Introduction
In women, breast cancer is the most frequently
diagnosed malignant disease, accounting for 25% of all cancer
cases, and presenting the highest mortality rate worldwide
(1). Chemotherapy can reduce the
risk of 10-year mortality by a third in patients with breast cancer
(2). Approximately 60–75% of
patients have hormone receptor (HR)-positive breast cancer, for
whom adjuvant endocrine therapy alone can significantly improve
clinical outcomes (3).
Traditionally, physicians make treatment decisions based on
clinicopathological characteristics; however, in the past decades,
several multigene signatures have been developed, which can provide
more precise prognostic and predictive information for early-stage
HR-positive breast cancer. Multigene signatures facilitate
individualized treatment and decrease adjuvant chemotherapy usage
in patients with breast cancer (4).
Among the identified multigene assays, the 21-gene
recurrence score (RS) assay, which is composed of 16 cancer-related
genes and 5 reference genes, is the most widely studied and used in
the clinic (5). The 21-gene RS assay
is performed on fixed, paraffin-embedded tumor tissues using
reverse transcription-quantitative PCR (RT-qPCR). The 21-gene RS
assay scores, ranging from 0 to 100, are classified into low-(RS
<18), intermediate-(18≤ RS <31) and high-(RS ≥31) risk groups
(5). Data from National Surgical
Adjuvant Breast and Bowel Project (NSABP) B-14 trial showed that RS
predicted distant recurrence for patients with HR-positive, human
epidermal growth factor receptor 2 (HER2)-negative and lymph
node-negative breast cancer who were treated with tamoxifen
(5). The NSABP B-20 trial further
demonstrated that patients in the high-risk group displayed a 27.6%
decrease in the 10-year distant recurrence rate due to
chemotherapy, which confirmed the necessity of adjuvant
chemotherapy for patients with RS ≥31 (6). The Southwest Oncology Group-8814 and
Eastern Cooperative Oncology Group E2197 studies extended the
application of the 21-gene RS assay to the lymph node-positive
population (7,8). With robust prognostic and predictive
value in breast cancer, the 21-gene RS assay has been recommended
by clinical practice guidelines, including those published by the
National Comprehensive Cancer Network (NCCN) (9), and has been increasingly used in the
clinic worldwide.
To lower the risk of undertreatment, which was
defined as controlling the 10-year recurrence risk of breast cancer
at a distant site to 10 and 20% for each cutoff, respectively, the
prospective trials TAILORx and West Germany Study Plan-B used the
21-gene RS assay with a different cutoff value compared with
previous studies (10,11). In the two aforementioned trials, the
risk group classification criteria were as follows: Low (RS
<11), intermediate (11≤ RS <26) and high (RS ≥26). The
TAILORx trial reported that endocrine therapy alone resulted in
improved disease-free survival for patients in the low-risk group
(10). For patients with RS=11-25,
adjuvant chemotherapy did not provide additional survival benefits,
especially for patients aged >50 years (12). Furthermore, for patients with
RS=26−100 receiving adjuvant chemotherapy, the clinical outcomes
were improved compared with those of patients treated with
endocrine therapy alone (13).
In the TAILORx study, patients with RS ≥26 received
adjuvant chemotherapy (10);
however, the NSABP B-20 study demonstrated that only patients with
RS ≥31 tumors benefited the most from adjuvant chemotherapy
(6). At present, there are limited
data available on the clinicopathological features and treatment
patterns of patients with RS=26−30.
The present study evaluated the clinicopathological
characteristics, adjuvant chemotherapy usage and disease outcomes
of patients with HR+/HER2−/lymph
node− breast cancer with RS=26−30 in comparison with
those of patients with RS=18−25 and RS ≥31. Furthermore, whether
21-gene RS testing lead to a treatment recommendation alteration
was investigated. The aim of the present study was to demonstrate
the clinical features and to identify the appropriate treatment
decision for patients with RS=26−30.
Materials and methods
Study population
Female patients diagnosed with invasive breast
cancer who received surgery between January 2014 and December 2017
at Ruijin Hospital, Shanghai Jiaotong University School of Medicine
(Shanghai, China) were retrospectively included in the present
study. Data regarding clinicopathological characteristics,
treatment decisions and survival events were retrieved from
Shanghai Jiaotong University Breast Cancer Database. The present
study was reviewed and approved by the Ethical Committee of Ruijin
Hospital. The inclusion criteria were as follows: i) Primary
invasive breast cancer; ii) HR positive and HER2 negative; iii)
lymph node-negative; and iv) RS ≥18. The exclusion criteria were as
follows: i) Male patients with breast cancer; and ii) patients with
incomplete data for immunohistochemical (IHC) results, chemotherapy
usage or survival.
Pathological, IHC analysis and 21-gene
RS assay testing
Histopathological examination of tumor histological
subtype, grade and presence of lymphovascular invasion was
conducted by experienced pathologists at the Department of
Pathology of Ruijin Hospital in concordance with the World Health
Organization classification (14).
The tumor tissue was fixed by 10% neutral buffered formalin at room
temperature overnight before embedding in paraffin. The 4-µm-thick,
formalin-fixed, paraffin-embedded (FFPE) tissue sections were
incubated with the peroxidase-blocking solution (cat. no. S2023;
Dako; Agilent Technologies, Inc.) for 3 min and blocked with the
blocking solution (cat. no. X0909; Dako; Agilent Technologies,
Inc.) for 10 min after dewaxing in xylene for 60 min and
rehydration in a descending alcohol series (100, 95 and 75%), all
at room temperature. Subsequently, IHC staining of estrogen
receptor (ER), progesterone receptor (PR) and Ki-67 index was
automatically performed using a Ventana BenchMark XT system
(Ventana Medical Systems, Inc.). Briefly, the FFPE tissue sections
were incubated for 32 min at 42°C with primary antibodies targeted
against ER (cat. no. IR657; clone 1D5; 1:100; rabbit monoclonal),
PR (cat. no. IR068; clone PR636; 1:100; mouse monoclonal) and Ki-67
(cat. no. IR626; clone MIB-1; 1:100; mouse monoclonal) (all Dako;
Agilent Technologies, Inc.). Subsequently, tissue sections were
incubated with secondary goat anti-mouse (cat. no. P0447) or goat
anti-rabbit (cat. no. P0448) (both 1:100; Dako; Agilent
Technologies, Inc.) antibodies for 30 min at room temperature. A
Dako automated immunohistochemistry system (Dako; Agilent
Technologies, Inc.) was used to interpret the IHC results, which
were checked by two experienced pathologists using a light
microscope (magnification, ×100). ER+ and PR+
tumors were defined as tumors with nuclear staining in ≥1% of tumor
cells. Ki-67 index was assessed in ≥1,000 invasive tumor cells, and
was characterized as the proportion of positively stained cells in
the nucleus vs. the total number of cells in the field. Luminal
subtype was determined according to the St Gallen 2013 expert panel
(15). Luminal A-like subtype was
defined as ER+, PR ≥20% and Ki-67 <14%, while luminal
B-like was defined as ER+ and PR <20% or Ki-67 ≥14%.
The 21-gene RS assay was performed on formalin-fixed,
paraffin-embedded tissue sections as described in our previous
study (16). The amount of tissue
was determined by assessing the percentage of tumor on hematoxylin
and eosin-stained slides. Briefly, the tissue slides were stained
with Harris hematoxylin solution for 10 min and then differentiated
in 1% acid alcohol, all at room temperature. Following bluing,
eosin solution was used for counterstaining for 30 sec at room
temperature, and then slides were dehydrated in 95 and 100%
alcohol, washed in xylene and mounted using a neutral balsam (data
not shown). Total RNA was extracted using the RNeasy FFPE RNA kit
(Qiagen GmbH) from two 10-µm unstained sections and was measured
after verifying the absence of DNA contamination, which was
assessed by a quantitative (q)PCR assay for β-actin DNA.
Gene-specific reverse transcription was performed at 65°C for 5 min
and 37°C for 60 min using the Omniscript RT kit (Qiagen GmbH).
Subsequently, standardized qPCR was performed using Premix Ex Taq™
(Takara Bio, Inc.) in 96-well plates using an Applied Biosystems
7500 Real-Time PCR system (Thermo Fisher Scientific, Inc.), with
the following thermocycling conditions: 95°C for 10 min, 95°C for
20 sec and 60°C for 45 sec (for 40 cycles). The primers and probes
used for qPCR are listed in Table
SI. The expression levels of each cancer-associated gene were
measured in triplicate and normalized to 5 reference genes,
including ACTB, GAPD, GUSB, RPLP0 and TFRC. The RS was calculated
using a specific algorithm as previously described (5). For patients with ipsilateral multifocal
or bilateral invasive cancer, the highest RS was recorded.
