Introduction
Colon cancer is the third most common cancer in men
and the second most common cancer in women (1). It is a heterogeneous disease (2). The annual incidence rate is
approximately 1.2 million and more than 600 patients die each year
from the colon cancer (3). Surgical
resection is an early method for the clinical treatment of colon
cancer. The surgical wound area is large and the patient's
postoperative pain is obvious. So the anesthetic management and
postoperative analgesia during surgery are important factors for
patients to recover quickly after surgery.
Dexmedetomidine is a selective adrenergic agonist
that has less effect on respiratory function, has anti-anxiety,
anti-sympathetic nerve and analgesic and sedative properties
(4). It is an important option for
short-term or long-term sedation in intensive care units (5). Previous studies have shown that
dexmedetomidine has good analgesic sedative effect, and also
significantly reduces the dose of anesthetic drugs required,
reduces the duration of coma and shorten duration of mechanical
ventilation (6). Sufentanil is an
effective analgesic that rapidly crosses the blood-brain barrier
and selectively activates central µ-opioid receptors (7), with a very high therapeutic index in
clinical studies. There is no active metabolite, but there is a
high degree of lipophilic property (8). Studies have shown that sufentanil is
commonly used in intravenous and intrathecal routes to treat acute
pain and can also be used for postoperative analgesia (9,10). In a
study on laryngectomy by Qin et al (11), dexmedetomidine combined with
sufentanil improved postoperative sleep quality, had good analgesic
effect and reduced the number of coughs in patients after
operation.
At present, there is scarce research on
dexmedetomidine combined with sufentanil in colon cancer resection
and on the immune function and coagulation function of patients.
This study provides more references for the treatment of patients
with colon cancer resection by observing the application and
anesthetic effect of dexmedetomidine combined with sufentanil in
colon cancer resection.
Materials and methods
Baseline data
Cancer patients (n=176) admitted to Xiangya Hospital
of Central South University, were selected for study. They were
divided into group A (92 cases) and group B (84 cases). There were
42 males and 50 females in group A, aged 29–66 years. The average
age was 52.51±6.11 years. TNM stage was 48 cases in I+II stage and
44 cases in stage III. There were 47 males and 37 females in group
B, aged 27–64 years. The average age was 53.13±6.05 years. TNM
staging was 43 cases in stages I+II and 41 cases in stage III. The
study was approved by the Ethics Committee of Xiangya Hospital
Central South University (Changsha, China). The patients or their
guardians sign a full informed consent.
Inclusion and exclusion criteria
Inclusion criteria: In line with the National
Comprehensive Cancer Network (NCCN) oncology clinical practice
guidelines (12); CT, color doppler
ultrasound, MRI and other tests to exclude distant metastases, TNM
staging criteria for stage I to III; no previous treatment or
radiation therapy, and this is the first diagnosis; no major organ
dysfunction, with detailed clinical and pathological data.
Exclusion criteria: Patients who were unwilling to participate in
this study; patients with other malignant tumors, those who did not
cooperate with follow-up and who were lost to follow-up; patients
with blood system diseases, comorbid with serious complications and
immune system diseases; patients with severe mental illness that
led to poor treatment compliance.
Operative methods
In both groups, patients underwent general
anesthesia intratracheal intubation. In the two groups, patients
routinely fasted before surgery, but did not receive preoperative
medication. After entering the operating room, venous transfusion
was opened routinely to closely monitor non-invasive arterial blood
pressure, blood oxygen saturation, end tidal carbon dioxide
tension, bispectral index and electrocardiogram. Then patients
received sufentanil (Yichang Humanwell Pharmaceutical Co., Ltd.;
item no. H20054256) of 0.5 µg/kg, etomidate (Shanghai Shifeng
Biotechnology Co., Ltd.; item no. EB03700) of 0.3 mg/kg and
atracurium (Jiangsu Hengrui Pharmaceutical Co., Ltd.; item no.
