Introduction
Pituitary adenomas are relatively common and account
for 10–15% of all intracranial tumors, with an incidence of ~3
cases per 100,000 people globally (1–4).
Meningioma is one of the most common intracranial tumor types, with
an estimated incidence of 7.86 cases per 100,000 people per year,
~80% of which are benign and World Health Organization
classification grade I (5–8). In contrast, pituitary adenoma
associated with meningioma (PAM) is a rare disease, with only 33
cases described before 2017 (9). The
tumorigenesis of PAM remains unknown and there are currently no
known epidemiological or well-characterized genetic associations
between meningioma and pituitary adenoma.
MEN1 is located on chromosome 11q13 and is
composed of 10 exons that encode a 610 amino acid protein called
menin. Mutated forms of MEN1 include nonsense mutations,
missense mutations, frameshifts and insertions. Multiple endocrine
neoplasia type 1 (MEN-1) syndrome is an autosomal dominant disease
caused by germline MEN1 mutations, which lead to the
development of multi-focal neoplastic endocrine lesions of the
parathyroid glands, endocrine pancreas, duodenum, anterior
pituitary, and less commonly, the stomach, adrenal cortex, thymus
and lungs (10–12). In addition, various non-endocrine
lesions may occur in the skin, central nervous system (CNS) and
soft tissues. Asgharian et al (13) reported that meningioma may be a
component tumor of MEN-1, and mutations in the MEN1 gene may
participate in its pathogenesis. The present study revealed that
5/23 samples from patients with PAM harbored the recurrent germline
mutation MEN1 c.1523G>A; p.G508D. However, none of these
patients exhibited MEN-1 syndrome-associated symptoms other than
the presence of the pituitary adenoma.
Materials and methods
Patients
There were 8,197 patients with pituitary adenoma
admitted to Beijing Tiantan Hospital (Beijing, China) between
January 1, 2005 and December 31, 2017 who were retrospectively
reviewed. The average age was 44.17±13.1 years (ranging from 4–84
years), with 4008 male patients (48.9%) and 4189 female patients
(51.1%), M/F ratio of 1:1.05). All patients were classified
according to preoperative imaging, including hormone, plain and
enhanced head magnetic resonance imaging, thin layer skull base
computed tomography scanning and three-dimensional reconstruction.
Patients who were diagnosed with meningioma and pituitary adenoma
simultaneously or successively were included in the present study.
The clinical data of patients with MEN-1 were referred by a
previous study (14). A total of 119
patients with sporadic pituitary adenoma (SPA) were selected from
the 8,197 patients. They were recruited randomly from Beijing
Tiantan Hospital between January 1, 2005 and December 31, 2017.
Written informed consent was obtained from all of these 119
patients with SPA.
The present study was conducted in accordance with
established ethical guidelines as outlined in the Declaration of
Helsinki and was approved by the Ethics Committee of Beijing
Tiantan Hospital. Written informed consent was obtained from all
participants.
Tissue samples and histology
Tissue samples were obtained from the department of
neurosurgery, Beijing Tiantan Hospital. Fresh tumor tissue samples
were immediately snap-frozen in liquid nitrogen and stored at
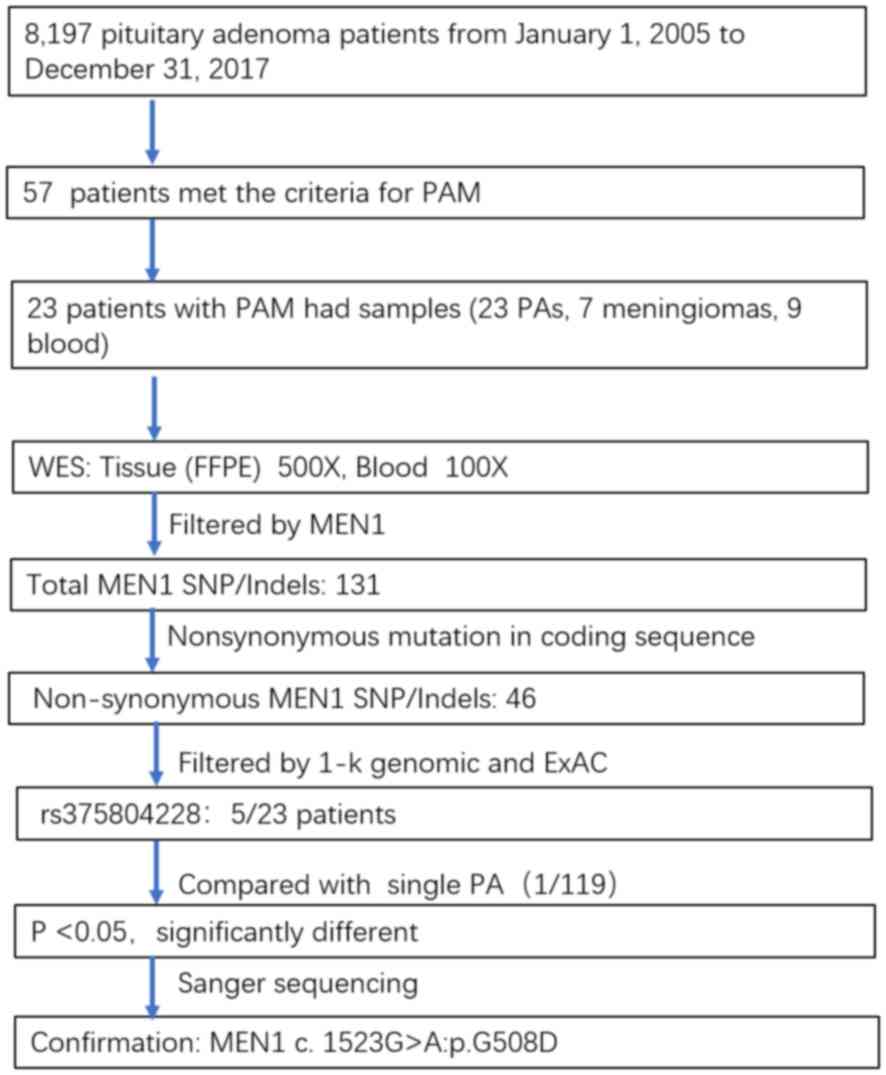
−80°C. A total of 23 patients with PAM (23 pituitary adenoma
tissues, nine peripheral blood samples and seven meningioma
tissues) were subjected to next generation and Sanger sequencing
(Table SI).
Genomic DNA preparation and whole
exome sequencing
The genomic DNA from blood and formalin-fixed and
paraffin-embedded (FFPE) samples was extracted using the DNeasy
