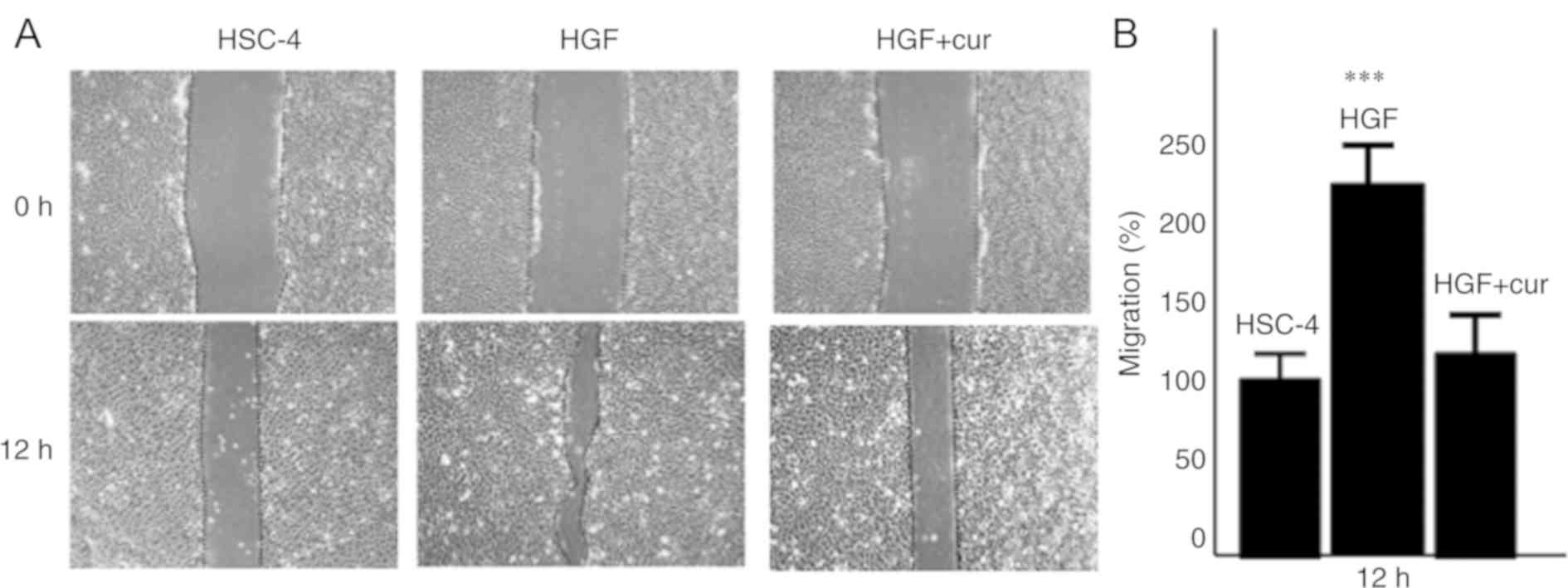
To further assess the effect of curcumin on
HGF-induced cell motility, we also examined cell migration using a
wound healing assay (Fig. 2). In
agreement with our cell migration data, the migration of
HGF-induced HSC-4 cells was increased >2-fold compared to
control cells (P<0.001). However, as in the case of cell
invasion, curcumin pre-treatment dramatically reduced the migration
of HGF-induced cells (P<0.05), which was similar to that
of control cells. Collectively, these findings strongly suggested
that curcumin pre-treatment could inhibit the HGF-induced motility
of HSC-4 cells, resulting in reduced cell invasion and
migration.
