Introduction
According to global cancer incidence and mortality
statistics in 2012, lung cancer has remained the most common
malignant tumor type worldwide and has accounted for the highest
number of cancer-associated mortalities (1). In 2015, >4 million new cases of
cancer and ~3 million cancer-associated mortalities occurred in
China, including ~733,000 new lung cancer cases and 610,000 lung
cancer-associated deaths (2). The
average 5-year survival rate for all cases of lung cancer combined
is just 16.8% (3), while it is 55%
for localized lung cancers, 27% for regional metastasis and 4% for
distant metastasis (4). However, the
majority of patients with lung cancer are diagnosed at an
intermediate or late stage and, thus, have missed the best
opportunity to be cured (4).
Therefore, to improve the overall survival rate of patients with
lung cancer, it is important to focus on early diagnosis and
treatment of lung cancer.
Recent advancements in the promising field of
epigenetics have identified a strong association between cancer and
epigenetics. DNA methylation is one of the earliest and most
important types of epigenetic modification and has an important
role in regulating growth, gene expression and genomic stability
(5). In previous years, numerous
studies have demonstrated that hypermethylation of CpG islands is
associated with gene silencing and is an important molecular change
during the development of cancer (6,7). In the
early stages of cancer, even prior to imaging scans, abnormal DNA
methylation may be detected. Therefore, analysis of DNA methylation
may be a powerful tool for early diagnosis of lung cancer (8).
Herman et al (9) described conventional
methylation-specific PCR (cMSP) that is able to rapidly assess the
methylation status of the majority of CpG sites within a CpG
island. This simple and sensitive method is currently the most
widely worldwide. However, during cMSP, bisulfite
modification-induced DNA damage and degradation can markedly reduce
the sensitivity of methylation detection. As technology advances,
quantitative PCR (qPCR) may replace conventional PCR and qPCR is
more sensitive and specific compared with cMSP and has a reduced
likelihood for operational contamination (10). Hulbert et al (11) modified the magnetic bead method to
extract DNA, reduced the degradation of DNA during the bisulfite
process and combined it with qPCR for detection with higher
sensitivity and specificity. This process was named
methylation-on-beads followed by quantitative methylation-specific
PCR (MOB-qMSP).
Cysteine dioxygenase type 1 (CDO1) is a mammalian
non-heme iron enzyme, the major functions of which are regulation
of cysteine levels and participation in metabolic pathways of
compounds, including pyruvate and taurine (12). CDO1 is also a tumor suppressor enzyme
and previous studies have demonstrated that CDO1 gene promoter
methylation leads to silencing of this gene in the development of
various types of cancer, including breast cancer (13), hepatocellular cancer (14), gallbladder cancer (15), colorectal cancer (16), gastric cancer (16), prostate cancer (17) and esophageal squamous cell carcinoma
(18). In studies investigating lung
cancer, methylation of the CDO1 promoter has been observed; Feng
et al (19) confirmed the
association of this with non-small cell lung cancer (NSCLC) and
Hulbert et al (11) indicated
that methylation of the CDO1 promoter has good sensitivity and
specificity for detecting lung cancer. However, the vast majority
of research available is based on European or American populations
and only few studies have been performed among Asian patients. To
the best of our knowledge, the present study was the first to use
MOB-qMSP to detect CDO1 methylation in China, providing an
important reference for the identification of CDO1 gene methylation
in Asian patients with NSCLC.
Materials and methods
Patients and samples
In the present study, 42 patients diagnosed with
NSCLC were included. The cohort included 30 males and 12 females
with a mean age of 60 years (range, 33–83 years). All patients were
enrolled from the Department of Thoracic Surgery, The Second
Xiangya Hospital of Central South University (Changsha, China)
between August 2017 and January 2018. Prior to surgery, all
patients received assessments including CT scan of the chest, MRI
scan of the brain, color Doppler ultrasound of the abdomen and a
radionuclide bone scan. Surgical resection and pathological
analyses were performed in all patients and staging was performed
according to the most recent Tumor-Node-Metastasis (TNM) guidelines
(20). Of the 42 tumor tissue
samples, 25 were adenocarcinoma and 17 were squamous cell
carcinoma. The matched adjacent normal lung tissues were confirmed
by pathologists. The present study was approved by The Ethics
Committee of the Second Xiangya Hospital (Changsha, China) and
written informed consent was provided from the patients. Tumor and
normal tissues were collected immediately after excision and stored
at −80°C.
