Introduction
Cervical cancer is a malignant tumour of the cervix
that can be divided into two histological types, adenocarcinoma
(AC) and squamous cell carcinoma (SCC) (1). SCC is more common and has an occurrence
rate of 70% (2). AC originates from
glandular cells that line the cervical canal (the endocervix),
whereas SCC originates from squamous cells lining the outer part of
the cervix that opens to the ectocervix. The region in which the
squamous and the thin, flat glandular cells are located is termed
the transformation zone, and the majority of tumours originate from
this zone (3).
The most common cause for the occurrence of cervical
cancer is a persisting infection with the sexually transmitted
human papilloma virus (HPV) (4). HPV
is accountable for 90–100% of cervical cancer cases amongst women,
especially those <35 years old (5). The types of HPV can be classified as
either high-risk (HR) or low-risk in terms of their association
with precancerous, benign or cancer lesions (6). HR HPV 16 and 18 subtypes are the most
prevalent subtypes of HPV, which are responsible for 70% of
cervical cancer cases (1,4). In addition, previous studies have
identified an association between the HPV 16 and 18 subtypes and
malignant tumours of the penis, vulva and anus (1,7).
Globally, cervical cancer is the fourth most
commonly diagnosed cancer amongst women, and it is especially
common in low- and middle-income countries (LMICs) such as South
Africa (SA), India, China and Brazil (8–10). A
total of ~569,000 new cases of cervical cancer and 311,000 deaths
linked to cervical cancer were reported worldwide in 2018 (1,8). In
total, 84% of the new cases and between 87 and 90% of the deaths
occur in LMICs (1,8). However, HPV infections and associated
malignancies are also common in regions of high socioeconomic
status. In 2008, 80 million individuals were estimated to be
infected with HPV in the USA (11).
Despite this, thousands more women from LMICs die prematurely from
cervical cancer compared with women from developed countries
(2,12).
The aim of the current review was to discuss the
types of genetic and epigenetic factors as well as socio-economic
factors that influence the occurrence, progression or suppression
of cervical cancer. These factors may assist in identifying
potential prognostic and diagnostic tools (e.g. biomarkers) for the
treatment of cervical cancer.
Cervical cancer incidence in low- to
middle-income countries
In 2010, Bruni et al (5) reported a 4-fold higher prevalence of
cervical cancer in LMICs compared with that in developed countries.
In countries ranked low in the Human Development Index, cervical
cancer is ranked as the second most common type of cancer and the
second highest cause of cancer-related mortality amongst women
after breast cancer. In Africa, cervical cancer is the most
commonly diagnosed type of cancer and the leading cause of
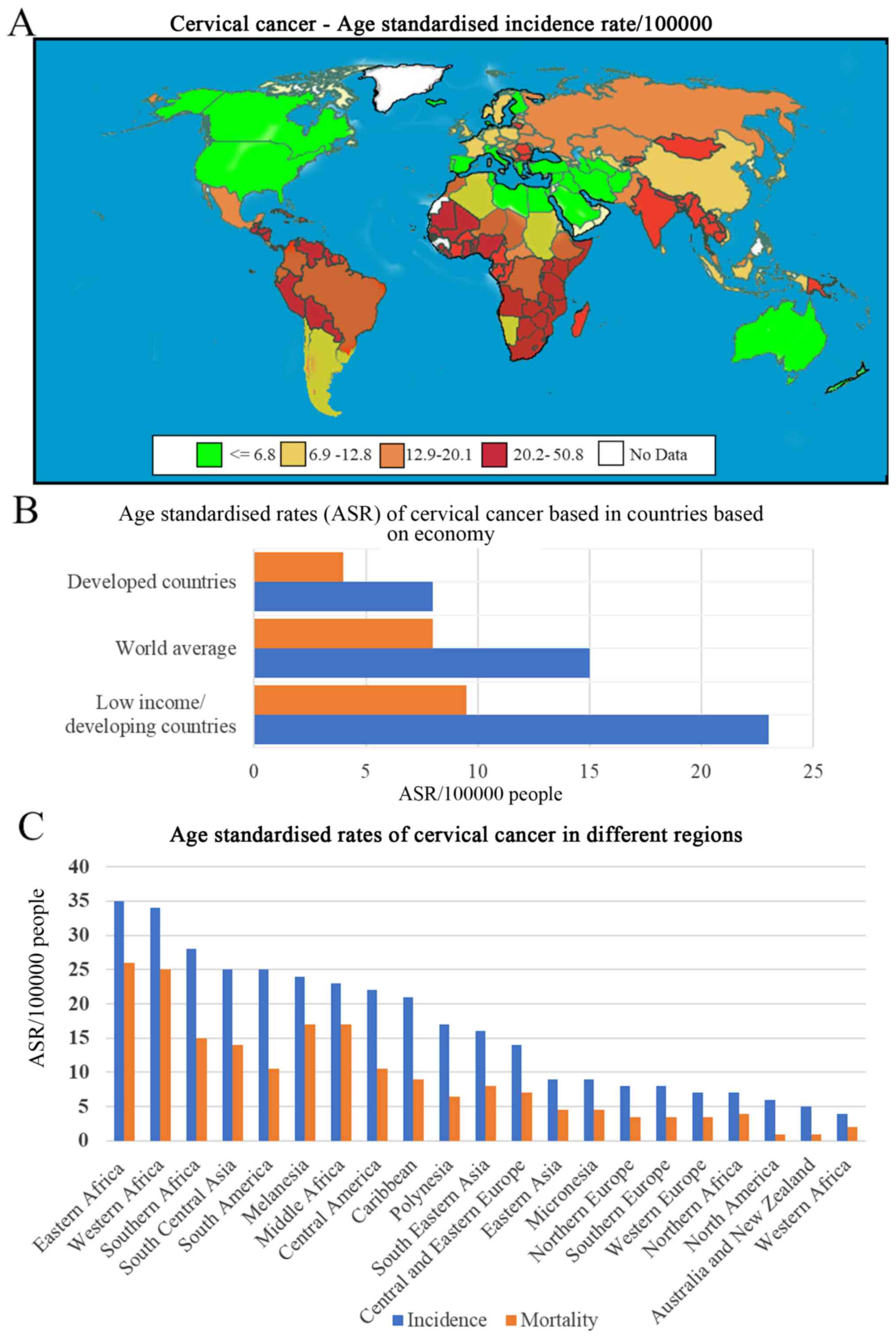
cancer-related death among women (13). Table I
and Fig. 1 (14–16)
present data from the GLOBOCAN 2018 report (15) and show the rate of cervical cancer in
LMICs compared with that in developed countries. In Sub-Saharan
Africa, the highest incidence of cervical cancer since 2012 was
observed in women aged between 15 and 44 years (15,17,18).
 | Table I.Region-specific incidence and
mortality ASRs for cancers of the cervix in 2018. |
Table I.
Region-specific incidence and
mortality ASRs for cancers of the cervix in 2018.
| Region | Incidence | Mortality |
|---|
| Southern
Africa | 43.1 | 20.0 |
| Eastern Africa | 40.1 | 30.0 |
| Western Africa | 29.6 | 23.0 |
| Melanesia | 27.7 | 19.0 |
| Middle Africa | 26.8 | 21.1 |
| South-Eastern
Asia | 17.2 | 10.0 |
| Eastern Europe | 16.0 | 6.1 |
| Caribbean | 15.5 | 8.5 |
| South America | 15.2 | 7.1 |
|
Micronesia/Polynesia | 14.2 | 6.3 |
| Central
America | 13.0 | 7.0 |
| South Central
Asia | 13.0 | 8.2 |
| Eastern Asia | 10.9 | 4.1 |
| Northern
Europe | 9.5 | 2.1 |
| Southern
Europe | 7.8 | 2.2 |
| Northern
Africa | 7.2 | 5.1 |
| Western Europe | 6.8 | 2.1 |
| Northern
America | 6.4 | 1.9 |
| Australia/New
Zealand | 6.0 | 1.7 |
| West Asia | 4.1 | 2.5 |
Gonidia and Sartorius (18) used three mathematical models to
predict the prevalence of HPV in Swaziland by applying age-specific
cervical cancer incidence rates from GLOBOCAN 2012 to the Swazi
female population in 2014 (13,18,19). The
results revealed that the published incidence rates were a gross
underestimation of the actual number of cervical cancer cases
(18). Another model was used to
regress age-standardised cervical cancer incidence (20) in Sub-Saharan African countries
against HR HPV prevalence among women with normal cervical cytology
(21). The model estimated that,
among women with normal cervical cytology, the age-standardised
cervical cancer incidence rate was 62.6 per 100,000 women; the
prevalence of HPV-HIV among women with normal cytology exhibited a
higher estimated age-standardised incidence of 101.1 per 100,000
women (21). South Africa has the
highest age-standardised incidence of cervical cancer globally,
with 32 cases per 100,000 women (22), whereas Paraguay (a middle-income
country) has the highest incidence (34.2 per 100,000 women) and a
mortality rate of 15.7 per 100,000 women (21,23).
These results indicated that women infected with HIV may be at a
higher risk of developing HPV infection, pre-invasive cervical
disease and invasive cervical cancer (ICC) (18,24,25).
Currently, South Africa has the highest incidence of HIV infection
worldwide, with ~7.2 million cases in a population of 58 million
(26); an estimated 13 million women
are infected with HIV in sub-Saharan Africa (25,27). The
treatment of cervical cancer in women infected with HIV presents
with great difficulties. For example HIV infection increases the
likelihood of the patient having a persistent HPV infection
resulting in cervical abnormalities and cervical cancer (7). A compromised immune system and
increased risk of HPV infection lowers the number of individuals
that have been successfully treated in this population (25). The cancer burden in developing
countries such as Swaziland is increased due to late diagnosis,
advanced stages of HIV and HPV infections when the cancer is
diagnosed, lack or inaccessibility of treatment, lack of treatment
facilities, and logistic and cultural obstacles to treatment
(28), which result in poor
prognosis (14).
These factors, which are common to the majority of
developing countries, resulted in the identification of an
association between cervical cancer and the level of development
(i.e. the socioeconomic status) of a country and its individuals.
Cervical cancer is significantly associated with the geographical
area; the highest incidence rates are prevalent in low-income
countries (10). The incidence in
2013 was 24% in sub-Saharan Africa, 21% in Eastern Europe and 16%
in Latin America (29). A prevalence
of >30% was also reported in Eastern Africa and the Caribbean
(30). Southern Africa has the
highest prevalence of HIV (19.9% out of a population of
66,401,000), and the second highest cervical cancer incidence
(18.4% out of a total population of 668,319 women with cancer)
after Asia and the Pacific (53.1%) (27). This suggested that Southern Africa
might have a leading number of HIV-associated cervical cancer
cases.
Countries representing specific
areas
As previously stated, areas where cervical cancer is
the leading cause of death amongst women include Sub-Saharan
Africa, Melanesia (Western Pacific), South Central and South East
Asia, the Caribbean and Latin America (31). In wealthier developed countries, it
is recommended that screening for cervical cancer with the
Papanicolaou (Pap) smear is initiated at 21 years and repeated
every year until the patient is 65 years old (32). Pap smears should be performed in
conjunction with HPV screening; if the test reveals no signs of
cervical cancer but presence of HPV, genotyping of the HPV should
be performed, which may either be followed by a colposcopy, or HPV
and cytology must be repeated at a later stage (33). Table
II presents the age-standardised rate (ASR) which is the number
per 100,000 people diagnosed with cervical cancer in the four
countries, which will be discussed below. These four LMICs (South
Africa, Tanzania, India and Brazil) are also compared against China
Despite its large number of new cervical cancer cases, cervical
cancer only comprises 2.5% of all cancer cases (15).
 | Table II.Cervical cancer statistics. |
Table II.
Cervical cancer statistics.
| Country | New cases
(no.) | All cancers
(%) | ASR per 100,000
women | (Refs.) |
|---|
| South Africa | 5,735 | 15.17 | 22.56 | (31) |
| Brazil | 16,370 | 8.1 | 12.2 | (32) |
| India | 96,922 | 17 | 14.7 | (26) |
| Tanzania | 9,772 | 37,9 | 59.1 | (26) |
| China | 106,430 | 2.5 | 10.7 | (26) |
South Africa
The United Nations defines South Africa as an upper
middle-income country with unequal distribution of wealth and a
high rate of poverty (34). The 2014
South African National Cancer Registry statistics (35) for cervical cancer cases and deaths
reported that the number of new observed cases in South African
women was 5,735 in 2014, accounting for 16.17% of all cancers
diagnosed in South African women; the ASR was 22.56 per 100,000
women (35). Analysis of the ASR and
new cases by ethnic group revealed that the black population was
more affected than any other group (Table III). The ‘white’ and ‘other’
population groups, which includes those of mixed ancestry, had
<10% of the number of cases and an ASR <50% compared with the
black population. The Asian population had the lowest number of
cases and the lowest ASR. This population group is also the most
economically disadvantaged and has poor access to healthcare
(36). Since South Africa has the
highest infection rate of HIV and the largest anti-retroviral
program in the world (37), it is
important to consider the influence of these two factors on
cervical cancer. The total levels of HIV-related cancers have
decreased following the introduction of anti-retroviral therapy;
despite this, the mortality due to Acquired Immune Deficiency
Syndrome (AIDS)-related illnesses is still high and has contributed
to a lower life expectancy in South Africa (38). HIV is associated with abnormal
cervical cytology and higher levels of HPV or numerous HPV types
(34). The high prevalence of HIV in
South Africa was predicted to lead to a marked increase in the
incidence of cervical cancer, as it is one of the AIDS-defining
illnesses, but such an increase has not occurred. Initially, prior
to the introduction of anti-retroviral treatment, this was due to
women succumbing to HIV/AIDS before they could develop cervical
cancer; following the introduction of retroviral therapy, a
decrease in the rates of all AIDS-defining cancers affected the
numbers of cervical cancer cases (36).
 | Table III.Cervical cancer statistics in
different population groups in South Africa (35). |
Table III.