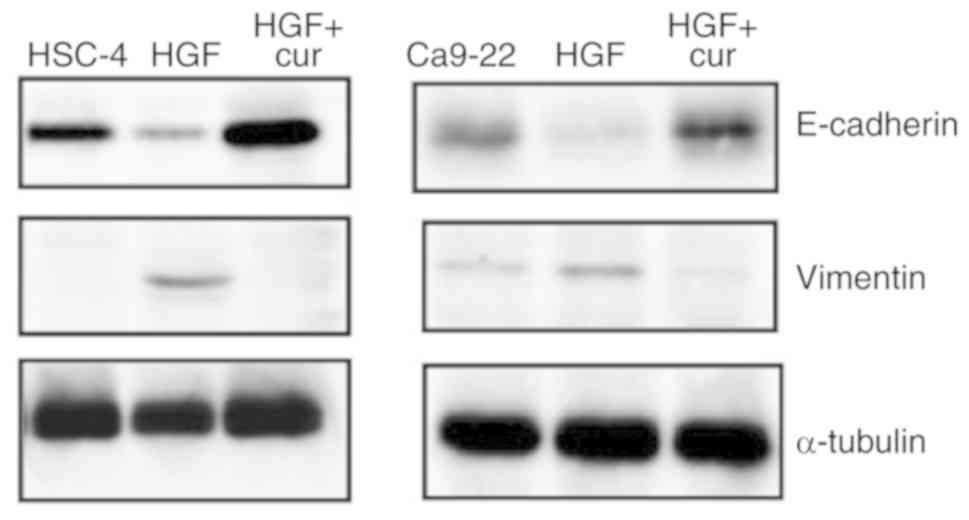
Curcumin suppresses HGF-induced EMT in
HSC-4 and Ca9-22 cells
The concomitant down-regulation of the epithelial
marker E-cadherin and up-regulation of the mesenchymal marker
vimentin is recognised as a hallmark of cells undergoing EMT
(25,26) and results in the loss of cell
polarity, an important step in EMT induction (27,28).
Therefore, we next performed western blot analyses to assess the
expression level of these markers and determine whether the
observed inhibitory effects of curcumin on HGF-induced cell
motility involved alterations in the EMT process (Fig. 3). As expected, E-cadherin and
vimentin expression levels were decreased and increased,
respectively, in response to HGF stimulation, which confirmed that
HGF-induced HSC-4 and Ca9-22 cells were undergoing EMT. Remarkably,
curcumin pre-treatment abrogated HGF-induced changes in E-cadherin
and vimentin expression, which strongly suggested that curcumin
could inhibit HGF-induced EMT in oral cancer cells.
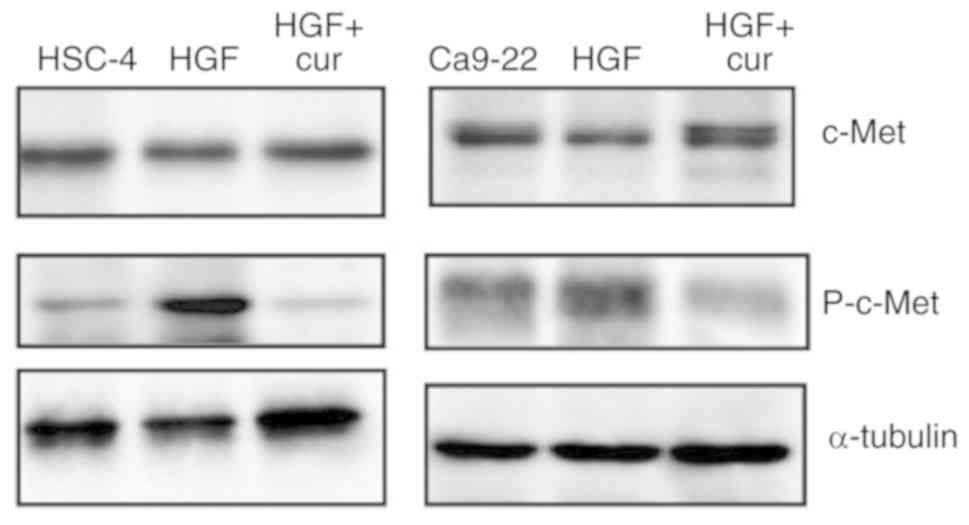
Curcumin represses HGF-induced
signalling in HSC4 cells through inhibition of the c-Met/ERK
pathway
As mentioned previously, HGF signalling plays an
important role in EMT induction. A crucial step is the
homodimerisation and autophosphorylation of the c-Met receptor
tyrosine kinase upon HGF binding (29,30).
