Introduction
In the last 30 years, advances in molecular biology
have led to the development of molecular targeted therapy as an
important and feasible approach to treat gastric cancer (GC). For
example, trastuzumab, a monoclonal antibody against human epidermal
growth factor receptor 2, and ramucirumab, a monoclonal antibody
against vascular endothelial growth factor receptor 2 (VEGFR2), are
used for the treatment of advanced GC (1,2).
However, despite a comprehensive treatment strategy based on
surgery, chemotherapy, radiotherapy and molecular targeted therapy,
the 5-year survival rate of patients with GC remains
unsatisfactory. Therefore, it is necessary to continue searching
for effective molecular therapeutic targets.
Cadherin 11 (CDH11) is a type II cadherin located on
human chromosome 16q22.1 (3); its
encoded protein, CDH11, contains an extracellular domain of five
repeats, a single membrane-spanning domain and a highly conserved
C-terminal cytoplasmic domain (4).
CDH11 mediates cell-cell and cell-extracellularmatrix adhesion
through Ca2+-dependent homophilic interactions. The role
of CDH11 in cancer progression has attracted increased attention
(5,6). Chu et al (7) have reported that CDH11 expression
gradually increases from primary prostate cancer to metastatic
lesions, particularly in the bone. The intracardiac injection of
prostate cancer PC3 cells results in the formation of bone
metastasis, which is inhibited by CDH11 knockout, in mice (7). Further mechanistic studies have
revealed that CDH11 not only facilitates the physical link between
cancer cells and osteoblasts through CDH11 homophilic interactions,
but also increases the metastatic ability of cancer cells by
promoting the expression of migration- and invasion-associated
genes induced by the juxtamembrane and β-catenin binding domains of
CDH11 (7,8).
Assefnia et al (9)analyzed human cancer microarray datasets
from The Cancer Genome Atlas (TCGA) and reported that CDH11 was
increased in breast cancer and brain malignancy compared with
normal tissues. In vitro assays revealed that CDH11
knockdown significantly inhibited the growth and metastasis of
breast cancer and glioblastoma cells (9). Nakajima et al (10) demonstrated that patients with
osteosarcoma and high expression of CDH11 exhibited significantly
longer overall survival (OS) time compared with those with low
CDH11 expression. Promoter CpG methylation is an important process
of gene inactivation (11). Carmona
et al (12) have demonstrated
that the CDH11 gene in the lymphatic metastases of melanoma and
head and neck tumors display notable methylation compared with
primary tumors, resulting in the epigenetic silencing of CDH11.
Cellular and mouse models have demonstrated that the restoration of
CDH11 expression decreases the growth, motility and dissemination
of metastatic head and neck cancer cells, whereas the depletion of
CDH11 expression enhances growth and motility.
CDH11 is one of the 13 previously identified genes
exhibiting significantly increased CpG methylation in GC compared
with the non-metaplastic gastric mucosa (13). However, the role of CDH11 in GC
progression remains unclear. The present study aimed to use public
cancer databases to explore the expression pattern of CDH11 and
analyze the potential function and prognostic value of CDH11 in
GC.
Materials and methods
Patients and tissues
A total of 30 pairs of frozenGC and matched
paracancerous tissues (≥6 cm away from the tumor) were collected
from patients with GC (21 men and 9 women; mean age, 60.6 years;
age range, 51–79 years) who were admitted to the First Affiliated
Hospital of Chongqing Medical University (Chongqing, China) between
June 2016 and October 2016. These samples were used for reverse
transcription-quantitative (RT-q)PCR. Another 82
paraffin-embeddedpairs of GC tissues and matched paracancerous
tissues were collected from patients with GC admitted to the First
Affiliated Hospital of Chongqing Medical University between January
2011 and September 2014, which were used for immunohistochemical
analysis. The patient cohort for immunohistochemistry comprised 55
men and 27 women with a mean age of 57.7 years (age range, 46–80
years). Tumor-Node-Metastasis (TNM) staging (14) was as follows: 14 cases of stage I, 28
cases of stage II, 35 cases of stage III and 5 cases of stage IV.
All patients underwent total or subtotal gastrectomy for the first
time and did not receive radiotherapy and chemotherapy prior to
surgery. Of the 112 patients with GC, 21 cases were highly
differentiated, 42 were moderately differentiated and 49 were
poorly differentiated. The use of human tissue samples and
experimental protocols were approved by the Medical Ethics Review
Committee of the First Affiliated Hospital of Chongqing Medical
University, and written informed consentwas obtained from all
patients.
RT-qPCR
Total RNA was extracted from 30 mg of frozen tissues
using the TRIzol® reagent (Takara Biotechnology Co.,
Ltd.) and reverse-transcribed into cDNA according to the
manufacturer's instructions. The reverse transcription conditions
were as follows: 37°C for 15 min and 85°C for 5 sec. Two-step PCR
was performed using a SYBR® Green assay (Takara
Biotechnology Co., Ltd.) on a CFX96 PCR machine (Bio-Rad
Laboratories, Inc.), according to the manufacturer's kit and PCR
machine instructions. The thermocycling conditions were as follows:
Pre-denaturation at 95°C for 30 sec; followed by 45 cycles of
denaturation at 95°C for 5 sec, annealing at 60°C for 30 sec and
extension at 65°C for 1 min. GAPDH was used as the endogenous
control, and the mRNA expression of CDH11 was analyzed using the
2−ΔΔCq method (15). The
primers used were as follows: CDH11 forward,
5′-CCCAGTACACGTTGATGCCT-3′ and reverse, 5′-GACGTTCCCACATTGGACCT-3′;
GAPDH forward, 5′-CTTTGGTATCGTGGAAGGACTC-3′ and reverse,
5′-GTAGAGGCAGGGATGATGTTCT-3′ (Sangon Biotech Co., Ltd.).
Immunohistochemical analysis
Tissues fixed with 4% paraformaldehyde at room
temperature for 12 h were embedded in paraffin, and a series of
4-µm sections were prepared. Sections were incubated at 60°C for 20
min, deparaffinized in xylene at room temperature for 25 min and
rehydrated in a descending ethanol series, prior to incubation in
3% H2O2 at room temperature for 10 min to
inhibit endogenous peroxidase activity. Nonspecific binding was
blocked with 5% goat serum (BIOSS) at room temperature for 20 min,
and the sections were incubated with a rabbit anti-human CDH11
antibody (1:50; cat. no 6444R; BIOSS) overnight at 4°C. The next
day, the sections were incubated with a HRP conjugated goat
anti-rabbit IgG (1:1,000; cat. no. 40295G; BIOSS) at 37°C for 45
min and stained with diaminobenzidine reagent (DAB) for 4 min at
room temperature. Five fields were randomly selected under a light
microscope for scoring (magnification, ×100). Staining intensity
scoring criteria were as follows: 0 points, no staining; 1 point,
light yellow; 2 points, brownish yellow; 3 points, brown. Staining
range scoring criteria were as follows: 0 points, <5%; 1 point,
5–25%; 2 points, 26–50%; 3 points, 51–75%; 4 points, >75%. The
total score was the sum of the staining intensity and range, and a
total score ≥4 points was considered as high expression.
Gene Expression Profiling Interactive
Analysis (GEPIA) and UALCAN analysis
GEPIA (http://gepia.cancer-pku.cn), an onlinecancer
microarray database, was used to analyze the differences in CDH11
expression in 408 GC samples from TCGA and 211 normal samples from
TCGA and GTEx (16). The cut-off
P-value and log2[fold-change (FC)] were defined as 0.01
and 1, respectively. UALCAN (http://ualcan.path.uab.edu) is an interactive web
resource for analyzing cancer transcriptome data from TCGA
(17). The expression of CDH11 in GC
samples was analyzed based on disease state (cancer or normal), TNM
stage, sex, age, tumor grade, histological subtype and
Helicobacter pylori infection status.
Kaplan-Meier plotter and online
survival analysis
Kaplan-Meier Plotter (http://kmplot.com), an online cancer microarray
database containing gene expression profiles and survival data from
876 patients with GC, was used to analyze the effects of CDH11 on
OS and progression-free survival (PFS) (18). Patients at different stages were
divided into a high and a low expression group according to the
automatically selected best cut-off to assess the OS and PFS.