Cervical cancer statistics in
different population groups in South Africa (35).
| Group | New cases
(no.) | All cancers
(%) | ASR per 100,000
women |
|---|
| Asian | 81 |
6.89 |
9.98 |
| Black | 4,870 | 30.46 | 27.01 |
| White | 407 |
3.45 | 13.10 |
| Other | 340 |
8.36 | 13.72 |
The screening policy for cervical cancer in South
Africa was established in 2000 and involves three Pap smears per
lifetime starting at 30 years of age and occurring at 10-year
intervals. The smear is repeated after 12 months if any abnormality
is observed. If the abnormality persists or if a high-grade lesion
is identified, the patient is referred for a colposcopy (39).
HPV is common among young, sexually active
individuals; the infection normally clears without treatment, but
recurrence is also common. Persistent infection with HR HPV
genotypes leads to precancerous lesions and ultimately cervical
carcinoma (39). Due to the high
prevalence of transient HPV infection in young women among certain
population groups, the usefulness of HPV screening as a tool for
identifying those at risk of cervical cancer is limited (34). One preventive strategy for HPV
infection used in South Africa is the introduction of the HPV
vaccine. In 2014, a national school-based program for the HPV
bivalent vaccine was introduced in all public schools, targeting
girls in grade 4 (aged ≥9 years old) with a two-dose (6 months
apart) schedule (40). Although a
high vaccination rate was achieved in these school based programs,
the effect on HPV infection rates is yet to be elucidated.
Tanzania
Cervical cancer accounts for 40% of all cancer cases
diagnosed in women in East Africa, with the highest ASR (50.6 per
100,000 women) in Tanzania. Tanzania also ranks 13th on
prevalence of HIV infection worldwide (15). Since women infected with HIV are at
an increased risk of developing cervical cancer, it is important
that they are screened early and often (23,41). To
combat cervical cancer, Tanzania has introduced a ‘screen and
treat’ policy; the most common form of screening is visual
inspection of the cervix with the naked eye following the
application of acetic acid, abbreviated as visual inspection with
acetic acid (VIA) (42). The acetic
acid solution swabbed on the surface of the cervix (cervical
epithelium) turns pre-cancerous lesions white (43). Following a positive diagnosis, the
lesions are immediately treated by cryotherapy (41). Similarly to other low-income,
developing countries, the rates of HPV infection are high in
Tanzania; this has led to the Tanzanian Ministry of Health to
initiate a school-based HPV vaccination program (44). Other factors that contribute to the
high incidence of cervical cancer in Tanzania include; resource
allocation (in term of distribution of trained personnel, funds and
equipment in urban and rural areas nationwide), lack of resources
to fulfil the needs of the entire population, sociocultural
influence and a lack of political will (where leaders do not
prioritise healthcare programmes and choose to divert funds and
resources from screening and treatment programmes) (45). Sociocultural factors such as folklore
and myths around cervical cancer screening influence the
willingness of people to undergo screening (41).
India
In 2015, cervical cancer was the second most common
cancer amongst women in India; an estimated 132,314 new cases were
diagnosed and 73,337 patients succumbed to cervical cancer
(46). Due to India's large
population of 1.2 billion, this accounted for nearly one-fifth of
all cervical cancer cases worldwide (47). It is difficult to determine an
accurate ASR for India, as different cancer registries cover
different areas, and ASRs vary between 9 and 40 per 100,000 women,
depending on the region (47). India
spends 0.9% of its gross domestic product (GDP) on healthcare, and
no organised cervical cancer screening programs are available
(48). Risk factors associated with
cervical cancer in India include illiteracy, lack of toilets or
running water inside the house, not washing genitals after sexual
intercourse, age at first sexual intercourse <15 years, multiple
lifetime sexual partners, widowhood and HPV (49). The prevalence of HR HPV in India
ranges between 7 and 13%, and HPV 16 and 18 are the most common
types of HPV in India (50). This
varies by region and is also possibly influenced by culture;
however, unlike in developed countries, the number of young women
diagnosed with HPV is not higher than that of older women (47), suggesting that the infection is
equally distributed across all age groups.
HPV testing is cheap, objective and reproducible in
rural areas in India (50). However,
the use of HPV testing in a ‘screen and treat strategy’ can cause
problems, as the majority of HPV infections in young women do not
result in cervical cancer (47). HPV
cytology-based cervical cancer screening is not possible for the
entire population of India due to its large size, as well as a lack
of resources, trained staff and facilities. This has led to the
adoption of other low-cost, easy-to-perform screening methods; VIA
is a popular option. Variants of this method include VIA with
magnification or with Lugol's iodine. These tests have been
demonstrated to be sensitive but not specific (46). The HPV vaccine is not widely
available in India at present, mainly due to its cost. A study of
the willingness of parents to vaccinate their daughters was
performed in Eastern India and reported that, initially, only 40%
of parents agreed to vaccinate their children; this increased to
80% following a short education session about the vaccine (50). The most common reason stated for
refusing the vaccine was the safety of the vaccine, followed by the
perception of the vaccine as permission to engage in sexual
activity (51). Effective
vaccination against HPV 16 and 18 in India is expected to result in
a 75% decrease in the number of cervical cancer cases (47).
Brazil
The poorest regions of Latin America with the lowest
resources have high rates of cervical cancer, possibly due to
economic, social, educational and geographical factors that limit
access to cervical cancer screening (52). Brazil is one of the few countries
that have implemented an organised screening program (52). It is predicted that >8,079 women
die every year in Brazil from cervical cancer (15). HPV infections have been estimated to
affect >5.7% of women in Brazil and account for 68% of cervical
cancer cases (53). Brazil has a
long history of cervical cancer screening programs, the first of
which was initiated in 1956 (54).
The screening strategy for preventing cervical cancer in Brazil
targets women between 25 and 64 years of age. Pap smears are
recommended to be repeated every 3 years (55). However, similarly to other developing
nations, there is a shortage of resources, especially in trained
healthcare professionals, that limits the coverage of the
population using cytology-based pap-smear screening. The physical
size of the country is another factor limiting screening coverage,
which is further complicated by the large population, as there are
>64 million women aged >15 years in Brazil (55). To lower HPV infection rates and
control the rates of cervical cancer, the National Health System of
Brazil has adopted the quadrivalent HPV vaccine as part of the
national vaccination program; the vaccine was adopted in 2014 and
is offered to girls between 9 and 13 years of age, and to women
infected with HIV between the ages of 9 and 26 years.
Factors that contribute to the development
of cervical cancer
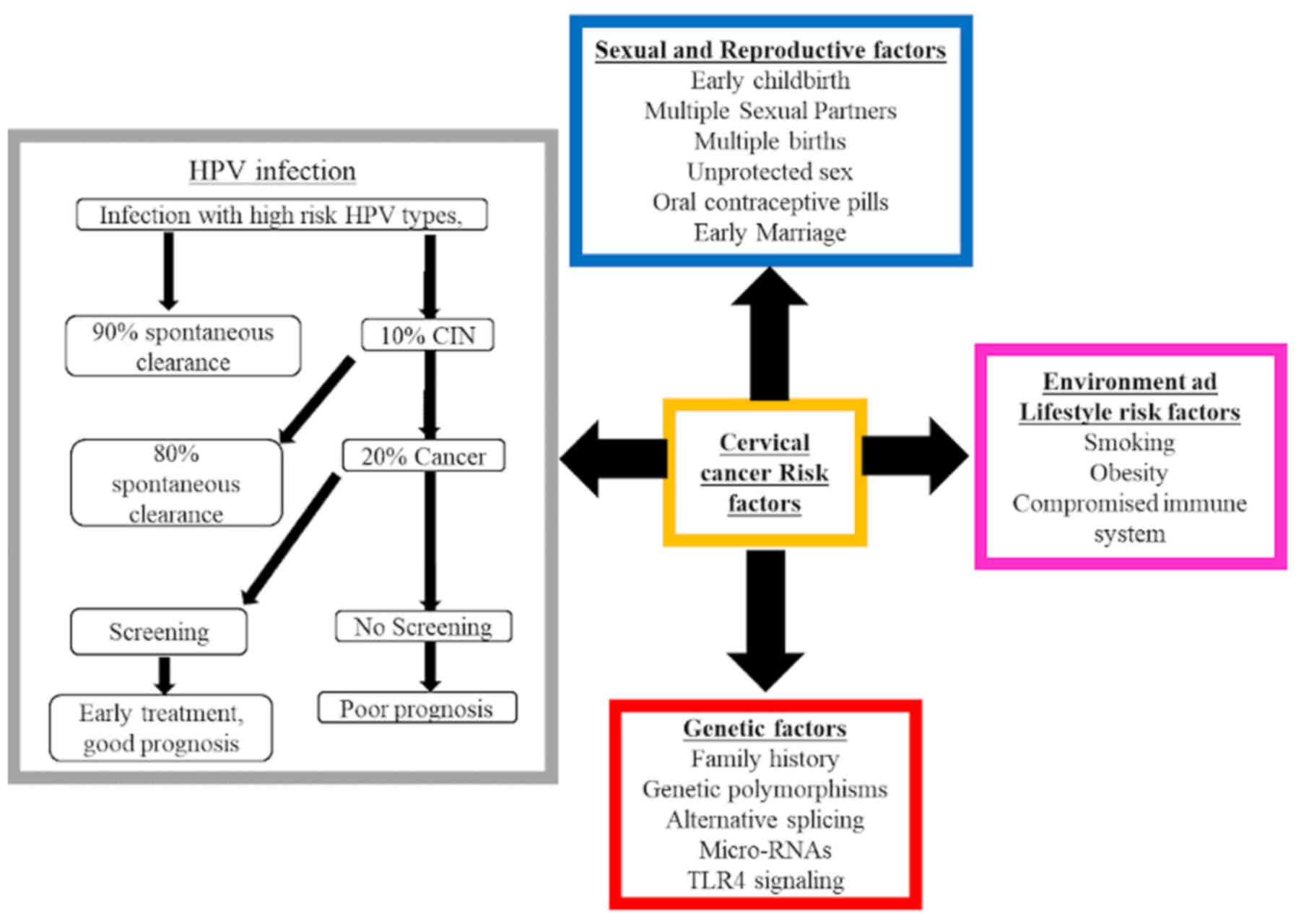
Epigenetic factors
Several factors other than persistent HPV infection
contribute to the development and/or progression of cervical
cancer, including smoking tobacco (including cigarettes, cigars,
pipes, hookah and shisha), high parity, continuous use of oral
hormonal contraceptives, promiscuity and co-infection with HIV
(Fig. 2) (1,16,56). Of
those, high parity and tobacco smoking are the most important
contributing factors, as they mediate HR HPV infection progression,
which results in high rates of cervical pre-cancer and cancer
(57–59). Chatzistamatiou et al (58) identified a significant association
between smoking and HR HPV DNA positivity, but not between smoking
and the viral HPV 16 E7 oncoprotein in HPV-associated
carcinogenesis (58). In addition,
the study displayed a higher odds ratio for smokers and ex-smokers
compared with that for non-smokers (58). In contrast to non-smokers, continuous
cigarette smokers have previously been reported to exhibit higher
risk of developing SCC; among women with an increased smoking
intensity, those who start smoking at a young age are at higher
risk of developing SCC but not AC (60,61).
However, Haverkos (62) observed no
significant association between cervical cancer and smoking.
Therefore, the influence of smoking on increasing the risk of
developing cervical cancer remains controversial. However, previous
studies have provided evidence of cigarette smoking derivatives
acting as causative agents of cervical and lung cancer (61,63).
Co-infection with Chlamydia trachomatis and
herpes simplex virus type-2, immunosuppression, and nutritional
challenges have also been demonstrated to contribute to cervical
cancer development and progression (1,16).
Several genetic and immunological host and viral factors are also
predicted as contributing factors (64). Goodson et al (65) identified >80 environmental
chemicals that serve roles in dysregulating host pathways in
carcinogenesis. Environmental risk factors for cervical cancer also
include compounds associated with cigarette smoke, such as coal tar
derivatives, tar-based vaginal sanitary products and smoke inhaled
from biomass-burning stoves (62).
