Introduction
Colorectal cancer (CRC) is the third most common
type of cancer in both men and women, and it is the fourth most
common cause of cancer-associated mortality in the United States
(1). In 2018, Siegel (2) reported that clinical outcomes of
American patients with CRC vary depending on the stage of cancer at
diagnosis, with a 5-year survival rate of ~90% for localized
disease, 71% for regional disease and only 14% for distantly
metastatic CRC (mCRC). Therefore, detecting CRC at an early stage
is crucial to reduce the cancer-specific mortality rate. Currently,
diagnostic imaging examinations, including CT and MRI, have the
ability to depict the size and location of CRC, but have limited
potential in detecting small primary tumours and metastatic lesions
(3). Colonoscopy and biopsy are
considered the gold standard for confirming the diagnosis of CRC.
However, biopsy specimens may fail to provide a definitive
diagnosis if the viable tumour tissue is difficult to obtain or if
the tissue samples are extensively ulcerated or necrotic (4). Additionally, several serum tumour
markers, including carcinoembryonic antigen (CEA), cancer antigen
19-9 (CA19-9) and cancer antigen 72-4 (CA72-4), are widely used for
the screening, diagnosis and postoperative surveillance of
gastrointestinal cancer, but have insufficient sensitivity and
specificity (5). In addition,
patients with CRC diagnosed in the early stages often receive
surgery to remove the original tumour and the nearby lymph nodes,
yet 20–30% of these patients suffer from recurrence or metastasis
within 5 years of radical resection, indicating the presence of
minimal residual disease (6)
Consequently, developing a less invasive and reliable method for
the early diagnosis and dynamic management of CRC is urgently
required.
Circulating tumour cells (CTCs) are tumour cells
that are shed from the primary tumour and metastatic foci, and
enter the peripheral bloodstream (7); they can be classified into signal CTCs
and CTC clusters (8). Unlike the
conventional theory that the metastatic dissemination of cancer
cells represents the final stage of a deteriorating process, CTCs
often disseminate at the beginning during the process of
tumorigenesis, with some of them invading distant organs and
eventually developing into overt metastatic lesions (9). Therefore, the detection of CTCs in the
circulation may represent a feasible way to improve the early
diagnosis and treatment of patients with CRC prior to metastasis.
Clinical studies have suggested that CTCs are significantly
associated with poor progression-free survival (PFS) and overall
survival (OS) time in patients with CRC. For example, Bork et
al (10) revealed that >1 CTC
per 7.5 ml blood in the blood was significantly associated with
worse OS time (38.4 months vs. 49.8 months; P<0.001) in
non-metastatic patients with CRC (UICC I–III), as well as in the
complete cohort (33.6 months vs. 48.4 months; P<0.001), compared
with non-detected group. Furthermore, Cohen et al (11) detected CTCs from 7.5 ml blood of 430
patients with metastatic CRC. It suggested that patients with >3
CTCs had shorter PFS time (4.4 months vs. 7.8 months, P=0.004) and
OS time (9.4 months vs. 20.6 months, P<0.0001) compared with
those whose CTCs was <3.
Since there may only be 1 CTC in 1×107
leukocytes per ml of blood, it is challenging to isolate CTCs from
peripheral blood. The principles of CTC isolation involve CTC
enrichment followed by detection. The former is achieved by means
of physical properties of the cells, such as size, density or
specific biological features, whereas the latter is commonly
achieved by immunostaining and microscopy, or by PCR-based methods
(12). The most frequently used CTC
detection technology reported in these studies is the
CellSearch® system (Janssen Diagnostics). This system
enriches CTCs using ferromagnetic beads coated with antibodies that
target epithelial cell adhesion molecule (EpCAM), and defines CTCs
according to their morphological characteristics, positive
expression of cytokeratin and absence of CD45 (also known as
leukocyte common antigen). However, certain CTCs may lose
epithelial cell markers during the process of
epithelial-to-mesenchymal transition, resulting in a reduced
positive rate and accuracy of the CellSearch® system
(13). The advantage of the Cyttel
method (14) is that the enrichment
of CTCs does not rely on the expression of EpCAM, and the enriched
cells can be used for subsequent experiments, including cell
culture and other tests. The Cyttel method involves a leukocyte
depletion mechanism. After collecting a peripheral blood sample,
erythrocytes can be removed by hypotonic haemolysis. Since all
leucocytes express CD45, these can be removed using anti-CD45
antibody-conjugated magnetic beads.
Abnormal chromosome numbers (aneuploidy) are
invariably found in the pleomorphic cells of malignant tumours and
have been recognized as a common feature of cancer. This type of
somatic copy number alteration has been proposed to drive
tumourigenesis (15). Aneuploidy of
chromosomes 7 and 8 is commonly observed in patients with CRC, with
a high frequency of numerical abnormalities of the entire
chromosome 7, as well as loss, gain or amplification of specific
regions of chromosome 8 in primary CRCs with associated metastases
(16). Detecting aneuploidy in
peripheral blood cells may represent a novel approach for CTC
detection, and assessing aneuploidy of chromosomes 7 and 8 at
diagnosis may be of great clinical significance in patients with
CRC. Therefore, the Cyttel method uses immunofluorescence and
fluorescence in situ hybridization (imFISH) on the remaining
cells, using DNA probes for chromosome 7 (CEP7), chromosome 8
(CEP8) and human CD45. Only cells that are CD45-negative and that
emit signals (>2) of CEP7 or CEP8 are recognized as CTCs. The
Cyttel method may allow the preservation of the rare karyocytes in
the peripheral blood. By combining the detection of multiple
molecular markers and abnormal chromosome alterations in cancer
cells, the detection rate and accuracy of diagnostic tests may be
improved.
In the present study, a total of 89 blood samples
from 59 patients diagnosed with CRC and 30 healthy individuals were
collected for single CTC identification using the Cyttel method.
Subsequently, the diagnostic sensitivities of CTCs and serum tumour
markers (CEA, CA19-9 and CA72-4) were compared to assess the
efficacy of CTCs in detecting CRC. Furthermore, the associations
between total and aneuploid CTCs and the clinicopathological
characteristics of patients with CRC were explored.
