Introduction
Lung cancer consists of non-small cell lung cancer
and small cell lung cancer. Non-small cell lung cancer accounts for
about 85% in lung cancer (1,2). The subtype of non-small cell lung
cancer is generally lung adenocarcinoma, which accounts for about
40% in lung cancer (3). At present,
the early diagnosis of patients with lung adenocarcinoma is still
poor. Many patients are in the advanced stage or have metastasis
when they are diagnosed, which makes the treatment of patients very
difficult (4,5). At present, small pulmonary nodules are
treated by non-invasive methods such as chest computed tomography
(CT) and MRI and invasive diagnostic methods such as biopsy and
puncture (6). Chest CT is an optimal
method to screen lung cancer, and has high resolution. The
detection rate of lung ground glass nodules (GGN) is increasing
with the application of chest CT (7).
GGN lesion can be divided into pure GGN (pGGN)
lesion and mixed GGN (mGGN) lesion in CT imaging. It is shown that
the malignant rate of GGN of early lung adenocarcinoma is high in
thin slice CT. A total of 18% of pGGN and 63% of mGGN are malignant
niduses (8). According to the
classification criteria of International Association for the Study
of Lung Cancer (IASLC), American Thoracic Society (ATS), and
European Respiratory Society (ERS), lung adenocarcinoma can be
divided into atypical adenomatous hyperplasia (AAH), adenocarcinoma
in situ (AIS), microinvasive adenocarcinoma (MIA), and
invasive adenocarcinoma (IAC) (9,10). AAH
and AIS are considered as pre-invasive lung adenocarcinoma, and MIA
and IAC as invasive lung adenocarcinoma. However, there are pGGN or
mGGN in AAH, AIS, MIA, and IAC and there are different solid
components in CT imaging (11,12).
There are significant differences between the operation of
pre-invasive lung adenocarcinoma and the operation of invasive lung
adenocarcinoma. Also, the lymph node structure of patients with
pre-invasive lung adenocarcinoma or invasive lung adenocarcinoma is
different (13,14). Therefore, it is crucial to
discriminate invasive lesions and invasive lung adenocarcinoma
accurately, especially in terms of operation plan, prognosis
assessment, and doctor-patient communication.
With the development of CT technology,
multi-detector CT (MDCT) is used to screen tumors, because its time
resolution is good, and it can process data in a same direction and
has various image processing functions (15). In the present study, MDCT was used to
examine GGN patients. The imaging features of MDCT and its
predictive value for pre-invasive lung adenocarcinoma and invasive
lung adenocarcinoma were investigated.
Patients and methods
General data
The data of 168 patients with pulmonary GGN were
analyzed. These patients were diagnosed in Shengli Oilfield Central
Hospital (Dongying, China) from January 2013 to June 2015.
According to the classification criteria of IASLC/ATS/ERS (16), there were 11 patients with AAH, 59
patients with AIS, 29 patients with MIA, and 69 patients with IAC.
Patients with MIA and IAC were included in group A (invasive lung
adenocarcinoma, n=98), and patients with AAH and AIS were included
in group B (pre-invasive lung adenocarcinoma, n=70). There were 38
patients with pGGN and 60 patients with mGGN in group A, including
41 males and 57 females, aged 36 to 81 years, with a mean age of
(57.3±6.2). There were 51 patients with pGGN and 19 patients with
mGGN in group B, including 32 males and 38 females, aged 35 to 79
years, with a mean age of (57.9±6.1).
This study was approved by the Ethics Committee of
Shengli Oilfield Central Hospital. Signed informed consent was
obtained from the patients or their guardians.
Inclusion and exclusion criteria
Inclusion criteria: Patients with lung
adenocarcinoma according to postoperative histopathological
diagnosis (17); with complete
medical record; without primary tumors and patients with blood
sugar levels at normal levels. Exclusion criteria: Patients with
history of iodine allergy and other drug allergies; with history of
thoracic surgery; with other tumors; complicated with severe liver
dysfunction and kidney dysfunction; with acute pulmonary infection,
connective tissue diseases, infectious diseases, metabolic
diseases, hematopoietic dysfunction and patients with mental
illness or a family history of mental illness, pregnant or
lactating women.