H20060869) of 0.15 mg/kg. After successful anesthesia induction, an
appropriate laryngeal mask was placed after the patient's muscles
were relaxed and they were unconscious. Then the anesthesia was
applied. The tidal volume was 8–10 ml/kg. The respiratory rate was
adjusted to 12 times/min. During operation, micro-pump continued to
inject remifentanil (Yichang Humanwell Pharmaceutical Co., Ltd.;
item no. H20030197) of 5–6 µg/(mg·h) and propofol (Sichuan Guorui
Pharmaceutical Co., Ltd.; item no. H20030115) of 3–6 mg/kg
intravenous pumping. Atracurium of 0.25–0.5 mg/kg was added to
maintain anesthesia. After completion of the operation, the tube
was extubated after the patient regained spontaneous breathing,
cough and swallowing reflex. During analgesia, patients received
200 µg of dexmedetomidine, sufentanil of 2.0 µg/kg and ramosetron
hydrochloride of 0.3 mg (Shanghai Yuanye Biotechnology Co., Ltd.;
item no. S61106) for analgesia in group A. Patients were given
analgesia with sufentanil of 2.0 µg/kg and ramosetron hydrochloride
of 0.3 mg in group B. In the two groups, analgesic drugs were
diluted in normal saline and to a dose of 100 ml.
Observation indexes
The time to effect of anesthetics, operation time,
awakening time, extubation time and recovery time of patients in
the two groups were recorded. Before induction, before and after
intubation, the MAP, CVP, HR levels and the incidence of
postoperative adverse reactions were measured and recorded in
patients of the two groups.
Five milliliters of venous blood was taken before
anesthesia induction and 30 min after surgery for 1 day. Blood
coagulation analyzer HF-6000 (Hunan Hukang Centrifuge Co., Ltd.;
item no. HF-6000) was used to detect four items of coagulation (PT,
APTT, TT, FIB).
Five milliliters of venous blood was taken before
anesthesia induction for 30 min and after surgery for 1 day.
FACSCalibur flow cytometry (BD Biosciences) was used to detect T
lymphocyte subsets in peripheral blood. Anticoagulated whole blood
of 100 µl was placed in a TruCOUNT tube. Twenty microliters each of
CD3-FITC, CD4-PE and CD8-PE (BD Biosciences) antibody was added,
mixed and allowed to stand at room temperature for 15 min.
Hemolysin of 370 µl was added (BD Biosciences), mixed and allowed
to stand at room temperature for 15 min. Samples were tested on a
flow cytometer and the peripheral blood CD3+,
CD4+, CD8+ and
CD4+/CD8+ values were read.
The pain scores of patients in the two groups were
measured and recorded after operation at 4, 24 and 48 h. The visual
analogue scale (VAS) was used. The scoring standard: the score was
recorded by using a slidable swimming ruler with a length of 10 cm.
The scale was: 0, painless and 10 points aggravated by pain.
Statistical analysis
The analysis was performed by using SPSS 21.0
statistical software (Easybio). Enumeration data were expressed as
cases/percentage [n(%)] within groups. Chi-square test was used to
compare the enumeration data between groups. In Chi-square test,
when the theoretical frequency was less than 5, continuity
correction Chi-square test was adopted. Measurement data were
expressed as mean number ± standard deviation (mean ± SD). The
t-test of independent samples was used to compare the measurement
data between groups. Paired t-test was used for sequential
comparisons within the group. Multiple time-points were observed
and compared by using repetitive measurement and analysis of
variance. The Bonferroni method was used for pairwise comparison
between different time-points in the group. The difference was
statistically significant at P<0.05.
Results
Baseline data
There were no significant differences in patients
regarding sex, age, body mass index, place of residence, nation,
educational background, smoking history, drinking history, movement
history, diabetes history, obesity status, TNM stage, or other
clinical baseline data between the two groups (P>0.05) (Table I).
 | Table I.Baseline data of patients in both
groups [n(%)] (mean ± SD). |
Table I.
