Introduction
Cancer, the second leading cause of death globally,
was responsible for an estimated 9.6 million deaths in 2018
(1). An increasing life expectancy
extends the period over which oncogenes act on cells and increases
the risk of cancer development. The formation and development of
cancer is caused by the accumulation of genetic mutations in cells.
Cancer is a genetic event in which normal cells accumulate genomic
instability and acquire the ability to replicate indefinitely,
which is the phenotype of immortality. Telomerase repression and/or
short telomeres in human cells are suggested to be a natural
evolutionary strategy in the fight against cancer; it functions as
a strong barrier to tumor transformation and prevents uncontrolled
cell proliferation (2). The basis of
oncogenesis is the infinite proliferation of malignant cells, which
in most cases is achieved by the activation of telomerase (3).
Telomeres are repeatable (TTAGGG) DNA-protein
complexes that protect the ends of chromosomes. They are reduced
during cell division in somatic cells. Dysfunctional telomeres may
arise as a result of their critical shortening that induce a DNA
damage responses (DDR) and cause cellular senescence. If the cells
inherit or acquire damage to detect short telomeres they will
continue to divide, and the telomeres will continue to shorten and
the cells will reach the next phase called the crisis. This leads
to the joining of the ends of various chromosomes, pathological
mitoses, genomic instability and apoptosis. Some of the cells avoid
crisis and activate the telomerase gene, telomerase reverse
transcriptase (TERT), which codes for telomerase, the
enzyme responsible for the synthesis of telomere. Telomerase
activity allows the cancer cell to have unlimited replication.
Although TERT is usually silenced in almost all somatic cells, it
is significantly expressed in 85–95% of human cancers (3,4). TERT
expression is up-regulated in tumors via multiple genetic and
epigenetic mechanisms including: TERT promoter mutations (mainly
C228T or C250T), alterations in alternative splicing of TERT
pre-mRNA, TERT amplification, epigenetic modifications through TERT
promoter methylation, and/or disruption of telomere position effect
(TPE) machinery (1). Rarely, another
DNA recombination mechanism called alternative lengthening of
telomeres (ALT) is used in ~5-15% of tumors mostly arising from
mesenchymal or epithelial tissues (including bone, soft tissues,
neuroendocrine systems, and nervous system) (5). The mechanisms that regulate TERT
expression and telomerase activity are extensively studied. In
addition, telomerase inhibition strategies are used to
progressively shorten telomeres and ultimately kill cancer cells.
Currently, many drugs that inhibit telomerase in various mechanisms
are being evaluated in cancer clinical trials. We present
achievements in the field of telomeres and telomerase biology,
mechanisms underlying cancer and the development of cancer
therapies (6).
History
In 1965, L. Hayflick showed that a human diploid
cell can divide only a limited number of times (Hayflick
limit-about 60 divisions) and there is a gradual inhibition of
mitotic activity, called replicative sencence (7). Ołownikow associated the problem of
chromosome shortening with sencence (8). Then Szostak and Blackburn (1982),
Blackburn (1991) and Greider 1991) described that telomeres
shortened 50–200 bp in each division until reaching the critical
limit (9–11). The sequential structure of telomeres
formed by 5′-TTAGGG-3′ repeats and its genomic DNA protection
function has been proposed by Moyzis et al (12), Makarov et al (13), Wellinger and Sen (14). In 2001, Blackburn introduced a
telomeric function to maintain chromosome integrity and genome
stability (15). In addition,
Greider and Blackburn (16), Lendvay
et al (17), Lingner and Cech
(18) presented two telomerase
subunits: The telomerase reverse transcriptase catalytic subunit
(TERT) and the RNA template (TERC). In 2004, Liu et al
(19) described that in somatic
cells, telomerase remains inactive, but its activity can be found
in germ cells and stem cells. In addition, reactivation of
telomerase in somatic cells is one way to acquire uninhibited
proliferation in cancer. Telomerase activity was detected gradually
in approximately 85% of malignant tumors (20,21). In
2013, the presence of C228T and C250T in the TERT promoter mutation
in melanoma was reported (22,23).
Further studies have shown the presence of these mutations in other
cancers, mainly in the central nervous system, bladder, liver,
thyroid, and others, described in the following sections of the
article. Recent reports explaining the reactivation of telomerase
and attempts to inhibit it in malignant cells give hope for its
potential use in cancer treatment.
Telomere structure and function
Telomeres are nucleoprotein structures located at
the ends of chromosomes in eukaryotic cells. Each chromosome has
two telomeres and there are 92 telomeres in a diploid human cell.
Human telomeric DNA is composed of tandem repeats [10-15 kilobases
(kb) at birth] of double-stranded DNA nucleotide sequence
5′-TTAGGG-3′, and the final 3′ G-rich single-stranded overhang
(150–200 nucleotide long), linked by telomere-binding proteins
(TBPs) (3).
The telomere spatial structure is created from the
3′G-rich overhang, which invades the homologous double-stranded
TTAGGG region and forms a smaller D-loop. Then the larger T-loop is
built using protein protective complex called shelterin.
Shelterin has three core subunits: Telomere repeat
factor (TRF)-1, TRF2, which recognize and bind duplex TTAGGG
repeats, and human protection of telomeres 1 (POT1) which is
responsible for recognizing single-stranded TTAGGG overhangs. These
three proteins are additionally connected by: TRF1-interacting
protein 2 (TIN2), TINT1/PTOP/PIP1 protein (TPP1), and
repressor-activator protein 1 (Rap1) (Fig. 1).
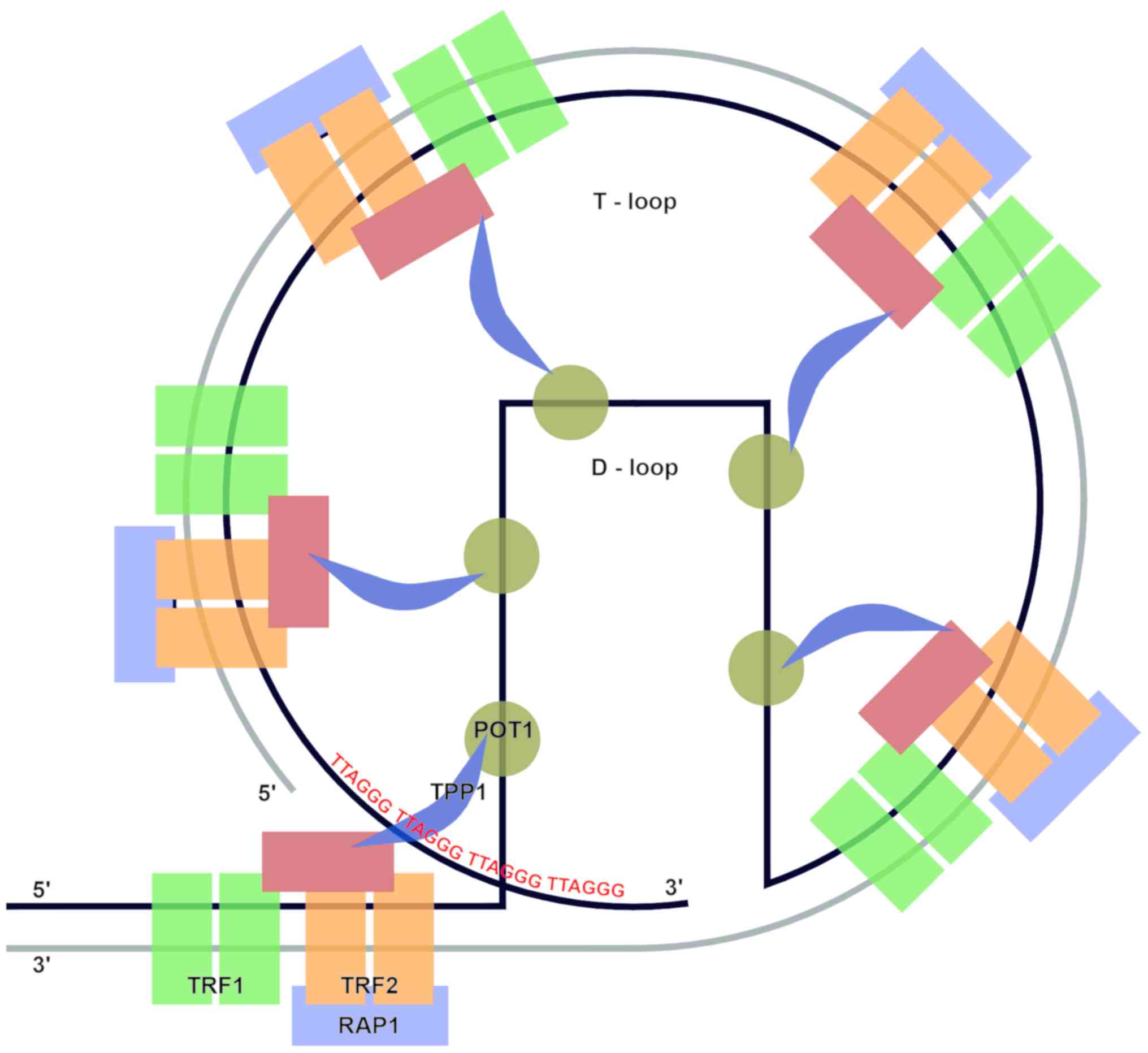 | Figure 1.Telomere structure. Telomeric DNA
contains tandem repeats of DNA sequence 5′-TTAGGG-3′, terminal 3′
G-rich overhang and shelterin complex of six subunits: TRF1, TRF2
and POT1 (proteins responsible for recognition of TTAGGG telomeric
repeats), and TIN2, TPP1 and RAP1 (complex stabilizing proteins).
The telomere structure forms two loops, the T-loop and the D-loop.
TRF1, telomere repeat factor-1; TRF2, telomere repeat factor-2;
POT1, protection of telomeres-1; TIN2, TRF1 interacting protein-2;
RAP1, repressor/activator protein 1; TPP1, TINT1/PTOP/PIP1 protein
(POT1-TIN2 organizing protein). |
TRF1 controls the replication of telomeric DNA, TRF2
participates in the formation of T-loops, prevents the activation
of DDR pathways and non-homologous end joining (NHEJ) of telomere
(24,25). POT1 (in association with TPP1)
combines with 3′ single-stranded overhang and inhibits ATR-mediated
DDR by preventing the recruitment of replication protein A
(26). RAP1 affects the selective
binding of TRF2 to telomeric DNA (27). TIN2 combines TRF1 and TRF2 with the
TPP1/POT1 heterodimer and with telomeric DNA, improves complex
stabilization (28).