DNA extraction and methylation
analysis
Two methods were used for DNA extraction from tissue
and bisulfite conversion (kits were used according to the
manufacturer's protocol: i) cMSP including the use of a traditional
DNA Purification kit (DP304 TIANamp Genomic DNAkit, Qiagen China
Co., Ltd.) and an EZ DNA Methylation kit (D5005 EZ DNA
Methylation-Gold kit, Zymo Research Corp.) and ii) MOB-qMSP,
including the use of silica super magnetic beads (cat. no. MD1471
MagneSil KF; Paramagnetic Particles; Promega Corporation) in the
process of DNA isolation and bisulfite conversion (11). The methylation and unmethylation CDO1
gene primers were designed using Methyl Primer Express 1.0 (Thermo
Fisher Scientific, Inc.) and a TaqMan probe and β-actin primers
were used (Table I).
 | Table I.PCR primers and TaqMan probes for
conventional methylation-specific PCR and methylation-on-beads
followed by quantitative methylation-specific PCR. |
Table I.
PCR primers and TaqMan probes for
conventional methylation-specific PCR and methylation-on-beads
followed by quantitative methylation-specific PCR.
| Gene | Primer sequence
(5′-3′) | Size, bp |
|---|
| CDO1,
methylation |
| 96 |
|
Forward |
TTTTTGGGACGTCGGAGATAAC |
|
|
Reverse |
CGAAAAAACCCTACGAACACG |
|
| CDO1,
unmethylation |
| 102 |
|
Forward |
GATTTTTGGGATGTTGGAGATAAT |
|
|
Reverse |
AAAACAAAAAAACCCTACAAACACA |
|
| CDO1 |
| 97 |
|
Forward |
AGGCGGGGAGATTTTGCG |
|
|
Reverse |
CCTAAAACGCCGAAAACAACG |
|
|
Probe |
6FAM-CGGTTTACGCGTATATTTTCGGTTTT-TAMRA |
|
| β-actin |
| 103 |
|
Forward |
TAGGGAGTATATAGGTTGGGGAAGTT |
|
|
Reverse |
AACACACAATAACAAACACAAATTCAC |
|
|
Probe |
6FAM-GTGGGGTGGTGATGGAGGAGGTTTAG-TAMRA |
|
DNA extraction from samples was performed using 30
mg of tissue and 40 µl Proteinase K (Qiagen China Co., Ltd.). After
digestion and multiple elution, CT Conversion reagent was used for
DNA bisulfite conversion to obtain the methylated DNA. cMSP was
performed in a 50-µl PCR mixture consisting of 9.25 µl Premix Ex
Taq (Takara Bio, Inc.), 1 µl forward primer, 1 µl reverse primer, 2
µl DNA and 36.75 µl water. The thermocycling conditions included 35
cycles of 98°C for 10 sec, 60°C for 30 sec and 72°C for 60 sec.
After amplification, each PCR product mixture was separated on a 2%
agarose gel, added nucleic acid gel stain (cat. no. GG1301; 500µl
GenGreen nucleic acid gel stain; Beijing Dingguo Changsheng
Biotechnology Co., Ltd.) and visualized under Blue Light Gel Imager
(wavelength, 440 nm; cat. no. G500312-0001; Sangon Biotech Co.,
Ltd.) in room temperature.
Modification technology-based magnetic beads were
used for DNA extraction and bisulfite conversion (11). For DNA extraction, 30 mg of tissue
was added to 300 µl Buffer AL and 40 µl Proteinase K (Qiagen China
Co., Ltd.), followed by incubation (50°C, overnight). After DNA
bisulfite conversion on magnetic beads, the methylated DNA was
obtained. MOB-qMSP was performed in 20-µl PCR mixture consisting of
10 µl Premix Ex Taq, 0.4 µl forward primer, 0.4 µl reverse primer,
0.8 µl probe, 0.4 µl ROX Reference Dye, 2 µl DNA and 6 µl water. An
ABI StepOnePlus Real-Time PCR system (Applied Biosystems; Thermo
Fisher Scientific, Inc.) was used for the reaction with the
following conditions: 95°C for 20 sec, 45 cycles at 95°C for 1 sec
and 60°C for 20 sec. After amplification, the results were directly
observed on the ABI StepOnePlus Real-Time PCR system.