Baseline data of patients in both
groups [n(%)] (mean ± SD).
| Classification | Group A (n=92) | Group B (n=84) | t/χ2
value | P-value |
|---|
| Sex |
|
| 1.864 | 0.172 |
| Male | 42 (45.65) | 47 (55.95) |
|
|
|
Female | 50 (54.35) | 37 (44.05) |
|
|
| Age/years | 52.51±6.11 | 53.13±6.05 | 0.676 | 0.500 |
| BMI
(kg/m2) | 22.84±2.15 | 22.42±1.71 | 1.425 | 0.156 |
| Place of
residence |
|
| 2.582 | 0.108 |
| City | 56 (60.87) | 41 (48.81) |
|
|
|
Rural | 36 (39.13) | 43 (51.19) |
|
|
| Ethnicity |
|
| 0.001 | 0.978 |
| Han | 48 (52.17) | 44 (52.38) |
|
|
|
Minority | 44 (47.83) | 40 (47.62) |
|
|
| Educational
background |
|
| 0.046 | 0.831 |
| ≥ High
school | 42 (45.65) | 37 (44.05) |
|
|
| < High
school | 50 (54.35) | 47 (55.95) |
|
|
| Smoking history |
|
| 2.366 | 0.124 |
| Yes | 43 (46.74) | 49 (58.33) |
|
|
| No | 49 (53.26) | 35 (41.67) |
|
|
| Drinking history |
|
| 0.175 | 0.676 |
| Yes | 53 (57.61) | 51 (60.71) |
|
|
| No | 39 (42.39) | 33 (39.29) |
|
|
| Movement history |
|
| 1.547 | 0.214 |
| Yes | 62 (67.39) | 49 (58.33) |
|
|
| No | 30 (32.61) | 35 (41.67) |
|
|
| Diabetes
history |
|
| 0.039 | 0.842 |
|
Yes | 49 (53.26) | 46 (54.76) |
|
|
| No | 43 (46.74) | 38 (45.24) |
|
|
| Obesity status |
|
| 0.007 | 0.934 |
|
Yes | 52 (56.52) | 48 (57.14) |
|
|
| No | 40 (43.48) | 36 (42.86) |
|
|
| TNM stage |
|
| 0.017 | 0.896 |
| Stage
I+II | 48 (52.17) | 43 (51.19) |
|
|
| Stage
III | 44 (47.83) | 41 (48.81) |
|
|
Comparison of anesthesia induced
intubation time of patients in the two groups
There was no significant difference in the time of
anesthesia taking effect of patients in the two groups (P>0.05).
The awakening time, extubation time and recovery time of group A
were lower than those of group B (P<0.05) (Table II).
 | Table II.Comparison of anesthesia induced
intubation time of patients in the two groups (mean ± SD, min). |
Table II.
Comparison of anesthesia induced
intubation time of patients in the two groups (mean ± SD, min).
