Introduction
Esophageal cancer is the sixth most common cause of
cancer-associated mortality worldwide in 2013, with an incidence
rate of 1/100,000 in China (1). The
majority of patients with esophageal cancer are diagnosed in the
progressive stage (2). Current
treatments available for esophageal cancer result in poor prognosis
(3), with a 5-year survival rate of
13% (4). Thus, development of novel
therapeutic targets for the treatment of esophageal cancer remains
critical, and research into the molecular mechanisms underlying
tumorigenesis are required.
microRNAs (miRNAs) are small endogenous non-coding
RNAs that play essential roles in several complex physiological
processes, including cell proliferation and apoptosis, growth and
development, organogenesis, immune defense and inflammation
(5,6). Aberrant expression of miRNAs has been
implicated in a number of human diseases, including cancer
(7–9). Some miRNAs were reported to be
associated with the origin and development of esophageal cancer,
such as miR-21, miR-375 and the miR-17–92 cluster (10). miR-203 functions as a tumor
suppressor in several malignancies, and its expression has been
reported to vary across different types of tumor (11–14). It
has been demonstrated that miR-203 is significantly downregulated
in esophageal cancer (15). A
previous study reported that overexpression of miR-203 notably
induces cell apoptosis, inhibits cell proliferation, migration and
invasion in vitro, and suppresses tumor growth in
vivo in esophageal cancer cells (16). However, to the best of our knowledge,
the molecular mechanisms underlying the regulatory activities of
miR-203 in esophageal cancer are not yet fully understood.
The present study aimed to determine whether
Mitogen-Activated Protein Kinase Kinase Kinase 1 (MAP3K1) is a
direct target of miR-203, and investigate the molecular mechanism
underlying the role of miR-203 in esophageal cancer.
Materials and methods
Cell culture and transfection
TE-1 cells and 293 cells were purchased from The
Cell Bank of Type Culture Collection of the Chinese Academy of
Sciences, and cultured in RPMI-1640 medium (Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.), at 37°C in a humidified atmosphere of 5%
CO2. Prior to transfection, cells
(1×106/well) were washed 3 times with PBS and added to
serum-free RPMI-1640 medium. A total of 2 µg of miR-203
mimic/inhibitor, MAP3K1 pcDNA3.1 and MAP3K1 siRNA were transfected
into TE-1 cells using Lipofectamine™ 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.), according to the manufacturer's protocol.
After 6 h, the medium was replaced with fresh medium and maintained
in the cultures for at least 24 h for further analysis.
Luciferase reporter assay
The 3′-untranslated region (UTR) segment of MAP3K1
and its mutant were amplified and inserted into the pGL3-control
luciferase reporter vector (Promega Corporation). Cells transfected
with wild-type or mutated MAP3K1 3′UTR were divided into two
groups: Control group and mimic group. Cells in the control group
were transfected with the miR-negative control. Reporter plasmids
containing 3′UTR MAP3K1 were co-transfected with the miR-203 mimic
into 293 cells using Lipofectamine™ 2000 reagent. 48 h following
transfection, luciferase activity was determined using the
Luciferase Reporter Assay System and Luciferase Assay kit (both
from Promega Corporation). Results were expressed as the Firefly
luciferase activity normalized to Renilla luciferase
activity.
Western blotting
In order to examine the effect of miR-203
mimic/inhibitor on MAP3K1 protein expression, cells were divided
into three groups: Control group, mimic group and inhibitor group.
Cells in the control group were transfected with the miR-negative
control. Cells were divided into control, MAP3K1 pcDNA3.1 and
MAP3K1 siRNA groups to detect MAP3K1 and Fas protein expression in
MAP3K1-overexpressed or MAP3K1-knockdown cells. Cells in the
control group were transfected with the empty vector + scrambled
siRNA. Cells were divided into control, mimic and mimic + MAP3K1
groups to examine whether MAP3K1 could reverse the effect of
miR-203 mimic on MAP3K1 and Fas protein expression. Cells in the
control group were transfected with the miR-negative control +
empty vector. Cells were divided into control, inhibitor and
inhibitor + MAP3K1 siRNA groups to determine whether MAP3K1 could
reverse the effect of miR-203 inhibitor on MAP3K1 and Fas protein
expression. Cells in the control group were transfected with the
miR-negative control + scramble siRNA.
Total protein was extracted from cells using
ice-cold lysis buffer [150 mM NaCl, 150 mM Tris-HCl (pH 7.4), 0.2%
SDS, 1% Nonidet P-40, 50 mM sodium fluoride, 100 mM sodium vanadate
and 1 mM phenylmethylsulfonyl fluoride]. Protein concentrations
were determined using the BCA method. A total of 50 µg of
protein/lane was separated via SDS-PAGE on a 12% gel and
subsequently transferred onto a nitrocellulose membrane (EMD
Millipore). The membranes were blocked with 3% BSA (Sigma-Aldrich;
Merck KGaA) overnight at 4°C, prior to being washed three times
with TBST. Subsequently, the membranes were incubated with primary
antibodies against MAP3K1 (dilution, 1:800; cat. no. sc-449), Fas
(dilution, 1:400; cat. no. sc-74540;) and GAPDH (dilution, 1:1,000;
cat. no. sc-51907) (all from Santa Cruz Biotechnology, Inc.) at
37°C for 1 h. Membranes were washed with TBST 3 times and
subsequently incubated with horseradish peroxidase-labeled
secondary antibodies (dilution, 1:1,000; cat. no. sc-2005; Santa
Cruz Biotechnology, Inc.) at 37°C for 1 h. Protein bands were
visualized using an ECL detection kit (Pierce; Thermo Fisher
Scientific, Inc.).
