Introduction
Macrophages are a group of immune cells that serve
essential roles in both physiological and pathological conditions
by being involved in inflammatory and immune responses (1,2). In
response to intracellular or extracellular stimulation, the
monocyte-macrophage system can transit to two major distinct
polarization patterns: The pro-inflammatory M1 type and the
anti-inflammatory M2 type, which exhibit contrasting cellular
phenotypes and functions (3). M2
type macrophages have a high phenotypic heterogeneity and can be
further divided into three subsets: M2a, M2b and M2c (4). The M2a subtype is defined as
alternatively activated macrophages, induced by fungal and helminth
infections, interleukin (IL)-4 and IL-13; the M2b subtype is
defined as type 2 macrophages, induced by immune complexes and
lipopolysaccharide; and the M2c subtype is defined as deactivated
macrophages, induced by IL-10, transforming growth factor-β and
glucocorticoids (4).
Glioma is a type of malignant tumor arising from
glial cells of the brain or the spine. The heterogeneity of
macrophages is high in the glioma microenvironment (5). Gliomas contain two subtypes of
macrophages, brain-resident microglia and circulating
monocyte-derived macrophages (6).
Both of these subtypes have been demonstrated to contribute to
glioma progression and maintenance (7). Macrophage transformation from the M1 to
the M2 type can promote glioma development (8,9).
However, to the best of our knowledge, there are no reports on the
polarization status of macrophage-like cells in the peripheral
blood of patients with glioma. Chitinase-3-like protein 1, also
termed YKL-40, is highly expressed in glioma tissues compared with
adjacent normal brain tissues (10).
YKL-40 is secreted by tumor cells and tumor-associated macrophages
into the blood and has a prognostic value in various types of
cancer, such as Hodgkin lymphoma and melanoma (11,12).
However, the association between the polarization status of
macrophage-like cells in the peripheral blood and tumor stage or
YKL-40 expression in patients with glioma remains unclear.
The development of diagnostic and therapeutic
strategies for glioma has greatly improved during the past decades,
but glioma remains one of the most malignant tumors worldwide (3–8
cases/100,000 individuals) (13).
Based on the advances in cancer immunotherapy and the role of
macrophages in glioma development, novel immunological markers and
potential therapeutic targets of macrophages should be considered
in glioma research. Therefore, the present study aimed to
investigate the polarization status of macrophage-like cells in the
peripheral blood of patients with glioma and to evaluate the
associations among macrophage-like cell polarization patterns,
glioma severity and the glioma marker YKL-40 in the peripheral
blood of patients with glioma.
Materials and methods
Patients
Blood samples were obtained from 40 patients with
glioma and 38 healthy controls (all Chinese) at The First
Affiliated Hospital of Anhui Medical University (Hefei, China).
Glioma tissues and adjacent normal tissues were obtained from 40
patients with glioma (all Chinese) upon excision surgery at the
Department of Neurosurgery of The First Affiliated Hospital of
Anhui Medical University. The patient characteristics are
summarized in Table I. Patients with
glioma (average age, 52.7 years; age range, 8–82 years) and healthy
controls (average age, 39.7 years; age range, 23–62 years) were
recruited and their blood and tumor samples were collected between
May 2017 and August 2018. Specifically, adjacent normal tissues
were excised from non-functional tissues within 2 cm from the tumor
tissues. Blood samples and tumor tissues were collected from the
same 40 patients. The staging of glioma was based on the 2016 World
Health Organization Classification of Tumors of the Central Nervous
System (14). The present study was
approved by the Ethics Committee of Anhui Medical University.
Informed consent was provided by all participants or their
guardians. The histological types of the included patients were
limited to glioma (including glioblastoma, mesoglioma,
ganglioglioma, astrocytic glioma and spongiocytoma). Patients with
incomplete information (age, sex, histological type, stage,
recurrence or received chemotherapy) were excluded.
 | Table I.Summary of patient
characteristics. |
Table I.
Summary of patient
characteristics.
| Characteristic | Patient no. | Percentage |
|---|
| Age, years |
|
|
|
<50 | 16 | 40.0 |
|
≥50 | 24 | 60.0 |
| Sex |
|
|
|
Male | 22 | 55.0 |
|
Female | 18 | 45.0 |
| Histopathological
type |
|
|
|
Glioblastoma | 23 | 57.5 |
|
Mesoglioma | 5 | 12.5 |
|
Ganglioglioma | 3 | 7.5 |
|
Astrocytic glioma | 5 | 12.5 |
|
Spongiocytoma | 4 | 10.0 |
| Stagea |
|
|
| 0 | 0 | 0.0 |
| I | 2 | 5.0 |
| II | 11 | 27.5 |
|
III | 7 | 17.5 |
| IV | 20 | 50.0 |
| Recurrence |
|
|
|
Yes | 9 | 22.5 |
| No | 31 | 77.5 |
| Received
chemotherapy |
|
|
|
Yes | 5 | 12.5 |
| No | 35 | 87.5 |
Blood samples
Peripheral venous blood (4 ml) was collected from
each participant. Peripheral blood mononuclear cells were isolated
using a PBMC isolation kit (cat. no. DKW-KLSH-0100; DAKEWE, Inc.)
for use in subsequent flow cytometry analysis. For patients with
glioma, blood samples were collected before the surgical
procedure.