The shelterin protein complex plays a fundamental
role in homeostasis and telomere end stabilization, and protects
chromosome ends from inappropriate DNA repair by preventing the
activation of DDR pathways and non-homologous end joining (NHEJ)
(29).
The basic function of telomeres is to protect the
ends of chromosomes against degradation and loss of genetic
information. During cell division, telomeres are shortened and do
not bear genetic information essential for the cell. The process is
the result of combining the phenomenon of end replication with DNA
processing at the ends of chromosomes. During semiconservative
replication, the delayed strand (resulting from the combination of
Okazaki fragments) after the removal of the RNA primer has an
incomplete 5′ end. The resulting gap cannot be filled because the
DNA polymerases responsible for the replication process synthesize
the polynucleotide chain only in the 5′ to 3′ direction.
Telomeres also protect chromosomes against abnormal
recombination, chromosome fusion (prevents chromosomal aberrations,
including translocations, duplications, and deletions), or their
degradation as a result of an exonuclease attack (30–32).
They are a molecular clock that, after exceeding the limit of
divisions, directs the cell to the path of replicative senescence
or apoptosis. With each division, the telomeric sequence is
shortened by approximately 50–150 bp in human somatic cells in cell
culture. The time at which the telomere will be critically
shortened may vary. It is a consequence of the heterogeneous
distribution of telomeres in different chromosome arms and the
specific rate of telomere shortening in individual cell lines
(33). Research results indicate
that the shortest telomere can be a factor stopping further cell
division. Telomere shortening causes telomere conformational
changes and loss of T-loop formation, promotes genomic instability
and, in combination with other oncogenic changes, can potentially
stimulate cancer initiation (34).
Telomeres are also involved in functions such as
regulation of gene expression through transcriptional silencing of
genes located close to the telomeres, which is called telomere
position effect (TPE), or those located at long distances from
telomeres, termed TPE over long distances (TPE-OLD) (35,36).
Telomerase
Most of the telomeric DNA is copied in the
replication process. However, telomere deficiency is supplemented
by telomerase-catalyzed elongation.
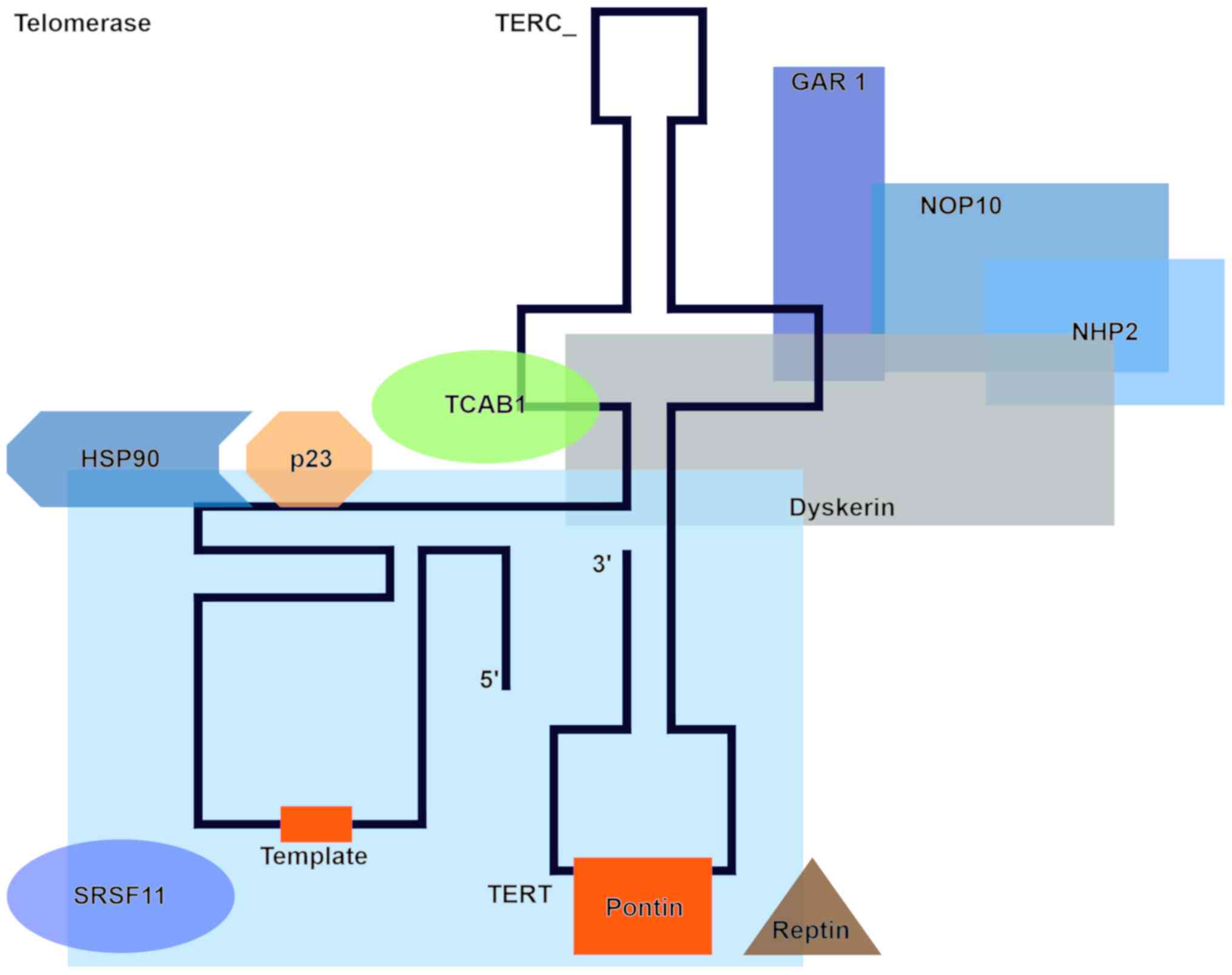
Telomerase, an enzyme made of protein subunits and
RNA, comprises a catalytic subunit with reverse transcriptase
activity (TERT-telomerase reverse transcriptase), an RNA template
(TERC-telomerase RNA component) with a sequence complementary to
the sequence of the telomere, and accessory proteins such as
discerin, NHP2 ribonucleoprotein, NOP10 ribonucleoprotein, and GAR1
(localization factor). The TERT subunit binds to TERC and the
protein complex. Telomerase is recruited to single stranded
telomeric DNA through interaction with the telomere-localizing
protein TPP1. Additional factors such as the chaperones HSP90 and
p23, a WD-repeat-containing protein 79 called TCAB1, as well as the
ATPases pontin and reptin are also involved in this process. SRSF11
(TERC-blinding protein) stabilizes the telomerase-telomere complex
(37) (Fig. 2).
TERT synthesizes telomeric sequences using TERC as a
template. The expression or activity of TERT is regulated at many
stages by various factors. Induction of TERT mRNA expression
requires binding of the transcription factors c-MYC and SP1 to the
E-box (5′-CACGTG-3′) and five GC boxes (5′-GGGCGG-3′) (38). Other transcription factors such as
E2F, AP-1, estrogen response element (ERE) for estrogen receptor α
binding, and CCCTC binding factor are involved in the activation of
TERT transcription (38). The
phosphatidylinositol-3 kinase (PI3K)/AKT pathway enhances TERT
activity at the post-translational level via TERT phosphorylation
by AKT (39).
Telomerase is activated in germline, hematopoietic,
stem and mitotically active, and rapidly regenerating cells. In
contrast, telomerase activity is very low or absent in somatic
cells, although telomerase activity has been found in normal human
blood cells, proliferative basal skin layer, endometrial tissue,
intestinal crypt proliferative zone, and hair follicles (40–44).
Telomerase repression and/or shorter telomeres in
human cells prevent uncontrolled cell proliferation (2). Lack of telomerase leads to a
progressive shortening of telomeres during division because of the
nature of DNA polymerase. When the telomere length reaches a
critical size, DNA damage occurs and the stage of growth arrest
called replicative senescence is activated.
By contrast, cells with strong proliferative
potential are characterized by high telomerase activity. These
cells include stem cells and embryonic and progenitor cells of the
hematopoietic system, skin, and intestinal crypts. Telomerase
activity is closely related to the life stages of the body. The
enzyme is active during embryonic development.
Cancer cells are characterized by high telomerase
activity, which enables cells to divide indefinitely. Telomerase is
active in 85–95% of cancers (3,4). The
exception is cancer cells possessing an active Alternative
Lengthening of Telomeres (ALT) pathway. ALT, which is the ability
of cancer cells to extend telomeres in the absence of telomerase,
is based on homologous recombination using telomeric DNA as a
matrix (45). ALT activation
correlates with the presence of mutations in the genes encoding
α-thalassemia/mental retardation X-linked chromatin remodeler and
death domain associated protein in both tumors and cell lines
(46). This process is observed in
aggressive, difficult to treat tumors of mesenchymal origin, which
account for approximately 5–15% of all cancers (47).
TERT induction and telomerase activation not only
create unlimited cancer cell proliferation potential by stabilizing
telomere length (telomere lengthening-dependent), but also cause
oncogenic effects independently of the telomere lengthening
function. The telomere lengthening-independent functions of TERT,
which significantly contribute to cancer initiation or progression,
include its effects on mitochondrial and ubiquitin-proteasomal
function, DNA damage repair, gene transcription, microRNA (miRNA)
expression, RNA-dependent RNA polymerase activity, and
epithelial-mesenchymal transition (48–56).
These TERT activities physiologically affect the processes that
ultimately lead to cell aging; however, they also drive cancer
development by conferring survival, proliferation, motherhood, and
invasive phenotypes.
Several signalling pathways (mainly c-MYC, NF-κB,
B-Catenin) are involved in the transcriptional reactivation of TERT
in cancer cells. Additionally PI3K/AKT kinase pathway enhances TERT
activity at the posttranslational level via phosphorylation. TERT
activation occurs as a result of c-MYC binding to the E-box
(5′-CACGTG-3′) in the TERT promoter region. There is an increase in
vascular cell viability and stimulation of c-MYC-dependent
oncogenesis potential as a result of induction of transcriptional
activity of TERT, stabilization of c-MYC levels on chromatin and
c-MYC ubiquitination and proteosomal degradation (57). NF-κB controls the transcription of
TERT via NF-κB binding sites in the TERT promoter specific for p50
and p65.
This pathway induces TERT transcription activities
and additionally leads to repression of ROS-dependent activation,
modulation of TERT nuclear translocation, recruitment of IL6 and
TNF alpha, and upregulation of MMP. Repression of ROS-dependent
activation, inflammation and cancer progression occurs as a result
of these activities (54,58,59).
