Introduction
Aging is a fundamental biological process
accompanied by alterations in the regulatory activities performed
by the endocrine system, including the thyroid gland (1). On the other hand, with aging, the
prevalence of hypo- and hyperthyroidism increases (2). Both hypo- and hyperpituitarism are
believed to affect longevity (3).
Defining a physiological norm for the thyroid hormones is
complicated in the elderly by the gradual resetting of the
hypothalamic-pituitary-thyroid axis, which leads to increased
levels of thyroid stimulating hormone (4).
The prevalence of thyroid neoplasms is increased in
the elderly; these tumors are more aggressive in men than in women
(5). The mortality rate of thyroid
cancer (TC) gradually increases with age, from the ages of 40–45
(6). Notably, young survivors of TC
have increased risks for other aging-related diseases, including
diabetes, disorders of lipid metabolism, eye disorders, ear
conditions and diseases of the musculoskeletal system and
connective tissue (7). A majority of
TC arise from the epithelial elements of the gland, including
thyrocytes and follicular cells. The classification of thyroid
tumors into two major groups, differentiated (including papillary,
follicular, and medullary) or undifferentiated (anaplastic)
carcinoma, based on clinical features and morphology was supported
by advances in molecular studies (8).
Importantly, a recent study on DNA methylation and
histone modification patterns in >2,000 tumors collected from
patients of various ages revealed that most tumor types did not
demonstrate age-associated changes in DNA methylation (9), which is in agreement with the theory
that the epigenetic clock in cancer cells is reprogrammed (9). Remarkably, this theory does not hold
for thyroid tumors, which displayed age-associated differential
methylation of CpGs (9). In fact,
age at diagnosis serves as a strong indicator of prognosis in TC,
particularly in well-differentiated tumors (10,11),
with an order of magnitude difference in hazard of death from
cancer between the youngest and oldest cohorts. It is also of note
that thyroid tissue displays the lowest level of DNA methylation
changes in cancer and one of the lowest with aging (9).
Overall, previous studies indicate the presence of
an association between thyroid carcinogenesis and aging. The
present study explored whether genes that promote aging may also
play a role in the development of TC, Therefore, besides the
identification of previously identified common genes, those that
are known to be associated with aging but not with TC are worthy of
further study. A mega-analysis of thyroid cancer datasets obtained
from Gene Expression Omnibus (GEO) identified two genes,
TNFRSF12A and CHI3L1, as likely contributors to both
the process of aging and the thyroid carcinogenesis.
Materials and methods
Study workflow
The workflow was organized as follows. Firstly, the
large-scale literature-based mining effort for thyroid cancer (TC)-
and aging-associated gene sets was undertaken; these gene sets were
compared. For each gene from the list implicated in aging alone, a
mega-analysis was conducted in 16 publicly available expression
datasets retrieved from GEO. For genes that showed significant
change in expression across analyzed datasets, functional pathway
analysis and protein-protein interaction (PPI) by co-expression
analysis were conducted, then conclusions on their pathogenic
significance in TC were made.
Literature-based relation data
Relation data for both aging and TC were extracted
and analyzed using Pathway Studio (www.pathwaystudio.com), and the results were
downloaded into a genetic database Aging_TC, hosted at http://database.gousinfo.com. The downloadable format
of the database was available at http://gousinfo.com/database/Data_Genetic/Aging_TC.xlsx,
with different section of the results presented in different
worksheets (e.g., age-specific genes were presented in workseheet
‘Aging_alone genes’). In this study, we refered to these worksheets
in the form of Aging_TC→‘worksheet name’. Beside the list of
analyzed genes (Aging_TC→Aging_alone genes, Aging_TC→TC_alone
genes, and Aging_TC→Common genes), supporting references for each
disease-gene relation are presented (Aging_TC→Ref for Aging_alone
genes, Aging_TC→Ref for TC_alone genes, and Aging_TC→Ref for Common
genes), including titles of the references and the sentences
describing identified disease-gene relationships. The information
could be used to locate a detailed description of an association of
a candidate gene with aging and/or TC. Please refer to
Aging_TC→DataNote for the decryption of each worksheet.
Data selection for mega-analysis
The relevant expression datasets available at GEO
(https://www.ncbi.nlm.nih.gov/geo/)
were retrieved with the keyword ‘thyroid cancer’ (n=91). The
following criteria were applied: i) Organism, Homo sapiens;
ii) data type, RNA expression; iii) sample size, ≥10; and iv) the
studies were performed according to case-control design. A total of
16 datasets remained available for the mega-analysis.
Mega-analysis models
To discern the effect sizes of the selected genes in
a case vs. control expression comparison, both fixed-effect and
random-effects models were employed. The expression log fold change
(LFC) was used as the effect size. The results derived from both
models were compared. In order to assess the variance within and
between different studies, the heterogeneity of the mega-analysis
was analyzed. In the case that total variance Q was equal to or
smaller than the expected between-study variance df, the statistic
ISq=100% × (Q-df)/Q was set as 0, and a fixed-effect model was
selected for the mega-analysis. Otherwise, a random-effects model
was selected. The genes with significant effects were identified
according to the following criteria: P<1×10−7 and
effect size (LFC) >1 or <-1.
Multiple linear regression
analysis
A multiple linear regression (MLR) model was
employed to study the possible influence of three factors on the
changes in gene expression in TC: Sample size, population region,
and study age. P-values and 95% confidence interval (CI) were
reported for each of the factors.
Pathway analysis
For the set of genes identified through expression
mega-analysis described above, a functional pathway analysis was
conducted with an aim to identify potential biological associations
between the selected genes and the TC. The analysis was performed
using the ‘Shortest Path’ module of Pathway Studio (www.pathwaystudio.com).
Co-expression analysis
For each pair of the genes/proteins identified in
the pathway analysis, a study of their co-expression was executed.