Treatment decision and prognosis
information
After surgery, the multidisciplinary team (MDT),
which consisted of breast surgeons, medical oncologists,
pathologists, radiation oncologists and specialized breast nurses,
discussed and determined the appropriate adjuvant treatment
recommendations for each patient. In the first-round of MDT voting,
a primary chemotherapy recommendation was made based on standard
clinicopathological and IHC results, which was recorded as the
chemotherapy recommendation pre-RS assay. After obtaining the
result of the 21-gene RS assay, the second-round of MDT voting was
organized to determine the final decision, which was recorded as
the chemotherapy recommendation post-RS assay. In both rounds of
voting, if the vote was not unanimous, the decision of the
attending physician who performed the surgery was recorded. The
actual chemotherapy usage was confirmed during follow-up. The most
frequently used chemotherapy regimens included docetaxel plus
cyclophosphamide, epirubicin plus cyclophosphamide and epirubicin
plus cyclophosphamide followed by docetaxel.
Statistical analysis
All clinicopathological characteristics were
presented as patient number and percentage [n, (%)] and analyzed as
categorical variables. The χ2 test was used to evaluate
the RS distribution and chemotherapy usage in patients with
different clinicopathological characteristics, and Fisher's exact
test was performed when had expected values less than 5. Multiple
logistic regression models were used to generate adjusted odds
ratios (ORs) with 95% confidence intervals (CIs) to assess factors
associated with RS distribution and chemotherapy. Kaplan-Meier with
Tarone-Ware tests (17) was used to
compare the recurrence-free survival rate, and pairwise comparisons
were performed for the recurrence-free survival. Recurrence events
included local, regional, distant and contralateral breast
recurrence. P<0.05 was considered to indicate a statistically
significant difference. Statistical analyses were performed using
SPSS software (version 22.0; IBM Corp.).
Results
Baseline clinicopathological
characteristics
A total of 821 patients diagnosed between January
2014 and December 2017 were reviewed, of which, 38 were excluded
and 783 were included in the present study. The number of patients
in the RS=18-25, =26−30 and ≥31 groups was 374 (47.8%), 200 (25.5%)
and 209 (26.7%), respectively. Baseline clinicopathological
characteristics are presented in Table
I. The mean age of the patients was 56.0±12.4 years, and 511
(65.3%) patients were aged >50 years. A total of 565 (72.2%)
patients had tumors ≤2 cm in size. Additionally, 138 (17.6%)
patients had grade-III tumors. The proportion of patients with ER
≥50%, PR ≥20% and Ki-67 ≥14% was 96.6, 63.9 and 53.0%,
respectively. Furthermore, 562 (71.8%) patients had luminal B-like
breast cancer.
 | Table I.Clinicopathological characteristics
of patients according to 21-gene RS classification. |
Table I.
Clinicopathological characteristics
of patients according to 21-gene RS classification.
| Variable | Total, n (%)
(n=783) | RS=18-25, n (%)
(n=374, 47.8%) | RS=26−30, n (%)
(n=200, 25.5%) | RS ≥31, n (%)
(n=209, 26.7%) | P-value |
|---|
| Age (years) |
|
|
|
| 0.030 |
|
≤50 | 272 (34.7) | 147 (39.3) | 58 (29.0) | 67 (32.1) |
|
|
>50 | 511 (65.3) | 227 (60.7) | 142 (71.0) | 142 (67.9) |
|
| BMI |
|
|
|
| 0.740 |
|
<25 | 585 (74.7) | 284 (75.9) | 148 (74.0) | 153 (73.2) |
|
|
≥25 | 198 (25.3) | 90 (24.1) | 52 (26.0) | 56 (26.8) |
|
| Comorbidity |
|
|
|
| 0.286 |
| No | 456 (58.2) | 217 (58.0) | 91 (45.5) | 130 (62.2) |
|
|
Yes | 327 (41.8) | 157 (42.0) | 109 (54.5) | 79 (37.8) |
|
| Tumor size
(cm) |
|
|
|
| 0.206 |
| ≤2 | 565 (72.2) | 281 (75.1) | 139 (69.5) | 145 (69.4) |
|
|
>2 | 218 (27.8) | 93 (24.9) | 61 (30.5) | 64 (30.6) |
|
| Histological
type |
|
|
|
| 0.123 |
|
IDC | 650 (83.0) | 301 (80.5) | 167 (83.5) | 182 (87.1) |
|
|
Non-IDC | 133 (17.0) | 73 (19.5) | 33 (16.5) | 27 (12.9) |
|
| Tumor grade |
|
|
|
| <0.001 |
|
I/II | 524 (66.9) | 268 (71.7) | 135 (67.5) | 121 (57.9) |
|
|
III | 138 (17.6) | 40 (10.7) | 34 (17.0) | 64 (30.6) |
|
|
Unknown | 121 (15.5) | 66 (17.6) | 31 (15.5) | 24 (11.5) |
|
| LVI |
|
|
|
| 0.994 |
|
Yes | 38 (4.9) | 18 (4.8) | 10 (5.0) | 10 (4.8) |
|
| No | 745 (95.1) | 356 (95.2) | 190 (95.0) | 199 (95.2) |
|
| ER status (%) |
|
|
|
| 0.001 |
|
<50 | 27 (3.4) | 7 (1.9) | 4 (2.0) | 16 (7.7) |
|
|
≥50 | 756 (96.6) | 367 (98.1) | 196 (98.0) | 193 (92.3) |
|
| PR status (%) |
|
|
|
| <0.001 |
|
<20 | 283 (36.1) | 82 (21.9) | 75 (37.5) | 126 (60.3) |
|
|
≥20 | 500 (63.9) | 292 (78.1) | 125 (62.5) | 83 (39.7) |
|
| Ki-67 index
(%) |
|
|
|
| <0.001 |
|
<14 | 368 (47.0) | 209 (55.9) | 95 (47.5) | 64 (30.6) |
|
|
≥14 | 415 (53.0) | 165 (44.1) | 105 (52.5) | 145 (69.4) |
|
| Luminal
subtype |
|
|
|
| <0.001 |
| Luminal
A-like | 221 (28.2) | 153 (40.9) | 49 (24.5) | 19 (9.1) |
|
| Luminal
B-like | 562 (71.8) | 221 (59.1) | 151 (75.5) | 190 (90.9) |
|
| Surgery type |
|
|
|
| 0.346 |
|
BCS | 371 (47.4) | 172 (46.0) | 91 (45.5) | 108 (51.7) |
|
|
Mastectomy | 412 (52.6) | 202 (54.0) | 109 (54.5) | 101 (48.3) |
|
Clinicopathological characteristics of
the different RS groups
Univariate analysis indicated that age (P=0.030),
tumor grade (P<0.001), ER status (P=0.001), PR status
(P<0.001), Ki-67 index (P<0.001) and luminal subtype
(P<0.001) were significantly different among the three RS groups
(Table I). Grade-III tumors were
present in 10.7, 17.0 and 30.6% of patients in the RS=18-25, =26−30
and ≥31 groups, respectively. Regarding luminal subtype, the
RS=18-25, =26−30 and ≥31 groups contained 59.1, 75.5 and 90.9%
luminal B-like tumors, respectively (Table I).
Multivariate analysis demonstrated that tumor grade,
PR expression and Ki-67 index were independently associated with RS
grouping (P<0.05). Compared with the RS ≥31 group, the RS=26−30
group was associated with higher PR expression (OR, 2.84; 95% CI,
1.69–4.79; P<0.001) and lower Ki-67 index (OR, 1.88; 95% CI,
1.06–3.34; P=0.032), and tended to display fewer grade-III tumors
(OR, 1.63; 95% CI, 0.96–2.76; P=0.070). There was no significant
difference between the RS=18−25 and =26−30 groups in terms of grade
(P=0.133), PR expression (P=0.063) or Ki-67 index (P=0.924;
Table II).
 | Table II.Baseline characteristics stratified
by 21-gene RS classification, with RS=26−30 as a reference. |
Table II.
