Introduction
Breast cancer is one of the most common types of
cancer worldwide among women (1).
Although progress has been made in the diagnosis and treatment of
breast cancer, it is the second leading cause of cancer-associated
death among women in the United States (1,2). To
reduce the mortality rate of breast cancer, early detection and
improved therapy is needed. It is important to identify more novel
sensitive prognostic biomarkers for breast cancer, especially for
invasive breast carcinoma.
Long non-coding RNAs (lncRNAs) are non-coding
transcripts, usually >200 nucleotides in length (3). Increasing evidence suggests that
lncRNAs are important regulators in multiple biological processes
and that lncRNAs with aberrant expression levels are involved in
cancer development and may be potential diagnostic biomarkers for
cancer (3–5). MicroRNAs (miRNAs) are a class of small
non-coding RNAs which serve an important role in the gene
regulation network (6). miRNAs
target the coding DNA sequence region or 3′untranslated region
(3′UTR) of mRNAs, inducing mRNA degradation or translational
repression (7,8).
lncRNAs have been identified as miRNA sponges that
compete for miRNA binding with mRNAs (9). lncRNA H19 was found to promote the
epithelial to mesenchymal transition in human colon cancer cells by
sponging miR-138 and miR-200a and eliminating the inhibition of
vimentin, ZEB1 and ZEB2 induced by the two miRNAs (9). Moreover, lncRNAs can exert their
biological effects by interacting with proteins. For example, it
was reported that lncRNA GATA6-AS negatively regulates nuclear
LOXL2 function and regulates endothelial gene expression via
interaction with the epigenetic regulator LOXL2 in endothelial
cells (10).
In the present study, differentially expressed
lncRNAs in invasive breast carcinoma from The Cancer Genome Atlas
(TCGA) database and cBioPortal (11,12) were
investigated, identifying 292 differentially expressed lncRNAs in
1,100 invasive breast carcinoma cases, including 10
prognosis-related lncRNAs. Subsequently, to explore the molecular
mechanisms of these 10 prognosis-related lncRNAs, bioinformatics
methods were used to predict the potential target miRNAs, mRNAs and
proteins, and to construct a lncRNA-miRNA-mRNA regulatory network
and lncRNA-protein interaction network. Finally, the target mRNAs
and proteins were annotated using Gene Ontology (GO) enrichment and
Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analyses
using The Database for Annotation, Visualization and Integrated
Discovery tools (DAVID) (13,14).
Materials and methods
Identification of differentially
expressed lncRNAs and prognosis-related lncRNAs
The HUGO Gene Nomenclature Committee (HGNC) database
(genenames.org/) is a resource for approved human
gene nomenclature and gene symbols of lncRNAs were obtained from
this site. The cBioPortal database (cbioportal.org/) was used to visualize, analyze and
download several large-scale cancer genomics datasets from
databases such as TCGA and GEO (11,12). In
this study, the TCGA Breast Invasive Carcinoma dataset (TCGA,
Firehose Legacy) was selected, which contains 1,100 invasive breast
carcinoma samples with RNA-seq data (11,12). The
subtypes of breast carcinoma were not distinguished in the present
study. The gene symbols of lncRNAs were submitted to the cBioPortal
database and the differentially expressed lncRNAs were obtained. To
investigate whether these differentially expressed lncRNAs were
correlated with invasive breast carcinoma prognosis, these lncRNAs
were analyzed using the Kaplan-Meier estimate of overall survival
and disease free survival rate in cBioPortal (11,12), and
the log-rank test were statistically significant at a P<0.05
level. The median overall survival time was also obtained from the
database.
lncRNA-miRNA-mRNA regulatory network
construction
lncRNA sequences were obtained from National Center
for Biotechnology Information database (ncbi.nlm.nih.gov/). To predict the miRNAs which could
be sponged by these lncRNAs, the lncRNA sequences were submitted to
the RegRNA database (http://regrna2.mbc.nctu.edu.tw/) (15), which selected the predicted miRNA
target sites according to the follow criteria: System score ≥150
and minimum folding free energy ≤-25. To reduce the number of false
positive results, the top 10 ranked miRNAs were selected as
potential targets.
Subsequently, the target mRNAs of miRNAs were
predicted using the TargetScan database (http://www.targetscan.org/vert_72/) (16), which is widely used in the prediction
of miRNA targets and has been updated with a modified set of
representative transcripts and different miRNA annotations in March
2018. Using the TargetScan database, predictions were ranked
according to the predicted efficacy of targeting and the
probability of conserved targeting. Then, the top 10 ranked mRNAs
were selected and submitted to the cBioPortal database to screen
for aberrantly expressed mRNAs (define as alternation frequency
≥5%) as potential targets of miRNAs. The lncRNA-miRNA-mRNA
regulatory network was constructed using Cytoscape version 3.6.1
(17).
lncRNA-protein interactions
prediction
In order to predict the lncRNA-binding proteins, the
lncRNA symbols were submitted to the RNAct database (https://rnact.crg.eu/) (18) and the top 10 ranked proteins were
selected based on the prediction score to minimize the likelihood
of false positive results. The lncRNA-protein interaction network
was constructed using Cytoscape version 3.6.1.
GO enrichment and KEGG pathway
analysis
GO enrichment and KEGG pathway analyses were
performed to further investigate the functions of the target genes
and proteins. GO and KEGG analyses were performed using DAVID
version 6.8 (https://david.ncifcrf.gov/) (13,14) and
GO analysis was limited to biological process and molecular
function. P<0.05 was considered to indicate a statistically
significant difference.