MDCT examination
Patients were scanned by a SOMATOM Definition AS+
64-row 128-slice spiral CT scanner (Siemens Healthcare GmbH). The
patients took a supine position, with their heads close to the
scanner, then they took a deep breath and held the breath. Scope:
Between the sternoclavicular joint and the base of lungs, including
the chest wall and armpits on both sides. The sternoclavicular
joint was used as a baseline. Scanning parameters: Tube current 170
mAs; tube voltage 120 kV; visual field 350 mm; layer thickness 5
mm; and reconstruction thickness 2 mm. Subsequently, an enhanced
examination was performed. The contrast agent, iopamidol with a
concentration of 1.5 ml/kg, was injected into the elbow vein of the
patients by a high pressure injector at a rate of 2.5 ml/sec, and
the dosage was between 80 and 100 ml (Shanghai Bracco Sine
Pharmaceutical Corp. Ltd.; H20053388). After this step was
finished, the patients took a deep breath and held the breath, then
their lungs were scanned. The window level of mediastinal imaging
was 220 HU, the window width was 40 HU. The width of the lung
window was 1,600 HU, the window level was −600 HU. After the
patients were scanned, the data were input into Extended
BrillianceTM workstation and the imaging was
rebuilt.
Imaging analysis
Data were observed, including type of nidus (single
and multiple), site of nidus (left, right), size of nidus (the
length, diameter, axis of nidus and the length of solid component),
size of solid component (the diameter of solid component), nature
of nidus (pGGN, mGGN), pleura, burr, lobe, periphery, vacuole, and
similar round. CT imaging was analyzed by two radiologists.
Statistical analysis
Statistical analysis was performed by SPSS 22.0 (IBM
Corp). The count data were expressed as [n (%)]. Chi-square test
was used to compare the count data between groups. The measurement
data were expressed as mean value ± standard deviation (mean ± SD).
Independent sample t-test was used to compare the measurement data
between groups. ROC curves of the size of nidus and size of solid
component were drawn. Logistic multivariate regression analysis was
used to analyze the risk factors that affect invasive lung
adenocarcinoma. P<0.05 indicates that the difference is
statistically significant.
Results
General data of the patients in two
groups
There was no significant difference in general data
of the patients in groups A and B, including sex, age, body mass
index, smoking history, respiratory symptoms, family history of
cancers, carcinoembryonic antigen (CEA), aspartate aminotransferase
(AST) and alanine aminotransferase (ALT) (P>0.05) (Table I).
 | Table I.General data of the patients in groups
A and B, n(%)/(mean ± SD). |
Table I.
General data of the patients in groups
A and B, n(%)/(mean ± SD).
| Categories | Group A (n=98) | Group B (n=70) | t/χ2 | P-value |
|---|
| Sex |
|
| 0.250 |
0.617 |
| Male | 41 (41.84) | 32 (45.71) |
|
|
|
Female | 57 (58.16) | 38 (54.29) |
|
|
| Age (years) | 57.3±6.2 | 57.9±6.1 | 0.534 |
0.623 |
| BMI
(kg/m2) | 22.26±3.59 | 22.89±3.47 | 1.137 |
0.257 |
| Smoking history |
|
| 0.428 |
0.513 |
| Yes | 47 (47.96) | 30 (42.86) |
|
|
| No | 51 (52.04) | 40 (57.14) |
|
|
| Respiratory
symptoms |
|
| 0.744 |
0.388 |
| Yes | 19 (19.39) | 10 (14.29) |
|
|
| No | 79 (80.61) | 60 (85.71) |
|
|
| Family history of
cancers |
|
| 0.520 |
0.471 |
| Yes | 15 (15.31) | 8 (11.43) |
|
|
| No | 83 (84.69) | 62 (88.57) |
|
|
| CEA (ng/ml) | 3.16±1.54 | 2.84±1.35 | 1.397 |
0.164 |
| AST (U/l) | 21.07±11.27 | 20.25±10.56 | 0.477 |
0.634 |
| ALT (U/l) | 27.63±9.48 | 28.41±9.24 | 0.531 |
0.596 |
Imaging features of MDCT of the
patients in two groups
Size of nidus of patients in group B was smaller,
many patients in group B had pGGN. Many patients in group A had
mGGN. There were no or only a few solid components in mGGN in the
plain scan. Solid components could be seen after CT was enhanced.