Baseline characteristics stratified
by 21-gene RS classification, with RS=26−30 as a reference.
|
| RS=18−25
(n=374) | RS ≥31 (n=209) |
|
|---|
|
|
|
|
|
|---|
| Variable | OR | 95% CI | P-value | OR | 95% CI | P-value | P-value |
|---|
| Age (years) |
|
| 0.111 |
|
| 0.142 | 0.209 |
|
≤50 | 1.37 | 0.93–2.02 |
| 1.41 | 0.89–2.23 |
|
|
|
>50 | 1 |
|
| 1 |
|
|
|
| Tumor grade |
|
|
|
|
|
| 0.007 |
|
I/II | 1 |
|
| 1 |
|
|
|
|
III | 0.67 | 0.39–1.13 | 0.133 | 1.63 | 0.96–2.76 | 0.070 |
|
|
Unknown | 1.07 | 0.66–1.74 | 0.781 | 0.90 | 0.49–1.64 | 0.724 |
|
| ER status (%) |
|
| 0.888 |
|
| 0.113 | 0.138 |
|
<50 | 1.10 | 0.31–3.94 |
| 2.54 | 0.80–8.04 |
|
|
|
≥50 | 1 |
|
| 1 |
|
|
|
| PR status (%) |
|
| 0.063 |
|
| <0.001 | <0.001 |
|
<20 | 0.61 | 0.37–1.03 |
| 2.84 | 1.69–4.79 |
|
|
|
≥20 | 1 |
|
| 1 |
|
|
|
| Ki-67 index
(%) |
|
| 0.924 |
|
| 0.032 | 0.032 |
|
<14 | 1 |
|
| 1 |
|
|
|
|
≥14 | 0.97 | 0.55–1.72 |
| 1.88 | 1.06–3.34 |
|
|
| Luminal
subtype |
|
| 0.212 |
|
| 0.950 | 0.342 |
| Luminal
A-like | 1 |
|
| 1 |
|
|
|
| Luminal
B-like | 0.65 | 0.33–1.28 |
| 1.03 | 0.45–2.35 |
|
|
Adjuvant chemotherapy usage in the
different RS groups
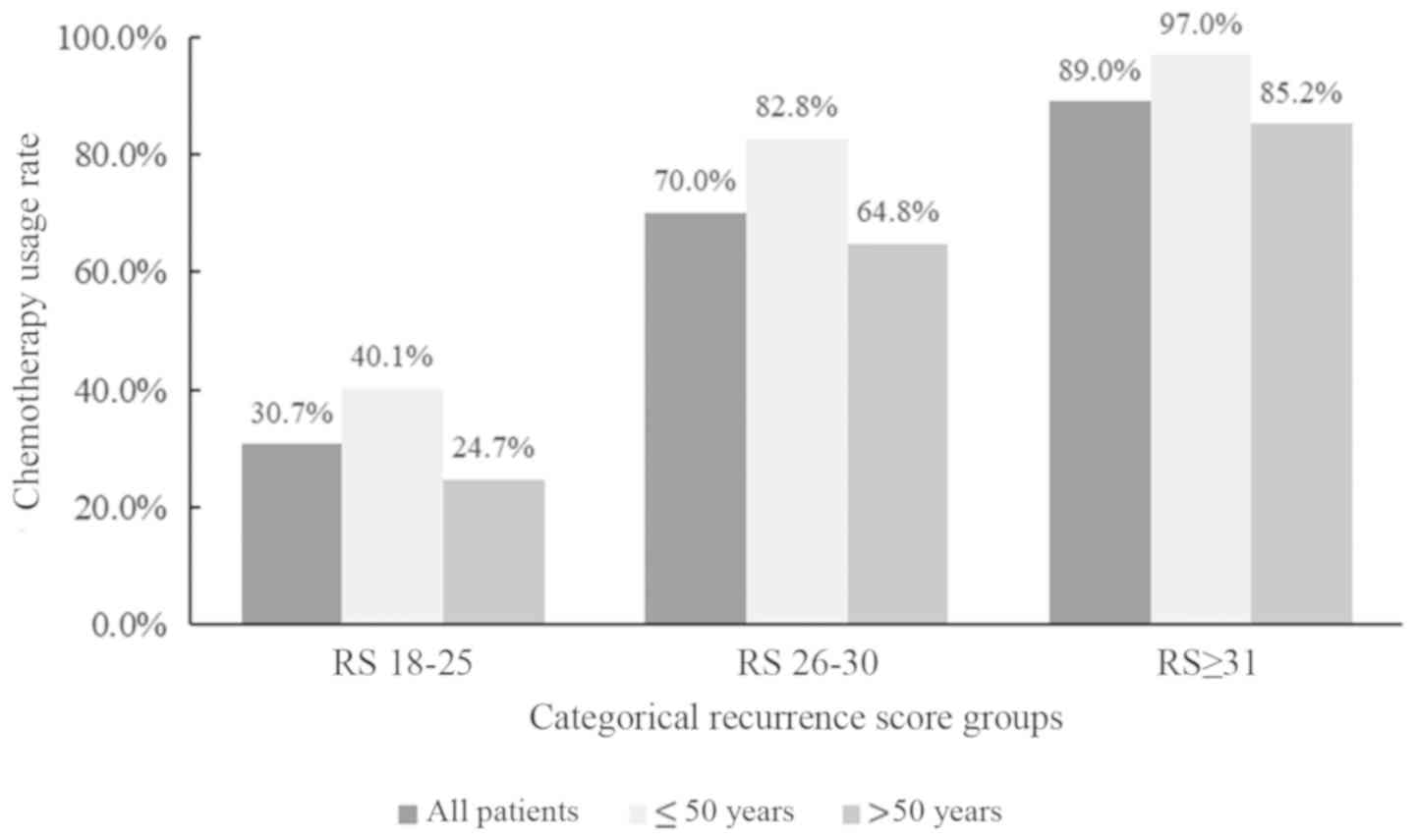
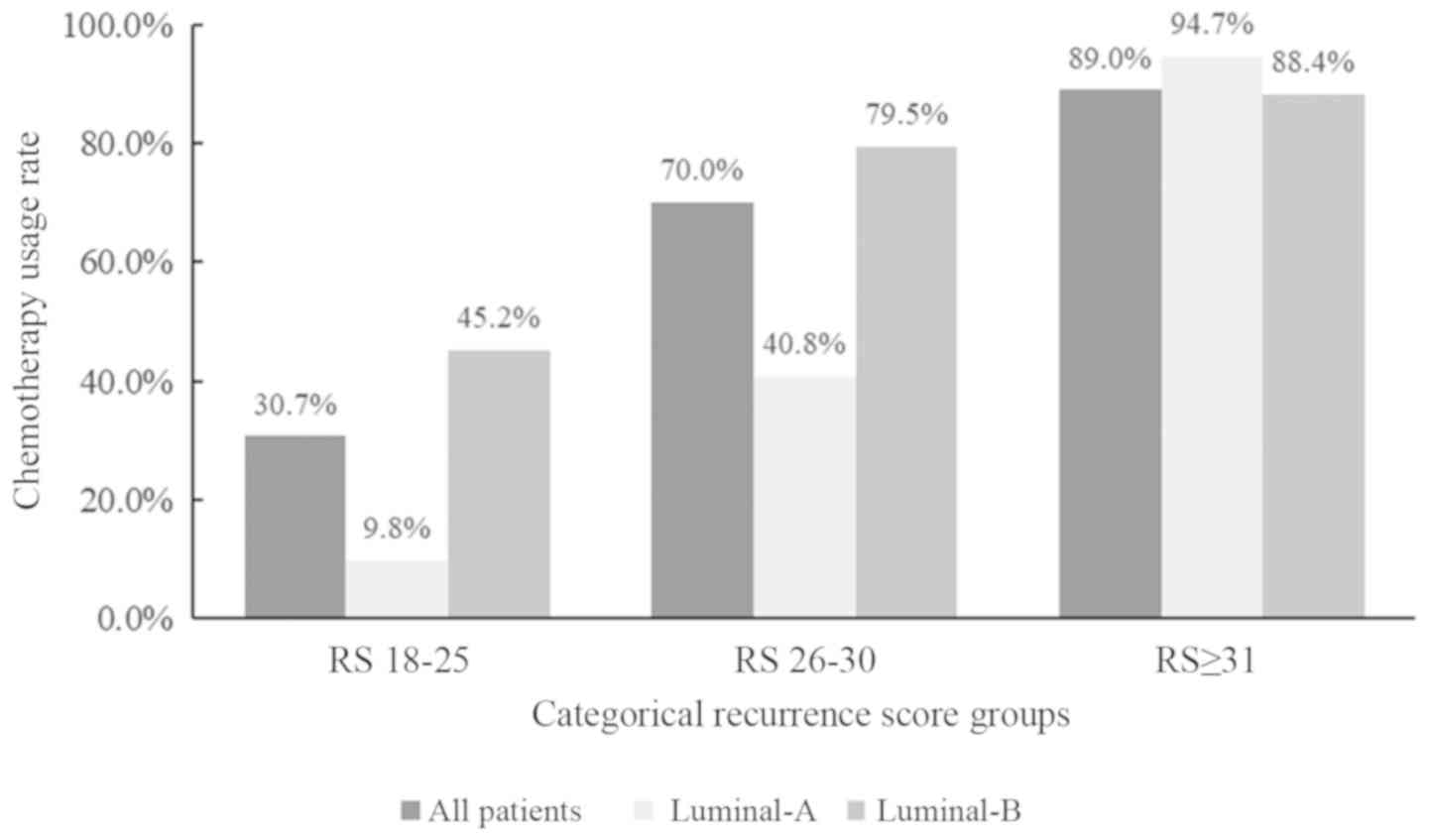
A total of 115 (30.7%), 140 (70.0%) and 186 (89.0%)
patients in the RS=18-25, =26−30 and ≥31 groups, respectively,
received chemotherapy (P<0.001). Table III presents the clinicopathological
parameters associated with chemotherapy usage in the whole
population.