The purpose of this analysis was to identify possible
protein-protein interaction (PPI) of the proteins involved. The
Fisher's Z was employed as the effect size for the mega-analysis,
as shown in the equation below:
FisherZ=0.5xlog(1+correlation1-correlation)
All co-expression associations with P-value
<1×10−4 and Fisher's Z-value ≥0.3 or ≤-0.3 were
identified as significant, and then presented as a Cytoscape-imaged
PPI network.
Results
Genes commonly affected by the process
of aging and by the carcinogenesis in the thyroid gland
The curated Aging_TC database identified 262 genes
with substantially increased expression or activity with aging
(supported by 1,495 scientific references) and 816 genes associated
with the pathogenesis of TC (supported by 4,169 references). A
total of 63 genes were identified to be involved in both aging and
TC (right tail Fisher's exact test, P=3.82×10−35;
Fig. S1), which accounts for about
a quarter of the genes associated with aging (24.05%). The
descriptions of these 63 genes are presented in Table I. Further information on these genes
was also provided in Aging_TC→Common genes and Aging_TC→Ref for
common genes.
 | Table I.Common genes associated with aging
and thyroid cancer. |
Table I.
Common genes associated with aging
and thyroid cancer.
| Name | Entrez Gene ID | Human chromosome
position |
|---|
|
SERPINC1 |
462;304917;11905 | 1q25.1 |
| IL17A |
16171;301289;3605 | 6p12.2;6p12 |
| EDN1 |
1906;24323;13614 | 6p24.1 |
| LCN2 |
16819;170496;3934 | 9q34.11;9q34 |
| PTGS2 |
19225;29527;5743 |
1q31.1;1q25.2-q25.3 |
| HLA-G |
24747;14991;3135 | 6p21.3;6p22.1 |
| CSF1 |
1435;12977;78965 | 1p13.3 |
| MIF |
4282;17319;81683 | 22q11.23 |
| PTH |
19226;24694;5741 |
11p15.3;11p15.3-p15.1 |
| CYP27B1 |
1594;13115;114700 | 12q14.1 |
| CYP24A1 |
25279;1591;13081 | 20q13;20q13.2 |
| HUWE1 | 59026;10075 | Xp11.22 |
| EIF2AK2 |
5610;54287;19106 |
2p22-p21;2p22.2 |
| PDGFRB |
5159;24629;18596 | 5q32;5q33.1 |
| APP |
54226;351;11820 | 21q21.3 |
| HYOU1 |
10525;12282;192235 |
11q23.1-q23.3;11q23.3 |
| EGF |
25313;1950;13645 | 4q25 |
| TNF |
24835;21926;7124 | 6p21.3;6p21.33 |
| HMOX1 |
15368;3162;24451 |
22q12.3;22q13.1 |
| MIR21 | 406991 | 17q23.1 |
| MMP9 |
81687;17395;4318 |
20q11.2-q13.1;20q13.12 |
| FAS |
246097;14102;355 |
10q23.31;10q24.1 |
| DPP4 |
25253;1803;13482 | 2q24.3;2q24.2 |
| KLK3 |
18048;18050;354;13648;16619;16618;16613;16624;16612;16623;16622;13646;16617;16616;16615 |
19q13.33;19q13.41 |
| PROS1 |
19128;81750;5627 | 3q11.2;3q11.1 |
| FASLG |
14103;356;25385 | 1q23;1q24.3 |
| GPX3 |
2878;64317;14778 | 5q23;5q33.1 |
| ALB |
11657;213;24186 | 4q13.3 |
| DCN |
1634;13179;29139 | 12q21.33 |
| B3GAT1 |
27087;76898;117108;964 | 11q25 |
| ADIPOQ |
9370;246253;11450 | 3q27;3q27.3 |
| ARG2 |
11847;29215;384 | 14q24.1 |
| RUNX3 |
12399;156726;864 | 1p36.11;1p36 |
| PRDX1 |
5052;18477;100363379;117254 | 1p34.1 |
| HTRA1 |
5654;65164;56213 |
10q26.13;10q26.3 |
| RCAN1 | 54720;1827 | 21q22.12 |
| CTNNB1 |
84353;12387;1499 | 3p21;3p22.1 |
| BAG3 |
9531;29810;293524 |
10q25.2-q26.2;10q26.11 |
| TGFB1 |
21803;7040;59086 |
19q13.1;19q13.2 |
| TGFA |
24827;21802;7039 | 2p13;2p13.3 |
| CTSD |
1509;171293;13033 | 11p15.5 |
| MUC1 | 4169;4582 | 1q21;1q22 |
| TRAP1 |
68015;10131;287069 | 16p13.3 |
| CDKN1A |
12575;114851;1026 | 6p21.2 |
| CCNA2 |
12428;114494;890 | 4q27 |
| CCR2 |
1231;12772;729230 | 3p21.31 |
| IL21 |
59067;365769;60505 | 4q26-q27;4q27 |
| BAX |
12028;24887;581 |
19q13.33;19q13.3-q13.4 |
| IL4 |
287287;3565;16189 | 5q31.1 |
| ENO1 |
2023;24333;433182;13806 | 1p36.23;1p36.2 |
| HGF |
3082;15234;24446 | 7q21.11;7q21.1 |
| TNFSF10 |
246775;22035;8743 | 3q26.31;3q26 |
| IL6 |
24498;3569;16193 | 7p15.3;7p21 |
| THRA |
7067;81812;21833 |
17q11.2;17q21.1 |
| ANGPT2 |
89805;11601;285 | 8p23.1 |
| EP300 |
170915;2033;328572 | 22q13.2 |
| GSTM1 |
2944;14863;24424 | 1p13.3 |
| CCL2 |
287562;6347;20293 |
17q12;17q11.2-q12 |
| TXNIP |
117514;56338;10628 | 1q21.1 |
| GSTT1 | 2952 | 22q11.23 |
| POSTN |
50706;361945;10631 | 13q13.3 |
| NQO1 |
18104;1728;24314 | 16q22.1 |
| IL10 |
16153;25325;3586 |
1q31-q32;1q32.1 |
In order to assess the functional profile of the 63
genes associated with both aging and TC, a gene set enrichment
analysis (GSEA) against the GO and Pathway Studio Ontology was
conducted. The GSEA showed that these common genes were mainly
involved in the protein kinase domain, cell proliferation, tissue
development, gland development and response to hormone processes.