These environmental factors can activate signalling pathways
responsible for the carcinogenesis of HPV-related cancers,
including SCC, in low-income countries (62). These pathways are similar to those
known as the hallmarks of cancer, and include hyperproliferative
signalling, insensitivity to growth-factor signals, evasion of
apoptosis, continuous angiogenesis, genomic unsteadiness, mutation,
advanced inflammation and the disruption of normal metabolic
functions (65).
In the past decade, the World Health Organization
(WHO) reported that billions of individuals from developing regions
such as Africa still utilize coal, crop residue, dung and wood for
heating and cooking purposes. This is unfortunate because
biomass-burning stoves are universally known as a vital source of
bio-carcinogens (65). Therefore,
women exposed to excessive smoke from these stoves are at greater
risk of developing cervical cancer (16,66).
This was further verified by Bennett et al (67), who identified an association between
the use of solid fuel and cervical cancer.
Coaltar derivatives, such as Lysol, are sometimes
used in vaginal sanitary products. The first evidence to associate
Lysol usage with cervical cancer was in 1931 when it was noted that
the majority of a group of New York women with cervical cancer were
continuous users of Lysol sanitizers (62). Lysol is a tar-based derivative, which
was initially used in animal research for carcinogenesis (68). A later study amongst Californian
women found a significant association between the use of Lysol and
cervical cancer (69). Goodson et
al (65) also reported that
chemical constituents of tobacco (e.g. benzyl pyrenes, polycyclic
aromatics and tobacco-based nitrosamines) and tar-based vaginal
sanitizers could affect the development of cancer, including
cervical cancer, since they penetrate cells and tissues, and
stimulate vital carcinogenic pathways. The precise understanding of
these pathways may lead to more effective immunotherapeutic
strategies and control measures of HPV-related tumours that are
usually induced in HR populations exposed to multiple carcinogens
due to a low socioeconomic background.
Role of genetic polymorphisms in
cervical cancer
Genetic polymorphisms are single nucleotide base
changes that occur between two genomes as a deletion, insertion or
a substitution of a single nucleotide. These alterations can either
be insignificant due to being silent, or they can be highly
significant, as they can lead to different types of disorders, such
as cervical cancer (70). HPV is
considered to be the primary contributory factor to cervical cancer
tumour angiogenesis, although a review of previous studies
(71) reported additional
contributors, including the regulation of long non-coding RNAs
(lncRNAs) and/or their gene polymorphisms, such as polymorphisms in
toll-like receptor (TLR) genes (72).
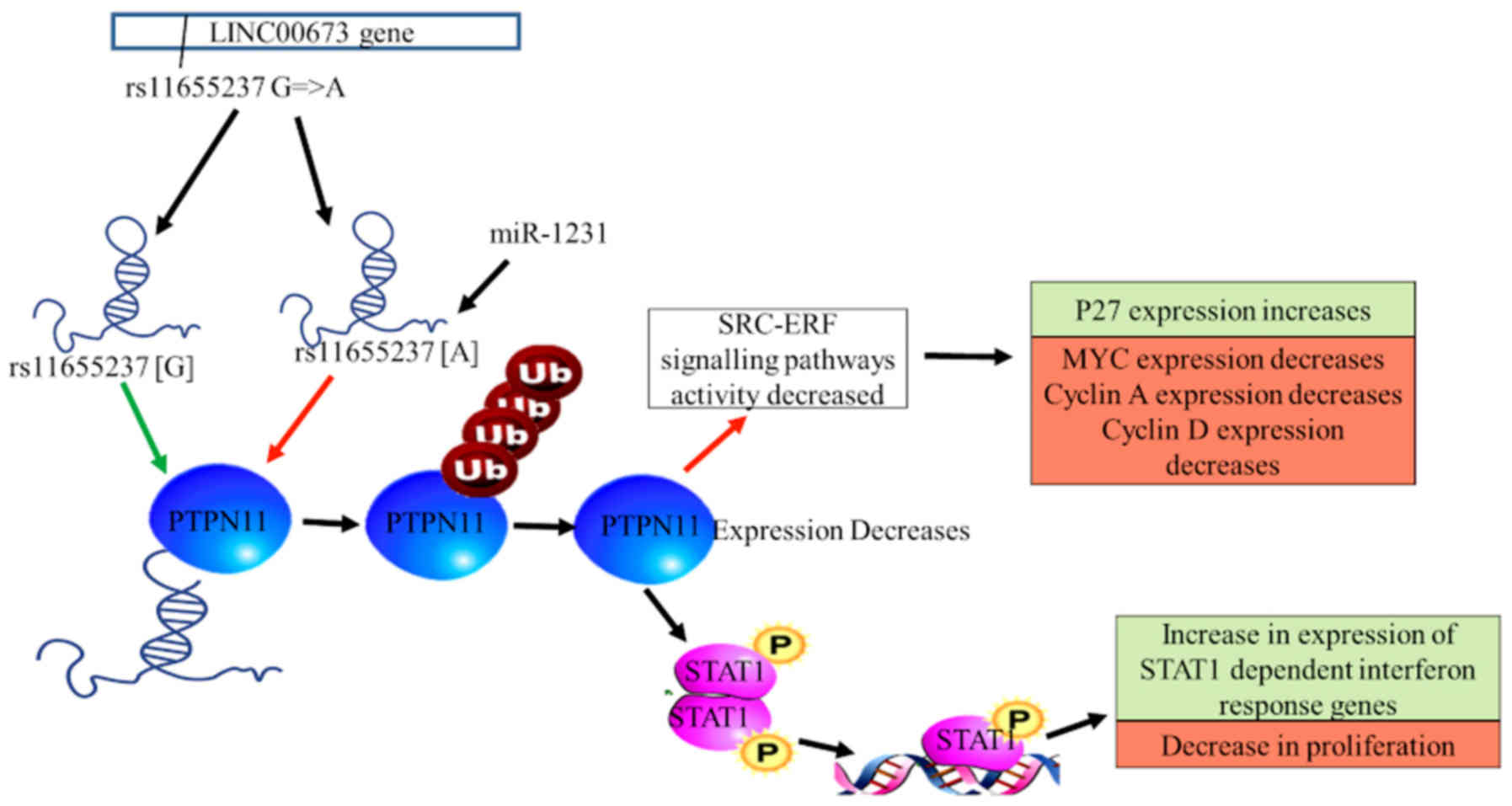
LncRNA LINC00673 has been implicated in the
development and prognosis of multiple tumours, including SCC
(73). In 2018, Wang and Luo
(72) demonstrated that the genetic
variant of LINC00673 rs11655237 increases vulnerability to cervical
cancer in the Chinese population, suggesting that this gene variant
is associated with the risk of cervical cancer The study further
suggested that downregulation of the transcription levels of
LINC00673 rs11655237 in cervical tissues may be the main reason for
the association between the genetic variant rs11655237 and the risk
of cervical cancer. In addition, a G-to-A nucleotide substitution
in the rs11655237 gene variant of LINC00673 may be the cause of
dysregulated transcription, as it generates a target locus for
microRNA (miRNA or miR)-1231 attachment, which in turn inhibits
LINC00673 (60,74). The A allele and the AA/AG genotype of
the rs11655237 gene variant have been demonstrated to be associated
with the risk of developing cervical cancer, since the gene variant
alters the transcription of LINC00673, which is vital for tumour
suppression (Fig. 3) (72).
In 2018, Weng et al (75) investigated the association between
the lncRNA HOX transcript antisense intergenic RNA (HOTAIR) genetic
polymorphisms and the recurrence of cancer, as well as the survival
rates of patients with cervical cancer. Although no significant
association was observed between HOTAIR gene polymorphisms and
patient clinicopathological characteristics, the study revealed
that GG-genotype carriers in the HOTAIR rs920778 gene variant
exhibited high risk of cervical cancer recurrence, low predicted
survival due to stomal and pelvic lymph node metastasis, and
increased mortality compared with carriers of the AA/AG genotype
(74–76). Although the AA genotype is not
associated with cervical cancer, it is significantly associated
with gastric and oesophageal squamous cancer in Chinese populations
(77). Therefore, the prognosis of a
patient may depend on the impact of HOTAIR expression on the
rs920778 gene variant. In addition, since the HOTAIR gene
polymorphism rs920778 has insignificant associations with cervical
cancer carcinogenesis, it cannot be described as a potential
predictive factor for patient prognosis (75).
A previous meta-analysis conducted by Yang et
al (78) identified an
association between TLR gene polymorphisms and the risk of cervical
cancer. The results revealed that white populations carrying the C
allele of the TLR 9 1486 T/C gene polymorphism and the A allele of
the TLR 9 G2848A gene polymorphism were significantly associated
with an increased risk of developing cervical cancer (78,79). TLR
9 has been previously reported to promote cervical intraepithelial
neoplasia (CIN) progression in women from Tunisia (80). These results suggested that TLR 9 may
represent a potential biomarker for the malignant transformation of
cervical squamous cells. The ability of genes and epigenetic
factors to act as contributors of cervical cancer carcinogenesis,
and the identification of these factors indicate their potential
use in diagnosis and treatment.
Role of alternative splicing in
cervical cancer
Alternative splicing mechanisms
Alternative splicing is a process by which introns
are excised (i.e. ‘spliced’) from pre-mRNA to allow the assembly of
exons for translation into a protein (81). The alternative splicing process is
imperative to all eukaryotic organisms for the production of
multiple alternative isoforms, which are needed for the functional
diversity of proteins (82,83). Alternative splicing can undergo
several interruptions, such as disturbances associated with miRNA
expression, which may lead to several human disorders, including
cancer (84,85). In addition, Skotheim and Nees
(86) have reported that cellular
activities such as cell proliferation, motility and drug
responsiveness can be negatively affected by the expression of
alternative splice variants and tumour-specific variants.
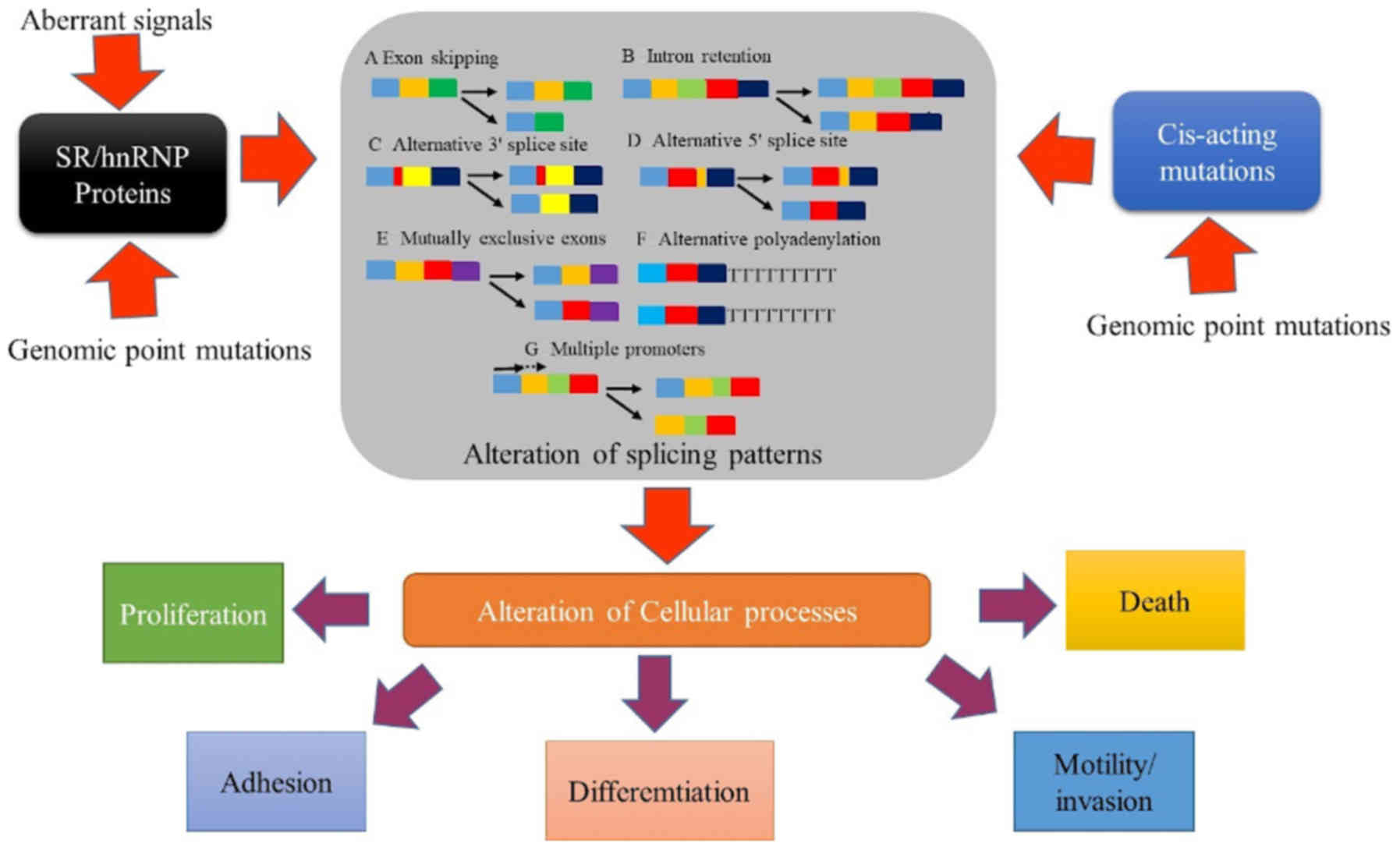
Alternative splicing abnormalities
Abnormalities in alternative splicing have been
demonstrated to lead to the development of multiple human diseases
such as cancer, heart disease, and age-associated diseases,
HIV-associated nephropathy and autoimmune disorders (87). Aberrant alternative splicing is
usually a result of genomic point mutations that occur in splicing
factors or elements that result in transcriptome alterations such
as exon alteration, intron retention and gene expression changes
(Fig. 4) (83). These mutations and alterations
normally affect multiple protein pathways and mRNA expression
(83,88). The influence of aberrant alternative
splicing in the majority of cancer types results from splicing
factor mutations in cancer genes or transcripts of non-mutated
genes (89). Genome-wide associated
studies have demonstrated the association of the splicing factor 3A
subunit 1 (SF3A1) gene (which is located on 22q12.3) with several
diseases, including lung and breast cancer, as well as inflammatory
bowel disease (90,91). Mutations in the SF3A1 coding region
are associated with susceptibility to multiple types of cancer,
including oesophageal AC, osteosarcoma, myxoid liposarcoma,
synovial sarcoma, ovarian carcinoma, glioblastoma, endometrial,
lung, breast and gastric cancer (92).