| Items | Group A (n=92) | Group B (n=84) | t value | P-value |
|---|
| Time of anesthesia
taking effect | 4.78±1.04 | 5.02±1.13 | 1.467 | 0.144 |
| Awakening time | 13.48±2.53 | 14.89±2.96 | 3.406 | 0.001 |
| Extubation
time | 11.26±3.12 | 12.56±3.45 | 2.625 | 0.009 |
| Recovery time | 10.46±2.78 | 11.56±2.83 | 2.600 | 0.010 |
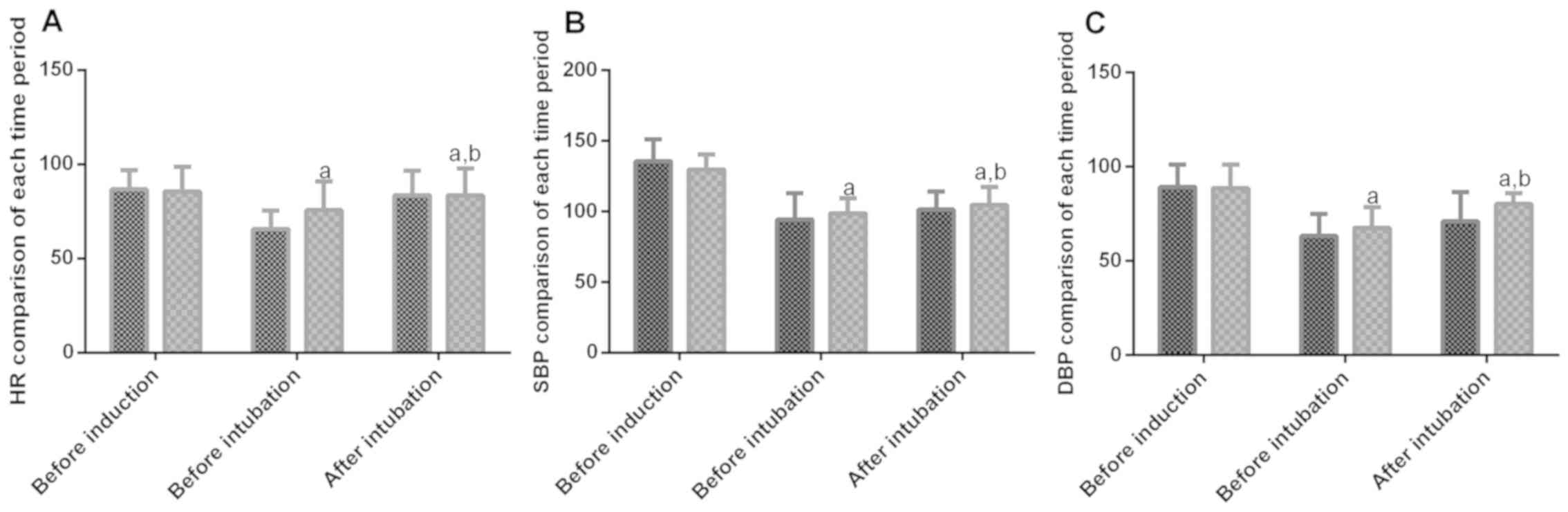
Comparison of HR, SBP and DBP in each
period of operation of patients in the two groups
The expression of HR, SBP and DBP in each time
period in patients of both groups were statistically significant
(P<0.05). There was no significant difference in HR, SBP and DBP
of patients in the two groups before induction (P>0.05). Before
and after intubation, HR, SBP and DBP of patients in the two groups
were lower than before induction (P<0.05). HR, SBP and DBP
before intubation were significantly lower than those after
intubation (P>0.05). Moreover, HR, SBP and DBP in group A before
and after intubation were significantly lower than those in group B
(P<0.05) (Fig. 1).
Comparison of preoperative and
postoperative coagulation functions of patients in two groups
There was no difference in the expression levels of
PT, APTT, TT and FIB in coagulation function of patients in two
groups before operation (P>0.05), but the expression levels of
coagulation function PT, APTT, TT and FIB in group A after
operation were lower than that in group B (P<0.05) (Table III).
 | Table III.Comparison of preoperative and
postoperative coagulation functions of patients in the two groups
(mean ± SD). |
Table III.
