Introduction
Sex hormone-binding globulin (SHBG) is a circulating
glycoprotein composed of 373 amino acids and 3 carbohydrate chains
that can bind to dihydrotestosterone, testosterone and estradiol,
especially C18 or C19 and 17-β-hydroxyl groups. SHBG regulates
plasma clearance and the uptake of sex hormones (1). High SHBG expression levels
theoretically decrease the uptake of sex hormones. Notably, only a
small percentage (<2%) of steroids are unbound in plasma, and
the remainder are primarily bound to SHBG and albumin (2). Therefore, SHBG may influence the
carcinogenesis and progression of hormone-dependent types of
cancer, such as prostate, ovary and breast cancer (3–5). There
are conflicting reports regarding the association between serum
levels of SHBG and the risk of prostate cancer development
(3,4). Grasso et al (5) identified that the plasma SHBG
expression levels in patients with prostate cancer were higher
compared with those with benign hyperplasia or healthy volunteers.
Moreover, circulating SHBG expression levels are higher in patients
with lymph node invasion (6) and
poor differentiation (7). In a
previous prospective study of lung cancer development, there were
no significant difference in the mean concentration of sex hormones
or SHBG between patients who had lung cancer and those who did not
have lung cancer (8). However,
another previous study identified that SHBG concentration was also
higher in patients with lung cancer (9).
Intracellular SHBG has been reported in liver,
placenta, endometrial, breast and prostate cancers (7–10).
Steroid-free SHBG can bind to the cell membrane and once bound,
SHBG can bind to steroids with equal affinity as it does in the
serum. This interaction is closely associated with estrogen
sensitivity to each cell (11).
Binding of estradiol to SHBG ultimately results in breast cancer
cell apoptosis and growth suppression. Therefore, SHBG serves a
protective role in the exposure of breast cells to estrogen
(12).
The SHBG expression levels in healthy postmenopausal
females has been reported to be lower compared with premenopausal
females, although the difference was not statistically significant
(13). In patients aged 50–64 years,
a decline of 10% was observed in SHBG expression levels compared
with premenopausal females (14). In
patients aged >65 years, SHBG expression levels returned to the
pre-menopause level (14). SHBG
expression levels were lower in patients with breast cancer
compared with in controls (15). In
pre-menopausal patients with breast cancer, the SHBG binding
capacity is in the normal range; however, it is decreased in
post-menopausal patients with breast cancer (16–18). The
free fraction of estradiol is increased while SHBG exhibits
relative or absolute decrement in post-menopausal patients with
breast cancer (19). It has been
suggested that different critical expression levels of SHBG must be
determined for pre-menopause and post-menopausal females because
the mean SHBG expression levels in these two groups differ
(20). Murayama et al
(20) identified that plasma
expression levels of SHBG in postmenopausal patients with
ER-positive breast cancer are higher compared with patients with
ER-negative breast cancer. On the contrary, there was a
considerable overlap of plasma SHBG expression levels between
patients with estrogen receptor (ER)+ and ER−
endometrial and cervical cancer (13). The major beneficial effect of
tamoxifen is that it can block estrogen at the receptor level and
decrease the level of biologically active estradiol by upregulating
SHBG expression (21). However,
there was no significant association between SHBG expression level
and treatment response in patients with breast cancer (22,23).
Lymph node metastasis and histological status in patients with high
SHBG expression levels are similar to patients with low SHBG
expression levels (24). The
recurrence rate between high- and low-SHBG expression level groups
was not significantly different and although the high SHBG group
had longer disease-free survival times, this difference was not
significant in premenopausal patients with breast cancer (20).
In the present study, SHBG reference range was
determined based on sex and age (by decade) of healthy male and
female volunteers. The serum SHBG expression levels of breast
cancer exhibited a different trend compared to healthy female
volunteers by age decade comparison.
Patients and methods
Collection of blood specimens
Peripheral blood samples were obtained from healthy
volunteers (40 males and 40 females) at Inha University Hospital
(Incheon, Republic of Korea) subsequent to obtaining approval, if
no laboratory (routine blood test, liver function test and tumor
markers) and imaging (plain X-ray and CT scan) abnormalities were
observed during the regular medical check-up. Blood samples from 34
female patients with breast cancer were obtained at 109 different
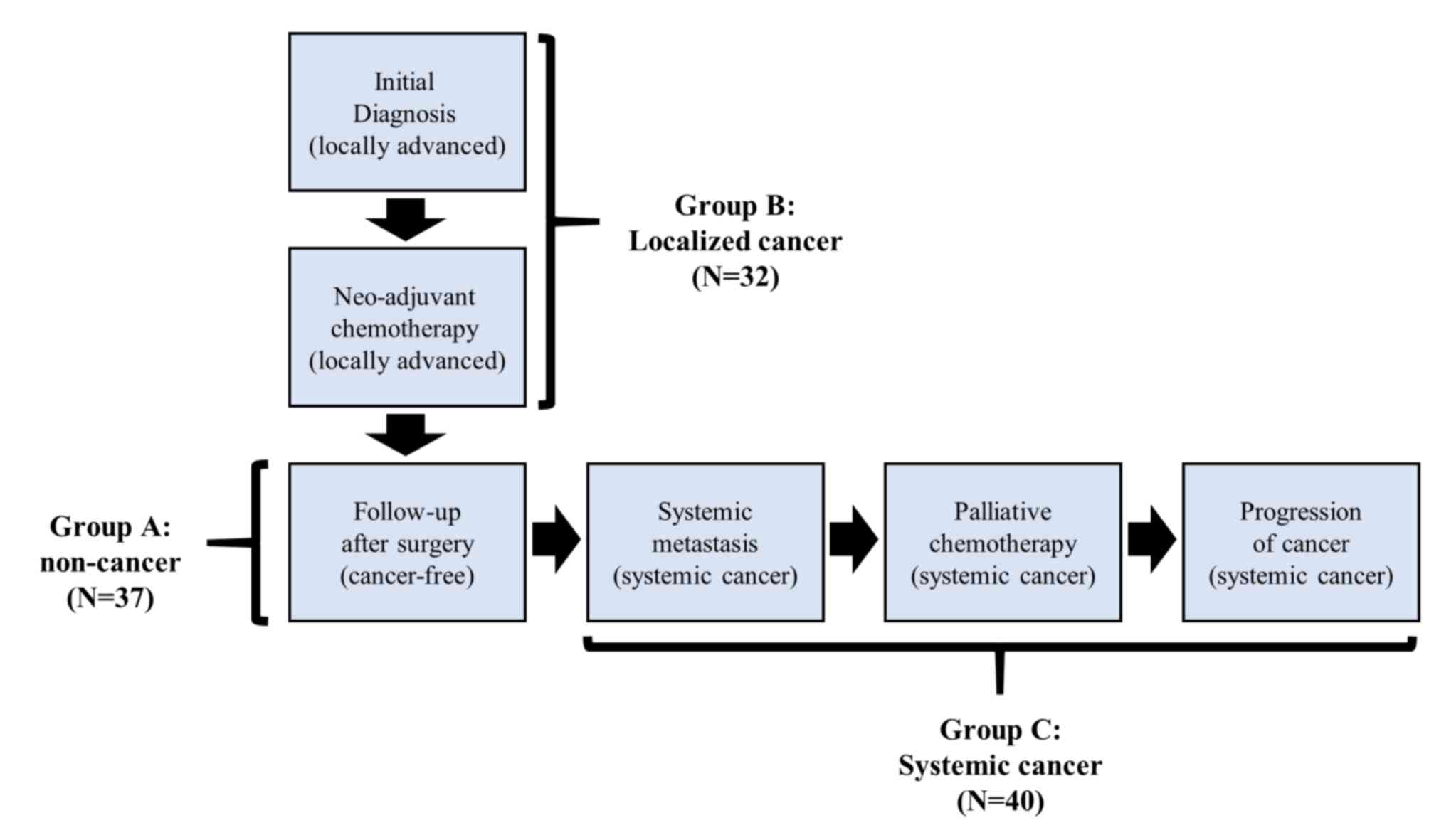
time points and grouped as follow: i) Group A, non-tumor state
after surgery (n=37); ii) Group B, localized tumor at diagnosis and
during pre-operative chemotherapy (n=32); and iii) Group C,
systemic metastasis (n=40) (Fig. 1).
