Introduction
Osteosarcoma (OS), the most frequent primary bone
malignancy affects adolescents and young adults, is characterized
by occurrence at the extremities of long bones and originating from
primitive osteogenic mesenchymal cells (1). OS is most common in pediatric bone
malignancies, accounting for ~5% of pediatric tumors (2). Currently, chemotherapeutic treatments
combined with surgical resection is one of the predominant
treatments for patients with OS (3,4). Despite
considerable progress in the diagnosis and treatment of OS, the
overall clinical efficacy of OS treatment remains unsatisfactory
(5,6). However, pulmonary metastasis is the
predominant site of osteosarcoma recurrence and the most general
cause of mortality (7). Thus, it is
necessary to look for new strategies for treatment of OS.
MicroRNAs (miRNAs) are a kind of regulatory RNA,
which are small endogenous non-coding RNAs consisting 18–25
nucleotides, and negatively regulate translation of the specific
target gene via directly binding to 3′UTR region of target mRNAs
(8). Increasing evidence has
demonstrated that miRNAs display important roles in modulating the
progression and development of various tumors (9–11).
Accumulating evidence confirms that dysregulated miRNAs contribute
to multiple physiological and pathological processes in different
malignancies (including osteosarcoma), such as apoptosis, cell
proliferation, and autophagy (12–14).
Extensive research has shown that microRNA-429 (miR-429)
deregulates and plays major roles in many tumors including
melanoma, esophageal squamous cell carcinoma, renal cell carcinoma,
and OS (15–18). Therefore, determining the exact role
of miR-93 in OS carcinogenesis might contribute to improving the
diagnosis and prognosis of patients with this tumor.
HOXA9, is a homeobox (HOX) gene, reported to
regulate several malignant diseases. For instance, increasing
expression of HOXA9 inhibited lung cancer cell invasion and
migration (19). Makabe et al
(20) found that HOXA9 was involved
in endometrial carcinogenesis. Furthermore, HOXA9 was reported to
promote leukemogenesis (21). In
addition, HOXA9 was reported as the direct target of some miRNAs in
regulation of tumor development and progression. A previous study
reported that HOXA9 acted as the target of miR-873 in regulating
the progression of OS (22). It was
also demonstrated as the target of miR-182 in regulating gastric
cancer cell proliferation and migration (23). However, whether HOXA9 is the target
of miR-429 in modulating OS cell viability, invasion and migration
has not been reported.
Wnt/β-catenin pathway is well known to participate
in tumorigenesis. Previous studies have stated that HOXA9 could
regulate Wnt/β-catenin pathway in glioma cell growth (24) and regulate OS cell proliferation and
apoptosis (25). In this study, we
aimed to test miR-429 functional role in OS progression and to
confirm whether miR-429 modulate OS progression by targeting HOXA9
through Wnt/β-catenin pathway.
Materials and methods
Samples
Fifty-six paired OS tissues and adjacent normal
tissues were collected from the patients who underwent surgery in
Weifang People's Hospitall (Weifang, China) between April 2011 and
October 2017. The mean age of all patients was 9.4 years, and the
age range 5–17 years. None of the patients received any treatment
prior to surgery. All the tissues were collected in the same
condition and were histopathologically verified carcinoma. The
fresh OS tissues were confirmed by pathologists and then stored at
−80°C for further experimental use. This study was conducted in
accordance with the Declaration of Helsinki. The Ethics Committee
of Weifang People's Hospital approved tissue sample collection and
use protocols. Written informed consent was obtained from each
patient and their parents before collecting the specimens. Tables I and II show the demographic features and
clinicopathological data.
 | Table I.Association between miR-429
expression and clinicopathological characteristics of patients with
OS. |
Table I.
Association between miR-429
expression and clinicopathological characteristics of patients with
OS.
|
|
| miR-429 |
|
|---|
|
|
|
|
|
|---|
|
Characteristics | Cases | Low | High | P-value |
|---|
| Age, years |
|
|
| 0.798 |
|
≥18 | 20 | 14 | 6 |
|
|
<18 | 36 | 24 | 12 |
|
| Sex |
|
|
| 0.176 |
|
Female | 30 | 18 | 12 |
|
|
Male | 26 | 20 | 6 |
|
| Tumor size, cm |
|
|
| 0.022a |
| ≤5 | 31 | 25 | 6 |
|
|
>5 | 25 | 13 | 12 |
|
| TNM stage |
|
|
| 0.012a |
| I | 26 | 22 | 4 |
|
|
II/III | 30 | 16 | 14 |
|
| Distant
metastasis |
|
|
| 0.013a |
| No | 29 | 24 | 5 |
|
|
Yes | 27 | 14 | 13 |
|
| Location |
|
|
| 0.022a |
|
Femur/Tibia | 28 | 23 | 5 |
|
|
Elsewhere | 28 | 15 | 13 |
|
 | Table II.Association between HOXA9 expression
and clinicopathological characteristics of patients with OS. |
Table II.
Association between HOXA9 expression
and clinicopathological characteristics of patients with OS.
|
|
| HOXA9 |
|
|---|
|
|
|
|
|
|---|
|
Characteristics | Cases | High | Low | P-value |
|---|
| Age, years |
|
|
| 0.472 |
|
≥18 | 30 | 18 | 12 |
|
|
<18 | 26 | 18 | 8 |
|
| Sex |
|
|
| 0.264 |
|
Female | 28 | 20 | 8 |
|
|
Male | 28 | 16 | 12 |
|
| Tumor size, cm |
|
|
| 0.012a |
| ≤5 | 32 | 25 | 7 |
|
|
>5 | 24 | 11 | 13 |
|
| TNM stage |
|
|
| 0.018a |
| I | 34 | 26 | 8 |
|
|
II/III | 22 | 10 | 12 |
|
| Distant
metastasis |
|
|
| 0.003a |
| No | 26 | 22 | 4 |
|
|
Yes | 30 | 14 | 16 |
|
| Location |
|
|
| 0.439 |
|
Femur/Tibia | 29 | 20 | 9 |
|
|
Elsewhere | 27 | 16 | 11 |
|
Cell culture
The OS cell lines (MG-63, U2OS, Saos-2) and normal
human fetal osteoblast (hFOB 1.19) cells were purchased from
Tianjin Sai'er Biotechnology Co., Ltd. The cells were cultured in
Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher
Scientific, Inc.) and DMEM/F-12 medium, respectively. The media
contained 10% fetal bovine serum (FBS; HyClone; GE Healthcare Life
Sciences), 100 IU/ml penicillin and 100 mg/ml streptomycin (both
from Baomanbio). The cells were maintained at 37°C with 5%
CO2.
