Introduction
Gliomas are the most frequently occurring type of
brain tumor in adults, accounting for 45–55% of primary nervous
system tumors, worldwide (1). They
are characterized by high aggressiveness, due to local diffuse
infiltration, and a poor prognosis (2,3).
High-grade gliomas, which are classified as grade III or IV by the
World Health Organization (WHO) classification system (4), are the brain tumor type with the
highest mortality rate, and exhibit heterogeneity at both the
microscopic and molecular levels (5,6). Despite
recent advancements in the treatment of glioma, the median survival
time of patients is <16 months (7). Therefore, a comprehensive understanding
of the mechanisms underpinning gliomagenesis is imperative, and may
provide new insights into the clinical management of glioma.
microRNAs (miRNAs) are short non-coding RNA
molecules that serve crucial roles in the regulation of numerous
fundamental cellular processes by binding to the 3′-untranslated
regions of target gene (8). Multiple
studies have indicated that various miRNAs are aberrantly expressed
in glioma tissues: Xiong et al (9) identified that miR-320a inhibits the
invasion and migration of glioma cells via the targeting of
aquaporin 4. Furthermore, Liu et al (10) demonstrated that miR-93 serves as an
oncogene in glioma by directly targeting retinoblastoma-like
protein 2. Previous studies have also reported that miR-9 can
influence the angiogenesis and apoptosis of glioma by regulating
MYC (11) and homocysteine inducible
ER protein with ubiquitin like domain 1 (12). However, the mechanism behind miR-9
regulation of glioma cell proliferation and apoptosis is yet to be
elucidated.
Forkhead box G1 (FOXG1), also known as brain factor
1, is an important member of the forkhead box transcription factor
family. FOXG1 is upregulated in a variety of malignant tumors and
is involved in multiple developmental pathways in tumor cells,
including proliferation, differentiation, cell cycle regulation and
apoptosis (13). Silencing or
down-regulation of FOXG1 inhibits the invasion and metastasis of
colorectal cancer cells (14), and
also can inhibit the proliferation of glioma cells (15). Furthermore, Shibata et al
(16) revealed that miR-9 regulates
Cajal-Retzius cell differentiation by targeting FOXG1 in the mouse
medial pallium. However, to the best of our knowledge, whether
miR-9 can inhibit the proliferation and apoptosis of glioma via the
targeting of FOXG1 is yet to be determined.
The present study aimed to investigate the
association between FOXG1 and miR-9-5p. It was identified that
miR-9-5p is expressed at a lower level in glioma tissues compared
with adjacent paracancerous tissues. In addition, FOXG1 was
identified as a direct target of miR-9-5p that mediates glioma cell
proliferation and apoptosis.
Materials and methods
Tissue samples
All clinical samples were obtained from the
Affiliated Hospital of Xizang Minzu University (Xianyang, China)
between May 2018 and February 2019. Glioma tissues from 5 patients
(age range, 24–60 years; sex, 3 males and 2 females) and
glioma-adjacent tissues from 5 individuals (age range, 23–57 years;
sex, 2 males and 3 females) were fixed with 4% paraformaldehyde for
48–72 h at room temperature and embedded in paraffin. For all
patients, the original diagnosis and tumor grading were conducted
in a blinded manner by two experienced pathologists, according to
the principles defined by the WHO classification system. Written
informed consent was obtained from all patients, prior to surgery.
The present study was approved by the Ethics Review Board of the
Affiliated Hospital of Xizang Minzu University.
Cell culture and transfection
The human glioblastoma U87 MG (CL-0238) and TG-905
(CL-0309) cell lines were purchased from Procell Life Science &
Technology Co., Ltd. (the origin of glioblastoma U87 MG cell is
unknown and the cell line is preserved at the ATCC). U87 MG cells
were cultured in MEM (Hyclone; Logan) supplemented with 10% FBS
(Gibco; Thermo Fisher Scientific, Inc.) and 1% penicillin and
streptomycin (Beijing Solarbio Science & Technology Co., Ltd.),
in a humidified atmosphere of 5% CO2 at 37°C. TG-905
cells were cultured in RPMI-1640 medium supplemented with 10% FBS
and 1% penicillin and streptomycin. For sub-culturing purpose,
cells were incubated with 0.25% trypsin at 37°C and cultures were
harvested at 80% confluency.
Small interfering (si)RNA against FOXG1
(FOXG1-siRNA), negative control siRNA (NC-siRNA), and the miR-9-3p
mimic and negative control molecules (NC mimic) were purchased from
Guangzhou RiboBio Co., Ltd. Cell transfection was performed using
riboFECT™ CP Transfection kit (Guangzhou RiboBio Co., Ltd.),
according to the manufacturer's protocol, with 50 pmol/ml miR-9-3p
mimic and NC mimic, and 40 pmol/ml FOXG1-siRNA and NC-siRNA. Cells
were seeded into 6-well plates at a density of 1×105
cells/well until they reached 50% confluence, 24 h before
transfection. Fresh medium was replaced after 6 h. Following
transfection for 24–48 h at 37°C, cells were collected for RT-qPCR
or western blotting analyses. The FOXG1-siRNA and NC-siRNA
sequences were as follows: FOXG1-siRNA forward,
5′-CGUUUUACACACAUUUGCATT-3′ and reverse,
5′-UGCAAAUGUGUGUAAAACGTT-3′; NC-siRNA forward,
5′-UUCUCCGAACGUGUCACGUTT-3′ and reverse,
5′-ACGUGACACGUUCGGAGAATT-3′.
Immunohistochemistry
The paraffin-embedded glioma and glioma-adjacent
tissues were cut into 4 µm thick slices, deparaffinized using
xylene and rehydrated with graded alcohol solutions (100% alcohol I
for 3 min, 100% alcohol II for 3 min, 95% alcohol for 3 min, 85%
alcohol for 3 min and 75% alcohol for 3 min, respectively).
Subsequently, antigen retrieval was performed using 3% hydrogen
peroxide at room temperature for 15 min. Cells were then blocked
with 5% bovine serum albumin (BSA; Beyotime Institute of
Biotechnology) at room temperature for 20 min. The sections were
incubated with primary antibody FOXG1 (1:500; cat. no. ab18987;
Abcam) at 4°C overnight, warmed for 30 min in a 37°C incubator, and
then incubated with goat anti-rabbit biotinylated secondary
antibodies (1:100; cat. no. BA1003; Wuhan Boster Biological
Technology, Ltd) for 30 min at 37°C. The sections were then stained
with Strept Avidin Biotin Enzyme Complex (Wuhan Boster Biological
Technology, Ltd.) for 30 min at 37°C, and then counterstained with
hematoxylin for 2 min at room temperature, before being treated
with 3,3′-diaminobenzidine, which was used as the chromogen, for 5
min at room temperature. In the negative control group, PBS was
used in place of the primary antibody. Five visual fields were
randomly selected and assessed for immunoreactive using a light
microscope (Nikon Corporation; magnification ×400). The images were
analyzed using Image-Pro Plus software (version 6.0; Media
Cybernetics, Inc.).