Therefore, we performed western blot analyses to examine the effect
of curcumin pre-treatment on the level of phospho-c-Met.
HGF-induced HSC-4 and Ca9-22 cells exhibited an increased level of
phospho-c-Met compared to control cells. Remarkably, curcumin
pre-treatment abolished the increase in phospho-c-Met level
resulting from HGF stimulation (Fig.
4), which strongly suggested that the inhibitory effects of
curcumin on HGF-induced EMT involve the down-regulation of HGF
signalling.
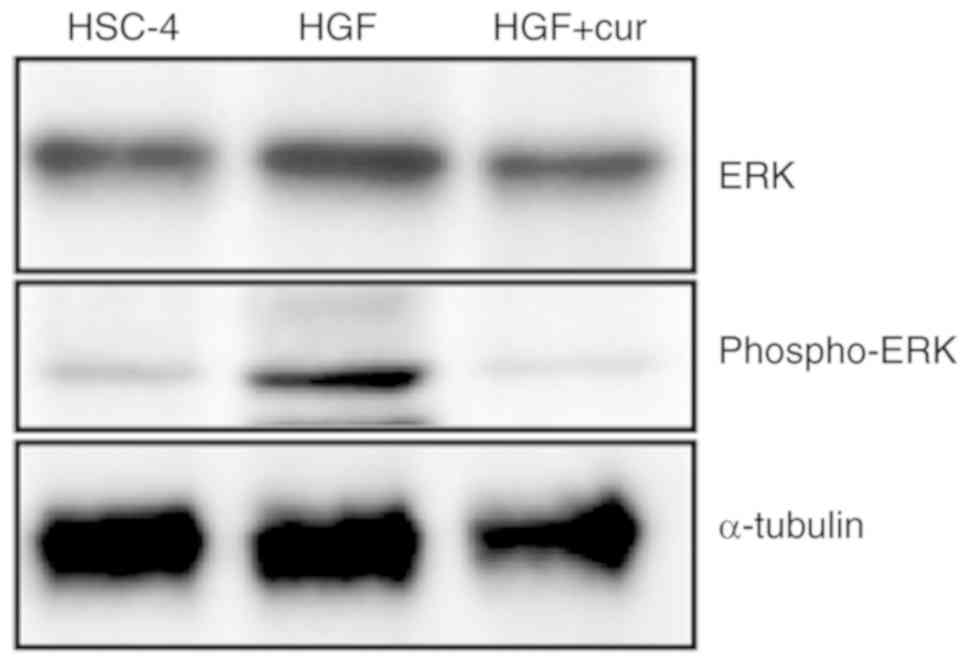
The role of HGF signalling in the induction of EMT
is mediated via the downstream activation of the AKT and ERK
effector pathways (31).
Accordingly, we assessed the activation status of the ERK pathway
to further dissect the mechanisms of EMT inhibition by curcumin.
Western blot analyses showed that ERK phosphorylation, a marker of
ERK activation, was increased in HGF-induced HSC-4 cells compared
to control cells (Fig. 5). In stark
contrast, curcumin pre-treatment completely blocked HGF-induced ERK
phosphorylation. Collectively, these findings indicated that
curcumin could inhibit HGF-induced EMT by repressing c-Met and ERK
activation.
