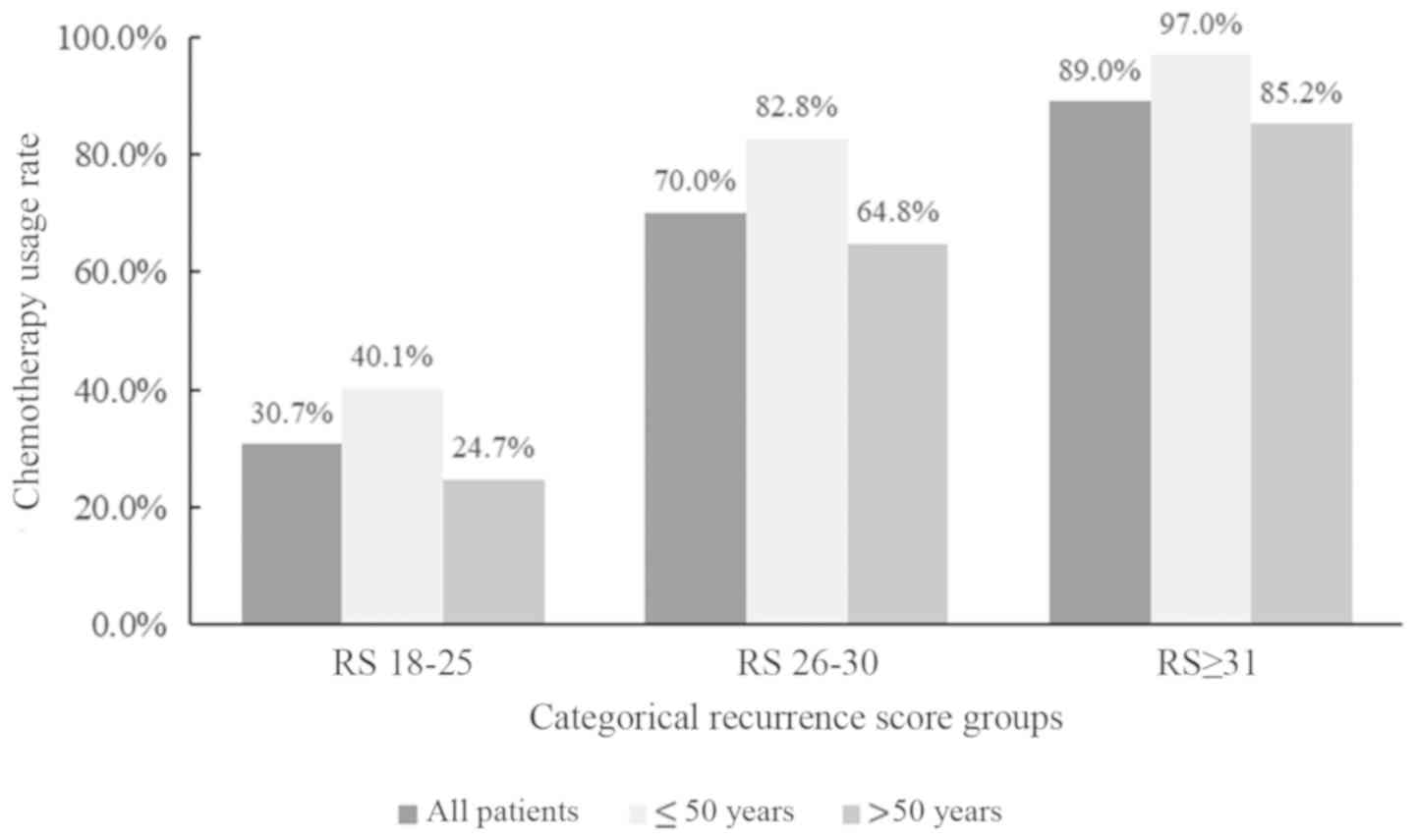
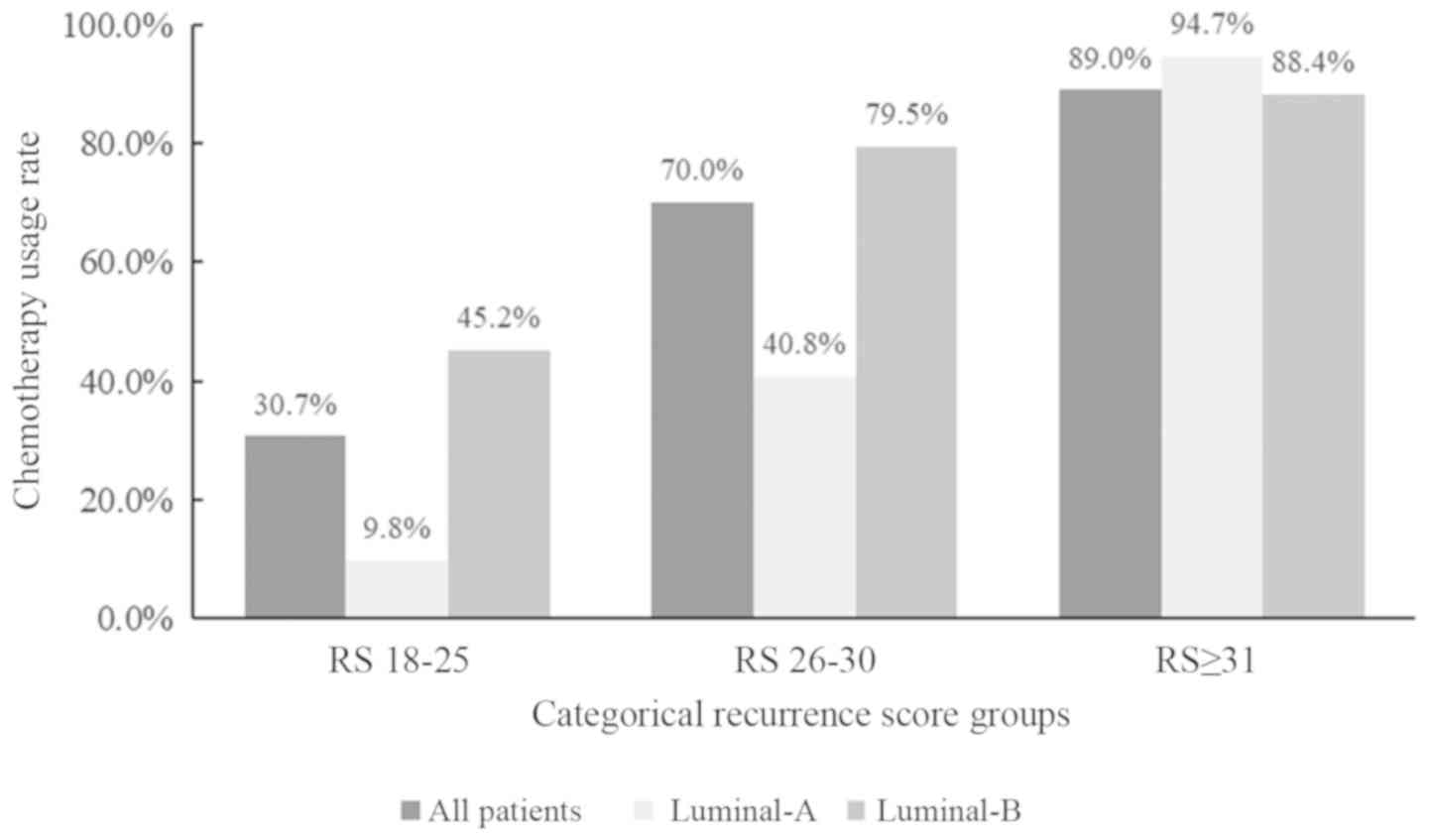
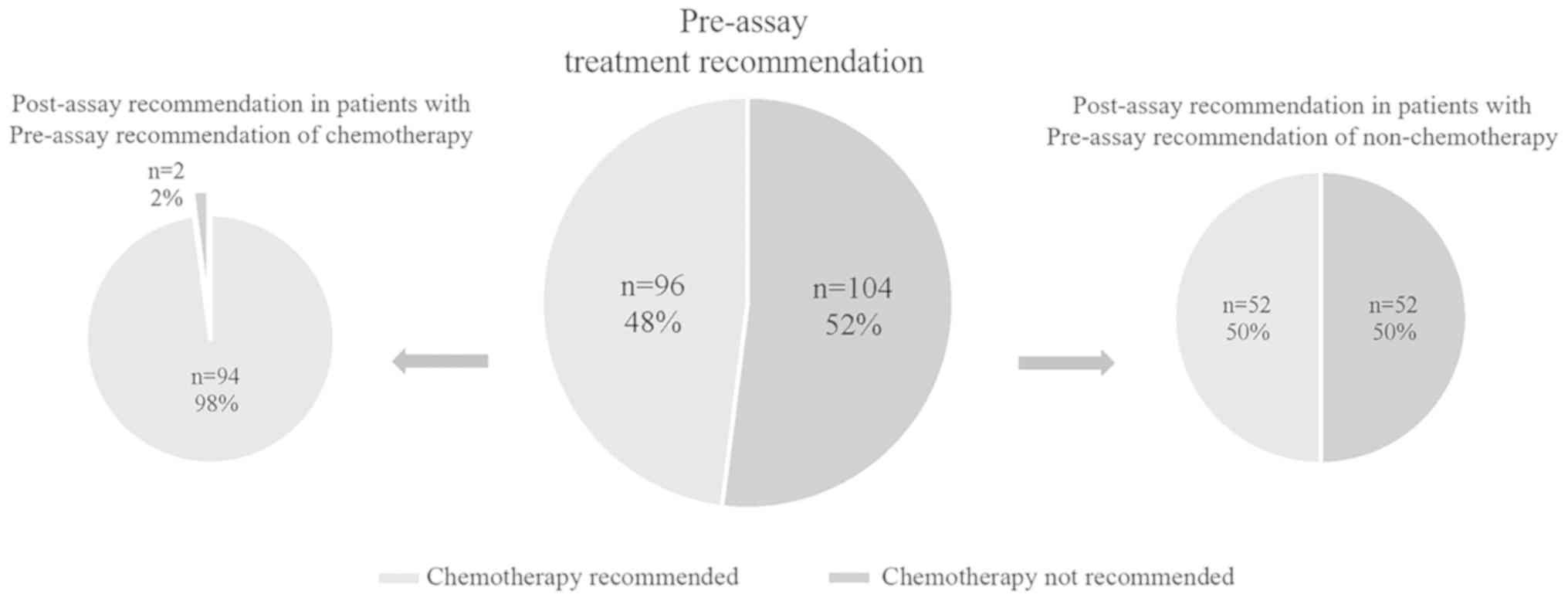
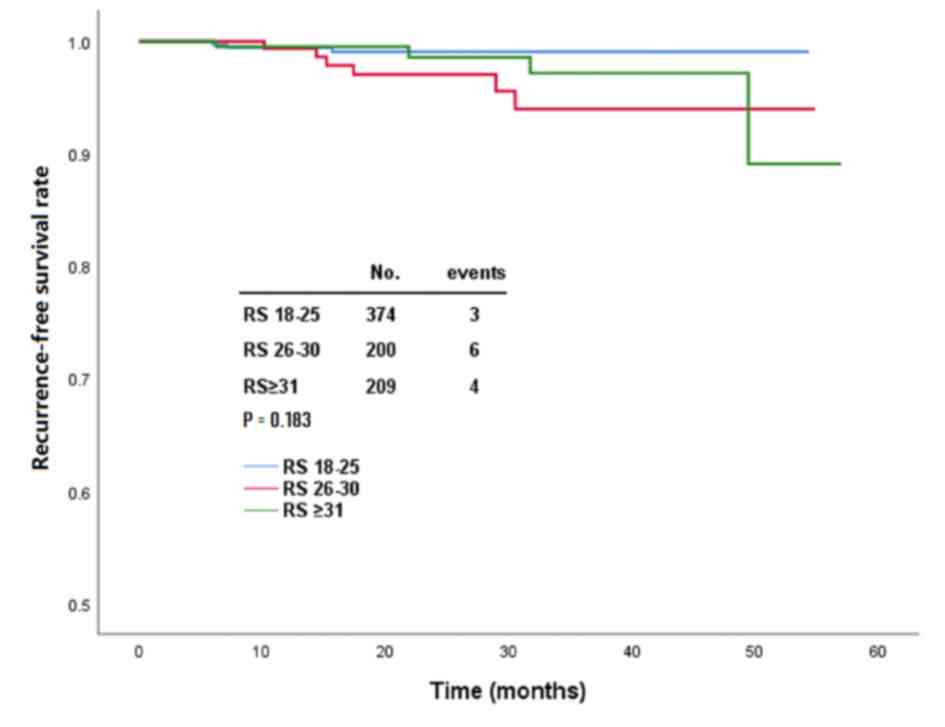
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Early Breast Cancer Trialists'
Collaborative Group (EBCTCG), . Peto R, Davies C, Godwin J, Gray R,
Pan HC, Clarke M, Cutter D, Darby S, McGale P, et al: Comparisons
between different polychemotherapy regimens for early breast
cancer: Meta-analyses of long-term outcome among 100,000 women in
123 randomised trials. Lancet. 379:432–444. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Early Breast Cancer Trialists'