Western blotting
48 h after transfection, harvested cells were washed
with PBS and lysed using radioimmunoprecipitation assay lysis
buffer (Wuhan Boster Biological Technology, Ltd). Total protein was
quantified using the bicinchoninic acid Protein Assay kit (Wuhan
Boster Biological Technology, Ltd.). Equal amount of protein (20
µg/lane) were separated using SDS-PAGE on a 10% gel and then
transferred onto a polyvinylidene fluoride membrane (EMD
Millipore). Subsequently, the membranes were blocked for 1 h at
room temperature using 5% skim milk, followed by incubation with
primary antibodies against β-actin (1:1,000; cat. no. ab8227;
Abcam) and FOXG1 (1:1,000; cat. no. ab18987; Abcam) overnight at
4°C. The membrane was then incubated with a biotin-conjugated goat
anti-rabbit IgG (1:5,000; cat. no. BA1003; Wuhan Boster Biological
Technology, Ltd.) at room temperature for 1 h. Protein bands were
visualized using an enhanced chemiluminescence kit (EMD Millipore)
and β-actin was considered as the internal control.
Reverse transcription-quantitative
(RT-q)PCR)
Total RNA was extracted from U87 and TG-905 cells
using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc) according to the manufacturer's protocol. For the
quantification of miRNA expression, RT and qPCR analysis were
performed using the Bulge-Loop™ miRNA qRT-PCR Starter kit
(Guangzhou RiboBio Co., Ltd) at 42°C for 60 min and 70°C for 10
min. For the quantification of FOXG1 expression, mRNA was converted
to cDNA using a PrimeScript™RT reagent kit (Takara Bio, Inc.) at
37°C for 15 min and 85°C for 5 sec. qPCR was performed using
SYBR® Premix Ex Taq™ (Takara Bio, Inc.). The
thermocycling conditions were as follows: Initial denaturation at
95°C for 3 min; 40 cycles of denaturation at 95°C for 5 sec,
annealing at 60°C for 30 sec and elongation at 72°C for 30 sec; and
a final extension at 72°C for 30 sec. The relative expressions
levels of FOXG1 and miR-9-3p were calculated using the
2−ΔΔCq method and normalized to the housekeeping gene
β-actin and U6 rRNA (17). The
specific primer sequences were as follows: FOXG1 forward,
5′-GGCTCACGCTCAACGGCATCTACGA-3′ and reverse,
5′-GCGGCACCTTCACGAAGCACTTGTT-3′; and β-actin forward,
5′-GAAGATCAAGATCATTGCTCCT-3′ and reverse,
5′-TACTCCTGCTTGCTGATCCA-3′. The catalog number of miR-9-3p primer
is MQPS0002283-1-100, and the catalog number of U6 is
MQPS0000002-1-100.
Cell proliferation assay
The growth curves of U87 and TG-905 cells were
determined using Cell Counting Kit-8 (CCK-8; Dojindo Molecular
Technologies, Inc.) 24 h after transfection, according to the
manufacturer's protocol. Cells (7×103/well) were
suspended and seeded in 96-well plates overnight. Following
incubation, the original culture medium was removed and 100 µl
fresh medium mixed with CCK-8 at a ratio of 10:1 was added to each
well. The absorbance at 450 nm was measured using a microplate
reader (Thermo Fisher Scientific, Inc.) to generate cell growth
curves.
Annexin-V/propidium iodide (PI)
double-staining assay
Following transection with miR-9-3p mimic or FOXG1
siRNA for 24 h, U87 and TG-905 cells were digested using 0.25%
trypsin and centrifuged at 148 × g for 5 min at room temperature,
prior to collection for apoptosis detection. The collected cells
were washed with PBS and digested using trypsin. Subsequently,
1×105 cells were resuspended in 100 µl binding buffer, 5
µl Annexin V-FITC and 5 µl PI staining solution (BD Biosciences),
followed by incubation at room temperature (20-25°C) for 15 min,
according to the manufacturer's protocol. Cell apoptosis was
analyzed using a flow cytometer (BD Biosciences) within 1 h of
treatment and the data were analyzed using FlowJo 10.07 software
(FlowJo LLC). Cells that were Annexin V-positive were considered to
indicate cells undergoing apoptosis.
Dual luciferase reporter assay
miR-9-3p targets were predicted using bioinformatics
software programs, including TargetScan (http://www.targetscan.org/), mirDB (http://mirdb.org/) and DIANA TOOLS (http://diana.imis.athena-innovation.gr).
U87 and TG-905 cells (4×104/well) were plated in 24-well
plates. When the cultures attained 50% confluence, cells were
co-transfected using the Renilla luciferase pRL-TK plasmid (100
ng/ml; Shanghai GenePharma Co., Ltd.) and a recombinant Firefly
luciferase pGL3 reporter containing the 3′-untranslated region
(3′-UTR) of human FOXG1 (2 µg/ml; Shanghai GenePharma Co., Ltd.).
This was followed by transfection with the miR-9-3p mimic and NC
mimic using Lipofectamine®2000 (Invitrogen; Thermo
Fisher Scientific, Inc.), according to the manufacturer's protocol.
The cells were then collected and lysed for a luciferase assay 24 h
after transfection using a Dual-Luciferase Reporter assay kit
(Promega Corporation). Firefly luciferase activity was normalized
to Renilla luciferase activity for each tested well.
Statistical analysis
Statistical analyses were conducted using SPSS 20.0
software (IBM Corp.). Data are presented as the mean ± standard
deviation, and each experiment was performed in triplicate.
Differences among multiple groups were compared using one-way
analysis of variance and Dunnett's or Tukey's post hoc test, and
differences between two groups were compared using Dunnett's
t-test. P<0.05 was considered to indicate a statistically
significant difference.