Statistical analysis
All data were analyzed with SPSS 21.0 statistical
software (IBM Corp.). The rate of DNA methylation was assessed
using the χ2 or Fisher's exact test. The two detection
methods for CDO1 gene methylation were compared using McNemar's
test. κ statistics were used to evaluate the concordance between
the two methods. P<0.05 was considered to indicate statistically
significant difference.
Results
Patient characteristics
The general data and pathological features of the 42
patients are summarized in Table
II. Subjects with smoking indexes of ≥400 and <400 were
equally represented. Among the pathological features,
adenocarcinoma accounted for 62% of cases, while squamous cell
carcinoma accounted for 38% of cases. According to the TNM staging
system, 32 cases were in the early stages (I/II) and 10 cases were
in the late stages (III/IV). Regarding the degree of tumor
differentiation, there were 20 cases of high/moderate
differentiation and 22 of poor differentiation.
 | Table II.Demographic characteristics of 42
patients with NSCLC. |
Table II.
Demographic characteristics of 42
patients with NSCLC.
| Characteristic | n (%) |
|---|
| Age, years |
|
| Mean ±
standard deviation | 60±9.51 |
|
Range | 33-83 |
| Sex |
|
|
Male | 30 (71) |
|
Female | 12 (29) |
| Smoking index |
|
|
≥400 | 21 (50) |
|
<400 | 21 (50) |
| Histology |
|
|
Adenocarcinoma | 26 (62) |
|
Squamous carcinoma | 16 (38) |
| TNM stage |
|
|
I/II | 32 (76) |
|
III/IV | 10 (24) |
|
Differentiation |
|
|
High/moderate | 20 (48) |
|
Poor | 22 (52) |
In the present study, the promoter DNA methylation
levels of the CDO1 gene in tissues from 42 patients with NSCLC were
compared; in 32 cases, cMSP was used and MOB-qMSP was used in 31
cases. The results obtained with the two methods indicated that
CDO1 methylation was significantly higher in tumor tissues compared
with normal tissues. Of the 32 cases assessed using cMSP, CDO1
methylation was detected in 19 tumor tissues and in 3 normal lung
tissues. Of the 31 cases assessed using MOB-qMSP, CDO1 methylation
was detected in 22 tumor tissues and in 0 normal lung tissues
(Fig. 1; Table III). Furthermore, 21 pairs of
samples were assessed with cMSP and MOB-qMSP and the κ statistical
result of 0.47 indicated a low concordance between the two methods
for assessing promoter DNA methylation. In addition, MOB-qMSP had a
higher positive detection rate for CDO1 hypermethylation compared
with cMSP (Table IV).