Expression studies by Liu et al (93) demonstrated the ability of HR HPV
oncoproteins E6 and E7 to induce the expression of serine- and
arginine-rich splicing factor 10 (SRSF10), which is crucial for HPV
16- and 18-positive cervical carcinogenesis. Increased levels of
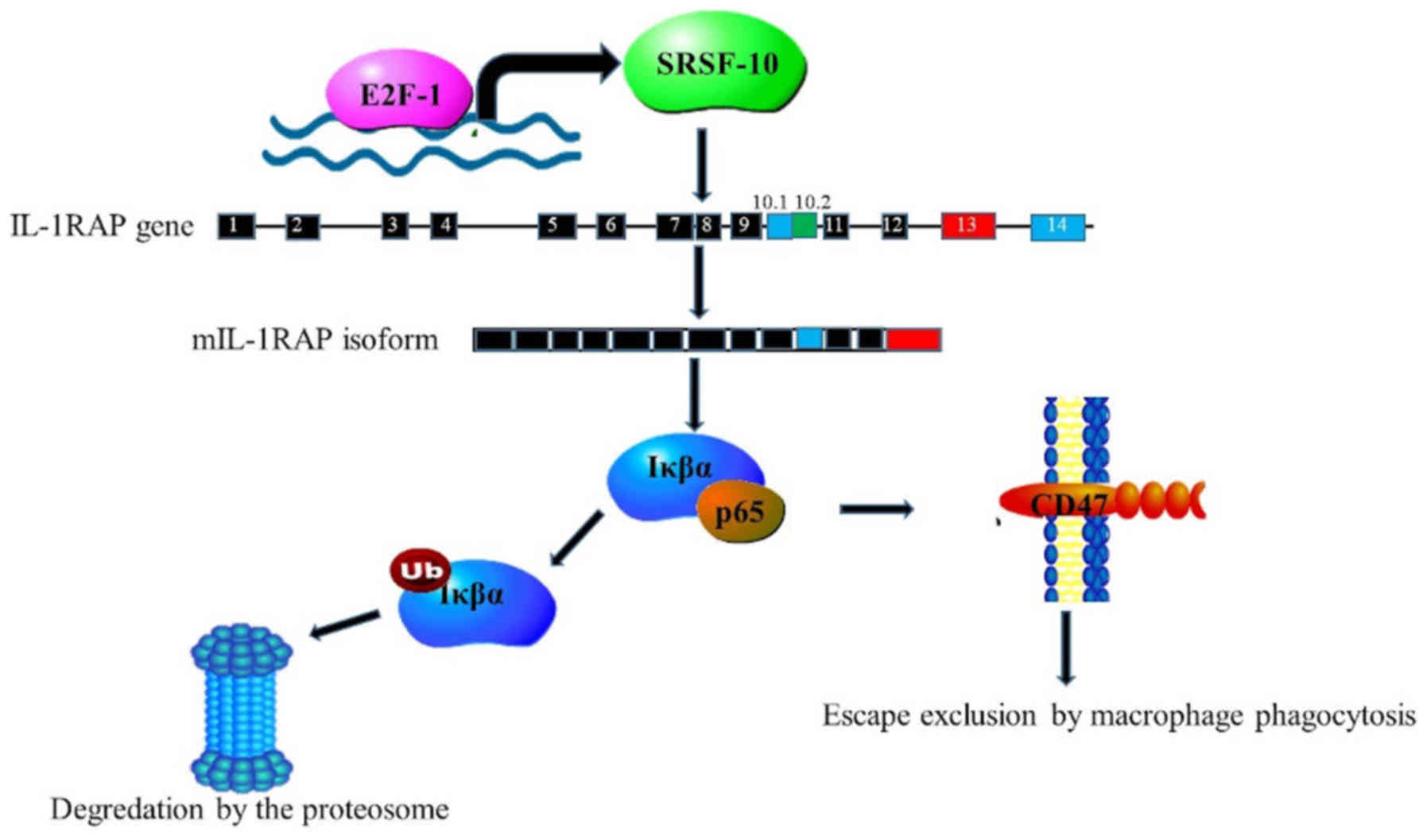
SRSF10 were induced by E2F transcription factor 1 (93). The synthesis of the membrane form of
interleukin-1-receptor accessory protein (mIL1RAP) is controlled by
SRSF10 through the modulation of alternative splicing of the IL1RAP
exon 13. This gives rise to the interleukin 1β isoform, which
induced NF-κB transcription of the CD47 receptor. This cell surface
molecule signals cells to escape macrophage phagocytosis (Fig. 5) (93). The study further revealed a
significant association amongst SRSF10, mIL1RAP and CD47 expression
in tissues of the uterus (93). This
revealed that alternative splicing, inflammatory molecules and
immune scrutiny served crucial roles in HPV 16/18-positive
carcinogenesis (93,94). These results suggested that proteins
associated with cervical cancer need to be researched for splice
site abnormalities, since this method can also be used as a
screening tool for the disease.
TLR mechanisms that lead to cervical cancer
TLRs are pattern recognition receptors that function
in innate immune responses by identifying conserved components of
microorganisms known as pathogen-associated molecular patterns.
Previous studies have indicated that TLRs are expressed in tumour
cells and in the tumour microenvironment of various types of cancer
(95). TLR 3 (96), TLR 4 (96), TLR 5 and TLR 9 (96) have been identified in cervical cancer
tissues, indicating that they may be involved in the occurrence of
the disease. TLRs are activated by ligands such as lipids, and have
been demonstrated to serve an important role in the development and
progression of cervical cancer (97). These protein receptors are also
expressed in other tumours (98),
indicating that they may bind different ligands (Table IV). TLRs are highly expressed in
immune and cancerous cells (80,99).
Persisting HPV infection is the major cause of cervical cancer;
however, alterations in expression levels of TLRs are suspected to
also serve a significant role in HPV infection-induced cervical
cancer (80). In support of this,
Fehri et al (80) analysed
the expression of TLR 9 in 53 samples from Sian women with ICC,
CIN, condyloma and normal cervical tissues; the results revealed
statistically significant differences in CIN and ICC between
condyloma and normal healthy tissues. Almost all patients with ICC
exhibited a higher level of TLR 9 expression in tumour epithelial
cells, whereas between 50–80% of patients with CIN and condyloma
exhibited weaker TLR expression (80). These results supported previous
studies on Korean, Chinese and Canadian populations, which also
revealed low TLR 9 expression in CIN and high TLR 9 expression in
ICC (100,101). High expression levels of TLR 9 were
mainly observed in the absence of HPV and/or its clearance
(102). Hasan et al
(103,104) reported that HPV subtype 16 proteins
E6 and E7 dysregulated TLR 9 expression and functions. This
suggested that HPV may use TLR 9 dysregulation to suppresses its
function of viral DNA recognition, as TLR 9 is a crucial
recognition receptor for DNA-introducing pathogens, particularly
DNA viruses; in addition, it promotes inflammatory and immune
responses (99,105). The results indicated the potential
role of TLR 9 in CIN progression among women from various
populations, suggesting that it may be used as a biomarker for SCC
transformation.
 | Table IV.TLR expression in different types of
cancer (97). |
Table IV.
TLR expression in different types of
cancer (97).
| Cancer type | TLRs |
|---|
| Cervical | TLR 3, TLR 4, TLR
5, TLR 9 |
| Gastric | TLR 2, TLR 4, TLR
5, TLR 9 |
| Colorectal | TLR 2, TLR 3, TLR
4, TLR 5, TLR 9 |
| Ovarian | TLR 2, TLR 3, TLR
4, TLR 5 |
| Lung | TLR 2, TLR 3, TLR
4, TLR 9 |
| Prostate | TLR 4, TLR 9 |
| Breast | TLR 2, TLR 3, TLR
4, TLR 9 |
| Liver | TLR 2, TLR 3, TLR
4, TLR 6, TLR 9 |
| Pancreatic | TLR 2, TLR 4, TLR
9 |
TLR 9 signalling induces tumour progression,
survival and immune evasion in various cancer types, including
cervical cancer (106). However,
whether TLR 9 promotes or suppresses tumour growth remains unknown.
Better understanding of the TLR 9 signalling pathway in cervical
cancer may aid in the development of new immunotherapeutic
strategies (80).
The combination of TLR 4 and the ligand
lipopolysaccharide may trigger lipid raft flow that leads to the
alteration of the lipid raft space conformation (Fig. 6A) (107). The change in the conformation of
lipid rafts results in the aggregation of NADH oxidase subunits on
lipid rafts, inducing a redox reaction of lipid rafts to produce
reactive oxygen species and the inhibition of hypoxia-inducible
factor 1α degradation, which may lead to the induction of cervical
cancer (Fig. 6B) (97).