 | Table III.Clinicopathological characteristics
according to chemotherapy usage in the overall population. |
Table III.
Clinicopathological characteristics
according to chemotherapy usage in the overall population.
| Variable | Total, n (%)
(n=783) | Chemo, n (%)
(n=441) | Non-chemo, n (%)
(n=342) | P-value |
|---|
| Age (years) |
|
|
| 0.004 |
|
≤50 | 272 (34.7) | 172 (63.2) | 100 (36.8) |
|
|
>50 | 511 (65.3) | 269 (61.0) | 242 (70.8) |
|
| Menstrual
status |
|
|
| 0.002 |
|
Premenopausal | 287 (36.7) | 182 (63.4) | 105 (36.6) |
|
|
Postmenopausal | 496 (63.3) | 259 (52.2) | 237 (47.8) |
|
| Comorbidity |
|
|
| <0.001 |
| No | 456 (58.2) | 287 (62.9) | 169 (37.1) |
|
|
Yes | 327 (41.8) | 154 (47.1) | 173 (52.9) |
|
| Surgery type |
|
|
| 0.891 |
|
BCS | 371 (47.4) | 208 (56.1) | 163 (43.9) |
|
|
Mastectomy | 412 (52.6) | 233 (56.6) | 179 (43.4) |
|
| Tumor size
(cm) |
|
|
| <0.001 |
| ≤2 | 565 (72.2) | 296 (52.4) | 269 (47.6) |
|
|
>2 | 218 (27.8) | 145 (66.5) | 73 (33.5) |
|
| Histological
type |
|
|
| <0.001 |
|
IDC | 650 (83.0) | 397 (61.1) | 253 (38.9) |
|
|
Non-IDC | 133 (17.0) | 44 (33.1) | 89 (66.9) |
|
| Tumor grade |
|
|
| <0.001 |
|
I/II | 524 (66.9) | 280 (53.4) | 244 (46.6) |
|
|
III | 138 (17.6) | 120 (87.0) | 18 (13.0) |
|
|
Unknown | 121 (15.5) | 41 (33.9) | 80 (66.1) |
|
| LVI |
|
|
| 0.027 |
|
Yes | 38 (4.9) | 28 (73.7) | 10 (26.3) |
|
| No | 745 (95.1) | 413 (55.4) | 332 (44.6) |
|
| ER status (%) |
|
|
| 0.008 |
|
<50 | 27 (3.4) | 22 (81.5) | 5 (18.5) |
|
|
≥50 | 756 (96.6) | 419 (55.9) | 330 (44.1) |
|
| PR status (%) |
|
|
| <0.001 |
|
<20 | 283 (36.1) | 202 (71.4) | 81 (28.6) |
|
|
≥20 | 500 (63.9) | 198 (39.6) | 302 (60.4) |
|
| Ki-67 index
(%) |
|
|
| <0.001 |
|
<14 | 368 (47.0) | 135 (36.7) | 233 (63.3) |
|
|
≥14 | 415 (53.0) | 306 (73.7) | 109 (26.3) |
|
| Luminal
subtype |
|
|
| <0.001 |
| Luminal
A-like | 221 (28.2) | 53 (24.0) | 168 (76.0) |
|
| Luminal
B-like | 562 (71.8) | 388 (69.0) | 174 (31.0) |
|
| RS |
|
|
| <0.001 |
|
18-25 | 374 (47.8) | 115 (30.7) | 259 (69.3) |
|
|
26-30 | 200 (25.5) | 140 (70.0) | 60 (30.0) |
|
|
≥31 | 209 (26.7) | 186 (89.0) | 23 (11.0) |
|
Multivariate analysis indicated that menstruation
(OR, 2.55; 95% CI, 1.62–4.02; P<0.001), larger tumor size (OR,
1.91; 95% CI, 1.23–2.98; P=0.004), histological grade III (OR,
2.35; 95% CI, 1.25–4.40; P=0.008), higher Ki-67 index (OR, 2.59;
95% CI, 1.40–4.81; P=0.002), luminal B-like tumor (OR, 2.29; 95%
CI, 1.09–4.83; P=0.029) and RS category (P<0.001) were
independently associated with chemotherapy usage. Compared with
patients with RS=18-25, patients with RS=26−30 (OR, 7.20; 95% CI,
4.55–11.42; P<0.001) or RS ≥31 (OR, 16.08; 95% CI, 9.19–28.14;
P<0.001) were more likely to receive adjuvant chemotherapy.
Furthermore, patients with comorbidities received chemotherapy less
often compared with patients without comorbidities (OR, 0.52; 95%
CI, 0.34–0.79; P=0.002; Table
IV).
 | Table IV.Multivariate analysis of factors
associated with chemotherapy in the overall population. |
Table IV.
Multivariate analysis of factors
associated with chemotherapy in the overall population.
| Variable | OR | 95% CI | P-value |
|---|
| Age (≤50 years vs.
>50 years) | 1.53 | 0.69–3.39 | 0.290 |
| Menstrual status
(premenopausal vs. postmenopausal) | 2.55 | 1.62–4.02 | <0.001 |
| Comorbidity (yes
vs. no) | 0.52 | 0.34–0.79 | 0.002 |
| Tumor size (>2
cm vs. ≤2 cm) | 1.91 | 1.23–2.98 | 0.004 |
| Histologic type
(non-IDC vs. IDC) | 0.33 | 0.10–1.12 | 0.076 |
| Tumor grade |
|
| 0.027 |
| III vs.
I/II | 2.35 | 1.25–4.40 | 0.008 |
| Unknown
vs. I/II | 0.92 | 0.27–3.18 | 0.899 |
| ER status (<50%
vs. ≥50%) | 1.60 | 0.43–5.95 | 0.487 |
| PR status (<20%
vs. ≥20%) | 1.78 | 1.00–3.18 | 0.052 |
| Ki-67 index (≥14%
vs. <14%) | 2.59 | 1.40–4.81 | 0.002 |
| Luminal subtype
(luminal B-like vs. luminal A-like) | 2.29 | 1.09–4.83 | 0.029 |
| RS |
|
| <0.001 |
| 26−30
vs. 18–25 | 7.20 | 4.55–11.42 | <0.001 |
| ≥31 vs.
18–25 | 16.08 | 9.19–28.14 | <0.001 |
In the RS=18−25 group, distribution of age,
menstrual status, comorbidity, lymphovascular invasion, tumor size,
histological type, tumor grade, PR status, Ki-67 index and luminal
subtype were significantly different between patients receiving
adjuvant chemotherapy and those not receiving it (Table SII). Multivariate analyses
demonstrated that menstruation (OR, 2.53; 95% CI, 1.38–4.65;
P=0.003), larger tumor size (OR, 2.21; 95% CI, 1.20–4.09; P=0.011),
grade-III tumors (OR, 2.75; 95% CI, 1.18–6.44; P=0.019), PR <20%
(OR, 4.36; 95% CI, 2.24–8.48; P<0.001) and Ki-67 ≥14% (OR, 6.90;
95% CI, 3.83–12.46; P<0.001) were factors independently
associated with chemotherapy usage (Table SIII).