Patients with locally advanced breast cancers with clinical stage
III with normal laboratory findings planned for pre-operative
chemotherapy were enrolled. The median age was 40 years (range,
25–66 years) for the 40 healthy males, 34 years (range, 21–56
years) for the 40 healthy female volunteers and 45 years (range,
32–65 years) for the 34 female patients with breast cancer. Median
follow-up duration was 14 months (range 2–48 months). The blood
samples were stored at −80°C. Heparinized vacuum tubes and needles
(BD Biosciences) were used to avoid platelet damage and venous
occlusion, as in the clinical setting.
Determination of the normal range of
serum SHBG
With 40 healthy male and 40 healthy female volunteer
blood samples, the normal cut-off expression levels of SHBG were
defined as the mean ±2 standard deviations (21–69 nmol/l) (25). Serum SHBG expression levels were
considered positive when SHBG expression levels were out of this
reference range (>69 nmol/l, elevated; <21 nmol/l,
decreased). Patients aged ≥50 years were defined as post-menopause
(26).
ELISA
A sandwich ELISA was performed to measure SHBG,
cancer antigen (CA)15-3 and CA125 expression levels according to
the manufacturer's instructions. Goat polyclonal antibody kit
specific for SHBG (cat. no. M-0700; 1:50; Alpha Diagnostic Intl.,
Inc.) and monoclonal antibody kits specific for CA15-3 (cat. no.
IS-F3329; 1:100; LifeSpan Biosciences, Inc.) and CA125 (cat. no.
CA239T; 1:100; Calbiotech, Inc.) were used to coat 96-well
microplates. Each blood sample was added to the plate and incubated
for 1 h at room temperature. Following washing 3 times with wash
buffer from the kit to remove unbound proteins, enzyme-linked
antibodies in each kit specific for SHBG, CA15-3 and CA125 were
added to wells and incubated for 1 h at room temperature and the
absorbance was measured at 450 nm. A standard curve was constructed
by plotting absorbance values versus SHBG, CA15-3 and CA125
concentrations of the standards. Concentrations in the test samples
were determined using this standard curve. All samples were run in
triplicate. The detection limit of SHBG was 0.2 nmol/l. Intra- and
inter-assay variations of SHBG were 4.3–8.5 and 7.3–11.5%,
respectively. The dilution linearity was 102% (range 96–108%). The
upper normal range was 25 IU/ml for CA15-3 and 35 IU/ml for
CA125.
Statistical analysis
The data were presented as mean ± standard
deviation. The Shapiro-Wilk test was performed to determine whether
variables were normally distributed or not. An independent t-test
was used to compare differences between two groups when variables
were normally distributed, while Wilcoxon rank-sum tests were
performed for non-normally distributed variables. One-way analysis
of variance (ANOVA) with Bonferroni post-hoc analysis was used to
examine differences for normally distributed variables among three
groups or more. If the normality assumption was violated,
Kruskal-Wallis with Dunn's post-hoc test was performed instead. The
correlation between variables was estimated using Spearman's rank
correlation coefficient. P<0.05 was considered to indicate a
statistically significant difference. All statistical analyses and
graphing were performed using SAS software version 9.4 (SAS
Institute, Inc., Cary, NC, USA) and R version 3.6.1. (27,28).