Collaborative Group (EBCTCG), . Effects of chemotherapy and
hormonal therapy for early breast cancer on recurrence and 15-year
survival: An overview of the randomised trials. Lancet.
365:1687–1717. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kwa M, Makris A and Esteva FJ: Clinical
utility of gene-expression signatures in early stage breast cancer.
Nat Rev Clin Oncol. 14:595–610. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Paik S, Shak S, Tang G, Kim C, Baker J,
Cronin M, Baehner FL, Walker MG, Watson D, Park T, et al: A
multigene assay to predict recurrence of tamoxifen-treated,
node-negative breast cancer. N Engl J Med. 351:2817–2826. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Paik S, Tang G, Shak S, Kim C, Baker J,
Kim W, Cronin M, Baehner FL, Watson D, Bryant J, et al: Gene
expression and benefit of chemotherapy in women with node-negative,
estrogen receptor-positive breast cancer. J Clin Oncol.
24:3726–3734. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Goldstein LJ, Gray R, Badve S, Childs BH,
Yoshizawa C, Rowley S, Shak S, Baehner FL, Ravdin PM, Davidson NE,
et al: Prognostic utility of the 21-gene assay in hormone
receptor-positive operable breast cancer compared with classical
clinicopathologic features. J Clin Oncol. 26:4063–4071. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Albain KS, Barlow WE, Shak S, Hortobagyi
GN, Livingston RB, Yeh IT, Ravdin P, Bugarini R, Baehner FL,
Davidson NE, et al: Prognostic and predictive value of the 21-gene
recurrence score assay in postmenopausal women with node-positive,
oestrogen-receptor-positive breast cancer on chemotherapy: A
retrospective analysis of a randomised trial. Lancet Oncol.
11:55–65. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Goetz MP, Gradishar WJ, Anderson BO,
Abraham J, Aft R, Allison KH, Blair SL, Burstein HJ, Dang C, Elias
AD, et al: NCCN guidelines insights: Breast cancer, version 3.2018.
