Introduction
The incidence of hepatocellular carcinoma (HCC),
which predominantly occurs in patients with a cirrhotic liver, is
increasing and has become the second leading cause of
cancer-associated death worldwide, accounting for 746,000 cases or
9.1% of all cancer death in 2012 (1). Amongst all the potential treatment
options, including local reginal therapy, resection and
chemotherapy, liver transplant has the most favorable outcome and
results in improved overall survival times (2). The United Network for Organ Sharing and
the Organ Procurement and Transplantation Network have increased
the priority allocation of liver transplants for patients with HCC
nodules between 1–2 cm (3). In
addition, resection of very early stage HCCs, with a diameter <2
cm, increases the overall 5-year survival rate of patients
(4). Therefore, diagnosing HCC at an
earlier stage, particularly for small HCC lesions is important.
Currently, dynamic contrast enhanced magnetic resonance imaging
(MRI) is the most accurate imaging modality for the diagnosis of
HCC (5). Notably, up to 45% of small
HCC cases may be misdiagnosed, according to the MR diagnostic
criteria (6). Diffusion weighted
imaging (DWI) MRI has been found to increase the sensitivity in the
diagnosis of small HCC when combined with conventional MRI
(7–10). However, all of these previous studies
were individual studies. Differences in population characteristics,
patient risk estimation, study design and imaging protocols reduce
the reliability of the results from individual studies; therefore,
additional studies are required to confirm its value. Thus, the
present meta-analysis was performed to determine the value of DWI
combined with contrast-enhanced MRI for small HCC, with diameters
≤2 cm.
Materials and methods
Literature search
A systematic literature search using PubMed
(https://pubmed.ncbi.nlm.nih.gov), Embase
(https://www.embase.com), Web of Science
(https://apps.webofknowledge.com) and
Cochrane Library databases (https://www.cochranelibrary.com) were performed
independently by three radiologists to identify articles published
prior to June 2019, using the key words ‘hepatocellular carcinoma’,
‘liver cancer’, ‘liver cell carcinoma’, ‘magnetic resonance
imaging’, ‘diffusion magnetic resonance imaging’, ‘diffusion MRI’
and ‘diffusion weighted MRI’. Related citations in eligible
articles were also assessed for inclusion.
Study selection
The three radiologists reviewed all 2447 abstracts
after duplication removal and subsequently the full text of the 119
articles was obtained if the following inclusion criteria was
fulfilled: i) Included the diagnostic accuracy of MRI with DWI for
HCC; ii) constituted original research rather than a meta-analysis,
a review article, case report or case series; iii) published in
English; and iv) results are from humans and not animals. For the
studies in which the full text was reviewed, the following
inclusion criteria was used: i) Included original data regarding
the detection of small HCC lesions, ≤2 cm; ii) included both
contrast-enhanced MRI and DWI; iii) included sufficient data, with
>20 patients to calculate true positive (TP), false positive
(FP), false negative (FN) and true negative (TN) for constructing a
2×2 contingency table; and iv) patients diagnosed with hepatic
lesion using pathological analysis (surgical resection, explant
and/or biopsy) or imaging from follow-up according to the
guidelines for standardization of liver imaging, diagnosis,
classification and reporting of hepatocellular carcinoma (3). In addition, articles from the same
institution, which included an overlap period of patient
recruitment were considered to have an overlapping population. In
these cases, the study, which had the larger number of small HCCs
cases, was included. If there were disagreements between the three
investigators, the consensus amongst the three radiologists was
used to resolve the disagreement. Disagreements were resolved
following discussions between the three investigators, until at
least two of the investigators reached the same conclusion.
Attempts were made to contact the authors of the article only if
data for the 2×2 contingency table was not fulfilled from the
inclusion criteria (authors of two articles had been contacted for
this study). A total of 109 studies were excluded according to the
following exclusion criteria: i) They were not relevant to the
present meta-analysis if they fit one of the followings conditions:
Cancer type includes malignant cancer other than HCC, such as
cholangiocarcinoma, hepatoepidermoid carcinoma and metastatic
cancer; diagnosis of HCC using a combination of multiple imaging
modalities; size of HCC lesions >2 cm; ii) the size of the HCCs
was not specified; iii) they evaluated previously treated HCCs; iv)
the specificity was not evaluated; v) there was a lack of
sufficient data to construct a 2×2 contingency table; and vi) there
was study population overlap. A total of 10 studies were included
for analysis. In addition, the reference list of these 10 studies
was reviewed. Once any of the studies fulfilled the inclusion
criteria but not the aforementioned exclusion, they were not
included for analysis. No studies were excluded in the process.