In the imaging of MDCT of patients in group A and B, there was no
significant difference in the type of nidus, site of nidus, pleural
indentation, clear periphery, vacuole, and the ratio of similar
round (P>0.05). There were significant differences in the nature
of nidus, burr, and lobes of patients in group A and B (P<0.05).
Sizes of nidus and solid components of patients in group A were
significantly bigger than those of patients in group B (P<0.05)
(Table II).
 | Table II.Comparison of the imaging features of
MDCT of the patients in groups A and B (mean ± SD). |
Table II.
Comparison of the imaging features of
MDCT of the patients in groups A and B (mean ± SD).
| Categories | Group A (n=98) | Group B (n=70) |
t/χ2 | P-value |
|---|
| Type of nidus |
|
| 0.049 | 0.826 |
|
Single | 77 (78.57) | 54 (77.14) |
|
|
|
Multiple | 21 (21.43) | 16 (22.86) |
|
|
| Site of nidus |
|
| 0.334 | 0.563 |
|
Right | 53 (54.08) | 41 (58.57) |
|
|
|
Left | 45 (45.92) | 29 (41.43) |
|
|
| Size of nidus
(mm) | 12.3±2.4 | 8.6±3.9 | 7.596 | <0.001 |
| Size of solid
component (mm) | 1.6±0.8 | 0.6±0.4 | 9.628 | <0.001 |
| Nature of
nidus |
|
| 19.040 | <0.001 |
|
pGGN | 38 (38.78) | 51 (72.86) |
|
|
|
mGGN | 60 (61.22) | 19 (27.14) |
|
|
| Pleural
indentation |
|
| 3.323 | 0.068 |
|
Yes | 10 (10.20) | 2 (2.86) |
|
|
| No | 88 (89.80) | 68 (97.14) |
|
|
| Burr |
|
| 21.661 | <0.001 |
|
Yes | 36 (36.73) | 4 (5.71) |
|
|
| No | 62 (63.27) | 66 (94.29) |
|
|
| Lobes |
|
| 23.851 | <0.001 |
|
Yes | 72 (73.47) | 25 (35.71) |
|
|
| No | 26 (26.53) | 45 (64.29) |
|
|
| Clear
periphery |
|
| 2.661 | 0.103 |
|
Yes | 81 (82.65) | 64 (91.43) |
|
|
| No | 17 (17.35) | 6 (8.57) |
|
|
| Vacuole |
|
| 3.680 | 0.055 |
|
Yes | 36 (36.73) | 16 (22.86) |
|
|
| No | 62 (63.27) | 54 (77.14) |
|
|
| Similar round |
|
| 1.721 | 0.190 |
|
Yes | 65 (66.33) | 53 (75.71) |
|
|
| No | 33 (33.67) | 17 (24.29) |
|
|
Diagnostic value of the size of nidus
and the size of solid component for invasive lung
adenocarcinoma
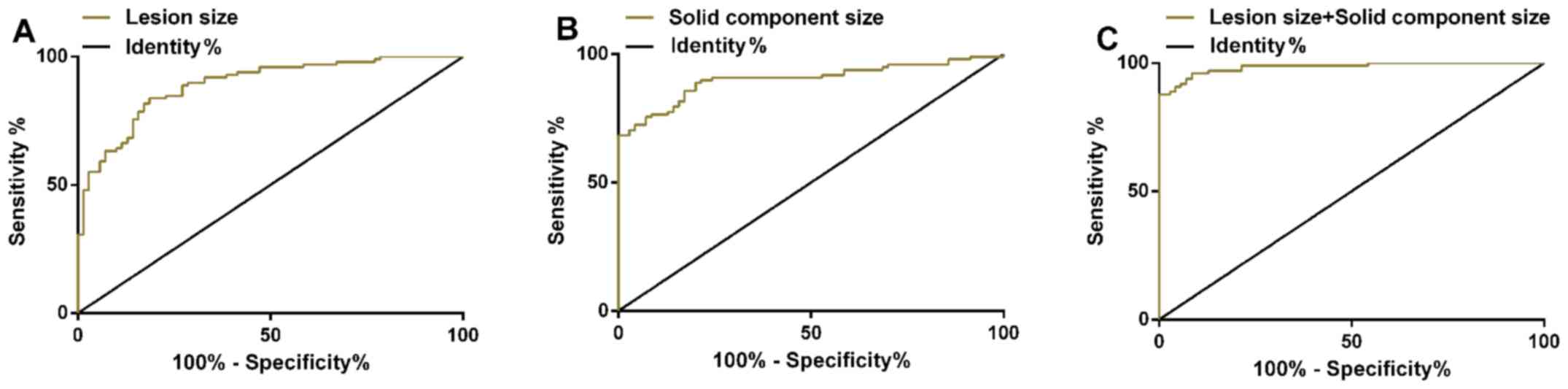