Results
Identification of differentially
expressed lncRNAs associated with prognosis in invasive breast
carcinoma
A total of 4,525 lncRNA gene symbols were obtained
from HGNC, of which 292 differentially expressed lncRNAs were
identified in 1,100 patients with invasive breast carcinoma. Among
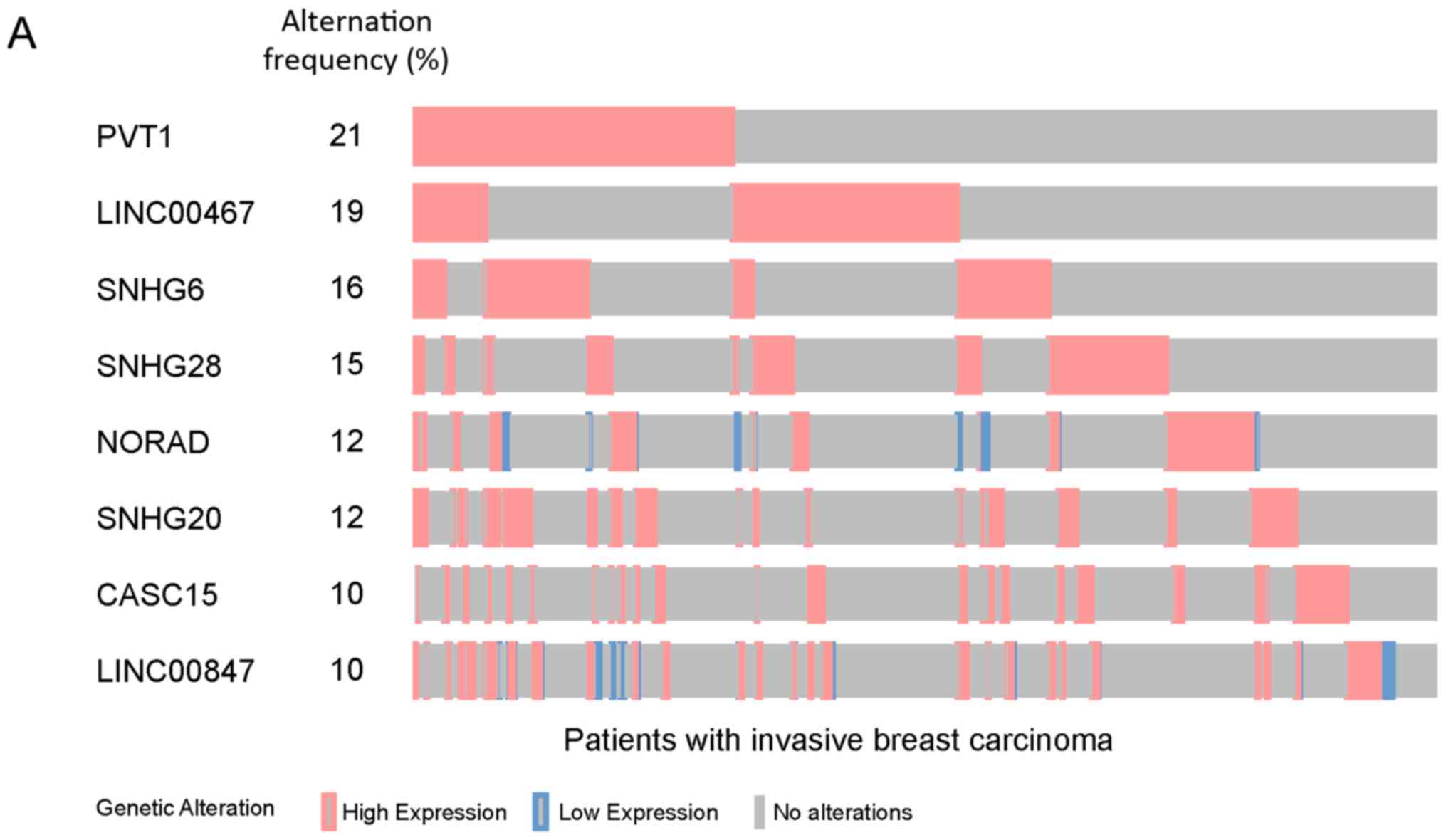
these, 8 lncRNAs (PVT1, LINC00467, SNHG6, SNHG28, NORAD, SNHG20,
CASC15 and LINC00847) exhibited aberrant expression levels in ≥10%
of cases, accounting for 65% of patients with invasive breast
carcinoma (Fig. 1A).
To investigate whether differentially expressed
lncRNAs were associated with the prognosis of invasive breast
carcinoma, the 292 differentially expressed lncRNAs were submitted
to the cBioPortal database and overall survival of patients with
invasive breast carcinoma was analyzed. As shown in Fig. 1B, the 10 lncRNAs (NORAD, SNHG1,
LINC00654, FAM157A, DLEU2, LINC01559, SPATA8, TCL6, LINC00632 and
PWRN1) with expression alterations were significantly associated
with poor prognosis in invasive breast carcinoma. The overall
survival rate in patients with expression alterations of the 10
lncRNAs were lower than in patients without expression alterations
(log-rank test P<0.05; Fig. 1C),
and the median overall survival time in cases with expression
alterations of these 10 lncRNAs were shorter compared with cases
without expression alterations (Table
I). Moreover, among the 10 lncRNAs, DLEU2, TCL6 and PWRN1
expression alterations were significantly correlated with disease
free survival rate, the disease free survival rate in cases with
expression alterations of the 3 lncRNAs were lower than cases
without expression alterations (log-rank test P<0.05; Fig. 1D).
 | Table I.The median overall survival of
patients with invasive breast carcinoma with and without
alterations in different genes. |
Table I.
The median overall survival of
patients with invasive breast carcinoma with and without
alterations in different genes.
| Gene | Median survival,
months | Log-rank test
P-value |
|---|
| NORAD |
|
5.69×10−3 |
| With
alteration | 91.92 |
|
| Without
alteration | 130.06 |
|
| SNHG1 |
| 0.0453 |
| With
alteration | 114.06 |
|
| Without
alteration | 129.6 |
|
| LINC00654 |
| 0.0242 |
| With
alteration | 94.15 |
|
| Without
alteration | 129.6 |
|
| FAM157A |
| 0.0232 |
| With
alteration | 114.72 |
|
| Without
alteration | 129.6 |
|
| DLEU2 |
|
2.17×10−03 |
| With
alteration | 93.76 |
|
| Without
alteration | 129.47 |
|
| LINC01559 |
| 0.0218 |
| With
alteration | 77.56 |
|
| Without
alteration | 129.47 |
|
| SPATA8 |
| 0.0444 |
| With
alteration | 74.67 |
|
| Without
alteration | 129.47 |
|
| TCL6 |
|
2.72×10−04 |
| With
alteration | 81.57 |
|
| Without
alteration | 129.47 |
|
| LINC00632 |
| 0.0104 |
| With
alteration | 33.9 |
|
| Without
alteration | 129.47 |
|
| PWRN1 |
| 0.0164 |
| With
alteration | 74.67 |
|
| Without
alteration | 129.6 |
|
lncRNA-miRNA-mRNA regulatory network
analysis
Firstly, to explore the underlying molecular
mechanisms of the 10 prognosis-related lncRNAs, 88 miRNAs which
could be sponged by these 10 lncRNAs were predicted using the
RegRNA database. Secondly, 765 target mRNAs of these 88 miRNAs were
predicted using the TargetScan database. Thirdly, to improve the
prediction accuracy, 322 aberrantly expressed target mRNAs were
screened as potential targets using the cBioPortal database.