Results
miR-9-3p is downregulated in glioma
tissues
miR-9-3p has been reported to be downregulated in
gastric carcinoma (18) and
colorectal cancer (19). To
investigate whether the expression of miR-9-3p is abnormal in
glioma, the present study performed RT-qPCR to detect the
expression level of miR-9-3p mRNA in glioma and adjacent
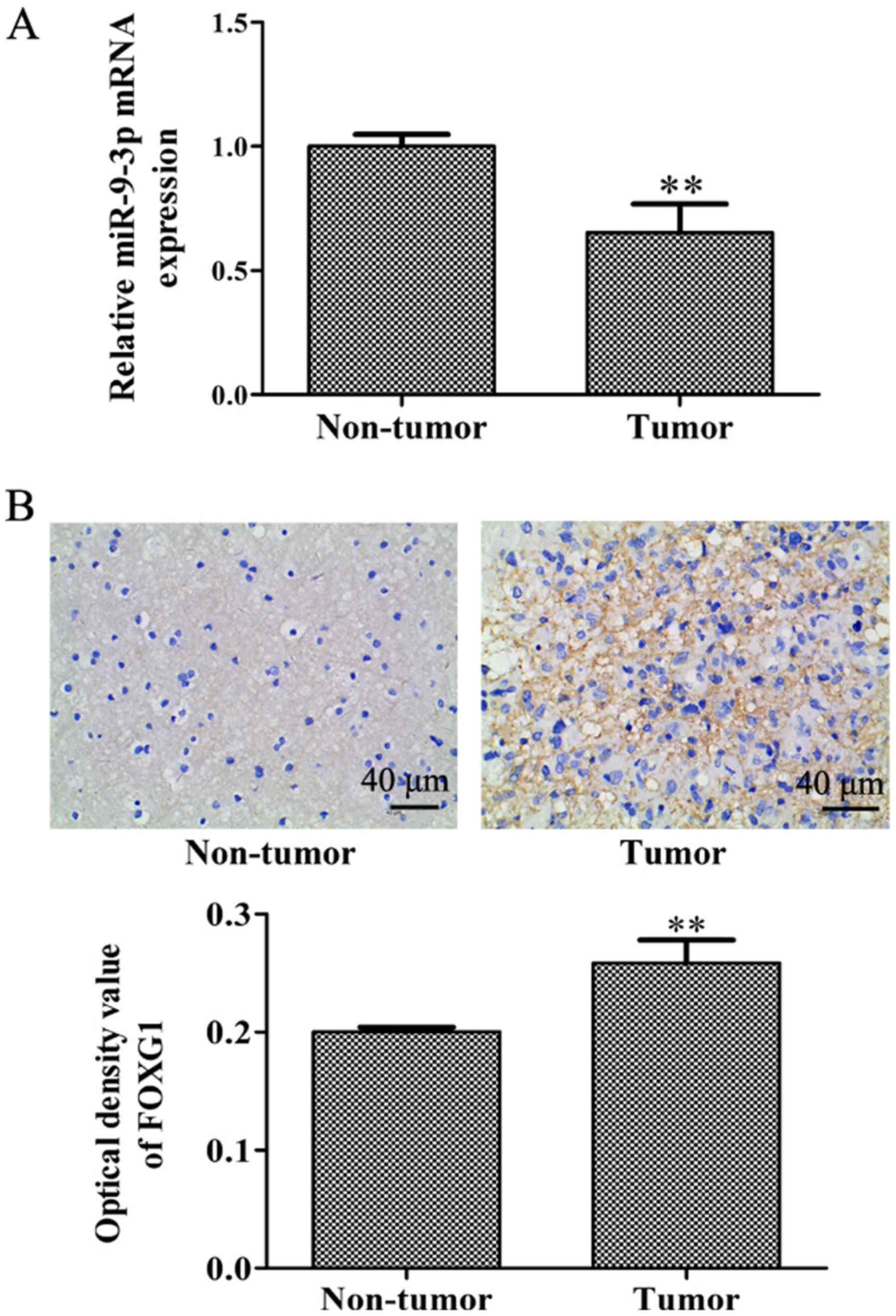
paracancerous glioma tissues. As exhibited in Fig. 1A, miR-9-3p expression was
significantly lower in glioma tissues compared with in
glioma-adjacent tissues, which indicates a potential role for
miR-9-3p in glioma tumorigenesis and progression. To investigate
the potential downstream mechanisms of miR-9-3p in glioma, an
immunohistochemistry assay was performed to detect the expression
level of FOXG1. As presented in Fig.
1B, the protein expression level of FOXG1 in glioma tissue was
significantly increased compared with that of glioma-adjacent
tissues. In view of the negative regulatory association between
miRNA and its target genes, this result reveals that miR-9-3p may
have a targeted regulatory association with FOXG1.