Results
Healthy volunteers and patients with
breast cancer
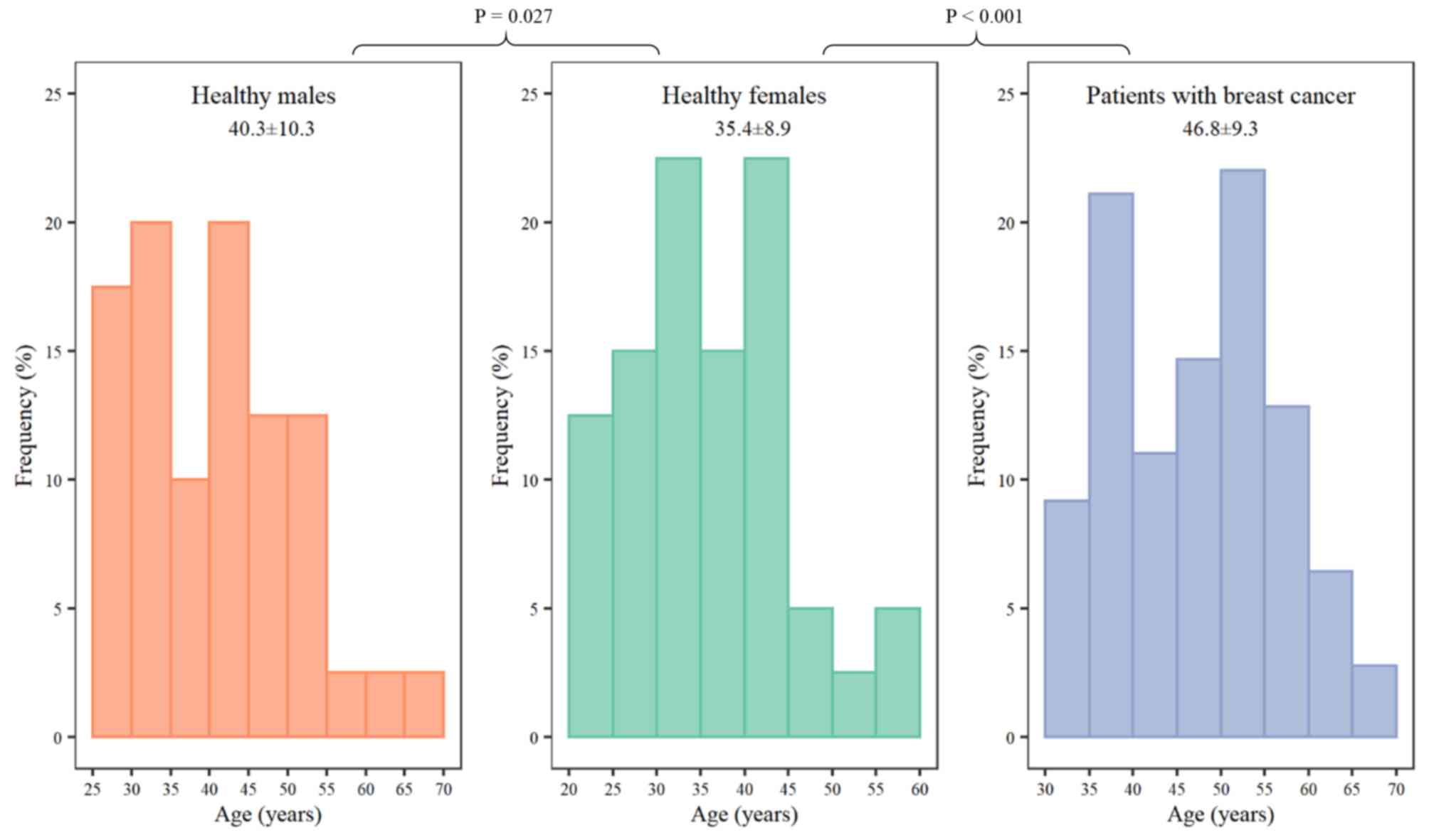
The median age of healthy female volunteers was
younger compared with healthy males (P=0.027) or patients with
breast cancer (P<0.001; Fig. 2).
A total of 109 samples were obtained from patients with breast
cancer in 3 different disease states (Group A, non-tumor state
after surgery, n=37; Group B, localized tumor at diagnosis and
during neo-adjuvant chemotherapy, n=32; and Group C, systemic
metastasis, n=40) during a long-term follow-up (Fig. 1).
Comparison of SHBG expression levels
with sex and age in healthy volunteers
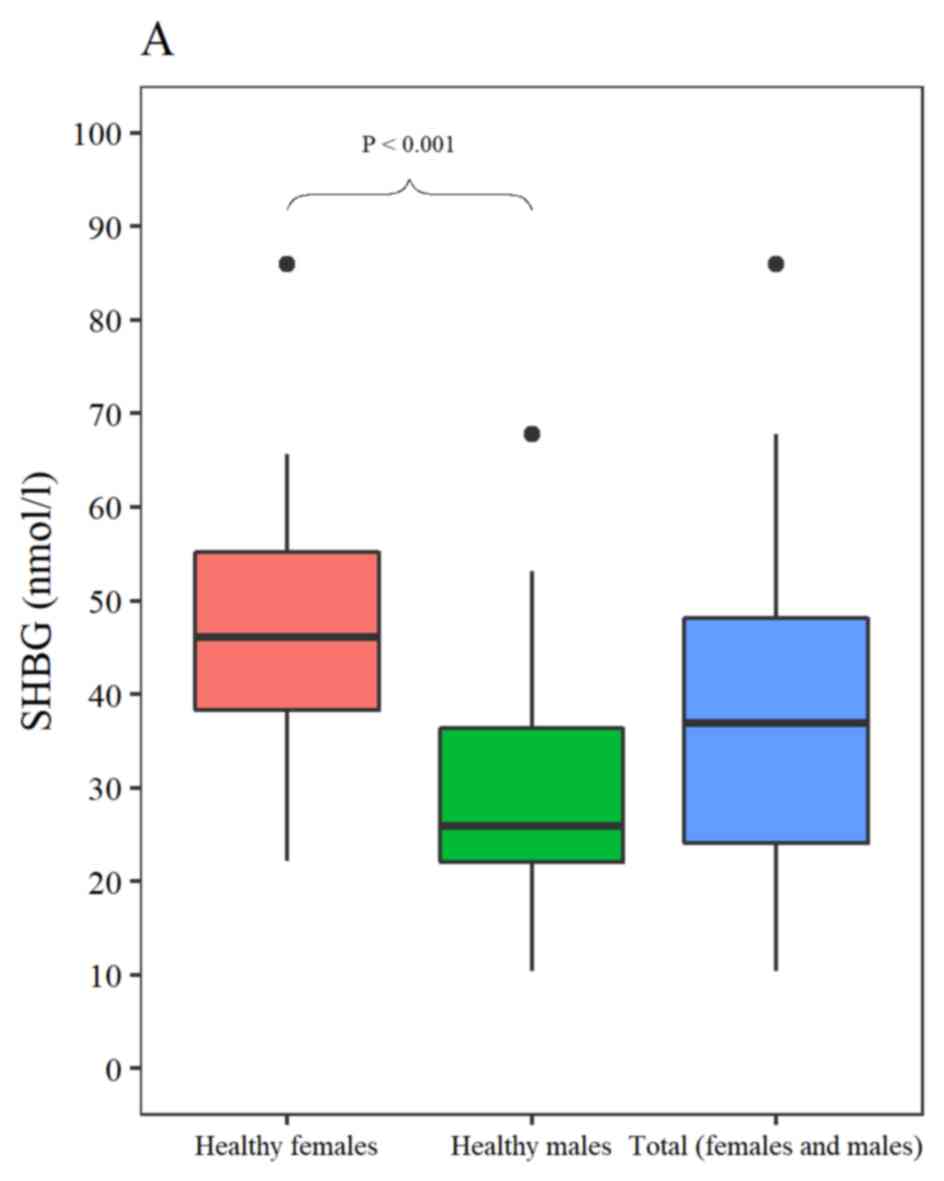
The mean expression levels of SHBG in male and
female healthy volunteers were 29.0±11.6 and 46.4±12.8 nmol/l,
respectively. The SHBG expression levels were significantly higher
in females compared with males (P<0.0001; Fig. 3A). The difference in SHBG expression
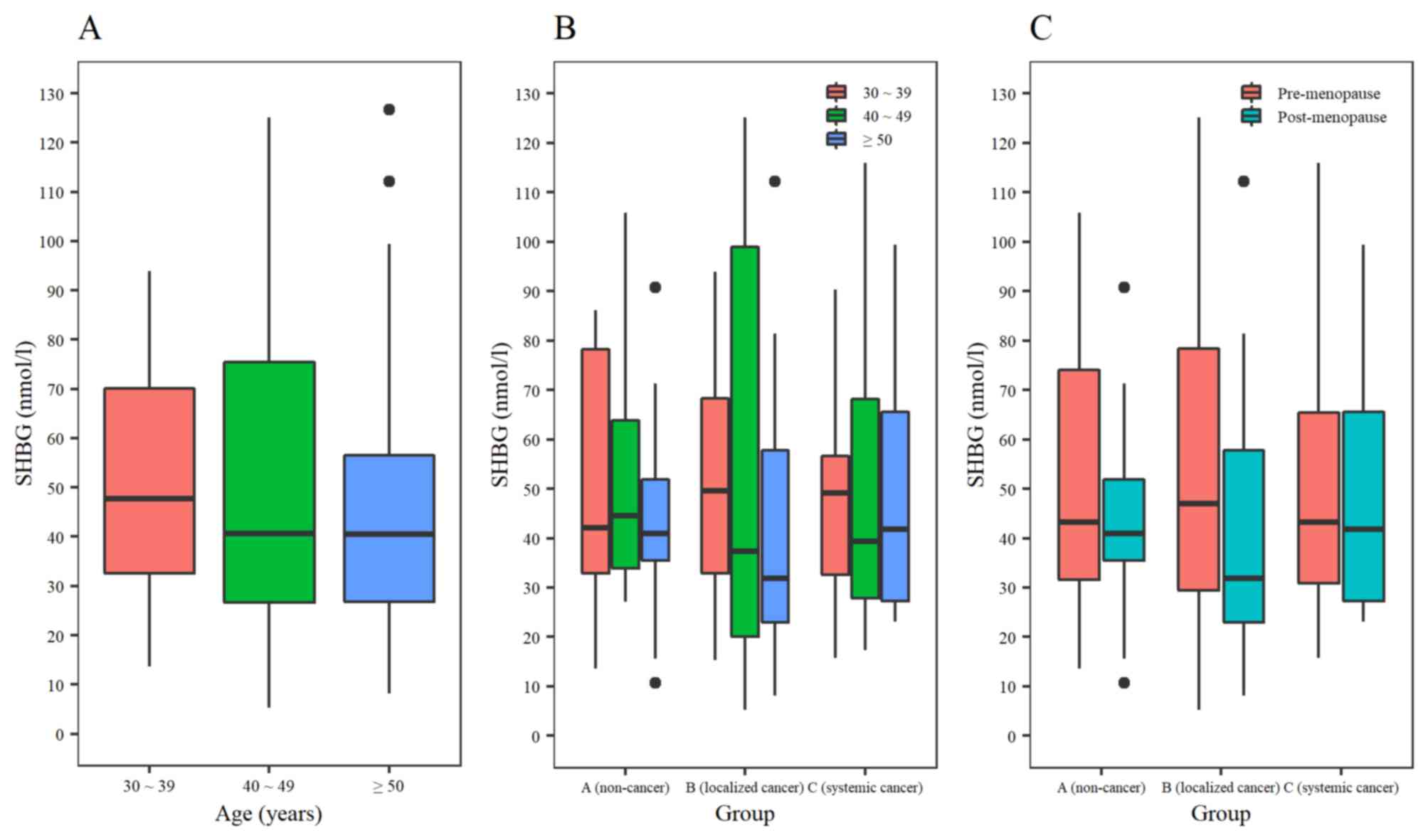
levels due to sex was compared in healthy volunteer subgroups
stratified by age; the difference between sexes was maintained in
people aged <30, 30–39 and ≥50 years, but not in the age group
of 40–49 years (Fig. 3B) (Table I). In men, SHBG expression levels
increased until the age of 49 and then decreased (P=0.01). In
females, SHBG expression levels decreased until the age of 49. An
increasing pattern in females ≥50 years was identified, which was
not a statistically significant difference (P=0.66; Fig. 3B) (Table