Cell transfection
Overexpression of miR-429 in MG-63 cells and
knockdown of miR-429 in Saos-2 cells due to its expression was
lower in Saos-2 cells than in MG-63 cells. miR-429 mimic, miR-429
inhibitor, control mimic and control inhibitor were used for
increasing or decreasing miR-429, and was obtained from Shanghai
GenePharma Co., Ltd. HOXA9 siRNA was synthesized by Shanghai
GeneChem Co., Ltd. When the cells reached growth phase, they were
collected for transfection. Transfections were finished by using
the Lipofectamine™ 2000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). miR-429 mimic, forward,
5′-UAAUACUGUCUGGUAAAACCGU-3′ and reverse,
5′-GGUUUUACCAGACAGUAUUAUU-3′; NC mimic, forward,
5′-UUCUCCGAACGUGUCACGUUT-3′ and reverse,
5′-ACGUGACACGUUCGGAGAATT-3′; miR-429 inhibitor,
5′-GCTGATTTAAAGGCTTAG-3′; NC inhibitor, 5′-CAAATGTAGGTAGAGGA-3′;
HOXA9 siRNA, 5′-ACGGCAUUACCAGACAGUAUUA-3′; NC siRNA,
5′-AGCGUGUAGCUAGCAGAGG-3′. The efficiency of knockdown and
upregulation was analyzed using qRT-PCR.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
TRIzol Reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) was applied for extracting total RNA from OS
clinical tissues and cell lines. TaqMan MicroRNA Reverse
Transcription kit (Takara) was applied for performing reverse
transfection. TaqMan miRNA qRT-PCR Kit (Takara) was used for
performing quantitative RT-PCR. U6 and GAPDH were applied for
normalizing miR-429 level and HOXA9, respectively. The
thermocycling conditions were as follows: Initial denaturation at
95°C for 3 min and 35 cycles of denaturation at 95°C for 15 sec and
annealing/elongation at 60°C for 30 sec. A melting curve analysis
was performed to detect products. The relative expression was
analyzed using the 2−ΔΔCq method (26). The primer sequences are shown in
Table III.
 | Table III.Primer sequences for real-time
fluorescence quantification PCR. |
Table III.
Primer sequences for real-time
fluorescence quantification PCR.
| Gene | Primer
sequences |
|---|
| GAPDH | F:
5′-GCACCGTCAAGGCTGAGAAC-3′ |
|
| R:
5′-ATGGTGGTGAAGACGCCAGT-3′ |
| U6 | F:
5′-CTCGCTTCGGCAGCACA-3′ |
|
| R:
5′-AACGCTTCACGAATTTGCGT-3′ |
| miR-429 | F:
5′-GTCGTATCCAGTGCAGGGTCCGAGGTA-3′ |
|
| R:
5′-AGTGCAGGGTCCGAGGTATT-3′ |
| HOXA9 | F: 5′-
CAACAAAGACCGAGCAAA-3′ |
|
| R: 5′-
CAACAAAGACCGAGCAAA-3′ |
MTT assay
3-(4, 5-Dimethylthiazolyl-2)-
2,5-diphenyltetrazolium bromide (MTT) assay was carried out for
assessing cell viability. The cells treated with miR-429 mimic,
miR-429 inhibitor, control mimic and control inhibitor were seeded
in 96-well plates at a density of 2,000 cells/well with three
duplicate wells per group and cultured for 1, 2, 3, 4 days. MTT
solution (20 µl) was added to each well and incubated for another 4
h at 37°C. Then, the medium was removed and dimethyl sulfoxide
(DMSO) was added to dissolve formazan crystals by swirling gently.
Finally, the absorbance was measured at 490 nm using a
spectrophotometric plate reader.
Transwell assays
Transwell assays were applied for detection of cell
migration and invasion. The steps performed for the migration and
invasion were similar, except for the top chamber coating with or
without Matrigel. The Transwell chambers (8 µm pore filter) were
coated with or without 100 ml Matrigel (5 mg/ml) (BD Biosciences).
In brief, cells were added into the top chamber and DMEM medium
containing 20% FBS was added to the lower chamber. The upper and
lower chamber was separated by polycarbonate film. After incubation
for 24 h at 37°C, the cells were invaded or migrated to the lower
chamber. The non-migrated and non-invaded cells on the upper
surface of the membrane were carefully scraped off using a cotton
swab. Migrated and invaded cells were fixed with methanol and
stained with 0.5% crystal violet for 10 min at room temperature.
Then, the cells in the lower chamber were fixed, stained and
finally counted with a microscope (Olympus Corporation).
Western blot analysis
Radio immunoprecipitation assay (RIPA) lysis buffer
(Sigma-Aldrich; Merck KGaA) was applied for lysing OS cells. The
protein concentration was determined using the BCA assay, the
proteins (10 µg) were separated by 15% SDS-PAGE, followed by
transferred to NC membranes. Then, the membranes were blocked for 1
h at 37°C with 5% skimmed milk overnight. Subsequently incubated
with primary antibodies (HOXA9, ab140631, 1:1,000; β-catenin,
ab32572, 1:1,000; c-myc, ab32072, 1:1,000; p-c-Jun, ab32385,
1:1,000; GAPDH, ab181602, 1:1,000; all from Abcam) at room
temperature for 3 h, and then incubated with sheep anti-mouse or
donkey anti-rabbit horseradish peroxidase-conjugated (HRP) antibody
(GENXA931-1ML and GENA934-1ML, Sigma-Aldric; Merck KGaA). The
enhanced chemiluminescence reagent (ECL; Pierce Biotechnology,
Inc.) was used to conduct autoradiography.
Dual-luciferase reporter assay
The putative targets of miR-130a-3p were predicted
using the TargetScan (http://www.targetscan.org/vert_72/). The wild-type
(WT) or mutant type (MuT) of HOXA9 3′UTR fragments were sub-cloned
into the pMIR-REPORT vector. Then, the MG-63 and Saos-2 cells were
co-transfected with miR-429 mimic, miR-429 inhibitor, control mimic
and control inhibitor along with the 3′UTR of HOXA9 using
Lipofectamine 2000 reagent. After transfection for 48 h, the
luciferase activity was measured by a luciferase reporter assay
system (Promega Corporation), and Renilla luciferase
activity was used to normalize the data.
Statistical analysis
All experimental conditions were repeated in
duplicate. Results are presented as mean ± SD. Statistic analysis
was performed using SPSS v.19.0 software (SPSS, Inc.). Unpaired
Student's t-test or Tukey's post hoc test of one-way analysis of
variance (one-way ANOVA) was applied for comparing the differences
between two groups or more than two groups. The clinical
association was analyzed with Chi-square test. P<0.05, was
considered statistically significant.