In the RS ≥31 group, univariate analysis indicated
that younger age (97.0% vs. 85.2%; P=0.009), menstruation (97.0%
vs. 85.2%; P=0.009), no comorbidity (94.6% vs. 79.7%; P<0.001)
and infiltrating ductal carcinoma (91.8% vs. 70.4%; P=0.001) were
associated with adjuvant chemotherapy administration (Table SIV). Multivariate analysis also
indicated that the aforementioned factors were independently
associated with chemotherapy usage (Table SV).
Adjuvant chemotherapy usage in
patients with RS=26−30
The baseline characteristics of patients with
RS=26−30 are presented in Table V.
According to the results of the univariate analysis, younger age
(P=0.012), menstruation (P=0.022), larger tumors (P=0.009),
grade-III tumors (P<0.001), high-level Ki-67 index (P<0.001)
and luminal B-like tumors (P<0.001) were associated with
chemotherapy. Chemotherapy use was higher in patients aged ≤50
years (48/58; 82.5%) compared with patients aged >50-years
(92/142; 64.8%; Fig. 1). Besides,
among 151 patients with luminal B-like breast cancer, 120 (79.5%)
patients received chemotherapy, which was higher compared with
patients in the luminal-A cohort (40.8%; Fig. 2).
 | Table V.Clinicopathological characteristics
according to chemotherapy usage in patients with a 21-gene
recurrence score of 26–30. |
Table V.
Clinicopathological characteristics
according to chemotherapy usage in patients with a 21-gene
recurrence score of 26–30.
| Variable | Total, n (%)
(n=200) | Chemo, n (%)
(n=140) | Non-chemo, n (%)
(n=60) | P-value |
|---|
| Age (years) |
|
|
| 0.012 |
|
≤50 | 58 (29.0) | 48 (82.8) | 10 (17.2) |
|
|
>50 | 142 (71.0) | 92 (64.8) | 50 (35.2) |
|
| Menstrual
status |
|
|
| 0.022 |
|
Premenopausal | 63 (31.5) | 51 (81.0) | 12 (19.0) |
|
|
Postmenopausal | 137 (68.5) | 89 (65.0) | 48 (35.0) |
|
| Comorbidity |
|
|
| 0.017 |
| No | 109 (54.5) | 84 (77.1) | 25 (22.9) |
|
|
Yes | 91 (45.5) | 56 (61.5) | 35 (38.5) |
|
| Surgery type |
|
|
| 0.173 |
|
BCS | 91 (45.5) | 61 (43.6) | 30 (56.4) |
|
|
Mastectomy | 109 (54.5) | 79 (72.5) | 30 (27.5) |
|
| Tumor size
(cm) |
|
|
| 0.009 |
| ≤2 | 139 (69.5) | 90 (64.7) | 49 (35.3) |
|
|
>2 | 61 (30.5) | 50 (82.0) | 11 (18.0) |
|
| LVI |
|
|
| 0.479 |
|
Yes | 10 (5.0) | 6 (60.0) | 4 (40.0) |
|
| No | 190 (95.0) | 134 (70.5) | 56 (29.5) |
|
| Histological
type |
|
|
| 0.001 |
|
IDC | 167 (83.5) | 126 (75.4) | 41 (24.6) |
|
|
Non-IDC | 33 (16.5) | 14 (42.4) | 19 (57.6) |
|
| Tumor grade |
|
|
| <0.001 |
|
I/II | 135 (67.4) | 95 (70.4) | 40 (29.6) |
|
|
III | 34 (17.0) | 32 (94.1) | 2 (5.9) |
|
|
Unknown | 31 (15.5) | 13 (41.9) | 18 (58.1) |
|
| ER status (%) |
|
|
| 0.319 |
|
<50 | 196 (98.0) | 136 (69.4) | 60 (30.6) |
|
|
≥50 | 4 (2.0) | 4 (100.0) | 0 (0.00) |
|
| PR status (%) |
|
|
| 0.151 |
|
<20 | 75 (37.5) | 57 (76.0) | 18 (24.0) |
|
|
≥20 | 125 (62.5) | 83 (66.4) | 42 (33.6) |
|
| Ki-67 index
(%) |
|
|
| <0.001 |
|
<14 | 95 (47.5) | 52 (54.7) | 43 (45.3) |
|
|
≥14 | 105 (52.5) | 88 (83.8) | 17 (16.2) |
|
| Luminal
subtype |
|
|
| <0.001 |
| Luminal
A-like | 49 (24.5) | 20 (40.8) | 29 (59.2) |
|
| Luminal
B-like | 151 (75.5) | 120 (79.5) | 31 (20.5) |
|
Multivariate analysis demonstrated that age and
luminal subtype were independent factors associated with adjuvant
chemotherapy usage. Patients aged ≤50 years were more likely to
receive adjuvant chemotherapy compared with patients aged >50
years (OR, 5.75; 95% CI, 2.08–15.90; P=0.001). Compared with
patients with luminal A-like tumors, a higher number of patients
with luminal B-like tumors received adjuvant chemotherapy (OR,
7.75; 95% CI, 3.28–18.32; P<0.001; Table SVI).
Alteration to chemotherapy
recommendation after 21-gene RS in patients with RS=26−30
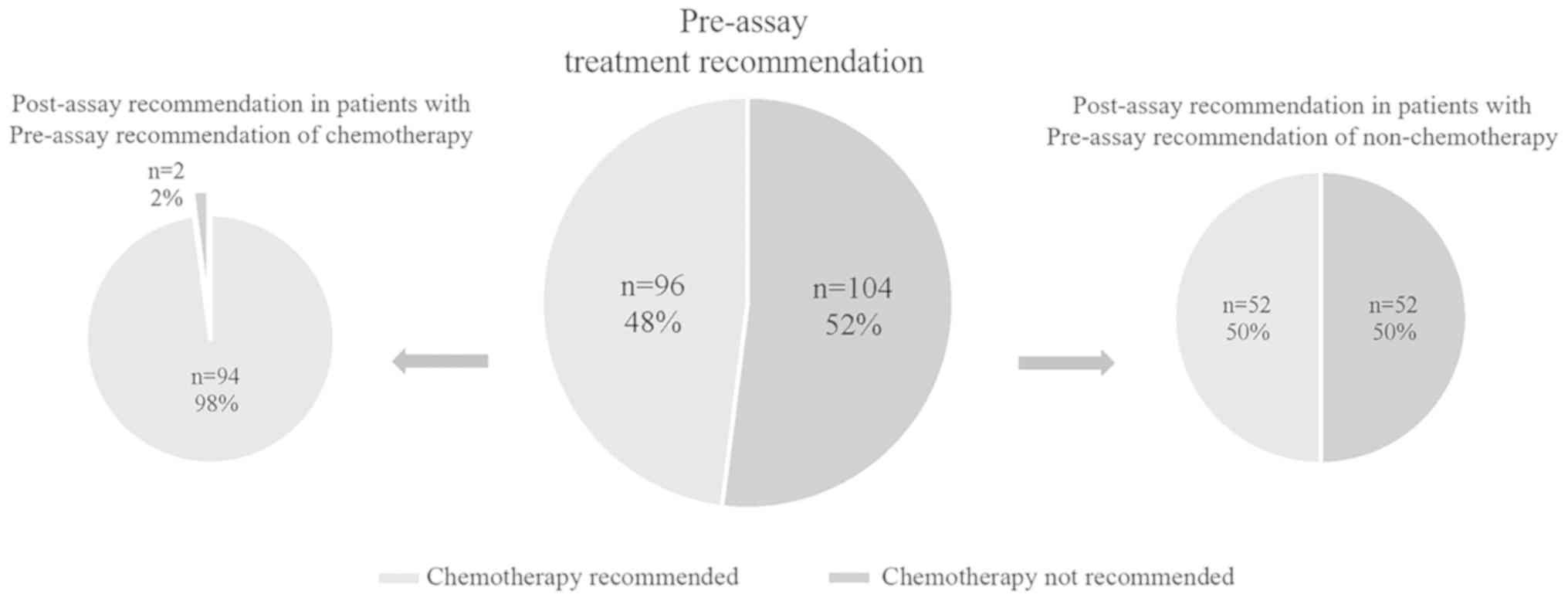
A total of 200 patients with RS=26−30 underwent two
rounds of MDT recommendations. Before 21-gene RS testing, endocrine
therapy alone was recommended by the MDT for 104 (52.0%) patients,
while chemoendocrine therapy was suggested for the remaining 96
(48.0%) patients. After RS testing, the adjuvant regimen of 54
(27.0%) patients was altered: 52 patients shifted from no
chemotherapy to chemotherapy, and 2 patients shifted from
chemotherapy to no chemotherapy (Table
VI and Fig. 3).
 | Table VI.Pre- and post-assay chemotherapy
decision and actual usage in patients with a 21-gene recurrence
score of 26–30. |
Table VI.