Overexpression of miR-9-3p inhibits
cell proliferation and increases cell apoptosis
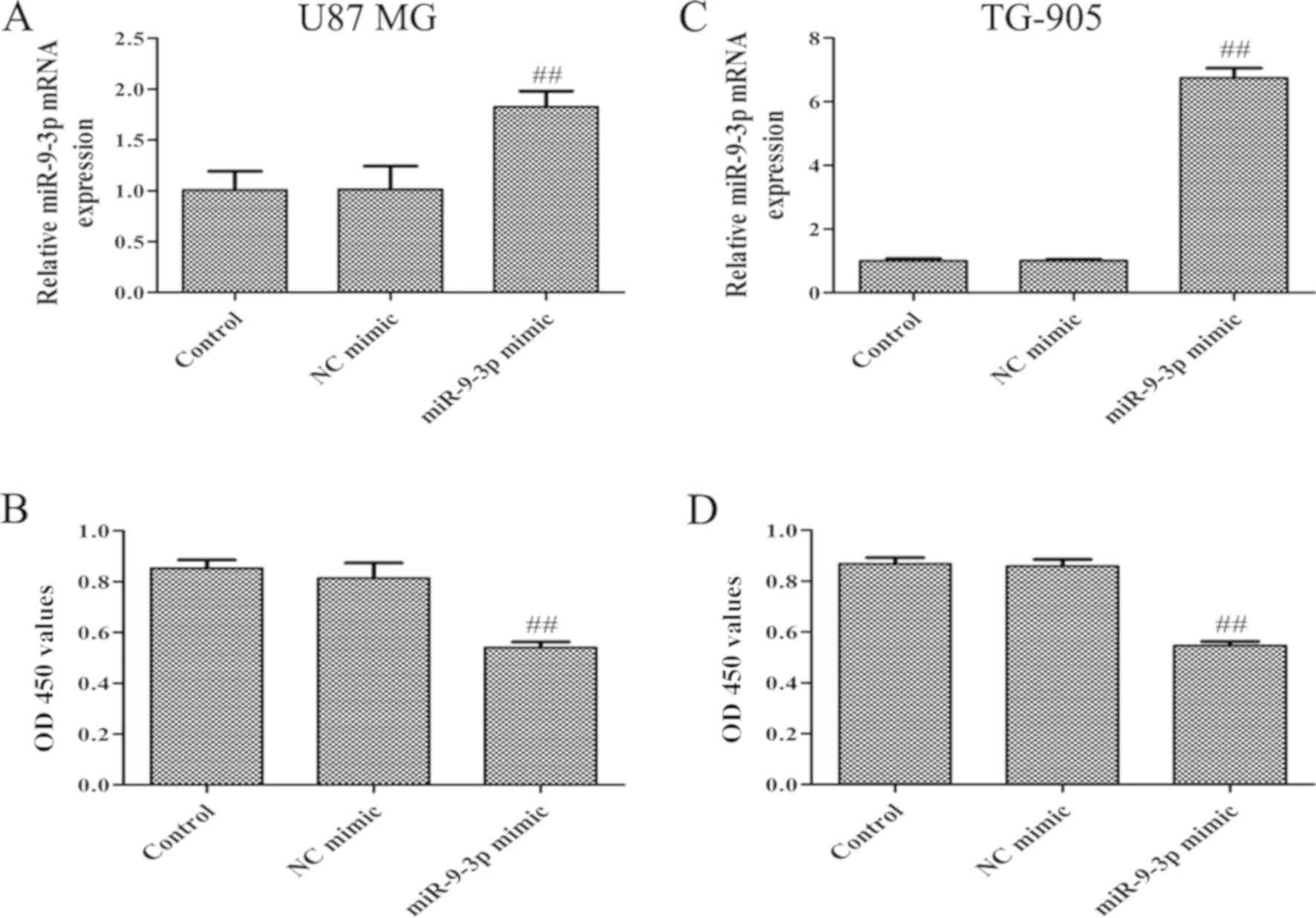
To further investigate the biological functions of
miR-9-3p in glioma, U87 MG and TG-905 cells were transfected with
miR-9-3p mimic and a NC mimic. RT-qPCR revealed that miR-9-3p
expression was significantly increased following transfection with
miR-9-3p mimic, compared with the control and NC mimic groups,
suggesting high transfection efficiency in U87 MG and TG-905 cells
(Fig. 2A and C). CCK-8 and
Annexin-V/PI double-staining assays were then performed to
determine the proliferation and apoptosis rate in U87 MG and TG-905
cells, and to investigate the influence of miR-9-3p on the
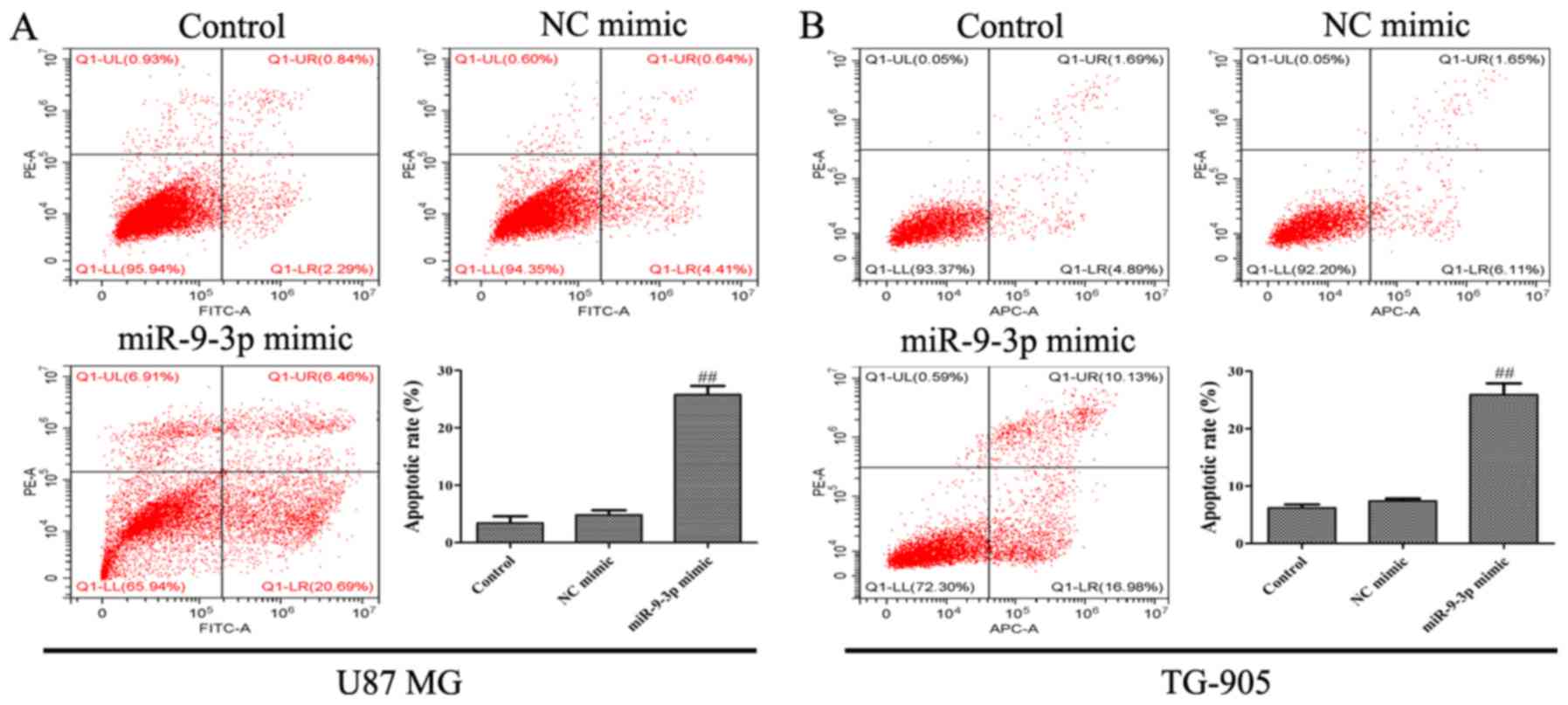
progression of glioma. It was revealed that miR-9-3p overexpression
significantly suppressed proliferation (Fig. 2B and D), whilst simultaneously
increasing the apoptosis of U87 MG and TG-905 cells (Fig. 3A and B). The present results indicate
that miR-9-3p may act as a tumor suppressor and regulate the
progression of glioma cells.