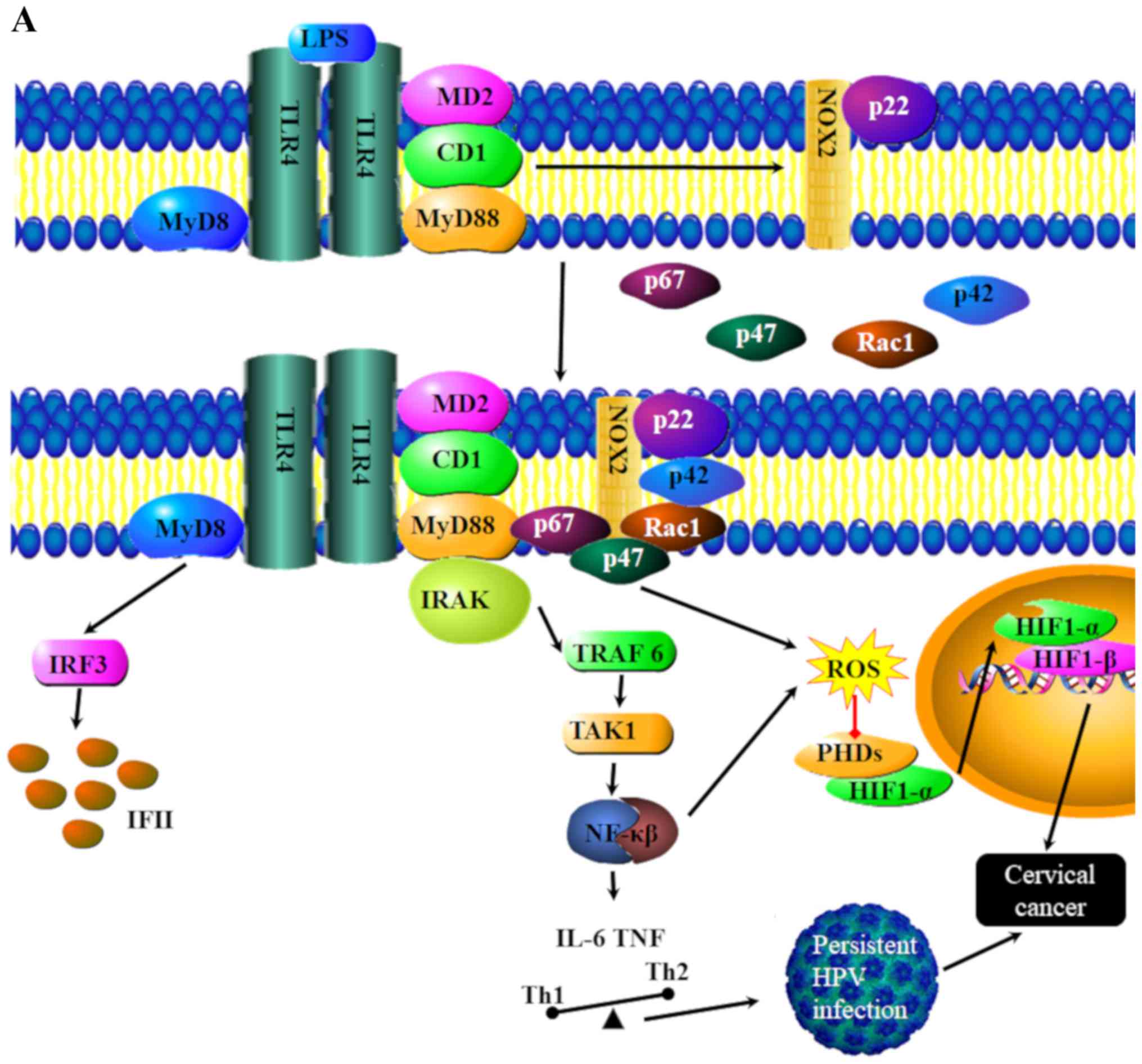 | Figure 6.TLR4 signaling pathways in cervical
cancer. (A) Binding of LPS to TLR4 leads to the transmission of
signals via MyD88, At the same time the activation of TLR5 leads to
increased Nox1 signaling. The primary role of NOX1 is to generate
ROS. NOX1 signaling also leads to the inhibition of HIF-1α
degradation, which increases the possibly of developing cervical
cancer MyD88 signaling in conjunction with NOX1 leads to the
activation of transcription factors and Interfrons (IFN), leading
to a pro-inflammatory and antiviral response. This pathway
demonstrates how inflammation can lead to cancer progression. CD1,
cluster differentiation 1; HIF-1α, hypoxia inducible factor 1α;
IL-6, interleukin 6; IFN, interferon; IRAK, interleukin-1
receptor-associated kinase; IRF3, interferon regulatory factor 3;
LPS, lipopolysaccharides; MyD8, myeloid differentiation primary
response 8; MyD88, myeloid differentiation primary response 88;
NF-κB, nuclear factor kappa-light-chain-enhancer of activated B
cells; Nox1, NADPH oxidase 1; PHDs, propyl hydroxylases; Rac1,
ras-related C3 botulinum toxin substrate 1; TAK1, transforming
growth factor beta-activated kinase 1; TLR, toll-like receptor;
Traf6, tumor necrosis factor receptor (TNFR)-associated factor 6;
NADH, nicotinamide adenine dinucleotide hydrogen. |
miRNA in cervical cancer
miRNAs are a group of small non-coding RNAs of 18–25
nucleotides in length that regulate gene expression at the
post-transcriptional level (108).
miRNAs also serve crucial functions in cell proliferation,
differentiation, migration, invasion and drug resistance (78,109–111).
The translation of mRNA can be suppressed or degraded when their
3′-untranslated regions (3′-UTRs) are targeted by miRNAs (93,112–114).
Abnormal miRNA expression has been reported to contribute to the
progression of multiple types of cancer, including breast, lung,
oesophageal and ovarian cancer (115–120).
Expression of miR-214 in cervical cancer
Several miRNAs serve significant roles in the
development of cancer, such as miR-98 (121), miR-98 (121), miR-146b-5p (122) and miR-214 (122). miR-214 has been demonstrated to be
associated with both the inhibition and progression of multiple
types of cancer, including cervical cancer (78). Yang et al (78) analysed the expression levels of
miR-214 in cervical cancer and non-cancerous tissues. The results
revealed that the inhibition of miR-214 expression in cancerous
tissue stimulated cervical cancer proliferation, whereas excessive
expression of miR-214 inhibited cancer progression (78). MTT assays were performed on cervical
cancer cells revealed a significant inversely proportional
association between the expression levels of miR-214 and enhancer
of zeste homolog 2 (EZH2); increased expression levels of miR-214
and reduced expression levels of EZH2 resulted in reduced cervical
cancer cell proliferation, whereas low expression of miR-214
combined with high EZH2 expression resulted in increased cervical
cancer cell proliferation in vitro (78). Thus, the differential expression
levels of miR-214 suggest that it may be a potential biomarker for
prognosis and diagnosis of cervical cancer, or a tool for treatment
of this disease. Statistical analysis determined an association
between miR-214 expression levels and the survival rates of
patients with cervical cancer (78).
Patients expressing low levels of of miR-214 exhibited enhanced
levels of cervical cancer proliferation. Moreover, patients with
higher expression levels of miR-214 exhibited improved survival
rates. A decrease in the EZH2 expression levels was significantly
associated with the inhibition of cervical cancer proliferation
(78).
Expression of miR-217 in cervical cancer
Previous studies have revealed that miRNAs act as
tumour suppressors in cervical cancer cells by regulating specific
target genes. For example, Dong et al (123) compared Rho-associated coiled-coil
containing protein kinase 1 (ROCK1) expression levels in cervical
cancer and non-cancerous tissues and cell lines. Significantly
lower expression levels of miR-217 were observed in metastatic and
non-metastatic cervical cancer tissues and cell lines compared with
those in non-cancerous tissues and cell lines (123). Reduced invasive capabilities of
SiHa and HeLa cell lines, as well as reduced colony formation, was
demonstrated when miR-217 was highly expressed; in addition, the
effect of high miR-217 expression on apoptosis in SiHa and HeLa
cell lines was analysed by flow cytometry. The flow cytometry
indicated that increased transcription of miR-217 in cells resulted
in a greater proportion of these cells undergoing apoptosis
(123). Overexpression of miR-217
significantly inhibited cell growth, whereas overexpression of
ROCK1 increased cell invasive abilities in the SiHa cell line due
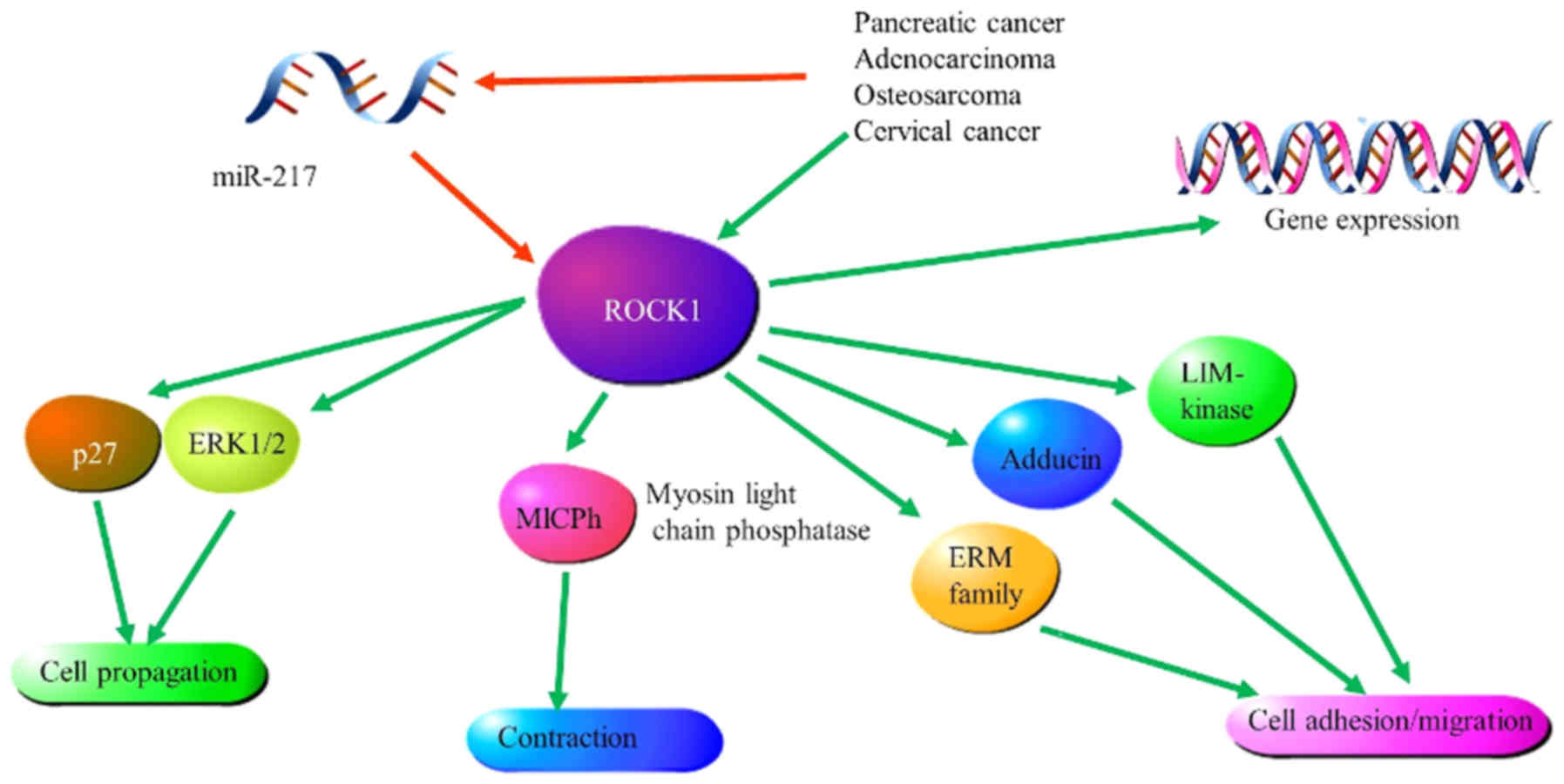
to decreased miR-217 expression (123). Therefore, the ability of miR-217 to
suppress cervical cancer may be modulated through ROCK1. This was
further supported by suppressed luciferase activity of
pmirGLO-ROCK1-3′UTR-1 and pmirGLO-ROCK1-3′UTR-2 following
overexpression of miR-217 (123).
Lastly, western blot analysis revealed that overexpression of
miR-217 significantly lowered ROCK1 expression levels, whereas
reduced levels of miR-217 expression resulted in increased ROCK1
expression levels (Fig. 7) (120). Therefore, miR-217 may target ROCK1
to inhibit cervical cancer proliferation (123). The level of miRNA transcription
serves an important role in cervical cancer progression, which in
turn highlights the importance of broadening our knowledge on
potential miRNA target genes, as well as the potential mechanisms
of migration and invasion for the identification of potential
prognostic and diagnostic tools, and for the development of novel
immunotherapeutic strategies for cervical cancer.
Prevention and diagnosis of cervical
cancer
Geographic variation in cervical cancer incidence
is based in part on differences in the availability of screening to
allow for the early identification and removal of precancerous
lesions. The diagnosis of HPV should also be considered as an
important and challenging causative factor for cervical cancer that
varies geographically (124).
Cervical cancer prevention: HPV
vaccination
In 2013, Adesina et al (125) estimated that 11 million women in
Sub-Saharan Africa would be diagnosed with cervical cancer within
the next 10–20 years, with ~1.7 million cases diagnosed in 2010
(126). Prevention of cervical
cancer is regarded as the best control method; however, treatment
is also an important intervention (127). A cost-effective HPV vaccine has
been reported as lifesaving amongst girls between 9 and 12 years of
age (50). The number of cervical
cancer cases are predicted to effectively decrease after five
decades of comprehensive vaccination (128). There are two types of HPV vaccines
used for targeting the predominant subtypes of HPV: The bivalent
vaccine, which targets the HPV 16 and 18 subtypes, and the
quadrivalent vaccine, which targets the HPV 6, 11, 16 and 18
subtypes (2,129). HPV vaccines have been reported to
effectively assist in preventing HPV infection, and result in the
prevention of CIN (130,131) HPV vaccination first became popular
in high-income countries but remained unpopular in low income
countries due to the high price of the vaccine. Since 2011, HPV
vaccinations have gained popularity in middle-income countries due
to a decrease in cost (2); since
2012, increased financial support for HPV vaccination projects in
low-income countries has enabled such countries to adopt
vaccination programs (2,132). HPV vaccination for women in South
Africa was first introduced in 2014 through the National HPV
Immunization Program. The primary target age for HPV vaccination in
South Africa is 9 years (pupils in grades 3 and 4) (6,17).
According to the European Medicines Agency, the standard dose for
girls <14 years of age is two doses at 0 and <6 months
depending on whether they are receiving the Cervarix or Gardasil
vaccine (21). Herrero et al
(2,7)
clinically demonstrated that three doses of HPV vaccine can provide
total protection against persisting HPV infection and associated
precancerous lesions in women aged between 15 and 26 years old,
provided that they have not been previously exposed to the virus
(7). Therefore, treatment (or
vaccination) and screening is crucial for lowering the incidence,
burden and mortality rates of cervical cancer (7).