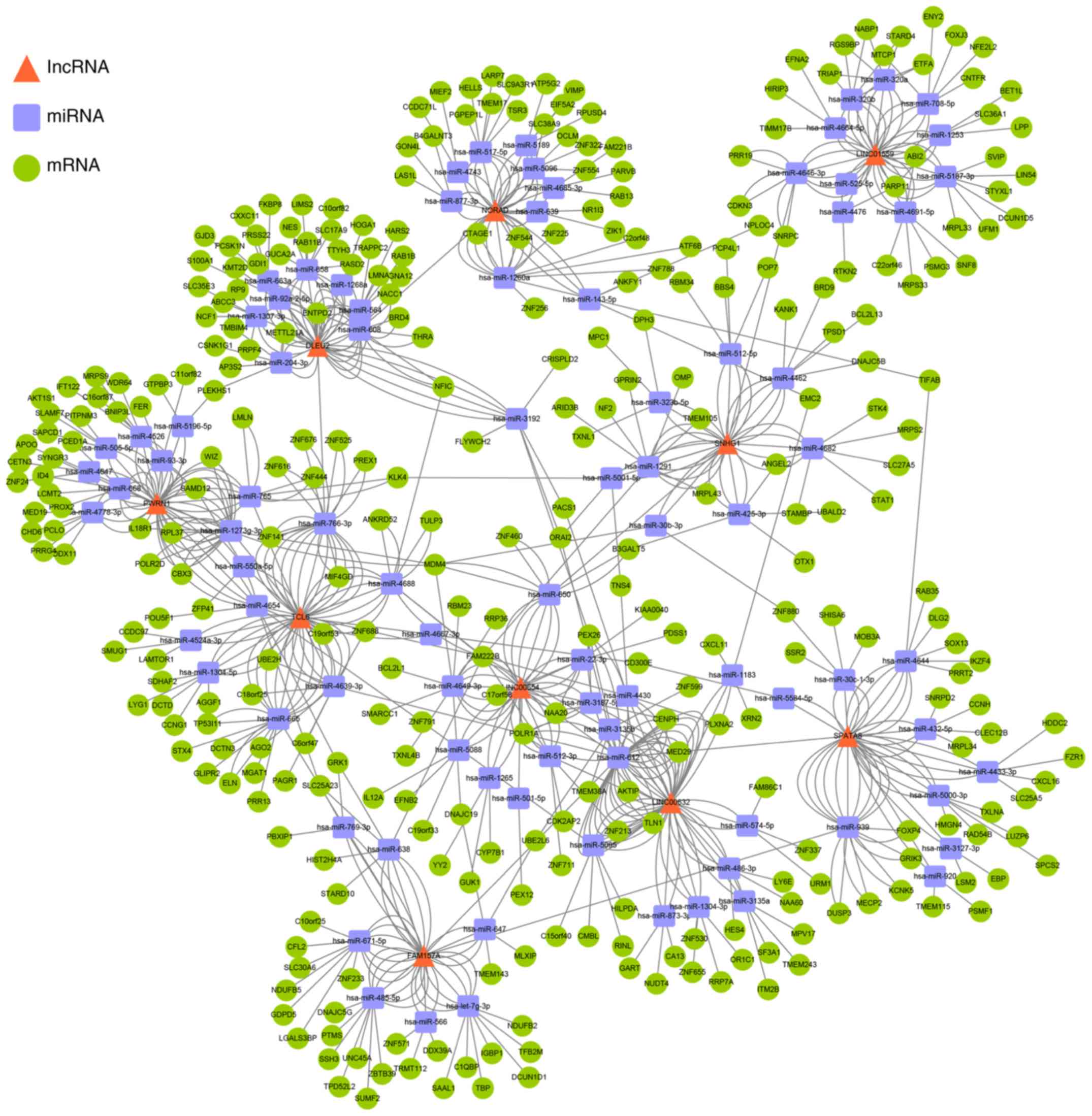
Overall, the lncRNA-miRNA-mRNA regulatory network was composed of
10 lncRNAs, 88 miRNAs and 322 mRNAs, the red triangles represent
lncRNAs, the blue squares represent miRNAs, and green circles
represent mRNAs (Fig. 2).