MethHC and cBioPortal
MethHC (http://methhc.mbc.nctu.edu.tw), a database of DNA
methylation and gene expression in human cancer, was used to
compare the average methylation level of the CDH11 promoter in GC
samples and matched normal samples (19). A total of 478 stomach adenocarcinoma
samples from the cBioPortal database (http://www.cbioportal.org) were used to analyze the
association between CDH11 mRNA expression and DNA methylation and
copy-number alterations (20).
Search Tool for the Retrieval of
Interacting Genes/Proteins (STRING), Kyoto Encyclopedia of Genes
and Genomes (KEGG) and Gene Ontology (GO) analyses
STRING (http://string-db.org), a database of known
protein-protein interactions, was used to predict proteins closely
associated with CDH11. The minimum required interaction score and
maximum number of interactors on the 1st shell were defined as 0.7
and 50, respectively. Subsequently, pathway-enrichment analysis was
performed for these proteins using KEGG (http://kobas.cbi.pku.edu.cn), and the maximum false
discovery rate was defined as 0.05 (21). GO analysis was performed using the
Database for Annotation, Visualization and Integrated Discovery
(version 6.8; http://david.ncifcrf.gov/home.jsp), and the maximum
adjusted P-value was defined as P<0.05 (22).
Prediction of upstream transcription
factors of the CDH11 gene
The UCSC Genome Browser (http://genome.ucsc.edu) was used to localize the CDS
region of CDH11, and its upstream 2,000 bp fragment was considered
to be the transcriptional promoter region of the CDH11 gene
(23). JASPAR (http://jaspardev.genereg.net) was used to predict the
transcription factors that may bind to the CDH11 promoter (24). Transcription factors that were highly
expressed and positively associated with the expression of CDH11 in
GC were further screened and considered as the upstream
transcription factors of CDH11.
Statistical analysis
The RT-qPCR experiments were repeated three times,
and the data were analyzed using SPSS 19.0 software (IBM Corp.).
Data are presented as the mean ± standard deviation. The
differences in CDH11 mRNA expression between GC and paracancerous
tissues from 30 patients with GC admitted to the First Affiliated
Hospital of Chongqing Medical University were compared using the
paired Student's t-test. The method for differential analysis from
GEPIA was one-way ANOVA, using disease state (tumor or normal) as
the variable for calculating differential expression. The method
for differential analysis from UALCAN was independent sample
t-test. The association between CDH11 protein expression levels and
the clinicopathological parameters of patients with GC was examined
using the χ2 test. Pearson correlation analysis was used
to determine thecorrelation between mRNACDH11 expressionand the
β-value of CDH11 methylation and CDH11 relative linear copy number,
within the cBioPortal database. The effects of CDH11 on the
prognosis of patients with GC was analyzed using the Kaplan-Meier
survival plot and log-rank test. Multivariate Cox analysis was used
to determine the ability of CDH11 to predict the prognosis of
patients. P<0.05 was considered to indicate a statistically
significant difference.
Results
CDH11 is upregulated in GC and is
associated with clinical parameters
The expression of CDH11 in GC tissues and normal
gastric tissues was analyzed using the GEPIA and UALCAN databases.
As demonstrated in Fig. 1A, in both
the GEPIA and UALCAN databases, CDH11 was upregulated in GC tissues
compared with normal gastric tissues, and the fold-changes were 5.7
and 3.2, respectively.
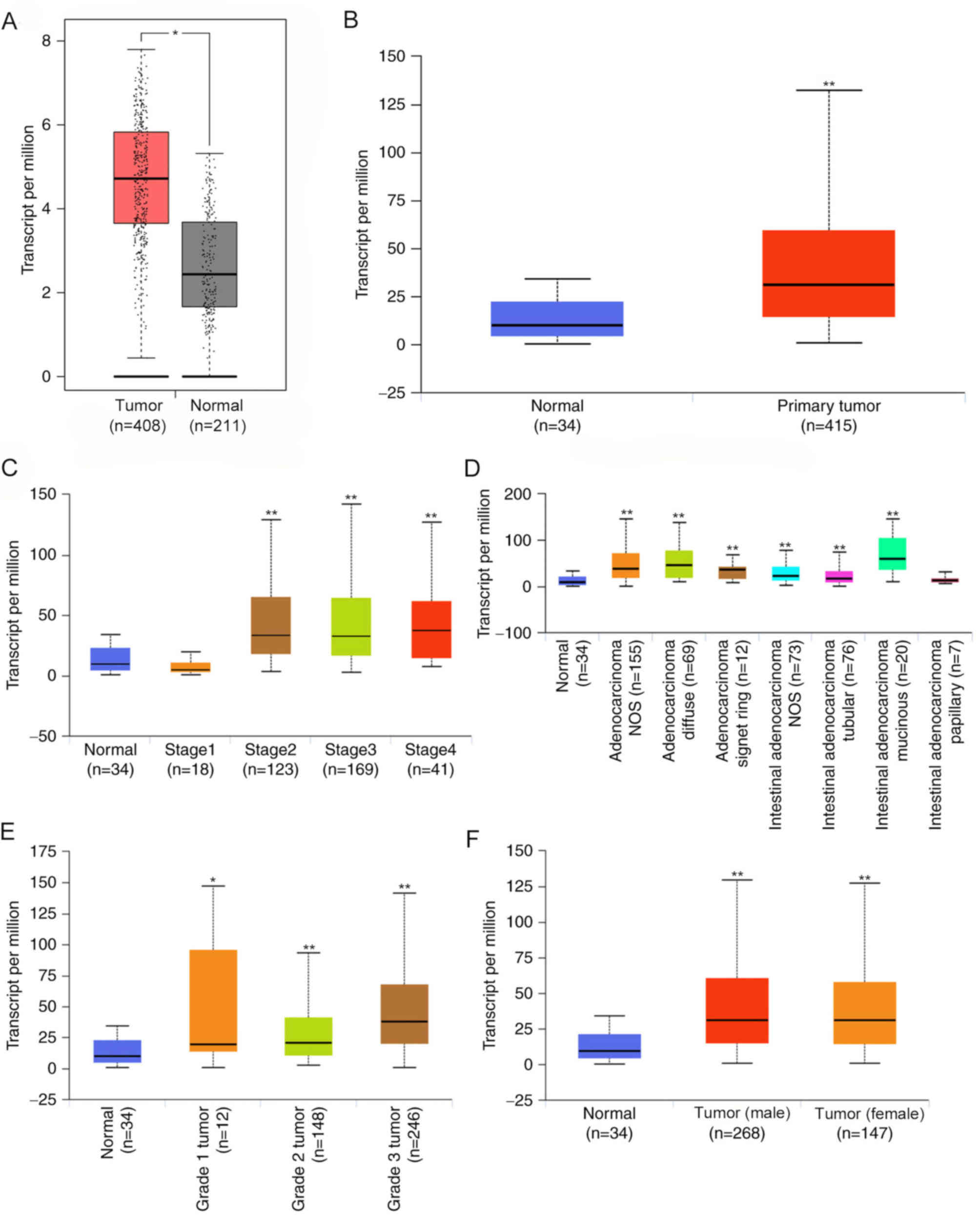 | Figure 1.CDH11 expression in GC and its
association with clinical parameters. (A) GEPIA and (B) UALCAN
databases were used to analyze the differences in CDH11 expression
between GC and normal gastric tissues. GEPIA included 408 GC
samples from TCGA database and 211 normal gastric samples from TCGA
and GTEx databases. UALCAN included 415 GC samples and 34 normal
gastric samples from TCGA database. Association between CDH11
expression and clinical parameters including (C)
Tumor-Node-Metastasis stage, (D) histological subtypes, (E) tumor
grade and (F) sex. GC, gastric cancer; T, tumor tissue; N, normal
gastric tissue CDH11, cadherin 11; GEPIA, Gene Expression Profiling
Interactive Analysis; TCGA, The Cancer Genome Atlas; STAD, stomach
adenocarcinoma. *P<0.05, **P<0.01 vs. the normal group. |
The association between CDH11 and pathological
parameters was analyzed using the UALCAN database. CDH11 expression
was significantly higher in stage II, III and IV GC tissues
compared with that in stage I GC tissues and normal gastric
tissues, whereas no significant differences in expression were
detected between stage I GC tissues and normal tissues. CDH11
expression was higher in mucinous intestinal adenocarcinoma and
diffuse adenocarcinoma compared with other histological subtypes.