Results
miR-429 expression is decreased and
HOXA9 expression is increased in OS
According to previous studies, miR-429 and HOXA9
were dysregulated in multiple tumors. Here, the aim was to test
miR-429 and HOXA9 expression in OS tissues and cell lines. As
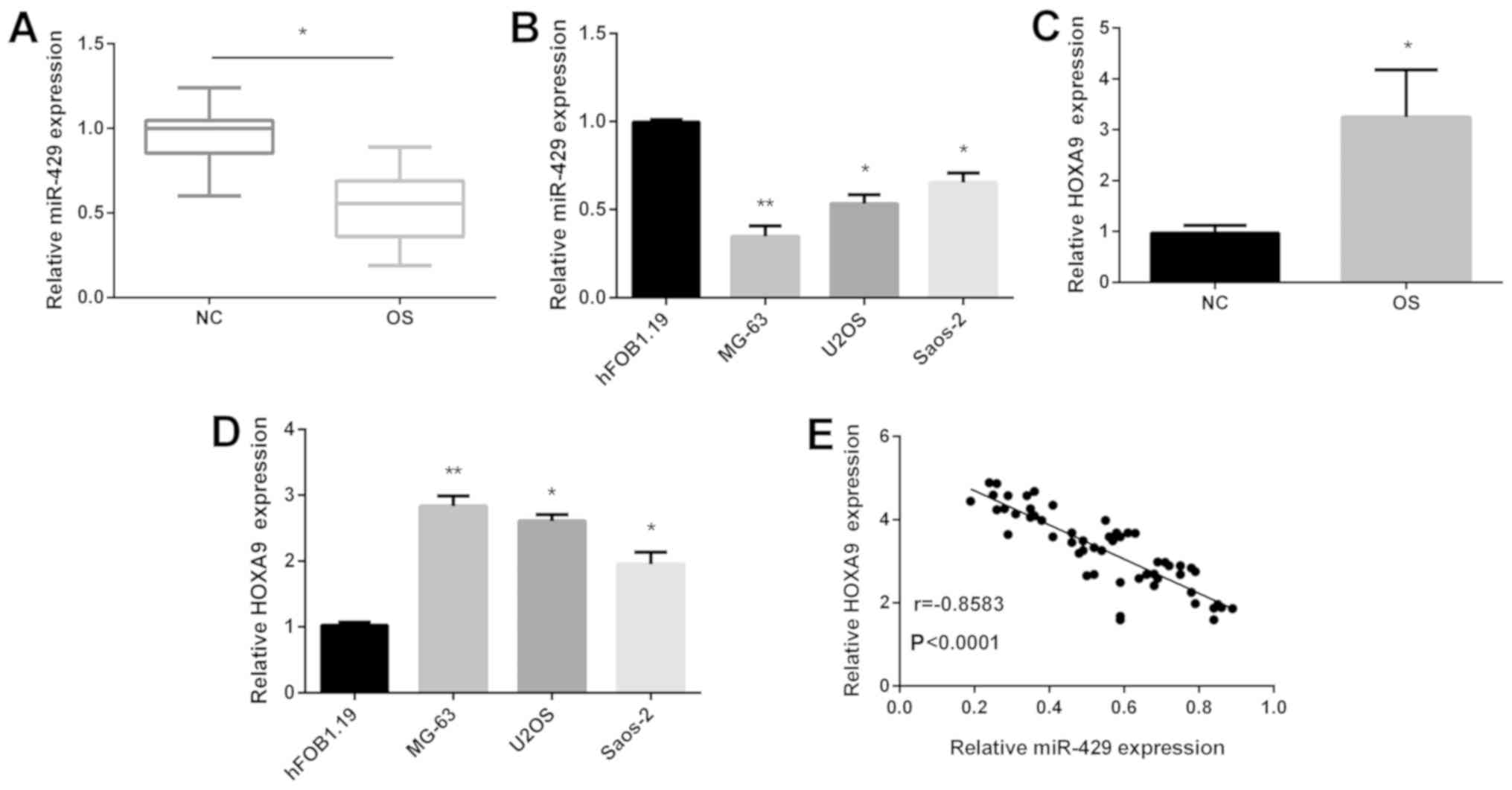
illustrated in Fig. 1A, the
expression of miR-429 was downregulated in OS tissues compared with
that in normal tissues. Subsequently, the expression of miR-429
expression was decreased in OS cell lines compared with that in
normal human fetal osteoblasts (hFOB 1.19) as shown in Fig. 1B. Moreover, miR-429 expression was
lowest in MG-63 cells compared with that in U2OS and Saos-2 cells
(Fig. 1B). Furthermore, the findings
also showed that HOXA9 was increased in OS tissues (Fig. 1C) and cell lines (Fig. 1D). Due to the opposite expression of
miR-429 and HOXA9 in OS, we detected the correlation between
miR-429 and HOXA9. Results showed that their relationship was
negative (Fig. 1E). To confirm their
clinical importance, we divided the subgroups (high/low) according
to the median value as a cutoff of miR-429 and HOXA9. In addition,
the expression of miR-429 (Table I)
and HOXA9 (Table II) were notably
associated with tumor size, TNM stage and distant metastasis.
Collectively, the above results indicated that miR-429 might serve
as a tumor suppressor while HOXA9 serves as an oncogene in OS.