FOXG1 is a direct target of miR-9-3p
in glioma cells
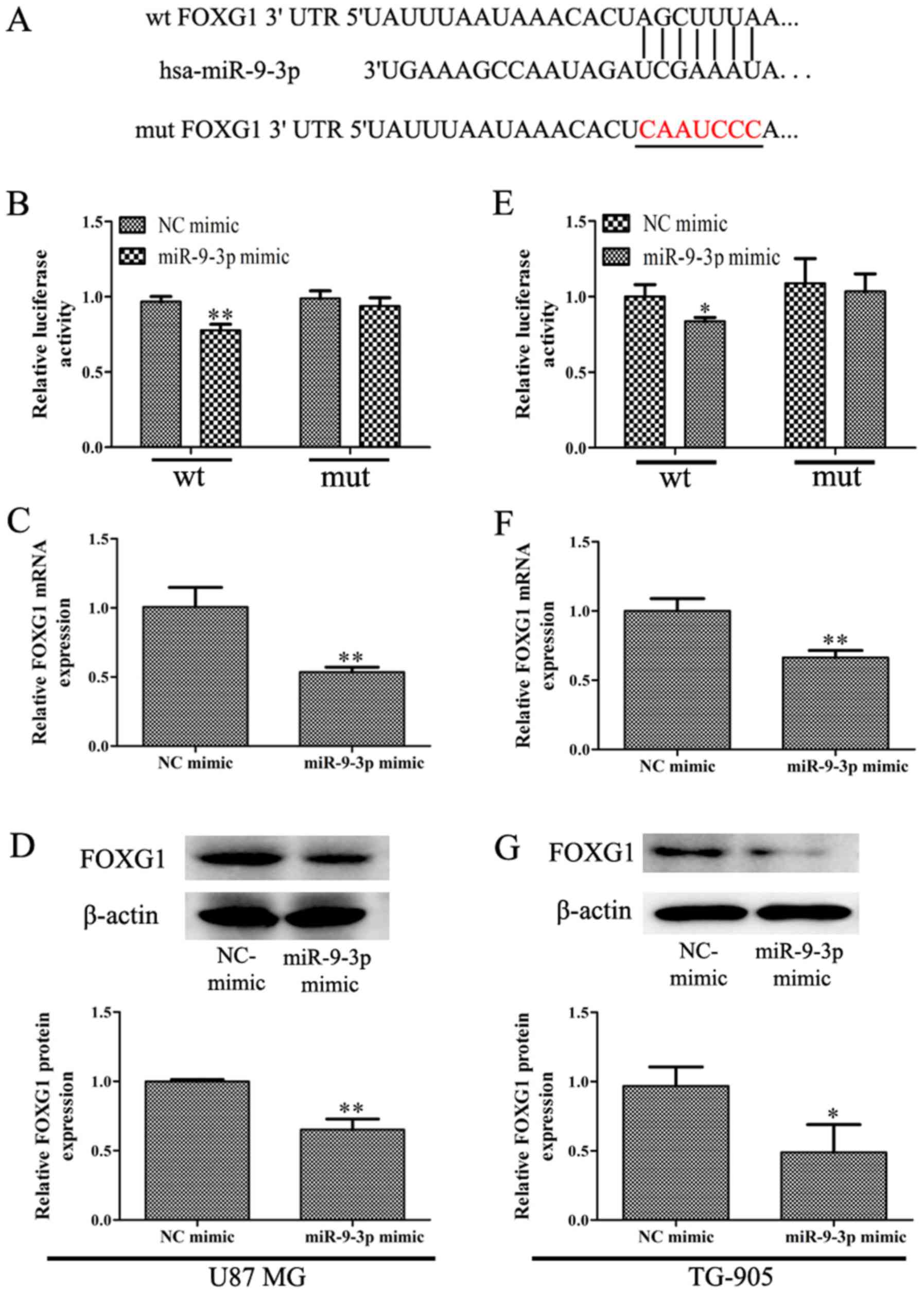
To determine the mechanism of miR-9-3p regulation of
glioma, the present study investigated potential miR-9-3p targets
using TargetScan, mirDB and DIANA TOOLS. FOXG1 was selected from
several putative miR-9-3p targets as it serves key roles in:
Cerebellar development (20), glioma
proliferation (21), apoptosis
(15) and also predicts prognosis
(22). As indicated in Fig. 4A, it was revealed that the 3′-UTR of
FOXG1 was contains a conserved putative target site for miR-9-3p.
Subsequently, dual-luciferase assays were performed to determine
whether FOXG1 is a direct target of miR-9-3p. The results indicated
that overexpression of miR-9-3p suppressed the activity of
luciferase reporters containing FOXG1 3′-UTR in U87 MG and TG-905
cells (Fig. 4B and E). In addition,
the RT-qPCR assay revealed that miR-9-3p overexpression was
significantly inhibited the expression of FOXG1 mRNA in U87 MG and
TG-905 cells (Fig. 4C and F).
Western blotting analysis revealed that the overexpression of
miR-9-3p significantly inhibited the expression of FOXG1 protein
(Fig. 4D and G). In summary, the
present study demonstrated that miR-9-3p targets FOXG1 and
suppresses its expression in glioma cells.
FOXG1 gene silencing enhances the
effect of miR-9-3p mimic on cell proliferation inhibition and
apoptosis
To determine whether FOXG1 influences the effect of
miR-9-3p on the proliferation and apoptosis of U87 cells, siRNA was
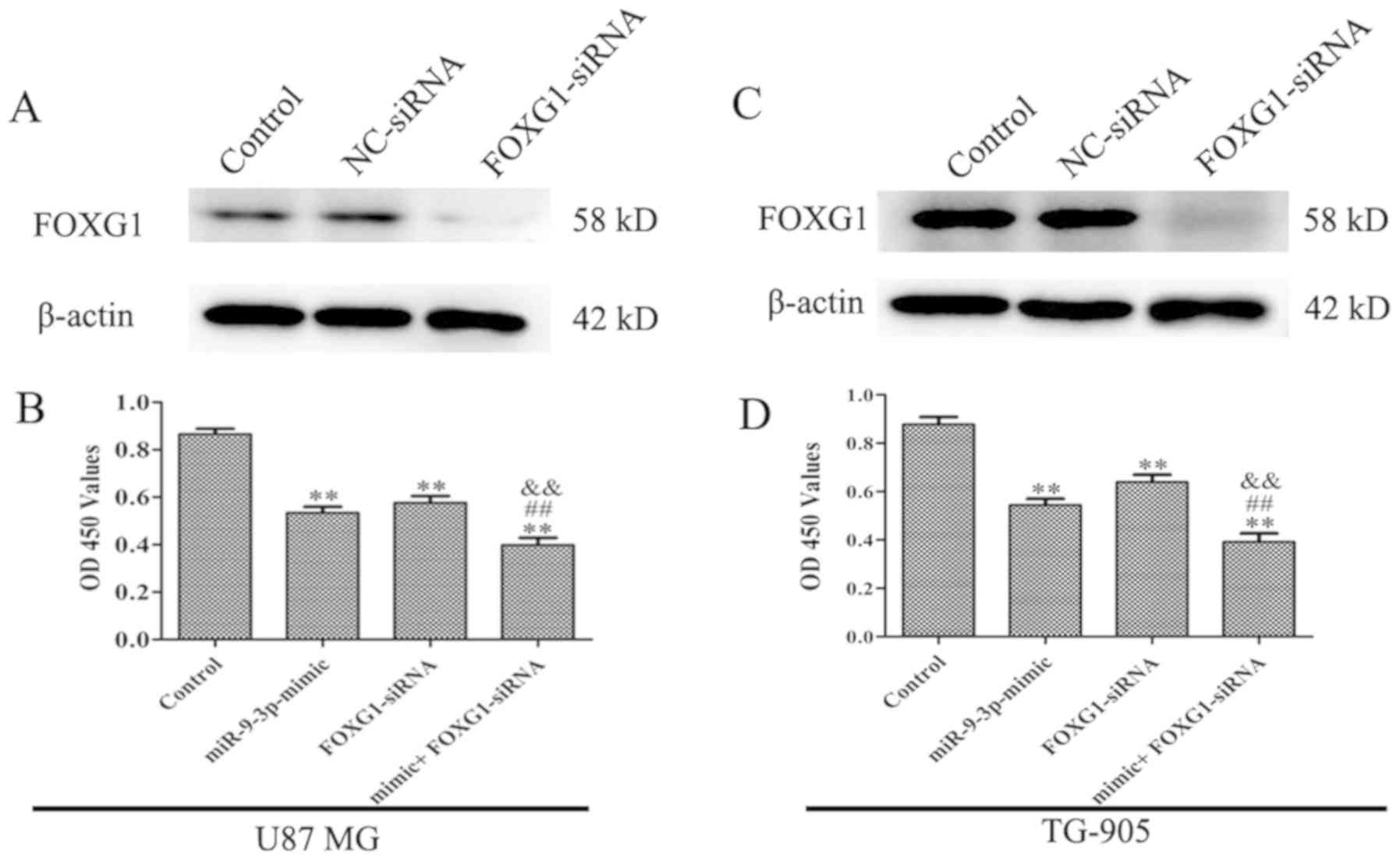
used to downregulate FOXG1 expression. As revealed in Fig. 5A and C, FOXG1 protein expression was
downregulated following transfection with FOXG1 siRNA, suggesting
high transfection efficiency of FOXG1 siRNA in U87 MG and TG-905
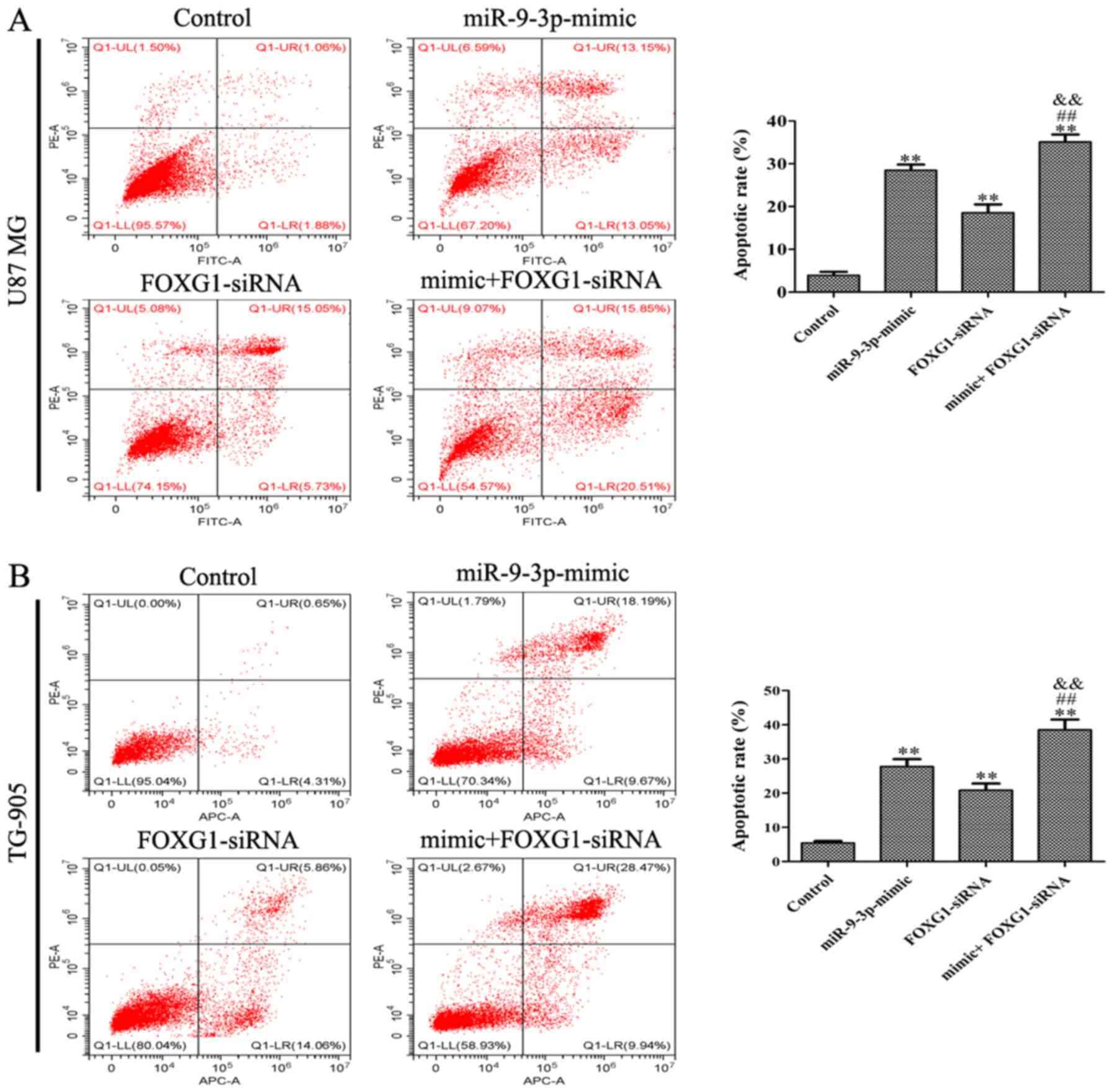
cells. CCK-8 and flow cytometry results revealed that siFOXG1
significantly decrease the proliferation and increased apoptosis in