Pre- and post-assay chemotherapy
decision and actual usage in patients with a 21-gene recurrence
score of 26–30.
| Post-assay | Pre-assay | Actual chemo
usage |
|
|---|
|
|
|
|
|---|
| Decision | N (%) | Chemo | Non-chemo | Chemo | Non-chemo | Adherence to MDT
decision |
|---|
| Chemo | 146 (73.0) | 94 | 52 | 138 | 8 | 190/200=95.0% |
| Non-chemo | 54 (27.0) | 2 | 52 | 2 | 52 |
|
Regarding the actual adjuvant treatment usage, 10
patients did not follow the treatment recommendation; therefore,
the rate of adherence to MDT recommendations was 95.0% (190/200;
Table VI). A total of 146 patients
received a recommendation for adjuvant chemotherapy after the RS
assay; however, only 138 (94.5%) patients received chemotherapy.
The eight patients who did not adhere to the MDT recommendations
displayed tumors with high ER expression and low Ki-67 index, which
were primarily T1-stage and grade I/II tumors (Table SVII).
Adjuvant chemotherapy usage and
disease outcomes
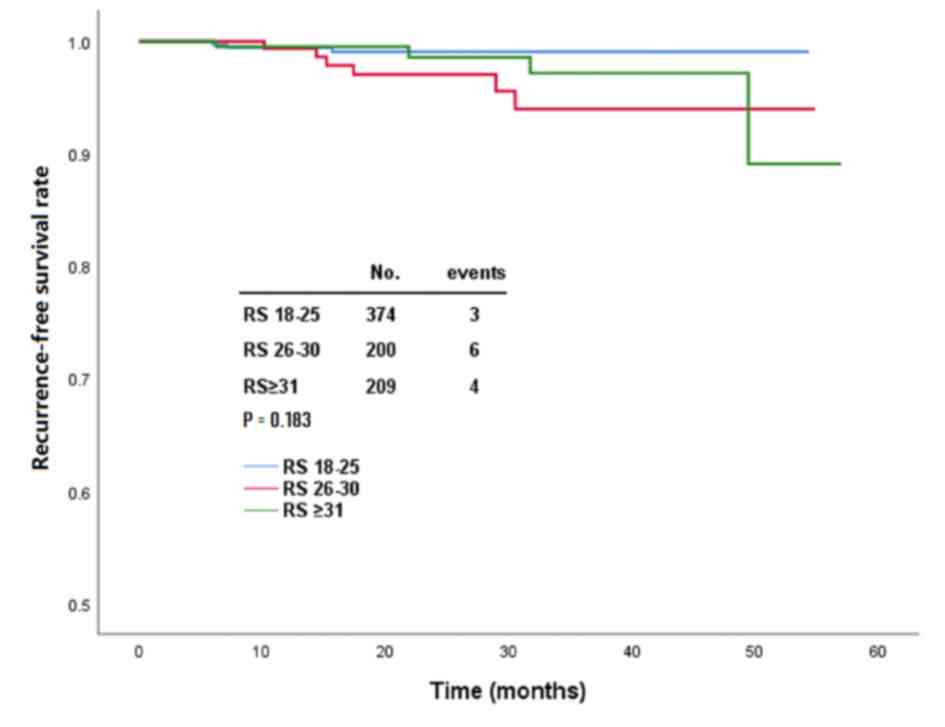
After a median follow-up of 21.5 months (range, 2–54
months), 13 (1.7%) patients experienced disease recurrence,
including 6 locoregional, 4 distant and 3 contralateral breast
recurrences. In the RS=18-25, =26−30 and ≥31 groups, there were 3,
6 and 4 patients, respectively (Table
SVIII), with disease recurrence (P=0.183; Fig. 4). Pairwise comparison indicated that
there were no significantly different clinical outcomes between
each pair of groups (data not shown). In the RS=26−30 group, 4
patients with disease relapse received chemotherapy, and there was
no significant difference compared with patients not receiving
chemotherapy (2 patients; P=0.764; Table SVIII).
Discussion
The present study included 783 patients with
HR+/HER2−/lymph node− breast
cancer with RS ≥18, and indicated that patients with RS=26−30
displayed higher PR expression and lower Ki-67 index compared with
patients with RS ≥31. Multivariate analysis suggested that age ≤50
years and luminal B-like tumors were independently associated with
chemotherapy usage in the RS=26−30 group. After 21-gene RS testing
and MDT discussion, the chemotherapy usage in patients with
RS=26−30 increased, and a high adjuvant chemotherapy compliance
rate of 95.0% was achieved. No survival difference was observed
between patients receiving chemotherapy and patients not receiving
chemotherapy in the RS=26−30 group, who displayed good prognoses
after a short follow-up period.
Routine clinicopathological factors associated with
RS have been widely studied, including age, tumor grade, PR
expression, Ki-67 level and luminal subtype. Patients with high
tumor grade (16,18,19) or
low PR expression (16,19) are associated with high-risk RS. The
similarities and differences between the RS=26−30 and RS ≥31 groups
have attracted increased attention, which may guide further
adjuvant chemotherapy selection (20). Park et al (20) reported that, compared with patients
with RS=18-25, patients with RS=26−30 displayed more aggressive
tumor characteristics. The present study suggested that patients in
the RS=26−30 group displayed higher PR expression (OR, 2.84) and
lower Ki-67 index (OR, 1.88) compared with those of patients in the
RS ≥31 group. Moreover, there was no significant difference between
the RS=18−25 and =26−30 groups, indicating that patients with
RS=26−30 may display similar biological behavior to patients with
RS=18-25, and cannot be managed in the same way as patients in the
RS ≥31 group.
RS has been reported to be the most important
independent factor associated with adjuvant chemotherapy usage in
patients with HR+/HER2−/node−
breast cancer (21). The usage of
the 21-gene RS testing has significantly reduced chemotherapy
administration (22,23). Based on the standard RS risk
classification (5), the adjuvant
usage rates are 4–7, 30–40 and >80% in patients with low-,
intermediate- and high-risk RS, respectively (24,25). In
the present study, the rates of chemotherapy were 30.7, 70.0 and
89.0% in the RS=18-25, =26−30 and ≥31 groups, respectively. In
patients with RS ≥18, RS displayed the highest adjusting OR value
in adjuvant chemotherapy selection (7.20 for RS=26−30 and 16.08 for
RS ≥31 vs. patients in the RS=18−25 group) compared with the OR
values of other clinicopathological parameters; this could reflect
the importance of the 21-gene RS assay over routine clinical
parameters. In the TAILORx trial, patients with RS=26−30 were
typically categorized into the intermediate-risk RS group, but were
recommended chemotherapy, which may have resulted in the high rate
of chemotherapy usage in these patients (10).
According to the NCCN guideline, adjuvant endocrine
therapy and adjuvant chemotherapy followed by endocrine therapy can
be considered for patients in the RS=26−30 group (9). Park et al (20) reported that, in the RS=26−30 group,
patients who were younger and displayed grade-III tumors could gain
survival benefit from adjuvant chemotherapy. Tsai et al
(26) reported that the 70-gene
signature could guide adjuvant chemotherapy in patients with
RS=18-30. Moreover, a previous study indicated that a nomogram
based on routine clinicopathological factors could also predict the
probability of chemotherapy recommendation (27). The present study conducted a
univariate analysis, which indicated that age, menstrual status,
comorbidity, tumor size, histological type, tumor grade, Ki-67
index and luminal subtype were associated with chemotherapy usage
in patients with RS 26–30, whereas only age and luminal subtype
remained significant in the multivariate analysis. The TAILORx
trial observed that patients aged ≤50 years with RS=16−25 could
benefit from chemotherapy (12).
Williams et al (28) reported
that patients aged <50 years were more likely to receive
adjuvant chemotherapy compared with those aged >50 years,
regardless of their RS. The Danish Breast Cancer Cooperative
Group-77B clinical trial demonstrated that patients with luminal
A-like breast cancer did not benefit from adjuvant chemotherapy
(hazard ratio=1.06; P=0.86) (29).