Among all tumor grades, grade 3 tumors exhibited the highest
expression of CDH11. CDH11 expression was not significantly
associated with sex (Fig. 1B), age
or H. pylori infection (data not shown). These results
suggested that CDH11 was significantly increased in GC, and
increased CDH11 was associated with TNM stage, differentiation
degree and tumor grade.
High expression of CDH11 as a
prognosticmarker of GC
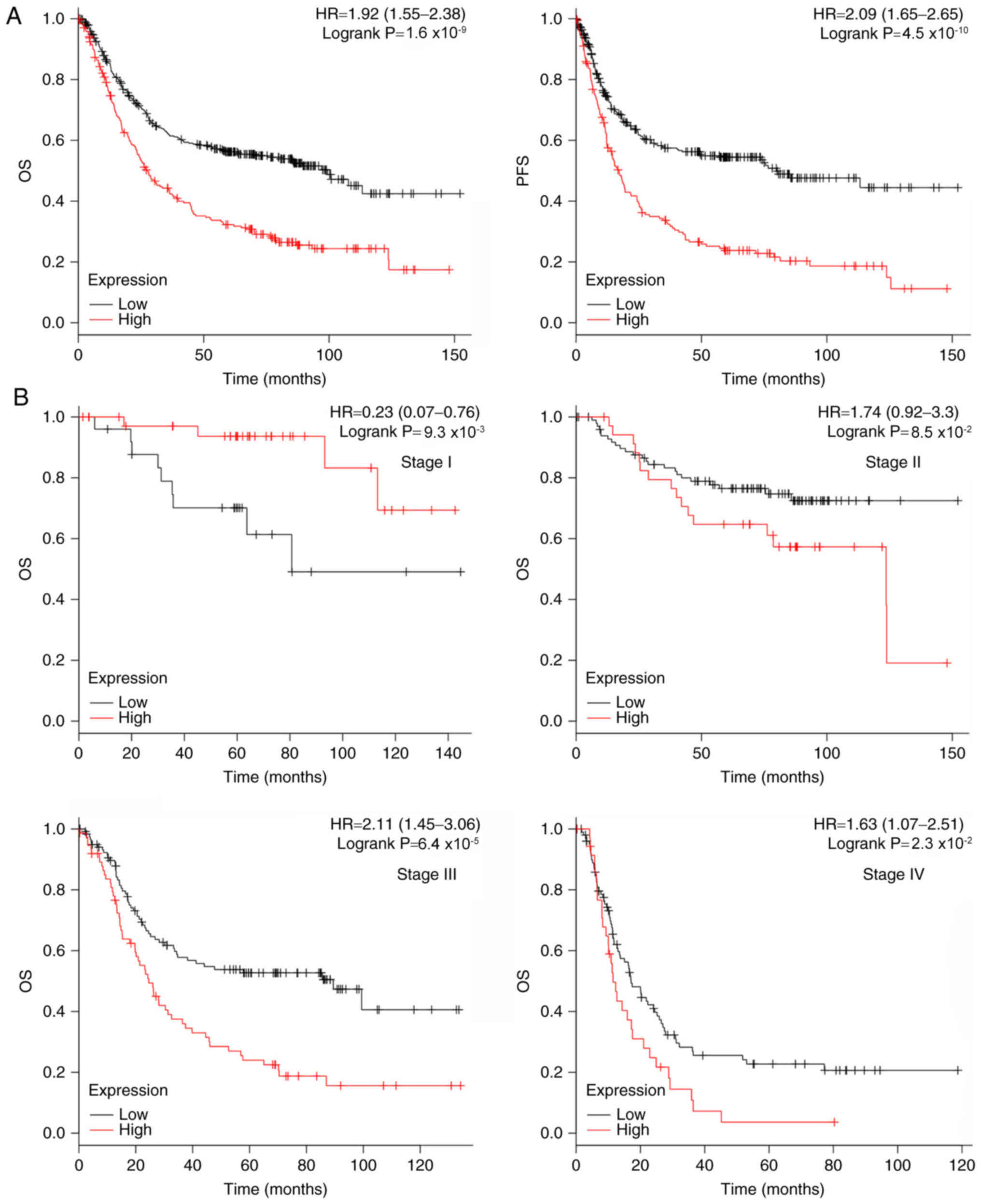
The prognostic significance of CDH11 was analyzed in
876 patients with GC using the Kaplan-Meier Plotter database.
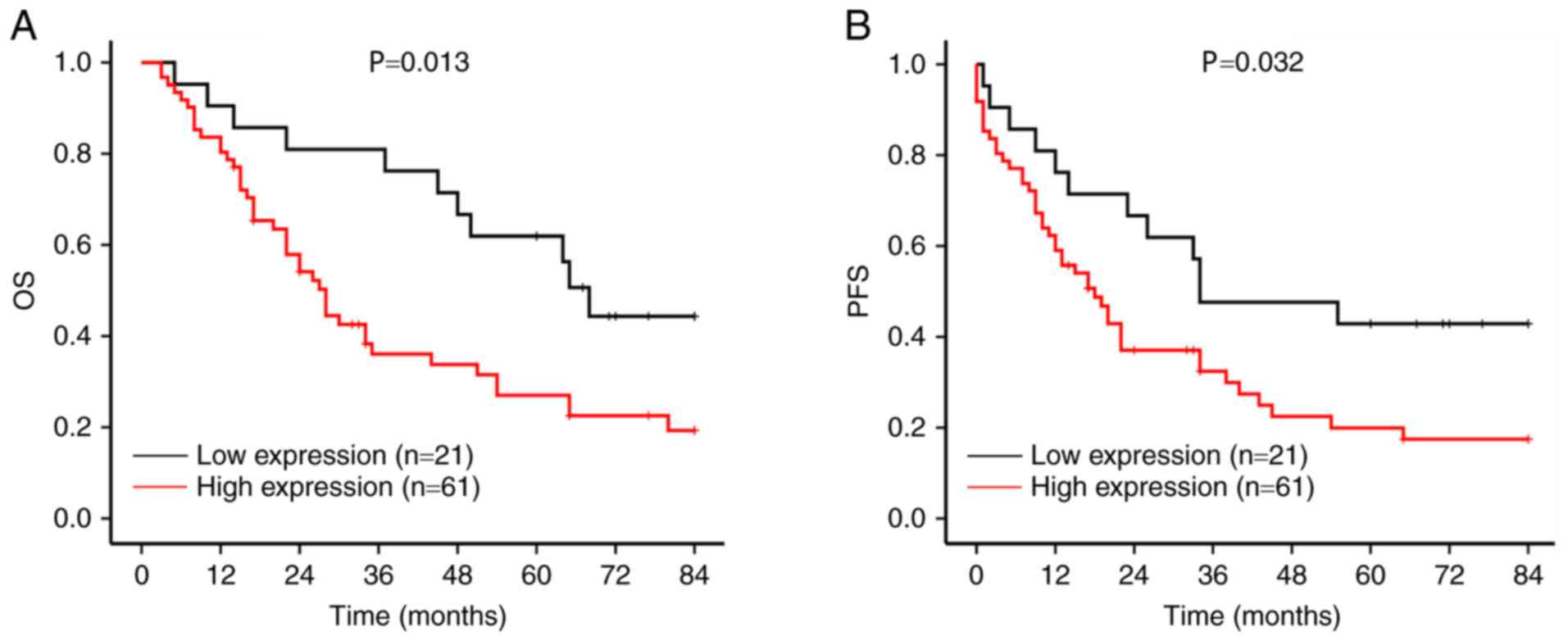
Patients with high CDH11 expression exhibited shorterOSandPFS times
(27.5 and 17.2 months, respectively) compared with those with low
CDH11 expression (99.4 and 80.1 months, respectively) (Fig. 2A).
The prognostic significance of CDH11 for patients
with GC at different stages was further analyzed. In patients with
stage III–IV GC, high CDH11 expression was associated with short OS
time. In patients with stage II GC, high CDH11 expression exhibited
a trend towards a short OStime (Fig.
2B). Patients with stage III GC with high expression of CDH11
exhibited shorter PFS time compared with those with low expression.