Materials and methods
Subjects and blood sample
collection
A total of 59 patients with newly diagnosed CRC were
enrolled in the present study at Beijing Shijitan Hospital
(Beijing, China) between July 2016 and August 2017. These patients
had no history of any other malignancies during the previous 5
years. Blood samples were collected at the diagnosis of CRC.
Patients received standard surgical resection of tumours and other
adjuvant therapy according to the National Comprehensive Cancer
Network (NCCN) guidelines for CRC (17). Patients with stage IV CRC also
received surgery due to bowel obstruction or intestinal
haemorrhage. The 59 patients with CRC were followed up until
December 2018 (primary endpoint). The medical records of these
patients were carefully reviewed to obtain clinicopathological data
and follow-up information. A total of 30 healthy donors, including
17 males and 13 females with an average age of 62.7 years (range,
44–84 years), were included as controls. None of the controls had
tumours, a family history of cancer or other systemic diseases.
Written informed consent was obtained from all subjects. The
present study was approved by the Ethics Review Committee of
Beijing Shijitan Hospital.
Peripheral blood (3.2 ml) was collected into acid
citrate dextrose (ACD) anticoagulant tubes (Becton, Dickinson and
Company) and stored at 4°C. The blood sample had to be processed
for CTC detection within 24 h. To avoid bias, CTC detection and
clinicopathological data collection were blindly and independently
performed by different researchers.
CTC detection using the Cyttel
method
The collected blood (4 ml, including 0.8 ml of ACD)
was added with PBS to 45 ml in a centrifuge tube and then
centrifuged at 600 × g for 5 min at 4°C to separate cells from
plasma. The supernatant was discarded, and the remaining cells were
resuspended in 45 ml erythrocyte lysis buffer (Beijing Solarbio
Science & Technology Co., Ltd.). The centrifuge tube was placed
in a vertical mixer to ensure the red blood cells would be fully
lysed by hypotonic haemolysis, at room temperature. The
aforementioned centrifugation process was repeated, and the
supernatant was discarded. Residual cell particles were resuspended
in 300 µl PBS and incubated with 20 µl anti-CD45
antibody-conjugated magnetic beads (Catalog no. 11153D, Thermo
Fisher Scientific, Inc.) for 30 min at room temperature, with
gentle tilting and rotation. The suspension was subsequently added
to 3 ml isolation buffer (Thermo Fisher Scientific, Inc.), prior to
centrifugation at 300 × g for 5 min at 4°C, and the supernatant was
subsequently discarded. The remaining suspension was added with 100
µl PBS and placed in a magnetic stand (Promega Corporation) for 2
min to separate the magnetic beads. Following centrifugation at
2,070 × g for 3 min at 4°C, the supernatant was discarded. The 100
µl suspension was mixed with 100 µl fixative solution (Thermo
Fisher Scientific, Inc.). The solution containing CTCs was
transferred onto superfrost plus slides (Thermo Fisher Scientific,
Inc.) and dried overnight at 33°C, in the oven. The prepared slides
were immersed in saline-sodium citrate (SSC) buffer (Beijing
Solarbio Science & Technology Co., Ltd.). at 37°C for 15 min,
and then dehydrated by increasing concentrations of ethanol (75, 85
and 100%) for 3 min each. A total of 10 µl hybridization solution
containing centromere DNA probes of chromosome 7 (green) and 8
(orange) (Abbott Pharmaceutical Co. Ltd.) was added to the slides,
which were mounted and incubated in a StatSpin ThermoBrite
hybridization oven (Abbott Pharmaceutical Co. Ltd.) at 37°C for 90
min. Following hybridization, the slides were put in the working
fluid of formamide at 43°C for 15 min to wash the probes, and also
washed with 2× SSC twice. The Alexa Fluor 594-conjugated anti-human
CD45 antibody (1:100; cat. no. FAB1430T; R&D Systems, Inc.) was
added to the slides, incubated at 33°C for 1 h in the oven and
subsequently removed. The nucleus was stained with 10 µl nuclear
dye DAPI (Vector Laboratories, Inc.; Maravai LifeSciences) at room
temperature for 5 min. The sealing slides were automatically
scanned and analysed using the Cyttel PathfinderTM system (Cyttel
Biosciences Inc.) in the high-power field (10×40).
Measurement of serum tumour
markers
Peripheral blood (3 ml) was collected into tubes
without anticoagulant and centrifuged at 1,500 × g and 4°C for 10
min. The expression levels of CEA, CA19-9 and CA72-4 in serum
samples were determined using an automatic immunoassay analyser
(Cobas e601; Roche Diagnostics). The normal reference values for
the three serum tumour markers were 0–5 ng/ml for CEA, 0–37.0 U/ml
for CA19-9 and 0–6.9 U/ml for CA72-4. Exceeding the upper limit of
the normal threshold was considered positive. For the combined
detection of two or more tumour markers, an elevated result of any
of the tumour markers was considered positive. The diagnostic
sensitivity was calculated by dividing the number of positive cases
by the total number of CRC cases.
Statistical analysis
Statistical analysis was performed using the SPSS
statistical software (v.19.0; IBM Corp.). Data are presented as the
mean ± standard deviation. A receiver operating characteristic
(ROC) curve was plotted to determine the sensitivity and
specificity of CTC detection for diagnosis. Differences between
groups were determined using two-tailed unpaired Student's t-test.
The χ2 test or Fisher's exact test was performed to
explore the associations between total and aneuploid CTCs and
patient clinicopathological characteristics. Kaplan-Meier analysis
and the log-rank test were employed to evaluate the prognostic
significance of CTCs in patient survival. P<0.05 was considered
to indicate a statistically significant difference.
Results
Detection of CTCs in patients with CRC
and in healthy individuals
Cells enriched from the peripheral blood of patients
with CRC and from healthy individuals were examined for the
presence of cell nuclei (DAPI-stained) and chromosome ploidy (CEP7-
and/or CEP8-stained). In addition, immunofluorescence staining of
CD45 was applied to exclude leukocytes. Based on the staining
results of DAPI, CD45, CEP7 and CEP8, cells were divided into five
groups: Group A, CD45−, DAPI+ and
CEP7>2/CEP8=2 (the number of hybridization signals for CEP7>2
or CEP8=2); Group B, CD45−, DAPI+ and
CEP7=2/CEP8>2; Group C, CD45−, DAPI+ and
CEP7>2/CEP8>2; Group D, CD45−, DAPI+
and CEP7=2/CEP8=2; and Group E, CD45+, DAPI+
and CEP7≥2/CEP8≥2 (Fig. 1). Cells in
groups A-C characterized as nucleated cells with aneuploidy of
chromosomes 7 and/or 8 but without CD45 expression were defined as
CTCs, whereas cells in groups D and E were defined as indeterminate
cells (normal leukocytes).