Wnt/B-Catenin is another pathway involved in the regulation of
TERT. Activation of Wnt-depend reporters results in stem cell
pluripotency, cell proliferation, and cancer progression (60).
Telomeres as tumor suppressors
Divisions of human cells that lack telomerase
activity lead to shortening of telomeres and disruption of their
spatial structure (they lose the ability to form T loops). Telomere
shortening is a natural consequence of cell division due to the
‘end replication problem’ and leads to critically shortened
telomeres that trigger DDR. The DNA damage detection pathways by
chromosomal instability and p53 or p16-RB pathway activation are
induced, and the cell enters a stage named replicative senescence.
This is thought to be an effective barrier against cancer by
blocking proliferation and genetic mutations resulting from DNA
replication. Telomeres act as tumor suppressors. If the cells
acquire a mutation in the gene encoding p53, which is responsible
for detecting short telomeres or other checkpoint proteins, they
can overcome senescence. The cells will continue to proliferate
until telomeres become critically short and then will be directed
to apoptosis. As a result of various disorders, some cells
(~1/107) activate telomerase or ALT and acquire the
potential for endless proliferation, which makes them immortal
(31). Most often this condition is
achieved by upregulation or reactivation of telomerase. The rare
telomerase negative immortalization pathway termed ALT involves DNA
recombination to maintain telomeres.
Mechanisms activating TERT transcription and
telomerase in human cancer
The TERT gene is located on the short arm of
chromosome 5. The 433-bp genomic region encompassing 52 CpG sites
located immediately upstream of the TERT core promoter
region may bind to transcription factors or repressors (61). This region upstream of the
TERT promoter is unmethylated in normal human cells, whereas
it is methylated in malignant cells. There is also evidence that
the unmethylated region upstream of the promoter core sequence is
responsible for binding to the repressor (62). Transcriptional regulation of the
TERT gene occurs at many levels and is mediated by various
positive and negative factors or signaling pathways (4). These factors control the TERT
gene and ensure inhibition of TERT activity in most normal cells,
as well as its expression at the right time and place in a small
number of cell types such as activated lymphocytes or stem cells.
This balance may be disturbed in malignant cells. A typical example
is the Myc/Max/Mad1 protein. Endogenous expression of the cellular
c-MYC oncogene may result in dissociation of the Mad1/Max repressor
from the E-box complex, leading to de-repression of the TERT
gene and telomerase activation (63).
Epigenetic factors responsible for DNA methylation,
histone acetylation, methylation, and phosphorylation are another
group of factors modulating TERT transcription. As mentioned,
TERT promoter methylation is required for the expression of
TERT and activation of telomerase in cancer cells (62).
Some viruses may code for proteins that act as
cofactors to stimulate TERT transcription as well. These
include Epstein-Barr virus, cytomegalovirus, Kaposi
sarcoma-associated herpesvirus, human papillomavirus, hepatitis B
virus, hepatitis C virus, and human T-cell leukemia virus-1
(64).
TERT expression and telomerase activity in
tumors are also affected by TERT promoter mutations, which occur
mainly at two active points of chromosome 5, C228T and C250T. The
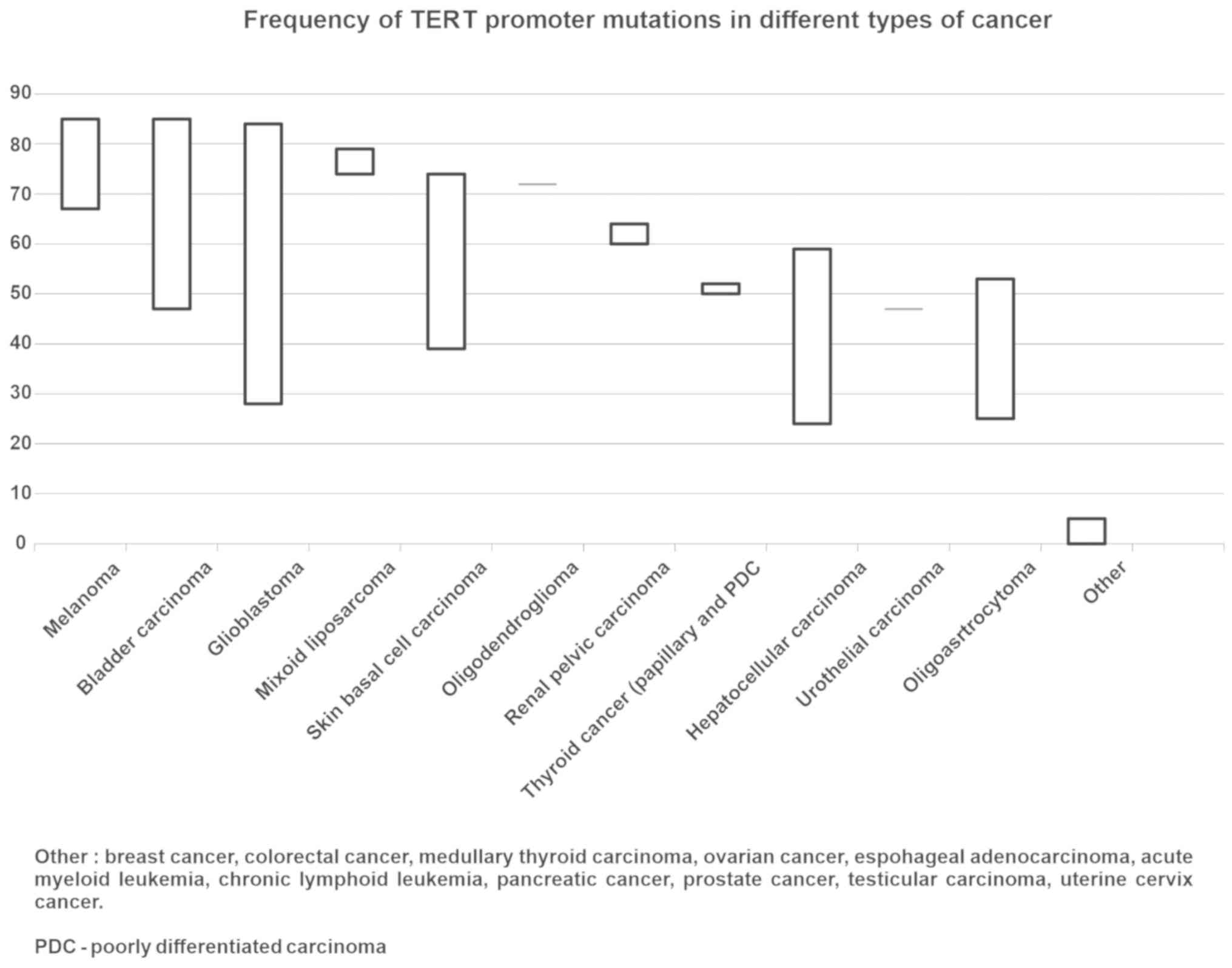
incidence of TERT promoter mutations varies from undetectable to
over 90% in various human malignancies. The highest TERT promoter
mutation rates (up to 80–90%) occur in glioblastoma, melanoma,
bladder urothelial carcinoma, and brain lower-grade glioma. An
intermediate mutation frequency range is observed in liver
hepatocellular carcinoma and thyroid carcinoma. The lowest level of
mutation frequency (<10%) is detected in kidney, lung, prostate,
and gastrointestinal cancers and in leukemia (65). The mutation frequency of the TERT
promoter varies depending on the type of cancer (Fig. 3) (22,37,66–72). The
C228T mutation is more common than the C250T mutation. These
mutations form the binding site for E-twenty six (ETS)
transcription factors. In addition, GABPA and GABPB1 (ETS family
members) binding proteins form heterotetramers that bind to the de
novo ETS site and activate TERT transcription (73).
The presence of the TERT promoter mutation is
negatively correlated with telomere length and is associated with
older patients (4). Telomere damage
causing their dysfunction and genomic instability, which are often
observed in old age, may be due to short telomeres (3).
Some viruses may code for proteins that act as
cofactors to stimulate TERT transcription as well. These include
Epstein-Barr virus, cytomegalovirus, Kaposi sarcoma-associated
herpesvirus, human papillomavirus, hepatitis B virus, hepatitis C
virus, and human T-cell leukemia virus-1 (64). Targeted activation is one of the key
mechanisms of carcinogenesis through a virus. For example, HPV E6
is a viral oncoprotein that forms a complex with E6AP and c-Myc.
This complex binds to the E-box in the TERT core promoter and
induces TERT activation. Another well-studied cofactor is the CMV
early protein 72, which by interacting with Sp1 activates TERT
transcription (64). Recent studies
indicate that rearrangements and insertions of the oncogenic viral
genome at the TERT locus are new mechanisms underlying increased
expression of TERT by hijacking enhancers (74). Recent evidence suggests that viral
DNA integration at the TERT locus may trigger an additional TERT
regulatory mechanism; an exogenous viral enhancer was found to
drive endogenous TERT transcription (74). Enhancers regulate gene transcription
by interacting with gene promoters, regardless of their position
relative to the place of transcription initiation. These factors
make DNA more accessible to the transcription machine.
Functionally, rearrangements and onco-viral DNA cause active
enhancers to affect the TERT gene and increase TERT expression
(75).
High-throughput new generation sequencing of human
cancers has also provided genomic information on TERT
amplification and the important role of this genomic aberration in
telomerase activation during cancer development. Barthel et
al (65) analyzed more than
6,000 cancer patients and found that 4% of the studied tumors show
TERT gene amplification with high frequency in ovarian
cancer, adrenal cortical cancer, esophageal cancer, and lung
cancer. In addition, the highest telomerase activity was found in
tumors with TERT amplification.
TERT promoter mutations-cancer-specific
biomarkers in diagnostics/screening
Telomerase repression in combination with shorter
telomeres are protective mechanisms against cancer. Human somatic
cells achieve malignant transformation through TERT gene
de-repression/telomerase reactivation in most cases. The assessment
of cancer-specific expression of TERT or telomerase activation is
the experimental field for the potential clinical application of
cancer tests (3).
However, there have been difficulties in using
reliable tests to evaluate TERT or telomerase activity for
diagnostic or screening purposes.
On the one hand, false positive results of TERT
expression found in lymphocytes associated with inflammation or
infiltrating tumors. On the other hand, telomerase and mRNA TERT
are temperature sensitive, so managing them is difficult and at the
same time requires high quality tissue samples. An additional
problem is the specificity of the available TERT antibodies for
immune-histochemical staining or immunoblotting (4).