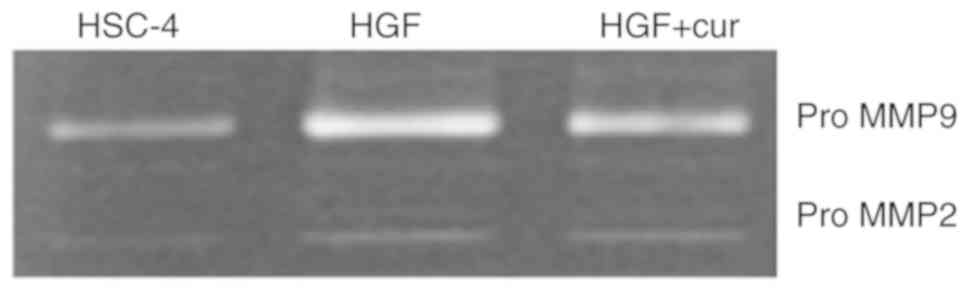
Curcumin represses the production of
gelatinolytic activity
Matrix metalloproteinases (MMPs) are major
regulators of the extracellular matrix and are known to play
important roles in tumour invasion and metastasis (32). Furthermore, activation of the MMP2
and MMP9 gelatinases has been associated with an induction of the
ERK pathway (33). Therefore, we
used gelatin zymography to evaluate gelatinolytic activity in the
conditioned medium of HSC-4 cells (Fig.
6). While control cells exhibited two major types of
gelatinolytic activity, which were consistent with pro-MMP9 and
pro-MMP2, HGF-induced cells exhibited a marked increase in pro-MMP9
production. Notably, curcumin pre-treatment inhibited the
production of pro-MMP9 by HGF-induced cells, whereas the production
of pro-MMP2 remained unaffected. This observation suggested that
inhibition of HGF signalling by curcumin might decrease cell
motility by repressing the production of gelatinolytic
activity.
Discussion
EMT is a highly conserved cellular program that
allows polarised, immotile epithelial cells to convert into motile
mesenchymal cells. Importantly, EMT plays physiological roles
during embryonic development, but also pathological roles in
cancer. HGF signalling plays an important role in EMT induction and
involves homodimerisation and autophosphorylation of its receptor
c-Met, which induces the transcription of downstream target genes.
Moreover, the activation of c-Met has been shown to promote
invasion and metastasis, as well as angiogenesis and tumorigenesis.
The functional diversity of HGF signalling has attracted much
interest in the clinical setting due to its potential prognostic
and therapeutic value (34).
In the present study, we demonstrated that HGF
signalling could induce EMT in the HSC-4 OSCC cell line and promote
both cell migration and invasion. Importantly, EMT in HSC-4 and
Ca9-22 cells involved down- and up-regulation of E-cadherin and
vimentin expression, respectively. Grotegut et al have
previously reported that HGF induces scattering of epithelial cells
via up-regulation of Snail, a transcriptional repressor involved in
EMT that represses the expression of E-cadherin and other
epithelial-related genes (35).
Importantly, HGF-induced up-regulation of Snail expression requires
the activation of the ERK pathway and its downstream effector early
growth response factor-1 (Egr-1). Furthermore, previous studies
have shown that the various oncogenic effects related to HGF
signalling are mediated by a complex downstream signalling network,
which prominently involves the AKT and ERK effector pathways
(31,36). In agreement with these findings, our
results indicated that the c-Met/ERK pathway mediated HGF-induced
EMT in HSC-4 cells. Therefore, we propose that in OSCC, HGF
signalling can activate the c-Met/ERK pathway, increasing Snail
expression, which induces EMT and increases the invasion and
migration of tumour cells.
MMPs are known to play important roles in tumour
invasion (31), and the activation
of the MMP2 and MMP9 gelatinases has been associated with induction
of the ERK pathway (33). We
previously reported that EGF increases the promoter activities of
MMP9 in oral cancer cells (37).
Moreover, it was previously reported that curcumin inhibits colon
cancer cell invasion via MMP9 (24).
The results of the current study also suggested that curcumin
inhibits OSCC cell invasion via MMP9.
In a previous study, Davies et al have shown
that inhibition of c-Met expression using a hammerhead ribozyme
transgene decreases invasion and metastasis of prostate cancer
cells (38). Hence, repression of
cell invasion and metastasis by c-Met blockade appears to be an
attractive therapeutic approach for oral cancer. In this study, we
investigated the potential beneficial effects of curcumin in OSCC.