I). In females, there was no significant difference in the SHBG
expression levels between the pre-menopause group <50 years and
the post-menopause group ≥50 years (46.2±13.2 vs. 48.3±8.8 nmol/l;
P=0.74; Table II) (Fig. 3C).
 | Table I.Comparison of SHBG levels by sex and
age in healthy volunteers. |
Table I.
Comparison of SHBG levels by sex and
age in healthy volunteers.
|
| Male | Female |
|
|---|
|
|
|
|
|
|---|
| Age (years) | Serum SHBG level,
mean ± SD | Patients, n | Serum SHBG level
mean ± SD | Patients, n | P-value |
|---|
| Range | 29.0±11.6 | 40 | 46.4±12.8 | 40 |
<0.0001a |
| <30 | 20.8±5.1 | 7 | 51.2±17.0 | 11 | 0.0008b |
| 30-39 | 25.6±7.9 | 12 | 44.4±11.3 | 15 |
<0.0001a |
| 40-49 | 36.6±13.1 | 13 | 43.8±10.8 | 11 | 0.17b |
| ≥50 | 28.9±11.7 | 8 | 48.3±8.8 | 3 | 0.05b |
| P-value |
| 0.01c |
| 0.66c |
 | Table II.Comparison of SHBG levels by
menopause state in female healthy volunteers. |
Table II.
Comparison of SHBG levels by
menopause state in female healthy volunteers.
|
| Serum SHBG level,
mean ± SD | Participants,
n | P-value |
|---|
| Pre-menopause
(<50 years) | 46.3±13.2 | 37 |
|
| Post-menopause (≥50
years) | 48.3±8.8 | 3 | 0.74a |
SHBG expression levels in patients
with breast cancer
Although there was a trend of increasing SHBG
expression levels with larger tumor volumes, there was no
statistically significant difference (P=0.86) in the SHBG
expression levels between the 3 different tumor states: Group A
(n=37), 47.3±23.7 nmol/l; Group B (n=32), 49.1±32.2 nmol/l; and
Group C (n=40), 51.4±29.0 nmol/l (Fig.