miR-429 suppresses OS cell viability,
invasion and migration
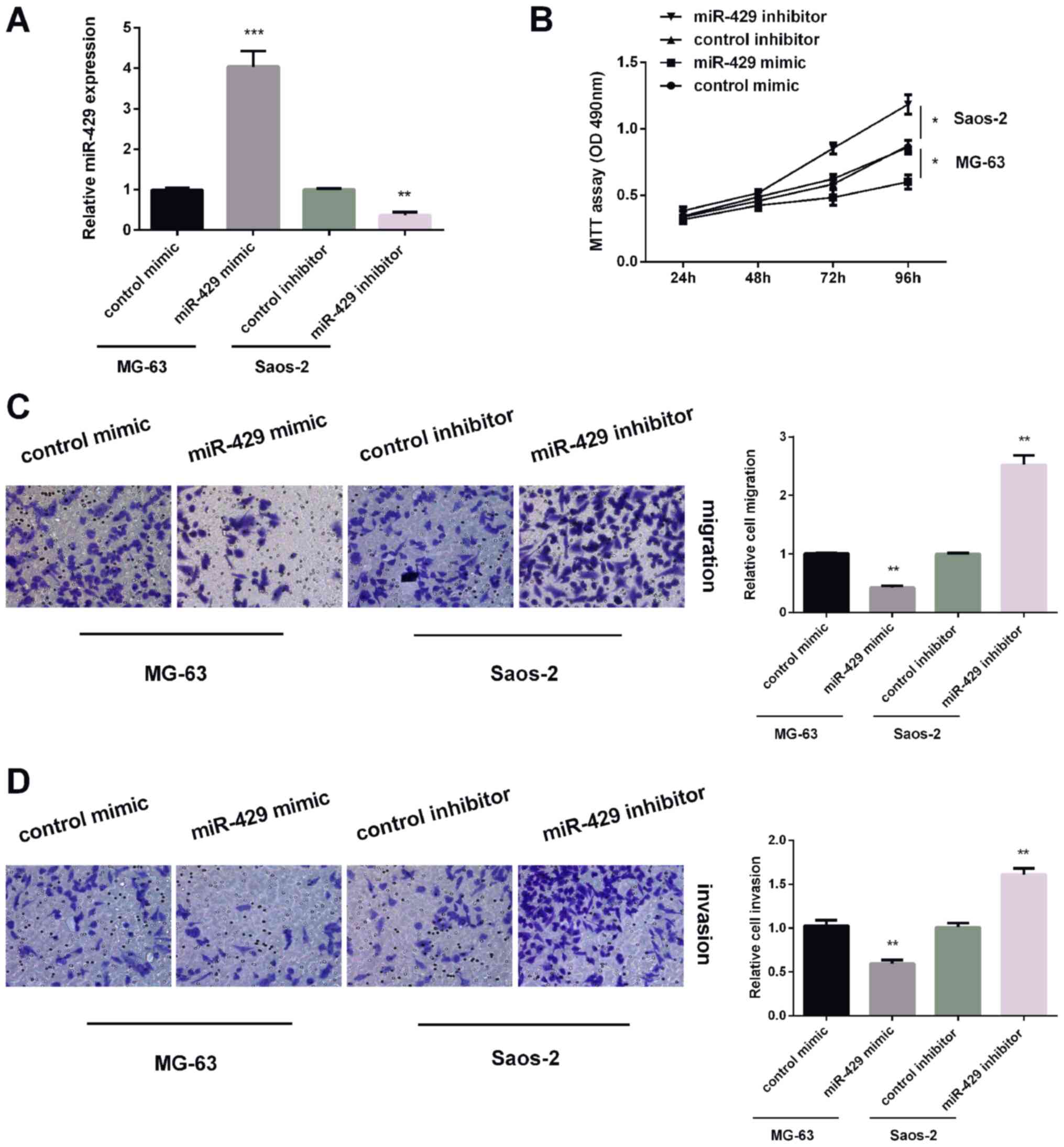
As stated, the expression of miR-429 was highest in
Saos-2 cells, the expression of miR-429 was lowest in MG-63 cells.
Thus, miR-429 inhibitor was transfected in Saos-2 cells and miR-429
mimic in MG-63 cells. Results of qRT-PCR revealed that miR-429
expression was increased by miR-429 mimic in MG-63 cells and
reduced by miR-429 inhibitor in Saos-2 cells (Fig. 2A). MTT assay was applied for testing
MG-63 cell viability after treated with miR-429 mimic or Saos-2
cells after treated with miR-429 inhibitor. The results of MTT
assay showed that miR-429 overexpression suppressed MG-63 cell
proliferation, while miR-429 knockdown remarkably promoted Saos-2
cell proliferation (Fig. 2B).
Furthermore, Transwell analysis showed that miR-429 overexpression
reduced MG-63 cell migration and miR-429 knockdown showed the
opposite effect on Saos-2 cells (Fig.
2C). For invasion, upregulating of miR-429 expression inhibited
MG-63 cell invasion, while downregulating of miR-429 expression
enhanced Saos-2 cell invasion (Fig.
2D). These findings demonstrated that miR-429 inhibited OS cell
proliferation, invasion and migration.
HOXA9 is the target of miR-429
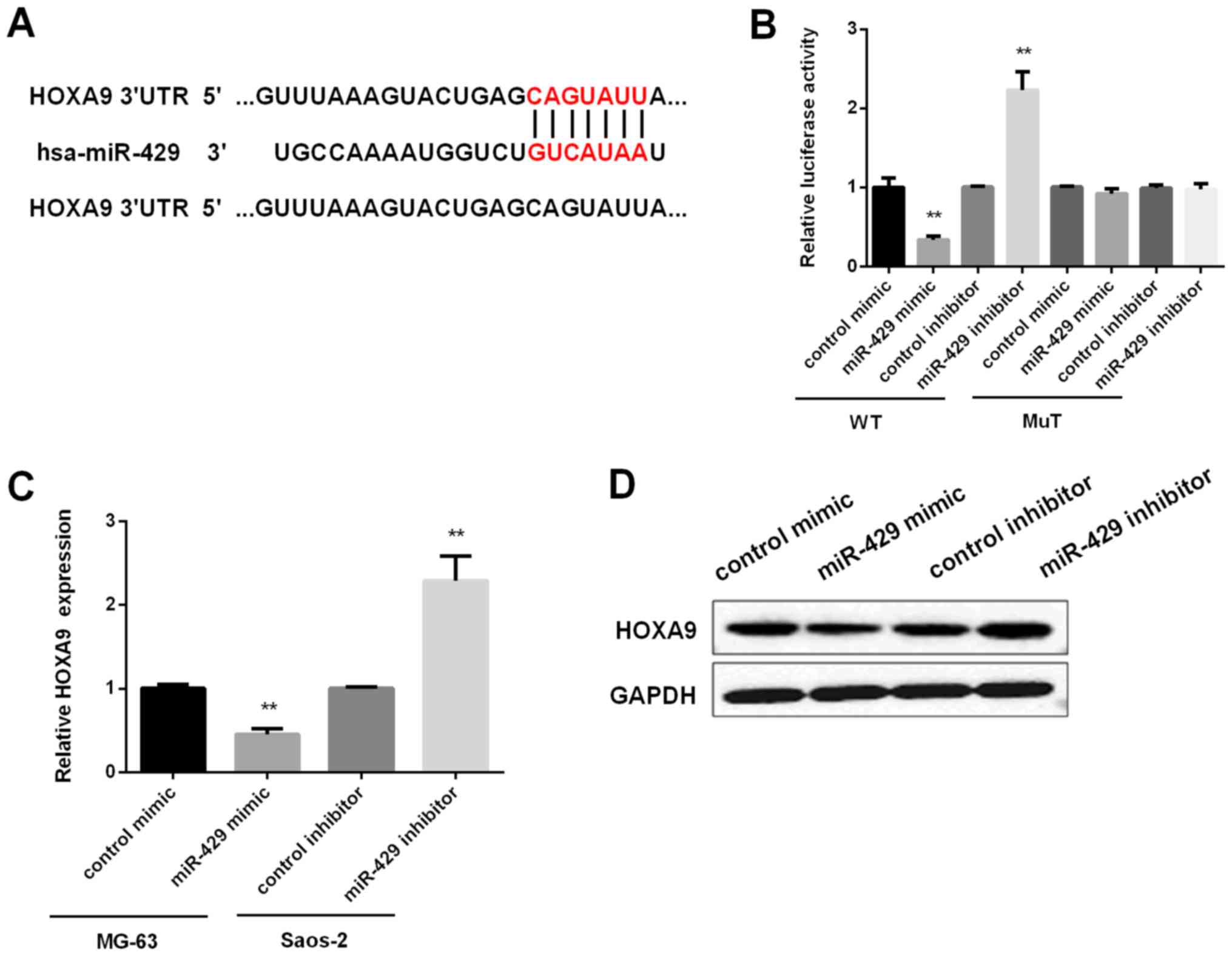
As we found that HOXA9 mRNA in tissues was inversely
correlated with miR-429 expression, we further investigated whether
HOXA9 was the target of miR-429. By searching both TargetScan
(http://www.targetscan.org) and Sanger
(http://microrna.sanger.ac.uk) databases,
it was found that the 3′UTR of HOXA9 contains conserved
miR-429-binding sites (Fig. 3A).