The chemopreventive and anti-tumour activities of curcumin have
been extensively documented, establishing it as a promising drug
for the prevention and treatment of cancer (39). Indeed, curcumin can modulate multiple
molecular pathways involved in carcinogenesis and exert its
chemopreventive and anti-tumour activities through several
mechanisms, which include induction of apoptosis, inhibition of
survival signals, scavenging of reactive oxidative species, and
reduction of the inflammatory cancer microenvironment (39). Previous studies have also
demonstrated that curcumin can inhibit tumour cell invasion and
metastasis. For example, Chen et al have reported that
curcumin can inhibit the invasion and metastasis of lung cancer
cells through the up-regulation of E-cadherin expression (40). In agreement with these observations,
we showed that curcumin pre-treatment could block HGF-induced
invasion and migration of HSC-4 cells by preventing EMT induction.
Furthermore, we demonstrated that in HSC-4 cells, curcumin could
inhibit c-Met and ERK phosphorylation in response to HGF
stimulation, which are essential steps in the signalling cascade
promoting EMT. Although further work is required to elucidate the
underlying mechanisms, we propose that the inhibitory effects of
curcumin on HGF-induced EMT and cell motility are mediated via
down-regulation of the c-Met/ERK pathway.
The HSC-4 and Ca9-22 human OSCC cell line was used
as an experimental model in this study. HSC-4 and Ca9-22 cells are
negative for cancer stemness (41)
and responsive to HGF and c-Met inhibitor SU11274 (23). Thus, the effects of curcumin on
HGF-induced EMT and the invasive and migratory potential of HSC-4
and Ca9-22 cells were investigated.
Moreover, Siddappa et al have recently
assessed a curcumin/metformin combination for the treatment of a
mouse model of induced oral carcinogenesis (42). They found that this drug combination
was an efficient chemopreventive treatment, as demonstrated by the
positive clinical response, that specifically inhibited CSCs
associated with cancer progression. In vitro studies also
showed that administration at an earlier disease stage resulted in
improved efficiency of these drugs. Our in vitro data on
HGF-induced HSC-4 cells further demonstrated that curcumin is a
promising drug that could target cells exhibiting CSC-like
characteristics. Nevertheless, additional studies will be necessary
to fully understand the molecular mechanisms involved in the
chemopreventive activity of curcumin in oral cancer.
In conclusion, this study demonstrated that curcumin
could inhibit HGF-induced EMT and cell motility in an OSCC cell
line via c-Met blockade and inhibition of the ERK effector pathway.
Importantly, these findings suggested that curcumin was a potent
drug targeting invasive oral cancer cells. Therefore, we believe
that our data provide a strong theoretical and experimental basis
for the development of novel approaches and drugs for the treatment
of oral cancer.
Acknowledgements
Not applicable.
Funding
The present study was supported by a KAKENHI grant
(grant no. JP18K09736) from The Japan Society for the Promotion of
Science.
Availability of data and materials
The datasets used and/or analysed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YO and MN contributed to the conception and design
of the experiments. YO, TS and HY performed the experiments. LZ, HH
and HK contributed to the study conception and design. All authors
read and approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
OSCC
|
oral squamous cell carcinoma
|
|
EMT
|
epithelial-mesenchymal transition
|
|
CSCs
|
cancer stem cells
|
|
HGF
|
hepatocyte growth factor
|
|
MMPs
|
matrix metalloproteinases
|
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cohen EE, Lingen MW and Vokes EE: The
expanding role of systemic therapy in head and neck cancer. J Clin
Oncol. 22:1743–1752. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kramer RH, Shen X and Zhou H: Tumor cell
invasion and survival in head and neck cancer. Cancer Metastasis
Rev. 24:35–45. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ziober AF, Falls EM and Ziober BL: The
extracellular matrix in oral squamous cell carcinoma: Friend or
foe? Head Neck. 28:740–749. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Acloque H, Adams MS, Fishwick K,
Bronner-Fraser M and Nieto MA: Epithelial-mesenchymal transitions:
The importance of changing cell state in development and disease. J
Clin Invest. 119:1438–1449. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Thiery JP: Epithelial-mesenchymal
transitions in tumor progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Thiery JP and Sleeman JP: Complex networks
orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell
Biol. 7:131–142. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Krisanaprakornkit S and Iamaroon A:
Epithelial-mesenchymal transition in oral squamous cell carcinoma.