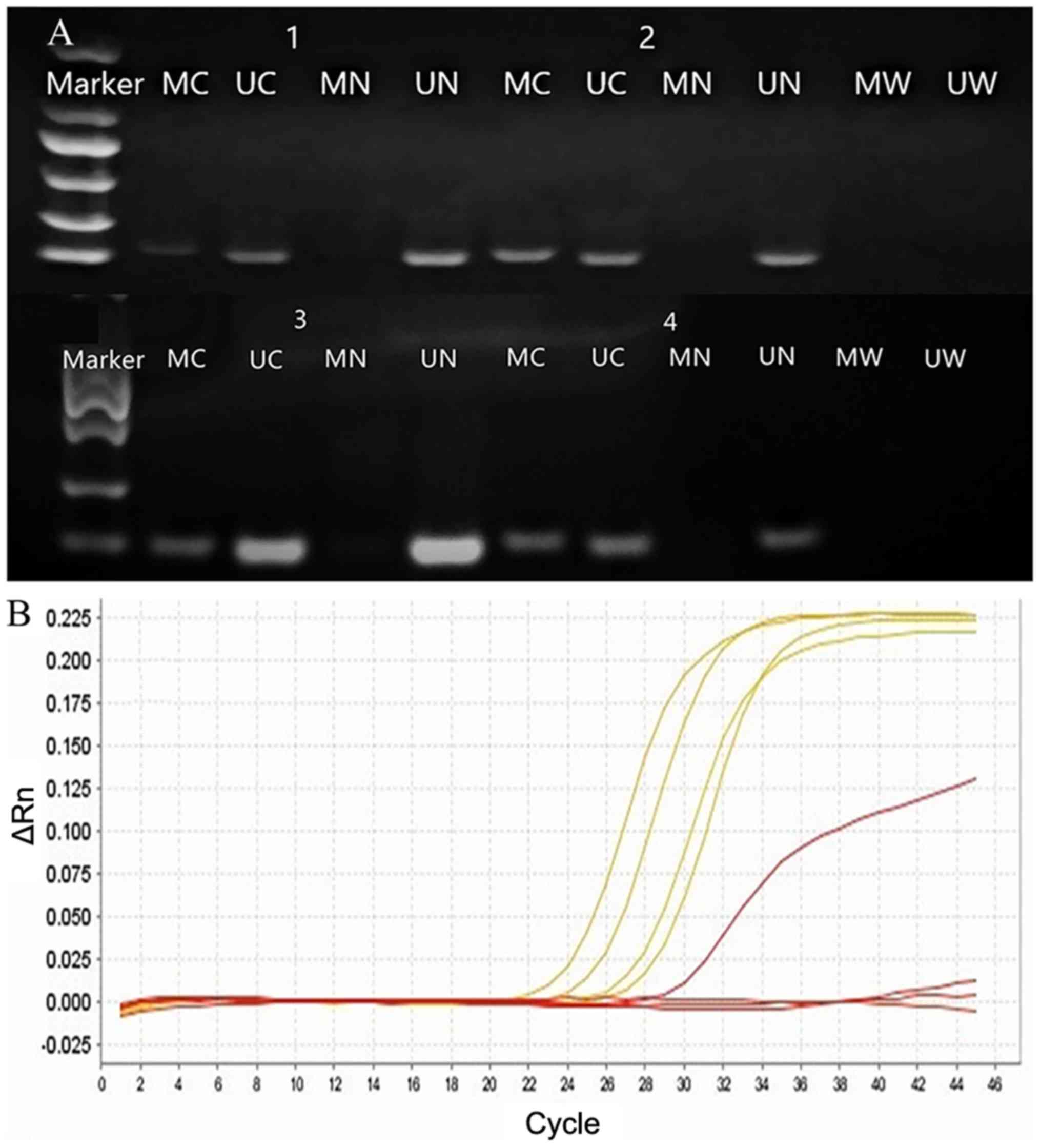 | Figure 1.CDO1 gene promoter methylation in
NSCLC was detected using gel electrophoresis and quantitative PCR.
Numbers 1–4 represent four groups of corresponding tissue samples
(tumor vs. normal lung tissue). The DL2000 DNA Marker contains six
discrete DNA fragments ranging in size from 100 bp to 2 kb (100,
250, 500, 750, 1,000 and 2,000 bp). (A) Amplification of
bisulfite-treated CDO1 from cancer and normal tissues. (B) β-actin,
yellow curve; CDO1 gene methylation in tumor tissue, red curve
above; CDO1 gene methylation in normal lung tissue, red curve at
the bottom. NSCLC, non-small cell lung cancer; CDO1, cysteine
dioxygenase type 1; MC or UC, methylation or unmethylation in tumor
tissue; MN or UN, methylation or unmethylation in normal lung
tissue; MW, methylation in water; UW, unmethylation in water. ΔRn,
normalized reporter value, fluorescence emission of the product at
each time point-fluorescence emission of the baseline. |
 | Table III.Promoter hypermethylation detection
using cMSP and MOB-qMSP in patients with NSCLC. |
Table III.