Then, luciferase reporter assay was applied for confirmation. The
findings revealed that overexpression of miR-429 significantly
suppressed firefly luciferase reporter activity of the WT HOXA9
3′UTR, while miR-429 knockdown increased its luciferase activity,
however, they did not affect the luciferase activity of MuT-HOXA9
3′UTR (Fig. 3B). Next, qRT-PCR and
Western blot analysis were carried out for detecting the effect of
miR-429 on HOXA9 expression in mRNA and protein level,
respectively. The findings demonstrated that miR-429 reversely
regulated the mRNA and protein expression of HOXA9 in OS cells
(Fig. 3C and D). Taken together, the
above results confirmed the inverse relationship between miR-429
and HOXA9.
Knockdown of HOXA9 reverses the
effects of miR-429 on OS cells
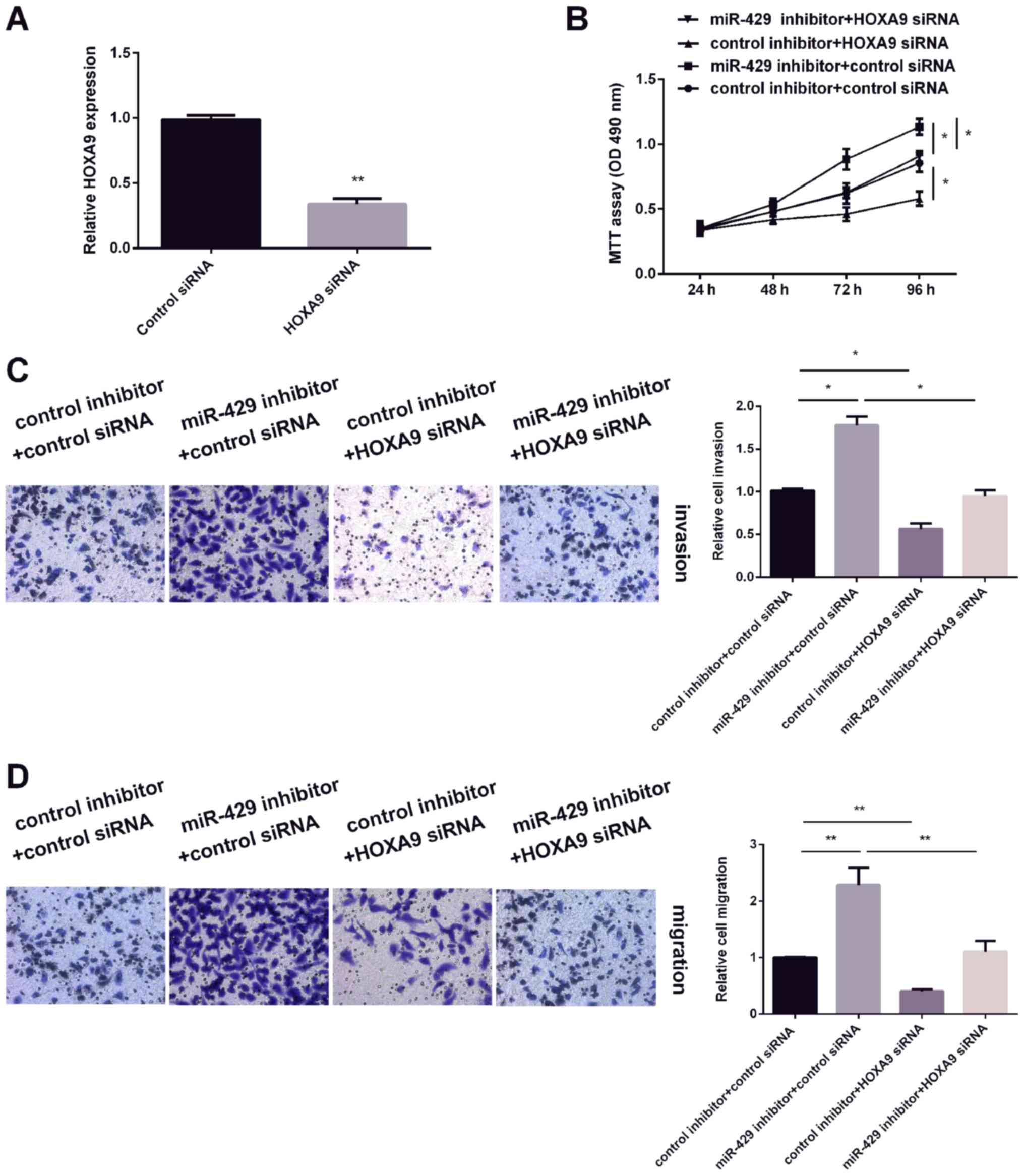
The effect of HOXA9 on OS cell proliferation,
invasion and migration were measured. As HOXA9 was overexpressed in
OS tissues and cell lines compared with normal tissues and human
fetal osteoblasts (hFOB 1.19), HOXA9 siRNA was tranfected into OS
cells to inhibit HOXA9 expression. As expected (Fig. 4A), HOXA9 expression was decreased
after transfection with HOXA9 siRNA. The results of MTT assay found
that knockdown of HOXA9 expression inhibited OS cell viability,
while decreasing miR-429 expression promoted cell expression.