Researchers have mainly focused on assessing TERT
promoter mutations in various cancers. The presence of the TERT
promoter mutation in human malignancies and its absence from normal
cells creates new cancer-specific markers. DNA stability enables
the routine use of mutation analysis. Detection of the mutant TERT
promoter in plasma, urine, and cerebral spinal fluid (CSF) serves
as a useful biomarker in hepatocellular carcinoma, bladder cancer,
and glioma, respectively (76–78). In
addition, the detection of methylated CpG in the TERT promoter
region in the feces may be useful for the diagnosis of
gastrointestinal cancer (79).
Evaluation of the methylated TERT promoter in CSF may be a useful
non-invasive diagnostics tool for predicting metastases to the
meninges (80). In addition,
analysis of circulating oncogenic miRNAs targeting TERT expression
can be a useful diagnostic tool. miRNA assessment in blood can be a
valuable biomarker in cancer (81).
TERT promoter mutations-prognostic factors
in cancer/sign of aggressiveness
Abnormal expression of TERT and hypermethylation of
the TERT promoter serve as prognostic factors in many types of
human cancer. The presence of the TERT promoter mutation is an
unfavorable prognostic factor (metastasis/survival) in melanoma and
glioma (82,83). In thyroid cancers, mutation of the
TERT promoter is associated with poor biological characteristics
and a low rate of survival, and includes differentiated thyroid
cancers with aggressive clinical behavior, poorly differentiated
thyroid cancers (PDTC), and anaplastic thyroid cancers (ATC)
(84,85). The association of TERT promoter
hypermethylation with poor results and cancer prognosis in brain
tumors and adrenal cortical cancer has also been reported (86,87).
The relationship between the coexistence of TERT
promoter and BRAF V600E mutations and a poor disease course
has been reported in thyroid cancer and melanoma (88,89). The
synergy in the coexistence of TERT promoter and BRAF V600E
mutations is most likely caused by the activation of the
mitogen-activated protein kinase (MAPK) and/or PI3-Akt pathways,
which upregulate ETS transcription factors and trigger the
expression of TERT. Recently, Liu et al (90) demonstrated that BRAF V600E
enhances the activation of the MAPK pathway, leading to the
FOS-mediated expression of GABPB, which binds to TERT mutated
promoters and induces TERT expression. In addition, a significant
relationship was observed between TERT promoter mutations and RAS
mutations that often occur in PDTC and ATC (85).
Liu et al (91) reported that KRAS mutations increase
TERT mRNA expression by activating the RAS/MEK pathway, which
contributes to the aggressive phenotype of non-small cell lung
cancer (NSCLC).
Telomere shortening in cancer and its
potential advantage
Although tumors with telomerase activation acquire
the ability to extend the telomere, telomere length is shorter in
prostate cancer than in normal tissues (92). Recent genome-wide analyses have shown
that 70% of the cohorts have shorter telomeres than normal samples
(65). Cancer cells with short
telomeres show upregulation of interferon-stimulated genes, which
is likely to contribute to the malignancy of the tumor. In
addition, truncated telomeres facilitate cancer evolution,
resulting in moderate chromosome instability (93). Theoretically, genetic or
pharmacological inhibition of telomerase activity in TERT-positive
cancer cells should have an antitumor effect. In addition, we would
expect the anticancer effect of telomerase inhibition to appear
earlier in cancer cells with shorter telomeres. In fact, a short
telomeric length may be a predictive biomarker of the efficacy of
telomerase inhibitors (94). The
Imetelstat telomerase inhibitor increases the median
progression-free survival and overall survival of NSCLC patients
with short telomeres (95).
Telomeres as a possible therapeutic
target
Telomerase is expressed in most types of cancer and
in cancer stem cells and is the focus of cancer treatments. Normal
human cells have lower telomerase activity and usually have longer
telomeres than cancer cells. The main point of anti-telomerase
therapy is the selective destruction of cancer cells while
minimizing the effect on normal cells (due to the presence of
longer telomeres compared to cancer cells). Many therapeutic
approaches have been adopted to achieve this goal (Table I).
 | Table I.Main research areas using telomerase
as a therapeutic target. |
Table I.
Main research areas using telomerase
as a therapeutic target.
| Author, year | Strategy |
| Factor | Target | Mechanism | Effect | (Refs.) |
|---|
| Frink et al,
2016; Burchett et al, 2014; Shammas et al, 2008;
Dikmen et al, 2005; Tokcaer-Keskin et al, 2010;
Burchett et al, 2017; Koziel et al, 2015; Wu et
al, 2017 | Antisense
oligonucleotides (ASO) | GRN163L
(Imetelstat) |
| RNA template of
telomerase component (TERC) | TERC competitive
antagonist. Blocking recruitment to telomeric DNA. Expression
inhibition. | Telomerase
inhibition | (94,96–102) |
| Chhabra et
al, 2018; Schrank et al, 2018 Pitman et al, 2013;
Puri et al, 2014; Wojdyla et al, 2014; Weng et
al, 2010 | Uncapping
mimicking | T-oligos |
| Telomere 3′
overhang region. | Shelterin complex
proteins dissociation. Ataxia telangiectasia mutated (ATM) pathway
activation. Cytotoxic effects-inducing DNA damage responses
(DDR). | Cycle arrest,
apoptosis | (103–108) |
| Guzman et
al, 2018; Zhou et al, 2016; Mitomo et al, 2008;
Zhang et al, 2015; Melnik et al, 2015; Yang et
al, 2015; Nguyen et al, 2017; | Expression
modulators | TERT/TERC-miRNA
TERT/TERC anti-miRNA |
| Suppressor miRNAs
Oncogenic miRNAs | TERT/TERC-targeted
suppressor miRNAs. TERT/TERC targeted oncogenic anti-miRNAs |
Post-transcriptional gene silencing.
Expression repression | (109–115) |
| Mizukoshi et
al, 2019; Staff et al, 2014; Fenoglio et al,
2013; Fenoglio et al, 2015; Lilleby et al, 2017;
Kotsakis et al, 2014 | Anti-telomerase
immunotherapy | Peptide
Vaccines | GV1001 GX301 UV1
Vx001 | HLA, HLA-A | CD4+/CD8+ T cells
stimulation | [116-121]
[116,126,127] | (116–121) |
| Mizukoshi et
al, 2019; Su et al, 2005; Khoury et al, 2017;
Galati et al, 2018; Salazar-Onfray et al, 2013 |
| GRNVAC1 TAPCells
vaccine |
| TERT-Targeting
Dendritic cells | Antigen
presentation by dendritic cells transfected with TERT-mRNA |
| (116,122–125) |
| Mizukoshi et
al, 2019; Thalmensi et al, 2016; Yan et al,
2013 |
| DNA Vaccines | phTERT INVAC-1 | HLA | CD4+/CD8+ T cells
stimulation |
| (116,126,127) |
| Jordheim et
al, 2013 | Reverse
transcriptase inhibitors | AZT
azidothymidine |
| DNA elongation | Replication
termination | Induction of
telomere shortening/Inhibition of tumor cell proliferation | (129) |
| Pascolo et
al, 2002 |
| BIBR 1532 |
| Reverse
transcriptase | Non-competitive
inhibition |
| (130) |
| Kim et al,
2002; Gomez et al, 2016 | G-quadruplex
stabilization | Telomestatin
BRACO-1910 RHPS4 |
| Telomeric DNA | Telomerase to
telomere endings access blocking | Telomeres erosion
induction/Cell cycle inhibition | (131,132) |
| Nemunaitis et
al, 2010; Schepelmann et al, 2007 | Gene therapy | Telomelysin |
| TERT positive
cells | Chimeric gene
introduction | Cancer cell
lysis | (133,134) |
Oligonucleotides
Therapies based on telomerase-targeted
oligonucleotides include GRN163L (Imetelstat). This 13-mer
oligonucleotide sequence is a competitive antagonist that binds to
the TERC template region. It blocks its recruitment to telomeric
DNA and leads to complete inhibition of telomerase activity.
Imetelstat has been studied in glioblastoma, bladder, breast,
liver, prostate, and pancreatic cancer and shows promising
antitumor activity, although it is too toxic as a stand-alone
therapy (94). GRN163L treated
pancreatic cancer and myeloma cells showed growth similar to
untreated cells for the first 3–8 and 3–5 weeks, respectively, but
later began to undergo progressive aging and apoptosis (96,97).
Significant reduction of rapid cellular attachment and reduction of
metastatic lesions has been demonstrated for lung cancer cells
expressing A549-luciferase treated with GRN163L (98). Despite promising anti-cancer effects,
the use of GRN163L in clinical settings is limited due to its
hematological toxicity. Neutropenia and thrombocytopenia require
frequent drug holidays, limiting the effectiveness of GRN163L as a
therapeutic agent (95). Another
problem associated with GRN163L is its harmful effect on
mesenchymal stem cells. A change in mesenchymal stem cell
morphology, loss of adhesion, and G1 phase arrest of the cell cycle
have been demonstrated (99).
Although the use of GRN163L alone is currently ineffective, it has
been shown to have promising effects in combination with other
molecularly targeted drugs or in the sensitization of cancer cells
to radiation therapy (100–102).
T-oligos homologous to the 3′-telomeric overhang are
also promising oligonucleotides that have demonstrated anticancer
activity. They dissociate shelterin complex proteins, activate the
ataxia telangiectasia mutated pathway, and exert cytotoxic effects
by inducing the DDR (103).
However, rapid degradation by nucleases and an incomplete
explanation of its mechanism of action remain obstacles to the
introduction of T-oligos into clinical trials (104). They dissociate shelterin complex
proteins, activate the ataxia telangiectasia mutated pathway (ATM)
and its downstream effectors p53, pRb, E2F1, cdk2, and p95/NBS1 and
exert cytotoxic effects by inducing the DDR. Two models explain the
mechanism of anti-cancer action by T-oligo. The first assumes that
T-oligo accumulating in the nucleus are detected by homology to the
telomere as damaged DNA and DDR activation occurs. The second model
assumes the action of T-oligos by dissociating shelter proteins,
thereby exposing the telomere overhang and inducing responses to
DNA damage (104). T-oligo
anti-tumor activity has been demonstrated in vitro in cancers such
as melanoma, lymphoma, lung, breast, prostate, pancreas, colorectal
and ovarian cancer (104–106). However, rapid degradation by
nucleases and an incomplete explanation of its mechanism of action
remain obstacles to the introduction of T-oligos into clinical
trials (104). Therefore, the
efforts of researchers are directed to the search for methods of
inhibition by nucleases. In addition, the use of T-oligo in
combined therapies has shown promising results. Additive inhibition
has been demonstrated in combination with an EGFR (gefitinib)
inhibitor in colorectal cancer (107). T-oligo also increases the
sensitivity of breast cancer cells to radiation therapy (108).
miRNAs are an endogenous group of oligonucleotides
that regulate gene expression at the post-transcriptional level,
effectively silencing genes by interacting with the RNA-Induced
Silencing Complex. Carcinogenic miRNAs may exist as oncogenic
miRNAs or suppressor miRNAs that promote or inhibit the development
of cancer, respectively. Several miRNAs (miR-128, miR-138,
miR-1182, miR-342, miR-491, and miR-541) negatively regulate the
expression of the TERT gene, thereby acting as tumor
suppressor miRNAs (109).