Reverse transcription-quantitative
(RT-q)PCR
In order to examine the effect of miR-203
mimic/inhibitor on miR-203 and MAP3K1 mRNA expression, cells were
divided into the following three groups: Control group, mimic group
and inhibitor group; control, mimic and mimic + MAP3K1 groups, and
control, inhibitor and inhibitor + MAP3K1 siRNA groups, as
previously described. miRNAs were isolated using the miRNeasy Mini
kit (Qiagen GmbH), according to the manufacturer's protocol. Total
RNA was extracted from TE-1 cells using TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc.). A total of 50 ng of
total RNA was reverse transcribed into cDNA using the First Strand
cDNA Synthesis kit (Fermentas; Thermo Fisher Scientific, Inc.).
qPCR was subsequently performed using the SYBR-Green PCR kit, in a
7900 Sequence Detection System (both from Applied Biosystems;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocols. Thermocycling conditions were as follows: Initial
denaturation at 95°C for 3 min, followed by 40 cycles of
denaturation at 95°C for 30 sec, annealing at 60°C for 30 sec and
elongation at 72°C for 45 sec, final extension at 72°C for 5 min.
The primers used were: miR-203 forward,
5′-agtggttcttaacagttcaacagtt-3′ and reverse 5′-tggtgtcgtggagtcg-3′;
U6 forward, 5′-ctcgcttcggcagcaca-3′ and reverse,
5′-aacgcttcacgaatttgcgt-3′; MAP3K1 forward,
5′-ccacagagaacagttcccct-3′ and reverse, 5′-ccattggctttggttgctct-3′;
GAPDH, forward, 5′-ctgacttcaacagcgacacc-3′ and reverse,
5′-gtggtccaggggtcttactc-3′. Relative expression levels were
measured using the 2−ΔΔCq method (17). miRNA levels were normalized to the
internal control U6, while MAP3K1 mRNA levels was normalized to the
internal reference gene GAPDH.
MTT assay
In order to examine the proliferation of
MAP3K1-overexpressed or MAP3K1-knockdown cells, cells were divided
into the respective groups: Control group, MAP3K1 pcDNA3.1 group
and MAP3K1 siRNA group; control, mimic and mimic + MAP3K1 groups,
and control, inhibitor and inhibitor + MAP3K1 siRNA groups, as
aforementioned. Cell proliferation was determined via an MTT assay.
Cells (1×104/well) were seeded into 96-well plates and
allowed to grow for the appropriate times (24, 48, 72 and 96 h) at
37°C with 5% CO2, prior to incubation with 10 µl of MTT
(Sigma-Aldrich; Merck KGaA) at 37°C for 4 h. Following MTT
incubation, the purple formazan crystals were dissolved using 200
µl of dimethyl sulfoxide (Sigma-Aldrich; Merck KGaA) and cell
proliferation was subsequently analyzed at a wavelength of 570 nm,
using a microplate reader (Molecular Devices, LLC).
Flow cytometry analysis of
apoptosis
In order to detect the apoptotic rate of
MAP3K1-overexpressed or MAP3K1-knockdown cells, cells were divided
into the respective groups: Control group, MAP3K1 pcDNA3.1 group
and MAP3K1 siRNA group; control, mimic and mimic + MAP3K1 groups,
and control, inhibitor and inhibitor + MAP3K1 siRNA groups, as
aforementioned. TE-1 cells were washed three times with PBS and
resuspended in binding buffer (Nanjing KeyGen Biotech Co., Ltd.).
The cells were subsequently stained with 5 µl Annexin-V and 5 µl
propidium iodide (Nanjing KeyGen Biotech Co., Ltd.) for 20 min at
room temperature in the dark. Apoptotic cells were subsequently
analyzed using a FACSCalibur flow cytometer (BD Biosciences) and
CellQuest Pro software (version 5.2; BD Biosciences).
Caspase 8/3 activity assay
In order to examine caspase 8/3 activity in
MAP3K1-overexpressed or MAP3K1-knockdown cells, cells were divided
into the following three groups: Control group, MAP3K1 pcDNA3.1
group and MAP3K1 siRNA group; control, mimic and mimic + MAP3K1
groups, and control, inhibitor and inhibitor+MAP3K1 siRNA groups,
as previously described. The activities of caspase 8 and caspase 3
in TE-1 cells were examined using the Caspase 8/Caspase 3
Colorimetric Assay kit (Nanjing KeyGen Biotech Co., Ltd.),
according to the manufacturer's protocol.