Promoter hypermethylation detection
using cMSP and MOB-qMSP in patients with NSCLC.
| Method | Tumor tissues, n
(%) | Normal tissues, n
(%) | P-value |
|---|
| cMSP |
|
Methylation | 19 (59.4) | 3
(9.4) | <0.001 |
|
Unmethylation | 13 (40.6) | 29 (90.6) |
|
| MOB-qMSP |
|
Methylation | 22 (71.0) | 0
(0.0) | <0.001 |
|
Unmethylation | 9
(29.0) | 31 (100.0) |
|
 | Table IV.Comparison of two methods to detect
CDO1 gene hypermethylation. |
Table IV.
Comparison of two methods to detect
CDO1 gene hypermethylation.
|
| MOB-qPCR |
|
|
|---|
|
|
|
|
|
|---|
| cMSP | Methylation | Unmethylation | P-value | κ |
|---|
| Methylation | 8 | 0 | 0.041 | 0.471 |
| Unmethylation | 6 | 7 |
|
|
Further analysis revealed that CDO1 gene
hypermethylation was significantly different between
highly/moderately and poorly differentiated tissues (P<0.05),
with the CDO1 methylation rate being higher in poorly
differentiated NSCLC tissue. However, no significant association
was observed regarding any of the other characteristics assessed
(Table V).
 | Table V.Association between promoter
hypermethylation of cysteine dioxygenase type 1 and clinical
characteristics. |
Table V.
Association between promoter
hypermethylation of cysteine dioxygenase type 1 and clinical
characteristics.
|
| cMSP | MOB-qMSP |
|---|
|
|
|
|
|---|
| Characteristic | M | U | M% | P-value | M | U | M% | P- value |
|---|
| Age, years |
|
|
|
0.149 |
|
|
|
0.054 |
|
≥60 | 13 | 5 | 72.2 |
| 14 | 2 | 87.5 |
|
|
<60 | 6 | 8 | 42.9 |
| 8 | 7 | 53.3 |
|
| Sex |
|
|
|
0.684 |
|
|
| >0.999 |
|
Male | 15 | 9 | 62.5 |
| 16 | 6 | 72.7 |
|
|
Female | 4 | 4 | 50.0 |
| 6 | 3 | 66.7 |
|
| Smoking index |
|
|
|
0.149 |
|
|
|
0.113 |
|
≥400 | 12 | 4 | 75.0 |
| 13 | 2 | 86.7 |
|
|
<400 | 7 | 9 | 43.8 |
| 9 | 7 | 56.3 |
|
| Histology |
|
|
|
0.713 |
|
|
| >0.999 |
|
LUAD | 11 | 9 | 55.0 |
| 14 | 6 | 70.0 |
|
|
LUSC | 8 | 4 | 66.7 |
| 8 | 3 | 72.7 |
|
| TNM stage |
|
|
|
0.703 |
|
|
| >0.999 |
|
I/II | 13 | 10 | 56.5 |
| 17 | 7 | 70.8 |
|
|
III/IV | 6 | 3 | 66.7 |
| 5 | 2 | 71.4 |
|
|
Differentiation |
|
|
| <0.001 |
|
|
|
0.045 |
|
High/moderate | 2 | 11 | 15.4 |
| 10 | 8 | 55.6 |
|
|
Poor | 17 | 2 | 89.5 |
| 12 | 1 | 92.3 |
|
Discussion
In somatic cells, abnormal hypermethylation of the
promoter region may lead to silencing and inactivation of tumor
suppressor genes, which is one of the important molecular changes
in tumor development. Increased levels of gene promoter methylation
may contribute to the initiation and progression of NSCLC,
indicating a close association between DNA methylation and NSCLC
(7). CDO1 gene hypermethylation has
been reported in various types of cancer and numerous studies have
assessed CDO1 gene promoter methylation levels in lung cancer
(21–23). CDO1 has been confirmed to be valuable
for the diagnosis of NSCLC (24).