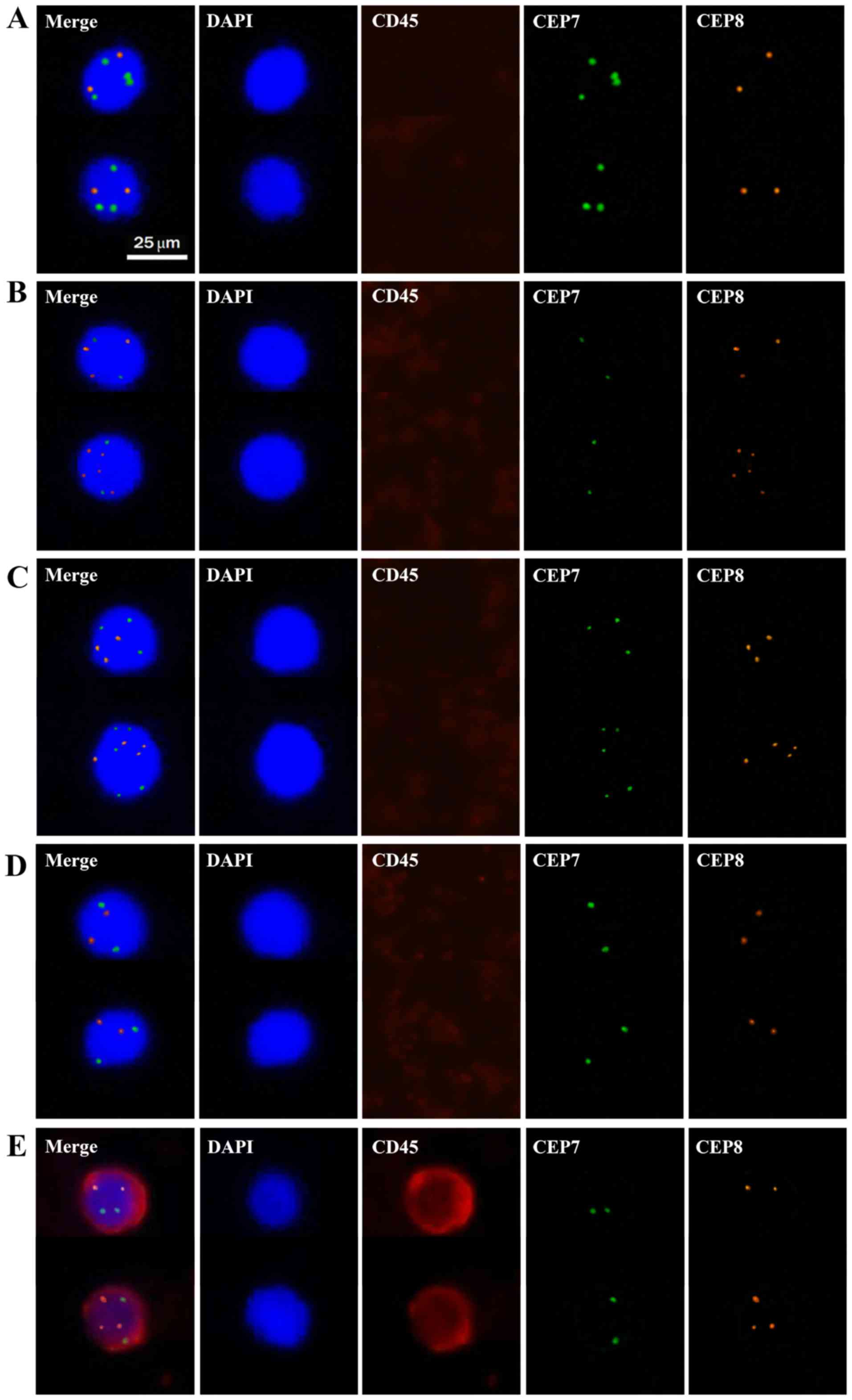 | Figure 1.Representative images of cells
detected in the peripheral blood of patients with colorectal
cancer. Cell nuclei stained with DAPI appear blue. Cytokines
stained with CD45 antibody appear red. Chromosomes stained with
CEP7 appear green. Chromosomes stained with CEP8 appear orange. (A)
CD45-, DAPI+ and CEP7>2/CEP8=2; (B) CD45-, DAPI+ and
CEP7=2/CEP8>2; (C) CD45-, DAPI+ and CEP7>2/CEP8>2; (D)
CD45-, DAPI+ and CEP7=2/CEP8=2; (E) CD45+, DAPI+ and CEP7≥2/CEP8≥2.