Cervical cancer screening
It is of great global interest to eliminate or
decrease the number of new cases and high mortality of cervical
cancer caused by persistent HPV infection. Therefore, Pap smear
screening for cervical cancer is highly encouraged during the HIV
diagnosis process. This procedure should be repeated every 3 years
for women who have been previously screened and diagnosed as
negative (50). Regular screening
for cervical cancer amongst women is predicted to lower the
lifetime risk of developing the disease (133). Population screening for cervical
cancer in regions of low socioeconomic status and low-resource
settings remains elusive; the cervical cancer screening coverage in
Southern Africa ranges between 4.1 and 38.0%. A study of 642
females in urban areas found that 17.3% had been screened, while of
580 females in rural areas 9.6% had been screened for HPV (130,134,135).
This low coverage is due to factors such as inaccessibility (due to
areas being remote), lack of funding, community awareness and
cost-effectiveness, complications of Pap smear, a lack of public
policy attention, or the implementation of ineffective public
policies (128,130,136).
HIV-associated cervical cancer incidence is
predicted to gradually increase in LMICs for the next 10 years
(137). Despite the incidence of
cervical cancer in many LMICs being lower than in many developed
countries, the cancer burden in LMICs is increasing due to the
advanced stage at diagnosis and inaccessible treatment (28). Thus, immediate action in the public
and global health sectors is advised (2,138).
Effective precancerous lesion detection using the
PapilloCheck® microarray and the HPV restriction
fragment length polymorphism (RFLP) PCR assay was achieved with
62.5 and 25.0% HPV co-infection detection, respectively (4). The RFLP PCR assay is suggested as a
primary HPV test for screening in LMICs, as it is cost-effective,
while the PapilloCheck® microarray assay is considered a
more sensitive and descriptive test (4). However, widespread access to screening
at the population level in LMICs remains challenging. For example,
in Uganda, cervical cancer is the leading type of cancer amongst
women, with 3,915 new cases and 2,275 deaths per year (21); however, <10% of Ugandan women have
ever been screened (139).
Several studies have reported multiple compounding
factors that contribute to non-participation of women in cervical
cancer screening, such as unemployment, lack of or low education
level, poor language proficiency (for example, the information and
education campaigns not being provided in the native language of
the women the programs are aimed at), being unmarried, lack of
knowledge of screening, previous negative experiences of screening,
cultural and traditional beliefs, and multiple other determinants
(140–142). Thus, the WHO and the European
Commission have recognised that equal access and equal utilization
of healthcare is essential to deal with inequity issues in
healthcare (143). This may improve
healthcare service practices such as screening and treatment
(144,145). In conclusion, appropriate cervical
cancer screening programs and good-quality cytological testing can
assist in lowering the incidence and mortality rates of cervical
cancer. The effectiveness of prevention and treatment programs is
dependent on equity in healthcare and good healthcare service
practices. A lack of cervical cancer screening is not the sole
cause for the high burden of cervical cancer in LMICs. Another
important consideration is the decision on whether to treat a
patient based on a positive result for an HPV test. Since most HPV
infections clear spontaneously, especially in younger women, if a
patient is treated without further screening, it may contribute to
unnecessary treatment costs (146).
Conclusions
Globally, Southern Africa displays the largest HIV
burden amongst women, contributing to it being a HR region for
developing an HPV infection, pre-invasive cervical disease and ICC.
Poor prognosis and diagnosis are common in low-income settings,
where women experience advanced stages of HPV infection and lack
treatment.
Control methods, including Pap smear screening and
HPV vaccines, have been used to help reducing the exponential
increase in new cervical cancer cases, although the low coverage
for screening remains a challenge in a number of LMICs. However,
these control methods were initially only available in developed
countries and have only recently become available in middle-income
countries, leaving women from low socioeconomic backgrounds
vulnerable. This is caused by the lack of funding and accessibility
to cost-effective treatment. Thus, the present review highlights
the importance of equity in the access and utilization of
healthcare services and products.
Although HPV vaccines have been introduced in
multiple LMICs, their prophylactic efficacy is only beneficial to
women who have not been exposed to the virus; therefore, this
identifies a need for further research into other possible
interventions, particularly for those already infected. The effect
of aberrant alternative splicing on cervical cancer requires
further research and may be a promising tool for treatment of the
disease. Similarly, TLRs and miRNAs may be explored further as
potential immunotherapeutic interventions.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Medical
Research Council of South Africa, through the SA-MRC/UP Precision
Prevention and Novel Drug Targets for HIV-Associated Cancers
Extramural Unit Grant and the SA-MRC SHIP Grant.
Availability of data and materials
Not applicable.
Authors' contributions
ZD was responsible for the Acquisition of funding.
RH, MM, RM and ZMK were responsible for the collection of data. RH,
MM, TM, RM and ZMK were responsible for writing the manuscript. RH,
TM, RM, ZMK, CH, SMW, RMR, GK and DOB were responsible for
editing/revising the manuscript. ZD is responsible for supervising
the research group. The review was written by RH, MM, TM, RM, ZMK
and ZD. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Herrero R, González P and Markowitz LE:
Present status of human papillomavirus vaccine development and
implementation. Lancet Oncol. 16:e206–e216. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Waggoner SE: Cervical cancer. Lancet.
361:2217–2225. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Golfetto L, Alves EV, Martins TR, Sincero
TCM, Castro JBS, Dannebrock C, Oliveira JG, Levi JE, Onofre ASC and
Bazzo ML: PCR-RFLP assay as an option for primary HPV test. Braz J
Med Biol Res. 51:e70982018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bruni L, Diaz M, Castellsagué X, Ferrer E,
Bosch FX and de Sanjosé S: Cervical human papillomavirus prevalence
in 5 continents: Meta-analysis of 1 million women with normal
cytological findings. J Infect Dis. 202:1789–1799. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kocjan BJ, Bzhalava D, Forslund O, Dillner
J and Poljak M: Molecular methods for identification and
characterization of novel papillomaviruses. Clin Microbiol Infect.
21:808–816. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ghebre RG, Grover S, Xu MJ, Chuang LT and
Simonds H: Cervical cancer control in HIV-infected women: Past,
present and future. Gynecol Oncol Rep. 21:101–108. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Idehen EE, Koponen P, Härkänen T,
Kangasniemi M, Pietilä AM and Korhonen T: Disparities in cervical
screening participation: A comparison of Russian, Somali and
Kurdish immigrants with the general Finnish population. Int J
Equity Health. 17:562018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Swanson M, Ueda S, Chen LM, Huchko MJ,
Nakisige C and Namugga J: Evidence-based improvisation: Facing the
challenges of cervical cancer care in Uganda. Gynecol Oncol Rep.
24:30–35. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Satterwhite CL, Torrone E, Meites E, Dunne
EF, Mahajan R, Ocfemia MC, Su J, Xu F and Weinstock H: Sexually
transmitted infections among US women and men: Prevalence and
incidence estimates, 2008. Sex Transm Dis. 40:187–193. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Say L, Chou D, Gemmill A, Tunçalp Ö,
Moller AB, Daniels J, Gülmezoglu AM, Temmerman M and Alkema L:
Global causes of maternal death: A WHO systematic analysis. Lancet
Glob Health. 2:e323–e333. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bray F, Ren JS, Masuyer E and Ferlay J:
Global estimates of cancer prevalence for 27 sites in the adult
population in 2008. Int J Cancer. 132:1133–1145. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Torre LA, Islami F, Siegel RL, Ward EM and
Jemal A: Global cancer in women: Burden and trends. Cancer Epidemol
Biomarkers Prev. 26:444–457. 2017. View Article : Google Scholar
|
|
15
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
World Health Organization, . WHO
guidelines for screening and treatment of precancerous lesions for
cervical cancer prevention. World Health Organization.
2013.March
16–2019
|
|
17
|
Karsa LV, Anttila A, Ronco G, et al:
Cancer screening in the European Union. Report on the
implementation of the Council Recommendation on cancer screening.
In: Cancer screening in the European Union. Report on the
implementation of the Council Recommendation on cancer screening.
European Commission. (Luxembourg). 1602008.
|
|
18
|
Ginindza TG and Sartorius B: Projected
cervical cancer incidence in Swaziland using three methods and
local survey estimates. BMC Cancer. 18:6392018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ferlay J, Colombet M, Soerjomataram I, et
al: Estimating the global cancer incidence and mortality in 2018:
GLOBOCAN sources and methods. Int J Cancer. 144:1941–1953. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sartorius K, Sartorius B, Aldous C,
Govender PS and Madiba TE: Global and country underestimation of
hepatocellular carcinoma (HCC) in 2012 and its implications. Cancer
Epidemiol. 39:284–290. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nakisige C, Schwartz M and Ndira AO:
Cervical cancer screening and treatment in Uganda. Gynecol Oncol
Rep. 20:37–40. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ferlay J, Ervik M, Lam F, Colombet M, Mery
L, Piñeros M, Znaor A, Soerjomataram I and Bray F: 2018.Global
Cancer Observatory, . Cancer Today. (Lyon, France). International
Agency for Research on Cancer. March
18–2019
|
|
23
|
International Agency for Research on
Cancer, . GLOBOCAN 2012: Estimated cancer incidence, mortality and
prevalence worldwide in 2012. 2012.
|
|
24
|
Chibwesha CJ, Goeieman B, Levin S, Mulongo
M, Faesen M, Swarts A, Ramotshela S, Williams S, Rakhombe N, Bruce
S, et al: Estimating the burden of cervical disease among
HIV-infected women accessing screening services in South Africa: A
model-based analysis. S Afr Med J. 108:235–239. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Simonds HM, Wright JD, du Toit N, Neugut
AI and Jacobson JS: Completion of and early response to
chemoradiation among human immunodeficiency virus (HIV)-positive
and HIV-negative patients with locally advanced cervical carcinoma
in South Africa. Cancer. 118:2971–2979. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
UNAIDS, . UNAIDS Terminology Guidelines.
June
13–2019
|
|
27
|
UNAIDS, . Report on the global HIV/AIDS
epidemic. UNAIDS2002. http://data.unaids.org/pub/report/2002/brglobal_aids_report_en_pdf_red_en.pdfJune
13–2019
|
|
28
|
Jacobson G, Chuang L and Pankow M:
Improving quality of care and timely access to radiation therapy
for patients with invasive cervical cancer at the National Cancer
Institute Paraguay. Gynecol Oncol Rep. 25:82–86. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bosch FX, Broker TR, Forman D, Moscicki
AB, Gillison ML, Doorbar J, Stern PL, Stanley M, Arbyn M, Poljak M,
et al: Comprehensive control of human papillomavirus infections and
related diseases. Vaccine. 31 (Suppl 7):H1–H31. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Forman D, de Martel C, Lacey CJ,
Soerjomataram I, Lortet-Tieulent J, Bruni L, Vignat J, Ferlay J,
Bray F, Plummer M, et al: Global burden of human papillomavirus and
related diseases. Vaccine. 30 (Suppl 5):F12–F23. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ginsburg O, Bray F, Coleman MP, Vanderpuye
V, Eniu A, Kotha SR, Sarker M, Huong TT, Allemani C, Dvaladze A, et
al: The global burden of women's cancers: A grand challenge in
global health. Lancet. 389:847–860. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fields MM: New cervical cancer screening
guidelines: Was the annual pap too much of a good thing? J Adv
Pract Oncol. 4:59–64. 2013.PubMed/NCBI
|
|
33
|
Moyer VA; U.S. Preventive Services Task
Force, : Screening for cervical cancer: U.S. Preventive Services
Task Force recommendation statement. Ann Intern Med. 156:880–891,
W312. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Botha MH and Dochez C: Introducing human
papillomavirus vaccines into the health system in South Africa.
Vaccine. 30 (Suppl 3):C28–C34. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
South African National Cancer Registry, .
Cancer in South Africa 2014. (Johannesburg). 2014.https://www.nicd.ac.za/wp-content/uploads/2019/12/2014-NCR-tables.pdfJanuary
7–2019
|
|
36
|
Denny L: Cervical cancer: The South
African perspective. FIGO 26th annual report on the results of
treatment in gynecological cancer. Int J Gynaecol Obstet. 95 (Suppl
1):S211–S214. 2006. View Article : Google Scholar
|
|
37
|
Bekker LG, Venter F, Cohen K, Goemare E,
Van Cutsem G, Boulle A and Wood R: Provision of antiretroviral
therapy in South Africa: The nuts and bolts. Antivir Ther. 19
(Suppl 3):S105–S116. 2014. View Article : Google Scholar
|
|
38
|
Dreyer G: Clinical implications of the
interaction between HPV and HIV infections. Best Pract Res Clin
Obstet Gynaecol. 47:95–106. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mbulawa ZZA, Coetzee D and Williamson AL:
Human papillomavirus prevalence in South African women and men
according to age and human immunodeficiency virus status. BMC
Infect Dis. 15:459. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tathiah N, Naidoo M and Moodley I: Human
papillomavirus (HPV) vaccination of adolescents in the South
African private health sector: Lessons from the HPV demonstration
project in KwaZulu-Natal. S Afr Med J. 105:9542015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lovgren K, Soliman AS, Ngoma T, Kahesa C
and Meza J: Characteristics and geographic distribution of
HIV-positive women diagnosed with cervical cancer in Dar es Salaam,
Tanzania. Int J STD AIDS. 27:1049–1056. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
McCree R, Giattas MR, Sahasrabuddhe VV,
Jolly PE, Martin MY, Usdan SL, Kohler C and Lisovicz N: Expanding
cervical cancer screening and treatment in Tanzania: Stakeholders'
perceptions of structural influences on scale-up. Oncologist.