Flow cytometry
A flow cytometric gating strategy
(CD115+CD1c−CD2−CD15−CD19−CD14+CD16+CD11b+)
was used to stain macrophage-like cells in the peripheral blood of
patients with glioma and healthy controls. CD115 was used to select
monocyte-lineage cells, dendritic cells were excluded using CD1c, B
cells were excluded using CD19, T and NK cells were excluded using
CD2, and granulocytes were excluded using CD15. Macrophage-like
cells were selected using the macrophage antibodies CD14, CD16 and
CD11b (all Miltenyi Biotec, Inc.). Macrophage-like cells were
divided into
CD115+CD1c−CD2−CD15−CD19−CD14+CD16+CD11bint
and
CD115+CD1c−CD2−CD15−CD19−CD14+CD16+CD11bhi
subsets for subsequent analyses; CD11bint (P6, Vioblue
1×102−1×104) and CD11bhi (P7,
Vioblue 1×104−1×106) were decided based on
the fluorescence-activated cell sorting (FACS) strategy shown in
Fig. S1. In CD11b+
macrophage-like cells, CCR7+CD86+ was used to
mark the M1 type, while CCR7−CXCR1+,
CCR7−CD86+ and
CCR7−CCR2+ were used to identify the M2a, M2b
and M2c macrophage-like cells, respectively (15). The cells were analyzed using the BD
FACSAria II flow cytometer and the BD FACSDiva software v8.0.2
(both BD Biosciences). Glioma tissues and adjacent normal tissues
were incubated with the following fluorescein isothiocyanate
(FITC)-, phycoerythrin (PE)-, allophycocyanin (APC), PE-Cy7-,
APC-Cy7- or Vioblue-conjugated monoclonal antibodies at 4°C for 1
h: Rat anti-human CD115-PE (2 µg/test; cat. no. 347303) was from
BioLegend, Inc., whereas mouse anti-human CD1c-FITC (5 µg/test;
cat. no. 130-113-863), CD2-FITC (5 µg/test; cat. no. 130-098-685),
CD15-FITC (5 µg/test; cat. no. 130-114-010), CD19-FITC (5 µg/test;
cat. no. 130-114-171), CD14-APC (2 µg/test; cat. no. 130-110-578),
CD16-APC-Cy7 (2 µg/test; cat. no. 130-113-952), CD11b-Vioblue (2
µg/test; cat. no. 130-110-616), CD86-APC (2 µg/test; cat. no.
130-114-095), CCR2-PE (2 µg/test; cat. no. 130-109-654), CCR7-FITC
(5 µg/test; cat. no. 130-117-700) and CXCR1-PE-Cy7 (2 µg/test; cat.
no. 130-115-950) were from Miltenyi Biotec, Inc.
Immunohistochemistry (IHC) and
hematoxylin and eosin (H&E) staining
IHC and H&E staining were performed as
previously described (16). Briefly,
paraffin-embedded tissue slides obtained from glioma and adjacent
tissues were deparaffinized in xylene twice for 5 min each at room
temperature. Subsequently, slides were transferred in 100% alcohol
twice for 3 min each, and then rehydrated in a descending alcohol
series (95, 70 and 50%) for 3 min each. The EDTA antigen retrieval
buffer (Beijing Solarbio Science & Technology Co., Ltd.) was
heated until boiling and added to the sections; this was repeated
two more times every 5–10 min. The slides were left to cool down at
room temperature and washed with double-distilled H2O.
Subsequently, the slides were blocked in 5% goat serum (Biological
Industries) for 60 min at room temperature, and subsequently
incubated with primary monoclonal rabbit anti-human anti-inducible
nitric oxide synthase (iNOS) (1:100; cat. no. sc-651) and
polyclonal sheep anti-human anti-CD206 (1:50; cat. no. sc-34577;
both from Santa Cruz Biotechnology, Inc.) antibodies at 4°C for 12
h. For H&E staining, sections were deparaffinized in xylene,
re-hydrated in absolute alcohol and washed briefly with distilled
water. Subsequently, they were stained with Harris hematoxylin
solution and washed with running tap water, differentiated in 1%
acid alcohol and washed with running tap water, blued in 0.2%
saturated lithium carbonate solution and washed with running tap
water, rinsed in 95% alcohol and counterstained with eosin-phloxine
solution, dehydrated in alcohol and cleared in xylene, and finally
mounted with mounting medium (xylene-based). ImageJ software
(version d 1.47; National Institutes of Health) was used for
quantification of IHC.
Calculation of M1 and M2 macrophage
infiltration
In areas of iNOS+ and CD206+
macrophage infiltration, individual macrophage infiltration was
measured on a ×200 magnification field using an Olympus BX53 light
microscope. An iNOS+ and CD206+ macrophage
cluster was counted as a single infiltrated macrophage. The ratio
of M1 and M2 infiltrated macrophages was calculated as the absolute
number of iNOS+ and CD206+ macrophages/total
cells per ×200 magnification field (n=8).