Luminal subtype was included in the nomogram model construction
that could predict the usage of adjuvant chemotherapy in patients
with RS=18−30 (27). The results of
the present study suggested that chemotherapy usage was more common
in patients aged ≤50 years vs. >50 years, or with luminal B-like
vs. luminal A-like tumors in the RS=26−30 group. The effect of
clinicopathological parameters on treatment decision had also been
confirmed by the updated results of the TAILORx trial, which
demonstrated that RS combined with clinical-risk stratification
helped to optimize treatment selection (30).
Previous studies reported that the adjuvant
chemotherapy recommendation in ~30% of cases, irrespective of RS
risk stratification, would be modified after the 21-gene assay
(23,25,31). In
the present study, the treatment recommendation of 54 (27.0%)
patients was altered once an RS of 26–30 was identified; among
them, 52 patients changed from being chemotherapy not recommended
prior to multigene testing to chemotherapy recommended afterwards.
A possible explanation may be that RS=26−30 is close to high-risk
RS (≥31), and therefore, physicians considered the patients to be
at risk in the present study. Furthermore, a high compliance rate
of 95.0% (190/200 patients) was achieved in the cohort of patients
included in the present study after 21-gene RS testing and MDT
discussion. Furthermore, the acceptance rate in the post-RS assay
chemotherapy-recommended cohort was 94.5% (138/146). Kuchel et
al (32) also reported that
patient decision conflicts decreased after 21-gene RS testing.
Additionally, for patients receiving 70-gene testing, a high
compliance rate was reported (91%) in terms of adjuvant
chemotherapy application (33).
The results from the Surveillance, Epidemiology and
End Results (SEER) database revealed a 5-year breast
cancer-specific mortality of 2.4 and 4.4% in RS=25−30 and >30
cohorts, respectively (34). The
TAILORx study indicated that, for patients with RS=11-25, =26−30
and ≥31 who received chemotherapy, the estimated rate of invasive
disease-free survival was 97.0, 90.5 and 78.0% at 5 years, and
92.9, 86.3 and 74.8% at 9 years, respectively (13). There was no significant survival
difference between the three RS groups within the short follow-up
period of the present study. However, the SEER data demonstrated
that patients with RS=26−30 had inferior breast cancer-specific
survival (BCSS; hazard ratio=1.81) and overall survival (OS; hazard
ratio=1.37) compared with those of patients with RS=18−25 (20). When the survival outcome was analyzed
according to chemotherapy usage, no benefit from chemotherapy was
observed in patients with RS=26−30 in the present study, which was
similar to the result reported for the Israeli population (35). Nevertheless, data from the National
Cancer Database indicated that there was a 1.8% absolute decrease
in the 5-year mortality risk by chemotherapy in the RS=26−30
population with lymph node-negative disease (hazard ratio=0.68;
P=0.029) (36). SEER data also
suggested that adjuvant chemotherapy was associated with a
decreased risk in BCSS (hazard ratio=0.68) and OS (hazard
ratio=0.58) (20). The relatively
short follow-up time and the small number of recurrence events in
the present study may have underestimated not only the long-term
real survival difference in different RS stratifications, but also
the influence of adjuvant chemotherapy in patients with
RS=26−30.
The present study was designed to evaluate the role
of 21-gene RS testing in patients with RS 26–30, for whom adjuvant
treatment has been unanimous until now. Additionally, the treatment
decision change due to 21-gene RS testing was evaluated in these
patients, which has been scarcely investigated in previous
literature. However, the present study had a number of limitations.
Firstly, the follow-up period was short; therefore, although the
survival benefits due to chemotherapy were greater in the early
years and the prognostic effect of the 21-gene RS assay was more
robust in the short term, further follow-up is required. Secondly,
the similarities and differences in regard to molecular biological
features of the RS=26−30 and other risk groups remain unknown. The
expression levels of every single gene in the 21-gene RS panel need
to be further compared across the three RS groups. Finally, the
retrospective design was subjected to confounding factors, and
although multivariate analysis was used to eliminate the
confounding effect, selection bias may still exist; therefore, the
results require cautious interpretation and further validation.
In conclusion, the present study suggested that PR
expression was higher and Ki-67 index was lower in the RS=26−30
group compared with those in the RS ≥31 group, and there was no
significant clinicopathological difference between the RS=18−25 and
=26−30 groups. For patients with RS=26−30, age ≤50 years and
luminal B subtype were independently associated with increased
chemotherapy usage. The results suggested that the 21-gene RS
testing could influence chemotherapy administration and improve the
adherence rate of adjuvant treatment. The short follow-up period
demonstrated that patients with RS=26−30 displayed promising
disease outcomes, and may receive little benefit from adjuvant
chemotherapy; however, further evaluation is required.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Ms. Yidong Du
(Comprehensive Breast Health Center, Ruijin Hospital, Shanghai
Jiaotong University School of Medicine) for her contribution during
the follow-up and Dr Min Jin (Institute of Health Science Shanghai
Institutes of Biological Sciences, Chinese Academy of Sciences) for
her help with statistical analysis.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81772797), the
Shanghai Municipal Education Commission-Gaofeng Clinical Medicine
Grant Support (grant no. 20172007) and the Shanghai Jiao Tong
University School of Medicine-‘Guangci Excellent Youth Training
Program’ (grant no. GCQN-2017-A18).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JY, XC and KS conceived the study. JY performed the
data analysis. JY wrote the manuscript. XC reviewed and edited the
manuscript. KS revised the manuscript critically for important
intellectual content. JW, OH, JH, LZ, WC and YL collected and
interpreted the data. KS and XC acquired the funding. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
All procedures were performed in accordance with the
ethical standards of the Ethical Committees of Ruijin Hospital and
the Declaration of Helsinki of 1964. The present study was reviewed
and approved by the Ethical Committee of Ruijin Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Early Breast Cancer Trialists'
Collaborative Group (EBCTCG), . Peto R, Davies C, Godwin J, Gray R,
Pan HC, Clarke M, Cutter D, Darby S, McGale P, et al: Comparisons
between different polychemotherapy regimens for early breast
cancer: Meta-analyses of long-term outcome among 100,000 women in
123 randomised trials. Lancet. 379:432–444. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Early Breast Cancer Trialists'
Collaborative Group (EBCTCG), . Effects of chemotherapy and
hormonal therapy for early breast cancer on recurrence and 15-year
survival: An overview of the randomised trials. Lancet.
365:1687–1717. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kwa M, Makris A and Esteva FJ: Clinical
utility of gene-expression signatures in early stage breast cancer.
Nat Rev Clin Oncol. 14:595–610. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Paik S, Shak S, Tang G, Kim C, Baker J,
Cronin M, Baehner FL, Walker MG, Watson D, Park T, et al: A
multigene assay to predict recurrence of tamoxifen-treated,
node-negative breast cancer. N Engl J Med. 351:2817–2826. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Paik S, Tang G, Shak S, Kim C, Baker J,
Kim W, Cronin M, Baehner FL, Watson D, Bryant J, et al: Gene
expression and benefit of chemotherapy in women with node-negative,
estrogen receptor-positive breast cancer. J Clin Oncol.
24:3726–3734. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Goldstein LJ, Gray R, Badve S, Childs BH,
Yoshizawa C, Rowley S, Shak S, Baehner FL, Ravdin PM, Davidson NE,
et al: Prognostic utility of the 21-gene assay in hormone
receptor-positive operable breast cancer compared with classical
clinicopathologic features. J Clin Oncol. 26:4063–4071. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Albain KS, Barlow WE, Shak S, Hortobagyi
GN, Livingston RB, Yeh IT, Ravdin P, Bugarini R, Baehner FL,
Davidson NE, et al: Prognostic and predictive value of the 21-gene
recurrence score assay in postmenopausal women with node-positive,
oestrogen-receptor-positive breast cancer on chemotherapy: A
retrospective analysis of a randomised trial. Lancet Oncol.
11:55–65. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Goetz MP, Gradishar WJ, Anderson BO,
Abraham J, Aft R, Allison KH, Blair SL, Burstein HJ, Dang C, Elias
AD, et al: NCCN guidelines insights: Breast cancer, version 3.2018.