Furthermore, HOXA9 siRNA reversed the promotion effect of miR-429
inhibitor on OS cell viability (Fig.
4B). In addition, similar effects were observed on the
migration and invasion capacity of OS cells (Fig. 4C and D). These findings demonstrated
that HOXA9 is involved in miR-429-mediated regulation of OS
cells.
miR-429 regulates Wnt/β-catenin
pathway in vitro
To further examine the mechanism of miR-429 in
modulating OS progression, the expression of the downstream genes
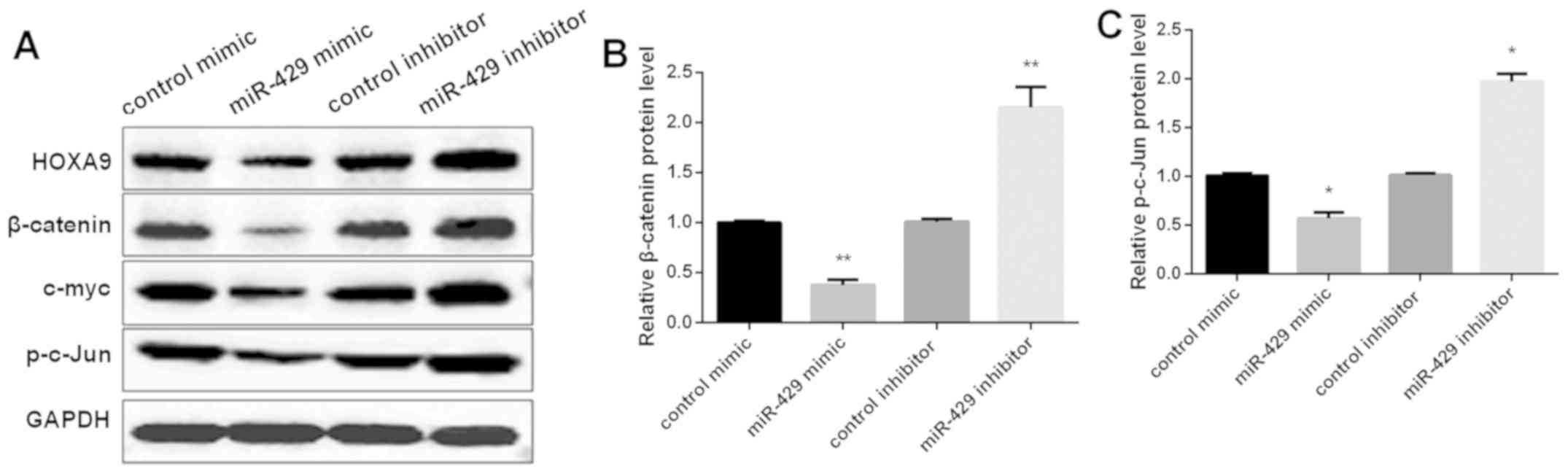
of Wnt/β-catenin pathway was tested by Western blot analysis. As
shown in Fig. 5A and B, miR-429
overexpression suppressed the expression level of β-catenin, c-myc
and p-c-Jun, as well as HOXA9 in MG-63 cells, while knockdown of
miR-429 exhibited the opposite effect on these levels. Taken
together, the findings indicated that miR-429 inhibited OS
progression by targeting HOXA9 and suppressing Wnt/β-catenin
pathway.
Discussion
OS is the most frequent primary bone malignancy, it
affects adolescents and young adults, and is characterized by high
incidence, great potential for metastasis and rapid progression
(27,28). Although years of continuous progress
have been obtained in OS therapy, the metastasis rates and
mortality of OS are still very high, and the clinical effect of OS
treatment remains unsatisfactory (29). Therefore, it is urgent to identify
targets for the development of therapeutics for OS. The molecular
mechanisms of OS carcinogenesis, especially regarding alterations
of miRNAs in OS have attracted much attention in recent
decades.
Increasing evidence has indicated that miRNAs play
vital roles as diagnostics biomarkers and therapeutic targets in
human malignancies, and expression of miRNAs are dysregulated,
including OS (30,31). miR-429, in combination with the other
four members (miR200a, miR-200b, miR-200c and miR-141), forms the
miR-200 family (32). miR-429 mostly
took part in the development of many tumors as a tumor suppressor.
For example, miR-429 expression was downregulated in human thyroid
cancer and it inhibited cell growth and promoted cell apoptosis
(33). Moreover, miR-429 served as a
suppressor in cervical cancer (34)
and in pancreatic cancer (35).
However, studies concerning miR-429 expression and its functional
effect in OS are still rare and it is therefore necessary to
investigate the underlying mechanisms of miR-429 in OS. Herein, it
was shown that miR-429 was decreased in OS tissues and cells, which
was in line with the studies reported by Liu et al and Deng
et al (18,36). Based on previous studies, we further
found that miR-429 suppressed OS cell proliferation, invasion and
migration. The low expression of miR-429 was obviously associated
with adverse clinical pathological issues of OS patients, including
OS tumor size, TNM stage and clinical stage.
Combined with the bioinformatic analysis tool and
dual-luciferase activity reporter assay, HOXA9 was validated a
putative target for miR-429. HOXA9 is a member of the HOX gene
family reported to have crucial roles in regulating hematopoiesis
(37). Previous studies have shown
that HOXA9 was the target of miRNAs in acute myeloid leukemia
(38), in cutaneous squamous cell
carcinoma (39) and in ovarian
cancer (40). Therefore, we
investigated whether HOXA9 was a mediator of miR-429 tumor
inhibition in OS cells. In this study, it is first displayed that
HOXA9 is a direct target of miR-429 in OS progression. Moreover, it
was found that HOXA9 acted as an oncogene in OS development. These
findings are consistent with previous research that HOXA9 level is
increased markedly in ovarian cancer patients (41) and it was upregulated in colon cancer
(42). The higher expression of
HOXA9 was associated with OS tumor size, TNM stage and clinical
stage. Furthermore, we showed that inhibiting HOXA9 suppressed OS
cell viability, invasion and migration and it could overturn
miR-429 inhibitor effect on OS progression. The above findings
suggested miR-429 may act as a tumor suppressor by increasing HOXA9
expression in OS progression. HOXA9 overexpression reverses the
inhibition effects of miR-429 on OS cells.