lncRNA-protein interaction
analysis
RNA-binding proteins are important to a number of
cellular processes, including mRNA splicing, export, stability and
translation (19), and lncRNAs could
bind to proteins forming RNA-binding proteins to exert their
biological functions. The RNAct database was a useful tool for
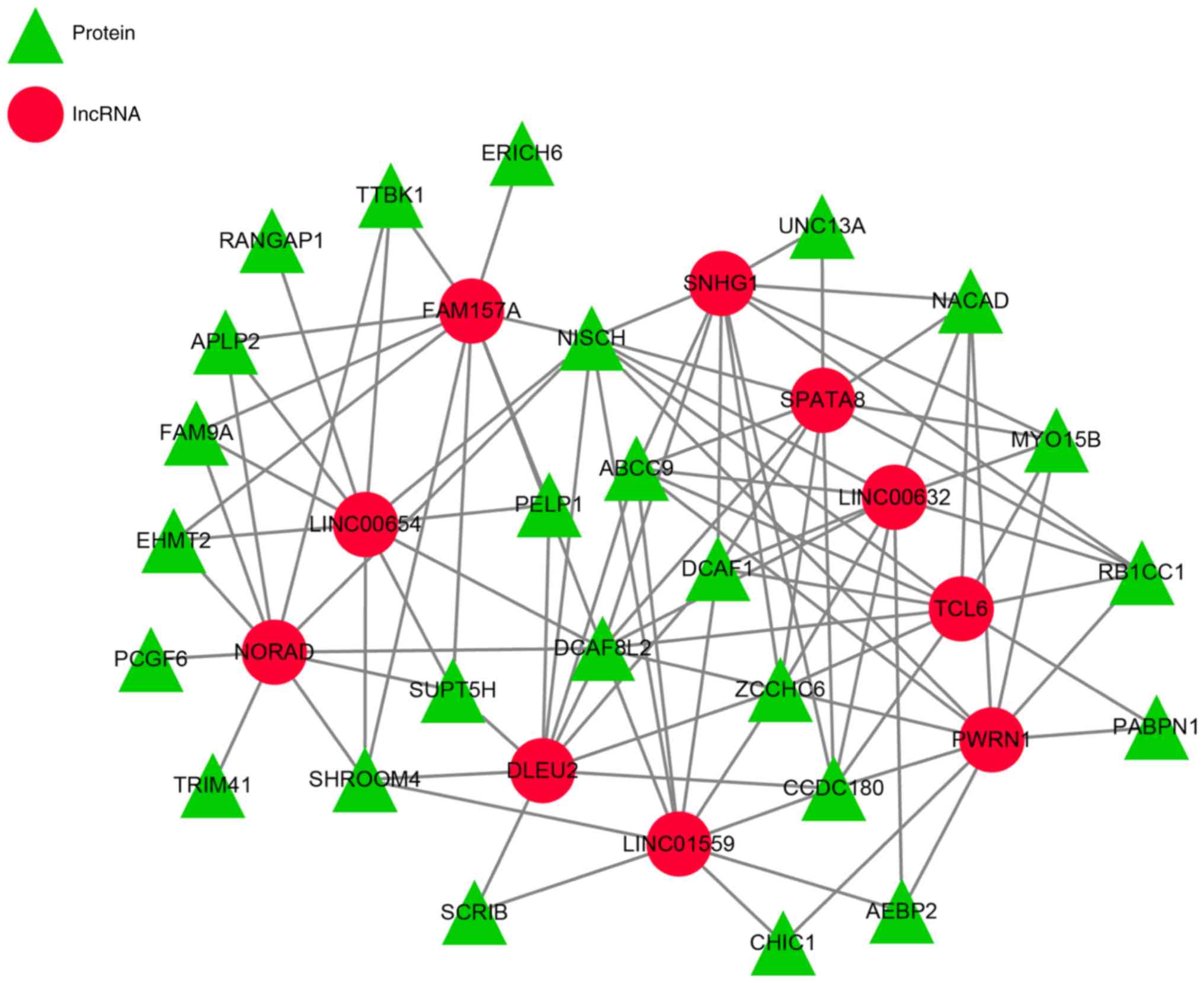
RNA-binding proteins prediction and the results showed that there
were 25 potential target proteins of the 10 lncRNAs (Fig. 3) and different lncRNAs might target
the same protein, such as, PWRN1, TCL6, LINC00632, SPATA8 and SNHG1
had the same target protein RB1CC1.
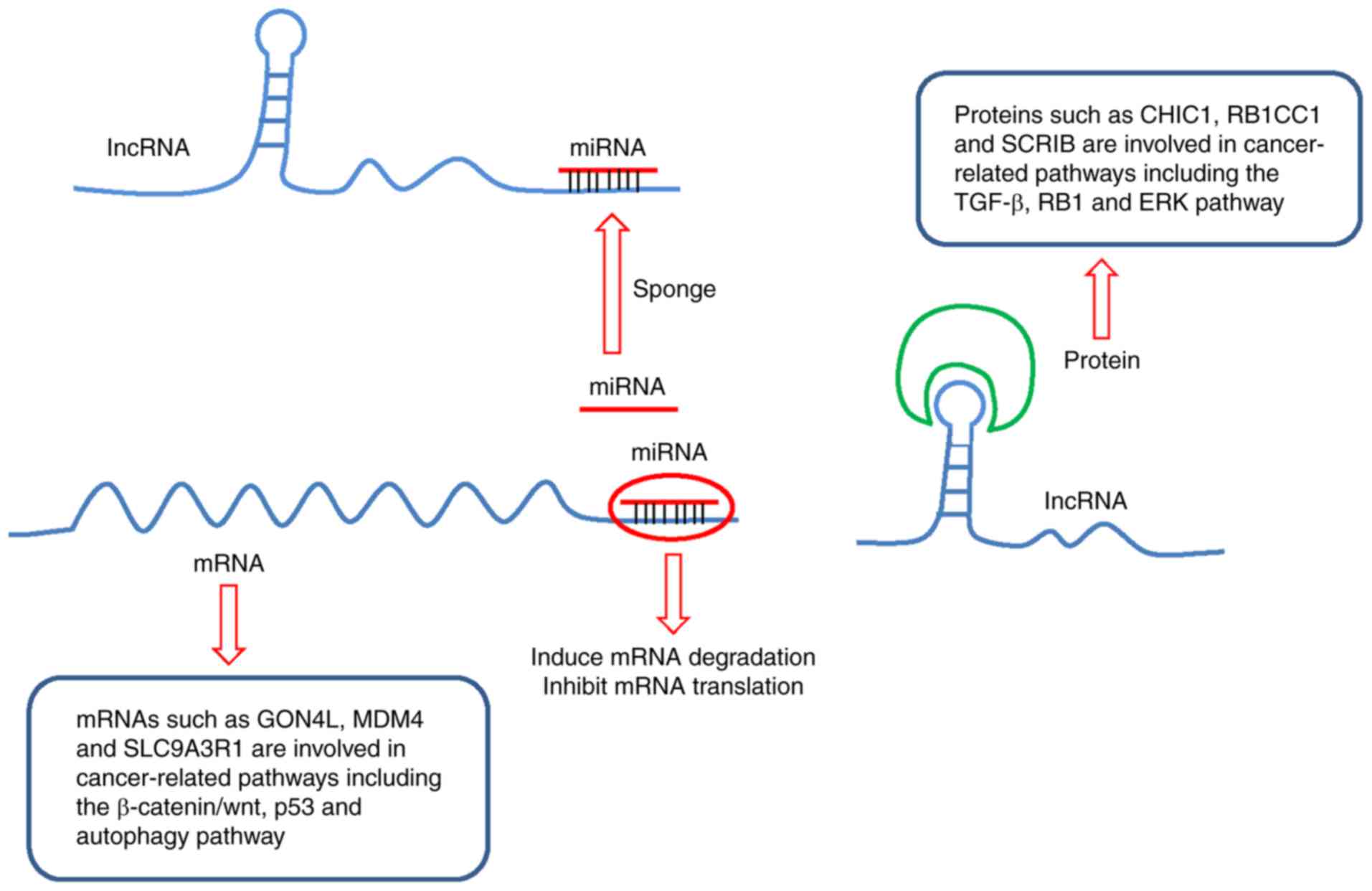
Combining the above two interaction networks,
lncRNAs could indirectly regulate mRNAs through sponging miRNAs,
abrogating the inhibition of mRNA expression levels or translation
induced by miRNAs. In addition, the lncRNAs could regulate the
target proteins by forming RNA-protein complexes (Fig. 4).