In patients with stage II and IV GC, high CDH11 expression was
mildly associated with shorter PFStimes compared with low
expression (Fig. 2C). However, high
CDH11 expression was associated with longer OS and PFS times in
patients with stage I GC.
Associations between CDH11 expression,
DNA methylation and copy number alterations in GC
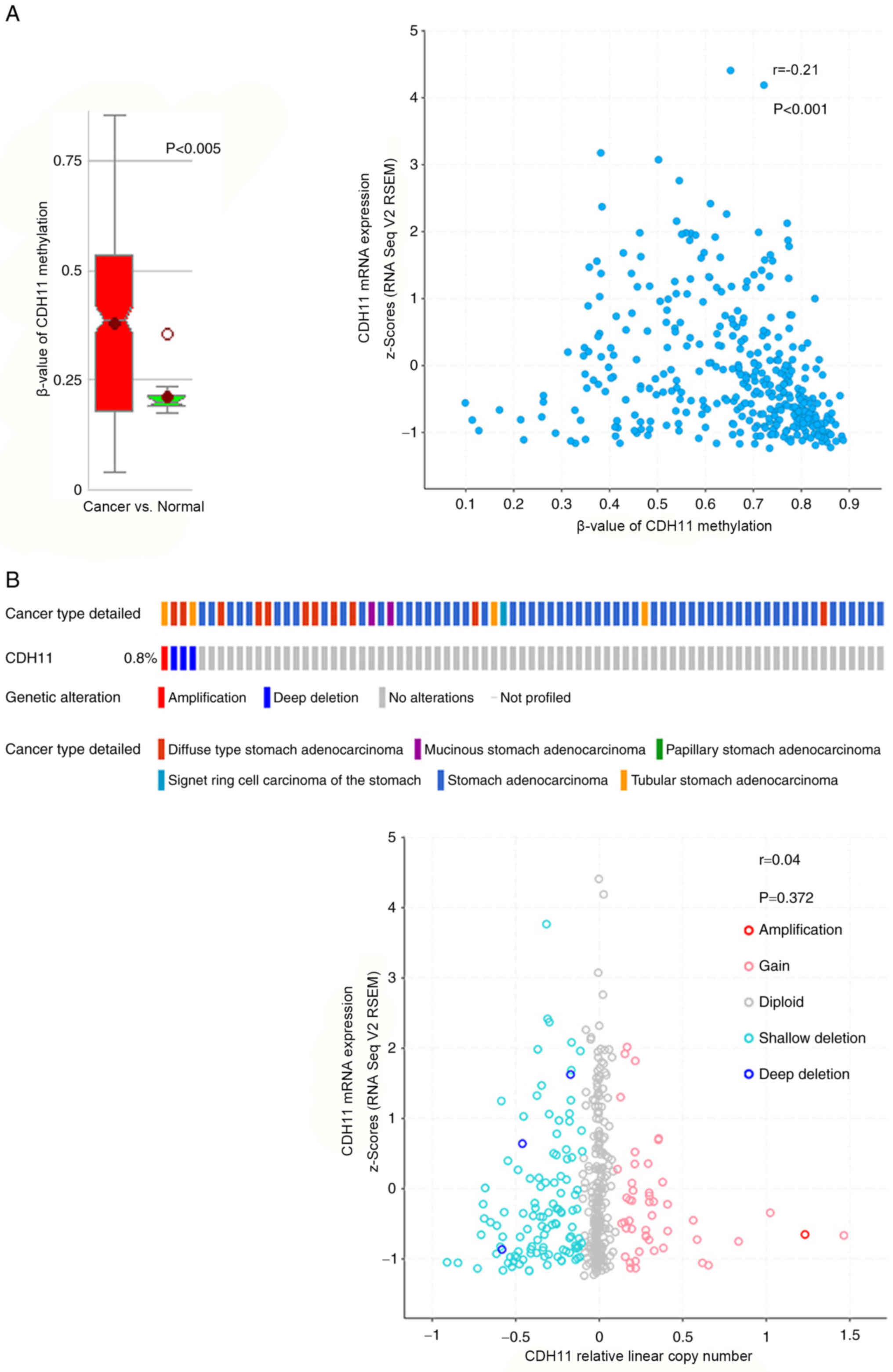
The MethHC database was used to analyze the
methylation status of CDH11 in GC tissues and normal gastric
tissues. Consistent with findings by Sepulveda et al
(13), the results of the present
study demonstrated that the level of methylation was markedly
increased in GC tissues compared with normal tissues (Fig. 3A). Although CDH11 expression was
significantly negatively associated with promoter methylation in GC
tissues, the correlation coefficient was only −0.21, indicating a
weak correlation (Fig. 3A). This may
explain the increased CDH11 promoter methylation in GC tissues,
which is not associated with lower CDH11 mRNA expression compared
with normal gastric tissues.
The association between increased CDH11 expression
in GC tissues and DNA copy number alteration was further analyzed.
Data from the cBioPortal database suggested that DNA copy number
alterations were not more prevalent in GC tissues compared with
normal gastric tissues. Only 1 of 478 patients exhibited
significant gene amplification. Furthermore, correlation analysis
indicated no correlation between copy number values and CDH11 mRNA
expression (Fig. 3B). These results
suggested that CDH11 was not regulated at the DNA level to mediate
its upregulation in GC tissues.
Identification of putative upstream
regulatory molecules of CDH11
The promoter sequence of CDH11 was acquired from
UCSC, and the transcription factors that could potentially bind to
the promoter sequences in vertebrates were analyzed using the
JASPAR database. A total of 342 transcription factors were
selected, and their expression patterns were analyzed. A total of
67 upregulated transcription factors in GC (data obtained from the
GEPIA database) were identified. Of these 67 transcription factors,
nine were positively associated with CDH11 mRNA expression, as
follows: Nuclear factor IA (NFIA), runt-related transcription
factor 2 (RUNX2), myocyte enhancer factor 2C (MEF2C), runt-related
transcription factor 1 (RUNX1), lymphoid enhancer-binding factor 1
(LEF1), nuclear factor IX (NFIX), transcription factor 4 (TCF4),
paired related homeobox 1 (PRRX1) and ETS proto-oncogene 1 (ETS1)
(data obtained from the GEPIA database; Fig. 4). Thus, it was speculated that these
nine transcription factors may mediate the upregulation of CDH11 in
GC.