Cell invasion assay
In order to investigate cell invasion ability of
MAP3K1-overexpressed or MAP3K1-knockdown cells, cells were divided
into the following groups: Control group, MAP3K1 pcDNA3.1 group and
MAP3K1 siRNA group; control, mimic and mimic + MAP3K1 groups, and
control, inhibitor and inhibitor + MAP3K1 siRNA groups, as
aforementioned. The cell invasion assay was performed using
Transwell inserts (Corning, Inc.) coated with Matrigel matrix (BD
Biosciences) at 37°C for 30 min. Cell suspension was prepared in
serum-free medium at a density of 5×104 cells/ml and
added to the upper chambers. A total of 1 ml of cell medium
containing 10% FBS was added to the lower chambers. Following
overnight incubation at 37°C, cotton swabs were used to gently
remove non-invaded cells. Cells on the lower surface of the
membrane were fixed in 95% ethanol for 20 min and subsequently
stained with hematoxylin for 10 min at room temperature. The number
of invaded cells was counted under an inverted light microscope
(Nikon Corporation; magnification, ×400).
Statistical analysis
Statistical analysis was performed using SPSS
software (version 19.0; SPSS, Inc.). Data are presented as the mean
± standard deviation. Unpaired two-tailed Student's t-test and
one-way analysis of variance, followed by Student-Keuls-Neuman
test, were used to analyze the statistical difference between the
respective groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
MAP3K1 is directly regulated by
miR-203
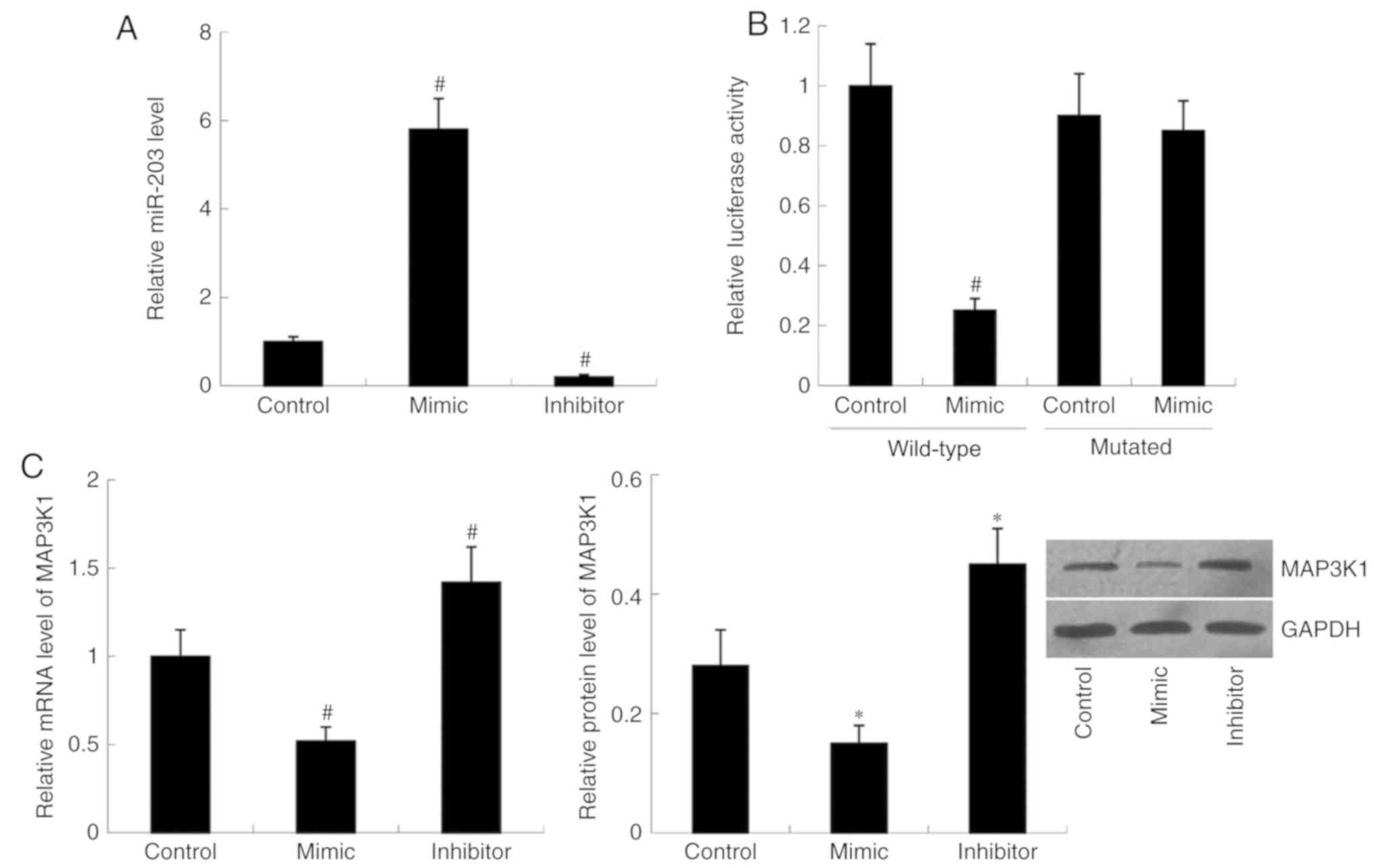
miR-203 mimic and miR-203 inhibitor were transfected
into cells, respectively. Relative miR-203 level was significantly
increased in the mimic group (P<0.01) but decreased in the
inhibitor group (P<0.01) compared with the control group
(Fig. 1A). Subsequently, MAP3K1
expression was examined in the cells following transfection with
miR-203 mimic and miR-203 inhibitor. RT-qPCR and western blot
analyses demonstrated that transfection with miR-203 mimic
decreased both MAP3K1 mRNA (P<0.01) and protein (P<0.05)
expression compared with the control group. Conversely, relative
MAP3K1 mRNA (P<0.05) and protein (P<0.01) levels were
significantly increased in cells transfected with miR-203 inhibitor
compared with the control group (Fig.