Functional analysis of target mRNAs
and proteins
To further investigate the functions of the target
genes and proteins, the official gene symbols of the targets were
submitted to the DAVID database, in which GO enrichment and KEGG
pathways analyses were performed. The result of GO analysis showed
that a total of 284 targets were enriched in 18 GO terms, including
‘transcription’, ‘regulation of transcription’, ‘translation’, ‘RNA
processing’, ‘mRNA splicing’, ‘negative regulation of release of
cytochrome c from mitochondria’, ‘covalent chromatin
modification’, ‘endosome localization’, ‘bile acid secretion’,
‘negative regulation of smoothened signaling pathway involved in
ventral spinal cord patterning’, ‘nucleic acid binding’, ‘DNA
binding’, ‘protein binding’, ‘metal ion binding’, ‘transcription
factor activity, sequence-specific DNA binding’, ‘structural
constituent of ribosome’, ‘protein tyrosine/serine/threonine
phosphatase’ and ‘single-stranded RNA binding’ (Table II). The results of KEGG pathways
analysis showed the targets were significantly enriched in ‘protein
processing in endoplasmic reticulum’.
 | Table II.GO enrichment analysis of target
mRNAs and proteins of patients with invasive breast carcinoma. |
Table II.
GO enrichment analysis of target
mRNAs and proteins of patients with invasive breast carcinoma.
| A, biological
process |
|---|
|
|---|
| GO term | Gene counts | P-value |
|---|
|
GO:0006351~transcription,
DNA-templated | 69 |
3.29×10−09 |
|
GO:0006355~regulation of transcription,
DNA-templated | 41 | 0.00258 |
|
GO:0006412~translation | 11 | 0.010969 |
| GO:0006396~RNA
processing | 6 | 0.024103 |
| GO:0090201~negative
regulation of release of cytochrome c from mitochondria | 3 | 0.032585 |
| GO:0000398~mRNA
splicing, via spliceosome | 9 | 0.034864 |
| GO:0016569~covalent
chromatin modification | 6 | 0.04242 |
| GO:0032439~endosome
localization | 2 | 0.049715 |
| GO:0032782~bile
acid secretion | 2 | 0.049715 |
| GO:0021914~negative
regulation of smoothened signaling pathway involved in ventral
spinal cord patterning | 2 | 0.049715 |
|
| B, molecular
function |
|
| GO term | Gene
counts | P-value |
| GO:0003676~nucleic
acid binding | 37 |
8.88×10−06 |
| GO:0003677~DNA
binding | 46 | 0.00099 |
| GO:0005515~protein
binding | 172 | 0.002573 |
| GO:0046872~metal
ion binding | 51 | 0.004791 |
|
GO:0003700~transcription factor activity,
sequence-specific DNA binding | 28 | 0.006082 |
|
GO:0003735~structural constituent of
ribosome | 10 | 0.012777 |
| GO:0008138~protein
tyrosine/serine/threonine phosphatase activity | 4 | 0.023806 |
|
GO:0003727~single-stranded RNA
binding | 4 | 0.035156 |
Discussion
In recent years, lncRNAs have been shown to be
regulators of onset and progression of different cancer types due
to their functions at both transcriptional and post-transcriptional
levels (19). At the transcriptional
level, lncRNAs have been reported to act as inducers of the
transcriptional activity of their targets (20). At post-transcriptional level, lncRNAs
can serve as ceRNAs to compete for miRNA binding sites on mRNAs
(9). Furthermore, lncRNAs can bind
to their target proteins, forming a localized complex at specific
targets or binding proteins together to form RNA-protein complexes
(21).
Aberrant expression levels of lncRNAs may lead to
abnormal miRNA and mRNA expression levels, which contributes to an
increased risk of diseases and several types of cancer (22,23). For
instance, lncRNA growth arrest-specific transcript 5 (GAS5) was
found to be downregulated in human breast cancer tissues and was
associated with advanced Tumor-Node-Metastasis stage and poor
prognosis (24). Moreover, GAS5 was
associated with tamoxifen resistance in breast cancer and
overexpression of GAS5 increased breast cancer cell sensitivity to
tamoxifen by regulating the expression levels of miR-222 (24).
In the present study, the expression levels of 4,525
human lncRNAs in 1,100 invasive breast carcinoma cases were
investigated and 292 differentially expressed lncRNAs were
identified, including 8 lncRNAs with expression alteration
frequency ≥10%. These results indicated that these 8 lncRNAs could
be potential biomarkers for invasive breast carcinoma
diagnosis.
Previously, studies have proven that lncRNAs could
predict the prognosis of breast cancer. He et al (25) used Cox regression analysis and a
robust likelihood-based survival model to screen prognosis-related
lncRNAs in 1,052 patients with breast cancer from TCGA database.
The group reported that 11 lncRNAs could classify the patients into
high and low risk groups with different overall survival rate. Sun
et al (26) demonstrated that
9 lncRNAs were associated with metastasis-free survival rate of
patients with breast cancer by analyzing the expression profiles of
lncRNAs in 916 breast cancer cases from the Gene Expression Omnibus
(GEO) database. Liu et al (27) investigated the prognostic value of
2,730 lncRNAs in ~1,000 invasive breast carcinoma cases from the
cBioPortal database, finding 11 lncRNAs with copy number
alterations, 4 lncRNAs with aberrant expression levels associated
with poor overall survival and 9 lncRNAs which could predict
recurrence of breast cancer. In addition, Liu et al
demonstrated that lncRNAs could also predict better prognosis of
breast cancer. Vishnubalaji et al (28) analyzed the lncRNA expression profiles
of 837 patients with invasive breast cancer and 105 healthy
patients from TCGA database, identifying 6 lncRNAs associated with
more favorable disease-free survival rate and 4 lncRNAs associated
with more favorable overall survival.