Comparison of preoperative and
postoperative coagulation functions of patients in the two groups
(mean ± SD).
|
| PT (sec) | APTT (sec) | TT (sec) | FIB (g/l) |
|---|
|
|
|
|
|
|
|---|
| Grouping | Before surgery | After surgery | Before surgery | After surgery | Before surgery | After surgery | Before surgery | After surgery |
|---|
| Group A (n=92) | 15.8±4.6 | 11.3±4.3 | 35.9±12.4 | 26.5±11.9 | 15.9±5.2 | 12.3±5.1 | 4.2±0.2 | 2.3±0.2 |
| Group B (n=84) | 15.9±4.5 | 13.2±4.2 | 36.2±12.1 | 31.3±12.0 | 16.7±5.3 | 14.2±5.0 | 4.3±0.6 | 3.1±0.3 |
| t value | 0.146 | 2.961 | 0.162 | 2.662 | 1.010 | 2.492 | 1.510 | 20.980 |
| P-value | 0.884 | 0.004 | 0.871 | 0.009 | 0.314 | 0.014 | 0.133 | <0.001 |
Comparison of immune function of
patients in the two groups before and after surgery
There were no significant differences in the
expression of CD3+, CD4+, CD8+,
CD4+/CD8+ of patients in the two groups
before surgery (P>0.05), but the expression of CD3+,
CD4+, CD8+, CD4+/CD8+
in group A after surgery was higher than that in group B
(P<0.05) (Table IV).
 | Table IV.Comparison of immune function of
patients in the two groups before and after surgery (mean ±
SD). |
Table IV.
Comparison of immune function of
patients in the two groups before and after surgery (mean ±
SD).
|
| CD3+
(%) | CD4+
(%) | CD8+
(%) |
CD4+/CD8+ |
|---|
|
|
|
|
|
|
|---|
| Grouping | Before surgery | After surgery | Before surgery | After surgery | Before surgery | After surgery | Before surgery | After surgery |
|---|
| Group A (n=92) | 55.88±9.29 | 45.74±7.47 | 33.49±5.71 | 24.11±5.89 | 21.44±4.11 | 20.99±3.71 | 1.57±0.24 | 1.19±0.26 |
| Group B (n=84) | 54.91±8.69 | 48.62±6.62 | 32.46±5.57 | 22.33±4.10 | 21.05±3.72 | 19.78±3.85 | 1.56±0.20 | 1.11±0.27 |
| t value | 0.714 | 2.697 | 1.209 | 2.306 | 0.658 | 2.123 | 0.299 | 2.002 |
| P-value | 0.477 | 0.008 | 0.228 | 0.022 | 0.512 | 0.035 | 0.766 | 0.046 |
Comparison of VAS scores of patients
in the two groups after operation
The VSA scores of patients in the two groups were
significantly decreased with time, with statistical significance
(P<0.05). The VAS scores of the patients in group A were lower
than those in group B after operation at 4, 24 and 48 h (P<0.05)
(Table V).
 | Table V.Comparison of VAS scores in each
period in the two groups after operation (mean ± SD). |
Table V.
Comparison of VAS scores in each
period in the two groups after operation (mean ± SD).
| Time | Group A (n=92) | Group B (n=84) | t value | P-value |
|---|
| After operation for
4 h | 4.27±0.52 | 6.32±1.12 | 15.790 | <0.001 |
| After operation for
24 h | 3.45±0.44 | 3.87±0.62 |
5.217 | <0.001 |
| After operation for
48 h | 2.78±0.35 | 3.12±0.41 |
5.932 | <0.001 |
| F-value | 262.100 | 390.600 | – | – |
| P-value | <0.001 | <0.001 | – | – |
Incidence of adverse reactions of
patients in the two groups after operation
In group A, there was 1 case with shivering (1.09%),
2 cases with nausea, vomiting and headache (2.17%) and 1 case with
respiratory depression (1.09%). The incidence of total adverse
reactions was 4.35%. In group B, there were 2 cases with shivering
(2.38%), 4 cases with nausea and vomiting (4.76%), 5 cases with
headache (5.95%), 5 cases with respiratory depression (5.95%). The
incidence of total adverse reactions was 19.05%. The total adverse
reaction rate of patients in group A was significantly lower than
that of patients in group B (P<0.05) (Table VI).
 | Table VI.Incidence of total adverse reactions
of patients in the two groups after operation [n(%)]. |
Table VI.