1C).
Luciferase-expressing plasmids containing the
wild-type MAP3K1-3′UTR or mutant MAP3K1-3′UTR were constructed and
co-transfected with miR-203 mimic or control. The results indicated
that translational activity of the wild-type MAP3K1-3′UTR was
significantly decreased in the miR-203 mimic group compared with
that of the control group (P<0.01). However, no significant
difference in translational activity of the mutant MAP3K1-3′UTR was
observed between the miR-203 mimic group and the control group
(Fig. 1B).
Effect of MAP3K1 on cell
proliferation, apoptosis and invasion
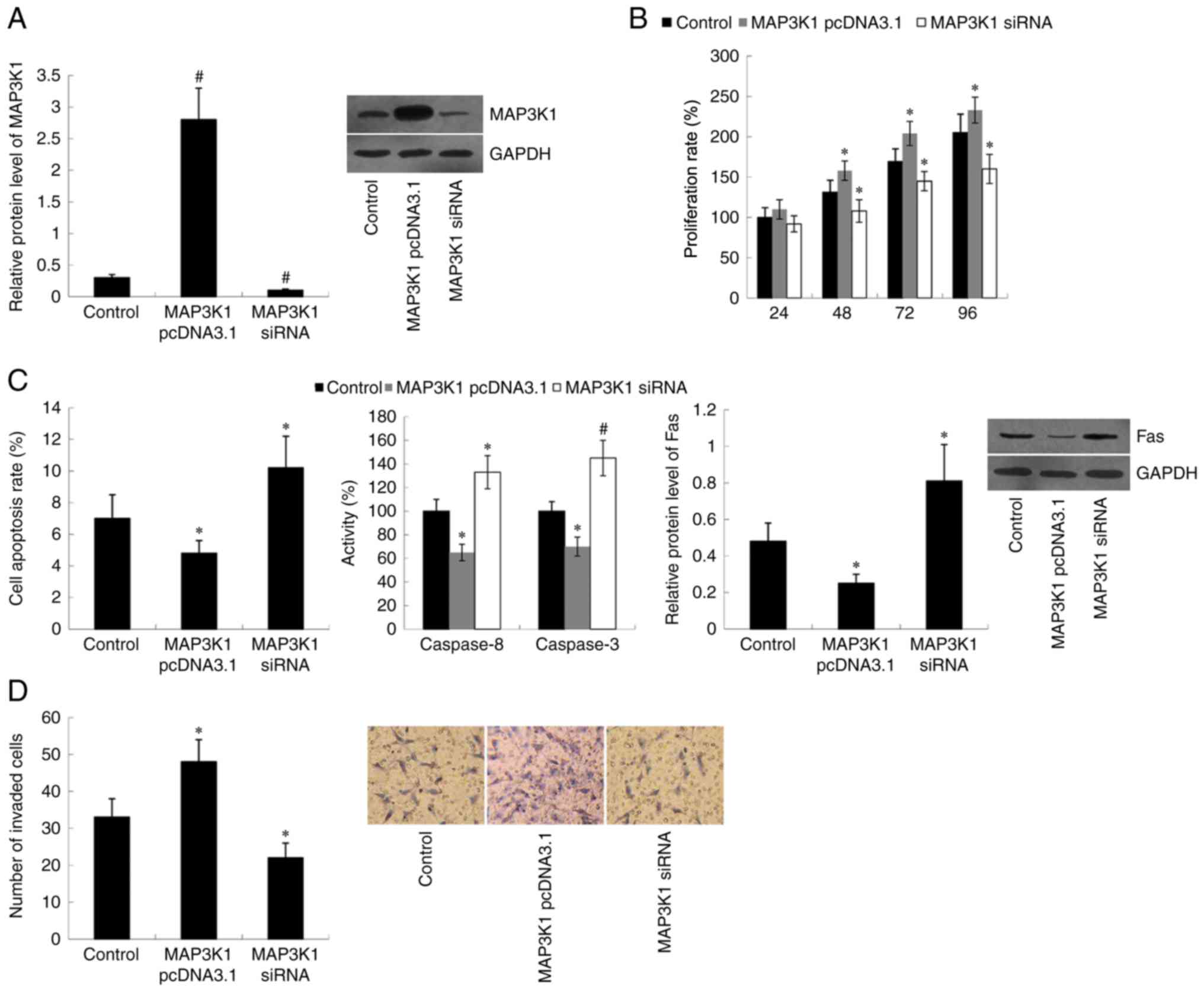
TE-1 cells were transfected with MAP3K1 pcDNA3.1 or
MAP3K1 siRNA to overexpress or knockdown MAP3K1, respectively. The
western blot analysis demonstrated that MAP3K1 protein expression
was significantly upregulated in MAP3K1 pcDNA3.1-transfected cells
(P<0.01) and downregulated in MAP3K1 siRNA-transfected cells
(P<0.01), compared with the control group (Fig. 2A). Subsequently, the effect of MAP3K1
on cell proliferation, apoptosis and invasion was assessed. Results
of the MTT assay indicated that transfection with MAP3K1 pcDNA3.1
significantly induced cell proliferation (P<0.05 at 48, 72 and
96 h), whereas transfection with MAP3K1 siRNA significantly
inhibited cell proliferation (P<0.05 at 48, 72 and 96 h)
compared with the control group (Fig.