U87 cells. Furthermore, silencing of FOXG1 enhanced the effect of
the miR-9-3p mimic on cell proliferation (Fig. 5B and D) and apoptosis (Fig. 6A and B). The present results
demonstrated that miR-9-3p regulates cell proliferation and
apoptosis via the inhibition of FOXG1.
Discussion
Glioma is the most common type of brain tumor, with
an incidence of 6 per 100,000 annually, worldwide (23). Numerous studies have focused on the
effects of miRNA dysregulation in various types of human cancer.
miRNAs have been revealed to influence a variety of biological
processes by negatively regulating the protein levels of target
onco- and tumor suppressor genes; therefore, miRNAs themselves
serve as oncogenes or tumor suppressor genes in various types of
human tumors (24,25). A number of studies have revealed the
aberrant regulation of miR-9 in multiple tumor types. For example,
upregulation of miR-9 was shown to promote tumor metastasis via the
targeting E-cadherin in serous ovarian cancer (26). Moreover, Tang et al (27) reported that miR-9 functions as a
tumor suppressor in ovarian serous carcinoma, as it inhibits cell
proliferation, migration and invasion via regulation of talin 1. By
contrast, Zhu et al (28)
demonstrated that miR-9 serves an oncogenic role by regulating the
proliferation of osteosarcoma cells via direct targeting of GCIP.
Additionally, miR-9 has been reported to be upregulated in certain
glioma specimens and cells, and may significantly promote
tumorigenesis and angiogenesis (11). However, Yang et al (12) reported that miR-9-3p is downregulated
in high-grade gliomas compared with non-tumor tissues, and the
overexpression of miR-9-3p increased apoptosis of glioma cells.
Therefore, further investigation is required to identify the
expression patterns and mechanisms of action of miR-9, in relation
to glioma. The present study revealed that the expression level of
miR-9-3p is significantly lower in glioma tissues compared with
glioma-adjacent tissues.