Specifically, this analysis uncovered a total of three
pathways/gene sets associated with cell apoptosis (26 unique genes)
and 2 pathways/gene sets associated with cell growth proliferation
(25 unique genes). A bar plot of the 39 pathways and enriched genes
out of the 63 common genes are presented in Fig. S2.
Gene expression analysis result
Although there was a significant overlap between
aging- and TC-associated gene sets (n=63; P=3.82×10−35),
a majority of the aging-associated genes (n=199 or 75.95%) have not
been yet implicated in the pathogenesis of TC. Therefore, the
association between each of these 199 genes with TC was examined,
using 16 gene expression datasets shown in Table II (12–26). The
detailed description of the results is presented in
Aging_TC→Mega-analysis. The significance of association criteria
(P<1×10−7 and absolute LFC>1) was met by two genes
and presented in Table III.
 | Table II.Datasets used for thyroid
cancer-aging relation mega-analysis. |
Table II.
Datasets used for thyroid
cancer-aging relation mega-analysis.
| Study | Dataset GEO ID | Control, n | Case, n | Country | Refs. |
|---|
| Jarzab et
al, 2015 | GSE35570 | 51 | 65 | Poland | (12) |
| Rusinek et
al, 2015 | GSE58545 | 18 | 27 | Poland | (13) |
| Swierniak et
al, 2015 | GSE58689 | 18 | 27 | Poland | (13) |
| Tarabichi et
al, 2015 | GSE60542 | 34 | 33 | Belgium | (14) |
| von Roemeling et
al, 2015 | GSE65144 | 13 | 12 | USA | (15) |
| Versteyhe et
al, 2013 | GSE39156 | 16 | 48 | Belgium | (16) |
| Pita et al,
2013 | GSE53157 | 3 | 24 | Portugal | (17) |
| Tomas et al,
2012 | GSE29265 | 20 | 29 | Belgium | N/A |
| Tomas et al,
2012 | GSE33630 | 45 | 60 | Belgium | (18,19) |
| Giordano et
al, 2011 | GSE27155 | 4 | 95 | USA | (20,21) |
| Yu et al,
2008 | GSE5364 | 58 | 270 | Singapore | (22) |
| Fontaine et
al, 2007 | GSE6339 | 135 | 48 | France | (23) |
| Salvatore et
al, 2007 | GSE9115 | 4 | 15 | USA | (24) |
| Reyes et al,
2006 | GSE3678 | 7 | 7 | USA | N/A |
| Vasko et al,
2006 | GSE6004 | 4 | 14 | USA | (25) |
| Liyanarachchi et
al, 2005 | GSE3467 | 9 | 9 | USA | (26) |
 | Table III.Significant genes from mega-analysis
[P<1×10−7 and abs(LFC)>1] involved in aging and
thyroid cancer. |
Table III.
Significant genes from mega-analysis
[P<1×10−7 and abs(LFC)>1] involved in aging and
thyroid cancer.
|
| Mega-analysis
results | Multiple linear
regression analysis for three factors |
|---|
|
|
|
|
|---|
| Gene name | Random effects
model | Datasets
included | LFC | STH of LFC | P-value | Sample size | Population
region | Year of study |
|---|
| CHI3L1 | 0 | 16 | 2.88 | 0.42 |
5.10×10−12 | 0.24 |
2.77×10−4 | 0.15 |
|
TNFRSF12A | 1 | 14 | 1.79 | 0.33 |
2.05×10−8 | 0.35 |
3.24×10−4 | 0.56 |
For each gene, a LFC was estimated from the majority
of the 16 studies (a total of 16 and 14 studies for CHI3L1
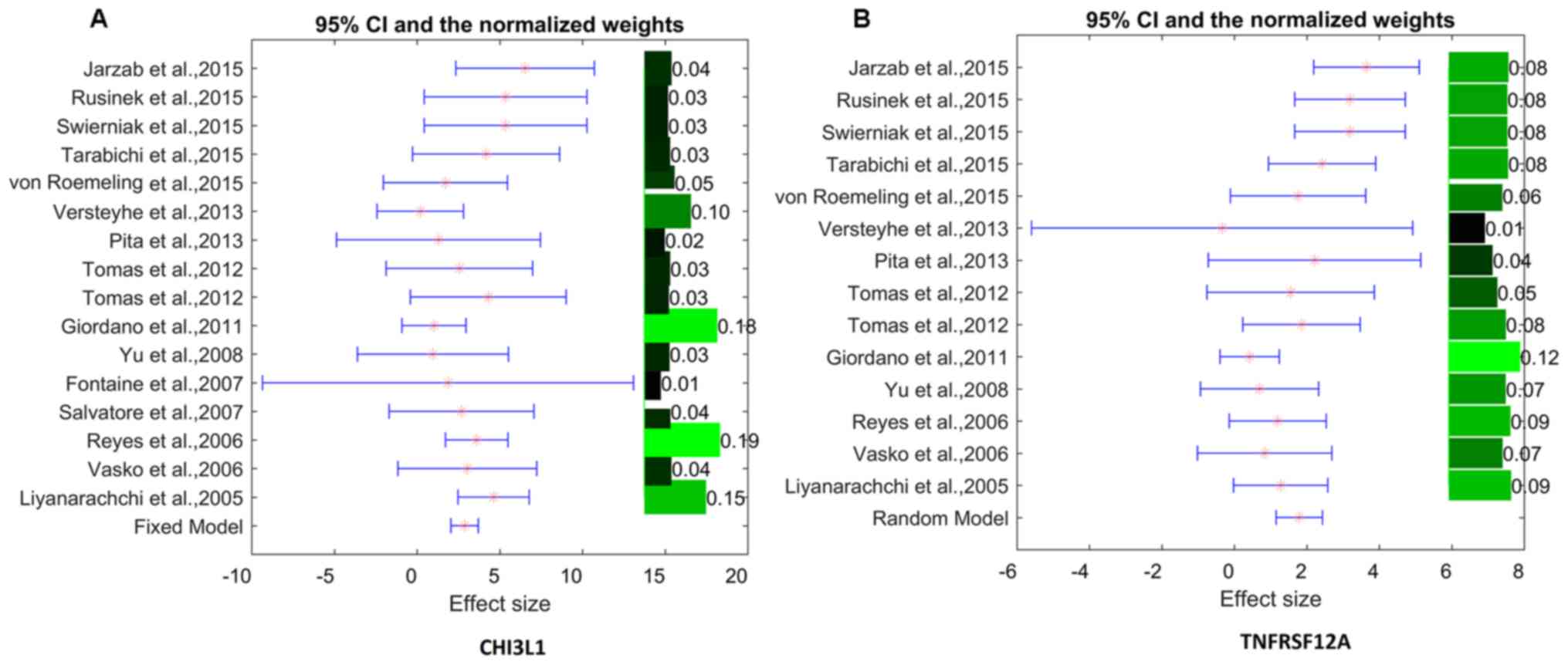
and TNFRSF12A, respectively). As shown in Fig. 1, the mRNA expression levels of
TNFRSF12A demonstrated strong between-study variances
(ISq=54.07% and Q test P=8.2×10−3); therefore, the
random-effects model was selected for the mega-analysis. In
contrast, no significant between-study variance was observed for
the gene CHI3L1 (ISq=14.73% and Q test P>0.28);
therefore, the fixed-effect model was selected for analysis of its
mRNA expression levels. This identified the sample population
region (country) as a significant factor that influences the LFC of
both genes in the case of TC (P<3.24×10−4; Table III).