3D). SHBG expression levels were compared by age in patients
with breast cancer regardless of cancer state, and these levels
exhibited a decreasing trend which revealed an increasing trend in
healthy females (Fig. 4A). SHBG
expression levels were compared by age between the 3 groups; there
was no significant difference in Group A by age; however, Groups B
and C exhibited a decrease in expression in the ≥50 years groups
(Fig. 4B). SHBG expression levels
were compared between pre-menopause and post-menopause groups; the
SHBG expression level exhibited a decreasing trend in
post-menopausal patients compared with pre-menopausal patients, in
all three groups (Table III;
Fig. 4C).
 | Table III.Comparison of SHBG levels by age in
different cancer states. |
Table III.
Comparison of SHBG levels by age in
different cancer states.
|
| Group A | Group B | Group C |
|
|---|
|
|
|
| Group C |
|
|---|
| Parameter | Serum SHBG (mean ±
SD) | Samples, n | Serum SHBG (mean ±
SD) | Samples, n | Serum SHBG (mean ±
SD) | Samples, n | P-value |
|---|
|
|---|
| A, age (years) |
|---|
| All patients | 47.3±23.7 | 37 | 49.1±32.2 | 32 | 51.4±29.0 | 40 | 0.86a |
| 30-39 | 49.5±25.4 | 13 | 51.9±24.2 | 11 | 49.9±23.0 | 9 | 0.97b |
| 40-49 | 53.3±28.0 | 7 | 57.9±48.2 | 7 | 51.9±32.0 | 14 | 0.85a |
| ≥50 | 43.2±21.0 | 17 | 42.4±29.1 | 14 | 51.8±30.9 | 17 | 0.55b |
| P-value | 0.72a |
| 0.59a |
| 0.94a |
|
|
|
| B, menopause
state |
|
| Pre-menopause | 50.8±25.7 | 20 | 54.3±34.2 | 18 | 51.1±28.3 | 23 | 0.98a |
| Post-menopause | 43.2±21.0 | 17 | 42.4±29.1 | 14 | 51.8±30.9 | 17 |
|
| P-value | 0.33c |
| 0.33d |
| 0.93d |
| 0.62 |
Comparison of serum SHBG positivity in
each decade of age in breast cancer
Using a cut-off point of the mean ±2 standard
deviations for SHBG positivity, a sensitivity of 36%, a specificity
of 65% and an accuracy of 46% were identified. For the
pre-menopause group, the sensitivity, specificity and accuracy were
44, 60 and 50%, respectively. In the post-menopause group, the
sensitivity, specificity and accuracy were 26, 71 and 42%,
respectively. When the sensitivity and specificity in each decade
were evaluated in whole breast cancers, the sensitivity and
specificity were as follows: 35 and 54% for age 30–39 years; 52 and
71% for age 40–49 years; and 26 and 71% for age ≥50 years,
respectively (Table IV).
 | Table IV.Comparison of SHBG positivity in each
decade of age in breast cancer. |
Table IV.
Comparison of SHBG positivity in each
decade of age in breast cancer.
| Biomarker | Sensitivity, % | Specificity, % | Accuracy, % |
|---|
| Total (n=109) | 36 | 65 | 46 |
| –39 (n=33) | 35 | 54 | 42 |
| –49 (n=28) | 52 | 71 | 57 |
| ≥50 (n=48) | 26 | 71 | 42 |
| Pre-menopause
(n=61) | 44 | 60 | 50 |
| Post-menopause
(n=48) | 26 | 71 | 42 |
Comparison of serum positivity among
SHBG, CA15-3 and CA125 in breast cancer
In 109 samples with different states of breast
cancer, sensitivity, specificity and accuracy of CA15-3 and CA125
were simultaneously compared (Table
V). Sensitivity increased when considering CA15-3 and CA125
together (75%). The highest sensitivity (79%) was obtained when all
three markers were used. When SHBG was measured alongside CA15-3 or
CA125 (64%), no benefit was identified regarding accuracy with
CA15-3 or CA125 (70%) (Table V).
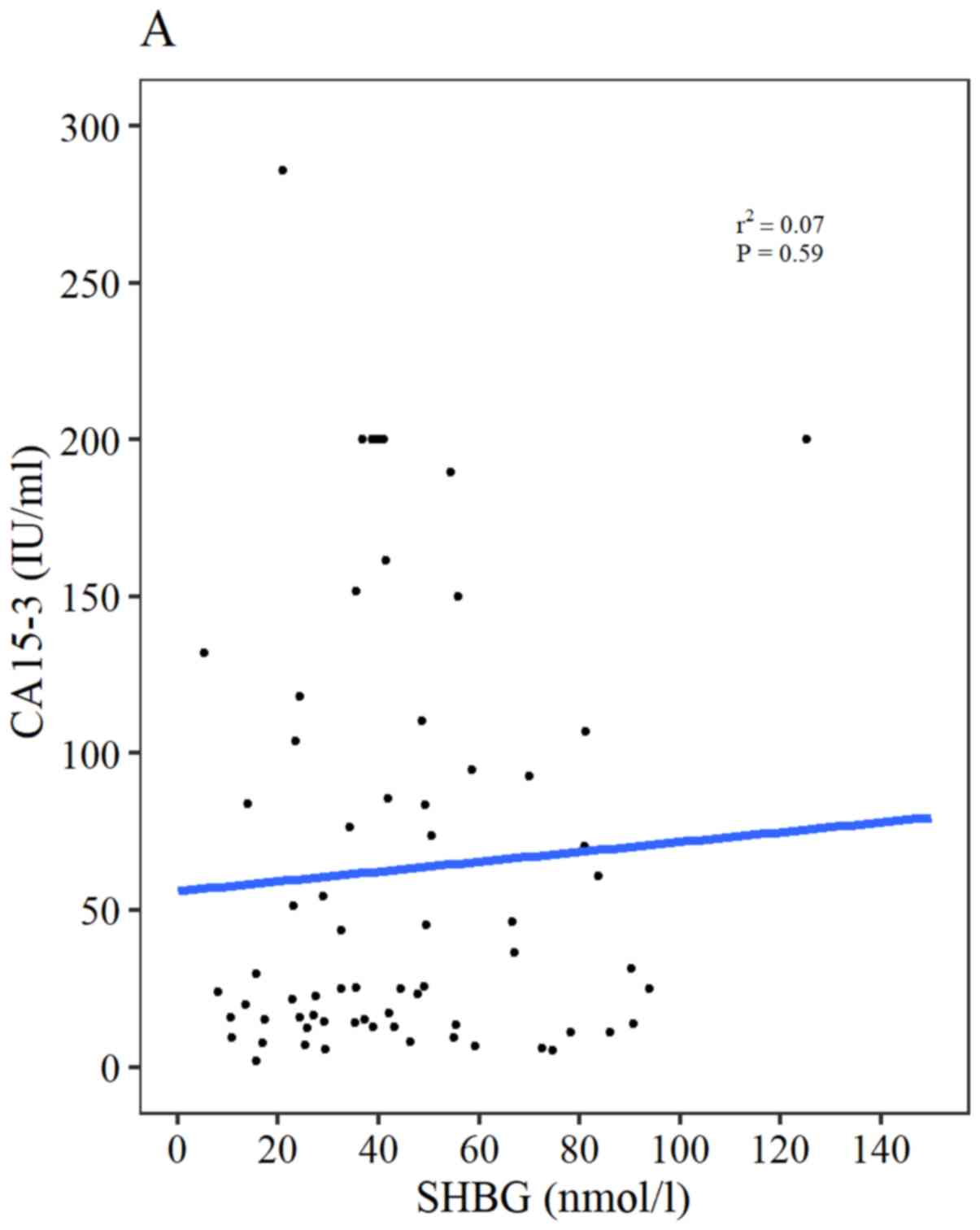
CA15-3 and CA125 levels were moderately correlated with each other
(r2=0.54; P<0.0001; data not shown). However, both
CA15-3 (r2=0.07; P=0.59) and CA125 (r2=−0.18;
P= 0.17) serum levels were not significantly correlated with SHBG
(Fig. 5A and B, respectively).