Statistical analysis
GraphPad Prism v7 (GraphPad Software, Inc.) was used
for statistical analysis. Variances were first assessed by
Bartlett's test. Subsequently, significant differences between two
groups were analyzed using a two-tailed unpaired Student's t-test
when equal variances were assumed, or a Welch's t-test for unequal
variances. P<0.05 was considered to indicate a statistically
significant difference.
Results
Detection of CD11bint and
CD11bhi peripheral blood macrophage-like cells in
patients with glioma
A total of 40 patients with glioma and 38 healthy
controls were recruited in the present study. The clinical and
pathological features of the patients are listed in Table I. Macrophage-like cells in the
peripheral blood were detected by flow cytometry with
CD115+CD1c−CD2−CD15−CD19−CD14+CD16+CD11b+
gating (Fig. S1). There were two
distinct populations of macrophage-like cells:
CD115+CD1c−CD2−CD15−CD19−CD14+CD16+CD11bint
and
CD115+CD1c−CD2−CD15−CD19−CD14+CD16+CD11bhi
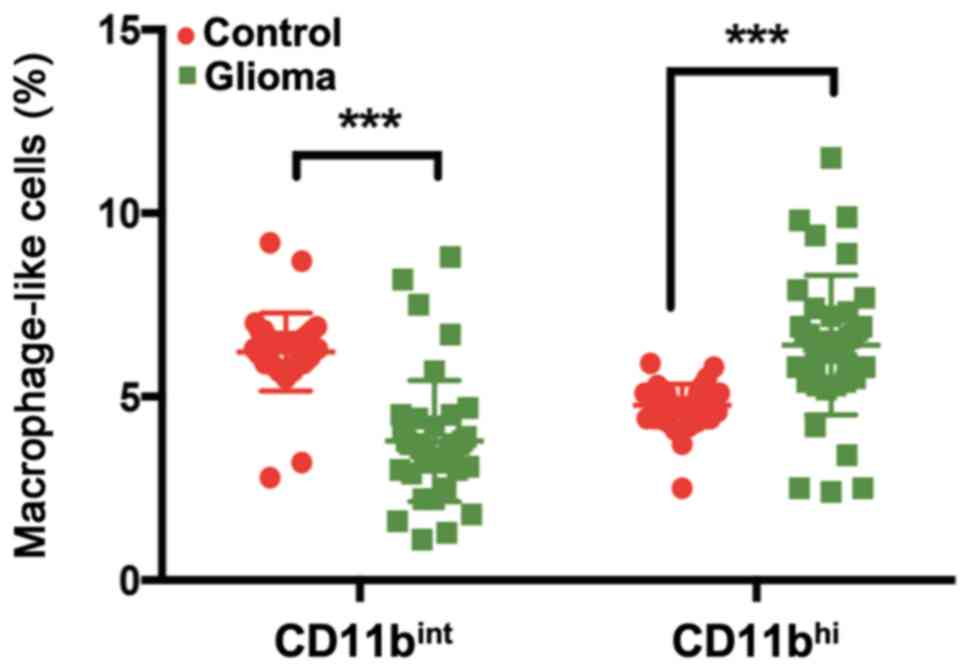
cells. The number and percentage of
CD115+CD1c−CD2−CD15−CD19−CD14+CD16+CD11bint
cells among peripheral blood monocytes from patients with glioma
were significantly decreased compared with those from healthy
controls (Fig. 1). By contrast, an
increased percentage of
CD115+CD1c−CD2−CD15−CD19−CD14+CD16+CD11bhi
cells was observed in the peripheral blood of patients with glioma
compared with those in the blood of healthy controls (Fig. 1).