Incidence of total adverse reactions
of patients in the two groups after operation [n(%)].
| Category | Group A (n=92) | Group B (n=84) | χ2
value | P-value |
|---|
| Shivering | 1 (1.09) | 2 (2.38) | 0.439 | 0.508 |
| Nausea and
vomiting | 0 (0.00) | 4 (4.76) | 4.483 | 0.034 |
| Headache | 2 (2.17) | 5 (5.95) | 1.641 | 0.200 |
| Respiratory
depression | 1 (1.09) | 5 (5.95) | 3.157 | 0.076 |
| Incidence of total
adverse reactions | 4 (4.35) | 16 (19.05) | 9.420 | 0.002 |
Discussion
Colon cancer is common (13). Most of the pathogenesis is slow and
occult and early symptoms are not obvious (14). Colon cancer is clinically treated
with surgical resection (15), but
postoperative pain is a common complication after surgery. It is of
great significance to improve the therapeutic effect of colon
cancer surgery and patient's postoperative recovery by applying
reasonable anesthesia in the surgical treatment (16).
Dexmedetomidine reduces the sympathetic nerves of
the heart and surrounding vasculature or enhances the tension of
parasympathetic nerve to alter hemodynamics (17). Previous studies have shown that
(18) dexmedetomidine can also
improve postoperative cellular immune function of patients with
malignant tumors. Sufentanil is a widely used analgesic that
improves the analgesic effect with a longer duration and a lighter
respiratory inhibition (19). It
also has a strong opioid receptor activation effect. Its obvious
analgesic effect and the possibility of cardiovascular events are
low (20). In this study, there was
no significant difference in the time of anesthesia taking effect
of patients in group A and group B. However, the awakening time,
extubation time and recovery time after anesthesia in group A was
lower than group B. MAP and CVP were significantly decreased before
intubation and HR was significantly slowed. However, after
intubation, it recovered to before induction level and group A was
lower than group B, indicating that dexmedetomidine combined with
sufentanil can provide more stable hemodynamics for patients with
colon cancer resection. The VAS scores of patients in group A were
significantly lower than those of patients in group B after surgery
for 4, 24 and 48 h, indicating that the awakening time language
statement and awakening time of dexmedetomidine combined with
sufentanil were faster in colon cancer resection, and the
postoperative analgesic effect was better. In the study of Dong
et al (21), the use of
dexmedetomidine and sufentanil in thoracotomy was shown to
successfully treat severe pain of patients after thoracotomy and to
maintain good hemodynamic stability, similarly to this study. We
observed the adverse reactions of patients after surgery and the
results showed that the incidence of total adverse reactions in
group A was significantly lower than that in group B, indicating
that dexmedetomidine and sufentanil were safer.
Blood coagulation of perioperative patients is
affected by various factors such as the size of the operation,
blood loss, anesthesia and blood transfusion (22). In the study of Chen et al
(23), the combination of anesthesia
and dexmedetomidine also improved the postoperative coagulation
status of patients. The results of the present study showed that
the expression levels of coagulation function PT, APTT, TT and FIB
in group A were lower than those in group B, indicating that
dexmedetomidine combined with sufentanil can effectively improve
postoperative coagulation function in patients with colon cancer
resection. Dexmedetomidine inhibits sympathetic nerve activity,
while sufentanil combined with dexmedetomidine can inhibit
sympathetic nerve activity and play the role of inhibiting stress
response, thereby preventing blood from becoming hypercoagulated
and accelerating blood flow.