 | Table V.Comparison of sensitivity,
specificity and accuracy of 3 biomarkers in breast cancer. |
Table V.
Comparison of sensitivity,
specificity and accuracy of 3 biomarkers in breast cancer.
| Biomarker | Sensitivity, % | Specificity, % | Accuracy, % |
|---|
| CA15-3 | 61 | 82 | 68 |
| CA125 | 51 | 65 | 56 |
| CA15-3 and
CA125 | 75 | 59 | 70 |
| CA15-3, CA125 and
SHBG | 79 | 32 | 64 |
Discussion
SHBG is a circulating glycoprotein that binds
dihydrotestosterone, testosterone and estradiol; notably, its
highest binding affinity is for dihydrotestosterone and
testosterone, with a lower affinity for estradiol (1). As a result, an increase in SHBG serum
concentration may result in lowering the percentage of unbound
dihydrotestosterone, testosterone and estradiol (2). Therefore, SHBG may be a useful
predictor of circulating total and bioavailable sex hormone levels
(2). By contrast to healthy females,
to the best of our knowledge, there has been no analysis of the
SHBG expression levels in healthy males. As revealed by the present
data, although there was a difference in age distribution of male
to female volunteers, healthy males exhibited a lower range of SHBG
expression levels compared with healthy females by age (decade of
age). This indicates that different normal reference values of SHBG
expression levels for males and females are needed to determine
abnormal expression levels of SHBG for risk evaluation of cancer or
for cancer status prediction. In males, the SHBG expression levels
increased from 30 to 49 years of age and then decreased ≥50 years,
at which age the incidence of prostate cancer typically increases
(3). This pattern was reversed in
females who exhibited a decreasing trend until 49 years. SHBG
expression levels increased in patients aged >50 years, when the
incidence of breast cancer typically increases (15,23).
There is controversy regarding the association
between the serum levels of SHBG and the risk of prostate cancer
development, which may come from fixed normal reference value
regardless of sex and age. In a Japanese population, SHBG
expression levels were not strongly associated with the risk of
prostate cancer, except in males age <60 years (3). However, a previous study in Spain
identified that low bioavailability of testosterone levels and high
SHBG expression levels were associated with a 4.9- and 3.2-fold
increase in the risk of prostate cancer, respectively (4). In localized prostate cancer, the
preoperative serum SHBG expression levels were associated with
prostatic extension and Gleason score (29). SHBG was not considered a biomarker
for high-grade disease (30). For
future prostate cancer studies, normal reference values of SHBG
should reflect sex and age. In lung cancer which is non-endocrine
cancer, the typical negative correlation between SHBG expression
levels and total dihydrotestosterone observed in healthy volunteers
and other endocrinological gynecology cancer was reversed, perhaps
due to the systemic manifestation of thyrotoxicosis, chronic liver
disease and disseminated cancer associated with liver metastasis
(8,9). In patients with cancer, different
reference ranges by age, sex, cancer type (endocrinological versus
non-endocrinological) and cancer stage (localized versus systemic
manifestations) can be applied to clarify these controversial
clinical results.
A previous study demonstrated that SHBG expression
levels are higher in the first 12 days of luteal phase compared
with the rest of the menstrual cycle (31,32). In
both pre-menopause and post-menopause groups, SHBG expression
levels decreased with increasing weight. SHBG level was lower in
single nulliparous compared with married nulliparous or parous
females (31). In post-menopause,
SHBG increased in the years following menopause (31). In the present study of healthy
volunteers, a similar trend was revealed as SHBG expression levels
exhibited a decreasing trend until age 49 and then an increasing
pattern >50 years. Therefore, it was suggested that the SHBG
expression levels must be determined for pre-menopausal and
post-menopausal females separately, as the mean SHBG expression
levels in these two groups were different (20). Serum SHBG expression levels are
regulated by a biologically active and unbound hormone fraction,
with androgens having an inhibitory effect and estrogens having a
stimulatory effect on the SHBG expression levels (1,16,33).
High SHBG expression levels were significantly associated with
decreased breast cancer risk and protective function in
post-menopausal females (34). In
the present study, in patients with breast cancer in Groups B and
C, the SHBG expression levels indicated a decreasing trend after
age 50, although it showed an increasing trend in healthy females
aged ≥50 years. Although the patients in this study were mostly
younger than Western patients (35),
receiving mainly chemotherapy instead of hormonal treatment and the
healthy volunteers were younger than the patients with cancer, the
trend was similar to that in Western patients when the SHBG
expression levels was compared by age (14,15).
SHBG expression levels were increased by estrogen but decreased by
testosterone, suggesting that upregulated SHBG may be an indicator
of an estrogenic environment (11).