J Natl Compr Cancer Netw. 17:118–126. 2019. View Article : Google Scholar
|
|
10
|
Sparano JA, Gray RJ, Makower DF, Pritchard
KI, Albain KS, Hayes DF, Geyer CE Jr, Dees EC, Perez EA, Olson JA
Jr, et al: Prospective validation of a 21-gene expression assay in
breast cancer. N Engl J Med. 373:2005–2014. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gluz O, Nitz UA, Christgen M, Kates RE,
Shak S, Clemens M, Kraemer S, Aktas B, Kuemmel S, Reimer T, et al:
West German study group phase III planB trial: First prospective
outcome data for the 21-gene recurrence score assay and concordance
of prognostic markers by central and local pathology assessment. J
Clin Oncol. 34:2341–2349. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sparano JA, Gray RJ, Makower DF, Pritchard
KI, Albain KS, Hayes DF, Geyer CE Jr, Dees EC, Goetz MP, Olson JA
Jr, et al: Adjuvant chemotherapy guided by a 21-gene expression
assay in breast cancer. N Engl J Med. 379:111–121. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sparano JA, Gray RJ, Makower DF, Albain
KS, Saphner TJ, Badve SS, Wagner LI, Kaklamani VG, Keane MM, Gomez
HL, et al: Clinical outcomes in early breast cancer with a high
21-gene recurrence score of 26 to 100 assigned to adjuvant
chemotherapy plus endocrine therapy: A secondary analysis of the
TAILORx randomized clinical trial. JAMA Oncol. 2019:(Epub ahead of
print).
|
|
14
|
Lakhani SR, Ellis IO, Schnitt SJ, Tan PH
and van de Vijver MJ: WHO classification of tumours of the breast.
4th. 4. IARC WHO Classification of Tumours; 2012
|
|
15
|
Goldhirsch A, Winer EP, Coates AS, Gelber
RD, Piccart-Gebhart M, Thürlimann B and Senn HJ; Panel members, :
Personalizing the treatment of women with early breast cancer:
Highlights of the St Gallen international expert consensus on the
primary therapy of early breast cancer 2013. Ann Oncol.
24:2206–2223. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu J, Fang Y, Lin L, Fei X, Gao W, Zhu S,
Zong Y, Chen X, Huang O, He J, et al: Distribution patterns of
21-gene recurrence score in 980 Chinese estrogen receptor-positive,
HER2-negative early breast cancer patients. Oncotarget.
8:38706–38716. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Agarwal GG: Statistics for
surgeons-understanding survival analysis. Indian J Surg Oncol.
3:208–214. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Singh K, He X, Kalife ET, Ehdaivand S,
Wang Y and Sung CJ: Relationship of histologic grade and histologic
subtype with oncotype Dx recurrence score; retrospective review of
863 breast cancer oncotype Dx results. Breast Cancer Res Treat.
168:29–34. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang JL, Kizy S, Marmor S, Altman A,
Blaes A, Beckwith H, Tuttle TM and Hui JYC: Tumor grade and
progesterone receptor status predict 21-gene recurrence score in
early stage invasive breast carcinoma. Breast Cancer Res Treat.
172:671–677. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Park S, Han Y, Liu Y, Toriola AT, Peterson
LL, Colditz GA, Kim SI, Cho YU, Park BW and Park Y: Adjuvant
chemotherapy and survival among patients 70 years of age and
younger with node-negative breast cancer and the 21-gene recurrence
score of 26–30. Breast Cancer Res. 21:1102019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jasem J, Amini A, Rabinovitch R, Borges
VF, Elias A, Fisher CM and Kabos P: 21-Gene recurrence score assay
as a predictor of adjuvant chemotherapy administration for
early-stage breast cancer: An analysis of use, therapeutic
implications, and disparity profile. J Clin Oncol. 34:1995–2002.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Parsons BM, Landercasper J, Smith AL, Go
RS, Borgert AJ and Dietrich LL: 21-Gene recurrence score decreases
receipt of chemotherapy in ER + early-stage breast cancer: An
analysis of the NCDB 2010–2013. Breast Cancer Res Treat.
159:315–326. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Albanell J, Svedman C, Gligorov J, Holt
SD, Bertelli G, Blohmer JU, Rouzier R, Lluch A and Eiermann W:
Pooled analysis of prospective European studies assessing the
impact of using the 21-gene recurrence score assay on clinical
decision making in women with oestrogen receptor-positive, human
epidermal growth factor receptor 2-negative early-stage breast
cancer. Eur J Cancer. 66:104–113. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Orucevic A, Heidel RE and Bell JL:
Utilization and impact of 21-gene recurrence score assay for breast
cancer in clinical practice across the United States: Lessons
learned from the 2010 to 2012 national cancer data base analysis.