ISRN Oncol. 2012:6814692012.PubMed/NCBI
|
|
10
|
Scheel C and Weinberg RA: Cancer stem
cells and epithelial-mesenchymal transition: Concepts and molecular
links. Semin Cancer Biol. 22:396–403. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan
A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al: The
epithelial-mesenchymal transition generates cells with properties
of stem cells. Cell. 133:704–715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Morel AP, Lièvre M, Thomas C, Hinkal G,
Ansieau S and Puisieux A: Generation of breast cancer stem cells
through epithelial-mesenchymal transition. PLoS One. 3:e28882008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang X, Gan G, Wang X, Xu T and Xie W:
The HGF-MET axis coordinates liver cancer metabolism and autophagy
for chemotherapeutic resistance. Autophagy. 15:1258–1279. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ismail NI, Othman I, Abas F, H Lajis N and
Naidu R: Mechanism of apoptosis induced by curcumin in colorectal
cancer. Int J Mol Sci. 20:E24542019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wong KE, Ngai SC, Chan KG, Lee LH, Goh BH
and Chuah LH: Curcumin nanoformulations for colorectal cancer: A
review. Front Pharmacol. 10:1522019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bahrami A, Majeed M and Sahebkar A:
Curcumin: A potent agent to reverse epithelial-to-mesenchymal
transition. Cell Oncol. 42:405–421. 2019. View Article : Google Scholar
|
|
18
|
Wang Q, Qu C, Xie F, Chen L, Liu L, Liang
X, Wu X, Wang P and Meng Z: Curcumin suppresses
epithelial-to-mesenchymal transition and metastasis of pancreatic
cancer cells by inhibiting cancer-associated fibroblasts. Am J
Cancer Res. 7:125–133. 2017.PubMed/NCBI
|
|
19
|
Liang Z, Lu L, Mao J, Li X, Qian H and Xu
W: Curcumin reversed chronic tobacco smoke exposure induced
urocystic EMT and acquisition of cancer stem cells properties via
Wnt/β-catenin. Cell Death Dis. 8:e30662017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jiao D, Wang J, Lu W, Tang X, Chen J, Mou
H and Chen QY: Curcumin inhibited HGF-induced EMT and angiogenesis
through regulating c-Met dependent PI3K/Akt/mTOR signaling pathways
in lung cancer. Mol Ther Oncolytics. 3:160182016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ohnishi Y, Yasui H, Kakudo K and Nozaki M:
Cetuximab-resistant oral squamous cell carcinoma cells become
sensitive in anchorage-independent culture conditions through the
activation of the EGFR/AKT pathway. Int J Oncol. 47:2165–2172.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ohnishi Y, Yasui H, Kakudo K and Nozaki M:
Regulation of cell migration via the EGFR signaling pathway in oral
squamous cell carcinoma cells. Oncol Lett. 13:930–936. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yasui H, Ohnishi Y, Nakajima M and Nozaki
M: Migration of oral squamous cell carcinoma cells are induced by
HGF/c-Met signalling via lamellipodia and filopodia formation.
Oncol Rep. 37:3674–3680. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tong W, Wang Q, Sun D and Suo J: Curcumin
suppresses colon cancer cell invasion via AMPK-induced inhibition
of NF-κB, uPA activator and MMP9. Oncol Lett. 12:4139–4146. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang J and Weinberg RA:
Epithelial-mesenchymal transition: At the crossroads of development
and tumor metastasis. Dev Cell. 14:818–829. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cano A, Pérez-Moreno MA, Rodrigo I,
Locasioa A, Blanco MJ, del Barrio MG, Portillo F and Nieto MA: The
transcription factor snail controls epithelial-mesenchymal
transitions by repressing E-cadherin expression. Nat Cell Biol.