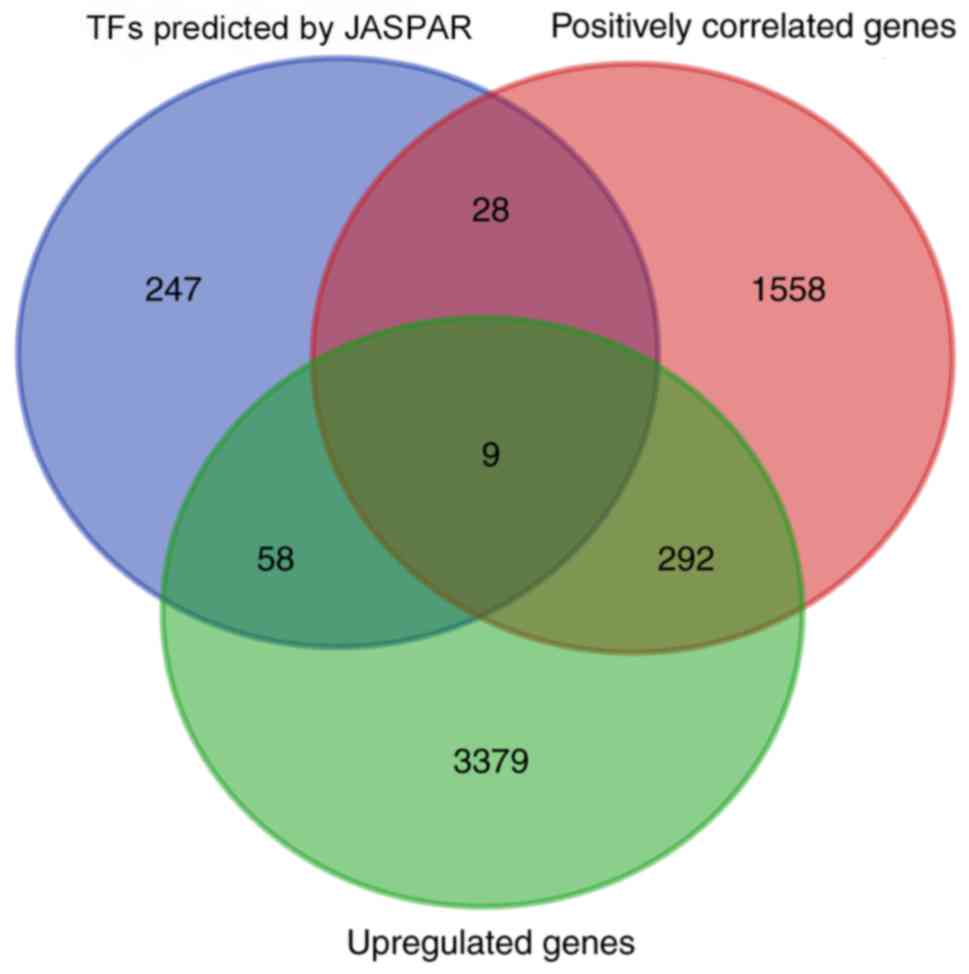 | Figure 4.Putative transcription factors
involved in CDH11 upregulation. The promoter sequence of CDH11 was
obtained from the UCSC database, and the transcription factors
capable of binding to the promoter sequence were predicted by the
JASPAR database. A total of 9 TFs (NFIA, RUNX2, MEF2C, RUNX1, LEF1,
NFIX, TCF4, PRRX1 and ETS1) were upregulated in gastric cancer
tissues and positively associated with CDH11 expression. CDH11,
cadherin 11; TFs, transcription factors. |
Potential molecular mechanism by which
CDH11 promotes GC
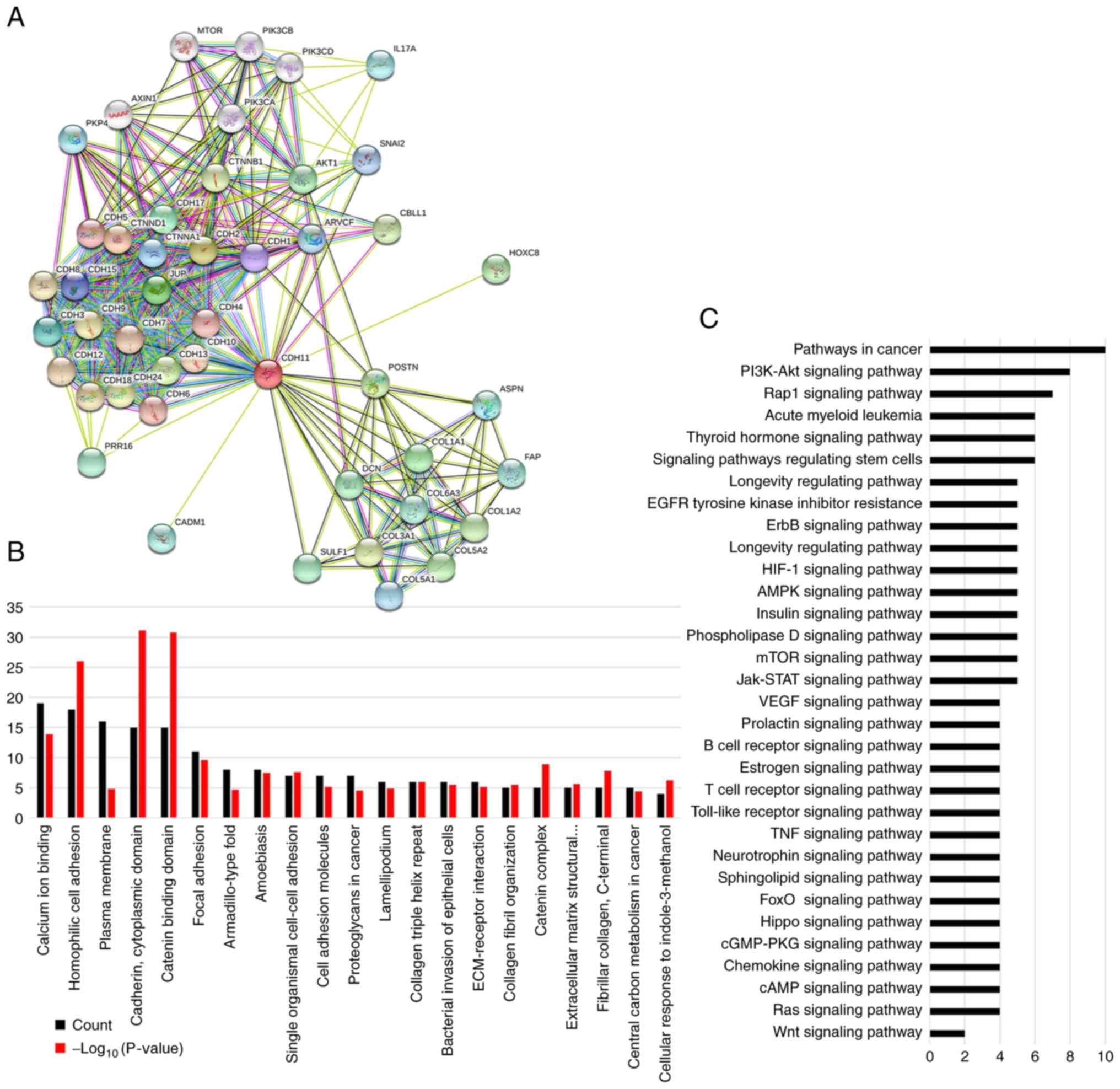
A total of 45 proteins associated with CDH11 were
identified using the STRING database (Fig. 5A) (http://string-db.org) (25). GO analysis revealed that most of
these proteins were involved in cell adhesion, extracellular matrix
recombination and cell movement (Fig.
5B). KEGG enrichment analysis demonstrated that these proteins
were enriched in various cancer signaling pathways such as
‘pathways in cancer’ (10 proteins), ‘PI3K-Akt signaling pathway’ (8
proteins), ‘HIF-1 signaling pathway’ (5 proteins), ‘mTOR signaling
pathway’ (5 proteins), ‘Jak-STAT signaling pathway’ (5 proteins)
and ‘VEGF signaling pathway’ (4 proteins) (Fig. 5C). These results suggested that CDH11
promoted GC progression by interacting with cell motility or
adhesion proteins and by activating the downstream
cancer-associated signaling pathways.