blood and tissue kit (Qiagen, Inc.) and the GeneRead DNA FFPE kit
(Qiagen, Inc.), respectively, according to the manufacturer's
protocol. DNA degradation and contamination were assessed using a
1% agarose gel and the concentration was measured using a
Qubit® DNA Assay kit and a Qubit® 2.0
Flurometer (both Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocols. For whole exome sequencing (WES), the
genomic DNA from 23 patients with PAM, including 23 pituitary
adenoma samples, nine peripheral blood samples and seven meningioma
tissues, were fragmented using a S220 Focused-ultrasonicator
(Covaris, Inc.), with resultant library fragments 180–280 bp in
length, according to the manufacturer's protocol.
Sequence data quality control
The original fluorescence image files obtained from
the HiSeq X10 platform were transformed to short reads by base
calling and recorded in FASTQ format, which contained sequence
information and corresponding sequencing quality information. After
excluding reads containing adapter contamination and
low-quality/unrecognizable nucleotides, clean data were used for
downstream analyses. Additionally, the total read number,
sequencing error rate, percentage of reads with average quality
>20 or >30, and GC content distribution were calculated.
Read mapping and somatic genetic
alteration detection
Valid sequencing data were mapped onto the reference
human genome (UCSC hg19) using Burrows-Wheeler Aligner software to
obtain the original mapping results in BAM format (15). SAMtools version 1.0 (16), Picard version 1.111(1901)
(broadinstitute.github.io/picard) and GATK version 3.1–1-g07a4bf8
(17) were subsequently used to
process the BAM files, and to perform duplicate marking, local
realignment and base quality recalibration to generate the final
BAM files for computing the sequence coverage and depth.
The tumor and blood samples were subjected to WES
and the mean coverage was ~500× for tumor samples and ~100× for
matched blood samples. MuTect version 1.1.4 and Strelka version
1.0.1 were used to identify somatic single nucleotide variations
(SNVs) and small insertions and deletions (InDels), respectively,
in the paired tumor and normal blood samples (18,19).
Cloning frequency analysis
Pituitary adenoma, meningioma and blood samples from
one patient with PAM were used for cloning frequency analysis and
phylogenetic tree construction. The cloning evolutionary analysis
was based on the purity of the tumor samples, the change in the
copy number and the allele frequency of the somatic mutation, and
was used to calculate the cancer cell fraction (CCF) and to
identify tumor sample subgroups. PyClone is a hierarchical Bayesian
model that infers the cellular prevalence of each variant (the
proportion of tumor cells in a sample that contains the variant),
and clusters variants based on the covariance of these prevalence
estimates across multiple samples obtained from the same patient
(20). PyClone software (version
13.0) (20) was used to perform
clonality and evolutionary analyses based on the information
obtained on genetic somatic mutations and copy number variations
(CNVs) of the pituitary tumor and meningioma. For each variant, the
CCF was defined as variant allele fraction (VAF)=p × CCF/[CT × p +
CN (1-p)], where CT is the copy number of the tumor sample, CN is
the copy number of the matched normal blood sample and p is the
tumor purity. Clonal status was defined according to the confidence
interval of the CCF. For each somatic mutation, the VAF was
calculated using the number of reads supporting the variant allele
(Rmut) and the number of reads supporting the reference allele
[Rnorm; namely, VAF=Rmut/(Rmut + Rnorm)].