2B). Furthermore, cell apoptotic rate, Caspase 8/3 activity and
Fas protein expression were significantly decreased in MAP3K1
pcDNA3.1-transfected cells (P<0.05) and increased in MAP3K1
siRNA-transfected cells (P<0.05 for cell apoptotic rate and Fas
protein expression; P<0.01 for Caspase 8/3 activity) compared
with the control group (Fig. 2C).
MAP3K1 failed to demonstrate effects on cell proliferation at 24 h
(Fig. 2B) following transfection
with MAP3K1 pcDNA3.1 or MAP3K1 siRNA, thus cell invasion ability
was assessed within 24 h (overnight, 12–16 h). Therefore, the
change in cell invasion ability could not be affected by cell
proliferation in the study. The results demonstrated that MAP3K1
overexpression promoted cell invasion (P<0.05), while MAP3K1
knockdown inhibited cell invasion (P<0.05) compared with the
control group (Fig. 2D).
MAP3K1 reverses the effect of miR-203
on cell proliferation, apoptosis and invasion
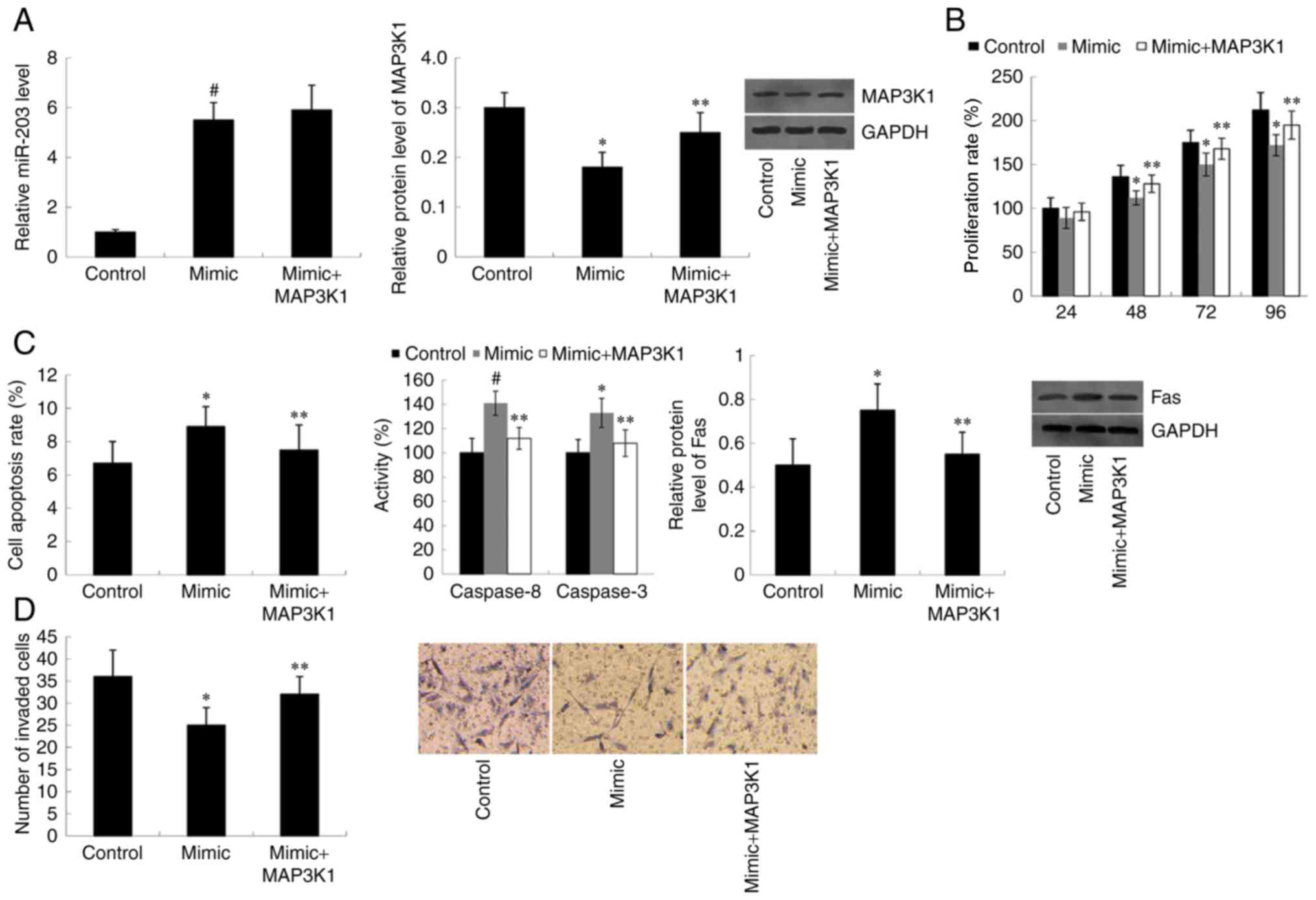
Cells were co-transfected with miR-203 mimic and
MAP3K1 pcDNA3.1, and RT-qPCR analysis was performed to detect
miR-203 expression. As presented in Fig.