Breast Cancer Res Treat. 157:427–435. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Carlson JJ and Roth JA: The impact of the
oncotype Dx breast cancer assay in clinical practice: A systematic
review and meta-analysis. Breast Cancer Res Treat. 141:13–22. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tsai M, Lo S, Audeh W, Qamar R, Budway R,
Levine E, Whitworth P, Mavromatis B, Zon R, Oldham D, et al:
Association of 70-gene signature assay findings with physicians'
treatment guidance for patients with early breast cancer classified
as intermediate risk by the 21-gene assay. JAMA Oncol.
4:e1734702018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Qu F, Chen X, Fei X, Lin L, Gao W, Zong Y,
Wu J, Huang O, He J, Zhu L, et al: A nomogram to predict adjuvant
chemotherapy recommendation in breast cancer patients with
intermediate recurrence score. Chin J Cancer Res. 30:222–230. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Williams AD, Reyes SA, Arlow RL, Tchou J
and De La Cruz LM: Is age trumping genetic profiling in clinical
practice? relationship of chemotherapy recommendation and oncotype
DX recurrence score in patients aged <50 years versus >/=50
years, and trends over time. Ann Surg Oncol. 25:2875–2883. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nielsen TO, Jensen MB, Burugu S, Gao D,
Jørgensen CL, Balslev E and Ejlertsen B: High-risk premenopausal
luminal a breast cancer patients derive no benefit from adjuvant
cyclophosphamide-based chemotherapy: Results from the DBCG77B
clinical trial. Clin Cancer Res. 23:946–953. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sparano JA, Gray RJ, Ravdin PM, Makower
DF, Pritchard KI, Albain KS, Hayes DF, Geyer CE Jr, Dees EC, Goetz
MP, et al: Clinical and genomic risk to guide the use of adjuvant
therapy for breast cancer. N Engl J Med. 380:2395–2405. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Levine MN, Julian JA, Bedard PL, Eisen A,
Trudeau ME, Higgins B, Bordeleau L and Pritchard KI: Prospective
evaluation of the 21-gene recurrence score assay for breast cancer
decision-making in ontario. J Clin Oncol. 34:1065–1071. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kuchel A, Robinson T, Comins C, Shere M,
Varughese M, Sparrow G, Sahu A, Saunders L, Bahl A, Cawthorn SJ and
Braybrooke JP: The impact of the 21-gene assay on adjuvant
treatment decisions in oestrogen receptor-positive early breast
cancer: A prospective study. Br J Cancer. 114:731–736. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kuijer A, Straver M, den Dekker B, van
Bommel ACM, Elias SG, Smorenburg CH, Wesseling J, Linn SC, Rutgers
EJT, Siesling S and van Dalen T: Impact of 70-gene signature use on
adjuvant chemotherapy decisions in patients with estrogen
receptor-positive early breast cancer: Results of a prospective
cohort study. J Clin Oncol. 35:2814–2819. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Petkov VI, Miller DP, Howlader N, Gliner
N, Howe W, Schussler N, Cronin K, Baehner FL, Cress R, Deapen D, et
al: Breast-cancer-specific mortality in patients treated based on
the 21-gene assay: A SEER population-based study. NPJ Breast
Cancer. 2:160172016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Stemmer SM, Steiner M, Rizel S,
Soussan-Gutman L, Ben-Baruch N, Bareket-Samish A, Geffen DB,
Nisenbaum B, Isaacs K, Fried G, et al: Clinical outcomes in
patients with node-negative breast cancer treated based on the
recurrence score results: Evidence from a large prospectively
designed registry. NPJ Breast Cancer. 3:332017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ibraheem AF, Press DJ, Olopade OI and Huo
D: Community clinical practice patterns and mortality in patients
with intermediate oncotype DX recurrence scores: Who benefits from
chemotherapy? Cancer. 125:213–222. 2019. View Article : Google Scholar : PubMed/NCBI
|