Increasing evidence shows that Wnt/β-catenin axis
was regulated, for example, by miR-25, −376c, −21, −34a miRNAs in
OS progression (43–45). Wnt/β-catenin signaling pathway
modulates various genes that in turn regulate a diversity of cell
functions such as proliferation, differentiation and morphogenesis,
and β-catenin was proved to enhance progression, invasion and
tumorigenesis in cancers (46).
Increasing evidence has suggested that the signaling pathway was
activated in OS cells and the aberrant regulation of the signaling
pathway results in tumorigenesis of OS cells (47–49). To
confirm the involvement of Wnt/β-catenin signaling pathway in
regulating migration of OS cells, Western blotting was performed to
analyze the nuclear fractions of β-catenin, c-myc and p-c-Jun. In
this study, results show that miR-429 upregulation deactivated the
Wnt/β-catenin pathway, while suppressing miR-429 promoted the
activation of this pathway.
In conclusion, miR-429 was underexpressed in OS and
repressed OS progression, whereas, HOXA9 was overexpressed in OS
and facilitated OS progression. Moreover, HOXA9 was shown as the
direct target of miR-429 in OS. Taken together, miR-429 suppressed
OS progression by targeting HOXA9 via Wnt/β-catenin signaling
pathway.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study areavailable from the corresponding author on reasonable
request.
Authors' contributions
LS and LW contributed to the conception of the
study. SL contributed significantly to the data analysis and study
preparation. YJ and QW performed the data analyses and wrote the
study. LS helped perform the analysis with constructive
discussions. All authors read and approved the final study.
Ethics approval and consent to
participate
The study was conducted in accordance with the
Declaration of Helsinki and was approved by the Ethics Committee of
Weifang People's Hospital (Weifang, China). Written informed
consent was obtained from each patient and their parents before
collecting the specimens.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Li J, Yang Z, Li Y, Xia J, Li D, Li H, Ren
M, Liao Y, Yu S, Chen Y, et al: Cell apoptosis, autophagy and
necroptosis in osteosarcoma treatment. Oncotarget. 7:44763–44778.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Endo-Munoz L, Cumming A, Sommerville S,
Dickinson I and Saunders NA: Osteosarcoma is characterised by
reduced expression of markers of osteoclastogenesis and antigen
presentation compared with normal bone. Br J Cancer. 103:73–81.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vos HI, Coenen MJ, Guchelaar HJ and Te Loo
DM: The role of pharmacogenetics in the treatment of osteosarcoma.
Drug Discov Today. 21:1775–1786. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Amankwah EK, Conley AP and Reed DR:
Epidemiology and therapies for metastatic sarcoma. Clin Epidemiol.
5:147–162. 2013.PubMed/NCBI
|
|
5
|
Bishop MW, Janeway KA and Gorlick R:
Future directions in the treatment of osteosarcoma. Curr Opin
Pediatr. 28:26–33. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Isakoff MS, Bielack SS, Meltzer P and
Gorlick R: Osteosarcoma: Current treatment and a collaborative
pathway to success. J Clin Oncol. 33:3029–3035. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mayer MN and Grier CK: Palliative
radiation therapy for canine osteosarcoma. Can Vet J. 47:707–709.
2006.PubMed/NCBI
|
|
8
|
Croce CM: Causes and consequences of
microRNA dysregulation in cancer. Nat Rev Genet. 10:704–714. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ning Q, Liu YF, Ye PJ, Gao P, Li ZP, Tang
SY, He DX, Tang SS, Wei H and Yu CY: Delivery of liver-specific
miRNA-122 using a targeted macromolecular prodrug toward
synergistic therapy for hepatocellular carcinoma. ACS Appl Mater
Interfaces. 11:10578–10588. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lin X, Wang S, Sun M, Zhang C, Wei C, Yang
C, Dou R, Liu Q and Xiong B: miR-195-5p/NOTCH2-mediated EMT
modulates IL-4 secretion in colorectal cancer to affect M2-like TAM
polarization. J Hematol Oncol. 12:202019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zuo S, Dai G, Wang L, Wen Y, Huang Z, Yang
W, Ma W and Ren X: Suppression of angiogenesis and tumor growth by
recombinant T4 phages displaying extracellular domain of vascular
endothelial growth factor receptor 2. Arch Virol. 164:69–82. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Weidhaas J: Using microRNAs to understand
cancer biology. Lancet Oncol. 11:106–107. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nana-Sinkam SP and Croce CM: MicroRNA
regulation of tumorigenesis, cancer progression and interpatient
heterogeneity: Towards clinical use. Genome Biol. 15:4452014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang D, Wang F, Wu W, Lian C and Liu E:
MicroRNA-429 inhibits cancer cell proliferation and migration by
targeting the AKT1 in melanoma. Cancer Biomark. 26:63–68. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zong M, Liu Y, Zhang K, J Y and Chen L:
The effects of miR-429 on cell migration and invasion by targeting
Slug in esophageal squamous cell carcinoma. Pathol Res Pract.
215:1525262019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Su Z, Jiang G, Chen J, Liu X, Zhao H, Fang
Z, He Y, Jiang X and Xu G: MicroRNA-429 inhibits cancer cell
proliferation and migration by targeting AKT1 in renal cell
carcinoma. Mol Clin Oncol. 12:75–80. 2020.PubMed/NCBI
|
|
18
|
Liu X, Liu Y, Wu S, Shi X, Li L, Zhao J
and Xu H: Tumor-suppressing effects of miR-429 on human
osteosarcoma. Cell Biochem Biophys. 70:215–224. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yu SL, Koo H, Lee HY, Yeom YI, Lee DC and
Kang J: Recombinant cell-permeable HOXA9 protein inhibits NSCLC
cell migration and invasion. Cell Oncol (Dordr). 42:275–285. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Makabe T, Arai E, Hirano T, Ito N,
Fukamachi Y, Takahashi Y, Hirasawa A, Yamagami W, Susumu N, Aoki D,
et al: Genome-wide DNA methylation profile of early-onset
endometrial cancer: Its correlation with genetic aberrations and
comparison with late-onset endometrial cancer. Carcinogenesis.
40:611–623. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sun Y, Zhou B, Mao F, Xu J, Miao H, Zou Z,
Phuc Khoa LT, Jang Y, Cai S, Witkin M, et al: HOXA9 reprograms the
enhancer landscape to promote leukemogenesis. Cancer Cell.