Another study indicated that CDO1 may potentially serve as a
molecular biomarker for multiple human cancers (25).
cMSP is one of the most widely used techniques to
detect DNA methylation of a locus of interest; it is able to detect
the methylation status rapidly and with high sensitivity (26). In the process of DNA methylation,
high bisulfite concentrations lead to DNA degradation and
inappropriate conversion (27),
reducing the sensitivity of methylation detection. In the present
study, MOB-qMSP was also used to detect the promoter methylation of
the target gene whilst reducing the degradation of DNA during the
bisulfite conversion process. This method has higher sensitivity
and specificity compared with cMSP (11). In the present study, the results
obtained with the two methods were consistent, as both methods
demonstrated that the methylation rates of CDO1 in tumor tissues
from patients with NSCLC were significantly higher compared with
those in normal lung tissues (P<0.05). In addition, the two
methods were applied to assess 21 samples in parallel and the
results showed that MOB-qMSP had a higher positive detection rate
compared with MSP (71.0 vs. 59.4%). This may be due to the method
of combining DNA with magnetic beads prior to bisulfite conversion,
which markedly reduces the degradation of DNA and increases the
sensitivity of detection (11).
Lung cancer is an age-associated disease (28–30) and
alterations in DNA methylation may be due to the harmful effects
exerted by tobacco smoking (31).
Levine et al (32) indicated
that the association between DNA methylation and lung cancer is
stronger among older patients and those who are current smokers.
Breitling et al (33)
suggested that DNA methylation serves a role in a variety of
smoking-associated outcomes. Although no statistically significant
differences were observed in the present study regarding the
association between age or smoking on CD01 methylation levels,
there still may be an association between these factors. The lack
of significance in the present study may be due to the study
limitations, such as a small sample size. High-quality studies with
a rational design, including large-scale controlled trials, are
required in the future.
In addition, in the present study, no significant
difference in CDO1 gene methylation was identified between
adenocarcinoma and squamous cell carcinoma, or between the early
and advanced stage, which was consistent with the results of Ooki
et al (23). However, it is
noteworthy that there were statistically significant differences in
CDO1 gene methylation between lung cancer tissues with
high/moderate and poor differentiation. This suggested that
methylation of the CDO1 gene may be an adjuvant marker for
evaluating the degree of malignancy of NSCLC; however, further
analysis is required to validate these results. For example, it
would be valuable to further verify the correlation between CDO1
hypermethylation and NSCLC using in vitro experiments.
In the present study, CDO1 gene promoter methylation
was more frequently observed in lung tumor tissues compared with
normal lung tissues and this high methylation frequency of the CDO1
gene was associated with the degree of differentiation of NSCLC. As
the degree of differentiation may be linked to the degree of
malignancy, CDO1 promoter hypomethylation may have value in
evaluating the prognosis of patients with NSCLC. However, further
high-quality studies including a larger sample size and regular
follow-ups are required to confirm these results.
In conclusion, the present study indicated that CDO1
promoter methylation has a role in NSCLC in the Chinese population
and that methylation levels are associated with the degree of
differentiation of NSCLC. MOB-qMSP has higher sensitivity and
specificity compared with cMSP and is more accurate method as it
reduces DNA degradation. If confirmed in further studies, detection
of CDO1 gene promoter methylation may be used as an adjunct to
NSCLC diagnosis, as well as identification of pathological
differentiation and guidance for the prognosis of patients with
NSCLC.
Acknowledgements
Not applicable.
Funding
This work was supported by Hunan Provincial Natural
Science Foundation of China (grant no. 2017JJ2364).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
CC and FY designed the study concept. WY, XW and MS
collected the data of patients. WY, YL, CC and BW analyzed the
data. WY drafted the manuscript. CC and AH provided technical and
material support. AH also participated in the acquisition of data.
CC, FY and AH reviewed or revised of manuscript. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by The Ethics
Committee of The Second Xiangya Hospital (Changsha, China; approval
no. 2014S006), and written informed consent was provided by all
patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wender R, Fontham ET, Barrera E Jr,
Colditz GA, Church TR, Ettinger DS, Etzioni R, Flowers CR, Gazelle
GS, Kelsey DK, et al: American Cancer Society lung cancer screening
guidelines. CA Cancer J Clin. 63:107–117. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Miller KD, Siegel RL, Lin CC, Mariotto AB,
Kramer JL, Rowland JH, Stein KD, Alteri R and Jemal A: Cancer
treatment and survivorship statistics, 2016. CA Cancer J Clin.