Validation of CDH11 expression and
prognostic effectin a local cohort of patients with GC
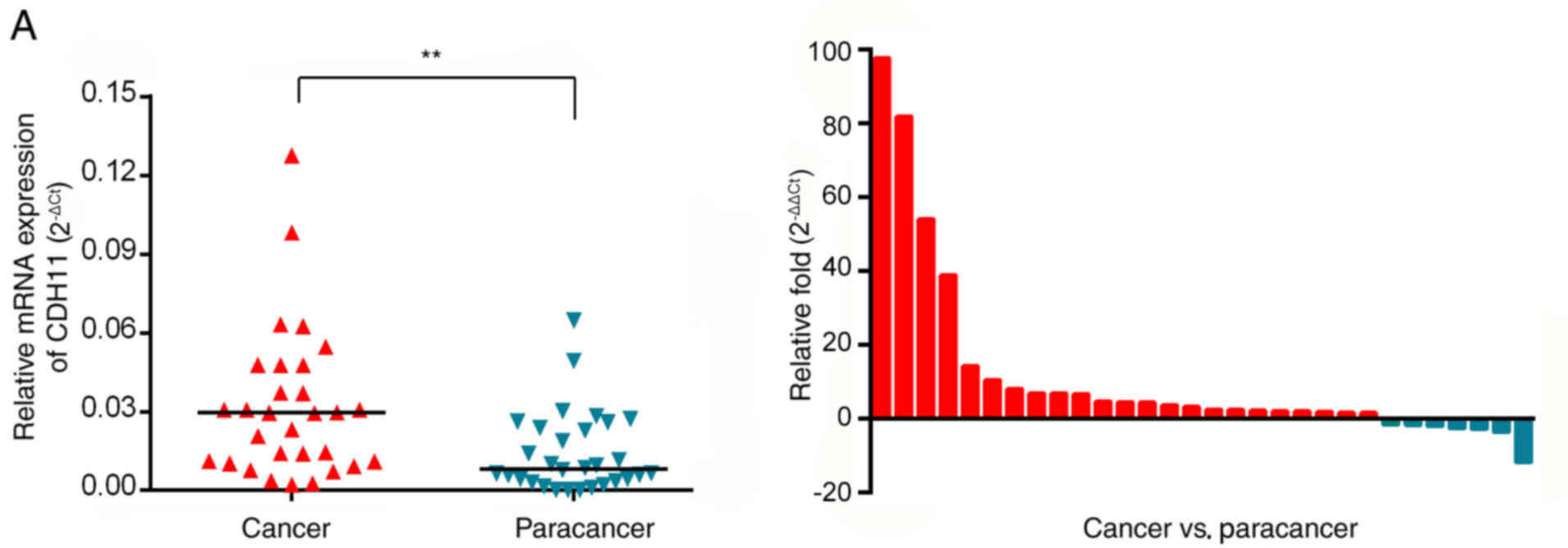
The mRNA expression levels of CDH11 in 30 GC tissues
and matchedparacancerous tissues from the First Affiliated Hospital
of Chongqing Medical University were detected by RT-qPCR. In 23
patients (76.7%), the mRNA expression of CDH11 was higher in GC
tissues compared with paracancerous tissues. The average CDH11 mRNA
expression in cancer tissues (mean ΔCt, 5.56) was 4.03-fold higher
compared with that in paracancerous tissues (mean ΔCt, 7.25)
(Fig. 6A). These results indicated
that the expression of CDH11 mRNA in GC tissues was significantly
higher compared with adjacent tissues. The expression of the CDH11
protein in 82 patients with GC was further detected by
immunohistochemistry. As demonstrated in Fig. 6B and C, CDH11 staining was mainly
detected in the cytoplasm, with lower staining in the cell membrane
(the negative control is presented in Fig. S1). Positive staining for the CDH11
protein was observed in 61 GC tissues (74.39%) in 82 patients
(staining score ≥4), and in only 27 (31.7%) paracancerous tissues.
The staining score (mean score, 4.69) in GC tissues was also
significantly higher compared withparacancerous tissues (mean
score, 2.44). High CDH11 expression was associated with poor
differentiation and a late stage (Table
I). Further analysis revealed that the expression of the CDH11
protein in patients with stage III and IV GC was higher compared
with that in patients with stage I and II GC (5.35 vs. 4.07,
respectively; Fig. 6C). These
results were consistent with the RT-qPCR data, suggesting that
CDH11 expression was increased in GC tissues and may be involved in
the progression of GC.
 | Table I.Association between CDH11 and
clinicopathological characteristics of patients with gastric
cancer. |
Table I.
Association between CDH11 and
clinicopathological characteristics of patients with gastric
cancer.
|
|
| CDH11 |
|
|
|---|
|
|
|
|
|
|
|---|
| Characteristic | Number | − | + | χ2 | P-value |
|---|
| Sex |
|
|
| 0.559 | 0.341 |
|
Female | 27 | 8 | 19 |
|
|
|
Male | 55 | 13 | 42 |
|
|
| Age, years |
|
|
| 0.479 | 0.489 |
|
<60 | 30 | 9 | 21 |
|
|
|
≥60 | 52 | 12 | 40 |
|
|
| Histological
grade |
|
|
| 6.488 | 0.011a |
| Grade
3 | 43 | 6 | 37 |
|
|
| Grade 1
or 2 | 39 | 15 | 24 |
|
|
| Tumor size, cm |
|
|
| 0.013 | 0.911 |
|
<5 | 36 | 9 | 27 |
|
|
| ≥5 | 46 | 12 | 34 |
|
|
| TNM stage |
|
|
| 7.045 | 0.008a |
|
1+2 | 42 | 16 | 26 |
|
|
|
3+4 | 40 | 5 | 35 |
|
|
| Vascular
invasion |
|
|
| 2.545 | 0.111 |
|
Absent | 64 | 19 | 45 |
|
|
|
Present | 18 | 2 | 16 |
|
|
| Distant
metastasis |
|
|
| 0.088 | 0.767 |
| No | 77 | 20 | 57 |
|
|
|
Yes | 5 | 1 | 4 |
|
|
High CDH11 protein indicates a poor
prognosis for patients with GC in the local cohort
The prognostic value of CDH11 was investigated in
the 82 patients with GC from the First Affiliated Hospital of
Chongqing Medical University. The median OS time of patients with
GC and high expression of CDH11 was 28.6 months (Fig. 7A), and the median PFS time was 18.4
months, which were significantly shorter compared with patients
with GC and low expression of CDH11 (OS time, 68.1 months; PFS
time, 34.5 months) (Fig. 7B).
Univariate analysis demonstrated that OS time was
significantlyassociated with histological grade (P<0.001), nodal
invasion (P=0.042), distantmetastasis (P=0.001), TNM stage
(P<0.001) and CDH11 expression (P=0.017) (Table II). PFS time was
significantlyassociated withhistological grade (P=0.001),
distantmetastasis (P=0.005), TNM stage (P=0.008) and CDH11
expression (P=0.038) (Table III).
However, the association between high CDH11 and OS and PFS wasnot
significant after adjusting for otherprognostic markers in the
multivariate analysis. These results indicated that CDH11 was
associated with a shorter OS and PFS times in patients with GC,
although high CDH11 expression was not an independent prognostic
factor.
 | Table II.Univariateanalysis and
multivariateanalysis of prognostic factors for overall survival in
gastric cancer. |
Table II.
Univariateanalysis and
multivariateanalysis of prognostic factors for overall survival in
gastric cancer.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variable | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Sex | 0.982 | 0.552–1.745 | 0.950 |
|
|
|
| Age | 1.09 | 0.627–1.895 | 0.759 |
|
|
|
| Histological
grade | 3.325 | 1.825–5.695 |
<0.001a | 3.122 | 1.752–5.564 |
<0.001a |
| Tumor size | 1.229 | 0.716–2.108 | 0.455 |
|
|
|
| Nodal invasion | 1.924 | 1.023–3.616 | 0.042a |
|
|
|
| Vascular
invasion | 1.564 | 0.833–2.935 | 0.164 |
|
|
|
| Distantmetastasis
a/Local/Youdao/Dict/ | 7.082 | 2.348–21.363 | 0.001a | 5.591 | 1.739–17.975 | 0.004a |
|
Application/7.5.0.0/resultui/dict/? |
|
keyword=metastasis |
| TNM stage | 2.499 | 1.143–1.329 | 0.001a | 2.124 | 1.195–3.788 | 0.010a |
| CDH11
expression | 2.236 | 1.157–2.425 | 0.017a |
|
| 0.354 |
 | Table III.Univariateand multivariateanalyses of
prognostic factors for progression-free survival in gastric
cancer. |
Table III.
Univariateand multivariateanalyses of
prognostic factors for progression-free survival in gastric
cancer.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variable | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Sex | 0.989 | 0.58–1.686 | 0.966 |
|
|
|
| Age | 1.125 | 0.638–1.985 | 0.685 |
|
|
|
| Histological
grade | 2.565 | 1.486–4.425 | 0.001a | 2.518 | 1.447–4.383 | 0.010a |
| Tumor size | 1.136 | 0.673–1.918 | 0.634 |
|
|
|
| Nodal invasion | 1.429 | 0.799–2.555 | 0.228 |
|
|
|
| Vascular
invasion | 1.508 | 0.823–2.764 | 0.184 |
|
|
|
|
Distantmetastasis | 4.509 | 1.569–12.9583 | 0.005a | 3.805 | 1.261–11.484 | 0.018a |
| TNM stage | 2.058 | 1.212–3.496 | 0.008a | 1.754 | 1.007–3.054 | 0.047a |
| CDH11
expression | 1.978 | 1.039–3.767 | 0.038a |
|
| 0.379 |
Discussion
In the present study, the upregulation of CDH11 in
GC was demonstrated, and high expression of CDH11 was associated
with the progression of GC. Using bioinformatics methods, the
overexpression of CDH11 was demonstrated to be mainly attributed to
transcriptional activation. Furthermore, it was revealed that CDH11
may exert its cancer-promoting effects by increasing cell movement
and activating multiple downstream signaling pathways.