Data extraction and quality
assessment
A total of two investigators reviewed the included
studies and extracted the relevant details for the meta-analysis.
The study characteristics extracted included the authors of the
study, year of publication, country of origin, number of overall
patients, overall size of HCC, cause of liver cirrhosis, study
design (prospective or retrospective image interpretation), study
period, b value of DWI, MRI field strength, number of HCCs which
were ≤2 cm, number of benign lesions, type of benign hepatic
lesions, reference standard and number of readers. The number of
readers is important as diagnosing HCC lesions by multiple readers
or radiologists increases the accuracy of the diagnosis, which
improves the reliability of the studies.
Data for the diagnostic value of DWI combined with
contrast-enhanced MRI for small HCC lesions were extracted to
construct a 2×2 contingency table. If the sensitivity and
specificity were reported by multiple radiologists, the average
sensitivity and specificity scores were reported to avoid under- or
overestimation of the diagnostic accuracy, which occurred in 1 out
of 10 of the included studies. In addition, raw data for the
diagnostic value of contrast-enhanced MRI was extracted if
available for the construction of a 2×2 contingency table, which
was available in 8 out of the 10 included studies. For studies with
DWI, the information of whether a preset apparent diffusion
coefficient (ADC) cutoff value to diagnose HCC was also extracted
for analysis, which was available in 1 out of 10 included studies).
All the data were analyzed using Stata version 14.0 (https://www.stata.com).
The quality of the included studies was assessed
using the Quality Assessment of Diagnostic Accuracy Studies
(QUADAS-2) (11). Exclusions of
patients with lesion smaller (<1 cm) was considered
inappropriate since this increased the selection bias. Any
reference standard to diagnose small hepatic lesions (≤2 cm), other
than pathological analysis (biopsy, surgical resection and
explant), for example, imaging follow-up, was considered unlikely
to lead to correct classification of the target condition and may
introduce bias. Diagnosis using reference standard based on imaging
follow-up or subsequent transcatheter arterial chemoembolization
were considered to include the knowledge of the results of the
index test. In addition, the risk of bias for reference standard
results based on biopsy or resection were considered unclear due to
the lack of information provided to the pathologist at the time of
assessment. An interval of >90 days between MRI scan and
reference standard examination was considered inappropriate since
during such a long interval, new tumorous growth adjacent to the
targeted mass identified by MRI scan or reference standard
examination may happen; the targeted mass may become larger over 2
cm; patients may receive treatment that may change the size and the
cell composition of the targeted mass. All the aforementioned
criteria were used by the two evaluators to specify the QUADAS-2,
which was generally developed for quality assessment for all
meta-analysis, to assess the quality of the included studies for
the present analysis.
Statistical analysis
All the data were analyzed using Stata software
(version 14.0; College Station, TX, USA). The sensitivity,
specificity and 95% confidence intervals (CIs) for the diagnosis of
small HCC lesions using contrast-enhanced MRI with DWI were
calculated using the bivariate random effects model (12), which were demonstrated via forest
plots. The assessment for the sensitivity and specificity was
performed on a per-lesion basis for all the included studies. The
summary receiver operating characteristic curves (SROCs) were
constructed and the area under the SROCs (AUC) of the conventional
MRI with DWI and conventional MRI alone were calculated to
determine the diagnostic performances. χ2 test
(P<0.05 indicating significant heterogeneity) and I2
was used to determine the heterogeneity. A random effects model was
used if I2 >50%; otherwise, a fixed effects model was
used. Univariate meta-regression analysis was performed according
to MRI field strength (1.5 T vs. 3.0 T), country of origin (Asia
vs. non-Asia), study design (prospective vs. retrospective) and
whether hepatobiliary phase was used in the diagnosis of small HCC.