Polarization patterns of
CD11bint and CD11bhi peripheral blood
macrophage-like cells in patients with glioma
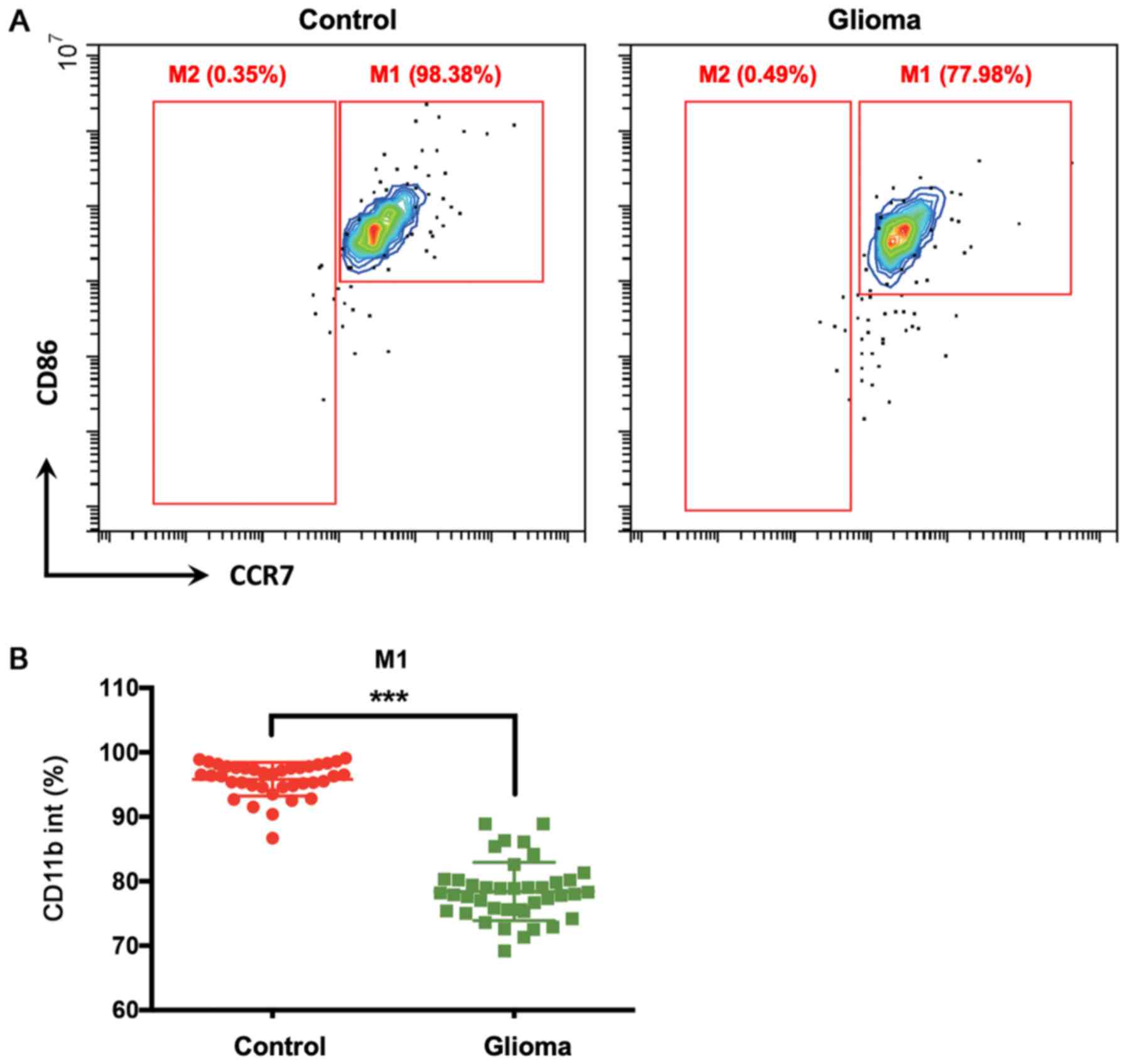
In CD11b+ (including both
CD11bint and CD11bhi) macrophage-like cells,
the percentage of M1 type cells (CCR7+CD86+)
was significantly lower in patients with glioma compared with that
in healthy controls (Figs. 2B and
3C). However, there were almost no
M2 type cells (CCR7−) in the CD11bint
population (Fig. 2A). In the
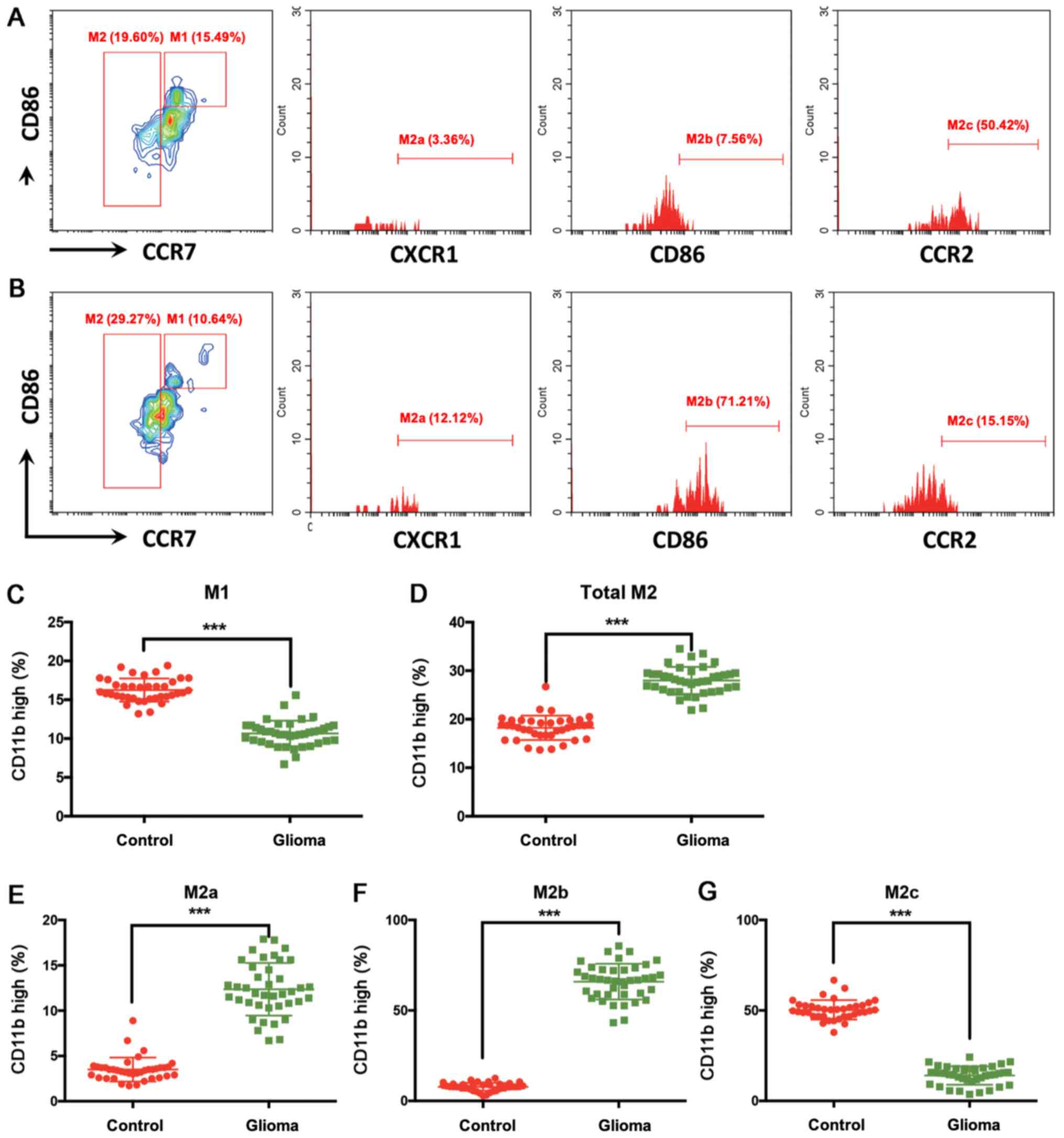
CD11bhi cell population, the percentage of total M2 type
cells was higher in patients with glioma compared with that in
healthy controls (Fig. 3A, B and D).
Specifically, M2a (CCR7−CXCR1+) and M2b
(CCR7−CD86+) type cells were upregulated,
whereas M2c type cells (CCR7−CCR2+) were
downregulated in the peripheral blood of patients with glioma
compared with those in the blood of healthy controls (Fig. 3E-G).
Polarization patterns of macrophages
in glioma tissues
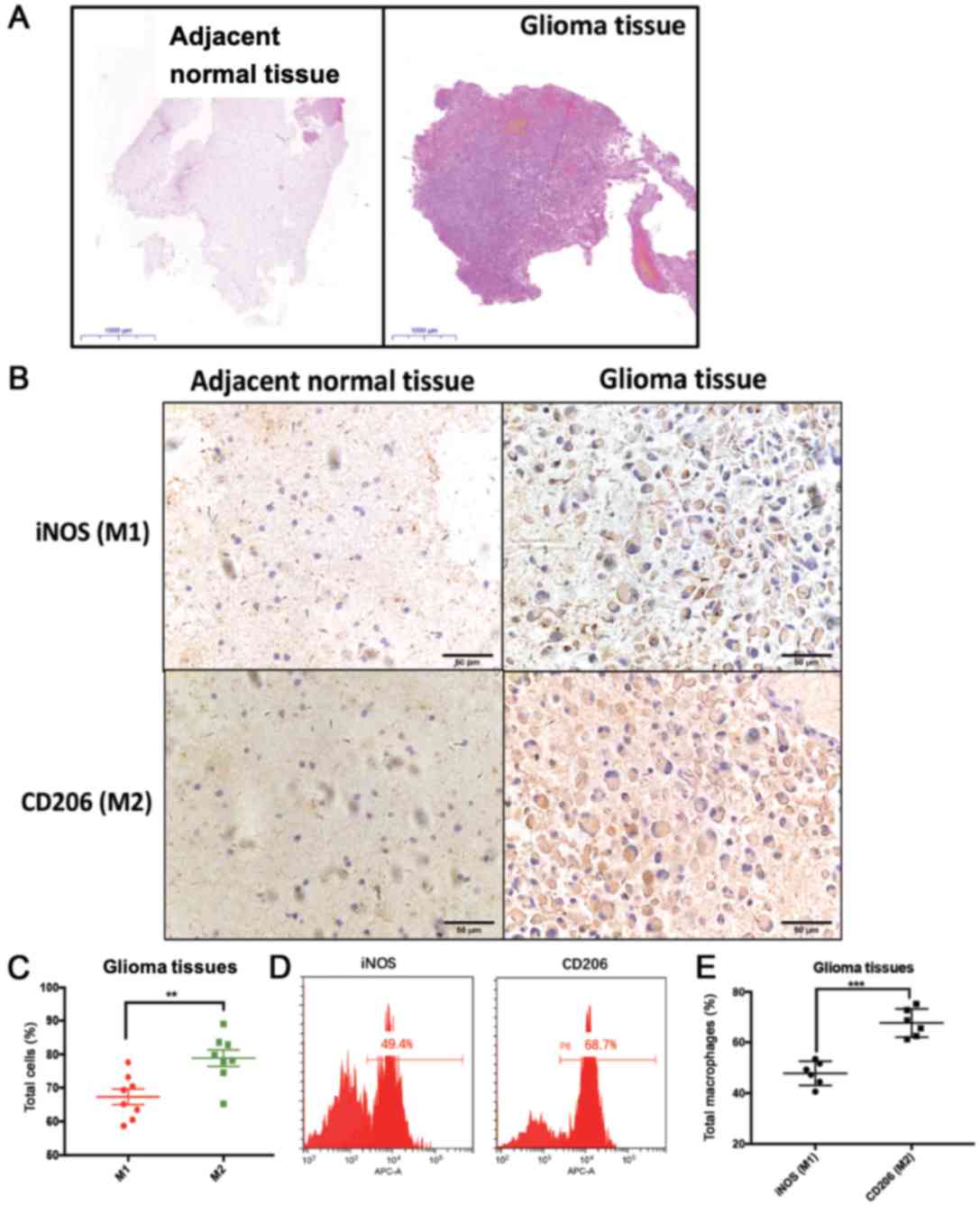
To validate this polarization pattern, glioma and
adjacent normal brain tissues were used for detecting the
well-established M1 (iNOS) and M2 (CD206) markers (Fig. 4). The typical morphology of glioma
and adjacent normal brain tissues is shown in Fig. 4A. Quantification of the IHC results
revealed that the percentage of the M2 marker CD206+
cells was higher than the percentage of the M1 marker
iNOS+ cells in glioma tumor tissues (Fig. 4B and C). Additionally, FACS analysis
validated that macrophages expressed higher levels of the M2 marker
CD206 than the M1 marker iNOS in glioma tissues (Fig. 4D and E). The present results