Functional pathway analysis
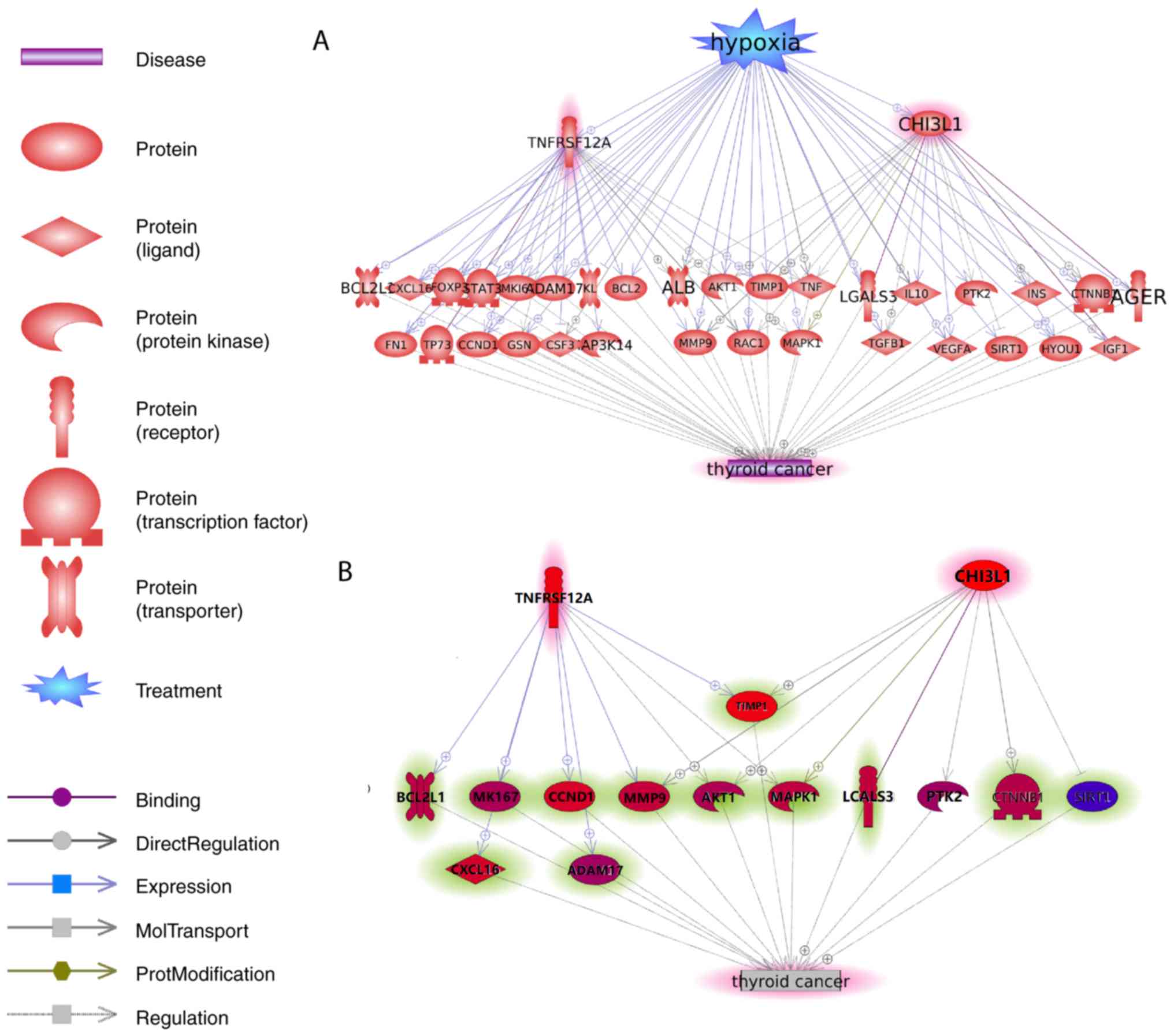
According to the de novo approach selected
for the identification of novel TC-associated genes, no prior
direct link to the pathogenesis of TC were known. However, Pathway
Studio-guided ‘shortest path analysis’ revealed plausible
associations of CHI3L1 and TNFRSF12A genes with TC,
with a set of common interactions (Fig.
2A).