Abnormal expression of cadherins is associated with
GC cancer progression. The loss or downregulation of E-cadherin
leads to the translocation of β-catenin into the nucleus, which in
turn promotes GC cell proliferation, drug resistance and metastasis
through the WNT/β-catenin signaling pathway (26). By contrast, N-cadherin acts as an
oncogene; high expression of N-cadherin contributes to the loss of
cell basal polarity and the disruption of cell adherens junctions,
which promotes the movement of tumor cells from the primary lesion
to the basement membrane, followed by the degradation of the
extracellular matrix, breaking through the tissue barrier structure
and eventually distant metastasis (27). However, the role of CDH11 in GC
remains unclear.
Shibata et al (28) have demonstrated that CDH11 is highly
expressed in gastric signet-ring cell carcinoma and surrounding
fibroblasts, suggesting that CDH11 serves a key role in the
formation of diffuse-type GC through cancer-stromal interactions.
In addition, Sandoval-Bórquez et al (29) analyzed 19 pairs of GC and
paracancerous samples and reported that CDH11 mRNA was highly
expressed in 17 of the samples. However, the aforementioned studies
were limited by the lack of large sample analysis, and thus the
role of CDH11 in GC needed further exploration. The present study
used the GEPIA and UALCAN databases to analyze the data obtained
from 408 and 415 patients with GC, respectively, and revealed that
the expression of CDH11 was significantly higher in GC tissues
compared with normal gastric tissues. No differences in CDH11
expression were observed between patients with stage I and normal
tissues, which exhibited significantly lower CDH11 expression
compared with patients with advanced GC. Follow-up survival
analysis using the Kaplan-Meier Plotter database demonstrated that
although high expression of CDH11 was associated with a shorter
survival time in patients with advanced GC, it was also associated
with a better prognosis in patients with stage I GC. A reasonable
explanation for this finding may be that CDH11-mediated homologous
adhesion promoted the formation of tight junction between cancer
cells and cancer nests in early GC (7,30). In
advanced GC, cancer cells infiltrated into the submucosa, where
stromal cells also expressed CDH11. Therefore, CDH11 mediated
heterologous adhesion between cancer cells and the stroma, thus
promoting invasion and migration of GC cells.
To verify the CDH11 expression pattern in GC and its
association with prognosis, specimens from the First Affiliated
Hospital of Chongqing Medical University were used in the present
study. The results demonstrated that the expression level of CDH11
was not only higher in GC tissues compared with paracancerous
tissues, but also higher in advanced GC tissues compared with early
GC. The survival analysis revealed that patients with high
expression of CDH11 exhibited significantly shorter OS and PFS
times compared with those with low CDH11 expression. Thus, the
results of the present study suggested that CDH11 protein
upregulation may be an indicator of poor prognosis in GC and
indicated an oncogenic role of CDH11 in GC.
The mechanism underlying the upregulation of CDH11
in GC remains unclear. Previous studies have demonstrated that the
CDH11 promoter is hypermethylated in bladder cancer, melanoma and
head and necktumors (31). The
present study analyzed the methylation status of CDH11 in GC and
normal gastric tissues. Consistent with the findings by Sepulveda
et al (13), the results of
the present study demonstrated higher promoter methylation of the
CDH11 gene in GC tissues compared with normal tissues, which
contradicted the high mRNA expression of CDH11 in GC tissues.
However, despite the high level of CDH11 promoter methylation in GC
tissues, the correlation coefficient between the CDH11 methylation
level and CDH11 mRNA expression level was only −0.21, which was a
weak correlation, suggesting that promoter methylation was not
sufficient to reverse the upregulation of CDH11 expression caused
by other reasons. The mechanism underlying the upregulation of
CDH11 in GC is not clear. DNA copy number variation is a mechanism
of gene upregulation; however, the copy number variation of CDH11
in GC tissues was only 0.8%, and was not associated with the mRNA
expression level of CDH11. These findings indicated that abnormal
CDH11 expression in GC tissues was not attributed to a regulatory
mechanism at the DNA level. Transcriptional activation is another
mechanism by which genes are upregulated; a total of 342
transcription factors were predicted to be able to bind to the
CDH11 promoter, of which nine (NFIA, RUNX2, MEF2C, RUNX1, LEF1,
NFIX, TCF4, PRRX1, and ETS1) were upregulated and positively
associated with the expression of CDH11 in GC. These nine
transcription factors may be the putative regulatory molecules of
CDH11, although this requires further functional validation.
CDH11 is a typical cell adhesion protein. One of its
basic functions is to mediate cell-cell and cell-matrix junctions,
thus regulating cell movement and the epithelial-mesenchymal
transition (32,33). In addition to its involvement in cell
movement, CDH11 is a positive regulator of NF-κB signaling
(34). Upregulated CDH11 in GC
increases NF-κB activity and promotes the nuclear localization of
NF-κB p65, leading to cell invasion and metastasis. In the present
study, to further determine how CDH11 promoted GC progression, 45
molecules associated with CDH11 were analyzed using the STRING
database, and the results demonstrated that these molecules were
primarily involved in cell adhesion (35,36) and
migration (37,38). KEGG analysis revealed that the
identified molecules were enriched in multiple cancer-associated
signaling pathways such as the PI3K-Akt (39), Wnt/β-catenin (40) and HIF-1 signaling pathway (41). This suggested that CDH11 promoted the
invasion and migration of GC cells by affecting the mechanical
connections with other adhesion molecules and cytoskeletal
proteins. However, the results of the present study indicated that
CDH11 may also promote cell proliferation, drug resistance and
metastasis by activating multiple downstream signaling
pathways.
In conclusion, the present study demonstrated that
CDH11 was upregulated in GC, and its upregulation may be used as a
direct prognostic indicator of a negative outcome. Although the
mechanism by which CDH11 promotes the progression of GC remains to
be determined, the present study demonstrated that CDH11
upregulation may serve an oncogenic role in GC. Considering the
anticancer effect of celecoxib in breast cancer cells (42), further studies on the function of
CDH11 may provide a promising approach for the treatment of GC.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets generated and/or analyzed during the
currentstudy are available from the corresponding author upon
reasonable request.
Authors' contributions
QW and CL conceived and designed the experiments. YJ
and XP conducted the experiments and analyzed the data. XP wrote
the manuscript. All authors read and approved thefinal
manuscript.
Ethics approval and consent to
participate
The use of human tissue samples and experimental
protocolswere approved by the Medical Ethics Review Committee of
the First Affiliated Hospital of Chongqing Medical University
andwritten informed consent was obtained from all patients
[approvalno. (2012)649].
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lordick F and Janjigian YY: Clinical
impact of tumour biology in the management of gastroesophageal
cancer. Nat Rev Clin Oncol. 13:348–360. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shah MA: Gastrointestinal cancer: Targeted
therapies in gastric cancer-the dawn of a new era. Nat Rev Clin
Oncol. 11:10–11. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kremmidiotis G, Baker E, Crawford J, Eyre
HJ, Nahmias J and Callen DF: Localization of human cadherin genes
to chromosome regions exhibiting cancer-related loss of
heterozygosity. Genomics. 49:467–471. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Okazaki M, Takeshita S, Kawai S, Kikuno R,
Tsujimura A, Kudo A and Amann E: Molecular cloning and
characterization of OB-cadherin, a new member of cadherin family
expressed in osteoblasts. J Biol Chem. 269:12092–12098.
1994.PubMed/NCBI
|
|
5
|
Kimura Y, Matsunami H, Inoue T, Shimamura
K, Uchida N, Ueno T, Miyazaki T and Takeichi M: Cadherin-11
expressed in association with mesenchymal morphogenesis in the
head, somite, and limb bud of early mouse embryos. Dev Biol.
169:347–358. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hoffmann I and Balling R: Cloning and
expression analysis of a novel mesodermally expressed cadherin. Dev
Biol. 169:337–346. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chu K, Cheng CJ, Ye X, Lee YC, Zurita AJ,
Chen DT, Yu-Lee LY, Zhang S, Yeh ET, Hu MC, et al: Cadherin-11
promotes the metastasis of prostate cancer cells to bone. Mol
Cancer Res. 6:1259–1267. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huang CF, Lira C, Chu K, Bilen MA, Lee YC,
Ye X, Kim SM, Ortiz A, Wu FL, Logothetis CJ, et al: Cadherin-11
increases migration and invasion of prostate cancer cells and
enhances their interaction with osteoblasts. Cancer Res.