ROC curves of the size of nidus and the size of
solid component in invasive lung adenocarcinoma were drawn. AUC of
the size of nidus in the treatment of invasive lung adenocarcinoma
was 0.891 (95% CI: 0.843–0.939), and sensitivity was 83.67%;
specificity was 81.43%, and optimal cut-off value was 9.8 mm. AUC
of the size of solid component in the treatment of invasive lung
adenocarcinoma was 0.902 (95% CI: 0.854–0.949), and sensitivity was
88.78%; specificity was 80.00%, and the optimal cut-off value was
0.9 mm. ROC curve was plotted to investigate the diagnosis of the
size of nidus combined with the size of the solid component for
invasive lung adenocarcinoma. The combined AUC was 0.984 (95% CI:
0.969–0.999); sensitivity was 87.76%; and specificity was 100.00%
(Table III and Fig. 1).
 | Table III.The diagnostic value of the size of
nidus and the size of solid component for invasive lung
adenocarcinoma. |
Table III.
The diagnostic value of the size of
nidus and the size of solid component for invasive lung
adenocarcinoma.
| Parameters | AUC | 95% CI | SE | Cut-off | Sensitivity
(%) | Specificity
(%) |
|---|
| Size of nidus | 0.891 | 0.843–0.939 | 0.024 | 9.8 (mm) | 83.67 | 81.43 |
| Size of solid
component | 0.902 | 0.854–0.949 | 0.024 | 0.9 (mm) | 88.78 | 80.00 |
| Size of nidus +
size of solid component | 0.984 | 0.969–0.999 | 0.001 | 0.9 | 87.76 | 100.00 |
Logistic multivariate regression
analysis of risk factors of invasive lung adenocarcinoma
Logistic multivariate regression analysis was used
to analyze different factors in pre-invasive lung adenocarcinoma
and invasive lung adenocarcinoma. The result showed that the size
of nidus (OR: 2.834, 95% CI: 1.577–5.092), the size of solid
component (OR: 16.605, 95% CI: 4.037–20.611), the nature of nidus
(OR: 2.998, 95% CI: 1.734–5.183), burr (OR: 12.033, 95% CI:
4.119–17.857), lobes (OR: 3.575, 95% CI: 1.836–6.085) were risk
factors that affected invasive lung adenocarcinoma (P<0.05)
(Tables IV and V).
 | Table IV.Logistic multivariate regression
analysis and assignments. |
Table IV.