Using Pathway Studio, the ‘shortest path’ analysis
was conducted to identify associated genes that link CHI3L1
and TNFRSF12A-encoded molecules to the pathogenesis of TC in
a unidirectional way. For example, the association between
CHI3L1 and transforming growth factor β1 (TGFB1) in TC was
identified. YKL-40, also known as chitinase-3-like protein 1
(CHI3L1), is a secreted glycoprotein that binds to
interleukin-13 receptor α2 (IL-13Rα2) and subsequently stimulates
the production of TGF-β1 (27), a
key molecule in thyroid carcinogenesis and considered as a new
prognostic and therapeutic target for TC (28,29). The
details for all other associations are presented in Fig. 2A, and are described in
Aging_TC→TC-2Genes_potential pathways. This reference information
included the type of association, the amount of underlying
supporting references, and the relevant sentences where these
relationships were identified and described. The shortest pathway
analysis was conducted using the Pathway Studio (www.pathwaystudio.com). All TC-associated genes
reported in the shortest pathway analysis were employed in the
identification of the TC-associated genes.
In order to confirm the associations depicted in
Fig. 2A, another mega-analysis was
conducted using 16 datasets, with the purpose to evaluate the
co-expression pattern of mRNAs encoded by CHI3L1 and
TNFRSF12A, and 31 genes that link the two genes with TC. The
association between CHI3L1 and TNFRSF12A and 13 of
the 31 genes presented in Fig. 2A
was validated (P<0.005; Fig. 2B).
Please refer to Aging_TC→MetaResults_ShortestPath for the detailed
description of the associations presented in Fig. 2B. In addition, a PPI network
connecting the products of CHI3L1 and TNFRSF12A and
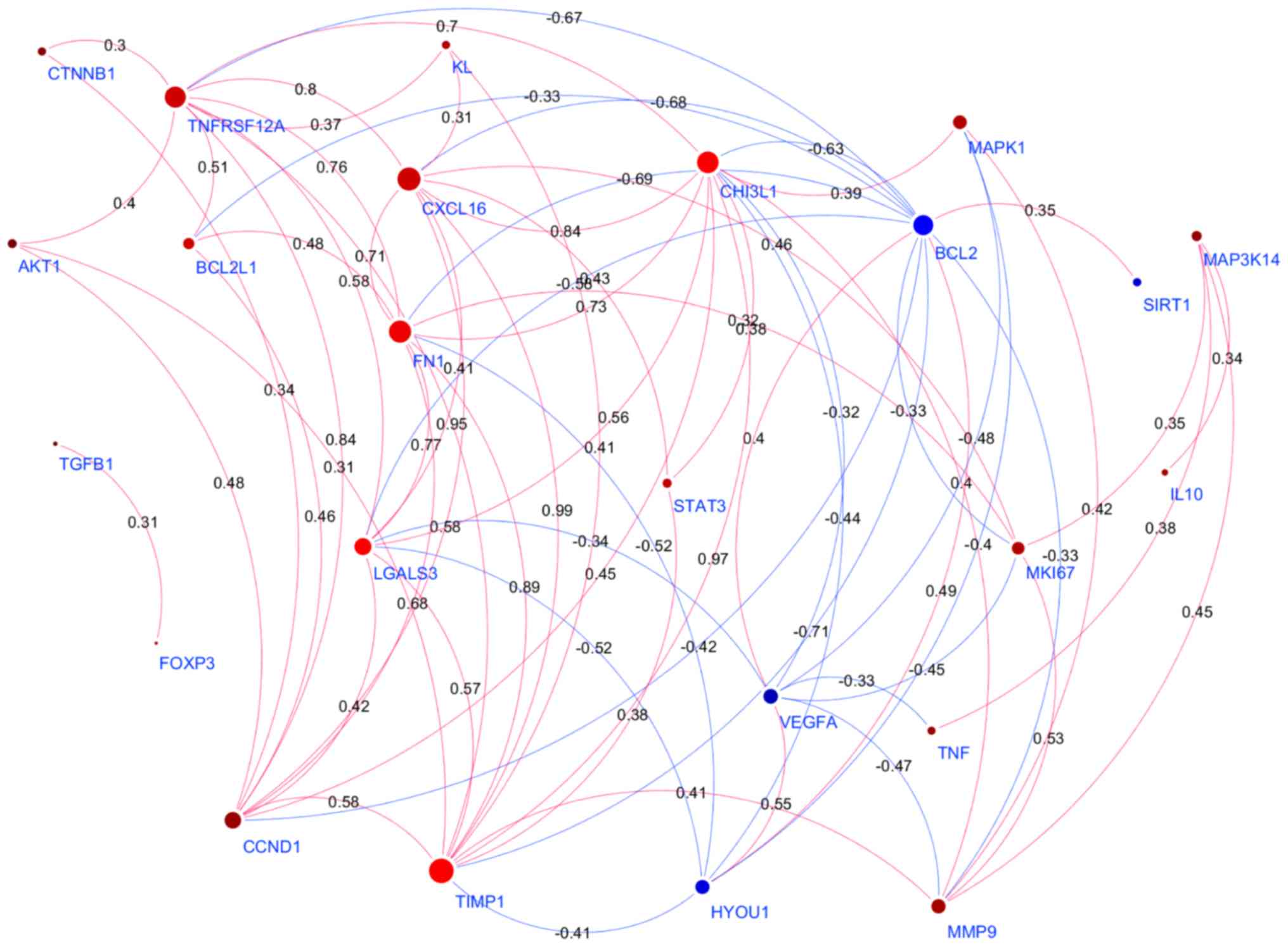
the ‘bridge’ genes (n=31) were generated using Cytoscape (Fig. 3). The detailed information of the
mega-analysis for the co-expression was presented in Aging-TC→PPI,
including a list of the genes (nodes) of the PPI network, and the
mega-analysis results for each pair of gene-gene correlation.
Discussion
The present study aimed to identify novel molecular
pathways, which connect the process of aging and the development of
TC. By removing all known associations between curated sets of
genes involved in aging and TC, uncovered aging-associated
contributors to TC were identified. According to the pre-selected
significance of association criteria (P<1×10−7 and
abs(LFC)>1), two aging-associated genes, TNFRSF12A and
CHI3L1, were found to be involved in the development of
TC.