Phylogenetic tree construction
Phylogenetic trees for the multiple tumor samples
obtained from the same patient were constructed manually. Only
somatic non-synonymous mutations were considered and point
mutations and InDels were incorporated. Somatic non-synonymous
alterations that were shared in both pituitary adenoma and
meningioma in the same case were counted towards the trunk;
likewise, private mutations, those mutations only present in either
the pituitary adenoma or meningioma, were counted towards the
branches. The length of the trunk and branches were proportional to
the number of somatic non-synonymous mutations.
Sanger validation
MEN1 candidate point mutations identified
from exome sequencing were validated using Sanger sequencing.
Genomic sequences around candidate mutations were obtained from the
National Center for Biotechnology Information. Primers were
designed using Primer 3 software (version 0.4.0;
bioinfo.ut.ee/primer3–0.4.0) and were as follows: MEN1,
forward 5′-CCGTGAGTTGCAGCTTGATG-3′ and reverse
5′-CAACCTTGCTCTCACCTTGC-3′.
The DNA samples obtained from the 23 patients were
subjected to PCR to validate the candidate mutation MEN1
c.1523G>A; p.G508D. The PCR mixture contained 25 µl 2X TSINGKE
Master mix (blue) (TSINGKE) (http://www.tsingke.net/shop/), 22 µl double distilled
H2O, 1 µl 10 µM forward and reverse primer, and 1 µl
template DNA at 10 ng/µl, resulting in a final volume of 50 µl. The
thermocycling conditions were as follows: 5 min at 94°C; 10 cycles
of 30 sec at 94°C, 30 sec at 61°C and 1 min at 72°C; 30 cycles of
30 sec at 94°C, 30 sec at 56°C and 1 min at 72°C; and 3 min at
72°C.
Following amplification of the DNA sequences, Sanger