Cells in groups A-C were defined as CTCs, whereas cells in groups D
and E were defined as normal leukocytes. Magnification, ×400. CTC,
circulating tumour cell; CEP7, chromosome 7; CEP8, chromosome
8. |
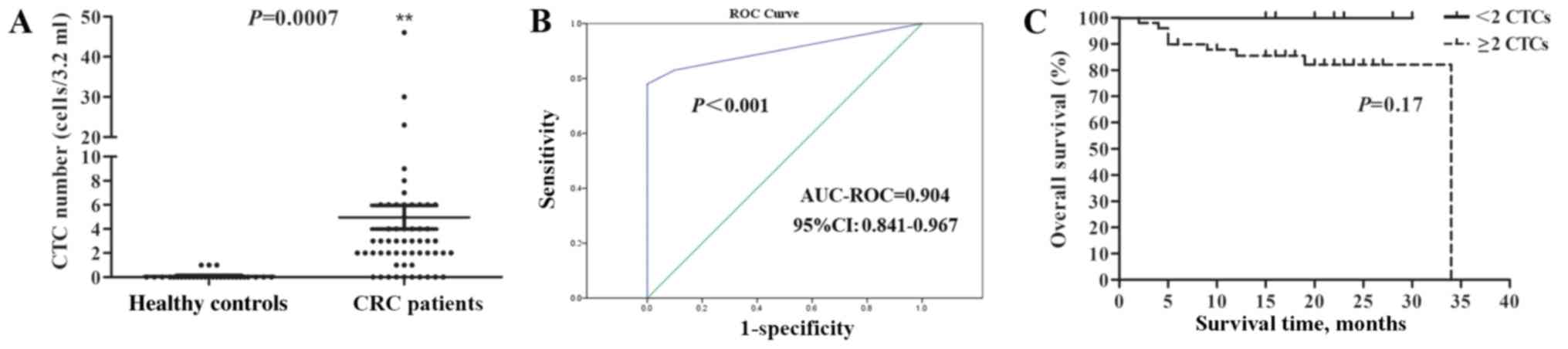
Based on the aforementioned CTC evaluation criteria,
CTCs were identified in the peripheral blood of 3/30 (10.00%)
healthy individuals, and their CTC count was 1. However, CTCs were
identified in 49/59 (83.05%) patients with CRC, with a range of
2–46 cells/3.2 ml, which was significantly higher than in the
control group (Fig. 2A). The ROC
curve was then plotted to characterize the discriminating power of
CTCs for the diagnosis of CRC. According to Youden's index, a
cut-off value of 2 cells/3.2 ml yielded a sensitivity of 83.05% and
a specificity of 100% (area under the curve, 0.904; 95% CI,
0.841–0.967; Fig. 2B). Although
there was no significant association observed between CTC increase
and overall survival according to the Kaplan-Meier analysis
(Fig. 2C), there was a trend showing
that patients with CRC with <2 CTCs may have an improved
prognosis compared with those with ≥2 CTCs, since all 10 patients
with <2 CTCs were alive at the primary endpoint. The association
between CTC numbers and TNM staging, and between CTCs with
multiploidy of chromosomes 7 or 8 and prognosis in patients with
CRC were also investigated, but there were no significant
differences identified (data not shown).
Diagnostic sensitivity of CTCs and
serum biomarkers in the diagnosis of CRC
Several serum tumour markers, such as CEA, CA19-9
and CA72-4, have been suggested as candidate biomarkers for
diagnosis, prognosis, monitoring recurrence and guiding treatment
in patients with CRC (4). Therefore,
the present study aimed to compare the sensitivities of CTCs and
tumour markers to diagnose CRC. The diagnostic sensitivity was used
to represent the rate of correctly classified positive cases. Among
the three individual serum tumour markers, CEA had the highest
diagnostic sensitivity (50.85%) in the 59 patients with CRC,
followed by CA72-4 (28.81%) and CA19-9 (23.73%; Table I). Combinations of any two or all
three of the serum tumour markers produced sensitivities that were
markedly improved (range, 40.68–64.41%) compared with those of the
single tumour markers. In addition, the diagnostic sensitivity of
CTCs was 83.05%, which was notably higher than that of each
traditional serum tumour marker and of the combinations. The
sensitivity reached 93.22% when combining CTCs and all three
biomarkers. Interestingly, the sensitivity of CTCs + CEA (91.53%)
was only slightly lower than the combined sensitivity of all four
markers, indicating that CTCs + CEA may be an effective combination
of serum tumour markers for the diagnosis and prognosis of CRC.
 | Table I.Diagnostic sensitivity of CTCs and
serum tumour markers in patients with colorectal cancer. |
Table I.
Diagnostic sensitivity of CTCs and
serum tumour markers in patients with colorectal cancer.
| Serum tumour
markers | Diagnostic
sensitivity (positive/total), % |
|---|
| CEA | 50.85 (30/59) |
| CA19-9 | 23.73 (14/59) |
| CA72-4 | 28.81 (17/59) |
| CEA + CA19-9 | 59.32 (35/59) |
| CEA + CA72-4 | 61.02 (36/59) |
| CA19-9 +
CA72-4 | 40.68 (24/59) |
| CEA + CA19-9 +
CA72-4 | 64.41 (38/59) |
| CTCs | 83.05 (49/59) |
| CTCs + CEA | 91.53 (54/59) |
| CTCs + CA19-9 | 84.75 (50/59) |
| CTCs + CA72-4 | 88.14 (52/59) |
| CTCs + CEA + CA19-9
+ CA72-4 | 93.22 (55/59) |
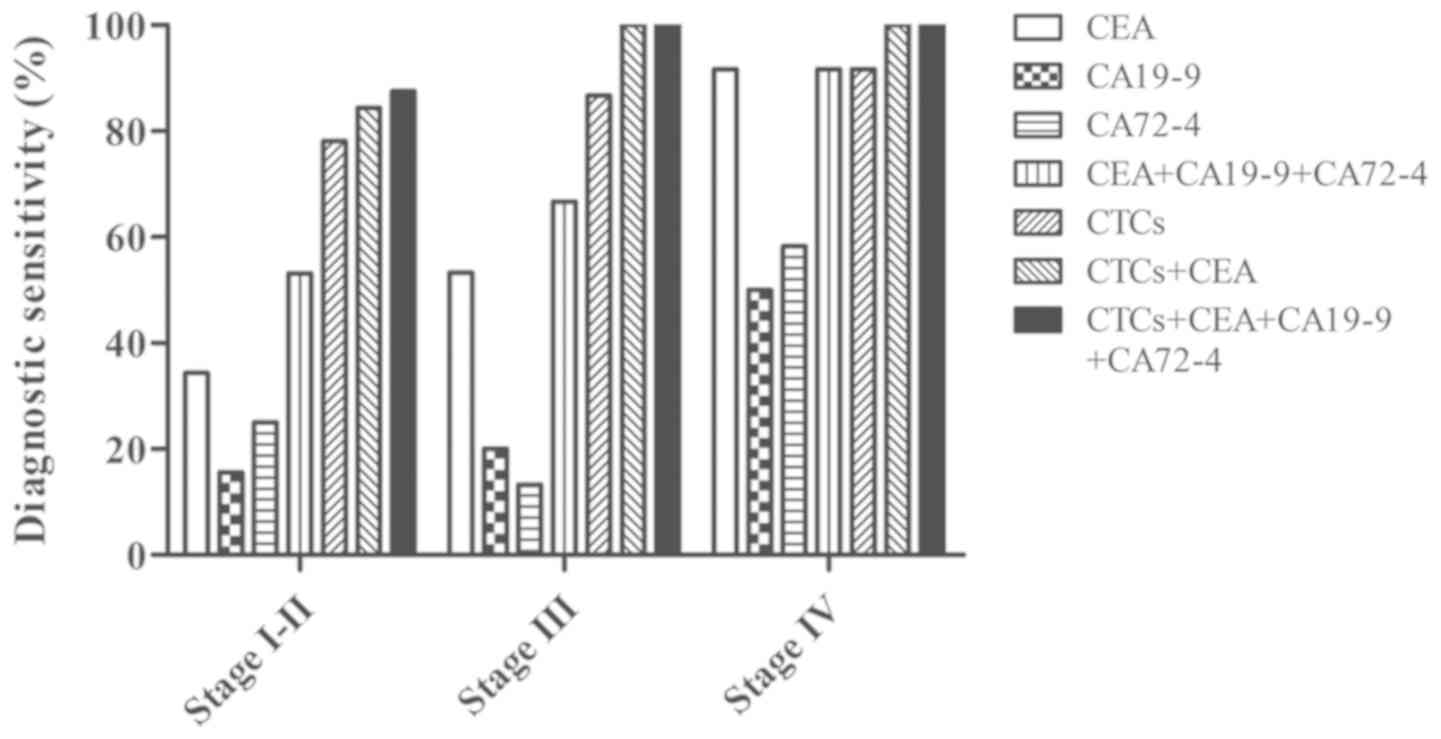
Furthermore, an association between tumour staging
and the detection rate of CTCs and tumour markers was observed. As
shown in Fig. 3, positive CEA
expression (≥5 ng/ml) were detected in 34.38% of stage I–II
patients with CRC, 53.33% of stage III patients and 91.67% of stage
IV patients, and these rates were higher than those for CA19-9 and
CA72-4 at all pathological stages. The combined diagnostic
sensitivity of CEA + CA19-9 + CA72-4 was further increased in
patients with stage I–II (53.13%), stage III (66.67%) and stage IV
(91.67%, same as CEA alone) CRC. Additionally, increased CTC levels
(≥2 cells/3.2 ml) were observed in patients with CRC compared with
normal individuals, with increased CTC number in 78.13% of patients