3A, overexpression of MAP3K1 did not affect the increased
miR-203 expression exhibited in miR-203 mimic-transfected cells.
However, western blot analysis demonstrated that the decreased
MAP3K1 protein expression observed in miR-203 mimic-transfected
cells was significantly rescued following transfection with MAP3K1
pcDNA3.1 (P<0.05). Furthermore, transfection with miR-203 mimic
suppressed the cell proliferation rate compared with the control
group (P<0.05 at 48, 72 and 96 h). However, overexpression of
MAP3K1 reversed the inhibitory effect of miR-203 mimic on cell
proliferation. Cells co-transfected with miR-203 mimic and the
MAP3K1 overexpression plasmid proliferated at a faster rate
compared with the miR-203 mimic-transfected cells (P<0.05 at 48,
72 and 96 h) (Fig. 3B). As presented
in Fig. 3C, the apoptotic rate,
Caspase 8/3 activity and Fas protein expression increased in cells
transfected with miR-203 mimic compared with the control group
(P<0.05 for cell apoptotic rate and Fas protein expression;
P<0.01 for Caspase 8/3 activity). However, the increased cell
apoptotic rate observed in miR-203 mimic-transfected cells was
significantly reversed following co-transfection with the MAP3K1
overexpression plasmid (P<0.05). Furthermore, overexpression of
MAP3K1 was demonstrated to reverse the inhibitory effect of miR-203
mimic on cell invasion (P<0.05) (Fig.
3D).
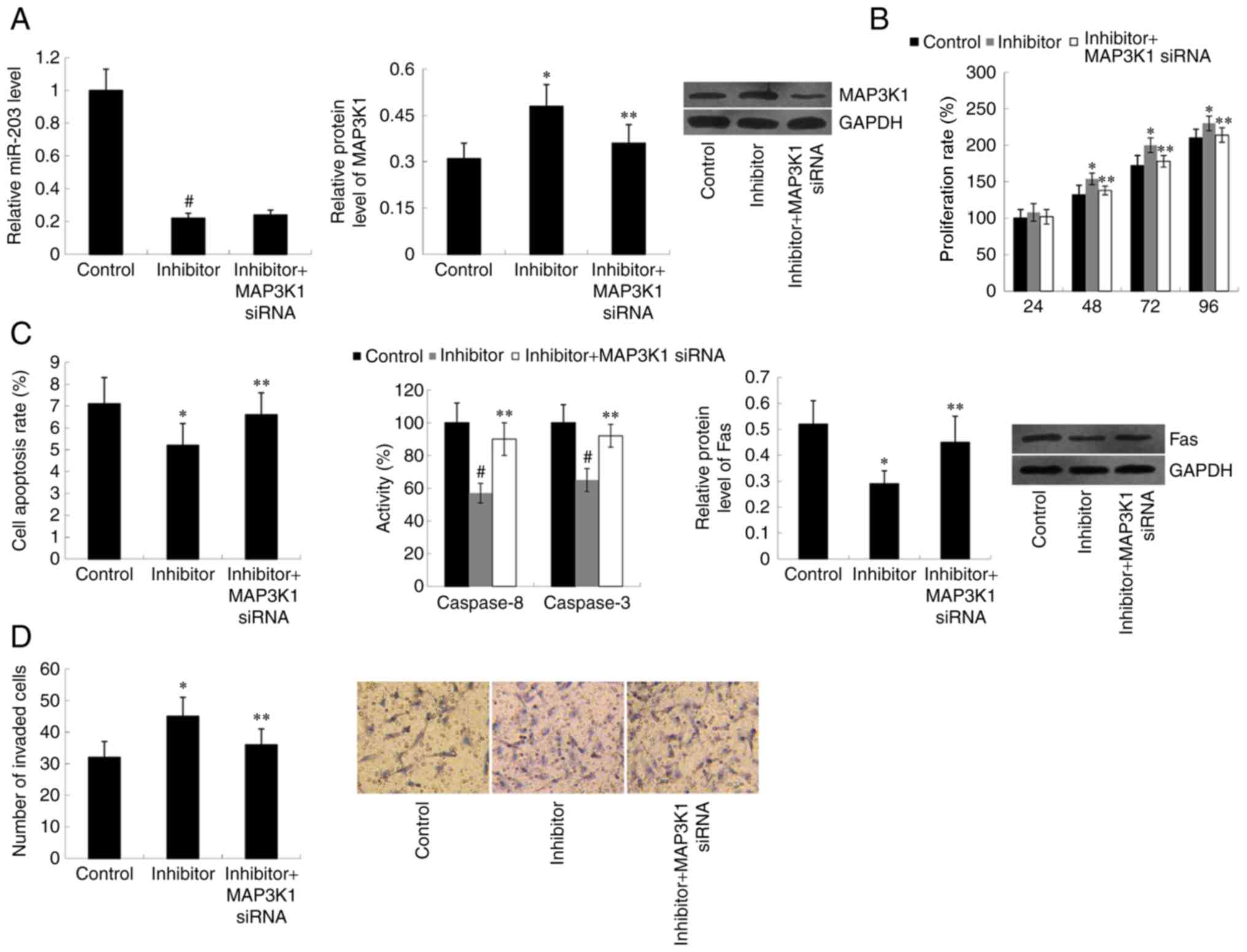
miR-203 and MAP3K1 expression in cells
co-transfected with miR-203 inhibitor and MAP3K1 siRNA was
subsequently assessed. MAP3K1 siRNA significantly decreased MAP3K1
protein expression in cells transfected with miR-203 inhibitor
(P<0.05); however, MAP3K1 siRNA was demonstrated to have no
effect on miR-203 expression compared with the control group
(Fig. 4A). As expected, the effect
of miR-203 inhibitor on cell proliferation, apoptosis and invasion
was weakened by MAP3K1 siRNA (P<0.05) (Fig. 4B-D).