sequencing was performed on all 23 samples according to the TSINGKE
protocol. The sequencing traces were visualized using Codon Code
Aligner software version 6.0.2 (TSINGKE) to confirm the presence of
candidate mutations.
Statistical analysis
Statistical analyses were performed using SPSS
software (v20.0; IBM Corp.). Statistical comparisons of MEN1
mutation status were performed using Fisher's exact test. The
χ2 test was used to analyze ordinal variables and
unpaired Student's t-test was used to test continuous data.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Clinical features
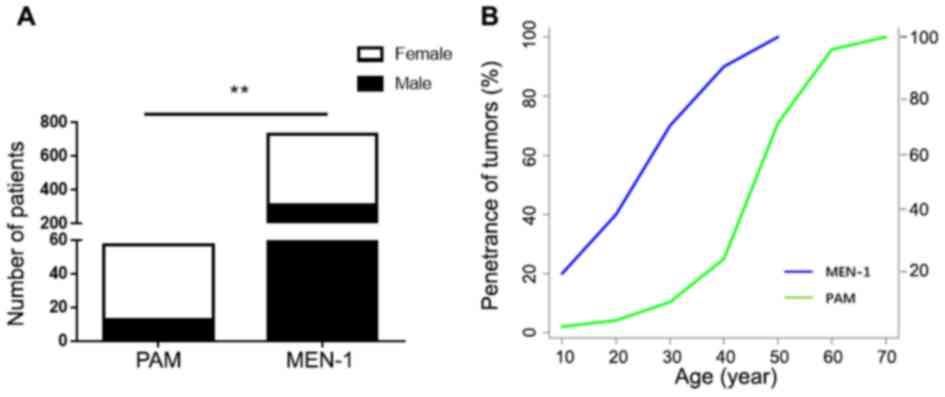
In the present study, 57 (0.7%) patients met the
criteria for PAM. The average age was 54.2±9.8 years (range 20–71
years), with 44 female and 13 male patients. Compared with patients
with MEN-1 (734 cases stated in the previous study) (14), there was a significant association
between PAM and the female sex (P=0.004; Fig. 1A). The age of tumor penetrance is the
age of patients at diagnosis. The age of tumor penetrance for PAM
was significantly higher than for patients with MEN-1 (14). The penetrance rate of MEN-1
syndrome-related tumors before the age of 40 was 94%, but only 25%
for PAM (Fig. 1B). Only one patient
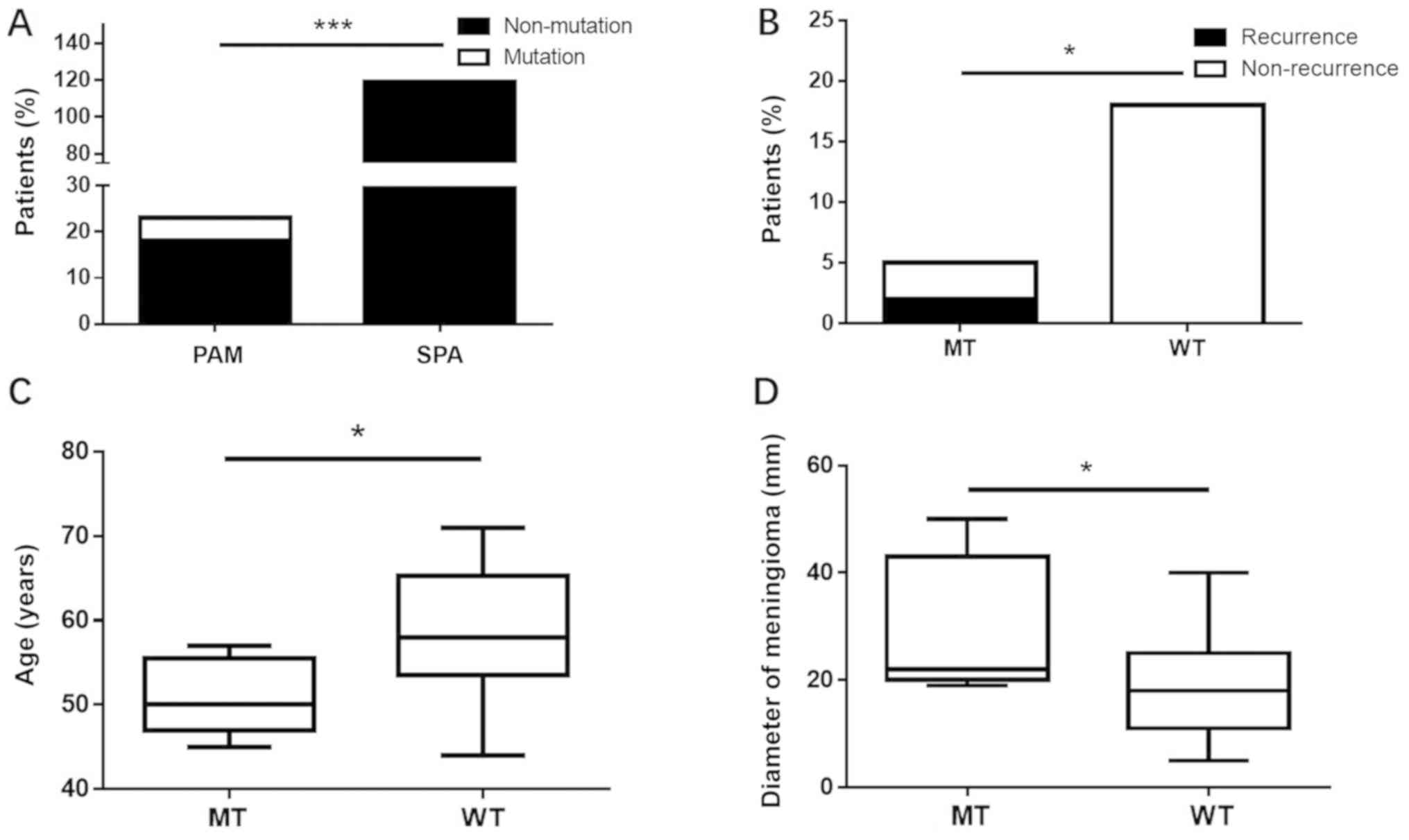
out of the 119 patients with SPA sequenced on the HiSeq X10
platform harbored the hotspot site (MEN1 c.1523G>A;
p.G508D). The difference was statistically significant (P=0.0004;
Fig. 2A). Compared with wild-type
MEN1, patients with a MEN1 mutation were more likely
to suffer from a recurrence of pituitary adenoma, develop a
pituitary adenoma at a younger age and suffer from a larger
diameter of the meningioma (P<0.05; Fig. 2B-D).