20:621–626. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ardahan M and Temel AB: Visual inspection
with acetic acid in cervical cancer screening. Cancer Nursing.
34:158–163. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gross K, Armstrong Schellenberg J, Kessy
F, Pfeiffer C and Obrist B: Antenatal care in practice: An
exploratory study in antenatal care clinics in the Kilombero
Valley, south-eastern Tanzania. BMC Pregnancy Childbirth.
11:362011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Runge AS, Bernstein ME, Lucas AN and
Tewari KS: Cervical cancer in Tanzania: A systematic review of
current challenges in six domains. Gynecol Oncol Rep. 29:40–47.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Mishra GA, Pimple SA and Shastri SS:
Prevention of cervix cancer in India. Oncology. 91 (Suppl 1):S1–S7.
2016. View Article : Google Scholar
|
|
47
|
Senapathy JG, Umadevi P and Kannika PS:
The present scenario of cervical cancer control and HPV
epidemiology in India: An outline. Asian Pac J Cancer Prev.
12:1107–1115. 2011.PubMed/NCBI
|
|
48
|
Gheit T, Vaccarella S, Schmitt M, Pawlita
M, Franceschi S, Sankaranarayanan R, Sylla BS, Tommasino M and
Gangane N: Prevalence of human papillomavirus types in cervical and
oral cancers in central India. Vaccine. 27:636–639. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Deodhar K, Gheit T, Vaccarella S, Romao
CC, Tenet V, Nene BM, Jayant K, Kelkar R, Malvi SG, Sylla BS, et
al: Prevalence of human papillomavirus types in cervical lesions
from women in rural Western India. J Med Virol. 84:1054–1060. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Chatterjee S, Chattopadhyay A, Samanta L
and Panigrahi P: HPV and cervical cancer epidemiology-current
status of HPV vaccination in India. Asian Pac J Cancer Prev.
17:3663–3673. 2016.PubMed/NCBI
|
|
51
|
Mattheij I, Pollock AM and Brhlikova P: Do
cervical cancer data justify HPV vaccination in India?
Epidemiological data sources and comprehensiveness. J R Soc Med.
105:250–262. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Lopez MS, Baker ES, Maza M, Fontes-Cintra
G, Lopez A, Carvajal JM, Nozar F, Fiol V and Schmeler KM: Cervical
cancer prevention and treatment in Latin America. J Surg Oncol.
115:615–618. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
HPV information centre, . Brazil human
papillomavirus and related cancers, fact sheet 2018 ICO/IARC
information centre on HPV and cancer, 2018. https://hpvcentre.net/statistics/reports/BRA_FS.pdfApril
17–2019
|
|
54
|
Napa LI: Cervical Cancer Screening:
Awareness and knowledge in Brazil. Clin Social Work Health
Intervention. 55–61. 2016. View Article : Google Scholar
|
|
55
|
Lorenzi AT, Syrjänen KJ and Longatto-Filho
A: Human papillomavirus (HPV) screening and cervical cancer burden.
A Brazilian perspective. Virol J. 12:112. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Cohen PA, Jhingran A, Oaknin A and Denny
L: Cervical cancer. Lancet. 393:169–182. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Castellsagué X and Muñoz N: Chapter 3:
Cofactors in human papillomavirus carcinogenesis-role of parity,
oral contraceptives, and tobacco smoking. J Natl Cancer Inst
Monogr. 20–28. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Chatzistamatiou K, Katsamagas T, Zafrakas
M, Zachou K, Orologa A, Fitsiou F, Theodoridis T, Konstantinidis T,
Konstantinidis TC and Agorastos T: Smoking and genital human
papilloma virus infection in women attending cervical cancer
screening in Greece. World J Obstet Gynecol. 2:53–61. 2013.
View Article : Google Scholar
|
|
59
|
Pista A, de Oliveira CF, Cunha MJ, Paixao
MT and Real O; CLEOPATRE Portugal Study Group, : Risk factors for
human papillomavirus infection among women in Portugal: The
CLEOPATRE Portugal Study. Int J Gynaecol Obstet. 118:112–116. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
International Collaboration of
Epidemiological Studies of Cervical Cancer, . Comparison of risk
factors for invasive squamous cell carcinoma and adenocarcinoma of
the cervix: Collaborative reanalysis of individual data on 8,097
women with squamous cell carcinoma and 1,374 women with
adenocarcinoma from 12 epidemiological studies. Int J Cancer.
120:885–891. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
International Collaboration of
Epidemiological Studies of Cervical Cancer, . Appleby P, Beral V,
Berrington de González A, Colin D, Franceschi S, Goodhill A, Green
J, Peto J, Plummer M and Sweetland S: Cervical cancer and hormonal
contraceptives: Collaborative reanalysis of individual data for
16,573 women with cervical cancer and 35,509 women without cervical
cancer from 24 epidemiological studies. Lancet. 370:1609–1621.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Haverkos HW: Multifactorial etiology of
cervical cancer: A hypothesis. MedGenMed. 7:572005.PubMed/NCBI
|
|
63
|
Haverkos HW, Haverkos GP and O'Mara M:
Co-carcinogenesis: Human papillomaviruses, coal tar derivatives,
and squamous cell cervical cancer. Front Microbiol. 8:22532017.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Muñoz N, Castellsagué X, de González AB
and Gissmann L: HPV in the etiology of human cancer. Vaccine. 24
(Suppl 3):S1–S10. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Goodson WH III, Lowe L, Carpenter DO,
Gilbertson M, Manaf Ali A, Lopez de Cerain Salsamendi A, Lasfar A,
Carnero A, Azqueta A, Amedei A, et al: Assessing the carcinogenic
potential of low-dose exposures to chemical mixtures in the
environment: The challenge ahead. Carcinogenesis. 36 (Suppl
1):S254–S296. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Velema JP, Ferrera A, Figueroa M, Bulnes
R, Toro LA, de Barahona O, Claros JM and Melchers WJ: Burning wood
in the kitchen increases the risk of cervical neoplasia in
HPV-infected women in Honduras. Int J Cancer. 97:536–541. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Bennett C, Kuhn AE and Haverkos HW: Human
papillomavirus and tar hypothesis for squamous cell cervical
cancer. J Biosci. 35:331–337. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Fujiki H: Gist of Dr. Katsusaburo
Yamagiwa's papers entitled ‘Experimental study on the pathogenesis
of epithelial tumors’ (I to VI reports). Cancer Sci. 105:143–149.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Rotkin ID: Epidemiology of cancer of the
cervix. 3. Sexual characteristics of a cervical cancer population.
Am J Public Health Nations Health. 57:815–829. 1967. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Karki R, Pandya D, Elston RC and Ferlini
C: Defining ‘mutation’ and ‘polymorphism’ in the era of personal
genomics. BMC Med Genomics. 8:37. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Mehta AM, Mooij M, Brankovic I, Ouburg S,
Morre SA and Jordanova ES: Cervical carcinogenesis and immune
response gene polymorphisms: A review. J Immunol Res.
2017:89138602017. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Wang Y and Luo T: LINC00673 rs11655237
polymorphism is associated with increased risk of cervical cancer
in a Chinese population. Cancer Control. 25:10732748188039422018.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Lu W, Zhang H, Niu Y, Wu Y, Sun W, Li H,
Kong J, Ding K, Shen HM, Wu H, et al: Long non-coding RNA linc00673
regulated non-small cell lung cancer proliferation, migration,
invasion and epithelial mesenchymal transition by sponging
miR-150-5p. Mol Cancer. 16:1182017. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Zheng J, Huang X, Tan W, Yu D, Du Z, Chang
J, Wei L, Han Y, Wang C, Che X, et al: Pancreatic cancer risk
variant in LINC00673 creates a miR-1231 binding site and interferes
with PTPN11 degradation. Nat Genet. 48:747–757. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Weng SL, Wu WJ, Hsiao YH, Yang SF, Hsu CF
and Wang PH: Significant association of long non-coding RNAs HOTAIR
genetic polymorphisms with cancer recurrence and patient survival
in patients with uterine cervical cancer. Int J Med Sci.
15:1312–1319. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Pan W, Liu L, Wei J, Ge Y, Zhang J, Chen
H, Zhou L, Yuan Q, Zhou C and Yang M: A functional lncRNA HOTAIR
genetic variant contributes to gastric cancer susceptibility. Mol
Carcinog. 55:90–96. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Chu H, Chen Y, Yuan Q, Hua Q, Zhang X,
Wang M, Tong N, Zhang W, Chen J and Zhang Z: The HOTAIR, PRNCR1 and
POLR2E polymorphisms are associated with cancer risk: A
meta-analysis. Oncotarget. 8:43271–43283. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Yang Y, Liu Y, Li G, Li L, Geng P and Song
H: microRNA-214 suppresses the growth of cervical cancer cells by
targeting EZH2. Oncol Lett. 16:5679–5686. 2018.PubMed/NCBI
|
|
79
|
Pandey NO, Chauhan AV, Raithatha NS, Patel
PK, Khandelwal R, Desai AN, Choxi Y, Kapadia RS and Jain ND:
Association of TLR4 and TLR9 polymorphisms and haplotypes with
cervical cancer susceptibility. Sci Rep. 9:9729. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Fehri E, Ennaifer E, Ardhaoui M, Ouerhani
K, Laassili T, Bel Haj Rhouma R, Guizani I and Boubaker S:
Expression of Toll-like receptor 9 increases with progression of
cervical neoplasia in Tunisian women-a comparative analysis of
condyloma, cervical intraepithelial neoplasia and invasive
carcinoma. Asian Pac J Cancer Prev. 15:6145–6150. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Stamm S, Ben-Ari S, Rafalska I, Tang Y,
Zhang Z, Toiber D, Thanaraj TA and Soreq H: Function of alternative
splicing. Gene. 344:1–20. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Chen M and Manley JL: Mechanisms of
alternative splicing regulation: Insights from molecular and
genomics approaches. Nat Rev Mol Cell Biol. 10:741–754. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Tazi J, Bakkour N and Stamm S: Alternative
splicing and disease. Biochim Biophys Acta. 1792:14–26. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Gonçalves V, Pereira JFS and Jordan P:
Signaling pathways driving aberrant splicing in cancer cells. Genes
(Basel). 9:E92017. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Irimia M and Roy SW: Origin of
spliceosomal introns and alternative splicing. Cold Spring Harb
Perspect Biol. 6:a0160712014. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Skotheim RI and Nees M: Alternative
splicing in cancer: Noise, functional, or systematic? Int J Biochem
Cell Biol. 39:1432–1449. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Faustino NA and Cooper TA: Pre-mRNA
splicing and human disease. Genes Dev. 17:419–437. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Bisognin A, Pizzini S, Perilli L, Esposito
G, Mocellin S, Nitti D, Zanovello P, Bortoluzzi S and Mandruzzato
S: An integrative framework identifies alternative splicing events
in colorectal cancer development. Mol Oncol. 8:129–141. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Pal S, Gupta R and Davuluri RV:
Alternative transcription and alternative splicing in cancer.
Pharmacol Ther. 136:283–294. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Chen X, Du H, Liu B, Zou L, Chen W, Yang
Y, Zhu Y, Gong Y, Tian J, Li F and Zhong S: The associations
between RNA splicing complex gene SF3A1 polymorphisms and
colorectal cancer risk in a Chinese population. PLoS One.
10:e01303772015. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Tian J, Liu Y, Zhu B, Tian Y, Zhong R,
Chen W, Lu X, Zou L, Shen N, Qian J, et al: SF3A1 and pancreatic
cancer: New evidence for the association of the spliceosome and
cancer. Oncotarget. 6:37750–37757. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Yoshida K, Sanada M, Shiraishi Y, Nowak D,
Nagata Y, Yamamoto R, Sato Y, Sato-Otsubo A, Kon A, Nagasaki M, et
al: Frequent pathway mutations of splicing machinery in
myelodysplasia. Nature. 478:64–69. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Liu F, Dai M, Xu Q, Zhu X, Zhou Y, Jiang
S, Wang Y, Ai Z, Ma L, Zhang Y, et al: SRSF10-mediated IL1RAP
alternative splicing regulates cervical cancer oncogenesis via
mIL1RAP-NF-κB-CD47 axis. Oncogene. 37:2394–2409. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Liu B, Tian Y, Li F, Zhao Z, Jiang X, Zhai
C, Han X and Zhang L: Tumor-suppressing roles of miR-214 and
miR-218 in breast cancer. Oncol Rep. 35:3178–3184. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Korneev KV, Atretkhany KN, Drutskaya MS,
Grivennikov SI, Kuprash DV and Nedospasov SA: TLR-signaling and
proinflammatory cytokines as drivers of tumorigenesis. Cytokine.