Changes of T lymphocyte subsets can assess the
immune function of the body. CD3+, CD4+ and
CD8+ T cells can be classified according to their
different functions and surface markers. The changes in the ratio
of the three are important markers reflecting the immune
dysfunction of the body (24). The
results of this study showed that the ratio of CD3+,
CD4+, CD8+ and
CD4+/CD8+ in peripheral blood of group A and
group B after operation was significantly lower than that before
operation. The ratio of CD3+, CD4+,
CD8+ and CD4+/CD8+ in group A was
significantly higher than that in group B, indicating that
anesthesia may have an impact on the immune function of patients
with colon cancer resection, while dexmedetomidine combined with
sufentanil have less effect on the immune function of patients. In
the study of Yang et al (25), dexmedetomidine, sufentanil and other
anesthesia were applied to patients receiving radical mastectomy,
which could effectively improve the immune function of patients.
This indicated that dexmedetomidine and sufentanil can improve the
immune function of patients.
This study confirmed that dexmedetomidine combined
with sufentanil is a viable anesthesia program in colon cancer
resection. However, further study is necessary to extend the study
time and add follow-up to further confirm the results.
In conclusion, dexmedetomidine combined with
sufentanil is a viable anesthetic regimen for colon cancer
resection, which can maintain good hemodynamic stability,
effectively relieve postoperative pain of patients and has fewer
postoperative complications. The coagulation function and immune
function also have a certain improvement effect in patients.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
LZ wrote the manuscript, interpreted and analyzed
the patient data. YL designed the study, performed the experiment,
and was responsible for the analysis and discussion of the data.
Both authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Xiangya Hospital Central South University (Changsha, China).
Patients who participated in this research had complete clinical
data. Signed informed consents were obtained from the patients
and/or the guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Marisa L, de Reyniès A, Duval A, Selves J,
Gaub MP, Vescovo L, Etienne-Grimaldi MC, Schiappa R, Guenot D,
Ayadi M, et al: Gene expression classification of colon cancer into
molecular subtypes: Characterization, validation, and prognostic
value. PLoS Med. 10:e10014532013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sinicrope FA, Shi Q, Smyrk TC, Thibodeau
SN, Dienstmann R, Guinney J, Bot BM, Tejpar S, Delorenzi M,
Goldberg RM, et al: Molecular markers identify subtypes of stage
III colon cancer associated with patient outcomes.
Gastroenterology. 148:88–99. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Warschkow R, Sulz MC, Marti L, Tarantino
I, Schmied BM, Cerny T and Güller U: Better survival in right-sided
versus left-sided stage I–III colon cancer patients. BMC Cancer.
16:5542016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Weerink MA, Struys MM, Hannivoort LN,
Barends CR, Absalom AR and Colin P: Clinical pharmacokinetics and
pharmacodynamics of dexmedetomidine. Clin Pharmacokinet.
56:893–913. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Keating GM: Dexmedetomidine: A review of
its use for sedation in the intensive care setting. Drugs.
75:1119–1130. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Grant MJ, Schneider JB, Asaro LA, Dodson
BL, Hall BA, Simone SL, Cowl AS, Munkwitz MM, Wypij D and Curley
MA; Randomized Evaluation of Sedation Titration for Respiratory
Failure Study Investigators, : Dexmedetomidine use in critically
ill children with acute respiratory failure. Pediatr Crit Care Med.
17:1131–1141. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
van de Donk T, Ward S, Langford R and
Dahan A: Pharmacokinetics and pharmacodynamics of sublingual
sufentanil for postoperative pain management. Anaesthesia.
73:231–237. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Willsie SK, Evashenk MA, Hamel LG, Hwang
SS, Chiang YK and Palmer PP: Pharmacokinetic properties of single-
and repeated-dose sufentanil sublingual tablets in healthy
volunteers. Clin Ther. 37:145–155. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Porela-Tiihonen S, Kokki M and Kokki H:
Sufentanil sublingual formulation for the treatment of acute,
moderate to severe postoperative pain in adult patients. Expert Rev
Neurother. 17:101–111. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ohnesorge H, Alpes A, Baron R and
Gierthmühlen J: Influence of intraoperative remifentanil and
sufentanil on sensory perception: A randomized trial. Curr Med Res
Opin. 32:1797–1805. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qin M, Chen K, Liu T and Shen X:
Dexmedetomidine in combination with sufentanil for postoperative
analgesia after partial laryngectomy. BMC Anesthesiol. 17:662017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Benson AB III, Venook AP, Cederquist L,
Chan E, Chen YJ, Cooper HS, Deming D, Engstrom PF, Enzinger PC,
Fichera A, et al: Colon cancer, version 1.2017, NCCN clinical
practice guidelines in oncology. J Natl Compr Canc Netw.