J Natl Compr Cancer Netw. 17:118–126. 2019. View Article : Google Scholar
|
|
10
|
Sparano JA, Gray RJ, Makower DF, Pritchard
KI, Albain KS, Hayes DF, Geyer CE Jr, Dees EC, Perez EA, Olson JA
Jr, et al: Prospective validation of a 21-gene expression assay in
breast cancer. N Engl J Med. 373:2005–2014. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gluz O, Nitz UA, Christgen M, Kates RE,
Shak S, Clemens M, Kraemer S, Aktas B, Kuemmel S, Reimer T, et al:
West German study group phase III planB trial: First prospective
outcome data for the 21-gene recurrence score assay and concordance
of prognostic markers by central and local pathology assessment. J
Clin Oncol. 34:2341–2349. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sparano JA, Gray RJ, Makower DF, Pritchard
KI, Albain KS, Hayes DF, Geyer CE Jr, Dees EC, Goetz MP, Olson JA
Jr, et al: Adjuvant chemotherapy guided by a 21-gene expression
assay in breast cancer. N Engl J Med. 379:111–121. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sparano JA, Gray RJ, Makower DF, Albain
KS, Saphner TJ, Badve SS, Wagner LI, Kaklamani VG, Keane MM, Gomez
HL, et al: Clinical outcomes in early breast cancer with a high
21-gene recurrence score of 26 to 100 assigned to adjuvant
chemotherapy plus endocrine therapy: A secondary analysis of the
TAILORx randomized clinical trial. JAMA Oncol. 2019:(Epub ahead of
print).
|
|
14
|
Lakhani SR, Ellis IO, Schnitt SJ, Tan PH
and van de Vijver MJ: WHO classification of tumours of the breast.
4th. 4. IARC WHO Classification of Tumours; 2012
|
|
15
|
Goldhirsch A, Winer EP, Coates AS, Gelber
RD, Piccart-Gebhart M, Thürlimann B and Senn HJ; Panel members, :
Personalizing the treatment of women with early breast cancer:
Highlights of the St Gallen international expert consensus on the
primary therapy of early breast cancer 2013. Ann Oncol.
24:2206–2223. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu J, Fang Y, Lin L, Fei X, Gao W, Zhu S,
Zong Y, Chen X, Huang O, He J, et al: Distribution patterns of
21-gene recurrence score in 980 Chinese estrogen receptor-positive,
HER2-negative early breast cancer patients. Oncotarget.
8:38706–38716. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Agarwal GG: Statistics for
surgeons-understanding survival analysis. Indian J Surg Oncol.
3:208–214. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Singh K, He X, Kalife ET, Ehdaivand S,
Wang Y and Sung CJ: Relationship of histologic grade and histologic
subtype with oncotype Dx recurrence score; retrospective review of
863 breast cancer oncotype Dx results. Breast Cancer Res Treat.
168:29–34. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang JL, Kizy S, Marmor S, Altman A,
Blaes A, Beckwith H, Tuttle TM and Hui JYC: Tumor grade and
progesterone receptor status predict 21-gene recurrence score in
early stage invasive breast carcinoma. Breast Cancer Res Treat.
172:671–677. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Park S, Han Y, Liu Y, Toriola AT, Peterson
LL, Colditz GA, Kim SI, Cho YU, Park BW and Park Y: Adjuvant
chemotherapy and survival among patients 70 years of age and
younger with node-negative breast cancer and the 21-gene recurrence
score of 26–30. Breast Cancer Res. 21:1102019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jasem J, Amini A, Rabinovitch R, Borges
VF, Elias A, Fisher CM and Kabos P: 21-Gene recurrence score assay
as a predictor of adjuvant chemotherapy administration for
early-stage breast cancer: An analysis of use, therapeutic
implications, and disparity profile. J Clin Oncol. 34:1995–2002.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Parsons BM, Landercasper J, Smith AL, Go
RS, Borgert AJ and Dietrich LL: 21-Gene recurrence score decreases
receipt of chemotherapy in ER + early-stage breast cancer: An
analysis of the NCDB 2010–2013. Breast Cancer Res Treat.
159:315–326. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Albanell J, Svedman C, Gligorov J, Holt
SD, Bertelli G, Blohmer JU, Rouzier R, Lluch A and Eiermann W:
Pooled analysis of prospective European studies assessing the
impact of using the 21-gene recurrence score assay on clinical
decision making in women with oestrogen receptor-positive, human
epidermal growth factor receptor 2-negative early-stage breast
cancer. Eur J Cancer. 66:104–113. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Orucevic A, Heidel RE and Bell JL:
Utilization and impact of 21-gene recurrence score assay for breast
cancer in clinical practice across the United States: Lessons
learned from the 2010 to 2012 national cancer data base analysis.
Breast Cancer Res Treat. 157:427–435. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Carlson JJ and Roth JA: The impact of the
oncotype Dx breast cancer assay in clinical practice: A systematic
review and meta-analysis. Breast Cancer Res Treat. 141:13–22. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tsai M, Lo S, Audeh W, Qamar R, Budway R,
Levine E, Whitworth P, Mavromatis B, Zon R, Oldham D, et al:
Association of 70-gene signature assay findings with physicians'
treatment guidance for patients with early breast cancer classified
as intermediate risk by the 21-gene assay. JAMA Oncol.
4:e1734702018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Qu F, Chen X, Fei X, Lin L, Gao W, Zong Y,
Wu J, Huang O, He J, Zhu L, et al: A nomogram to predict adjuvant
chemotherapy recommendation in breast cancer patients with
intermediate recurrence score. Chin J Cancer Res. 30:222–230. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Williams AD, Reyes SA, Arlow RL, Tchou J
and De La Cruz LM: Is age trumping genetic profiling in clinical
practice? relationship of chemotherapy recommendation and oncotype
DX recurrence score in patients aged <50 years versus >/=50
years, and trends over time. Ann Surg Oncol. 25:2875–2883. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nielsen TO, Jensen MB, Burugu S, Gao D,
Jørgensen CL, Balslev E and Ejlertsen B: High-risk premenopausal
luminal a breast cancer patients derive no benefit from adjuvant
cyclophosphamide-based chemotherapy: Results from the DBCG77B
clinical trial. Clin Cancer Res. 23:946–953. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sparano JA, Gray RJ, Ravdin PM, Makower
DF, Pritchard KI, Albain KS, Hayes DF, Geyer CE Jr, Dees EC, Goetz
MP, et al: Clinical and genomic risk to guide the use of adjuvant
therapy for breast cancer. N Engl J Med. 380:2395–2405. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Levine MN, Julian JA, Bedard PL, Eisen A,
Trudeau ME, Higgins B, Bordeleau L and Pritchard KI: Prospective
evaluation of the 21-gene recurrence score assay for breast cancer
decision-making in ontario. J Clin Oncol. 34:1065–1071. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kuchel A, Robinson T, Comins C, Shere M,
Varughese M, Sparrow G, Sahu A, Saunders L, Bahl A, Cawthorn SJ and
Braybrooke JP: The impact of the 21-gene assay on adjuvant
treatment decisions in oestrogen receptor-positive early breast
cancer: A prospective study. Br J Cancer. 114:731–736. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kuijer A, Straver M, den Dekker B, van
Bommel ACM, Elias SG, Smorenburg CH, Wesseling J, Linn SC, Rutgers
EJT, Siesling S and van Dalen T: Impact of 70-gene signature use on
adjuvant chemotherapy decisions in patients with estrogen
receptor-positive early breast cancer: Results of a prospective
cohort study. J Clin Oncol. 35:2814–2819. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Petkov VI, Miller DP, Howlader N, Gliner
N, Howe W, Schussler N, Cronin K, Baehner FL, Cress R, Deapen D, et
al: Breast-cancer-specific mortality in patients treated based on
the 21-gene assay: A SEER population-based study. NPJ Breast
Cancer. 2:160172016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Stemmer SM, Steiner M, Rizel S,
Soussan-Gutman L, Ben-Baruch N, Bareket-Samish A, Geffen DB,
Nisenbaum B, Isaacs K, Fried G, et al: Clinical outcomes in
patients with node-negative breast cancer treated based on the
recurrence score results: Evidence from a large prospectively
designed registry. NPJ Breast Cancer. 3:332017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ibraheem AF, Press DJ, Olopade OI and Huo
D: Community clinical practice patterns and mortality in patients
with intermediate oncotype DX recurrence scores: Who benefits from
chemotherapy? Cancer. 125:213–222. 2019. View Article : Google Scholar : PubMed/NCBI
|