with stage I–II disease, 86.67% of patients with stage III disease
and 91.67% of patients with stage IV disease. This corresponded to
the elevated diagnostic sensitivity of different stages of cancer.
Furthermore, when combining CTCs and CEA, the detection rate in
stage I–II patients reached 84.38%. The increase in the detection
sensitivity of CTCs suggested that CTCs may be used as an efficient
biomarker in the detection of all stages of CRC, particularly early
stage tumours.
Association of total or aneuploid CTCs
with clinicopathological characteristics of patients with CRC
As shown in Table
II, there was a significant difference in CTC status between
the well-, moderately and poorly differentiated CRC groups. CTCs
were more likely to be detected in poorly differentiated CRC
tumours (12/12) than in well- or moderately differentiated tumours.
Furthermore, the results suggested that there was a significant
association between CTCs and nerve invasion (pathological
diagnosis). The detection rate of CTCs could be remarkably
increased with cancer progression, such as invasion and
metastasis.
 | Table II.Association of total or aneuploid
CTCs with the clinicopathological characteristics of patients
(n=59) with colorectal cancer. |
Table II.
Association of total or aneuploid
CTCs with the clinicopathological characteristics of patients
(n=59) with colorectal cancer.
|
|
| Total CTCs, n | CTCs with
multiploidy of chromosome 7, n |
|---|
|
|
|
|
|
|---|
|
Characteristics | Number of
patients | Negative | Positive | P-value | Negative | Positive | P-value |
|---|
| Age, years |
|
|
| 0.396 |
|
| 0.157 |
|
<60 | 23 | 3 | 20 |
| 7 | 16 |
|
|
≥60 | 36 | 7 | 29 |
| 17 | 19 |
|
| Sex |
|
|
| 0.307 |
|
| 0.075 |
|
Male | 34 | 7 | 27 |
| 17 | 17 |
|
|
Female | 25 | 3 | 22 |
| 7 | 18 |
|
| Tumour size,
cm |
|
|
| 0.604 |
|
| 0.533 |
|
<5 | 36 | 6 | 30 |
| 15 | 21 |
|
| ≥5 | 23 | 4 | 19 |
| 9 | 14 |
|
| Locationa |
|
|
| 0.262 |
|
| 0.115 |
|
Left-sided | 43 | 6 | 37 |
| 20 | 23 |
|
|
Right-sided | 16 | 4 | 12 |
| 4 | 12 |
|
| Histological
differentiation |
|
|
| 0.026b |
|
| 0.163 |
|
Well | 9 | 4 | 5 |
| 4 | 5 |
|
|
Moderate | 38 | 6 | 32 |
| 18 | 20 |
|
|
Poor | 12 | 0 | 12 |
| 2 | 10 |
|
| Primary tumour |
|
|
| 0.894 |
|
| 0.841 |
|
T1-T2 | 8 | 1 | 7 |
| 4 | 4 |
|
| T3 | 26 | 5 | 21 |
| 10 | 16 |
|
| T4 | 25 | 4 | 21 |
| 10 | 15 |
|
| Lymph node |
|
|
| 0.350 |
|
| 0.111 |
| N0 | 35 | 7 | 28 |
| 17 | 18 |
|
|
N1-N2 | 24 | 3 | 21 |
| 7 | 17 |
|
| Distant
metastasis |
|
|
| 0.343 |
|
| 0.406 |
| M0 | 47 | 9 | 38 |
| 20 | 27 |
|
| M1 | 12 | 1 | 11 |
| 4 | 8 |
|
| TNM staging |
|
|
| 0.229 |
|
| 0.031b |
|
I–II | 32 | 7 | 25 |
| 17 | 15 |
|
|
III–IV | 27 | 3 | 24 |
| 7 | 20 |
|
| Venous
invasion |
|
|
| 0.293 |
|
| 0.311 |
|
Absent | 46 | 9 | 37 |
| 20 | 26 |
|
|
Present | 13 | 1 | 12 |
| 4 | 9 |
|
| Nerve invasion |
|
|
| 0.008b |
|
| 0.306 |
|
Absent | 45 | 4 | 41 |
| 17 | 28 |
|
|
Present | 14 | 6 | 8 |
| 7 | 7 |
|
| CEA, ng/ml |
|
|
| 0.612 |
|
| 0.438 |
|
<5 | 29 | 5 | 24 |
| 11 | 18 |
|
| ≥5 | 30 | 5 | 25 |
| 13 | 17 |
|
| CA19-9, U/ml |
|
|
| 0.248 |
|
| 0.020b |
|
<37 | 45 | 9 | 36 |
| 22 | 23 |
|
|
≥37 | 14 | 1 | 13 |
| 2 | 12 |
|
| CA72-4, U/ml |
|
|
| 0.600 |
|
| 0.177 |
|
<6.9 | 42 | 7 | 35 |
| 15 | 27 |
|
|
≥6.9 | 17 | 3 | 14 |
| 9 | 8 |
|
| Ki-67 index, % |
|
|
| 0.602 |
|
| 0.257 |
|
<70 | 11 | 2 | 9 |
| 3 | 8 |
|
|
≥70 | 48 | 8 | 40 |
| 21 | 27 |
|
| PD-L1
expression |
|
|
| 0.507 |
|
| 0.493 |
|
Negative | 21 | 4 | 17 |
| 8 | 13 |
|
|
Positive | 38 | 6 | 32 |
| 16 | 22 |
|
As shown in Fig. 1,
CTCs detected in the peripheral blood of patients with CRC had
triploidy, tetraploidy or multiploidy (≥5 copies of chromosomes 7
or 8), indicating the existence of heterogeneous polysomic
chromosomes in CTCs. The association between CTCs with multiploidy
of chromosomes 7 or 8 and clinicopathological characteristics was
evaluated. CTCs with aneuploidy of chromosome 8 were not
significantly associated with any of the clinicopathological
characteristics presented in Table
II (data not shown). However, CTCs with multiploidy of
chromosome 7 were significantly associated with TNM stage and serum
CA19-9 levels (Table II). This type
of CTC was more commonly observed in the peripheral blood of
patients with late-stage CRC.