Overexpression of miR-138 has been shown to inhibit cell
proliferation, invasion and induce apoptosis in cervical cancer
(110). In contrast, a decrease in
the level of miR-138 is observed in anaplastic thyroid cancer
(111). Another study found that
miR-1182 reduced the proliferation and migration of gastric cancer
cells (112). In contrast, miR-128
can act as either oncogenic or suppressor miRNA, depending on
interaction with various targets (109). TERC-targeted miRNAs may act as
telomerase inhibitors and are being tested in clinical
applications. Unfortunately, inhibition of telomerase induces an
anti-tumor effect when they lead to a critical shortening of
telomere, usually after weeks of treatment. In addition, the effect
of miRNA reducing telomerase activity in stem cells requires
further study. Oncogenic miRNAs such as miR-21 that cause tumor
transformation by regulating TERT expression have been identified.
The participation of miR-21 in melanoma, colorectal cancer or
glioma has been described (113,114).
The use of anti-miRNAs that are antisense to target miRNAs and
block their action has been reported. miRNA inhibition in cancer
treatment remains in the preclinical stages (115).
Anti-telomerase immunotherapy
Telomerase-based vaccines sensitize immune cells to
cancer cells expressing TERT peptides as surface antigens via the
human leukocyte antigen (HLA) class I and class II pathways. The
expansion of CD4+ and CD8+ cytotoxic T
lymphocytes (CTL) specific for oncogenic telomerase causes T cells
to kill telomerase-positive tumor cells (116).
TERT-targeted peptide vaccines
GV1001 is the most advanced of all TERT vaccines. It
induces specific T cell responses in pancreatic cancer, NSCLC, and
melanoma (117). It is a MHC class
II restricted peptide vaccine that elicits strong CD4+
and CD8+ T cell responses and cytotoxic T lymphocytes
(CTL) activation. Clinical studies have shown that it induces T
cell responses in 50–80% of patients with advanced pancreatic
cancer and lung cancer without clinical toxicity. The vaccine did
not affect bone marrow cells (116). GX301, a vaccine consisting of four
peptides derived from TERT, is more effective than single-peptide
vaccines; it has been tested in patients with prostate cancer and
kidney cancer (118). The results
of these studies indicate that multi-peptide vaccines are more
effective because they enhance the immune response in more
responders than single-peptide vaccines (119). Immune responses to UV1 or Vx-001
vaccines were demonstrated in prostate cancer and NSCLC,
respectively (120,121). The first of these induced an immune
response in 85.7% of patients and reduced prostate-specific antigen
(PSA) levels in 64% of patients with metastatic prostate cancer
(120). Similarly, the second
vaccine elicited a strong TERT-specific immune response in NSCLC
and had a good tolerance profile (121).
Immunotherapy using TERT-targeting
dendritic cells (DC)
DCs are the strongest antigen presenting cells and
play an important role in inducing immunity. GRNVAC1 is a DC-based
cancer vaccine that elicits a polyclonal immune response. Previous
clinical studies have shown that GRNVAC1 is effective, safe and
well tolerated in patients with prostate cancer and acute myeloid
leukemia (122,123).
Another DC-based approach is to produce therapeutic
dendritic-like cells called tumor antigen presenting cells
(TAPCells) (124). This vaccine has
been evaluated in patients with advanced stage melanoma and
castration-resistant prostate cancer. It increased the survival of
patients with melanoma and extended the doubling time of PSA
(125).
DNA vaccines
The genome encoding the TERT peptide can be created
using recombinant DNA technology. Plasmids containing these genomes
can be delivered to antigen presenting cells, which improves the
efficiency of epitope presentation in T lymphocytes. phTERT is a
DNA-based vaccine that codes for TERT, whereas INVAC-1 is a plasmid
that codes for the inactive form of TERT (126). The phTERT and INVAC-1 vaccines
inhibited tumor proliferation and prolonged survival in the
HPV-related and melanoma-related tumor model, respectively
(126,127).
Gene-modified T-cell therapy
This is another promising method based on the use of
T cells genetically modified to produce the T-cell receptor, which
recognizes tumor antigens and their epitopes (128).
Nucleosides
Azidothymidine (AZT) was the first reported
telomerase inhibitor. AZT inhibits telomerase irreversibly. AZT has
a synergistic effect with paclitaxel and significantly enhances its
effect. It is used to treat many human cancers associated with
viruses, whereas in non-viral tumors, it causes some degree of
regression (129). Acyclic
nucleoside analogs such us acyclovir, ganciclovir, and penciclovir
have been identified as inhibitors or antagonists of
telomerase.
Small molecule inhibitors
The BIBR1532 acid is a non-nucleotide small molecule
compound that selectively inhibits telomerase activity by
non-competitive binding to the active site of TERT (130). Although preclinical studies on
breast and prostate cancer cell lines have shown good results, no
further inclusion in clinical trials has been reported.
Stabilization of G quadruplexes
Stabilization of G-quadruplexes prevents TERC from
recognizing the unfolded single-stranded telomere overhang, thereby
inhibiting telomerase activity. Compounds that stabilize
G-quadruplexes include telomestatin, RHPS4, and BRACO19 (131). Other compounds that stabilize
G-quadruplexes and inhibit telomerase are being investigated.
Daunomycin, distamycin A, ascididemin and meridine, berberine,
cryptolepine derivatives, and cationic porphyrins are being studied
as possible telomerase inhibitors because of their ability to bind
and stabilize G-quadruplexes (132).
Gene therapy
Imetelstat (GRN163L) is one of the best known gene
therapy molecules, it was previously described in paragraph 10
(133). Other strategies include
the introduction of a chimeric gene, namely, the coding sequence of
a proapoptotic protein under the control of the TERT gene promoter.
Telomelysine is an attenuated adenovirus-5 vector that induces
lysis of cancer cells overexpressing TERT (133). Another approach involves the use of
viral vectors that have been genetically modified to encode a
cytotoxic prodrug activating enzyme (134).
Targeting telomeres and
telomerase-associated proteins
Therapeutic approaches based on targeting
telomerase-related proteins have been investigated. One of these
strategies is targeting tankyrase (the enzyme responsible for the
correct separation of chromosomes) with PARP inhibitors. Also
interesting is the inhibition of the chaperone HSP90 (required for
maturation and activation of telomerase) by Geldanamycin (135). Molecules against TRF1, TRF2, and
TIN2 or POT1 have also been analyzed (136).
Telomerase inhibitors derived from
natural products and microbial sources
Natural products capable of telomerase inhibition as
potential chemotherapeutic agents for cancer treatment have been
identified. Natural products from plants with activity against
telomerase include polyphenols (curcumin, quercetin, resveratrol,
and tannic acid), alkaloids (boldine and berberine), terpenoids
(pristimerin and oleanane), and xanthones (gambogic acid and
gambogenic acid) (137). Oleic acid
is a fatty acid that occurs in various animal and vegetable oils,
is classified as omega-9 monounsaturated fatty acid, and has been
identified as a human telomerase inhibitor (138). Actinomycetes spp. are
microorganisms containing benzofuran and benzodipyrane rings that
act as telomerase inhibitors. Rubromycins isolated from
Streptomyces collinus that have telomerase inhibitory
activity have recently been studied (138).
Telomerase and oxidative stress
Studies have reported telomerase damage induced by
reactive oxygen species (ROS). Antioxidant enzymes such as
peroxiredoxin 1 (PRDX1) and the nudix phosphohydrolase superfamily
enzyme (MTH1) protect telomeres against oxidative stress. Cancer
cells are more vulnerable to ROS than noncancer cells. Combination
therapy with ROS-inducing chemotherapeutic agents and inhibitors of
proteins that protect telomeres from ROS (such as PRDX1 and MTH1)
could selectively target telomere maintenance in cancer cells
(139).
Conclusions
Understanding the structure of telomeres and the
mechanism of action of telomerase provides hope for the
identification of new forms of cancer treatment. The high
specificity of telomerase and the potential for inhibiting its
activity at various stages underlie its value as a target for
cancer therapy. Many in vitro studies of telomerase
inhibition have been conducted to date, and clinical trials are in
the early stages. The combination of telomerase inhibitory agents
with cytostatic drugs or radiation therapy increases the
effectiveness of existing therapies. The results so far point to
the huge possibilities of using telomerase for the development of
new targeted therapies and for improving the efficacy of current
anticancer drugs. Further studies are required to develop
TERT-based cancer therapeutic interventions.
Acknowledgements
Not applicable.
Funding
The project was financed under the program of the
Minister of Science and Higher Education called ‘Regional
Initiative of Excellence’ (project no. 024/RID/2018/19).
Availability of data and materials
Not applicable.
Authors' contributions
TT and AlK developed the concept. TT and AlK
developed the methodology. TT, ArK, SG and AlK were involved in
validation. TT, ArK, SG and AlK prepared the original draft. TT,
ArK, SG and AlK reviewed and edited the manuscript. TT, ArK and AlK
were responsible for visualization. TT and AlK supervised the
study. TT and AlK were involved in project administration. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
WHO: Cancer, .
|
|
2
|
Seluanov A, Gladyshev VN, Vijg J and
Gorbunova V: Mechanisms of cancer resistance in long-lived mammals.
Nat Rev Cancer. 18:433–441. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shay JW and Wright WE: Telomeres and
telomerase: Three decades of progress. Nat Rev Genet. 20:299–309.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu T, Yuan X and Xu D: Cancer-specific
telomerase reverse transcriptase (TERT) promoter mutations:
Biological and clinical implications. Genes (Basel). 7(pii):
E382016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yuan X, Larsson C and Xu D: Mechanisms
underlying the activation of TERT transcription and telomerase
activity in human cancer: Old actors and new players. Oncogene.