Therefore, it was suggested that SHBG expression levels were an
improved predictor of hormone treatment compared with the estrogen
receptor, as higher SHBG expression levels were identified in
ER+ patients compared with ER− patients
(20,33,36).
Notably, the SHBG expression levels in postmenopausal patients with
ER+ were higher compared with patients with
ER− endometrial and cervical cancer, even if the SHBG
level revealed a high overlapping range between ER+ and
ER− groups (1). Whether
the considerable overlap of SHBG expression levels between
ER+ and ER− gynecological cancer is due to
other factors, such as heterogeneity of tumor stage, varying degree
of illness or weight, needs to be studied in the future (13).
A novel biomarker should be an independent predictor
of the selected outcome; it must increase the multivariable
predictive accuracy of a model (24). Despite controversy surrounding the
correlation of SHBG with ER status, suggesting that plasma SHBG has
little value as a predictive index in breast cancer, Dimou et
al (37) recently reported a
potentially causal inverse association between SHBG expression
levels and risk of ER positive breast cancer. In the present study,
although specificity was good ≥50, the sensitivity was too low in
patients with breast cancer to confirm the role of SHBG as a tumor
suppressor. SHBG expression levels were not correlated with known
tumor markers CA15-3 or CA125. No additive effect of the biomarkers
was identified using all three biomarkers for cancer prediction. A
larger study using an age-specific reference value with estrogen
level may resolve this issue in the future. A non-synonymous single
nucleotide polymorphism in exon 8 can result in an amino acid
substitution of asparaginase for aspartic acid (D356N, rs6259) in
the SHBG protein (38). The
asparagine allele of SHBG was associated with elevated circulating
SHBG in postmenopausal females (38). This genotype may be applied in future
studies as a biomarker.
Genistein not only increases SHBG expression in
Hep-G2 cells, but also suppresses Hep-G2 cell proliferation
(2). As genistein is an inhibitor of
tyrosine-specific protein kinases, isoflavonoid may serve a role in
the prevention of malignant tumors, including hormone-dependent
cancers in countries with high consumption of soy products, such as
Japan and Korea (2). Genotyping and
diet analysis must be combined in the future to determine the
protective role of SHBG in female breast and gynecological
cancer.
In conclusion, there was a significant difference in
the SHBG expression levels between male and female healthy
volunteers. There was also a different pattern in the SHBG
expression levels between female healthy volunteers and female
patients with breast cancer ≥50 years. Although SHBG itself cannot
be used as a biomarker, different reference values stratified by
age and sex may help to determine its role in predicting a
high-risk group for hormone-dependent cancer, and guide treatment
in post-menopausal patients with breast cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JK designed the present study, interpreted the data
and wrote the first draft of the manuscript. SP interpreted the
data and performed the statistical analysis. TK, KP and WK
performed the experiments, interpreted the data, and revising the
draft. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Patients provided written informed consent to
participate in the present study, which was approved from The
Institutional Review Board of Inha University Hospital (approval
no. 10-617) and Severance Hospital, Yonsei University College of
Medicine (approval no. 4-2009-0256).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Selby C: Sex hormone binding globulin:
Origin, function and clinical significance. Ann Clin Biochem.
27:532–541. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mousavi Y and Adlercreutz H: Genistein is
an effective stimulator of sex hormone-binding globulin production
in hepatocellular carcinoma human liver cancer cells and suppresses
proliferation of these cells in culture. Steriods. 58:301–304.
1993. View Article : Google Scholar
|
|
3
|
Sawada N, Iwasaki M, Inoue M, Sasazuki S,
Yamaji T, Shiazu T and Tsugane S; Japan Public Health Center-based
Prospective Study Group, : Plasma testosterone and sex
hormone-binding globulin concentrations and the risk of prostate
cancer among Japanese men: A nested case-control study. Cancer Sci.
101:2652–2657. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Garcia-Closas M, Brinton LA, Lissowsk J,
Richesson D, Sherman ME, Szeszenia-Dabrowska N, Peplonska B, Welch
R, Yeager M, Zatonski W and Chanock SJ: Ovarian cancer risk and
common variation in the sex hormone binding globulin gene: A
population-based case-control study. BMC Cancer. 7:602007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Grasso M, Buonaguidi A, Mondina R,
Borsellino G, Lania C, Banfi G and Rigatti P: Plasma sex hormone
binding globulin in patients with prostatic carcinoma. Cancer.
66:354–357. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Salonia A, Briganti A, Gallina A,
Karakiewicz P, Shariat S, Freschi M, Zann G, Caoitanio U, Bosi E,
Rigatti P and Montorsi F: Sex hormone-binding globulin: A novel
marker for nodal metastases prediction in prostate cancer patients
undergoing extended pelvic lymph node dissection. Urology.
73:850–855. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Haapianen R, Rannikko S, Adlercreutz H and
Alfthan O: Correlation of pretreatment plasma levels of estradiol
and sex-hormone binding globulin-binding capacity with clinical
stage and survival of patients with prostate cancer. The Prostate.
8:127–137. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Calciano A, Khaw KT, Barrett-Connor E and
Garland C: Sex hormones, sex hormone binding globulin, and lung
cancer: A 12 year prospective study in a cohort of malesaged 50-79.
Br Med J. 296:16401988. View Article : Google Scholar
|
|
9
|
Corbishley TP, Keating JJ, Johnson PJ and
Williams R: Serum concentrations of sex hormone binding globulin in
lung cancer. Br Med J. 293:792–793. 1986. View Article : Google Scholar
|
|
10
|
Fortunati N, Catalano MG, Boccuzzi G and
Fairia R: Sex hormone binding globulin, estradiol and breast
cancer. Mol Cell Endocrinol. 316:86–92. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dayalu NSL, Suresh H, Anil KB, Renuka S,
Sudershan K and Amitabha R: Sex hormone binding globulin in breast
cancer. Indian J Clin Biochemist. 23:25–254. 2008.
|
|
12
|
Catalano MG, Frairia R, Boccuzzi G and
Fortunati N: Sex hormone-binding globulin antagonizes the
anti-apoptotic effect of estradiol in breast cancer cells. Mol Cell
Endocrinol. 230:31–37. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ratajczak T, Twaddle E and Hähnel R: Sex
hormone-binding globulin and estrogen receptor in endometrial and
cervical cancer. Gynecol Oncol. 10:262–266. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Moore JW, Key TJ, Clark GM, Bulbrook RD,
Allen DS, Wang DY and Pike MC: Concentrations of sex
hormone-binding globulin (SHBG) in a population of normal females:
Relationship to risk factors for breast cancer. Steroids.