In Asia countries, Hepatitis B virus infection is the leading cause
of cirrhosis which results in HCC (13). In non-Asia country, the etiology of
HCC varies (13). The difference in
the etiology of HCC may be the cause of the heterogeneity. This was
the reason that country of origin was divided into Asia vs.
non-Asia in the present study to assess the potential cause of
heterogeneity. In addition, the diagnostic value of DWI combined
with contrast-enhanced MRI was assessed by comparing the diagnostic
performance of DWI with contrast-enhanced MRI to contrast-enhanced
MRI only. The calculation of TP, FP, TN and FN for the
contrast-enhanced MRI was only available for 8 of the 10 included
studies (7,8,14–19).
Multilevel mixed-effects logistic regression analysis was used to
compare the summary paired sensitivity/specificity data with a
significance level of P<0.05. Publication bias was assessed
using a Deeks' funnel plot.
Results
Study selection
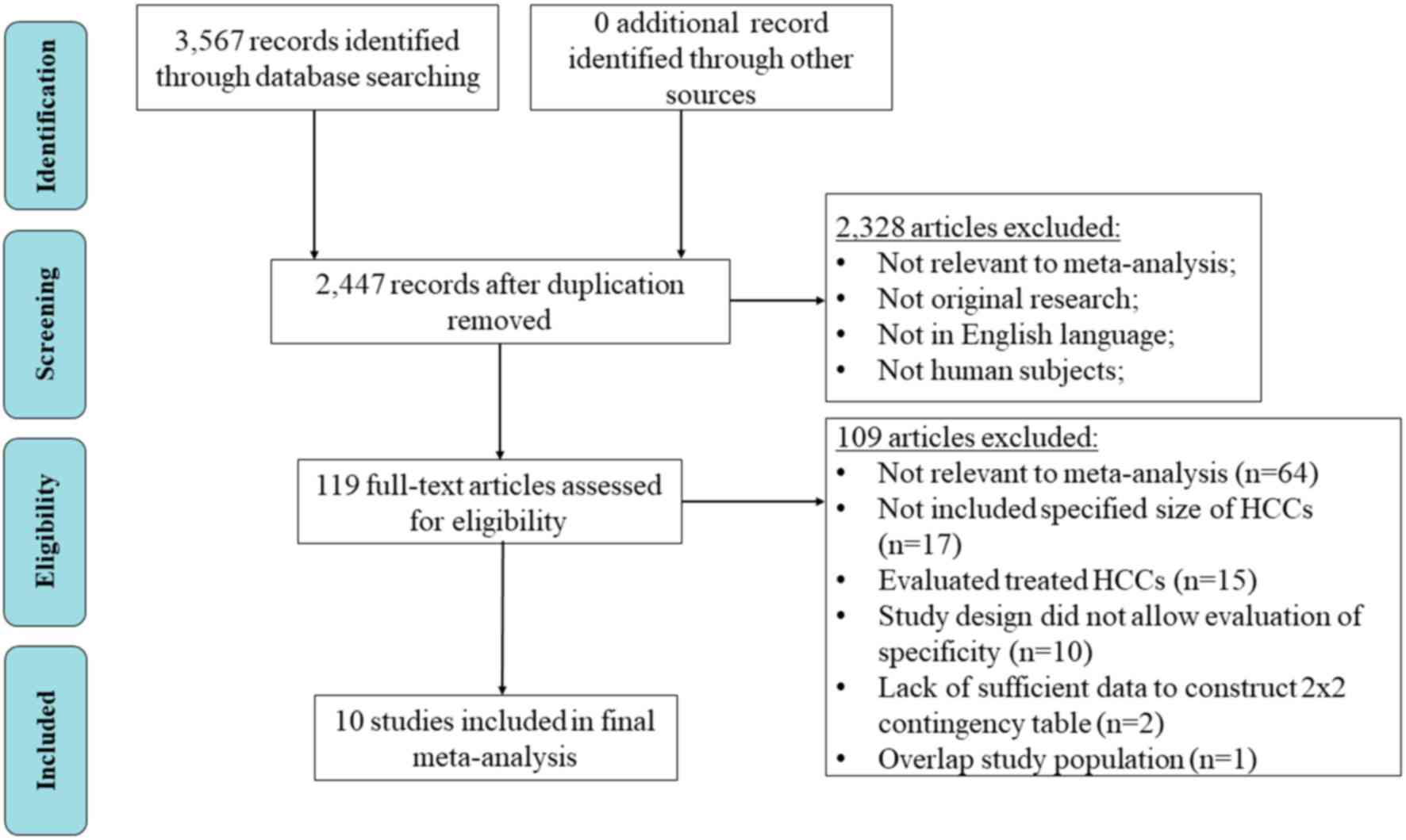
A flow chart following the Preferred Reporting Items
for Systematic Reviews and Meta-analysis principles was used to
demonstrate the selection procedure (Fig. 1). A total of 3,567 articles were
initially identified. There was a total of 2,447 articles remaining
following the removal of duplicates and a further 2,328 articles
were excluded, following screening of the abstract. Amongst the
remaining 119 studies, a total of 10 studies were included in the
meta-analysis using the inclusion criteria (7,8,14–21).