38:6172–6183. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dilley RL and Greenberg RA: ALTernative
telomere maintenance and cancer. Trends Cancer. 1:145–156. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hayflick L: The limited in vitro lifetime
of human diploid cell strains. Exp Cell Res. 37:614–636. 1965.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Olovnikov AM: A theory of marginotomy. The
incomplete copying of template margin in enzymic synthesis of
polynucleotides and biological significance of the phenomenon. J
Theor Biol. 41:181–190. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Szostak JW and Blackburn EH: Cloning yeast
telomeres on linear plasmid vectors. Cell. 29:245–255. 1982.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Blackburn EH: Structure and function of
telomeres. Nature. 350:569–573. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Greider CW: Telomerase is processive. Mol
Cell Biol. 11:4572–4580. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Moyzis RK, Buckingham JM, Cram LS, Dani M,
Deaven LL, Jones MD, Meyne J, Ratliff RL and Wu JR: A highly
conserved repetitive DNA sequence, (TTAGGG)n, present at the
telomeres of human chromosomes. Proc Natl Acad Sci USA.
85:6622–6626. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Makarov VL, Hirose Y and Langmore JP: Long
G tails at both ends of human chromosomes suggest a C strand
degradation mechanism for telomere shortening. Cell. 88:657–666.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wellinger RJ and Sen D: The DNA structures
at the ends of eukaryotic chromosomes. Eur J Cancer. 33:735–749.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Blackburn EH: Switching and signaling at
the telomere. Cell. 106:661–673. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Greider CW and Blackburn EH: A telomeric
sequence in the RNA of Tetrahymena telomerase required for telomere
repeat synthesis. Nature. 337:331–337. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lendvay TS, Morris DK, Sah J,
Balasubramanian B and Lundblad V: Senescence mutants of
Saccharomyces cerevisiae with a defect in telomere replication
identify three additional EST genes. Genetics. 144:1399–1412.
1996.PubMed/NCBI
|
|
18
|
Lingner J and Cech TR: Purification of
telomerase from Euplotes aediculatus: Requirement of a primer 3′
overhang. Proc Natl Acad Sci USA. 93:10712–10717. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu L, Lai S, Andrews LG and Tollefsbol
TO: Genetic and epigenetic modulation of telomerase activity in
development and disease. Gene. 340:1–10. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim NW, Piatyszek MA, Prowse KR, Harley
CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL and Shay
JW: Specific association of human telomerase activity with immortal
cells and cancer. Science. 266:2011–2015. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shay JW and Bacchetti S: A survey of
telomerase activity in human cancer. Eur J Cancer. 33:787–791.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Horn S, Figl A, Rachakonda PS, Fischer C,
Sucker A, Gast A, Kadel S, Moll I, Nagore E, Hemminki K, et al:
TERT promoter mutations in familial and sporadic melanoma. Science.
339:959–961. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang FW, Hodis E, Xu MJ, Kryukov GV, Chin
L and Garraway LA: Highly recurrent TERT promoter mutations in
human melanoma. Science. 339:957–959. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zimmermann M, Kibe T, Kabir S and de Lange
T: TRF1 negotiates TTAGGG repeat-associated replication problems by
recruiting the BLM helicase and the TPP1/POT1 repressor of ATR
signaling. Genes Dev. 28:2477–2491. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Arnoult N and Karlseder J: Complex
interactions between the DNA-damage response and mammalian
telomeres. Nat Struct Mol Biol. 22:859–866. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Denchi EL and de Lange T: Protection of
telomeres through independent control of ATM and ATR by TRF2 and
POT1. Nature. 448:1068–1071. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Janoušková E, Nečasová I, Pavloušková J,
Zimmermann M, Hluchý M, Marini V, Nováková M and Hofr C: Human Rap1
modulates TRF2 attraction to telomeric DNA. Nucleic Acids Res.
43:2691–2700. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Frescas D and de Lange T: TRF2-tethered
TIN2 can mediate telomere protection by TPP1/POT1. Mol Cell Biol.
34:1349–1362. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Stewart JA, Chaiken MF, Wang F and Price
CM: Maintaining the end: Roles of telomere proteins in
end-protection, telomere replication and length regulation. Mutat
Res. 730:12–19. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Martínez P and Blasco MA: Telomeric and
extra-telomeric roles for telomerase and the telomere-binding
proteins. Nat Rev Cancer. 11:161–176. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kowalska A and Kowalik A: Telomeres and
telomerase in oncogenesis. Contemp Oncol (Pozn). 10:485–496.
2006.
|
|
32
|
Shore D and Bianchi A: Telomere length
regulation: Coupling DNA end processing to feedback regulation of
telomerase. EMBO J. 28:2309–2322. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bourgeron T, Xu Z, Doumic M and Teixeira
MT: The asymmetry of telomere replication contributes to
replicative senescence heterogeneity. Sci Rep. 5:153262015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hemann MT, Strong MA, Hao LY and Greider
CW: The shortest telomere, not average telomere length, is critical
for cell viability and chromosome stability. Cell. 107:67–77. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pedram M, Sprung CN, Gao Q, Lo AWI,
Reynolds GE and Murnane JP: Telomere position effect and silencing
of transgenes near telomeres in the mouse. Mol Cell Biol.
26:1865–1878. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Robin JD, Ludlow AT, Batten K, Magdinier
F, Stadler G, Wagner KR, Shay JW and Wright WE: Telomere position
effect: Regulation of gene expression with progressive telomere
shortening over long distances. Genes Dev. 28:2464–2476. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jafri MA, Ansari SA, Alqahtani MH and Shay
JW: Roles of telomeres and telomerase in cancer, and advances in
telomerase-targeted therapies. Genome Med. 8:692016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kyo S, Takakura M, Fujiwara T and Inoue M:
Understanding and exploiting hTERT promoter regulation for
diagnosis and treatment of human cancers. Cancer Sci. 99:1528–1538.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kang SS, Kwon T, Kwon DY and Do SI: Akt
protein kinase enhances human telomerase activity through
phosphorylation of telomerase reverse transcriptase subunit. J Biol
Chem. 274:13085–13090. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Broccoli D, Young JW and de Lange T:
Telomerase activity in normal and malignant hematopoietic cells.
Proc Natl Acad Sci USA. 92:9082–9086. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Härle-Bachor C and Boukamp P: Telomerase
activity in the regenerative basal layer of the epidermis inhuman
skin and in immortal and carcinoma-derived skin keratinocytes. Proc
Natl Acad Sci USA. 93:6476–6481. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kyo S, Takakura M, Kohama T and Inoue M:
Telomerase activity in human endometrium. Cancer Res. 57:610–614.
1997.PubMed/NCBI
|
|
43
|
Hiyama K, Hirai Y, Kyoizumi S, Akiyama M,
Hiyama E, Piatyszek MA, Shay JW, Ishioka S and Yamakido M:
Activation of telomerase in human lymphocytes and hematopoietic
progenitor cells. J Immunol. 155:3711–3715. 1995.PubMed/NCBI
|
|
44
|
Ramirez RD, Wright WE, Shay JW and Taylor
RS: Telomerase activity concentrates in the mitotically active
segments of human hair follicles. J Invest Dermatol. 108:113–117.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Cesare AJ and Reddel RR: Alternative
lengthening of telomeres: Models, mechanisms and implications. Nat
Rev Genet. 11:319–330. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Heaphy CM, de Wilde RF, Jiao Y, Klein AP,
Edil BH, Shi C, Bettegowda C, Rodriguez FJ, Eberhart CG, Hebbar S,
et al: Altered telomeres in tumors with ATRX and DAXX mutations.
Science. 333:4252011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Henson JD, Hannay JA, McCarthy SW, Royds
JA, Yeager TR, Robinson RA, Wharton SB, Jellinek DA, Arbuckle SM,
Yoo J, et al: A robust assay for alternative lengthening of
telomeres in tumors shows the significance of alternative
lengthening of telomeres in sarcomas and astrocytomas. Clin Cancer
Res. 11:217–225. 2005.PubMed/NCBI
|
|
48
|
Im E, Yoon JB, Lee HW and Chung KC: Human
telomerase reverse transcriptase (hTERT) positively regulates 26s
proteasome activity. J Cell Physiol. 232:2083–2093. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Hu C, Ni Z, Li BS, Yong X, Yang X, Zhang
JW, Zhang D, Qin Y, Jie MM, Dong H, et al: hTERT promotes the
invasion of gastric cancer cells by enhancing FOXO3a ubiquitination
and subsequent ITGB1 upregulation. Gut. 66:31–42. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Saretzki G: Extra-telomeric functions of
human telomerase: Cancer, mitochondria and oxidative stress. Curr
Pharm Des. 20:6386–6403. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Masutomi K, Possemato R, Wong JM, Currier
JL, Tothova Z, Manola JB, Ganesan S, Lansdorp PM, Collins K and
Hahn WC: The telomerase reverse transcriptase regulates chromatin
state and DNA damage responses. Proc Natl Acad Sci USA.
102:8222–8227. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Liu Z, Li Q, Li K, Chen L, Li W, Hou M,
Liu T, Yang J, Lindvall C, Björkholm M, et al: Telomerase reverse
transcriptase promotes epithelial-mesenchymal transition and stem
cell-like traits in cancer cells. Oncogene. 32:4203–4213. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zhang K, Guo Y, Wang X, Zhao H, Ji Z,
Cheng C, Li L, Fang Y, Xu D, Zhu HH and Gao WQ: WNT/β-catenin
directs self-renewal symmetric cell division of hTERThigh prostate
cancer stem cells. Cancer Res. 77:2534–2547. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Ding D, Xi P, Zhou J, Wang M and Cong YS:
Human telomerase reverse transcriptase regulates MMP expression
independently of telomerase activity via NF-κB-dependent
transcription. FASEB J. 27:4375–4383. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Lassmann T, Maida Y, Tomaru Y, Yasukawa M,
Ando Y, Kojima M, Kasim V, Simon C, Daub CO, Carninci P, et al:
Telomerase reverse transcriptase regulates microRNAs. Int J Mol
Sci. 16:1192–1208. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Drevytska TI, Nagibin VS, Gurianova VL,
Kedlyan VR, Moibenko AA and Dosenko VE: Silencing of TERT decreases
levels of miR-1, miR-21, miR-29a and miR-208a in cardiomyocytes.
Cell Biochem Funct. 32:565–570. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Koh CM, Khattar E, Leow SC, Liu CY, Muller
J, Ang WX, Li Y, Franzoso G, Li S, Guccione E and Tergaonkar V:
Telomerase regulates MYC-driven oncogenesis independent of its
reverse transcriptase activity. J Clin Invest. 125:2109–2122. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Mattiussi M, Tilman G, Lenglez S and
Decottignies A: Human telomerase represses ROS-dependent cellular
responses to tumor necrosis factor-α without affecting NF-κB
activation. Cell Signal. 24:708–717. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Ghosh A, Saginc G, Leow SC, Khattar E,
Shin EM, Yan TD, Wong M, Zhang Z, Li G, Sung WK, et al: Telomerase
directly regulates NF-κB-dependent transcription. Nat Cell Biol.