2:76–83. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nelson WJ and Nusse R: Convergence of Wnt,
beta-catenin, and cadherin pathways. Science. 303:1483–1487. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shimada S, Mimata A, Sekine M, Mogushi K,
Akiyama Y, Fukamachi H, Jonkers J, Tanaka H, Eishi Y and Yuasa Y:
Synergistic tumour suppressor activity of E-cadherin and p53 in a
conditional mouse model for metastatic diffuse-type gastric cancer.
Gut. 61:344–353. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Organ SL and Tsao MS: An overview of the
c-MET signaling pathway. Ther Adv Med Oncol. 3:S7–S19. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Baldanzi G and Graziani A: Physiological
signaling and structure of the HGF receptor MET. Biomedicines.
3:1–31. 2015. View Article : Google Scholar
|
|
31
|
Parikh RA, Wang P, Beumer JH, Chu E and
Appleman LJ: The potential roles of hepatocyte growth factor
(HGF)-MET pathway inhibitors in cancer treatment. Onco Targets
Ther. 11:969–983. 2014.
|
|
32
|
Stamenkovic I: Matrix metalloproteinases
in tumor invasion and metastasis. Semin Cancer Biol. 10:415–433.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Moulik S, Pal S, Biswas J and Chatterjee
A: Role of ERK in modulating MMP 2 and MMP 9 with respect to tumour
invasiveness in human cancer cell line MCF-7 and MDA-MB-231. J
Tumor. 2:87–98. 2014.
|
|
34
|
Martin TA, Mason MD and Jiang WG:
Hepatocyte growth factor signaling in cancer metastasis. Curr
Signal Transduct Ther. 6:180–190. 2011. View Article : Google Scholar
|
|
35
|
Grotegut S, von Schweinitz D, Christofori
G and Lehembre F: Hepatocyte growth factor induces cell scattering
through MAPK/Egr-1-mediated upregulation of Snail. EMBO J.
25:3534–3545. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Migliore C and Giordano S: Molecular
cancer therapy: Can our expectation be MET? Eur J Cancer.
44:641–651. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ohnishi Y, Lieger O, Attygalla M, Iizuka T
and Kakudo K: Effects of epidermal growth factor on the invasion
activity of the oral cancer cell lines HSC3 and SAS. Oral Oncol.
44:1155–1159. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Davies G, Watkins G, Mason MD and Jiang
WG: Targeting the HGF/SF receptor c-met using a hammerhead ribozyme
transgene reduces in vitro invasion and migration in prostate
cancer cells. Prostate. 60:317–324. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Park W, Amin AR, Chen ZG and Shin DM: New
perspectives of curcumin in cancer prevention. Cancer Prev Res
(Phila). 6:387–400. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chen HW, Lee JY, Huang JY, Wang CC, Chen
WJ, Su SF, Huang CW, Ho CC, Chen JJ, Tsai MF, et al: Curcumin
inhibits lung cancer cell invasion and metastasis through the tumor
suppressor HLJ1. Cancer Res. 68:7428–7438. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ohnishi Y, Yasui H, Kakudo K and Nozaki M:
Lapatinib-resistant cancer cells possessing epithelial cancer stem
cell properties develop sensitivity during sphere formation by
activation of the ErbB/AKT/cyclin D2 pathway. Oncol Rep.
36:3058–3064. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Siddappa G, Kulsum S, Ravindra DR, Kumar
VV, Raju N, Raghavan N, Sudheendra HV, Sharma A, Sunny SP, Jacob T,
et al: Curcumin and metformin-mediated chemoprevention of oral
cancer is associated with inhibition of cancer stem cells. Mol
Carcinog. 56:2446–2460. 2017. View Article : Google Scholar : PubMed/NCBI
|