Discussion
miRNA is known to regulate several target genes
(18). Previous studies have
reported a number of target genes of miR-203, including twist 1,
RGS17, PBOV1, EGR1 and FGF2 (13,12,19,20).
Using prediction software, the present study hypothesized that
MAP3K1 may be a target gene of miR-203. Results of the luciferase
reporter assay demonstrated that miR-203 directly binds to the
3′UTR of MAP3K1, while western blotting analysis indicated that
MAP3K1 protein expression was upregulated by miR-203.
MAP3K1 is a serine/threonine kinase belonging to the
MAP3K family (21,22). It is activated by various stimuli,
such as cellular stress, growth factors and cytokines, and is part
of several signal transduction cascades, including the ERK, JNK and
NF-κB signaling pathways (23).
Previous studies have demonstrated that MAP3K1 plays critical roles
in multiple aspects of cell physiology (24,25),
including cell proliferation, apoptosis and motility in both normal
and tumor cells (22,26). For example, MAP3K1-targeting
artificial miRNA suppresses the growth and invasion of breast
cancer both in vivo and in vitro (27). Furthermore, depletion of MAP3K1
inhibits survival, invasion and migration of human pancreatic
cancer cells (28,29). Bian et al (30) reported that MAP3K1 activity is
essential for Lysophosphatidic acid-stimulated ovarian cancer cell
migration (30). Taken together,
these findings indicate that MAP3K1 plays a facilitating role in
the development of several types of tumor. However, the role of
MAP3K1 in esophageal cancer remains to be elucidated.
The present study performed in vitro
gain-of-function and loss-of-function experiments, in order to
investigate the effect of MAP3K1 on TE-1 cell proliferation,
apoptosis and invasion. The results demonstrated that MAP3K1
promoted cell proliferation and invasion, and inhibited cell
apoptosis, thus exerting a tumor-promoting role in esophageal
cancer. These results are consistent with those of a previous
study, which reported that MAP3K1 acts as a tumor promoter in
EC9706 cells (31).
Furthermore, MAP3K1 overexpression plasmid or MAP3K1
siRNA were transfected into TE-1 cells in the presence of miR-203
mimic or miR-203 inhibitor, in order to determine whether MAP3K1
was involved in the varying effects of miR-203 on cell
proliferation, apoptosis and invasion. It was demonstrated that
overexpression of MAP3K1 reversed the suppressed cell proliferation
and invasion abilities induced by miR-203 mimic, and the inhibitory
effect of miR-203 mimic on cell apoptosis. Furthermore, MAP3K1
siRNA weakened the effect of miR-203 inhibitor on cell
proliferation, apoptosis and invasion. Theses results suggest that
MAP3K1 plays a notable role in mediating the effect of miR-203 on
esophageal cancer cell proliferation, apoptosis and invasion.
The present study demonstrated that the cell
apoptosis rates were <10%. Regarding flow cytometry analysis,
cell debris existed, which may have affected accurate measurement
of the cell apoptotic rate. A limitation of the present study is
that cell proliferation was only detected via an MTT assay. Future
studies will aim to include cell cycle analysis to accurately
investigate cell proliferation.
Taken together, the results of the present study
provide a novel molecular mechanism underlying the role of miR-203
in esophageal cancer, and suggest that MAP3K1 is a direct target of
miR-203. miR-203 was able to suppress esophageal cancer cell
proliferation and invasion, and induce cell apoptosis at least
partially by inhibiting MAP3K1 expression.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
WY and MZ designed the present study. MZ, WF, LW and
XY performed the experiments and analyzed the data. MZ drafted the
initial manuscript. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Global Burden of Disease Cancer
Collaboration, . Fitzmaurice C, Dicker D, Pain A, Hamavid H,
Moradi-Lakeh M, MacIntyre MF, Allen C, Hansen G, Woodbrook R, et
al: The global burden of cancer 2013. JAMA Oncol. 1:505–527. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yokota T, Igaki H, Kato K, Tsubosa Y,
Mizusawa J, Katayama H, Nakamura K, Fukuda H and Kitagawa Y:
Accuracy of preoperative diagnosis of lymph node metastasis for
thoracic esophageal cancer patients from JCOG9907 trial. Int J Clin
Oncol. 21:283–288. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kamangar F, Dores GM and Anderson WF:
Patterns of cancer incidence, mortality, and prevalence across five
continents: Defining priorities to reduce cancer disparities in
different geographic regions of the world. J Clin Oncol.
24:2137–2150. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mohr AM and Mott JL: Overview of microRNA
biology. Semin Liver Dis. 35:3–11. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vienberg S, Geiger J, Madsen S and
Dalgaard LT: MicroRNAs in metabolism. Acta Physiol (Oxf).
219:346–361. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Reddy KB: MicroRNA (miRNA) in cancer.