In the present study, altered expression levels of
10 lncRNAs (NORAD, SNHG1, LINC00654, FAM157A, DLEU2, LINC01559,
SPATA8, TCL6, LINC00632 and PWRN1) were significantly correlated
with shorter overall survival time in patients with invasive breast
carcinoma, suggesting that these 10 lncRNAs may be independent
prognostic markers for the disease. Zhou et al (29) demonstrated that lncRNA NORAD serves a
carcinogenic role in breast cancer. By analyzing the overall
survival time of 21 patients with breast cancer, Zhou et al
(29) demonstrated that patients
with high expression levels of NORAD had less favorable survival
compared with the low expression group, which supports the findings
of the present study. Studies have reported that increased SNHG1
expression levels could predict less favorable overall survival
rate in various types of cancer, such as gastric cancer,
hepatocellular carcinoma, colorectal cancer and non-small cell lung
cancer (30). In the present study,
among the 10 prognosis associated lncRNAs, DLEU2, TCL6 and PWRN1
were significantly correlated with shorter disease-free survival
time of patients with invasive breast carcinoma. Recently, it was
reported that DLEU2 was involved in several types of cancer and
DLEU2 exon 9 was an independent marker of poor prognosis in
patients with esophageal adenocarcinoma (31). Aberrant expression levels of TCL6
were involved in clear cell renal carcinoma by regulating the
PI3K/AKT pathway and PWRN1 inhibits gastric cancer cell
proliferation and metastasis via p53 signaling pathway (32,33).
For a better understanding of the 10 lncRNAs in the
present study, further investigation of the underlying molecular
mechanisms are necessary. Competitive endogenous RNA (ceRNA)
networks have been reported in several types of cancer, including
breast cancer. For example, lncRNA CDC6 was upregulated in breast
cancer tissues and its high expression levels were associated with
more advanced clinical stages of patients with breast cancer
(34). In addition, overexpression
of CDC6 promoted proliferation and migration of breast cancer cells
by directly sponging of miR-215 (34). The present study constructed a
lncRNA-miRNA-mRNA regulatory network, which was composed of 10
lncRNAs, 88 miRNAs and 322 mRNAs. Overall, 11 target mRNAs (EMC2,
TFB2M, ENY2, RAD54B, ANGEL2, RBM34, AGO2, GON4L, NPLOC4, ZFP41 and
SSR2) were aberrantly expressed in ≥20% patients. Previously,
several target mRNAs, such as GON4L, MDM4 and SLC9A3R1, were
reported to be involved in cancer-related signaling pathways
(35–37). For instance, MDM4, a regulator of p53
tumor suppressor protein, suppressed the activity of p53 to promote
tumor development and some molecular agents such as SJ-172550 and
XI-006 have been developed to eliminate the inhibition of p53 by
MDM4 (38). The findings of the
present study indicated that the target mRNAs could be potential
diagnostic markers or therapeutic targets for invasive breast
carcinoma.
lncRNA-protein interactions and lncRNA-protein
complexes serve important roles in a variety of biological
processes and abnormal expression levels of RNA-binding proteins
can contribute to tumor development (39). lncRNA HULC was reported to function
as an oncogene and interact with the ATG7 protein in epithelial
ovarian carcinoma, wherein overexpression of HULC promoted ovarian
carcinoma cells proliferation, migration and invasion by
suppressing mRNA and protein expression of ATG7 (40). In the present study, the proteins
which could interact with the 10 lncRNAs were predicted and there
were 25 potential target proteins, including CHIC1, RB1CC1 and
SCRIB. Previous findings have shown that these target proteins are
involved in several cancer-related pathways, including the TGF-β,
RB1 and ERK pathways (41–43). Therefore, the 10 lncRNAs identified
in the present study may be potentially novel targets for invasive
breast carcinoma therapy. To better understand the function of
these lncRNAs and provide further insights into the ceRNA and
lncRNA-protein regulatory networks, function enrichment analysis
was performed on the 322 target mRNAs and 25 target proteins,
reporting that the targets were part of a number of functional
classes, including ‘transcription’, ‘regulation of transcription’,
‘translation’, ‘RNA processing’, ‘mRNA splicing and protein
processing’.
In conclusion, the present study analyzed the
association between lncRNA expression level alterations and
prognosis of 1,100 patients with invasive breast carcinoma,
identifying 10 prognosis-related lncRNAs, whose expression level
alterations predicted lower overall survival rate and shorter
overall survival time. Therefore, therapeutically targeting these
lncRNAs might be effective in improving the treatment of invasive
breast carcinoma. To further understand the molecular basis of the
lncRNAs, a regulatory network was constructed and the target mRNAs
and proteins were predicted, of which some targets were associated
with well-known signaling pathways. Overall, this regulatory
network could identify novel targets to aid in the development of
new drugs to improve the efficacy of breast cancer therapy.
However, breast cancer is divided into four main subtypes: Luminal
A, luminal B, ERBB2 positive and triple negative, and gene
expression profiles are not the same for all these subtypes
(44). As all these four subtypes
were included in the present study, it would be important to
explore the differentially expressed lncRNAs in each subtype of
breast cancer, allowing for the identification of more diagnostic
and prognostic biomarkers. In the present study, the relevance of
lncRNA expression levels to invasive breast carcinoma were based on
RNA sequencing (RNA-Seq) data, while in some other databases, the
cancer-related lncRNAs are identified using microarrays (45). For example, the lnCAR database
extracts clinical information and gene expression microarray data
from the GEO database (46). RNA-Seq
and microarrays are two of the most common methods of transcript
expression detection. Microarrays are rapid, accurate, sensitive,
cost-effective and specific to detect transcript expressions, and
RNA-Seq is a high-throughput sequencing technology, which can be
used to quantify, profile, and discover RNA transcripts, and the
transcripts can be mapped to the reference genome to get
comprehensive genetic information (47). Due to different analysis methods,
heterogeneity of samples and different data processing algorithms,
the results obtained from different databases may differ, therefore
the results of the present study need to be further validated using
in vitro and in vivo experiments. The primary
limitation of the study present was the lack of validation of the
findings in vitro and in vivo. To confirm the
prognostic role of the lncRNAs, expression levels of the lncRNAs in
a number of patients with invasive breast carcinoma with follow-up
records should be analyzed. To validate the underlying molecular
mechanisms of the lncRNAs, a luciferase reporter assay should be
performed and lncRNA knockdown cell models and a tumor xenograft
model should also be established to further identify the function
of the lncRNAs (48,49).