66:271–289. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sharma S, Kelly TK and Jones PA:
Epigenetics in cancer. Carcinogenesis. 31:27–36. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sandoval J, Peiró-Chova L, Pallardó FV and
García-Giménez JL: Epigenetic biomarkers in laboratory diagnostics:
Emerging approaches and opportunities. Expert Rev Mol Diagn.
13:457–471. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim Y and Kim D-H: CpG island
hypermethylation as a biomarker for the early detection of lung
cancer. Cancer Epigenetics. Humana Press. (NY, USA). 141–171. 2015.
View Article : Google Scholar
|
|
8
|
Leygo C, Williams M, Jin HC, Chan MWY, Chu
WK, Grusch M and Cheng YY: DNA methylation as a noninvasive
epigenetic biomarker for the detection of cancer. Dis Markers.
2017:37265952017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Herman JG, Graff JR, Myöhänen S, Nelkin BD
and Baylin SB: Methylation-specific PCR: A novel PCR assay for
methylation status of CpG islands. Proc Natl Acad Sci USA.
93:9821–9826. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Navarro E, Serrano-Heras G, Castaño MJ and
Solera J: Real-time PCR detection chemistry. Clin Chim Acta.
439:231–250. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hulbert A, Jusue-Torres I, Stark A, Chen
C, Rodgers K, Lee B, Griffin C, Yang A, Huang P, Wrangle J, et al:
Early Detection of Lung Cancer Using DNA Promoter Hypermethylation
in Plasma and Sputum. Clin Cancer Res. 23:1998–2005. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Brait M, Ling S, Nagpal JK, Chang X, Park
HL, Lee J, Okamura J, Yamashita K, Sidransky D and Kim MS: Cysteine
dioxygenase 1 is a tumor suppressor gene silenced by promoter
methylation in multiple human cancers. PLoS One. 7:e449512012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Minatani N, Waraya M, Yamashita K, Kikuchi
M, Ushiku H, Kojo K, Ema A, Nishimiya H, Kosaka Y, Katoh H, et al:
Prognostic significance of promoter DNA hypermethylation of
cysteine dioxygenase 1 (CDO1) gene in primary breast cancer. PLoS
One. 11:e01448622016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Choi JI, Cho E-H, Kim SB, Kim R, Kwon J,
Park M, Shin HJ, Ryu HS, Park SH and Lee KH: Promoter methylation
of cysteine dioxygenase type 1: Gene silencing and tumorigenesis in
hepatocellular carcinoma. Ann Hepatobiliary Pancreat Surg.
21:181–187. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Igarashi K, Yamashita K, Katoh H, Kojima
K, Ooizumi Y, Nishizawa N, Nishiyama R, Kawamata H, Tajima H, Kaizu
T, et al: Prognostic significance of promoter DNA hypermethylation
of the cysteine dioxygenase 1 (CDO1) gene in primary gallbladder
cancer and gallbladder disease. PLoS One. 12:e01881782017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vedeld HM, Andresen K, Eilertsen IA,
Nesbakken A, Seruca R, Gladhaug IP, Thiis-Evensen E, Rognum TO,
Boberg KM and Lind GE: The novel colorectal cancer biomarkers CDO1,
ZSCAN18 and ZNF331 are frequently methylated across
gastrointestinal cancers. Int J Cancer. 136:844–853. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Meller S, Zipfel L, Gevensleben H,
Dietrich J, Ellinger J, Majores M, Stein J, Sailer V, Jung M,
Kristiansen G, et al: CDO1 promoter methylation is associated with
gene silencing and is a prognostic biomarker for biochemical
recurrence-free survival in prostate cancer patients. Epigenetics.
11:871–880. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kwon J, Park M, Kim JH, Lee HW, Kang MC
and Park JH: Epigenetic regulation of the novel tumor suppressor
cysteine dioxygenase 1 in esophageal squamous cell carcinoma.
Tumour Biol. 36:7449–7456. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Feng N, Wang Y, Zheng M, Yu X, Lin H, Ma
RN, Shi O, Zheng X, Gao M, Yu H, et al: Genome-wide analysis of DNA
methylation and their associations with long noncoding RNA/mRNA
expression in non-small-cell lung cancer. Epigenomics. 9:137–153.