Logistic multivariate regression
analysis and assignments.
| Factors | Variate | Assignments |
|---|
| Size of nidus
(mm) | X1 | ≥9.8=1,
<9.8=0 |
| Size of solid
component (mm) | X2 | ≥0.9=1,
<0.9=0 |
| Nature of
nidus | X3 | mGGN=1, pGGN=0 |
| Burr | X4 | Yes=1, No=0 |
| Lobes | X5 | Yes=1, No=0 |
 | Table V.Logistic multivariate regression
analysis of risk factors of invasive lung adenocarcinoma. |
Table V.
Logistic multivariate regression
analysis of risk factors of invasive lung adenocarcinoma.
| Factors | β | SE | Wals | P-value | OR (95% CI) |
|---|
| Size of nidus
(mm) | 1.042 | 0.299 | 12.128 | <0.001 | 2.834
(1.577–5.092) |
| Size of solid
component (mm) | 4.036 | 1.347 | 8.975 | 0.003 | 16.605
(4.037–20.611) |
| Nature of
nidus | 1.098 | 0.279 | 15.457 | <0.001 | 2.998
(1.734–5.183) |
| Burr | 4.344 | 1.426 | 9.284 | 0.002 | 12.033
(4.119–17.857) |
| Lobes | 2.808 | 0.816 | 11.831 | 0.001 | 3.575
(1.836–6.085) |
Discussion
At present, when CT is used to scan the lungs of
patients, its imaging features are deficient for the diagnosis of
GGN. AAH, AIS, MIA, and IAC can be manifested as pGGN or mGGN with
a small amount of solid components in CT imaging. It is difficult
to diagnose the nature of nidus accurately before operation only
according to the imaging features of niduses in CT imaging
(18,19).
Histological biopsy is an important method to
diagnose whether GGN that existed in the patients' body for a long
time is an IAC, but biopsy and histological examination may cause
some trauma (20). The operation
plans of pre-invasive lesion of lung adenocarcinoma and invasive
lung adenocarcinoma are different. Diagnosing and timely treatment
of pre-invasive lesion of lung adenocarcinoma and invasive lung
adenocarcinoma can avoid unnecessary lymph node dissection and
reduce the trauma of patients. Thus, the early cure rate can reach
up to 100% (21). AAH, AIS, and MIA
grow along respiratory bronchiolar wall and alveolar wall, and most
of them are pGGN. If there are obvious local fibroblast
proliferation, local accumulation of tumor cells or alveolar wall
collapse, AAH, AIS, and MIA are often manifested as mGGN (22,23). In
this study, there were significant differences between the nature
of nidus, burr, and lobes of pre-invasive lung adenocarcinoma and
those of invasive lung adenocarcinoma. Pre-invasive lung
adenocarcinoma manifests as pGGN, while invasive lung
adenocarcinoma manifests as mGGN. The morphological features of
non-lobulated periphery and non-burr periphery of some pre-invasive
GGN lesions were similar to those in previous studies (24,25). In
pulmonary nodules, it is known that large nodules are more
malignant than small nodules, and that the solid component of GGN
represents a region of fibroblast proliferation or an invasive
component of tumors (26,27). In this study, the size of nidus and
the size of solid component of patients in group A were
significantly larger than those of patients in group B. This
conclusion is similar to the conclusion in the study of Yoon et
al (28). The sizes of nodules
and solid components of MIA and IAC are larger than those of
pre-invasive lung adenocarcinoma. ROC curves of nidus size and
solid component size for the diagnosis of invasive lung
adenocarcinoma were plotted, and it was found that the AUCs of the
size of nidus, the size of solid component and the combination for
the diagnosis of invasive lung adenocarcinoma were 0.891, 0.902 and
0.984, respectively. It suggested that the size of nidus and the
size of solid component can be used as diagnostic parameters of
invasive lung adenocarcinoma, and the combination of the two has
better diagnostic efficiency. Logistic multivariate regression
analysis was used to analyze risk factors of invasive lung
adenocarcinoma. The result showed that the size of nidus, the size
of solid component, the nature of nidus, burr, and lobes were risk
factors of invasive lung adenocarcinoma. In the study of Lee et
al (29), it was shown that the
nidus with size of <10 mm could distinguish pre-invasive lesions
and invasive lesions; the smaller nidus size, the smaller solid
proportion, the borderline without fissure and the borderline
without spots were the significant distinguishing factors of the
preinfiltrating lesion. In this study, it was found that the
optimal cut-off values for lesion size and solid component size for
invasive lung adenocarcinoma were 9.8 mm and 0.9 mm, respectively.