The present study is not without limitations. For
example, paraffin-embedded in situ hybridization was unable
to be performed, which may have been useful in determining direct
miR-9 expression. Prospective studies will focus on determining the
difference between tumor and adjacent normal tissue samples
collected from the same patient, in order to accurately elucidate
the differential expression of miR-9.
Overexpression of miR-9 has been revealed to inhibit
cell proliferation and invasion in pancreatic cancer (29), hepatocellular carcinoma (30) and nasopharyngeal carcinoma (31). The present study discovered that an
overexpression of miR-9-3p markedly inhibited the proliferation,
and increased the apoptosis, of U87 cells and the current results
demonstrated that miR-9-3p may act as a tumor suppressor, and its
overexpression may suppress glioma development, potentially
improving patient prognosis.
As previously described, miR-9 has numerous targets;
it can suppress the gene expression of StAR related lipid transfer
domain containing 13 (32), BLCAP
apoptosis inducing factor (33),
SRY-box transcription factor 7 (34)
and ATP binding cassette subfamily B member 1 (35), by serving either an oncogenic role or
acting as a tumor suppressor gene. The FOXG1 gene is located in the
q12 region of chromosome 14 and the protein it encodes contains 489
amino acids, is an important transcription factor and serves an
important role in the regulation of telencephalic development,
neuronal differentiation and neurogenesis (36,37).
Studies have identified that FOXG1 serves a role similar to that of
oncogenes in the occurrence and development of certain tumors
types, including medulloblastoma (38), non-small cell lung cancer (39) and ovarian cancer (40). In addition, a low expression of FOXG1
serves as an important indicator of good prognosis in patients with
glioma (22). Bredenkamp et
al (41) demonstrated that the
FOXG1 3′UTR has a conserved recognition site specific to miR-9, in
mammals. However, to the best of our knowledge, whether miR-9 can
regulate the progression of glioma via targeting FOXG1 has not been
reported. The present study identified that the 3′-UTR of FOXG1
contained a conserved putative target site specific to miR-9-3p in
U87 cells, and a dual-luciferase reporter assay revealed that
miR-9-3p overexpression suppressed luciferase activity.
Furthermore, it was identified that miR-9-3p overexpression
inhibited the mRNA and protein expression levels of FOXG1. The
present results suggest that FOXG1 is a direct functional target of
miR-9-3p. Additionally, the present study demonstrated that the
inhibition of FOXG1 not only inhibited cell proliferation and
increased cell apoptosis, but also enhanced the effect of miR-9-3p
mimic on the proliferation and apoptosis of U87 MG and TG-905
cells, indicating that miR-9-3p regulates cell proliferation and
apoptosis via targeting FOXG1 in glioma.
In summary, the present study demonstrated that
miR-9-3p functions as a tumor suppressor in glioma. It was
identified that miR-9-3p inhibits glioma progression via targeting
FOXG1. Although miRNA-based therapeutics are still under
development, the present results are support the hypothesis that
miR-9-3p and FOXG1 may represent novel and effective therapeutic
targets for glioma treatment in the future.
Acknowledgements
Not applicable.
Funding
The present study was funded by the National Natural
Science Foundation of China (grant no. 81560732).
Availability of data and materials
The datasets used or analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
JZ and XT conceived and designed the present study.
JZ, HZ and HD performed the experiments and analyzed the data. JZ
drafted the initial manuscript. XT critically revised the
manuscript for important intellectual content and supervised the
study. All authors have read and approved the final version of this
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics Review
Board of the Affiliated Hospital of Xizang Minzu University
(Xianyang, China), and written informed consent was obtained from
all patients prior to the study start (approval number:
201505).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Di Stefano AL, Enciso-Mora V, Marie Y,
Desestret V, Labussière M, Boisselier B, Mokhtari K, Idbaih A,
Hoang-Xuan K, Delattre JY, et al: Association between glioma
susceptibility loci and tumour pathology defines specific molecular
etiologies. Neuro Oncol. 15:542–547. 2013. View Article : Google Scholar
|
|
2
|
Li G, Shen J, Cao J, Zhou G, Lei T, Sun Y,
Gao H, Ding Y, Xu W, Zhan Z, et al: Alternative splicing of human
telomerase reverse transcriptase in gliomas and its modulation
mediated by CX-5461. J Exp Clin Cancer Res. 37:782018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Parker RG, Janjan NA and Selch MT:
Malignant tumors of the central nervous system. Radiation Oncology
for Cure and Palliation. Springer; Berlin, Heidelberg: 2003,
View Article : Google Scholar
|
|
4
|
Fuller GN and Scheithauer BW: The 2007
revised world health organization (WHO) classification of tumours
of the central nervous system: Newly codified entities. Brain
Pathol. 17:304–307. 2010. View Article : Google Scholar
|
|
5
|
Theeler BJ and Groves MD: High-grade
gliomas. Curr Treat Options Neurol. 13:386–399. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ke C, Tran K, Chen Y, Di Donato AT, Yu L,
Hu Y, Linskey ME, Wang PH, Limoli CL and Zhou YH: Linking
differential radiation responses to glioma heterogeneity.
Oncotarget. 5:1657–1665. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fischer U, Struss AK, Hemmer D, Pallasch
CP, Steudel WI and Meese E: Glioma-expressed antigen 2 (GLEA2): A
novel protein that can elicit immune responses in glioblastoma
patients and some controls. Clin Exp Immunol. 126:206–213. 2010.
View Article : Google Scholar
|
|
8
|
Kwok HH, Poon PY, Mak KH, Zhang LY, Liu P,
Zhang H, Mak NK, Yue PY and Wong RN: Role of G3BP1 in
glucocorticoid receptor-mediated microRNA-15b and microRNA-23a
biogenesis in endothelial cells. Cell Mol Life Sci. 74:3613–3630.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xiong W, Ran J, Jiang R, Guo P, Shi X, Li
H, Lv X, Li J and Chen D: MIRNA-320a inhibits glioma cell invasion
and migration by directly targeting aquaporin 4. Oncol Rep.
39:1939–1947. 2018.PubMed/NCBI
|
|
10
|
Liu DK, Wei YJ, Guo Y, Wang J and Wang GH:
MiRNA-93 functions as an oncogene in glioma by directly targeting
RBL2. Eur Rev Med Pharmacol Sci. 22:2343–2350. 2018.PubMed/NCBI
|
|
11
|
Chen X, Yang F, Zhang T, Wang W, Xi W, Li
Y, Zhang D, Huo Y, Zhang J, Yang A and Wang T: MiR-9 promotes
tumorigenesis and angiogenesis and is activated by MYC and OCT4 in
human glioma. J Exp Clin Cancer Res. 38:992019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang L, Mu Y, Cui H, Liang Y and Su X:
MiR-9-3p augments apoptosis induced by H2O2 through down regulation
of Herpud1 in glioma. PLoS One. 12:e01748392017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Golson ML and Kaestner KH: Fox
transcription factors: From development to disease. Development.
143:4558–4570. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Creagh EM, Conroy H and Martin SJ:
Caspase-activation pathways in apoptosis and immunity. Immunol Rev.