Pituitary adenoma and meningioma
components do not share a common clone origin in PAM
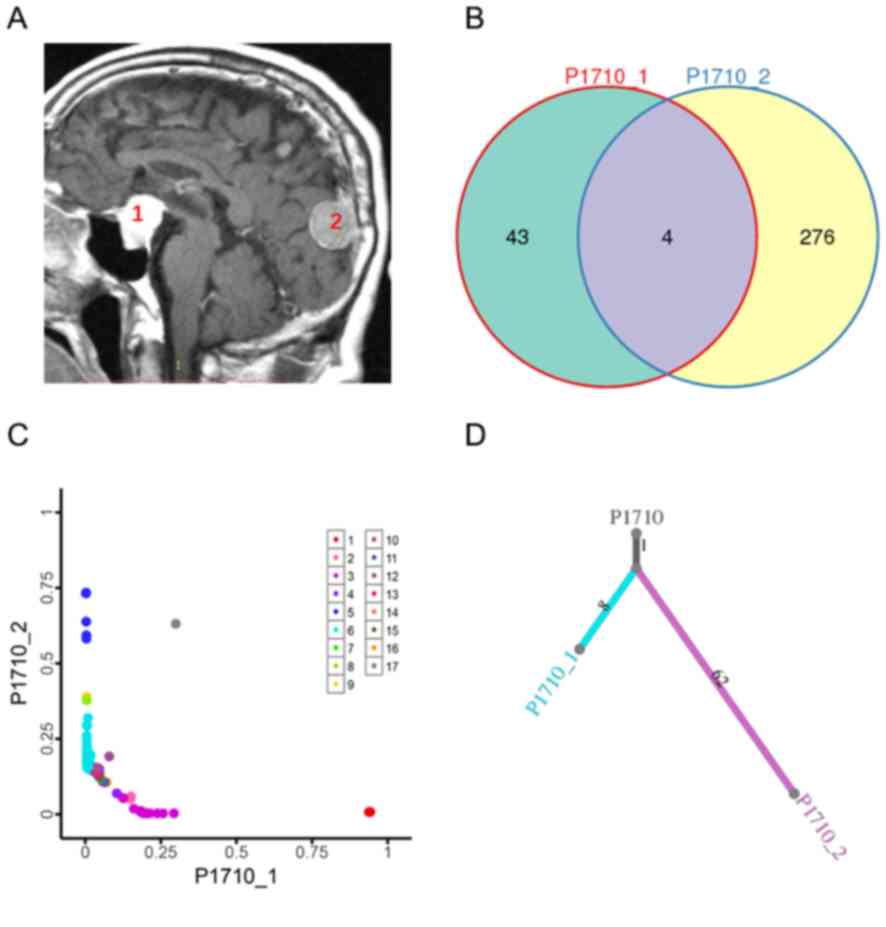
Pituitary adenoma and meningioma samples obtained
from one patient with PAM, indicated in Fig. 3A, were subjected to WES. The average
depths of WES were 500× for the tumor samples and 100× for the
peripheral blood samples. There were 47 somatic SNVs in the
pituitary adenoma and 280 somatic SNVs in the meningioma. To
explore the relationship between pituitary adenoma and meningioma
components in the same patient, somatic SNVs were classified as
ubiquitous (present in both tumor components) or private (only
present in a single tumor). Only four somatic SNVs were present in
both the pituitary adenoma and meningioma samples (Fig. 3B). These data indicated that it is
likely that SNVs in pituitary adenoma and meningioma components
accumulated independently. In addition, clonal analyses suggested
different mutation clusters for the patient with PAM after
adjusting for CCF in the samples using PyClone (Fig. 3C). To further explore the clonal
structure of PAM, phylogenetic trees were constructed using somatic
non-synonymous SNVs. The results revealed a short trunk length,
which suggested that PAM may be of polyclonal origin (Fig. 3D).
The aforementioned results, obtained by analyzing
somatic mutations, clonal analyses and phylogenetic tree
construction, implied that pituitary adenoma and PAM do not
originate from a common progenitor.