Discussion
CTC detection is a non-invasive approach that has
potential utility for clinical diagnosis, prognosis and evaluation
of therapeutic responses for different solid tumours. Therefore, it
is important to establish a reliable CTC detection strategy.
Various CTC detection methods, including the CellSearch®
system, the AdnaTest, isolation by size of epithelial tumour cells
(ISET®) and nested reverse transcription-quantitative
(RT-q)PCR-based techniques, have been reported in CRC studies
(10,12). The CellSearch® system is
currently the only method for CTC detection approved by the FDA.
However, the CTC-positive rates obtained using the
CellSearch® system are low, ranging between 18.8 and 33%
in patients with mCRC according to different studies (10,18).
Furthermore, CTCs are barely detectable using this system in
patients with non-mCRC (10). Gorges
et al (19) identified CTCs
using the CellSearch® technology and/or the AdnaTest in
parallel, and demonstrated that a combined analysis with both
methods leads to an elevated rate of CTC detection in patients with
mCRC (positive rate, 33% for CellSearch®; 30% for the
AdnaTest; 50% for both combined). The methods for CTC detection
have been recently improved. Chen et al (20) introduced a novel isolation method,
known as the ISET® system, involving automatic isolation
and staining procedures to capture CTCs, and revealed that CTCs
were detected in 38/72 (52.8%) patients with CRC. In another study
based on nested RT-qPCR, epithelial cell transforming 2 (ECT2) was
screened as a candidate marker gene for quantifying CTCs, with a
detection rate of ECT2 of ~60% in patients with different stages of
CRC (21).
Combining negative enrichment and imFISH, a strategy
that is independent of epithelial marker expression and tumour
size, has shown great potential for CTC detection, yielding
diagnostic sensitivities of 84, 76.2 and 69.4% for detecting CTCs
in lung, ovarian and ampullary cancer, respectively (22–24). In
the present study, the Cyttel method (combining probes for CEP7 and
CEP8 with an anti-CD45 antibody) was designed to detect CTCs in
patients with CRC and in healthy controls. This strategy achieved a
diagnostic sensitivity of 83.05% and a specificity of 100%, when
using a cut-off value of 2 CTCs/3.2 ml of peripheral blood. The
detection rate of CTCs with this method was higher than that of the
CellSearch® system, which has been reported to be 15.33%
(10), higher than that of the
ISET® method, which has been reported to be 35%
(20), and higher than that of the
approach using RT-qPCR and ECT2, which has been reported to be 59%
(21). Furthermore, the Cyttel
method requires less peripheral blood (3.2 ml) than the
CellSearch® system (7 ml). The present study suggested
that the Cyttel method may be a sensitive and convenient strategy
that could be helpful in improving the CTC detection rate in
CRC.
In the present study, CTCs were identified in 3/30
(10.00%) healthy donors. In theory, CTCs should not be present in
healthy individuals. However, Miller et al (25) demonstrated that CTCs detected by the
CellSearch® technique are extremely rare in healthy
volunteers (<3.5% for a threshold of ≥1 CTC per 7.5 ml of blood)
and patients with benign disease (<7.5% for a threshold of ≥1
CTC per 7.5 ml of blood), and Ilie et al (26) also successively detected CTCs in
patients with chronic obstructive pulmonary disease 1–4 years
before they were diagnosed with lung cancer. To explain the above
question, it is hypothesised that the epithelial cells isolated
using different CTC detection methods may not all be the real CTCs,
but instead include few cells with metabolic abnormality. These
cells may come from donors with unhealthy lifestyles and eating
habits, such as long-term smoking or high fat diet. Thus, it is
possible to identify CTC-like cells in healthy people. However, the
number of these abnormal cells is very low. Only one CTC was
identified in the three healthy donors in the present study, and a
cut-off value (2 CTCs/3.2 ml blood) was determined for the accurate
distinction of tumour patients from healthy individuals. In
conclusion, CTCs may be a useful tool for the early diagnosis and
risk assessment of patients with cancer and healthy
individuals.