The role of TNFRSF12A and CHI3L1 in
the aging-associated disease is well described, whereas no apparent
connections to the malignant processes in the thyroid were reported
thus far. TNFRSF12A encodes for an exclusive receptor for
tumor necrosis factor-related weak inducer of apoptosis
(TWEAK), an interacting pair of molecules involved in
age-associated pathological changes in skeletal muscle and other
organs (30–32). CHI3L1 encodes for YKL-40, a
glycoprotein upregulated in a variety of inflammatory conditions
commonly found in the elderly, including chronic obstructive
pulmonary disease and neurodegenerative diseases (33,34).
Employing a Pathway Studio-guided ‘shortest path
analysis’ revealed plausible associations of TNFRSF12A and
CHI3L1 with TC, simultaneously highlighting a set of common
interactors that included the signaling molecules AKT1, RAC1,
MAPK1 and the soluble proteins TNF-α, albumin,
TIMP1 and MMP9.
Among these, the association of TNFRSF12A and
CHI3L1 with AKT1 was notable, as this molecule was
both overexpressed and over-activated in TC (35). Moreover, increased Akt signaling led
to increased Bcl-2 promoter activity and cell survival (36). The interaction between
TNFRSF12A and TWEAK also positively regulated
pro-survival molecules of the Bcl2 family (37,38),
and, therefore augmented the pro-survival signal of AKT1
(39).
Among the soluble molecules, angiogenic matrix
metalloproteinase (MMP)9 was found to be associated with
TNFRSF12A and CHI3L1, which participates in
follicular TC cell invasion (40).
TIMP-1, acts as an inhibitor for MMP9 expression that
is often co-expressed with this molecule in thyroid tumors and
serves as a reliable surrogate marker for BRAF-mutated status and
likely aggressiveness (41).
Notably, TIMP−1 serves as a prominent PPI hub gene in the
Cytoscape network built upon co-expression of TNFRSF12A and
CHI3L1, along with LGALS3, a specific biomarker of
well-differentiated thyroid carcinomas (42), CXCL16, which mediated the
involvement of macrophages in the invasion of papillary TCs
(43) and fibronectin, an EMT
biomarker which promoted migration and invasion of papillary
thyroid cancers (44).
Importantly, many of the molecules discussed above
were upregulated in response to hypoxia (45), or stimulated the expression of key
mediators of the antihypoxic response (46), or both (47,48).
Both HIF-1α and HIF-2α were reported to be expressed
in TC (49). Moreover, a growing
evidence points out that hypoxia plays a significant role in the
maintenance of thyroid cancer stem cells (CSC) (50). General hypoxia due to diminished
vascularization is a well-known characteristic of aging tissues,
possibly due to endothelial dysfunction as well decreased function
of endothelial progenitor cell function (51,52). For
more information regarding the hypoxia-gene regulation presented in
Fig. 2A, please refer to
Aging_TC→TC_2Genes_potential pathway. Therefore, it can be
speculated that the age-dependent increase in hypoxia may also
contribute to the increased aggressiveness of TC observed in the
elderly (10,11).
The results could be influenced by multiple factors,
including sample size and sample source. Considering the fact that
the pathology of disease could change with time, the publication
date/age was checked in the present study. The MLR analysis showed
that country region was a significant factor that could influence
the gene expression levels of TC genes, but not the other two
factors. Other influencing factors of TC gene activities could
include mortality or epigenetics. However, due to be the limitation
of the metadata, these factors were not amiable for analysis in the
datasets employed in the present study, which would be valuable for
future studies.
In conclusion, the present study conducted a
functional pathway and co-expression analysis, and mined a set of
genes associated with aging. TNFRSF12A and CHI3L1
were identified as previously unrecognized contributors to the
development of thyroid tumors, which are known for unusual increase
in aggressiveness in the elderly. An analysis of the Cytoscape
network built upon co-expression of TNFRSF12A and
CHI3L1 points towards tissue hypoxia as a bridging factor,
which is common for the pathophysiology of aging and the
development of TC.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and material
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
ML, HC, AB and QS developed the study design,
analyzed the data, and wrote the original manuscript. KCK, LH, SH
and JF contributed to data analysis and manuscript writing and
revision. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Da Costa VM and Rosenthal D: Effects of
aging on thyroidal function and proliferation. Curr Aging Sci.
1:101–104. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cooper DS: Thyroid disease in the oldest
old: The exception to the rule. JAMA. 292:2651–2654. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jones CM and Boelaert K: The endocrinology
of aging: A mini-review. Gerontology. 61:291–300. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pasqualetti G, Caraccio N, Dell Agnello U
and Monzani F: Cognitive function and the aging process: The
peculiar role of mild thyroid failure. Recent Pat Endocr Metab
Immune Drug Discov. 10:4–10. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rukhman N and Silverberg A: Thyroid cancer
in older men. Aging Male. 14:91–98. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mazzaferri EL and Kloos RT: Current
approaches to primary therapy for papillary and follicular thyroid
cancer. J Clin Endocrinol Metab. 86:1447–1463. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Blackburn BE, Ganz PA, Rowe K, Snyder J,
Wan Y, Deshmukh V, Newman M, Fraser A, Smith K, Herget K, et al:
Aging-related disease risks among young thyroid cancer survivors.