89:127–135. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Zidi S, Sghaier I, Gazouani E, Mezlini A
and Yacoubi-Loueslati B: Evaluation of Toll-Like receptors 2/3/4/9
gene polymorphisms in cervical cancer evolution. Pathol Oncol Res.
22:323–330. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Yang X, Cheng Y and Li C: The role of TLRs
in cervical cancer with HPV infection: A review. Signal Transduct
Target Ther. 2:170552017. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Urban-Wojciuk Z, Khan MM, Oyler BL,
Fåhraeus R, Marek-Trzonkowska N, Nita-Lazar A, Hupp TR and Goodlett
DR: The role of TLRs in anti-cancer immunity and tumor rejection.
Front Immunol. 10:23882019. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Zhou Q, Zhu K and Cheng H: Toll-like
receptors in human papillomavirus infection. Arch Immunol Ther Exp
(Warsz). 61:203–215. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Hasimu A, Ge L, Li QZ, Zhang RP and Guo X:
Expressions of Toll-like receptors 3, 4, 7, and 9 in cervical
lesions and their correlation with HPV16 infection in Uighur women.
Chin J Cancer. 30:344–350. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
DeCarlo CA, Rosa B, Jackson R, Niccoli S,
Escott NG and Zehbe I: Toll-like receptor transcriptome in the
HPV-positive cervical cancer microenvironment. Clin Dev Immunol.
2012:7858252012. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Daud II, Scott ME, Ma Y, Shiboski S,
Farhat S and Moscicki AB: Association between toll-like receptor
expression and human papillomavirus type 16 persistence. Int J
Cancer. 128:879–886. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Hasan UA, Bates E, Takeshita F, Biliato A,
Accardi R, Bouvard V, Mansour M, Vincent I, Gissmann L, Iftner T,
et al: TLR9 expression and function is abolished by the cervical
cancer-associated human papillomavirus type 16. J Immunol.
178:3186–3197. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Hasan UA, Zannetti C, Parroche P, Goutagny
N, Malfroy M, Roblot G, Carreira C, Hussain I, Müller M,
Taylor-Papadimitriou J, et al: The human papillomavirus type 16 E7
oncoprotein induces a transcriptional repressor complex on the
Toll-like receptor 9 promoter. J Exp Med. 210:1369–1387. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Zolini GP, Lima GK, Lucinda N, Silva MA,
Dias MF, Pessoa NL, Coura BP, Cartelle CT, Arantes RM, Kroon EG and
Campos MA: Defense against HSV-1 in a murine model is mediated by
iNOS and orchestrated by the activation of TLR2 and TLR9 in
trigeminal ganglia. J Neuroinflammation. 11:202014. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Parroche P, Roblot G, Le Calvez-Kelm F,
Tout I, Marotel M, Malfroy M, Durand G, McKay J, Ainouze M,
Carreira C, et al: TLR9 re-expression in cancer cells extends the
S-phase and stabilizes p16(INK4a) protein expression. Oncogenesis.
5:e2442016. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Kaur G and Sethi RS: Multiple exposures to
poultry barn air and lipopolysaccharide synergistically increase
the pulmonary expression of TLR-4 and IL-1β. J Occup Health. Oct
27–2019.(Epub ahead of print). doi: 10.1002/1348-9585.12094.
PubMed/NCBI
|
|
108
|
Felekkis K, Touvana E, Stefanou C and
Deltas C: microRNAs: A newly described class of encoded molecules
that play a role in health and disease. Hippokratia. 14:236–240.
2010.PubMed/NCBI
|
|
109
|
Calin GA, Dumitru CD, Shimizu M, Bichi R,
Zupo S, Noch E, Aldler H, Rattan S, Keating M, Rai K, et al:
Frequent deletions and down-regulation of micro- RNA genes miR15
and miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad
Sci USA. 99:15524–15529. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Deng Y, Xiong Y and Liu Y: miR-376c
inhibits cervical cancer cell proliferation and invasion by
targeting BMI1. Int J Exp Pathol. 97:257–265. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Zhang B, Pan X, Cobb GP and Anderson TA:
microRNAs as oncogenes and tumor suppressors. Dev Biol. 302:1–12.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Fan Z, Cui H, Yu H, Ji Q, Kang L, Han B,
Wang J, Dong Q, Li Y, Yan Z, et al: MiR-125a promotes paclitaxel
sensitivity in cervical cancer through altering STAT3 expression.
Oncogenesis. 5:e2232016. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Fang W, Shu S, Yongmei L, Endong Z, Lirong
Y and Bei S: miR-224-3p inhibits autophagy in cervical cancer cells
by targeting FIP200. Sci Rep. 6:332292016. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Huang P, Xi J and Liu S: MiR-139-3p
induces cell apoptosis and inhibits metastasis of cervical cancer
by targeting NOB1. Biomed Pharmacother. 83:850–856. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Cheng X, Chen J and Huang Z: miR-372
promotes breast cancer cell proliferation by directly targeting
LATS2. Exp Ther Med. 15:2812–2817. 2018.PubMed/NCBI
|
|
116
|
Liao J, Lin J, Lin D, Zou C, Kurata J, Lin
R, He Z and Su Y: Down-regulation of miR-214 reverses erlotinib
resistance in non-small-cell lung cancer through up-regulating LHX6
expression. Sci Rep. 7:7812017. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Lu Q, Xu L, Li C, Yuan Y, Huang S and Chen
H: miR-214 inhibits invasion and migration via downregulating
GALNT7 in esophageal squamous cell cancer. Tumour Biol.
37:14605–14614. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Xia D, Li X, Niu Q, Liu X, Xu W, Ma C, Gu
H, Liu Z, Shi L, Tian X, et al: MicroRNA-185 suppresses pancreatic
cell proliferation by targeting transcriptional coactivator with
PDZ-binding motif in pancreatic cancer. Exp Ther Med. 15:657–666.
2018.PubMed/NCBI
|
|
119
|
Yu G, Wang J, Xu K and Dong J: Dynamic
regulation of uncoupling protein 2 expression by microRNA-214 in
hepatocellular carcinoma. Biosci Rep. 36:e003352016. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Zhu X, Ju S, Yuan F, Chen G, Shu Y, Li C,
Xu Y, Luo J and Xia L: microRNA-664 enhances proliferation,
migration and invasion of lung cancer cells. Exp Ther Med.
13:3555–3562. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Chen Z, Wang M, He Q, Li Z, Zhao Y, Wang
W, Ma J, Li Y and Chang G: MicroRNA-98 rescues proliferation and
alleviates ox-LDL-induced apoptosis in HUVECs by targeting LOX-1.
Exp Ther Med. 13:1702–1710. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Zhu Y, Wu G, Yan W, Zhan H and Sun P:
miR-146b-5p regulates cell growth, invasion, and metabolism by
targeting PDHB in colorectal cancer. Am J Cancer Res. 7:1136–1150.
2017.PubMed/NCBI
|
|
123
|
Dong J, Wang M, Ni D, Zhang L, Wang W, Cui
X, Fu S and Yao S: MicroRNA-217 functions as a tumor suppressor in
cervical cancer cells through targeting Rho-associated protein
kinase 1. Oncoly Lett. 16:5535–5542. 2018.
|
|
124
|
Scarinci IC, Garcia FAR, Kobetz E,
Partridge EE, Brandt HM, Bell MC, Dignan M, Ma GX, Daye JL and
Castle PE: Cervical cancer prevention: New tools and old barriers.
Cancer. 116:2531–2542. 2010.PubMed/NCBI
|
|
125
|
Adesina A, Chumba D, Nelson AM, Orem J,
Roberts DJ, Wabinga H, Wilson M and Rebbeck TR: Improvement of
pathology in sub-Saharan Africa. Lancet Oncol. 14:e152–e157. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Gelband H, Sankaranarayanan R, Gauvreau
CL, Horton S, Anderson BO, Bray F, Cleary J, Dare AJ, Denny L,
Gospodarowicz MK, et al: Costs, affordability, and feasibility of
an essential package of cancer control interventions in low-income
and middle-income countries: Key messages from Disease Control
Priorities, 3rd edition. Lancet. 387:2133–2144. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Goss PE, Lee BL, Badovinac-Crnjevic T,
Strasser-Weippl K, Chavarri-Guerra Y, St Louis J, Villarreal-Garza
C, Unger-Saldaña K, Ferreyra M, Debiasi M, et al: Planning cancer
control in Latin America and the Caribbean. Lancet Oncol.
14:391–436. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Jit M, Brisson M, Portnoy A and Hutubessy
R: Cost-effectiveness of female human papillomavirus vaccination in
179 countries: A PRIME modelling study. Lancet Glob Health.
2:e406–e414. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Schiller JT, Castellsagué X and Garland
SM: A review of clinical trials of human papillomavirus
prophylactic vaccines. Vaccine. 30:F123–F138. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
FUTURE II Study Group, : Quadrivalent
vaccine against human papillomavirus to prevent high-grade cervical
lesions. N Engl J Med. 356:1915–1927. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Joura EA, Giuliano AR, Iversen OE,
Bouchard C, Mao C, Mehlsen J, Moreira ED Jr, Ngan Y, Petersen LK,
Lazcano-Ponce E, et al: A 9-valent HPV vaccine against infection
and intraepithelial neoplasia in women. N Engl J Med. 372:711–723.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Levin A, Wang SA, Levin C, Tsu V and
Hutubessy R: Costs of introducing and delivering HPV vaccines in
low and lower middle income countries: Inputs for GAVI policy on
introduction grant support to countries. PLoS One. 9:e1011142014.
View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Goldie SJ, Gaffikin L, Goldhaber-Fiebert
JD, Gordillo-Tobar A, Levin C, Mahé C and Wright TC; Alliance for
Cervical Cancer Prevention Cost Working Group, : Cost-effectiveness
of cervical-cancer screening in five developing countries. N Engl J
Med. 353:2158–2168. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Cronjé HS and Beyer E: Screening for
cervical cancer in an African setting. Int J Gynaecol Obstet.
98:168–171. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Gakidou E, Nordhagen S and Obermeyer Z:
Coverage of cervical cancer screening in 57 countries: Low average
levels and large inequalities. PLoS Med. 5:e1322008. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Wake RM, Rebe K and Burch VC: Patient
perception of cervical screening among women living with human
immuno-deficiency virus infection attending an antiretroviral
therapy clinic in urban South Africa. J Obstet Gynaecol. 29:44–48.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Gopal S, Achenbach CJ, Yanik EL, Dittmer
DP, Eron JJ and Engels EA: Moving forward in HIV-associated cancer.
J Clin Oncol. 32:876–880. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Bruggmann D, Kayser L, Jaque J, Bundschuh
M, Klingelhöfer D and Groneberg DA: Human papilloma virus: Global
research architecture assessed by density-equalizing mapping.
Oncotarget. 9:21965–21977. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Ndejjo R, Mukama T, Musabyimana A and
Musoke D: Uptake of cervical cancer screening and associated
factors among women in Rural Uganda: A cross sectional study. PLoS
One. 11:e01496962016. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Idehen EE, Korhonen T, Castaneda A,
Juntunen T, Kangasniemi M, Pietilä AM and Koponen P: Factors
associated with cervical cancer screening participation among
immigrants of Russian, Somali and Kurdish origin: A
population-based study in Finland. BMC Women Health. 17:192017.
View Article : Google Scholar
|
|
141
|
Marlow LA, Wardle J and Waller J:
Understanding cervical screening non-attendance among ethnic
minority women in England. Br J Cancer. 113:833–839. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Olsson E, Lau M, Lifvergren S and
Chakhunashvili A: Community collaboration to increase foreign-born
women's participation in a cervical cancer screening program in
Sweden: A quality improvement project. Int J Equity Health.
13:622014. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Raine R, Fitzpatrick R, Barratt H, Bevan
G, Black N, Boaden R, Bower P, Campbell M, Denis JL, Devers K, et
al: Challenges, solutions and future directions in the evaluation
of service innovations in health care and public health. Health
Services and Delivery Researc. 4:162016.
|
|
144
|
Karl-Trummer U and Sardadvar S: The
interplay of health, migrant status and socioeconomic status in
eight EU countries. Health inequalities and risk factors among
migrants and ethnic minorities. COST Series Health Div. 1:79–92.
2012.
|
|
145
|
Simon J, Kiss N, Łaszewska A and Mayer S:
Public health aspects of migrant health: a review of the evidence
on health status for labour migrants in the European Region. World
Health Organisation Regional Office for Europe. 2015.
|
|
146
|
Kunckler M, Schumacher F, Kenfack B,
Catarino R, Viviano M, Tincho E, Tebeu PM, Temogne L, Vassilakos P
and Petignat P: Cervical cancer screening in a low-resource
setting: A pilot study on an HPV-based screen-and-treat approach.
Cancer Med. 6:1752–1761. 2017. View Article : Google Scholar : PubMed/NCBI
|