Therefore, we consider that <10 and 1 mm may be helpful in the
diagnosis of invasive lung adenocarcinoma. Whether 10 and 1 mm can
be used as alternatives need to be studied in the future. It was
found in this study that nidus size combined with solid component
size could improve the diagnostic efficacy of invasive lung
adenocarcinoma, and confirmed that burr and lobes were risk factors
for IAC, which were not reported before. Hu et al (30) used CT and positron emission
tomography (PET) to show that the appearance of vacuole signs,
pleural indentation signs, lobes, and burr-like appearance
indicates that the lesion is more likely to be malignant than
benign. For smaller GGN, the detection rate of PET-CT is lower,
while for larger GGN the detection rate is higher. This study
mainly used MDCT to distinguish between MIA, IAC and AAH, in AIS
patients, and did not observe the CT signs of group A and B, which
is one of the limitations of this study.
This study confirmed the diagnostic value of MDCT in
the treatment of pre-invasive lung adenocarcinoma and invasive lung
adenocarcinoma. However, the imaging features of MDCT of patients
with AAH, AIS, MIA and IAC were not observed. There are some
overlaps in the imaging features of MDCT of pre-invasive lung
adenocarcinoma and invasive lung adenocarcinoma. Therefore, in
clinical practice, clinical symptoms, signs, cytology, and
histological examination should be jointly used to reduce
misdiagnosis rate.
In conclusion, in GGN patients, nidus size and solid
component size can be used as excellent diagnostic parameters for
invasive lung adenocarcinoma, while nidus size (≥9.8 mm), solid
component size (≥0.9 mm), mGGN nature of lesion, burr and lobes can
distinguish invasive lung adenocarcinoma and preinvasive
lesions.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JL and HT conceived and designed the study, and
drafted the manuscript. JL, XY, LL and MP collected, analyzed and
interpreted the experimental data. MP and HT contributed to the
imaging analysis and revised the manuscript for important
intellectual content. All authors read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Shengli Oilfield Central Hospital (Dongying, China). Signed
informed consent was obtained from the patients or their
guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hirsch FR, Scagliotti GV, Mulshine JL,
Kwon R, Curran WJ Jr, Wu YL and Paz-Ares L: Lung cancer: Current
therapies and new targeted treatments. Lancet. 389:299–311. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gridelli C, Rossi A, Carbone DP, Guarize
J, Karachaliou N, Mok T, Petrella F, Spaggiari L and Rosell R:
Non-small-cell lung cancer. Nat Rev Dis Primers. 1:150092015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wei X, Zhang K, Qin H, Zhu J, Qin Q, Yu Y
and Wang H: GMDS knockdown impairs cell proliferation and survival
in human lung adenocarcinoma. BMC Cancer. 18:6002018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Guo T, Zhao S, Wang P, Xue X, Zhang Y,
Yang M, Li N, Li Z, Xu L, Jiang L, et al: YB-1 regulates tumor
growth by promoting MACC1/c-Met pathway in human lung
adenocarcinoma. Oncotarget. 8:48110–48125. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yu H, Zhang C, Liu S, Jiang G, Li S, Zhang
L, Kang E, Zhang B and Xu W: Application value of coaxial biopsy
system in needle cutting biopsy for focal ground glass-like density
nodule. J Cancer Res Ther. 14:1509–1514. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tao G, Jingying Y, Tan G, Xiaotao D and
Min C: A novel CT-guided technique using medical adhesive for
localization of small pulmonary ground-glass nodules and mixed
ground-glass nodules (≤20 mm) before video-assisted thoracoscopic
surgery. Diagn Interv Radiol. 24:209–212. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Qiu ZX, Cheng Y, Liu D, Wang WY, Wu X, Wu
WL and Li WM: Clinical, pathological, and radiological
characteristics of solitary ground-glass opacity lung nodules on
high-resolution computed tomography. Ther Clin Risk Manag.