Gene screening for PAM
predisposition
SNVs/InDels in a single tumor sample lacking a
matched control were obtained through alignment with the UCSC Human
Genome Reference hg19 using SAMtools. A total of 131 MEN1
SNVs/InDels were identified in all the samples. The 46 variants
that were non-synonymous mutations in protein-coding regions were
selected for further investigation. In addition, polymorphisms of
somatic SNVs and InDels referenced in the 1000 Genomes Project
(21) or Exome Aggregation
Consortium (22) with a minor allele
frequency >1% were removed. The hotspot site rs375804228
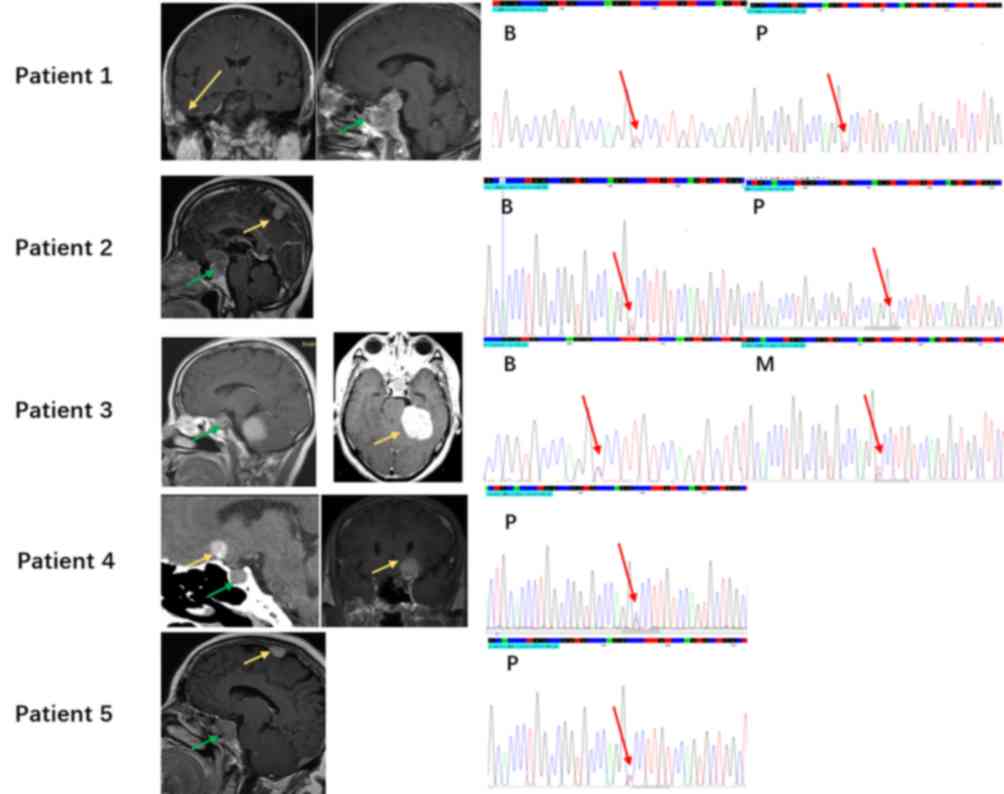
(MEN1 c.1523G>A; p.G508D), which was found in 5 of 23
patients with PAM, was further investigated (Fig. 4). However, the patients did not have
MEN-1 syndrome-related symptoms, and the imaging examination and
laboratory investigations were not indicative of MEN-1 syndrome.
Five patients with MEN1 germline mutation (MEN1
c.1523G>A; p.G508D) were analyzed using Sanger sequencing. Two
patients had a pituitary adenoma sample and a matched blood sample,
one patient had a meningioma sample and a matched blood sample, and
one patient had only pituitary adenoma sample. MEN1 germline
mutations (MEN1 c.1523G>A; p.G508D) were detected in
these samples. Patient 2 had a loss of heterozygosity on
MEN1 c.1523G>A; p.G508D in the pituitary adenoma sample,
which may contribute to tumorigenesis in PAM (Fig. 5).
Discussion
Pituitary adenoma and meningioma are the most common
benign tumors in the CNS (9). A
total of 57 novel PAM cases were identified from 8,197 pituitary
adenoma cases in the present study. Khandwala et al
(23) reported that the levels of
growth hormone (GH) and insulin-like growth factor 1 may
play an important role in the tumorigenesis of PAM. However, in the
present study, there were only six patients with GH pituitary
adenoma. There may be a common genetic mechanism leading to PAM,
particularly mutations of tumor suppressor genes. The present study
used somatic mutation and clonal analyses to reveal that, in PAM,
the pituitary adenoma and meningioma were not found to originate
from a common progenitor. Therefore, the most likely explanation
for the development of PAM is a genetic predisposition.
The present study revealed that 5/23 patients with
PAM had the same germline mutation in MEN1 (c.1523G>A;
p.G508D), compared with 1/119 patients with SPA. Therefore, the
germline mutation may be a genetic risk factor for the development
of PAM. However, the results of the present study showed that there
were significant differences between PAM and MEN-1 syndrome
(Table SII). Both sexes are equally
affected, with no geographical, racial or ethnic differences
(13). Mutated forms of MEN1
include nonsense mutations, missense mutations, frameshifts and
insertions. In addition, previous studies revealed that certain
endocrine tumors, as well as non-endocrine tumors, such as skin
tumors, lipomas and carcinoids, are associated with patients with
MEN-1 syndrome (24,25). CNS tumors, including ependymomas,
schwannomas and meningiomas, have rarely been reported in patients
with MEN-1 or MEN-1 variant syndromes (13,26). For
patients with PAM, related tumors include pituitary adenoma and
meningioma, and the age of tumor penetrance for PAM is
significantly higher than that for patients with MEN-1 syndrome
(14). In the present study, the
penetrance rate of tumors related to MEN-1 syndrome was 94% in
patients <40 years old, but was only 25% for patients with PAM.