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YH and YS searched the literature and designed the
study; XG screened the lncRNAs; YD and YS constructed the
interaction networks and performed the functional analysis; XX
reviewed the data and drafted the manuscript. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Colzani E, Liljegren A, Johansson AL,
Adolfsson J, Hellborg H, Hall PF and Czene K: Prognosis of patients
with breast cancer: Causes of death and effects of time since
diagnosis, age, and tumor characteristics. J Clin Oncol.
29:4014–4021. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Avgeris M, Tsilimantou A, Levis PK, Tokas
T, Sideris DC, Stravodimos K, Ardavanis A and Scorilas A: Loss of
GAS5 tumour suppressor lncRNA: An independent molecular cancer
biomarker for short-term relapse and progression in bladder cancer
patients. Brit J Cancer. 119:1477–1486. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chakraborty S, Andrieux G, Hasan AMM,
Ahmed M, Hosen MI, Rahman T, Hossain MA and Boerries M: Harnessing
the tissue and plasma lncRNA-peptidome to discover peptide-based
cancer biomarkers. Sci Rep. 9:123222019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yarmishyn AA, Batagov AO, Tan JZ, Sundaram
GM, Sampath P, Kuznetsov VA and Kurochkin IV: HOXD-AS1 is a novel
lncRNA encoded in HOXD cluster and a marker of neuroblastoma
progression revealed via integrative analysis of noncoding
transcriptome. BMC Genomics. (15 Suppl 9):S72014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sakaue S, Hirata J, Maeda Y, Kawakami E,
Nii T, Kishikawa T, Ishigaki K, Terao C, Suzuki K, Akiyama M, et
al: Integration of genetics and miRNA-target gene network
identified disease biology implicated in tissue specificity.
Nucleic Acids Res. 46:11898–11909. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fu X, Mao X, Wang Y, Ding X and Li Y:
Let-7c-5p inhibits cell proliferation and induces cell apoptosis by
targeting ERCC6 in breast cancer. Oncol Rep. 38:1851–1856. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hausser J, Syed AP, Bilen B and Zavolan M:
Analysis of CDS-located miRNA target sites suggests that they can
effectively inhibit translation. Genome Res. 23:604–615. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shuwen H, Qing Z, Yan Z and Xi Y:
Competitive endogenous RNA in colorectal cancer: A systematic
review. Gene. 645:157–162. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Neumann P, Jaé N, Knau A, Glaser SF,
Fouani Y, Rossbach O, Kruger M, John D, Bindereif A, Grote P, et
al: The lncRNA GATA6-AS epigenetically regulates endothelial gene
expression via interaction with LOXL2. Nat Commun. 9:2372018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cerami E, Gao J, Dogrusoz U, Gross BE,
Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, et
al: The cBio cancer genomics portal: An open platform for exploring
multidimensional cancer genomics data. Cancer Discov. 2:401–404.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gao J, Aksoy BA, Dogrusoz U, Dresdner G,
Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, et al:
Integrative analysis of complex cancer genomics and clinical
profiles using the cBioPortal. Sci Signal. 6:pl12013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang da W, Sherman BT and Lempicki RA:
Systematic and integrative analysis of large gene lists using DAVID
bioinformatics resources. Nat Protoc. 4:44–57. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang DW, Sherman BT, Tan Q, Kir J, Liu D,
Bryant D, Guo Y, Stephens R, Baseler MW, Lane HC, et al: DAVID
Bioinformatics Resources: Expanded annotation database and novel
algorithms to better extract biology from large gene lists. Nucleic
Acids Res. 35:W169–W175. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chang TH, Huang HY, Hsu JB, Weng SL, Horng
JT and Huang HD: An enhanced computational platform for
investigating the roles of regulatory RNA and for identifying
functional RNA motifs. BMC Bioinformatics. 14 (Suppl):S42013.
View Article : Google Scholar
|
|
16
|
Agarwal V, Bell GW, Nam JW and Bartel DP:
Predicting effective microRNA target sites in mammalian mRNAs.
ELife. 4:2015. View Article : Google Scholar
|
|
17
|
Shannon P, Markiel A, Ozier O, Baliga NS,
Wang JT, Ramage D, Amin N, Schwikowski B and Ideker T: Cytoscape: A
software environment for integrated models of biomolecular
interaction networks. Genome Res. 13:2498–2504. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lang B, Armaos A and Tartaglia GG: RNAct:
Protein-RNA interaction predictions for model organisms with
supporting experimental data. Nucleic Acids Res. 47:D601–D606.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Guo J, Li P, Liu X and Li Y: NOTCH
signaling pathway and non-coding RNAs in cancer. Pathol Res Pract.
215:1526202019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Youness RA and Gad MZ: Long non-coding
RNAs: Functional regulatory players in breast cancer. Non-coding
RNA Res. 4:36–44. 2019. View Article : Google Scholar
|
|
21
|
Kazan H, Ray D, Chan ET, Hughes TR and
Morris Q: RNAcontext: A new method for learning the sequence and
structure binding preferences of RNA-binding proteins. PLoS Comput
Boil. 6:e10008322010. View Article : Google Scholar
|
|
22
|
Weir BA, Woo MS, Getz G, Perner S, Ding L,
Beroukhim R, Lin WM, Province MA, Kraja A, Johnson LA, et al:
Characterizing the cancer genome in lung adenocarcinoma. Nature.
450:893–898. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sanchez Calle A, Kawamura Y, Yamamoto Y,
Takeshita F and Ochiya T: Emerging roles of long non-coding RNA in
cancer. Cancer Sci. 109:2093–2100. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gu J, Wang Y, Wang X, Zhou D, Shao C, Zhou
M and He Z: Downregulation of lncRNA GAS5 confers tamoxifen
resistance by activating miR-222 in breast cancer. Cancer Lett.