12:1445–1453. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kadota K, Villena-Vargas J, Yoshizawa A,
Motoi N, Sima CS, Riely GJ, Rusch VW, Adusumilli PS and Travis WD:
Prognostic significance of adenocarcinoma in situ, minimally
invasive adenocarcinoma, and nonmucinous lepidic predominant
invasive adenocarcinoma of the lung in patients with stage I
disease. Am J Surg Pathol. 38:448–460. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang Y, Tang J, Xu J, Cheng J and Wu H:
Analysis of pulmonary pure ground-glass nodule in enhanced dual
energy CT imaging for predicting invasive adenocarcinoma: comparing
with conventional thin-section CT imaging. J Thorac Dis.
9:4967–4978. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hiramatsu M, Inagaki T, Inagaki T, Matsui
Y, Satoh Y, Okumura S, Ishikawa Y, Miyaoka E and Nakagawa K:
Pulmonary ground-glass opacity (GGO) lesions-large size and a
history of lung cancer are risk factors for growth. J Thorac Oncol.
3:1245–1250. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chae HD, Park CM, Park SJ, Lee SM, Kim KG
and Goo JM: Computerized texture analysis of persistent part-solid
ground-glass nodules: Differentiation of preinvasive lesions from
invasive pulmonary adenocarcinomas. Radiology. 273:285–293. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liang J, Xu XQ, Xu H, Yuan M, Zhang W, Shi
ZF and Yu TF: Using the CT features to differentiate invasive
pulmonary adenocarcinoma from pre-invasive lesion appearing as pure
or mixed ground-glass nodules. Br J Radiol. 88:201408112015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Son JY, Lee HY, Lee KS, Kim JH, Han J,
Jeong JY, Kwon OJ and Shim YM: Quantitative CT analysis of
pulmonary ground-glass opacity nodules for the distinction of
invasive adenocarcinoma from pre-invasive or minimally invasive
adenocarcinoma. PLoS One. 9:e1040662014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Oda S, Awai K, Murao K, Ozawa A, Yanaga Y,
Kawanaka K and Yamashita Y: Computer-aided volumetry of pulmonary
nodules exhibiting ground-glass opacity at MDCT. AJR Am J
Roentgenol. 194:398–406. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yoshizawa A, Motoi N, Riely GJ, Sima CS,
Gerald WL, Kris MG, Park BJ, Rusch VW and Travis WD: Impact of
proposed IASLC/ATS/ERS classification of lung adenocarcinoma:
Prognostic subgroups and implications for further revision of
staging based on analysis of 514 stage I cases. Modern pathology.