Summary of included studies
The summarized characteristics and the diagnostic
performance of DWI combined with contrast-enhanced MRI for the
included 10 studies are shown in Tables
I and II, respectively. A total
of 837 small HCCs with a diameter ≤2 cm and 545 benign liver
lesions, with a diameter ≤2 cm was included in the meta-analysis.
The TP, FP, FN and TN were all calculated on a per-lesion basis. Of
the included studies, 6 originated from Asia, 3 from Europe and one
from Egypt. In addition, seven of the studies were retrospective,
and three were prospective. The reference standard for the
diagnosis of HCC included pathological analysis (surgical
resection, explant and/or biopsy) and imaging from follow-up. MR
imaging field strength was all ≥1.5 T.
 | Table I.Summary of the patient cohorts and
characteristics of MRI protocols for the included studies. |
Table I.
Summary of the patient cohorts and
characteristics of MRI protocols for the included studies.
| Author, year | Country | No. of
patients | Size of HCCs,
cm | Cause of liver
cirrhosis | MRI
interpretation | Study period | MRI field, T | DWI b value | (Refs.) |
|---|
| Basha et al,
2019 | Egypt | 185 | ≤2 | HBV, HCV,
alcoholism, cryptogenic | Prospective | January 2017 to May
2018 | 1.5 | 600, 1,000 | (14) |
| Di et al,
2013 | Italy | 70 | 0.5–2 | HBV, HCV,
alcoholism, autoimmune hepatitis | Prospective | December 2009 to
July 2010 | 1.5 | 0, 50, 400,
800 | (15) |
| Hwang et al,
2014 | Korea | 63 | 0.5–7.8 | HBV, HCV,
alcoholism, hepatitis, Wilson's disease | Retrospective | April 2008 to
October 2013 | 3.0 | 0, 100, 800 | (16) |
| Kwon et al,
2015 | Korea | 280 | 0.5–2 | NA | Retrospective | November 2009 to
June 2011 | 1.5 | 0, 50, 500,
900 | (17) |
| Le et al,
2012 | France | 62 | 0.8–2 | HBV, HCV,
alcoholism, non-alcoholic fatty liver disease | Retrospective | November 2008 to
August 2010 | 1.5 | 50, 400, 800 | (7) |
| Park et al,
2012 | Korea | 130 | 0.6–2 | Chronic liver
disease | Retrospective | May 2009 to July
2010 | 3.0 | 0, 100, 800 | (18) |
| Rhee et al,
2012 | Korea | 34 | ≤3 | HBV, HCV,
alcoholism | Retrospective | January 2008 to
December 2009 | 3.0 | 50, 800 | (21) |
| Vandecaveye et
al, 2009 | Belgium | 55 | 0.7–14 | HBV, HCV,
hemochromatosis, Budd-Chiari, primary biliary cirrhosis | Prospective | NA | 1.5 | 0, 100, 600,
1,000 | (20) |
| Xu et al,
2009 | China | 37 | 0.7–2 | Chronic liver
disease | Retrospective | February 2005 to
August 2005 | 1.5 | 500 | (8) |
| Zhao et al,
2014 | China | 33 | 0.5–2 | HBV, HCV,
alcoholism | Retrospective | August 2011 to
December 2012 | 3.0 | 0, 600 | (19) |
 | Table II.Summary of diffusion weighted imaging
in combination with contrast-enhanced magnetic resonance
imaging. |
Table II.