52:391–392. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Adami HO, Johansson EDB, Vegelius J and
Victor A: Serum concentrations of estrone, androstenedione,
testosterone and sex hormone-binding globulin in postmenopausal
females with breast cancer and in age-matched controls. Upsala J
Med Sci. 84:259–274. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Moore JW, Clark GM, Bulbrook RD, Hayward
JL, Murai JT, Hammond GL and Siiteri PK: Serum concentrations of
total and non-protein-bound estradiol in patients with breast
cancer and on normal controls. Int J Cancer. 29:17–21. 1982.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Toniolo PG, Levitz M, Zeleniuch-Jacquotte
A, Banerjee S, Koenig KL, Shore RE, Strax P and Pasternack BS: A
prospective study of endogenous estrogens and breast cancer in
postmenopausal females. J Natl Cancer Inst. 87:190–197. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zeleniucg-Jacquotte A, Shore RE, Koenig
KL, Akhmedkhanov A, Afanasyeva Y, Kato I, Kim MY, Rinaldi S, Kaaks
R and Toniolo P: Postmenopausal levels of estrogen, androgen, and
SHBG and breast cancer: Longterm results of a prospective study. Br
J Cancer. 90:153–159. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lipworth L, Adami HO, Trichopoulos D,
Carlstrom K and Mantzoros C: Serum steroid hormone levels,
sex-hormone binding globulin and body mass index in the etiology of
postmenopausal breast cancer. Epidemiology. 7:96–100. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Murayama Y, Utsunomiya J, Takahashi I,
Kitamura M and Tominaga T: Sex hormone binding globulin as a
reliable indicator of hormone dependence in human breast cancer.
Ann Surg. 190:133–138. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sakai F, Cheix F, Clavel M, Colon J, Mayer
M, Pommatau E and Saez S: Increases in steroid binding globulins
induced by tamoxifen in patients with carcinoma of the breast. J
Endocrinol. 76:219–226. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang DY, Rubend RD, Clark GMG, Moore JW
and Bulbrook RD: Effects of prednisolone on sex hormone binding
globulin during primary endocrine treatment of advanced breast
cancer. Breast Cancer Res Treat. 11:67–70. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Beattie MS, Costantino JP, Cummings SR,
Wickerham DL, Vogel VG, Dowsett M, Folkerd EJ, Willett WC, Wolmark
N and Hankinson SE: Endogenous sex hormones, breast cancer risk,
and tamoxifen response: An ancillary study in the NSABP Breast
Cancer Prevention Trial (P-1). J Natl Cancer Inst. 98:110–115.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kattan MW: Judging new markers by their
ability to improve predictive accuracy. J Natl Cancer Inst.
95:634–635. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rha SY, Yang WI, Gong SJ, Kim JJ, Yoo NC,
Roh JK, Min JS, Lee KS, Kim BS and Chung HC: Correlation of tissue
and blood plasminogen activation system in breast cancer. Cancer
Lett. 150:137–145. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Park HA, Park JK, Park SA and Lee JS: Age,
menopause, and cardiovascular risk factors among Korean middle-aged
women: The 2005 Korea National Health and Nutrition Examination
Survey. J Womens Health (Larchmt). 19:869–876. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
R Core Team, . R: A language and
environment for statistical computing. R Foundation for Statistical
Computing. (Vienna). 2012.http://www.R-project.org/
|
|
28
|
RStudio Team, . RStudio: Integrated
development for R. RStudio, Inc. (Boston, MA). 2019.http://www.rstudio.com/
|
|
29
|
Lee SE, Chung JS, Han BK, Park CS, Moon
KH, Byun SS, Choe GY and Hong SK: Pre-operative serum sex
hormone-binding globulin as a predictive marker for extra-prostatic
extension of tumor in patients with clinically localized prostate
cancer. Eur J Urol. 54:1324–1332. 2008. View Article : Google Scholar
|
|
30
|
Nunzio CD, Lombardo R, Albisinni S, Gacci
M and Tubaro A: Serum levels of sex hormone binding globulin (SHBG)
are not predictive of prostate cancer diagnosis and aggressiveness:
results from an Italian biopsy cohort. Int Braz J Urol. 39:793–799.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Moore JW, Key TJA, Bulvrook RD, Clark GMG,
Allen DS, Wang DY and Pike MC: Sex hormone binding globulin and
risk factors for breast cancer in a population of normal females
who had never used exogenous sex hormones. Br J Cancer. 56:661–666.
1987. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pugeat M, Cousin P, Baret C, Lejeune H and
Forest MG: Sex hormone-binding globulin during puberty in normal
and hyperandrogenic girls. J Pediatr Endocrinol Metab. 13
(Suppl):1277–1279. 2000.PubMed/NCBI
|
|
33
|
Plymate SR, Stutz FH and Fariss BL:
Relationship between sex hormone-binding globulin and estrogen
receptors in breast cancer. J Clin Oncol. 2:652–654. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
He XY, Liao YD, Zhang Y and Wang R: Sex
hormone binding globulin and risk of breast cancer in
postmenopausal females: A meta-analysis of prospective studies.
Horm Metab Res. 47:485–490. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kim Z, Min SY, Yoon CS, Lee HJ, Lee JS,
Youn HJ, Park HK, Noh DY and Hur MH; Korean Breast Cancer Society,
: The basic facts of Korean breast cancer in 2011: Results of a
nationwide survey and breast cancer registry database. J Breast
Cancer. 17:99–106. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fortunati N, Becchis M, Catalano MG, Comba
A, Ferrera P, Raineri M, Berta L and Frairia R: Sex hormone-binding
globulin, its membrane receptor, and breast cancer: A new approach
to the modulation of estradiol action in neoplastic cells. J
Steroid Biochem Mol Biol. 69:473–479. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dimou NL, Papadimitriou N, Gill D,
Christakoudi S, Murphy N, Gunter MJ, Travis RC, Key TJ, Fortner RT,
Haycock PC, et al: Sex hormone binding globulin and risk of breast
cancer: A Mendelian randomization study. Int J Epidemiol.
48:807–816. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhou JY, Shi R, Yu HL, Zheng WL and Ma WL:
Association between SHBG Asp327Asn (rs6259) polymorphism and breast
cancer risk: A meta-analysis of 10,454 cases and 13,111 controls.
Mol Biol Rep. 39:8307–8314. 2012. View Article : Google Scholar : PubMed/NCBI
|