434:1–10. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
He Y, Li X, Meng Y, Fu S, Cui Y, Shi Y and
Du H: A prognostic 11 long noncoding RNA expression signature for
invasive breast carcinoma. J Cell Biochem. 120:16692–16702. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sun J, Chen X, Wang Z, Guo M, Shi H, Wang
X, Cheng L and Zhou M: A potential prognostic long non-coding RNA
signature to predict metastasis-free survival of breast cancer
patients. Sci Rep. 5:165532015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu H, Li J, Koirala P, Ding X, Chen B,
Wang Y, Wang Z, Wang C, Zhang X and Mo YY: Long non-coding RNAs as
prognostic markers in human breast cancer. Oncotarget.
7:20584–20596. 2016.PubMed/NCBI
|
|
28
|
Vishnubalaji R, Shaath H, Elkord E and
Alajez NM: Long non-coding RNA (lncRNA) transcriptional landscape
in breast cancer identifies LINC01614 as non-favorable prognostic
biomarker regulated by TGFβ and focal adhesion kinase (FAK)
signaling. Cell Death Discov. 5:1092019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhou K, Ou Q, Wang G, Zhang W, Hao Y and
Li W: High long non-coding RNA NORAD expression predicts poor
prognosis and promotes breast cancer progression by regulating
TGF-β pathway. Cancer Cell Int. 19:632019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yu J, Yan Y, Hua C and Ming L:
Upregulation of lncRNA SNHG1 is associated with metastasis and poor
prognosis in cancers: A meta-analysis. Medicine. 98:e151962019.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ma W, Zhang CQ, Dang CX, Cai HY, Li HL,
Miao GY, Wang JK and Zhang LJ: Upregulated long-non-coding RNA
DLEU2 exon 9 expression was an independent indicator of unfavorable
overall survival in patients with esophageal adenocarcinoma. Biomed
Pharmacother. 113:1086552019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen Z, Ju H, Yu S, Zhao T, Jing X, Li P,
Jia J, Li N, Tan B and Li Y: Prader-Willi region non-protein coding
RNA 1 suppressed gastric cancer growth as a competing endogenous
RNA of miR-425-5p. Clin Sci (Lond). 132:1003–1019. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Su H, Sun T, Wang H, Shi G, Zhang H, Sun F
and Ye D: Decreased TCL6 expression is associated with poor
prognosis in patients with clear cell renal cell carcinoma.
Oncotarget. 8:5789–5799. 2017.PubMed/NCBI
|
|
34
|
Kong X, Duan Y, Sang Y, Li Y, Zhang H,
Liang Y, Liu Y, Zhang N and Yang Q: lncRNA-CDC6 promotes breast
cancer progression and function as ceRNA to target CDC6 by sponging
microRNA-215. J Cell Physiol. 234:9105–9117. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xia F, Jin P, Ding Y, Li F and Shi C:
GON4L drives nasopharyngeal carcinoma growth and proliferation
through regulation of β-catenin/wnt singling pathway. Biomed Res.
28:4348–4353. 2017.
|
|
36
|
Lam S, Lodder K, Teunisse AF, Rabelink MJ,
Schutte M and Jochemsen AG: Role of Mdm4 in drug sensitivity of
breast cancer cells. Oncogene. 29:2415–2426. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu H, Ma Y, He HW, Wang JP, Jiang JD and
Shao RG: SLC9A3R1 stimulates autophagy via BECN1 stabilization in
breast cancer cells. Autophagy. 11:2323–2334. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li Q and Lozano G: Molecular pathways:
Targeting Mdm2 and Mdm4 in cancer therapy. Clin Cancer Res.
19:34–41. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Busà R, Paronetto MP, Farini D,
Pierantozzi E, Botti F, Angelini DF, Attisani F, Vespasiani G and
Sette C: The RNA-binding protein Sam68 contributes to proliferation
and survival of human prostate cancer cells. Oncogene.
26:4372–4382. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chen S, Wu DD, Sang XB, Wang LL, Zong ZH,
Sun KX, Liu BL and Zhao Y: The lncRNA HULC functions as an oncogene
by targeting ATG7 and ITGB1 in epithelial ovarian carcinoma. Cell
Death Dis. 8:e31182017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Souchelnytskyi S: Proteomics of TGF-beta
signaling and its impact on breast cancer. Expert Rev Proteomics.
2:925–935. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chano T, Ikebuchi K, Ochi Y, Tameno H,
Tomita Y, Jin Y, Inaji H, Ishitobi M, Teramoto K, Nishimura I, et
al: RB1CC1 activates RB1 pathway and inhibits proliferation and
cologenic survival in human cancer. PLoS One. 5:e114042010.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Young LC, Hartig N, Muñoz-Alegre M,
Oses-Prieto JA, Durdu S, Bender S, Vijayakumar V, Vietri Rudan M,
Gewinner C, Henderson S, et al: An MRAS, SHOC2, and SCRIB complex
coordinates ERK pathway activation with polarity and tumorigenic
growth. Mol Cell. 52:679–692. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Mathias C, Zambalde EP, Rask P, Gradia DF
and de Oliveira JC: Long non-coding RNAs differential expression in
breast cancer subtypes: What do we know? Clin Genet. 95:558–568.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Klinge CM: Non-coding RNAs in breast
cancer: Intracellular and intercellular communication. Non-Coding
RNA. 4(pii): E402018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zheng Y, Xu Q, Liu M, Hu H, Xie Y, Zuo Z
and Ren J: lnCAR: A comprehensive resource for lncRNAs from cancer
arrays. Cancer Res. 79:2076–2083. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chen L, Sun F, Yang X, Jin Y, Shi M, Wang
L, Shi Y, Zhan C and Wang Q: Correlation between RNA-Seq and
microarrays results using TCGA data. Gene. 628:200–204. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zheng S, Li M, Miao K and Xu H: SNHG1
contributes to proliferation and invasion by regulating miR-382 in
breast cancer. Cancer Manag Res. 11:5589–5598. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhang YL, Li XB, Hou YX, Fang NZ, You JC
and Zhou QH: The lncRNA XIST exhibits oncogenic properties via
regulation of miR-449a and Bcl-2 in human non-small cell lung
cancer. Acta Pharmacol Sin. 38:371–381. 2017. View Article : Google Scholar : PubMed/NCBI
|