34:643–658, e5. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu Y, Wang Y, Yang H, Zhao L, Song R, Tan
H and Wang L: MicroRNA 873 targets HOXA9 to inhibit the aggressive
phenotype of osteosarcoma by deactivating the Wnt/β catenin
pathway. Int J Oncol. 54:1809–1820. 2019.PubMed/NCBI
|
|
23
|
Yu J, Tian X, Chang J, Liu P and Zhang R:
RUNX3 inhibits the proliferation and metastasis of gastric cancer
through regulating miR-182/HOXA9. Biomed Pharmacother. 96:782–791.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zheng DH, Wang X, Lu LN, Chen DL, Chen JM,
Lin FM and Xu XB: MiR-638 serves as a tumor suppressor by targeting
HOXA9 in glioma. Eur Rev Med Pharmacol Sci. 22:7798–7806.
2018.PubMed/NCBI
|
|
25
|
Zhang ZF, Wang YJ, Fan SH, Du SX, Li XD,
Wu DM, Lu J and Zheng YL: MicroRNA-182 downregulates Wnt/β-catenin
signaling, inhibits proliferation, and promotes apoptosis in human
osteosarcoma cells by targeting HOXA9. Oncotarget. 8:101345–101361.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gianferante DM, Mirabello L and Savage SA:
Germline and somatic genetics of osteosarcoma - connecting
aetiology, biology and therapy. Nat Rev Endocrinol. 13:480–491.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lindsey BA, Markel JE and Kleinerman ES:
Osteosarcoma overview. Rheumatol Ther. 4:25–43. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gorlick R and Khanna C: Osteosarcoma. J
Bone Miner Res. 25:683–691. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu X, Zhang C, Wang C, Sun J, Wang D,
Zhao Y and Xu X: miR-210 promotes human osteosarcoma cell migration
and invasion by targeting FGFRL1. Oncol Lett. 16:2229–2236.
2018.PubMed/NCBI
|
|
31
|
Wang L, Hu K and Chao Y: MicroRNA-1301
inhibits migration and invasion of osteosarcoma cells by targeting
BCL9. Gene. 679:100–107. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Alvarez-Garcia I and Miska EA: MicroRNA
functions in animal development and human disease. Development.
132:4653–4662. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wu G, Zheng H, Xu J, Guo Y, Zheng G, Ma C,
Hao S, Liu X, Chen H, Wei S, et al: miR-429 suppresses cell growth
and induces apoptosis of human thyroid cancer cells by targeting
ZEB1. Artif Cells Nanomed Biotechnol. 47:548–554. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shen F, Zheng H, Zhou L, Li W and Xu X:
Overexpression of MALAT1 contributes to cervical cancer progression
by acting as a sponge of miR-429. J Cell Physiol. 234:11219–11226.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu D, Song L, Dai Z, Guan H, Kang H,
Zhang Y, Yan W, Zhao X and Zhang S: MiR-429 suppresses
neurotrophin-3 to alleviate perineural invasion of pancreatic
cancer. Biochem Biophys Res Commun. 505:1077–1083. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Deng Y, Luan F, Zeng L, Zhang Y and Ma K:
MiR-429 suppresses the progression and metastasis of osteosarcoma
by targeting ZEB1. EXCLI J. 16:618–627. 2017.PubMed/NCBI
|
|
37
|
Pineault N, Helgason CD, Lawrence HJ and
Humphries RK: Differential expression of Hox, Meis1, and Pbx1 genes
in primitive cells throughout murine hematopoietic ontogeny. Exp
Hematol. 30:49–57. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Schneider E, Staffas A, Röhner L, Malmberg
ED, Ashouri A, Krowiorz K, Pochert N, Miller C, Wei SY, Arabanian
L, et al: Micro-ribonucleic acid-155 is a direct target of Meis1,
but not a driver in acute myeloid leukemia. Haematologica.
103:246–255. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhou L, Wang Y, Zhou M, Zhang Y, Wang P,
Li X, Yang J, Wang H and Ding Z: HOXA9 inhibits HIF-1α-mediated
glycolysis through interacting with CRIP2 to repress cutaneous
squamous cell carcinoma development. Nat Commun. 9:14802018.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chong GO, Jeon HS, Han HS, Son JW, Lee YH,
Hong DG, Park HJ, Lee YS and Cho YL: Overexpression of
microRNA-196b accelerates invasiveness of cancer cells in recurrent
epithelial ovarian cancer through regulation of homeobox A9. Cancer
Genomics Proteomics. 14:137–141. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Thomsen CB, Andersen RF, Steffensen KD,
Adimi P and Jakobsen A: Delta tocotrienol in recurrent ovarian
cancer. A phase II trial. Pharmacol Res. 141:392–396. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bhatlekar S, Ertel A, Gonye GE, Fields JZ
and Boman BM: Gene expression signatures for HOXA4, HOXA9, and
HOXD10 reveal alterations in transcriptional regulatory networks in
colon cancer. J Cell Physiol. 234:13042–13056. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yoshida A, Fujiwara T, Uotani K, Morita T,
Kiyono M, Yokoo S, Hasei J, Nakata E, Kunisada T and Ozaki T:
Clinical and functional significance of intracellular and
extracellular microRNA-25-3p in osteosarcoma. Acta Med Okayama.
72:165–174. 2018.PubMed/NCBI
|
|
44
|
Kureel J, John AA, Prakash R and Singh D:
MiR 376c inhibits osteoblastogenesis by targeting Wnt3 and
ARF-GEF-1-facilitated augmentation of beta-catenin transactivation.
J Cell Biochem. 119:3293–3303. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kushlinskii NE, Fridman MV and Braga EA:
Molecular mechanisms and microRNAs in osteosarcoma pathogenesis.
Biochemistry (Mosc). 81:315–328. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Liu X, Li L, Lv L, Chen D, Shen L and Xie
Z: Apigenin inhibits the proliferation and invasion of osteosarcoma
cells by suppressing the Wnt/β-catenin signaling pathway. Oncol
Rep. 34:1035–1041. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhang J, Yu XH, Yan YG, Wang C and Wang
WJ: PI3K/Akt signaling in osteosarcoma. Clin Chim Acta.
444:182–192. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Graziano AC, Cardile V, Avola R, Vicario
N, Parenti C, Salvatorelli L, Magro G and Parenti R: Wilms' tumor
gene 1 silencing inhibits proliferation of human osteosarcoma MG-63
cell line by cell cycle arrest and apoptosis activation.
Oncotarget. 8:13917–13931. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Perry JA, Kiezun A, Tonzi P, Van Allen EM,
Carter SL, Baca SC, Cowley GS, Bhatt AS, Rheinbay E, Pedamallu CS,
et al: Complementary genomic approaches highlight the PI3K/mTOR
pathway as a common vulnerability in osteosarcoma. Proc Natl Acad
Sci USA. 111:E5564–E5573. 2014. View Article : Google Scholar : PubMed/NCBI
|