Cancer Epidemiol Biomarkers Prev. 26:1695–1704. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
De Lellis RA, Lloyd RV, Heitz PU and Eng
C: World Health Organization classification of tumours. Pathology
and genetics of tumours of endocrine organs. IARC Press; Lyon,
France: 2004
|
|
9
|
Pérez RF, Tejedor JR, Bayón GF, Fernández
AF and Fraga MF: Distinct chromatin signatures of DNA
hypomethylation in aging and cancer. Aging Cell. 17:e127442018.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Krook KA, Fedewa SA and Chen AY:
Prognostic indicators in well-differentiated thyroid carcinoma when
controlling for stage and treatment. Laryngoscope. 125:1021–1027.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ganly I, Nixon IJ, Wang LY, Palmer FL,
Migliacci JC, Aniss A, Sywak M, Eskander AE, Freeman JL, Campbell
MJ, et al: Survival from differentiated thyroid cancer: What has
age got to do with it? Thyroid. 25:1106–1114. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Handkiewicz-Junak D, Swierniak M, Rusinek
D, Oczko-Wojciechowska M, Dom G, Maenhaut C, Unger K, Detours V,
Bogdanova T, Thomas G, et al: Gene signature of the post-Chernobyl
papillary thyroid cancer. Eur J Nucl Med Mol Imaging. 43:1267–1277.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rusinek D, Swierniak M, Chmielik E, Kowal
M, Kowalska M, Cyplinska R, Czarniecka A, Piglowski W, Korfanty J,
Chekan M, et al: BRAFV600E-associated gene expression profile:
Early changes in the transcriptome, based on a transgenic mouse
model of papillary thyroid carcinoma. PLoS One. 10:e01436882015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tarabichi M, Saiselet M, Trésallet C,
Hoang C, Larsimont D, Andry G, Maenhaut C and Detours V: Revisiting
the transcriptional analysis of primary tumours and associated
nodal metastases with enhanced biological and statistical controls:
application to thyroid cancer. Br J Cancer. 112:1665–1674. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
von Roemeling CA, Marlow LA, Pinkerton AB,
Crist A, Miller J, Tun HW, Smallridge RC and Copland JA: Aberrant
lipid metabolism in anaplastic thyroid carcinoma reveals stearoyl
CoA desaturase 1 as a novel therapeutic target. J Clin Endocrinol
Metab. 100:E697–E709. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Versteyhe S, Driessens N, Ghaddhab C,
Tarabichi M, Hoste C, Dumont JE, Miot F, Corvilain B and Detours V:
Comparative analysis of the thyrocytes and T cells: responses to
H2O2 and radiation reveals an
H2O2-induced antioxidant transcriptional
program in thyrocytes. J Clin Endocrinol Metab. 98:E1645–E1654.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pita JM, Banito A, Cavaco BM and Leite V:
Gene expression profiling associated with the progression to poorly
differentiated thyroid carcinomas. Br J Cancer. 101:1782–1791.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dom G, Tarabichi M, Unger K, Thomas G,
Oczko-Wojciechowska M, Bogdanova T, Jarzab B, Dumont JE, Detours V
and Maenhaut C: A gene expression signature distinguishes normal
tissues of sporadic and radiation-induced papillary thyroid
carcinomas. Br J Cancer. 107:994–1000. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tomás G, Tarabichi M, Gacquer D, Hébrant
A, Dom G, Dumont JE, Keutgen X, Fahey TJ III, Maenhaut C and
Detours V: A general method to derive robust organ-specific gene
expression-based differentiation indices: application to thyroid
cancer diagnostic. Oncogene. 31:4490–4498. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Giordano TJ, Au AY, Kuick R, Thomas DG,
Rhodes DR, Wilhelm KG Jr, Vinco M, Misek DE, Sanders D, Zhu Z, et
al: Delineation, functional validation, and bioinformatic
evaluation of gene expression in thyroid follicular carcinomas with
the PAX8-PPARG translocation. Clin Cancer Res. 12:1983–1993. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Giordano TJ, Kuick R, Thomas DG, Misek DE,
Vinco M, Sanders D, Zhu Z, Ciampi R, Roh M, Shedden K, et al:
Molecular classification of papillary thyroid carcinoma: distinct
BRAF, RAS, and RET/PTC mutation-specific gene expression profiles
discovered by DNA microarray analysis. Oncogene. 24:6646–6656.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yu K, Ganesan K, Tan LK, Laban M, Wu J,
Zhao XD, Li H, Leung CH, Zhu Y, Wei CL, et al: A precisely
regulated gene expression cassette potently modulates metastasis
and survival in multiple solid cancers. PLoS Genet. 4:e10001292008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fontaine JF, Mirebeau-Prunier D, Franc B,
Triau S, Rodien P, Houlgatte R, Malthièry Y and Savagner F:
Microarray analysis refines classification of non-medullary thyroid
tumours of uncertain malignancy. Oncogene. 27:2228–2236. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Salvatore G, Nappi TC, Salerno P, Jiang Y,
Garbi C, Ugolini C, Miccoli P, Basolo F, Castellone MD, Cirafici
AM, et al: A cell proliferation and chromosomal instability
signature in anaplastic thyroid carcinoma. Cancer Res.
67:10148–10158. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vasko V, Espinosa AV, Scouten W, He H,
Auer H, Liyanarachchi S, Larin A, Savchenko V, Francis GL, de la
Chapelle A, et al: Gene expression and functional evidence of
epithelial-to-mesenchymal transition in papillary thyroid carcinoma
invasion. Proc Natl Acad Sci USA. 104:2803–2808. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
He H, Jazdzewski K, Li W, Liyanarachchi S,
Nagy R, Volinia S, Calin GA, Liu CG, Franssila K, Suster S, et al:
The role of microRNA genes in papillary thyroid carcinoma. Proc
Natl Acad Sci USA. 102:19075–19080. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
He CH, Lee CG, Dela Cruz CS, Lee CM, Zhou
Y, Ahangari F, Ma B, Herzog EL, Rosenberg SA, Li Y, et al:
Chitinase 3-like 1 regulates cellular and tissue responses via
IL-13 receptor α2. Cell Rep. 4:830–841. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pisarev MA, Thomasz L and Juvenal GJ: Role
of transforming growth factor beta in the regulation of thyroid
function and growth. Thyroid. 19:881–892. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhong J, Liu C, Zhang QH, Chen L, Shen YY,
Chen YJ, Zeng X, Zu XY and Cao RX: TGF-β1 induces HMGA1 expression:
The role of HMGA1 in thyroid cancer proliferation and invasion. Int
J Oncol. 50:1567–1578. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tajrishi MM, Sato S, Shin J, Zheng TS,
Burkly LC and Kumar A: The TWEAK-Fn14 dyad is involved in
age-associated pathological changes in skeletal muscle. Biochem
Biophys Res Commun. 446:1219–1224. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Van Kirk CA, VanGuilder HD, Young M,
Farley JA, Sonntag WE and Freeman WM: Age-related alterations in
retinal neurovascular and inflammatory transcripts. Mol Vis.