14:1270–1281. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Zhang Y, Toh L, Lau P and Wang X: Human
telomerase reverse transcriptase (hTERT) is a novel target of the
Wnt/β-catenin pathway in human cancer. J Biol Chem.
287:32494–32511. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Cong YS, Wen J and Bacchetti S: The human
telomerase catalytic subunit hTERT: Organization of the gene and
characterization of the promoter. Hum Mol Genet. 8:137–142. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Lee DD, Leão R, Komosa M, Gallo M, Zhang
CH, Lipman T, Remke M, Heidari A, Nunes NM, Apolónio JD, et al: DNA
hypermethylation within TERT promoter upregulates TERT expression
in cancer. J Clin Invest. 129:223–229. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Casillas MA, Brotherton SL, Andrews LG,
Ruppert JM and Tollefsbol TO: Induction of endogenous telomerase
(hTERT) by c-Myc in WI-38 fibroblasts transformed with specific
genetic elements. Gene. 316:57–65. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Bellon M and Nicot C: Regulation of
telomerase and telomeres: Human tumor viruses take control. J Natl
Cancer Inst. 100:98–108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Barthel FP, Wei W, Tang M,
Martinez-Ledesma E, Hu X, Amin SB, Akdemir KC, Seth S, Song X, Wang
Q, et al: Systematic analysis of telomere length and somatic
alterations in 31 cancer types. Nat Genet. 49:349–357. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Killela PJ, Reitman ZJ, Jiao Y, Bettegowda
C, Agrawal N, Diaz LA Jr, Friedman AH, Friedman H, Gallia GL,
Giovanella BC, et al: TERT promoter mutations occur frequently in
gliomas and a subset of tumors derived from cells with low rates of
self-renewal. Proc Natl Acad Sci USA. 110:6021–6026. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Rachakonda PS, Hosen I, de Verdier PJ,
Fallah M, Heidenreich B, Ryk C, Wiklund NP, Steineck G, Schadendorf
D, Hemminki K and Kumar R: TERT promoter mutations in bladder
cancer affect patient survival and disease recurrence through
modification by a common polymorphism. Proc Natl Acad Sci USA.
110:17426–17431. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Wang K, Liu T, Liu L, Liu J, Liu C, Wang
C, Ge N, Ren H, Yan K, Hu S, et al: TERT promoter mutations in
renal cell carcinomas and upper tract urothelial carcinomas.
Oncotarget. 5:1829–1836. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Cevik D, Yildiz G and Ozturk M: Common
telomerase reverse transcriptase promoter mutations in
hepatocellular carcinomas from different geographical locations.
World J Gastroenterol. 21:311–317. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Liu X, Bishop J, Shan Y, Pai S, Liu D,
Murugan AK, Sun H, El-Naggar AK and Xing M: Highly prevalent TERT
promoter mutations in aggressive thyroid cancers. Endocr Relat
Cancer. 20:603–610. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Koelsche C, Renner M, Hartmann W, Brandt
R, Lehner B, Waldburger N, Alldinger I, Schmitt T, Egerer G, Penzel
R, et al: TERT promoter hotspot mutations are recurrent in myxoid
liposarcomas but rare in other soft tissue sarcoma entities. J Exp
Clin Cancer Res. 33:332014. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Vinagre J, Pinto V, Celestino R, Reis M,
Pópulo H, Boaventura P, Melo M, Catarino T, Lima J, Lopes JM, et
al: Telomerase promoter mutations in cancer: An emerging molecular
biomarker? Virchows Arch. 465:119–133. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Bell RJA, Rube HT, Kreig A, Mancini A,
Fouse SD, Nagarajan RP, Choi S, Hong C, He D, Pekmezci M, et al:
Cancer. The transcription factor GABP selectively binds and
activates the mutant TERT promoter in cancer. Science.
348:1036–1039. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Chen X, Kost J, Sulovari A, Wong N, Liang
WS, Cao J and Li D: A virome-wide clonal integration analysis
platform for discovering cancer viral etiology. Genome Res.
29:819–830. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Strååt K, Liu C, Rahbar A, Zhu Q, Liu L,
Wolmer-Solberg N, Lou F, Liu Z, Shen J, Jia J, et al: Activation of
telomerase by human cytomegalovirus. J Natl Cancer Inst.
101:488–497. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Labgaa I, Villacorta-Martin C, D'Avola D,
Craig AJ, von Felden J, Martins-Filho SN, Sia D, Stueck A, Ward SC
and Fiel MI: A pilot study of ultra-deep targeted sequencing of
plasma DNA identifies driver mutations in hepatocellular carcinoma.
Oncogene. 37:3740–3752. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Hurst CD, Platt FM and Knowles MA:
Comprehensive mutation analysis of the TERT promoter in bladder
cancer and detection of mutations in voided urine. Eur Urol.
65:367–369. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Juratli TA, Stasik S, Zolal A, Schuster C,
Richter S, Daubner D, Juratli MA, Thowe R, Hennig S, Makina M, et
al: TERT promoter mutation detection in cell-free tumor-derived DNA
in patients with IDH wild-type glioblastomas: A pilot prospective
study. Clin Cancer Res. 24:5282–5291. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Liu L, Liu C, Fotouhi O, Fan Y, Wang K,
Xia C, Shi B, Zhang G, Wang K, Kong F, et al: TERT promoter
hypermethylation in gastrointestinal cancer: A potential stool
biomarker. Oncologist. 22:1178–1188. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Bougel S, Lhermitte B, Gallagher G, de
Flaugergues JC, Janzer RC and Benhattar J: Methylation of the hTERT
promoter: A novel cancer biomarker for leptomeningeal metastasis
detection in cerebrospinal fluids. Clin Cancer Res. 19:2216–2223.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Bianchi F, Nicassio F, Marzi M, Belloni E,
Dall'olio V, Bernard L, Pelosi G, Maisonneuve P, Veronesi G and Di
Fiore PP: A serum circulating miRNA diagnostic test to identify
asymptomatic high-risk individuals with early stage lung cancer.
EMBO Mol Med. 3:495–503. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Nagore E, Heidenreich B, Rachakonda S,
Garcia-Casado Z, Requena C, Soriano V, Frank C, Traves V, Quecedo
E, Sanjuan-Gimenez J, et al: TERT promoter mutations in melanoma
survival. Int J Cancer. 139:75–84. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Yuan Y, Qi C, Maling G, Xiang W, Yanhui L,
Ruofei L, Yunhe M, Jiewen L and Qing M: TERT mutation in glioma:
Frequency, prognosis and risk. J Clin Neurosci. 26:57–62. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Melo M, da Rocha AG, Vinagre J, Batista R,
Peixoto J, Tavares C, Celestino R, Almeida A, Salgado C, Eloy C, et
al: TERT promoter mutations are a major indicator of poor outcome
in differentiated thyroid carcinomas. J Clin Endocrinol Metab.
99:E754–765. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Liu R and Xing M: TERT promoter mutations
in thyroid cancer. Endocr Relat Cancer. 23:R143–R155. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Castelo-Branco P, Choufani S, Mack S,
Gallagher D, Zhang C, Lipman T, Zhukova N, Walker EJ, Martin D,
Merino D, et al: Methylation of the TERT promoter and risk
stratification of childhood brain tumours: An integrative genomic
and molecular study. Lancet Oncol. 14:534–542. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Svahn F, Paulsson JO, Stenman A, Fotouhi
O, Mu N, Murtha TD, Korah R, Carling T, Bäckdahl M, Wang N, et al:
TERT promoter hypermethylation is associated with poor prognosis in
adrenocortical carcinoma. Int J Mol Med. 42:1675–1683.
2018.PubMed/NCBI
|
|
88
|
Liu X, Qu S, Liu R, Sheng C, Shi X, Zhu G,
Murugan AK, Guan H, Yu H, Wang Y, et al: TERT promoter mutations
and their association with BRAF V600E mutation and aggressive
clinicopathological characteristics of thyroid cancer. J Clin
Endocrinol Metab. 99:E1130–E1136. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Macerola E, Loggini B, Giannini R,
Garavello G, Giordano M, Proietti A, Niccoli C, Basolo F and
Fontanini G: Coexistence of TERT promoter and BRAF mutations in
cutaneous melanoma is associated with more clinicopathological
features of aggressiveness. Virchows Arch. 467:177–184. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Liu R, Zhang T, Zhu G and Xing M:
Regulation of mutant TERT by BRAF V600E/MAP kinase pathway through
FOS/GABP in human cancer. Nat Commun. 9:5792018. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Liu W, Yin Y, Wang J, Shi B, Zhang L, Qian
D, Li C, Zhang H, Wang S, Zhu J, et al: Kras mutations increase
telomerase activity and targeting telomerase is a promising
therapeutic strategy for Kras-mutant NSCLC. Oncotarget. 8:179–190.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Meeker AK, Hicks JL, Platz EA, March GE,
Bennett CJ, Delannoy MJ and De Marzo AM: Telomere shortening is an
early somatic DNA alteration in human prostate tumorigenesis.
Cancer Res. 62:6405–6409. 2002.PubMed/NCBI
|
|
93
|
Pestana A, Vinagre J, Sobrinho-Simões M
and Soares P: TERT biology and function in cancer: Beyond
immortalisation. J Mol Endocrinol. 58:R129–R146. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Frink RE, Peyton M, Schiller JH, Gazdar
AF, Shay JW and Minna JD: Telomerase inhibitor imetelstat has
preclinical activity across the spectrum of non-small cell lung
cancer oncogenotypes in a telomere length dependent manner.
Oncotarget. 7:31639–31651. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Chiappori AA, Kolevska T, Spigel DR, Hager
S, Rarick M, Gadgeel S, Blais N, Von Pawel J, Hart L, Reck M, et
al: A randomized phase II study of the telomerase inhibitor
imetelstat as maintenance therapy for advanced non-small-cell lung
cancer. Ann Oncol. 26:354–362. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Burchett KM, Yan Y and Ouellette MM:
Telomerase inhibitor Imetelstat (GRN163L) limits the lifespan of
human pancreatic cancer cells. PLoS One. 9:e851552014. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Shammas MA, Koley H, Bertheau RC, Neri P,
Fulciniti M, Tassone P, Blotta S, Protopopov A, Mitsiades C, Batchu
RB, et al: Telomerase inhibitor GRN163L inhibits myeloma cell
growth in vitro and in vivo. Leukemia. 22:1410–1418. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Dikmen ZG, Gellert GC, Jackson S, Gryaznov
S, Tressler R, Dogan P, Wright WE and Shay JW: In vivo inhibition
of lung cancer by GRN163L: A novel human telomerase inhibitor.