suggested that patients with glioma exhibited a distinct pattern of
macrophage polarization in the peripheral blood, namely a lower
percentage of M1 type cells and a higher percentage of M2 type
cells (especially M2a and M2b type cells) compared with healthy
controls.
Subsequently, the patterns of macrophage
polarization in patients with different stages of glioma were
evaluated. The percentages of macrophage-like cells in the
peripheral blood were not significantly different between patients
with advanced glioma (stages III and IV) and those with early
glioma (stages I and II) (Fig. S2).
Since previous studies have suggested that serum YKL-40 is a
candidate marker for glioma (17–19), the
present study investigated the association between the patterns of
macrophage polarization and serum YKL-40 status in patients with
glioma. However, the percentages of macrophage-like cells were not
significantly different between patients with low YKL-40 expression
and those with high YKL-40 expression (Fig. S3).
Discussion
Currently, a large proportion of the mortality of
patients with glioma is caused by tumor recurrence, indicating an
urgent requirement for improved diagnosis and therapy (20,21).
However, no specific diagnostic clinical marker for glioma has yet
been identified. For example, YKL-40 is a potential specific marker
of prognosis in high-grade glioma, but not in low-grade glioma
(17,22,23). A
previous study has demonstrated that tumor-associated macrophages
(TAMs) are infiltrated in the glioma microenvironment and can
facilitate survival, migration and neovascularization of glioma;
therefore, TAMs are considered as a potential therapeutic target
for glioma treatment (7). Although a