Summary of diffusion weighted imaging
in combination with contrast-enhanced magnetic resonance
imaging.
| Author, year | HCC cases, n | Benign lesions,
n | Type of benign
hepatic lesions | TP | FN | TN | FP | Reference
standard | No. of readers | (Refs.) |
|---|
| Basha et al,
2019 | 67 | 121 | DN, RN | 64 | 3 | 100 | 21 | Biopsy,
surgery | 2 | (14) |
| Di Martino et
al, 2013 | 93 | 39 | APS, DN, RN | 73 | 20 | 37 | 2 | Biopsy, surgical
resection, explant, imaging follow-up | 2 | (15) |
| Hwang et al,
2014 | 68 | 46 | APS, DN, RN,
hemangioma, bile duct adenoma | 47 | 21 | 43 | 3 | Explant | 2 | (16) |
| Kwon et al,
2015 | 222 | 61 | DN | 202 | 20 | 52 | 9 | Biopsy,
surgery | 2 | (17) |
| Le et al,
2012 | 66 | 16 | DN, RN, focal
fibrosis, FNH-like nodule, solitary necrosis | 58 | 8 | 12 | 4 | Biopsy, surgical
resection, explant | 2 | (7) |
| Park et al,
2012 | 179 | 144 | DN, RN, hemangioma,
eosinophilic abscess | 165 | 14 | 140 | 4 | Biopsy, surgical
resection, explant | 3 | (18) |
| Rhee et al,
2012 | 27 | 31 | DN, RN, FNH-like
nodules | 15 | 12 | 31 | 0 | Surgical
specimens | 2 | (21) |
| Vandecaveye et
al, 2009 | 34 | 41 | DN, RN, pseudo-FNH,
inflammatory pseudo-tumor, regenerative nodular hyperplasia | 31 | 3 | 34 | 7 | Surgical
resection | 2 | (20) |
| Xu et al,
2009 | 47 | 6 | Cirrhotic
nodule | 46 | 1 | 5 | 1 | Biopsy, explant,
imaging follow-up | 2 | (8) |
| Zhao et al,
2014 | 34 | 20 | Cirrhotic nodule,
DN, atypical hemangioma | 30 | 4 | 12 | 8 | Biopsy, surgical
resection, imaging follow-up | 2 | (19) |
Quality assessment and publication
bias
Fig. 2 demonstrates
the overall evaluation for the quality of the included studies
using QUADAS-2. The quality of the index test was high (90%, 9/10
studies); however, patient selection had a low score (70%, 7/10
studies), which could be due to a lack of avoidance of a
case-control design or the inappropriate exclusions during patient
selection. This also increased concerns regarding the applicability
of patient selection. For all the 10 included studies, some of them
used pathological finding as the only reference standard to
diagnose HCC. For the others, imaging follow up was used as a
reference standard for patients when pathology analysis was not
available. A low score was found for the reference standard (60%, 6
of 10 studies) due to a lack of using pathological analysis as a
reference standard. However, these studies used imaging follow-up
as one of the reference standards for those without pathological
confirmation, which has been shown to be effective for the
diagnosis of HCC (22). Therefore,
the concerns of bias for applicability of reference standards was
low for studies using imaging follow up as one of the reference
standards for patients when pathology was not used. The risk of
bias for flow and timing was high for 1 study since the interval
between MRI scan and the pathological analysis exceeded 90 days for
some of the patients, and was unclear for 2 studies for the lack of
information regarding the time interval between MRI scan and the
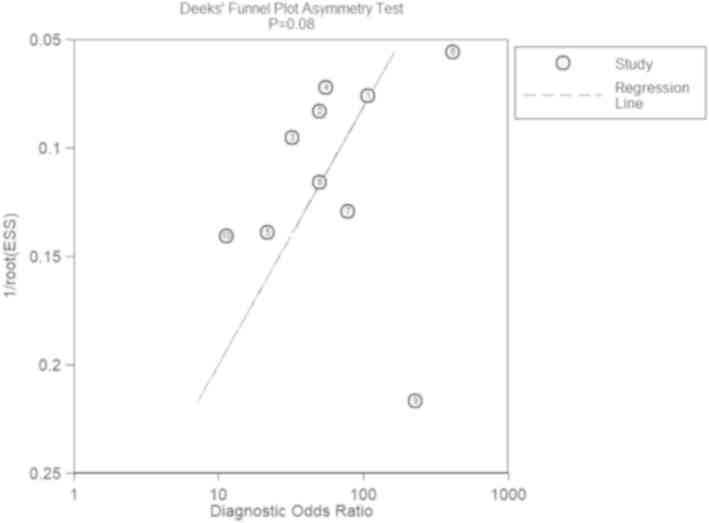
reference standard. The Deeks' funnel plot (Fig. 3) suggested that there was no
significant publication bias (P>0.05).