Cancer Res. 65:7866–7873. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Tokcaer-Keskin Z, Dikmen ZG,
Ayaloglu-Butun F, Gultekin S, Gryaznov SM and Akcali KC: The effect
of telomerase template antagonist GRN163L on bone-marrow-derived
rat mesenchymal stem cells is reversible and associated with
altered expression of cyclin d1, cdk4 and cdk6. Stem Cell Rev Rep.
6:224–233. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Burchett KM, Etekpo A, Batra SK, Yan Y and
Ouellette MM: Inhibitors of telomerase and poly(ADP-ribose)
polymerases synergize to limit the lifespan of pancreatic cancer
cells. Oncotarget. 8:83754–83767. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Koziel JE and Herbert BS: The telomerase
inhibitor imetelstat alone, and in combination with trastuzumab,
decreases the cancer stem cell population and self-renewal of HER2+
breast cancer cells. Breast Cancer Res Treat. 149:607–618. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Wu X, Zhang J, Yang S, Kuang Z, Tan G,
Yang G, Wei Q and Guo Z: Telomerase antagonist imetelstat increases
radiation sensitivity in esophageal squamous cell carcinoma.
Oncotarget. 8:13600–13619. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Chhabra G, Wojdyla L, Frakes M, Schrank Z,
Leviskas B, Ivancich M, Vinay P, Ganapathy R, Ramirez BE and Puri
N: Mechanism of action of G-quadruplex-forming oligonucleotide
homologous to the telomere overhang in melanoma. J Invest Dermatol.
138:903–910. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Schrank Z, Khan N, Osude C, Singh S,
Miller RJ, Merrick C, Mabel A, Kuckovic A and Puri N:
Oligonucleotides targeting telomeres and telomerase in cancer.
Molecules. 23(pii): E22672018. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Pitman RT, Wojdyla L and Puri N: Mechanism
of DNA damage responses induced by exposure to an oligonucleotide
homologous to the telomere overhang in melanoma. Oncotarget.
4:761–771. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Puri N, Pitman RT, Mulnix RE, Erickson T,
Iness AN, Vitali C, Zhao Y and Salgia R: Non-small cell lung cancer
is susceptible to induction of DNA damage responses and inhibition
of angiogenesis by telomere overhang oligonucleotides. Cancer Lett.
343:14–23. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Wojdyla L, Stone AL, Sethakorn N, Uppada
SB, Devito JT, Bissonnette M and Puri N: T-oligo as an anticancer
agent in colorectal cancer. Biochem Biophys Res Commun.
446:596–601. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Weng D, Cunin MC, Song B, Price BD, Eller
MS, Gilchrest BA, Calderwood SK and Gong J: Radiosensitization of
mammary carcinoma cells by telomere homolog oligonucleotide
pretreatment. Breast Cancer Res. 12:R712010. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Guzman H, Sanders K, Idica A, Bochnakian
A, Jury D, Daugaard I, Zisoulis DG and Pedersen IM: miR-128
inhibits telomerase activity by targeting TERT mRNA. Oncotarget.
9:13244–13253. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Zhou N, Fei D, Zong S, Zhang M and Yue Y:
MicroRNA-138 inhibits proliferation, migration and invasion through
targeting hTERT in cervical cancer. Oncol Lett. 12:3633–3639. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Mitomo S, Maesawa C, Ogasawara S, Iwaya T,
Shibazaki M, Yashima-Abo A, Kotani K, Oikawa H, Sakurai E, Izutsu
N, et al: Downregulation of miR-138 is associated with
overexpression of human telomerase reverse transcriptase protein in
human anaplastic thyroid carcinoma cell lines. Cancer Sci.
99:280–286. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Zhang D, Xiao YF, Zhang JW, Xie R, Hu CJ,
Tang B, Wang SM, Wu YY, Hao NB and Yang SM: miR-1182 attenuates
gastric cancer proliferation and metastasis by targeting the open
reading frame of hTERT. Cancer Lett. 360:151–159. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Melnik BC: miR-21: An environmental driver
of malignant melanoma? J Transl Med. 13:2022015. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Yang Y, Yang JJ, Tao H and Jin WS:
MicroRNA-21 controls hTERT via PTEN in human colorectal cancer cell
proliferation. J Physiol Biochem. 71:59–68. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Nguyen DD and Chang S: Development of
novel therapeutic agents by inhibition of oncogenic microRNAs. Int
J Mol Sci. 19(pii): E652017. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Mizukoshi E and Kaneko S:
Telomerase-targeted cancer immunotherapy. Int J Mol Sci. 20(pii):
E18232019. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Staff C, Mozaffari F, Frödin JE, Mellstedt
H and Liljefors M: Telomerase (GV1001) vaccination together with
gemcitabine in advanced pancreatic cancer patients. Int J Oncol.
45:1293–1303. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Fenoglio D, Traverso P, Parodi A,
Tomasello L, Negrini S, Kalli F, Battaglia F, Ferrera F, Sciallero
S, Murdaca G, et al: A multi-peptide, dual-adjuvant telomerase
vaccine (GX301) is highly immunogenic in patients with prostate and
renal cancer. Cancer Immunol Immunother. 62:1041–1052. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Fenoglio D, Parodi A, Lavieri R, Kalli F,
Ferrera F, Tagliamacco A, Guastalla A, Lamperti MG, Giacomini M and
Filaci G: Immunogenicity of GX301 cancer vaccine: Four (telomerase
peptides) are better than one. Hum Vaccin Immunother. 11:838–850.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Lilleby W, Gaudernack G, Brunsvig PF,
Vlatkovic L, Schulz M, Mills K, Hole KH and Inderberg EM: Phase
I/IIa clinical trial of a novel hTERT peptide vaccine in men with
metastatic hormone-naive prostate cancer. Cancer Immunol
Immunother. 66:891–901. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Kotsakis A, Papadimitraki E, Vetsika EK,
Aggouraki D, Dermitzaki EK, Hatzidaki D, Kentepozidis N, Mavroudis
D and Georgoulias V: A phase II trial evaluating the clinical and
immunologic response of HLA-A2(+) non-small cell lung cancer
patients vaccinated with an hTERT cryptic peptide. Lung Cancer.
86:59–66. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Su Z, Dannull J, Yang BK, Dahm P, Coleman
D, Yancey D, Sichi S, Niedzwiecki D, Boczkowski D, Gilboa E and
Vieweg J: Telomerase mRNA-transfected dendritic cells stimulate
antigen-specific CD8+ and CD4+ T cell responses in patients with
metastatic prostate cancer. J Immunol. 174:3798–3807. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Khoury HJ, Collins RH Jr, Blum W, Stiff
PS, Elias L, Lebkowski JS, Reddy A, Nishimoto KP, Sen D, Wirth ED
III, et al: Immune responses and long-term disease recurrence
status after telomerase-based dendritic cell immunotherapy in
patients with acute myeloid leukemia. Cancer. 123:3061–3072. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Galati D and Zanotta S: Empowering
dendritic cell cancer vaccination: The role of combinatorial
strategies. Cytotherapy. 20:1309–1323. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Salazar-Onfray F, Pereda C, Reyes D and
López MN: TAPCells, the Chilean dendritic cell vaccine against
melanoma and prostate cancer. Biol Res. 46:431–440. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Thalmensi J, Pliquet E, Liard C, Escande
M, Bestetti T, Julithe M, Kostrzak A, Pailhes-Jimenez AS, Bourges
E, Loustau M, et al: Anticancer DNA vaccine based on human
telomerase reverse transcriptase generates a strong and specific T
cell immune response. Oncoimmunology. 5:e10836702016. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Yan J, Pankhong P, Shin TH, Obeng-Adjei N,
Morrow MP, Walters JN, Khan AS, Sardesai NY and Weiner DB: Highly
optimized DNA vaccine targeting human telomerase reverse
transcriptase stimulates potent antitumor immunity. Cancer Immunol
Res. 1:179–189. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Ohta R, Demachi-Okamura A, Akatsuka Y,
Fujiwara H and Kuzushima K: Improving TCR affinity on 293T cells. J
Immunol Methods. 466:1–8. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Jordheim LP, Durantel D, Zoulim F and
Dumontet C: Advances in the development of nucleoside and
nucleotide analogues for cancer and viral diseases. Nat Rev Drug
Discov. 12:447–464. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Pascolo E, Wenz C, Lingner J, Hauel N,
Priepke H, Kauffmann I, Garin-Chesa P, Rettig WJ, Damm K and
Schnapp A: Mechanism of human telomerase inhibition by BIBR1532, a
synthetic, non-nucleosidic drug candidate. J Biol Chem.
277:15566–15572. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Kim MY, Vankayalapati H, Shin-Ya K,
Wierzba K and Hurley LH: Telomestatin, a potent telomerase
inhibitor that interacts quite specifically with the human
telomeric intramolecular g-quadruplex. J Am Chem Soc.
124:2098–2099. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Gomez DL, Armando RG, Cerrudo CS,
Ghiringhelli PD and Gomez DE: Telomerase as a cancer target.
Development of new molecules. Curr Top Med Chem. 16:2432–2440.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Nemunaitis J, Tong AW, Nemunaitis M,
Senzer N, Phadke AP, Bedell C, Adams N, Zhang YA, Maples PB, Chen
S, et al: A phase I study of telomerase-specific replication
competent oncolytic adenovirus (telomelysin) for various solid
tumors. Mol Ther. 18:429–434. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Schepelmann S, Ogilvie LM, Hedley D,
Friedlos F, Martin J, Scanlon I, Chen P, Marais R and Springer CJ:
Suicide gene therapy of human colon carcinoma xenografts using an
armed oncolytic adenovirus expressing carboxypeptidase G2. Cancer
Res. 67:4949–4955. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Picard D: Intracellular dynamics of the
Hsp90 co-chaperone p23 is dictated by Hsp90. Exp Cell Res.
312:198–204. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Ning X, Yang S, Wang R, Zhang R, Guo L,
Tie J, Cheng Y, Wang G, Wan S and Fang D: POT1 deficiency alters
telomere length and telomere-associated gene expression in human
gastric cancer cells. Eur J Cancer Prev. 19:345–351. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Ganesan K and Xu B: Telomerase inhibitors
from natural products and their anticancer potential. Int J Mol
Sci. 19(pii): E132017. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Mizushina Y, Takeuchi T, Sugawara F and
Yoshida H: Anti-cancer targeting telomerase inhibitors:
β-rubromycin and oleic acid. Mini Rev Med Chem. 12:1135–1143. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Smith S: Telomerase can't handle the
stress. Genes Dev. 32:597–599. 2018. View Article : Google Scholar : PubMed/NCBI
|