large proportion of TAMs in glioma come from the peripheral
circulation, to the best of our knowledge, the polarization of
macrophage-like cells in the peripheral blood of patients with
glioma, or the association between macrophage-like cells from the
peripheral blood and TAMs infiltrated in the glioma environment,
have not yet been systemically evaluated. In the present study, an
abnormal polarization pattern of macrophage-like cells was observed
in the peripheral blood of patients with glioma. The percentages of
CD115+CD1c−CD2−CD15−CD19−CD14+CD16+CD11bint
and
CD115+CD1c−CD2−CD15−CD19−CD14+CD16+CD11bhi
macrophage-like cells were significantly altered in patients with
glioma compared with those in healthy controls, suggesting that
these cells may be potential markers for the diagnosis and
monitoring of glioma.
Classical M1 polarization of the monocyte-macrophage
system has demonstrated anticancer activity in different types of
cancer (24,25). Alternatively activated M2 macrophages
generally act as tumor promoters and include three subtypes: M2a,
M2b and M2c (26). Different M2
subtypes serve different roles in tumorigenesis (27). The present study demonstrated that
the numbers of M1 macrophages in the peripheral blood of patients
with glioma were decreased compared with those in healthy controls,
indicating poor antitumor capacity of circulating macrophage-like
cells in patients with glioma. By contrast, the numbers of M2
macrophages were increased in patients with glioma compared with
those in healthy controls, indicating that more M2 macrophage-like
cells may be recruited in glioma and may infiltrate the glioma
microenvironment in the brain.