193:10–21. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen J, Wu X, Xing Z, Ma C, Xiong W, Zhu X
and He X: FOXG1 Expression is elevated in glioma and inhibits
glioma cell apoptosis. J Cancer. 9:778–783. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shibata M, Kurokawa D, Nakao H, Ohmura T
and Aizawa S: MicroRNA-9 modulates Cajal-Retzius cell
differentiation by suppressing Foxg1 expression in mouse medial
pallium. J Neurosci. 28:10415–10421. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Luo H, Zhang H, Zhang Z, Zhang X, Ning B,
Guo J, Nie N, Liu B and Wu X: Down-regulated miR-9 and miR-433 in
human gastric carcinoma. J Exp Clin Cancer Res. 28:822009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhu M, Xu Y, Ge M, Gui Z and Yan F:
Regulation of UHRF1 by microRNA-9 modulates colorectal cancer cell
proliferation and apoptosis. Cancer Sci. 106:833–839. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kersigo J, D'Angelo A, Gray BD, Soukup GA
and Fritzsch B: The role of sensory organs and the forebrain for
the development of the craniofacial shape as revealed by
Foxg1-cre-mediated microRNA loss. Genesis. 49:326–341. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang L, Wang J, Jin T, Zhou Y and Chen Q:
FoxG1 facilitates proliferation and inhibits differentiation by
downregulating FoxO/Smad signaling in glioblastoma. Biochem Biophys
Res Commun. 504:46–53. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schäfer S, Behling F, Skardelly M, Koch M,
Ott I, Paulsen F, Tabatabai G and Schittenhelm J: Low FoxG1 and
high Olig-2 labelling indices define a prognostically favourable
subset in isocitrate dehydrogenase (IDH)-mutant gliomas. Neuro Appl
Neurobiol. 44:207–223. 2018. View Article : Google Scholar
|
|
23
|
Goldbrunner R, Ruge M, Kocher M, Lucas CW,
Galldiks N and Grau S: The treatment of gliomas in adulthood. Dtsch
Arztebl Int. 115:20–21. 2018.
|
|
24
|
Wei Y, Schober A and Weber C: Pathogenic
arterial remodeling: The good and bad of microRNAs. Am J Physiol
Heart Circu Physiol. 304:H1050–H1059. 2013. View Article : Google Scholar
|
|
25
|
Li B, Liu YH, Sun AG, Huan LC, Li HD and
Liu DM: MiR-130b functions as a tumor promoter in glioma via
regulation of ERK/MAPK pathway. Eur Rev Med Pharmacol Sci.
21:2840–2846. 2017.PubMed/NCBI
|
|
26
|
Zhou B, Xu H, Xia M, Sun C, Li N, Guo E,
Guo L, Shan W, Lu H, Wu Y, et al: Overexpressed miR-9 promotes
tumor metastasis via targeting E-cadherin in serous ovarian cancer.
Front Med. 11:214–222. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tang H, Yao L, Tao X, Yu Y, Chen M, Zhang
R and Xu C: Mir-9 functions as a tumor suppressor in ovarian serous
carcinoma by targeting tln1. Int J Mol Med. 32:381–388. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhu SW, Li JP, Ma XL, Ma JX, Yang Y, Chen
Y and Liu W: MiR-9 modulates osteosarcoma cell growth by targeting
the GCIP tumor suppressor. Asian Pac J Cancer Prev. 16:4509–4513.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang J, Wang B, Ren H and Chen W: Mir-9-5p
inhibits pancreatic cancer cell proliferation, invasion and
glutamine metabolism by targeting GOT1. Biochem Biophy Res Commun.
509:241–248. 2019. View Article : Google Scholar
|
|
30
|
Han Y, Liu Y, Fu X, Zhang Q, Huang H,
Zhang C, Li W and Zhang J: MiR-9 inhibits the metastatic ability of
hepatocellular carcinoma via targeting beta galactoside
alpha-2,6-sialyltransferase 1. J Physiol Biochem. 74:491–501. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ding Y, Pan Y, Liu S, Jiang F and Jiao J:
Elevation of MiR-9-3p suppresses the epithelial-mesenchymal
transition of nasopharyngeal carcinoma cells via down-regulating
FN1, ITGB1 and ITGAV. Cancer Biol Ther. 18:414–424. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen L, Hu W, Li G, Guo Y, Wan Z and Yu J:
Inhibition of miR-9-5p suppresses prostate cancer progress by
targeting StarD13. Cell Mol Biol Lett. 24:202019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen Y, Zhang S, Zhao R, Zhao Q and Zhang
T: Upregulated miR-9-3p promotes cell growth and inhibits apoptosis
in medullary thyroid carcinoma by targeting BLCAP. Oncol Res.
25:1215–1222. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Han L, Wang W, Ding W and Zhang L: MiR-9
is involved in TGF-β1-induced lung cancer cell invasion and
adhesion by targeting SOX7. J Cell Mol Med. 21:2000–2008. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li Y, Zhao L, Li N, Miao Y, Zhou H and Jia
L: MiR-9 regulates the multidrug resistance of chronic myelogenous
leukemia by targeting ABCB1. Oncol Rep. 37:2193–2200. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Manuel MN, Martynoga B, Molinek MD, Quinn
JC, Kroemmer C, Mason JO and Price DJ: The transcription factor
Foxg1 regulates telencephalic progenitor proliferation cell
autonomously, in part by controlling Pax6 expression levels. Neural
Dev. 6:92011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Brancaccio M, Pivetta C, Granzotto M,
Filippis C and Mallamaci A: Emx2 and Foxg1 inhibit gliogenesis and
promote neuronogenesis. Stem Cells. 28:1206–1218. 2010.PubMed/NCBI
|
|
38
|
Adesina AM, Nguyen Y, Mehta V, Takei H,
Stangeby P, Crabtree S, Chintagumpala M and Gumerlock MK: FOXG1
dysregulation is a frequent event in medulloblastoma. J Neurooncol.
85:111–122. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ji KX, Cui F, Qu D, Sun RY, Sun P, Chen
FY, Wang SL and Sun HS: MiR-378 promotes the cell proliferation of
non-small cell lung cancer by inhibiting FOXG1. Eur Rev Med
Pharmacol Sci. 22:1011–1019. 2018.PubMed/NCBI
|
|
40
|
Chan DW, Liu VW, To RM, Chiu PM, Lee WY,
Yao KM, Cheung AN and Ngan HY: Overexpression of FOXG1 contributes
to TGF-β resistance through inhibition of p21WAF1/CIP1expression in
ovarian cancer. Br J Cancer. 101:1433–1443. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bredenkamp N, Seoighe C and Illing N:
Comparative evolutionary analysis of the FoxG1 transcription factor
from diverse vertebrates identifies conserved recognition sites for
microRNA regulation. Dev Genes Evol. 217:227–233. 2007. View Article : Google Scholar : PubMed/NCBI
|