Heterogeneity between studies
The 10 included studies demonstrated significant
heterogeneity with P<0.001 using χ2 test. The
heterogeneity for the sensitivity (I2 of 85.7) was
higher compared with that for specificity (I2 of 78.11).
In addition, there was no threshold effect found (correlation,
−0.65; proportion of heterogeneity due to threshold effect,
0.42).
Synthesis of general diagnostic
parameters
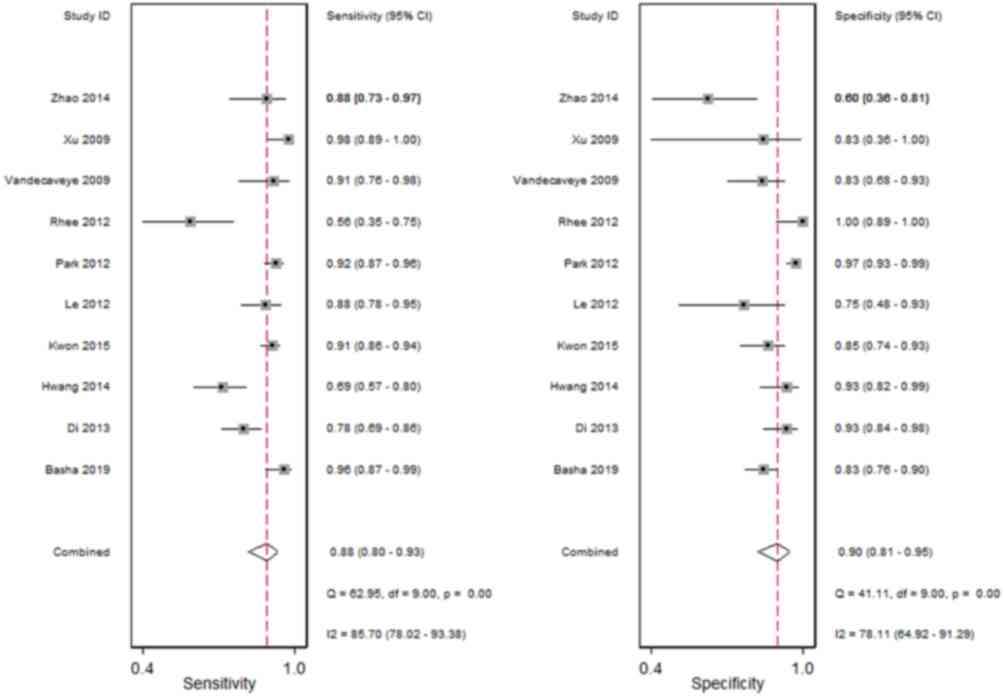
Fig. 4 demonstrates
the forest plots of sensitivity and specificity for DWI combined
with conventional MRI for the diagnosis of small HCC lesions, with
a diameter ≤2 cm. The pooled sensitivity and specificity were 0.88
(95% CI, 0.80–0.93) and 0.90 (95% CI, 0.81–0.95), respectively. The
positive and negative likelihood ratio was 8.4 (95% CI, 4.6–15.3)
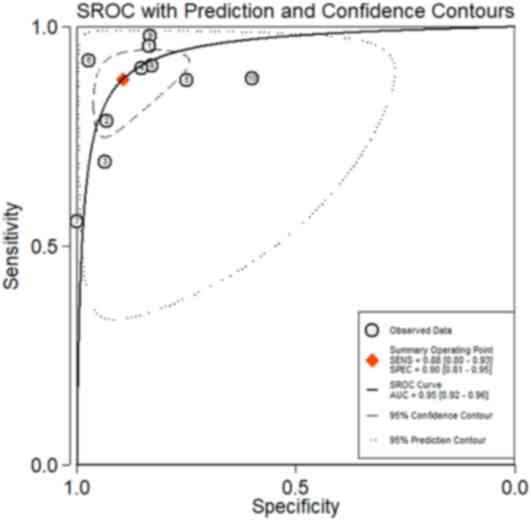
and 0.13 (95% CI, 0.08–0.22), respectively. Fig. 5 shows the summary ROC curve with an
AUC of 0.95.