Finally, the associations of macrophage-like cell
polarization patterns with glioma stage or with the glioma marker
YKL-40 were evaluated in the present study. Of note, no significant
association was identified between different tumor stages and the
polarization patterns of circulating macrophage-like cells. YKL-40
is abundantly expressed in glioma, and various studies have
reported the oncogenic role of this gene (17–19).
Additionally, it has been demonstrated that YKL-40 is secreted by
both tumor cells and tumor-associated macrophages (28,29).
However, the present study revealed that neither the percentage nor
the polarization status of macrophages in the
CD115+CD1c−CD2−CD15−CD19−CD14+CD16+CD11bint
and
CD115+CD1c−CD2−CD15−CD19−CD14+CD16+CD11bhi
subsets was different between patients with high YKL-40 expression
and those with low YKL-40 expression. The present results should be
further confirmed by evaluating the association between infiltrated
macrophages and YKL-40 expression within the tumor
microenvironment. In addition, other monocyte-derived cells, such
as dendritic cells (DCs), are recruited to the tumor tissue
(30) and express YKL-40 (31). Therefore, the function of
YKL-40-secreting DCs should not be neglected in glioma.
Efficient clinical biomarkers and targets are
required for timely and effective glioma diagnosis and therapy.
Although the present study only recruited 40 patients with glioma,
the aberrant polarization patterns of macrophage-like cells in the
peripheral blood may represent potential serum biomarkers for
glioma diagnosis and monitoring. Specifically, the upregulation of
M2a and M2b cells, and the downregulation of M2c cells in the
peripheral blood of patients with glioma require further validation
in glioma tissues using a large cohort of clinical samples.
Additionally, future studies should evaluate the specific function
of circulating macrophage-like cells in the brain glioma tissue.
For example, co-culturing primary infiltrated macrophages and
glioma cells could illustrate the communication between these two
types of cells. Finally, the effects of immunoregulatory drugs and
agents on the polarization status of macrophages should be examined
in glioma, such as the interaction between the programmed death
1(PD-1)/programmed death ligand 1 (PD-L1) pathway and the
polarization of macrophage-like cells in the peripheral blood of
patients with glioma. The polarization status of macrophages may be
a potential marker for the diagnosis and monitoring of patients who
received PD-1/PD-L1 checkpoint therapy (32). Overall, the present study revealed
the polarization status of macrophage-like cells in the peripheral
blood of patients with glioma. The potential prognostic application
of the abnormal polarization patterns of circulating
macrophage-like cells in glioma should be further evaluated in
future studies.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81673444 and
31900616), the Natural Science Foundation of Anhui Province for
young scholars (grant no. 1908085QH379) and Grants for Scientific
Research of BSKY from Anhui Medical University (grant no.
4501041101).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YG and WH performed the experiments. PZ, DH, YF, JT
and WW interpreted the results and drafted the manuscript. YG
revised the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Anhui Medical University, and informed consent was
obtained from all participants or their guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ariel A, Maridonneau-Parini I,
Rovere-Querini P, Levine JS and Mühl H: Macrophages in inflammation
and its resolution. Front Immunol. 3:2–3. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu YC, Zou XB, Chai YF and Yao YM:
Macrophage polarization in inflammatory diseases. Int J Biol Sci.
10:520–529. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Murray PJ: Macrophage polarization. Annu
Rev Physiol. 79:541–566. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Roszer T: Understanding the mysterious M2
macrophage through activation markers and effector mechanisms.
Mediators Inflamm. 2015:16–18. 2015. View Article : Google Scholar
|
|
5
|
Kurowska-Stolarska M and Alivernini S:
Synovial tissue macrophages: Friend or foe? RMD Open.
3:e0005272017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Müller S, Kohanbash G, Liu SJ, Alvarado B,
Carrera D, Bhaduri A, Watchmaker PB, Yagnik G, Di Lullo E,
Malatesta M, et al: Single-cell profiling of human gliomas reveals
macrophage ontogeny as a basis for regional differences in
macrophage activation in the tumor microenvironment. Genome Biol.
18:2342017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hambardzumyan D, Gutmann DH and Kettenmann
H: The role of microglia and macrophages in glioma maintenance and
progression. Nat Neurosci. 19:20–27. 2015. View Article : Google Scholar
|
|
8
|
Rhee I: Diverse macrophages polarization
in tumor microenvironment. Arch Pharm Res. 39:1588–1596. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yuan R, Li S, Geng H, Wang X, Guan Q, Li
X, Ren C and Yuan X: Reversing the polarization of tumor-associated
macrophages inhibits tumor metastasis. Int Immunopharmacol.
49:30–37. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tanwar MK, Gilbert MR and Holland EC: Gene
expression microarray analysis reveals YKL-40 to be a potential
serum marker for malignant character in human glioma. Cancer Res.
62:4364–4368. 2002.PubMed/NCBI
|
|
11
|
Biggar RJ, Johansen JS, Smedby KE,
Rostgaard K, Chang ET, Adami HO, Glimelius B, Molin D,
Hamilton-Dutoit S, Melbye M and Hjalgrim H: Serum YKL-40 and
interleukin 6 levels in Hodgkin lymphoma. Clin Cancer Res.