2017. View Article : Google Scholar
|
|
20
|
Goldstraw P, Chansky K, Crowley J,
Rami-Porta R, Asamura H, Eberhardt WE, Nicholson AG, Groome P,
Mitchell A, Bolejack V, et al; International Association for the
Study of Lung Cancer Staging and Prognostic Factors Committee,
Advisory Boards, Participating Institutions; International
Association for the Study of Lung Cancer Staging and Prognostic
Factors Committee Advisory Boards and Participating Institutions, .
The IASLC Lung Cancer Staging Project: Proposals for Revision of
the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the
TNM Classification for Lung Cancer. J Thorac Oncol. 11:39–51. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kwon YJ, Lee SJ, Koh JS, Kim SH, Lee HW,
Kang MC, Bae JB, Kim YJ and Park JH: Genome-wide analysis of DNA
methylation and the gene expression change in lung cancer. J Thorac
Oncol. 7:20–33. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wrangle J, Machida EO, Danilova L, Hulbert
A, Franco N, Zhang W, Glöckner SC, Tessema M, Van Neste L, Easwaran
H, et al: Functional identification of cancer-specific methylation
of CDO1, HOXA9, and TAC1 for the diagnosis of lung cancer. Clin
Cancer Res. 20:1856–1864. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ooki A, Maleki Z, Tsay JJ, Goparaju C,
Brait M, Turaga N, Nam HS, Rom WN, Pass HI, Sidransky D, et al: A
Panel of Novel Detection and Prognostic Methylated DNA Markers in
Primary Non-Small Cell Lung Cancer and Serum DNA. Clin Cancer Res.
23:7141–7152. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Diaz-Lagares A, Mendez-Gonzalez J, Hervas
D, Saigi M, Pajares MJ, Garcia D, Crujerias AB, Pio R, Montuenga
LM, Zulueta J, et al: A Novel Epigenetic Signature for Early
Diagnosis in Lung Cancer. Clin Cancer Res. 22:3361–3371. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Brait M, Ling S, Nagpal JK, Chang X, Park
HL, Lee J, Okamura J, Yamashita K, Sidransky D and Kim MS: Cysteine
dioxygenase 1 is a tumor suppressor gene silenced by promoter
methylation in multiple human cancers. PLoS One. 7:e449512012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ramalho-Carvalho J, Henrique R and
Jerónimo C: Methylation-specific PCR. DNA Methylation Protocols.
1708. Tost J: Springer, Humana Press; New York, NY: pp. 447–472.
2018, View Article : Google Scholar
|
|
27
|
Holmes EE, Jung M, Meller S, Leisse A,
Sailer V, Zech J, Mengdehl M, Garbe LA, Uhl B, Kristiansen G, et
al: Performance evaluation of kits for bisulfite-conversion of DNA
from tissues, cell lines, FFPE tissues, aspirates, lavages,
effusions, plasma, serum, and urine. PLoS One. 9:e939332014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
López-Otín C, Blasco MA, Partridge L,
Serrano M and Kroemer G: The hallmarks of aging. Cell.
153:1194–1217. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Derhovanessian E, Solana R, Larbi A and
Pawelec G: Immunity, ageing and cancer. Immun Ageing. 5:112008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rodier F and Campisi J: Four faces of
cellular senescence. J Cell Biol. 192:547–556. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bakulski KM and Fallin MDJE: Epigenetic
epidemiology: Promises for public health research. Environ Mol
Mutagen. 55:171–183. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Levine ME, Hosgood HD, Chen B, Absher D,
Assimes T and Horvath S: DNA methylation age of blood predicts
future onset of lung cancer in the women's health initiative. Aging
(Albany NY). 7:690–700. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Breitling LP, Yang R, Korn B, Burwinkel B
and Brenner H: Tobacco-smoking-related differential DNA
methylation: 27K discovery and replication. Am J Hum Genet.
88:450–457. 2011. View Article : Google Scholar : PubMed/NCBI
|