Subgroup analysis and
meta-regression
The results of the univariate meta-regression
analysis are shown in Table III.
Sensitivity was significantly higher for studies not using
hepatobiliary phase compared with those using hepatobiliary phase
(P<0.001). Specificity was significantly higher for studies
using a 3 T magnetic field compared with those using 1.5 T magnetic
field (P=0.03). There were no significant differences in either the
sensitivity or in specificity for the remaining study
characteristics (all P>0.05).
 | Table III.Subgroup analysis and
meta-regression. |
Table III.
Subgroup analysis and
meta-regression.
| Characteristic | No. of studies | (Refs.) | Pooled sensitivity
(CI) | P-value | Pooled specificity
(CI) | P-value |
|---|
| MRI field strength,
T |
|
|
| 0.76 |
| 0.03 |
|
1.5 | 6 | (7,8,15,16,18,21) | 0.91
(0.85–0.97) |
| 0.85
(0.75–0.95) |
|
|
3.0 | 4 | (17,19,20,22) | 0.81
(0.68–0.93) |
| 0.94
(0.87–1.00) |
|
| Country of
origin |
|
|
| 0.10 |
| 0.85 |
|
Asia | 6 |
(8,17-19,20,22) | 0.87
(0.78–0.95) |
| 0.92
(0.84–0.99) |
|
|
Non-Asia | 4 | (7,15,16,21) | 0.90
(0.81–0.98) |
| 0.86
(0.74–0.98) |
|
| Study design |
|
|
| 0.49 |
| 0.34 |
|
Prospective | 3 | (15,16,21) | 0.90
(0.80–1.00) |
| 0.88
(0.75–1.00) |
|
|
Retrospective | 7 |
(7,8,17-19,20,22) | 0.87
(0.79–0.95) |
| 0.90
(0.82–0.98) |
|
| Hepatobiliary phase
imaging |
|
|
| <0.001 |
| 0.92 |
|
Yes | 5 | (16–19,22) | 0.81
(0.72–0.91) |
| 0.94
(0.91–0.98) |
|
| No | 5 | (7,8,15,20,21) | 0.93
(0.88–0.98) |
| 0.79
(0.69–0.88) |
|
Additional value of DWI for
contrast-enhanced MRI
The comparisons in the diagnostic performance of the
different combinations of MRI protocols in the diagnosis of small
HCC lesions are shown in Table IV.
The sensitivity of DWI with contrast-enhanced MRI was significantly
higher compared with that in contrast-enhanced MRI alone (0.89 vs.
0.78; P=0.01). However, there was no significant difference for the
specificity between DWI with contrast-enhanced MRI and conventional
MRI alone (P=0.603).
 | Table IV.Comparison of the diagnostic
performance of DWI+ CE MRI with CE MRI alone. |
Table IV.