14:6974–6978. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schmidt H, Johansen JS, Gehl J, Geertsen
PF, Fode K and von der Maase H: Elevated serum level of YKL-40 is
an independent prognostic factor for poor survival in patients with
metastatic melanoma. Cancer. 106:1130–1139. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tamura Y, Kato H, Oh A, Kisanuki K,
Tsuchiya S, Terashima G and Shimasaki Y: Glioblastoma and other
malignant gliomas a clinical review. JAMA. 310:1842–1850. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Louis DN, Perry A, Reifenberger G, von
Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD,
Kleihues P and Ellison DW: The 2016 world health organization
classification of tumors of the central nervous system: A summary.
Acta Neuropathol. 131:803–820. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hung CH, Chen FM, Lin YC, Tsai ML, Wang
SL, Chen YC, Chen YT and Hou MF: Altered monocyte differentiation
and macrophage polarization patterns in patients with breast
cancer. BMC Cancer. 18:3662018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tu J, Cheung HH, Lu G, Chen Z and Chan WY:
MicroRNA-10a promotes granulosa cells tumor development via
PTEN-AKT/Wnt regulatory axis. Cell Death Dis. 9:10762018.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nutt CL, Betensky RA, Brower MA, Batchelor
TT, Louis DN and Stemmer-Rachamimov AO: YKL-40 is a differential
diagnostic marker for histologic subtypes of high-grade gliomas.
Clin Cancer Res. 11:2258–2264. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hormigo A, Gu B, Karimi S, Riedel E,
Panageas KS, Edgar MA, Tanwar MK, Rao JS, Fleisher M, DeAngelis LM
and Holland EC: YKL-40 and matrix metalloproteinase-9 as potential
serum biomarkers for patients with high-grade gliomas. Clin Cancer
Res. 12:5698–5704. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ku BM, Lee YK, Ryu J, Jeong JY, Choi J,
Eun KM, Shin HY, Kim DG, Hwang EM, Yoo JC, et al: CHI3L1 (YKL-40)
is expressed in human gliomas and regulates the invasion, growth
and survival of glioma cells. Int J Cancer. 128:1316–1326. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ludwig K and Kornblum HI: Molecular
markers in glioma. J Neurooncol. 134:505–512. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Agnihotri S, Burrell KE, Wolf A, Jalali S,
Hawkins C, Rutka JT and Zadeh G: Glioblastoma, a brief review of
history, molecular genetics, animal models and novel therapeutic
strategies. Arch Immunol Ther Exp (Warsz). 61:25–41. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Iwamoto FM, Hottinger AF, Karimi S, Riedel
E, Dantis J, Jahdi M, Panageas KS, Lassman AB, Abrey LE, Fleisher
M, et al: Serum YKL-40 is a marker of prognosis and disease status
in high-grade gliomas. Neuro Oncol. 13:1244–1251. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhao YH, Pan ZY, Wang ZF, Ma C, Weng H and
Li ZQ: YKL-40 in high-grade glioma: Prognostic value of protein
versus mRNA expression. Glioma. 1:104–110. 2018. View Article : Google Scholar
|
|
24
|
Ostuni R, Kratochvill F, Murray PJ and
Natoli G: Macrophages and cancer: From mechanisms to therapeutic
implications. Trends Immunol. 36:229–239. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cheng H, Wang Z, Fu L and Xu T: Macrophage
polarization in the development and progression of ovarian cancers:
An overview. Front Oncol. 9:4212019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mantovani A, Sica A, Sozzani S, Allavena
P, Vecchi A and Locati M: The chemokine system in diverse forms of
macrophage activation and polarization. Trends Immunol. 25:677–686.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Martinez FO, Sica A, Mantovani A and
Locati M: Macrophage activation and polarization. Bioscience.
13:453–461. 2008.
|
|
28
|
Shao R: YKL-40 acts as an angiogenic
factor to promote tumor angiogenesis. Front Physiol. 4:1222013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Horbinski C, Wang G and Wiley CA: YKL-40
is directly produced by tumor cells and is inversely linked to EGFR
in glioblastomas. Int J Clin Exp Pathol. 3:226–237. 2010.PubMed/NCBI
|
|
30
|
Ricard C, Tchoghandjian A, Luche H, Grenot
P, Figarella-Branger D, Rougon G, Malissen M and Debarbieux F:
Phenotypic dynamics of microglial and monocyte-derived cells in
glioblastoma-bearing mice. Sci Rep. 6:263812016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Di Rosa M, Tibullo D, Saccone S, Distefano
G, Basile MS, Di Raimondo F and Malaguarnera L: CHI3L1 nuclear
localization in monocyte derived dendritic cells. Immunobiology.
221:347–356. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang Y, Du W, Chen Z and Xiang C:
Upregulation of PD-L1 by SPP1 mediates macrophage polarization and
facilitates immune escape in lung adenocarcinoma. Exp Cell Res.
359:449–457. 2017. View Article : Google Scholar : PubMed/NCBI
|