Cancer Cell Int. 15:382015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Iorio MV and Croce CM: MicroRNA
dysregulation in cancer: Diagnostics, monitoring and therapeutics.
A comprehensive review. EMBO Mol Med. 4:143–159. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kiselev FL: MicroRNA and cancer. Mol Biol
(Mosk). 48:232–242. 2014.(In Russian). PubMed/NCBI
|
|
10
|
Mei LL, Qiu YT, Zhang B and Shi ZZ:
MicroRNAs in esophageal squamous cell carcinoma: Potential
biomarkers and therapeutic targets. Cancer Biomark. 19:1–9. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liang Y, Yang W, Zhu Y and Yuan Y:
Prognostic role of microRNA-203 in various carcinomas: Evidence
from a meta-analysis involving 13 studies. SpringerPlus.
5:15382016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chi Y, Jin Q, Liu X, Xu L, He X, Shen Y,
Zhou Q, Zhang J and Jin M: miR-203 inhibits cell proliferation,
invasion, and migration of non-small-cell lung cancer by
downregulating RGS17. Cancer Sci. 108:2366–2372. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shen J, Zhang J, Xiao M, Yang J and Zhang
N: miR-203 suppresses bladder cancer cell growth and targets
twist1. Oncol Res. 26:1155–1165. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen LZ, Ding Z, Zhang Y, He ST and Wang
XH: MiR-203 over-expression promotes prostate cancer cell apoptosis
and reduces ADM resistance. Eur Rev Med Pharmacol Sci.
22:3734–3741. 2018.PubMed/NCBI
|
|
15
|
Hezova R, Kovarikova A, Srovnal J,
Zemanova M, Harustiak T, Ehrmann J, Hajduch M, Svoboda M, Sachlova
M and Slaby O: Diagnostic and prognostic potential of miR-21,
miR-29c, miR-148 and miR-203 in adenocarcinoma and squamous cell
carcinoma of esophagus. Diagn Pathol. 10:422015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang F, Yang Z, Cao M, Xu Y, Li J, Chen
X, Gao Z, Xin J, Zhou S, Zhou Z, et al: MiR-203 suppresses tumor
growth and invasion and down-regulates MiR-21 expression through
repressing Ran in esophageal cancer. Cancer Lett. 342:121–129.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang SY, Gao F, Peng CG, Zheng CJ and Wu
MF: Hsa-miR-203 inhibits fracture healing via targeting PBOV1. Eur
Rev Med Pharmacol Sci. 22:5797–5803. 2018.PubMed/NCBI
|
|
20
|
Shi K, Qiu X, Zheng W, Yan D and Peng W:
MiR-203 regulates keloid fibroblast proliferation, invasion, and
extracellular matrix expression by targeting EGR1 and FGF2. Biomed
Pharmacother. 108:1282–1288. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pham TT, Angus SP and Johnson GL: MAP3K1:
Genomic alterations in cancer and function in promoting cell
survival or apoptosis. Genes Cancer. 4:419–426. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hagemann C and Blank JL: The ups and downs
of MEK kinase interactions. Cell Signal. 13:863–875. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bonvin C, Guillon A, van Bemmelen MX,
Gerwins P, Johnson GL and Widmann C: Role of the amino-terminal
domains of MEKKs in the activation of NF kappa B and MAPK pathways
and in the regulation of cell proliferation and apoptosis. Cell
Signal. 14:123–131. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kyriakis JM and Avruch J: Mammalian
mitogen-activated protein kinase signal transduction pathways
activated by stress and inflammation. Physiol Rev. 81:807–869.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Uhlik MT, Abell AN, Cuevas BD, Nakamura K
and Johnson GL: Wiring diagrams of MAPK regulation by MEKK1, 2, and
3. Biochem Cell Biol. 82:658–663. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lange-Carter CA, Pleiman CM, Gardner AM,
Blumer KJ and Johnson GL: A divergence in the MAP kinase regulatory
network defined by MEK kinase and Raf. Science. 260:315–319. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu C, Wang S, Zhu S, Wang H, Gu J, Gui Z,
Jing J, Hou X and Shao Y: MAP3K1-targeting therapeutic artificial
miRNA suppresses the growth and invasion of breast cancer in vivo
and in vitro. SpringerPlus. 5:112016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hirano T, Shino Y, Saito T, Komoda F,
Okutomi Y, Takeda A, Ishihara T, Yamaguchi T, Saisho H and
Shirasawa H: Dominant negative MEKK1 inhibits survival of
pancreatic cancer cells. Oncogene. 21:5923–5928. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Su F, Li H, Yan C, Jia B, Zhang Y and Chen
X: Depleting MEKK1 expression inhibits the ability of invasion and
migration of human pancreatic cancer cells. J Cancer Res Clin
Oncol. 135:1655–1663. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bian D, Su S, Mahanivong C, Cheng RK, Han
Q, Pan ZK, Sun P and Huang S: Lysophosphatidic acid stimulates
ovarian cancer cell migration via a ras-MEK kinase 1 pathway.
Cancer Res. 64:4209–4217. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zang WQ, Yang X, Wang T, Wang YY, Du YW,
Chen XN, Li M and Zhao GQ: MiR-451 inhibits proliferation of
esophageal carcinoma cell line EC9706 by targeting CDKN2D and
MAP3K1. World J Gastroenterol. 21:5867–5876. 2015. View Article : Google Scholar : PubMed/NCBI
|