At present, traditional serum tumour markers, such
as CEA, CA19-9 and CA72-4, are widely used in the clinical
management of CRC. According to the American Society of Clinical
Oncology, CEA detection is recommended to monitor recurrence
following curative surgery in patients with stage II and III CRC
(27). The preoperative serum CA19-9
levels have been reported to be a prognostic indicator in patients
with CRC (28). Additionally, a
recent study demonstrated that CA72-4 can supplement CEA in
evaluating CRC recurrence and metastasis (29). Early detection of tumour markers
before distal metastasis occurs may have a significant impact on
medical decision making and clinical outcomes of patients,
particularly in the early stages. Furthermore, preoperative or
postoperative serial CTC measurements may provide a method to
predict or monitor early relapse in patients with non-mCRC
(10). Based on the aforementioned
results, the detection sensitivities of CTCs and three serum tumour
markers were analysed simultaneously in patients with different
stages of CRC to develop a non-invasive, effective and reliable
biomarker detection strategy for the early diagnosis of CRC.
In the present study, the diagnostic sensitivities
of single and combined serum tumour markers ranged between 40.68
and 64.41%, respectively, which was consistent with the findings of
Gao et al (30). However, the
sensitivity of the CTC detection method reached 83.05%, which was
higher than that of the traditional tumour markers, even when
combining CEA, CA19-9 and CA72-4. A similar result was obtained in
a previous study using the same CTC detection method in patients
with lung cancer (22). For patients
with stage I–II CRC, CEA had the highest sensitivity (34.38%) among
the three individual serum tumour markers, while the detection rate
of the CTC method was 78.13%. It was clear that the CTC method was
more sensitive for detecting early stage CRC compared with the use
of other serum biomarkers. Furthermore, combining the CTC method
with CEA achieved a sensitivity of 91.53%, which was only slightly
lower than that for the combination of the four biomarkers
(93.22%). This finding suggests that the combination of CTCs and
CEA may be an effective and convenient routine detection strategy
for diagnosing CRC, and for predicting and monitoring early
recurrence and metastasis in patients with CRC.
The current study revealed novel insights into the
potential diagnostic application of a CTC detection method based on
aneuploidy detection. Davoli et al (31) revealed that an abnormally high number
of chromosomes was commonly associated with high-grade tumours and
poor prognosis. Papazachariou et al (32) have identified that the number of CTCs
with chromosome 7 aneuploidy is significantly involved with late
stage tumours and poor prognosis. In the present study, the
association between total and aneuploid CTCs and patient
clinicopathological characteristics was carefully analysed. Total
CTCs were found to be significantly associated with tumour
differentiation and nerve invasion. This finding indicated that the
detection rate of CTCs increased along with tumour progression,
which was consistent with the aforementioned results regarding the
detection rate of CTCs in different stages of CRC. Additionally,
there was a significant difference in CTCs with multiploidy of
chromosome 7 between different TNM stages. These CTCs were more
likely to be detected in patients with stage III–IV CRC than in
patients with stage I–II CRC. The present study suggested that
detection of CTCs based on aneuploidy detection could be more
specific for predicting highly malignant and invasive tumours in
CRC management than detection using current serum markers. A
careful classification of aneuploid CTCs may provide novel insights
for improved prediction of CRC.
In conclusion, the current data fully support the
potential clinical value of total and aneuploid CTCs as identified
with the Cyttel method in CRC diagnosis. The combination of CTCs
and CEA has the potential to become an effective and convenient
routine screening strategy for predicting CRC at different stages.
However, the present study was performed at a single medical centre
with a small sample size, which may limit the power of the
statistical analysis. The dynamic change in the number of CTCs at
different stages of treatment and the association between CTCs and
patient survival should be studied in future research.
Acknowledgements
Not applicable.
Funding
This research was supported by grants from the
National Natural Science Foundation of China (grant nos. 81372585,
81772557 and 81902360), the Beijing Health System High Level
Training Plan of Health Technical Personnel (grant no. 2014-3-048),
the Beijing Municipal Administration of Hospital's Clinical
Medicine Development of Special Funding Support (grant no.
XMLX201708), the Scientific Research and Development Program of
Beijing Railway Corporation of China (grant no. J2017Z605), the
Science Nurturing Foundation of Capital Medical University (grant
no. PYZ2017151) and the Youth Fund of Beijing Shijitan Hospital
(grant nos. 2017-q02 and q13).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HG and LD designed the study. HY and LM performed
the experiments and wrote the initial draft of the manuscript. WL
and YZ contributed to the analysis and interpretation of data. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics Review
Committee of Beijing Shijitan Hospital [Beijing, China; approval
no. 2016KY(55)]. Research has been conducted in accordance with the
Declaration of Helsinki. Written informed consent was obtained from
all subjects.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kekelidze M, D'Errico L, Pansini M,
Tyndall A and Hohmann J: Colorectal cancer: Current imaging methods
and future perspectives for the diagnosis, staging and therapeutic
response evaluation. World J Gastroenterol. 19:8502–8514. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ponz de Leon M and Di Gregorio C:
Pathology of colorectal cance. Dig Liver Dis. 33:372–388. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shah R, Jones E, Vidart V, Kuppen PJ,
Conti JA and Francis NK: Biomarkers for early detection of
colorectal cancer and polyps: Systematic review. Cancer Epidemiol
Biomarkers Prev. 23:1712–1728. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ryuk JP, Choi GS, Park JS, Kim HJ, Park
SY, Yoon GS, Jun SH and Kwon YC: Predictive factors and the
prognosis of recurrence of colorectal cancer within 2 years after
curative resection. Ann Surg Treat Res. 86:143–151. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Masuda T, Hayashi N, Iguchi T, Ito S,
Eguchi H and Mimori K: Clinical and biological significance of
circulating tumor cells in cancer. Mol Oncol. 10:408–417. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gkountela S, Castro-Giner F, Szczerba BM,
Vetter M, Landin J, Scherrer R, Krol I, Scheidmann MC, Beisel C,
Stirnimann CU, et al: Circulating tumor cell clustering shapes DNA
methylation to enable metastasis seeding. Cell. 176:98–112.e14.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Massague J and Obenauf AC: Metastatic
colonization by circulating tumour cells. Nature. 529:298–306.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bork U, Rahbari NN, Schölch S, Reissfelder
C, Kahlert C, Büchler MW, Weitz J and Koch M: Circulating tumour
cells and outcome in non-metastatic colorectal cancer: A
prospective study. Br J Cancer. 112:1306–1313. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cohen SJ, Punt CJA, Iannotti N, Saidman
BH, Sabbath KD, Gabrail NY, Picus J, Morse MA, Mitchell E, Miller
MC, et al: Prognostic significance of circulating tumor cells in
patients with metastatic colorectal cancer. Ann Oncol.