Furthermore, compared with patients with MEN-1 syndrome, there was
a significant association between PAM and the female sex.
Additionally, the mutated form of PAM only included a missense
mutation.
The present study has some limitations. This is a
retrospective study and the incidence of PAM is low. The number of
meningioma specimens from patients with PAM collected for exome
sequencing is also low. Samples will continue to be collected for
further study. More experiments and data may be needed to support
this study in the future.
The present study revealed that the pituitary
adenoma and meningioma tissues obtained from one patient with PAM
did not originate from a common progenitor. The germline mutation
MEN1 (c.1523G>A; p.G508D) may serve as a genetic
predisposition for PAM. Compared with wild-type MEN1, there
was a significant association between MEN1 mutation and
recurrence of pituitary adenoma, young age and larger diameter of
the meningioma.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Dr Chengcheng Wang
(Etiology Laboratory, National Cancer Center/Cancer Hospital
Chinese Academy of Medical Sciences) for experimental guidance, Dr
Zhexuan Li (Epidemiological Laboratory, Beijing Cancer
Hospital/Beijing Institute For Cancer Research) for support with
the statistical analysis and Mr. Lei Gong and Mrs. Hongyun Wang
(Cell Laboratory, Beijing Neurosurgical Institute) for support with
technique.
Funding
The present study was supported by grants from the
National High Technology Research and Development Program of China
863 Program (grant no. 2014AA020610), the National Natural Science
Foundation of China (grant no. 81771489), the Beijing Municipal
Science & Technology Commission (grant no. Z171100000117002)
and the China National Key Research and Development Program (grant
no. 2017YFC0908300).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
HBZ, CZL and YZZ designed the study. HBZ, YZM, JG
and YTS collected the samples and clinical data. HBZ, SDZ and WYX
performed the experiments. HBZ and CZL contributed to manuscript
writing and submission. HBZ, YZM, JG and WD performed the
statistical analyses and figure formatting. CZL and YZZ
participated in the funding application and management of the
project. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Institutional
Research Board of Beijing Tiantan Hospital, Capital Medical
University. Written informed consent was obtained from all
participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
PAM
|
pituitary adenoma associated with
meningioma
|
|
SPA
|
sporadic pituitary adenoma
|
|
SM
|
sporadic meningioma
|
|
MEN1
|
multiple endocrine neoplasia 1
|
|
CNS
|
central nervous system
|
|
FFPE
|
formalin-fixed and
paraffin-embedded
|
|
CCF
|
cancer cell fraction
|
References
|
1
|
McDowell BD, Wallace RB, Carnahan RM,
Chrischilles EA, Lynch CF and Schlechte JA: Demographic differences
in incidence for pituitary adenoma. Pituitary. 14:23–30. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lecoq AL, Kamenický P, Guiochon-Mantel A
and Chanson P: Genetic mutations in sporadic pituitary
adenomas-what to screen for? Nat Rev Endocrinol. 11:43–54. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ezzat S, Asa SL, Couldwell WT, Barr CE,
Dodge WE, Vance ML and McCutcheon IE: The prevalence of pituitary
adenomas: A systematic review. Cancer. 101:613–619. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Melmed S: Pathogenesis of pituitary
tumors. Nat Rev Endocrinol. 7:257–266. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Amirjamshidi A, Mortazavi SA, Shirani M,
Saeedinia S and Hanif H: ‘Coexisting pituitary adenoma and
suprasellar meningioma-a coincidence or causation effect: Report of
two cases and review of the literature’. J Surg Case Rep.
2017:rjx0392017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ruiz-Juretschke F, Iza B, Scola-Pliego E,
Poletti D and Salinero E: Coincidental pituitary adenoma and planum
sphenoidale meningioma mimicking a single tumor. Endocrinol Nutr.
62:292–294. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bondy M and Ligon BL: Epidemiology and
etiology of intracranial meningiomas: A review. J Neurooncol.
29:197–205. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Curto L, Squadrito S, Almoto B, Longo M,
Granata F, Salpietro F, Torre ML, Marini F, Trimarchi F and Cannavo
S: MRI finding of simultaneous coexistence of growth
hormone-secreting pituitary adenoma with intracranial meningioma
and carotid artery aneurysms: Report of a case. Pituitary.
10:299–305. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Herrero-Ruiz A, Villanueva-Alvarado HS,
Corrales-Hernandez JJ, Higueruela-Minguez C, Feito-Perez J and
Recio-Cordova JM: Coexistence of GH-producing pituitary
macroadenoma and meningioma in a patient with multiple endocrine
neoplasia type 1 with hyperglycemia and ketosis as first clinical
sign. Case Rep Endocrinol. 2017:23907972017.PubMed/NCBI
|
|
10
|
Concolino P, Costella A and Capoluongo E:
Multiple endocrine neoplasia type 1 (MEN1): An update of 208 new
germline variants reported in the last nine years. Cancer Genet.