17:1261–1274. 2011.PubMed/NCBI
|
|
32
|
Hénaut L, Sanz AB, Martin-Sanchez D,
Carrasco S, Villa-Bellosta R, Aldamiz-Echevarria G, Massy ZA,
Sanchez-Nino MD and Ortiz A: TWEAK favors phosphate-induced
calcification of vascular smooth muscle cells through canonical and
non-canonical activation of NFκB. Cell Death Dis. 7:e23052016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tong X, Wang D, Liu S, Ma Y, Li Z, Tian P
and Fan H: The YKL-40 protein is a potential biomarker for COPD: A
meta-analysis and systematic review. Int J Chron Obstruct Pulmon
Dis. 13:409–418. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Baldacci F, Lista S, Cavedo E, Bonuccelli
U and Hampel H: Diagnostic function of the neuroinflammatory
biomarker YKL-40 in Alzheimer's disease and other neurodegenerative
diseases. Expert Rev Proteomics. 14:285–299. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ringel MD, Hayre N, Saito J, Saunier B,
Schuppert F, Burch H, Bernet V, Burman KD, Kohn LD and Saji M:
Overexpression and overactivation of Akt in thyroid carcinoma.
Cancer Res. 61:6105–6111. 2001.PubMed/NCBI
|
|
36
|
Sen P, Mukherjee S, Ray D and Raha S:
Involvement of the Akt/PKB signaling pathway with disease
processes. Mol Cell Biochem. 253:241–246. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tran NL, McDonough WS, Savitch BA, Sawyer
TF, Winkles JA and Berens ME: The tumor necrosis factor-like weak
inducer of apoptosis (TWEAK)-fibroblast growth factor-inducible 14
(Fn14) signaling system regulates glioma cell survival via NFkappaB
pathway activation and BCL-XL/BCL-W expression. J Biol Chem.
280:3483–3492. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ameri H, Liu H, Liu R, Ha Y,
Paulucci-Holthauzen AA, Hu S, Motamedi M, Godley BF, Tilton RG and
Zhang W: TWEAK/Fn14 pathway is a novel mediator of retinal
neovascularization. Invest Ophthalmol Vis Sci. 55:801–813. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Whitsett TG, Cheng E, Inge L, Asrani K,
Jameson NM, Hostetter G, Weiss GJ, Kingsley CB, Loftus JC, Bremner
R, et al: Elevated expression of Fn14 in non-small cell lung cancer
correlates with activated EGFR and promotes tumor cell migration
and invasion. Am J Pathol. 181:111–120. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kalhori V and Törnquist K: MMP2 and MMP9
participate in S1P-induced invasion of follicular ML-1 thyroid
cancer cells. Mol Cell Endocrinol. 404:113–122. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Maeta H, Ohgi S and Terada T: Protein
expression of matrix metalloproteinases 2 and 9 and tissue
inhibitors of metalloproteinase 1 and 2 in papillary thyroid
carcinomas. Virchows Arch. 438:121–128. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Trimboli P, Virili C, Romanelli F,
Crescenzi A and Giovanella L: Galectin-3 performance in histologic
a cytologic assessment of thyroid nodules: A systematic review and
meta-analysis. Int J Mol Sci. 18:E17562017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cho SW, Kim YA, Sun HJ, Kim YA, Oh BC, Yi
KH, Park DJ and Park YJ: CXCL16 signaling mediated macrophage
effects on tumor invasion of papillary thyroid carcinoma. Endocr
Relat Cancer. 23:113–124. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Xia S, Wang C, Postma EL, Yang Y, Ni X and
Zhan W: Fibronectin 1 promotes migration and invasion of papillary
thyroid cancer and predicts papillary thyroid cancer lymph node
metastasis. Onco Targets Ther. 10:1743–1755. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Semenza GL: The hypoxic tumor
microenvironment: A driving force for breast cancer progression.
Biochim Biophys Acta. 1863:382–391. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yu X, Zhao R, Lin S, Bai X, Zhang L, Yuan
S and Sun L: CXCL16 induces angiogenesis in autocrine signaling
pathway involving hypoxia-inducible factor 1α in human umbilical
vein endothelial cells. Oncol Rep. 35:1557–1565. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zheng J, Lu W, Wang C, Xing Y, Chen X and
Ai Z: Galectin-3 induced by hypoxia promotes cell migration in
thyroid cancer cells. Oncotarget. 8:101475–101488. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Cui H, Grosso S, Schelter F, Mari B and
Krüger A: On the pro-metastatic stress response to cancer
therapies: Evidence for a positive co-operation between TIMP-1,
HIF-1α, and miR-210. Front Pharmacol. 3:1342012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Burrows N, Resch J, Cowen RL, von
Wasielewski R, Hoang-Vu C, West CM, Williams KJ and Brabant G:
Expression of hypoxia-inducible factor 1 alpha in thyroid
carcinomas. Endocr Relat Cancer. 17:61–72. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Mahkamova K, Latar N, Aspinall S and
Meeson A: Hypoxia increases thyroid cancer stem cell-enriched side
population. World J Surg. 42:350–357. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Hoenig MR, Bianchi C, Rosenzweig A and
Sellke FW: Decreased vascular repair and neovascularization with
aging: Mechanisms and clinical relevance with an emphasis on
hypoxia-inducible factor-1. Curr Mol Med. 8:754–767. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Valli A, Harris AL and Kessler BM: Hypoxia
metabolism in aging. Aging (Albany NY). 7:465–466. 2015. View Article : Google Scholar : PubMed/NCBI
|