20:1223–1229. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Krebs MG, Metcalf RL, Carter L, Brady G,
Blackhall FH and Dive C: Molecular analysis of circulating tumour
cells-biology and biomarkers. Nat Rev Clin Oncol. 11:129–144. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yu M, Bardia A, Wittner BS, Stott SL, Smas
ME, Ting DT, Isakoff SJ, Ciciliano JC, Wells MN, Shah AM, et al:
Circulating breast tumor cells exhibit dynamic changes in
epithelial and mesenchymal composition. Science. 339:580–584. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang L, Zhao M and He S: Study on the
clinical significance of detection of Cyttel circulating tumor
cells in peripheral blood of patients with gastric cancer. J Clin
Exp Med. 7:772–774. 2018.
|
|
15
|
Rajagopalan H: Aneuploidy and cancer.
Nature. 432:338–341. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sayagués JM, Abad MM, Melchor HB,
Gutiérrez ML, González-González M, Jensen E, Bengoechea O, Fonseca
E, Orfao A and Muñoz-Bellvis L: Intratumoural cytogenetic
heterogeneity of sporadic colorectal carcinomas suggests several
pathways to liver metastasis. J Pathol. 221:308–319. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Provenzale D, Gupta S, Ahnen DJ, Markowitz
AJ, Chung DC, Mayer RJ, Regenbogen SE, Blanco AM, Bray T, Cooper G,
et al: NCCN guidelines insights: Colorectal cancer screening,
Version 1.2018. J Natl Compr Canc Netw. 16:939–949. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang Y, Li J, Jin L, Wang D, Zhang J, Wang
J, Zhao X, Wu G, Yao H and Zhang Z: Independent correlation between
Ki67 index and circulating tumor cells in the diagnosis of
colorectal cancer. Anticancer Res. 37:4693–4700. 2017.PubMed/NCBI
|
|
19
|
Gorges TM, Stein A, Quidde J, Hauch S,
Röck K, Riethdorf S, Joosse SA and Pantel K: Improved detection of
circulating tumor cells in metastatic colorectal cancer by the
combination of the CellSearch® system and the
AdnaTest®. PLoS One. 11:e1551262016. View Article : Google Scholar
|
|
20
|
Chen F, Wang S, Fang Y, Zheng L, Zhi X,
Cheng B, Chen Y, Zhang C, Shi D, Song H, et al: Feasibility of a
novel one-stop ISET device to capture CTCs and its clinical
application. Oncotarget. 8:3029–3041. 2017.PubMed/NCBI
|
|
21
|
Chen CJ, Sung WW, Chen HC, Chern JY, Hsu
TH, Min Lin Y, Lin SH, Peck K and Yeh TK: Early assessment of
colorectal cancer by quantifying circulating tumor cells in
peripheral blood: ECT2 in diagnosis of colorectal cancer. Int J Mol
Sci. 18:7432017. View Article : Google Scholar
|
|
22
|
Chen YY and Xu GB: Effect of circulating
tumor cells combined with negative enrichment and CD45-FISH
identification in diagnosis, therapy monitoring and prognosis of
primary lung cancer. Med Oncol. 31:2402014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ning N, Zhan T, Zhang Y, Chen Q, Feng F,
Yang Z, Liu Z, Xu D, Wang F, Guo Y, et al: Improvement of specific
detection of circulating tumor cells using combined CD45 staining
and fluorescence in situ hybridization. Clin Chim Acta. 433:69–75.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sun B, Liu H, Wang S, Xiang J and Liu X:
Prognostic impact of circulating tumor cells in patients with
ampullary cancer. J Cell Physiol. 233:5014–5022. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Miller MC, Doyle GV and Terstappen LW:
Significance of circulating tumor cells detected by the CellSearch
system in patients with metastatic breast, colorectal and prostate
cancer. J Oncol. 2010:6174212010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ilie M, Hofman V, Long-Mira E, et al:
Clinical and pathological characteristics of CTC-positive COPD
patients). PLoS One. 9:e1115972014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Meyerhardt JA, Mangu PB, Flynn PJ, Korde
L, Loprinzi CL, Minsky BD, Petrelli NJ, Ryan K, Schrag DH, Wong SL,
et al: Follow-up care, surveillance protocol, and secondary
prevention measures for survivors of colorectal cancer: American
Society of Clinical Oncology clinical practice guideline
endorsement. J Clin Oncol. 31:4465–4470. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Abe S, Kawai K, Ishihara S, Nozawa H, Hata
K, Kiyomatsu T, Tanaka T and Watanabe T: Prognostic impact of
carcinoembryonic antigen and carbohydrate antigen 19-9 in stage IV
colorectal cancer patients after R0 resection. J Surg Res.
205:384–392. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li Q, Dai W, Li Y, Xu Y, Li X and Cai S:
Nomograms for predicting the prognostic value of serological tumor
biomarkers in colorectal cancer patients after radical resection.
Sci Rep. 7:463452017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gao Y, Wang J, Zhou Y, Sheng S, Qian SY
and Huo X: Evaluation of serum CEA, CA19-9, CA72-4, CA125 and
ferritin as diagnostic markers and factors of clinical parameters
for colorectal cancer. Sci Rep. 8:27322018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Davoli T, Uno H, Wooten EC and Elledge SJ:
Tumor aneuploidy correlates with markers of immune evasion and with
reduced response to immunotherapy. Science. 355(pii): eaaf83992017.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Papazachariou I, Tsiambas E, Koliopoulou
A, Tsounis D, Manaios M, Fotiades PP, Karameris A and Patsouris E:
Chromosomes 7, 16 numerical aberrations are poor prognostic factors
in colorectal adenocarcinoma: A tissue microarray analysis. Basic
App Pathol. 1:125–130. 2008. View Article : Google Scholar
|