24:653–664. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Russell PA, Wainer Z, Wright GM, Daniels
M, Conron M and Williams RA: Does lung adenocarcinoma subtype
predict patient survival? A clinicopathologic study based on the
new International Association for the Study of Lung Cancer/American
Thoracic Society/European Respiratory Society international
multidisciplinary lung adenocarcinoma classification. J Thorac
Oncol. 6:1496–1504. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang Y, Shen Y, Qiang JW, Ye JD, Zhang J
and Zhao RY: HRCT features distinguishing pre-invasive from
invasive pulmonary adenocarcinomas appearing as ground-glass
nodules. Eur Radiol. 26:2921–2928. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gao F, Li M, Sun Y, Xiao L and Hua Y:
Diagnostic value of contrast-enhanced CT scans in identifying lung
adenocarcinomas manifesting as GGNs (ground glass nodules).
Medicine (Baltimore). 96:e77422017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li M, Gao F, Jagadeesan J, Gill RR, Hua Y
and Zheng X: Incremental value of contrast enhanced computed
tomography on diagnostic accuracy in evaluation of small pulmonary
ground glass nodules. J Thorac Dis. 7:1606–1615. 2015.PubMed/NCBI
|
|
21
|
Travis WD, Brambilla E, Noguchi M,
Nicholson AG, Geisinger KR, Yatabe Y, Beer DG, Powell CA, Riely GJ,
Van Schil PE, et al: International association for the study of
lung cancer/American thoracic society/European respiratory society
international multidisciplinary classification of lung
adenocarcinoma. J Thorac Oncol. 6:244–285. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Perandini S, Soardi GA, Motton M, Augelli
R, Dallaserra C, Puntel G, Rossi A, Sala G, Signorini M, Spezia L,
et al: Enhanced characterization of solid solitary pulmonary
nodules with Bayesian analysis-based computer-aided diagnosis.
World J Radiol. 8:729–734. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
She Y, Zhao L, Dai C, Ren Y, Zha J, Xie H,
Jiang S, Shi J, Shi S, Shi W, et al: Preoperative nomogram for
identifying invasive pulmonary adenocarcinoma in patients with pure
ground-glass nodule: A multi-institutional study. Oncotarget.
8:17229–17238. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhou J, Li Y, Zhang Y, Liu G, Tan H, Hu Y,
Xiao J and Shi H: Solitary ground-glass opacity nodules of stage IA
pulmonary adenocarcinoma: Combination of 18F-FDG PET/CT and
high-resolution computed tomography features to predict invasive
adenocarcinoma. Oncotarget. 8:23312–23321. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lim HJ, Ahn S, Lee KS, Han J, Shim YM, Woo
S, Kim JH, Yie M, Lee HY and Yi CA: Persistent pure ground-glass
opacity lung nodules ≥10 mm in diameter at CT scan: Histopathologic
comparisons and prognostic implications. Chest. 144:1291–1299.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Goo JM, Park CM and Lee HJ: Ground-glass
nodules on chest CT as imaging biomarkers in the management of lung
adenocarcinoma. AJR Am J Roentgenol. 196:533–543. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang T, Pu XH, Yuan M, Zhong Y, Li H, Wu
JF and Yu TF: Histogram analysis combined with morphological
characteristics to discriminate adenocarcinoma in situ or minimally
invasive adenocarcinoma from invasive adenocarcinoma appearing as
pure ground-glass nodule. Eur J Radiol. 113:238–244. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yoon HE, Fukuhara K, Michiura T, Takada M,
Imakita M, Nonaka K and Iwase K: Pulmonary nodules 10 mm or less in
diameter with ground-glass opacity component detected by
high-resolution computed tomography have a high possibility of
malignancy. Jpn J Thorac Cardiovasc Surg. 53:22–28. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lee SM, Park CM, Goo JM, Lee HJ, Wi JY and
Kang CH: Invasive pulmonary adenocarcinomas versus preinvasive
lesions appearing as ground-glass nodules: differentiation by using
CT features. Radiology. 268:265–273. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hu L, Pan Y, Zhou Z and Gao J: Application
of positron emission tomography-computed tomography in the
diagnosis of pulmonary ground-glass nodules. Exp Ther Med.
14:5109–5113. 2017.PubMed/NCBI
|