70:4580–4589. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Assefnia S, Dakshanamurthy S, Guidry Auvil
JM, Hampel C, Anastasiadis PZ, Kallakury B, Uren A, Foley DW, Brown
ML, Shapiro L, et al: Cadherin-11 in poor prognosis malignancies
and rheumatoid arthritis: Common target, common therapies.
Oncotarget. 5:1458–1474. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nakajima G, Patino-Garcia A, Bruheim S, Xi
Y, San Julian M, Lecanda F, Sierrasesumaga L, Müller C, Fodstad O
and Ju J: CDH11 expression is associated with survival in patients
with osteosarcoma. Cancer Genomics Proteomics. 5:37–42.
2008.PubMed/NCBI
|
|
11
|
Rodríguez-Paredes M and Esteller M: Cancer
epigenetics reaches mainstream oncology. Nat Med. 17:330–339. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Carmona FJ, Villanueva A, Vidal A, Muñoz
C, Puertas S, Penin RM, Gomà M, Lujambio A, Piulats JM, Mesía R, et
al: Epigenetic disruption of cadherin-11 in human cancer
metastasis. J Pathol. 228:230–240. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sepulveda JL, Gutierrez-Pajares JL, Luna
A, Yao Y, Tobias JW, Thomas S, Woo Y, Giorgi F, Komissarova EV,
Califano A, et al: High-definition CpG methylation of novel genes
in gastric carcinogenesis identified by next-generation sequencing.
Mod Pathol. 29:182–193. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Washington K: 7th edition of
the AJCC cancer staging manual: Stomach. Ann Surg Oncol.
17:3077–3079. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tang Z, Li C, Kang B, Gao G, Li C and
Zhang Z: GEPIA: A web server for cancer and normal gene expression
profiling and interactive analyses. Nucleic Acids Res. 45:W98–W102.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chandrashekar DS, Bashel B, Balasubramanya
SAH, Creighton CJ, Ponce-Rodriguez I, Chakravarthi BVSK and
Varambally S: UALCAN: A portal for facilitating tumor subgroup gene
expression and survival analyses. Neoplasia. 19:649–658. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nagy Á, Lánczky A, Menyhárt O and Győrffy
B: Validation of miRNA prognostic power in hepatocellular carcinoma
using expression data of independent datasets. Sci Rep. 8:92272018.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang W, Hsu S and Huang H, Sun Y, Chou C,
Weng S and Huang H: MethHC: A database of DNA methylation and gene
expression in human cancer. Nucleic Acids Res. 43:D856–D861. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gao J, Aksoy BA, Dogrusoz U, Dresdner G,
Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, et al:
Integrative analysis of complex cancer genomics and clinical
profiles using the cBioPortal. Sci Signal. 6:l12013. View Article : Google Scholar
|
|
21
|
Xie C, Mao X, Huang J, Ding Y, Wu J, Dong
S, Kong L, Gao G, Li C and Wei L: KOBAS 2.0: A web server for
annotation and identification of enriched pathways and diseases.
Nucleic Acids Res. 39:W316–W322. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sherman BT and Lempicki RA: Systematic and
integrative analysis of large gene lists using DAVID bioinformatics
resources. Nat Protoc. 4:442009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kent WJ, Sugnet CW, Furey TS, Roskin KM,
Pringle TH, Zahler AM and Haussler D: The human genome browser at
UCSC. Genome Res. 12:996–1006. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Stormo GD: DNA binding sites:
Representation and discovery. Bioinformatics. 16:16–23. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Szklarczyk D, Gable AL, Lyon D, Junge A,
Wyder S, Huerta-Cepas J, Simonovic M, Doncheva NT, Morris JH, Bork
P, et al: STRING v11: Protein-protein association networks with
increased coverage, supporting functional discovery in genome-wide
experimental datasets. Nucleic Acids Res. 47:D607–D613. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yanaka Y, Muramatsu T, Uetake H, Kozaki K
and Inazawa J: miR-544a induces epithelial-mesenchymal transition
through the activation of WNT signaling pathway in gastric cancer.
Carcinogenesis. 36:1363–1371. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gao P, Xing AY, Zhou GY, Zhang TG, Zhang
JP, Gao C, Li H and Shi DB: The molecular mechanism of microRNA-145
to suppress invasion-metastasis cascade in gastric cancer.
Oncogene. 32:491–501. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shibata T, Ochiai A, Gotoh M, Machinami R
and Hirohashi S: Simultaneous expression of cadherin-11 in
signet-ring cell carcinoma and stromal cells of diffuse-type
gastric cancer. Cancer Lett. 99:147–153. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sandoval-Bórquez A, Polakovicova I,
Carrasco-Véliz N, Lobos-González L, Riquelme I, Carrasco-Avino G,
Bizama C, Norero E, Owen GI, Roa JC and Corvalán AH:
MicroRNA-335-5p is a potential suppressor of metastasis and
invasion in gastric cancer. Clin Epigenetics. 9:1142017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Alimperti S and Andreadis ST: CDH2 and
CDH11 act as regulators of stem cell fate decisions. Stem Cell Res.
14:270–282. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lin YL, Gui SL and Ma JG: Aberrant
methylation of CDH11 predicts a poor outcome for patients with
bladder cancer. Oncol Lett. 10:647–652. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yao J, Deng B, Zheng L, Dou L, Guo Y and
Guo K: miR-27b is upregulated in cervical carcinogenesis and
promotes cell growth and invasion by regulating CDH11 and
epithelial-mesenchymal transition. Oncol Rep. 35:1645–1651. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lee Y, Bilen MA, Yu G, Lin S, Huang C,
Ortiz A, Cho H, Song JH, Satcher RL, Kuang J, et al: Inhibition of
cell adhesion by a cadherin-11 antibody thwarts bone metastasis.
Mol Cancer Res. 11:1401–1411. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang JX, He WL, Feng ZH, Chen DL, Gao Y,
He Y, Qin K, Zheng ZS, Chen C, Weng HW, et al: A positive feedback
loop consisting of C12orf59/NF-κB/CDH11 promotes gastric cancer
invasion and metastasis. J Exp Clin Canc Res. 38:1642019.
View Article : Google Scholar
|
|
35
|
Ceteci F, Ceteci S, Karreman C, Kramer BW,
Asan E, Götz R and Rapp UR: Disruption of tumor cell adhesion
promotes angiogenic switch and progression to micrometastasis in
RAF-driven murine lung cancer. Cancer Cell. 12:145–159. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang Y, Li L, Zhu J, Kuang H, Dong S,
Wang H, Zhang X and Zhou Y: In vitro observations of self-assembled
ECM-mimetic bioceramic nanoreservoir delivering rFN/CDH to modulate
osteogenesis. Biomaterials. 33:7468–7477. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jia D, Jing Y, Zhang Z, Liu L, Ding J,
Zhao F, Ge C, Wang Q, Chen T, Yao M, et al: Amplification of
MPZL1/PZR promotes tumor cell migration through Src-mediated
phosphorylation of cortactin in hepatocellular carcinoma. Cell Res.
24:204–217. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Moon H, Ju H, Chung SI, Cho KJ, Eun JW,
Nam SW, Han K, Calvisi DF and Ro SW: Transforming growth factor-β
promotes liver tumorigenesis in mice via up-regulation of snail.
Gastroenterology. 153:1378–1391. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Janku F, Yap TA and Meric-Bernstam F:
Targeting the PI3K pathway in cancer: Are we making headway? Nat
Rev Clin Oncol. 15:273–291. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ordóñez-Morán P, Dafflon C, Imajo M,
Nishida E and Huelsken J: HOXA5 counteracts stem cell traits by
inhibiting Wnt signaling in colorectal cancer. Cancer Cell.
28:815–829. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Semenza GL: Targeting HIF-1 for cancer
therapy. Nat Rev Cancer. 3:721–732. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jendrossek V: Targeting apoptosis pathways
by Celecoxib in cancer. Cancer Lett. 332:313–324. 2013. View Article : Google Scholar : PubMed/NCBI
|