209:36–41. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Agarwal SK: The future: Genetics advances
in MEN1 therapeutic approaches and management strategies. Endocr
Relat Cancer. 24:T119–T134. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Weber F and Mulligan LM: Happy 20th
anniversary MEN1: From positional cloning to gene function
restoration. Endocr Relat Cancer. 24:E7–E11. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Asgharian B, Chen YJ, Patronas NJ, Peghini
PL, Reynolds JC, Vortmeyer A, Zhuang Z, Venzon DJ, Gibril F and
Jensen RT: Meningiomas may be a component tumor of multiple
endocrine neoplasia type 1. Clin Cancer Res. 10:869–880. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Goudet P, Bonithon-Kopp C, Murat A,
Ruszniewski P, Niccoli P, Ménégaux F, Chabrier G, Borson-Chazot F,
Tabarin A, Bouchard P, et al: Gender-related differences in MEN1
lesion occurrence and diagnosis: A cohort study of 734 cases from
the groupe d'etude des tumeurs endocrines. Eur J Endocrinol.
165:97–105. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li H and Durbin R: Fast and accurate
long-read alignment with Burrows-Wheeler transform. Bioinformatics.
26:589–595. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li H, Handsaker B, Wysoker A, Fennell T,
Ruan J, Homer N, Marth G, Abecasis G and Durbin R; 1000 Genome
Project Data Processing Subgroup, : The Sequence Alignment/Map
format and SAMtools. Bioinformatics. 25:2078–2079. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
DePristo MA, Banks E, Poplin R, Garimella
KV, Maguire JR, Hartl C, Philippakis AA, del Angel G, Rivas MA,
Hanna M, et al: A framework for variation discovery and genotyping
using next-generation DNA sequencing data. Nat Genet. 43:491–498.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cibulskis K, Lawrence MS, Carter SL,
Sivachenko A, Jaffe D, Sougnez C, Gabriel S, Meyerson M, Lander ES
and Getz G: Sensitive detection of somatic point mutations in
impure and heterogeneous cancer samples. Nat Biotechnol.
31:213–219. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Saunders CT, Wong WS, Swamy S, Becq J,
Murray LJ and Cheetham RK: Strelka: Accurate somatic small-variant
calling from sequenced tumor-normal sample pairs. Bioinformatics.
28:1811–1817. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Roth A, Khattra J, Yap D, Wan A, Laks E,
Biele J, Ha G, Aparicio S, Bouchard-Côté A and Shah SP: PyClone:
Statistical inference of clonal population structure in cancer. Nat
Methods. 11:396–398. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
1000 Genomes Project Consortium, .
Abecasis GR, Auton A, Brooks LD, DePristo MA, Durbin RM, Handsaker
RE, Kang HM, Marth GT and McVean GA: An integrated map of genetic
variation from 1,092 human genomes. Nature. 491:56–65. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lek M, Karczewski KJ, Minikel EV, Samocha
KE, Banks E, Fennell T, O'Donnell-Luria AH, Ware JS, Hill AJ,
Cummings BB, et al: Analysis of protein-coding genetic variation in
60,706 humans. Nature. 536:285–291. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Khandwala HM, McCutcheon IE, Flyvbjerg A
and Friend KE: The effects of insulin-like growth factors on
tumorigenesis and neoplastic growth. Endocr Rev. 21:215–244. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ito T, Igarashi H, Uehara H, Berna MJ and
Jensen RT: Causes of death and prognostic factors in multiple
endocrine neoplasia type 1: A prospective study: Comparison of 106
MEN1/Zollinger-Ellison syndrome patients with 1613 literature MEN1
patients with or without pancreatic endocrine tumors. Medicine
(Baltimore). 92:135–181. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Brandi ML, Gagel RF, Angeli A, Bilezikian
JP, Beck-Peccoz P, Bordi C, Conte-Devolx B, Falchetti A, Gheri RG,
Libroia A, et al: Guidelines for diagnosis and therapy of MEN type
1 and type 2. J Clin Endocrinol Metab. 86:5658–5671. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Giraud S, Choplin H, Teh BT, Lespinasse J,
Jouvet A, Labat-Moleur F, Lenoir G, Hamon B, Hamon P and Calender
A: A large multiple endocrine neoplasia type 1 family with clinical
expression suggestive of anticipation. J Clin Endocrinol Metab.
82:3487–3492. 1997. View Article : Google Scholar : PubMed/NCBI
|