15:370–398. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schweiger MR, Hussong M, Röhr C and
Lehrach H: Genomics and epigenomics of colorectal cancer. Wiley
Interdiscip Rev Syst Biol Med. 5:205–219. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Soler M, Estevez MC, Villar-Vazquez R,
Casal JI and Lechuga LM: Label-free nanoplasmonic sensing of
tumor-associate autoantibodies for early diagnosis of colorectal
cancer. Anal Chim Acta. 930:31–38. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Arnarson Ö, Butt-Tuna S and Syk I:
Postoperative complications following colonic resection for cancer
are associated with impaired long-term survival. Colorectal Dis.
21:805–815. 2019.PubMed/NCBI
|
|
16
|
Li L, Jin J, Min S, Liu D and Liu L:
Compliance with the enhanced recovery after surgery protocol and
prognosis after colorectal cancer surgery: A prospective cohort
study. Oncotarget. 8:53531–53541. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Devcic A, Schmeling WT, Kampine JP and
Warltier DC: Oral dexmedetomidine preserves baroreceptor function
and decreases anesthetic requirements of halothane-anesthetized
dogs. Anesthesiology. 81:419–430. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen J, Fang JI, Lei Z, Zhang Z, Yiheshan
A and Zhang B: Effect of dexmedetomidine on postoperative cellular
immune function in patients with malignant tumor: A meta-analysis.
Chin J Anesthesiol. 4:454–457. 2018.(In Chinese).
|
|
19
|
Feng M, Chen X, Liu T, Zhang C, Wan L and
Yao W: Dexmedetomidine and sufentanil combination versus sufentanil
alone for postoperative intravenous patient-controlled analgesia: A
systematic review and meta-analysis of randomized controlled
trials. BMC Anesthesiol. 19:812019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ma XX, Luo F and Lan R: Effects of
dexmedetomidine combined with sufentanil on P2X7 receptor
expression in peripheral blood mononuclear cells in patients with
burn pain. Acta Microscopica. 28:2019.
|
|
21
|
Dong CS, Zhang J, Lu Q, Sun P, Yu JM, Wu C
and Sun H: Effect of dexmedetomidine combined with sufentanil for
post-thoracotomy intravenous analgesia:a randomized, controlled
clinical study. BMC Anesthesiol. 17:332017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liuboshevskiĭ PA, Artamonova NI and
Ovechkin AM: Haemostasis disturbances as the component of the
surgical stress-response and possibilities of their correction.
Anesteziol Reanimatol. 3:44–48. 2012.(In Russian).
|
|
23
|
Chen Z, Shao D, Mao Z, Shi L, Zheng Y and
Zhang D: Effect of dexmedetomidine on blood coagulation function
following radical gastrectomy. J Clin Anesthesiol. 33:1086–1090.
2017.
|
|
24
|
Sun HZ, Song YL and Wang XY: Effects of
different anesthetic methods on cellular immune and neuroendocrine
functions in patients with hepatocellular carcinoma before and
after surgery. J Clin Lab Anal. 30:1175–1182. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang XH, Bai Q, Lv MM, Fu HG, Dong TL and
Zhou Z: Effect of dexmedetomidine on immune function of patients
undergoing radical mastectomy: a double blind and placebo control
study. Eur Rev Med Pharmacol Sci. 21:1112–1116. 2017.PubMed/NCBI
|