Comparison of the diagnostic
performance of DWI+ CE MRI with CE MRI alone.
| Diagnostic
methods | Pooled sensitivity
(CI) | P-value | Pooled specificity
(CI) | P-value |
|---|
| DWI+ CE MRI | 0.89
(0.83–0.94) | 0.01 | 0.88
(0.78–0.94) | 0.603 |
| CE MRI | 0.78
(0.74–0.83) |
| 0.90
(0.79–0.95) |
|
Discussion
The aim of the present meta-analysis was to assess
the diagnostic performance of DWI combined with conventional MRI
for the diagnosis of small HCC lesions, with a diameter ≤2 cm. The
results suggested that DWI with conventional MRI had a high
sensitivity of 88% and specificity of 90%. The meta-regression
analysis revealed that the heterogeneity of the pooled sensitivity
may be partially attributed to whether hepatobiliary phase was used
in the diagnosis of small HCC. In addition, the heterogeneity for
the pooled specificity may be caused by the different magnetic
fields used. However, a threshold effect was not identified.
Non-contrast enhanced ultrasonography (US) is a
common choice for HCC screening in patients who with chronic liver
disease, as it is cost-effective (23). However, there is low sensitivity when
compared with that in contrast-enhanced computer tomography (CT)
and MRI (24). Contrast-enhanced US
has emerged as a promising method to diagnose small HCCs (25); however, additional studies are
required to confirm its clinical value (23). Multiple meta-analyses have found that
contrast-enhanced MRI outperforms contrast enhanced CT in the
diagnosis of small HCCs with higher sensitivity and overall
accuracy (26,27). Previous meta-analysis indicated that
contrast-enhanced MRI had moderately high sensitivity and high
specificity in the diagnosis of small HCC (28). The present meta-analysis suggested
that DWI combined with conventional MRI increased the sensitivity
in the diagnosis of small HCC, whilst maintaining a high
specificity. It is hypothesized that the ability to suppress the
background signal of the liver parenchyma underlies the improved
ability of DWI to detect smaller lesions (29,30).
Li et al (31), found that DWI combined with gadoxetic
acid disodium-enhanced MRI was beneficial to diagnose HCC and
improved the specificity. However, the capability of
contrast-enhanced MRI with DWI to diagnose small HCC lesions was
not compared, which was investigated in the present meta-analysis.
The present analysis found an increased sensitivity while
maintaining high specificity using a combined method to diagnose
small HCCs compared with using contrast-enhanced MRI alone. HCC
lesions <2 cm are less frequently presented during imaging
compared with larger HCC lesions, including arterial enhancement,
portal/equilibrium washout and T2 hyperintensity (32). The increase in sensitivity using the
combined method could be due to the small HCCs presenting with
hyperintensity in DWI (33).
ADC has been used to diagnose benign and malignant
hepatic lesions (34). A previous
study suggested that ADC was lower in malignant lesions, such as
HCC and metastases, compared with that in benign lesions, such as
cysts and hemangiomas (35).
However, it is difficult to define a threshold of ADC value for
benign and malignant liver lesions differentiation (36). An increasing number of studies have
suggested that ADC is more accurate in grading smaller HCCs
(37,38), and for monitoring early treatment
responses of HCC to radiofrequency ablation (39). In the 10 studies included in the
present meta-analysis, only one study used a predetermined
threshold ADC value to diagnose small HCC (20), and no difference was found in the ADC
value between benign and malignant hepatic lesions. The remaining 9
studies used hyperintensity of the lesion compared with that in the
liver background in the DWI, as one of the diagnostic criteria for
HCC. The present analysis suggested that DWI may be used
straightaway, in different diagnostic centers, without using a
cut-off ADC value, which may differ between studies.
The value of DWI for the diagnosis of HCC ≤1 cm
requires further investigation as only 2 of the 10 studies compared
the diagnostic performance of DWI for HCC ≤1 cm (16,18).
Both studies found that the sensitivity could be increased by
adding DWI, which suggested the importance of using DWI in the
diagnosis of HCCs with smaller lesions.
There were several limitations in the present
meta-analysis. Notable heterogeneity among the included studies was
found, which may affect the applicability of the summery estimates.
In addition, the majority of the included studies were
retrospective studies (7 out of 10), in which confounding factors
and bias are more common compared with that in prospective
studies.
In conclusion, DWI in combination with conventional
MRI is beneficial for the diagnosis of small HCC, which may
increase the diagnostic sensitivity whilst maintaining high
specificity.
Acknowledgements
The authors would like to thank Professor Xiaojia
Zhang (Taiyuan Centers for Disease Control and Prevention, Taiyuan
City, China) for her statistical assistance.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
HL, GL and WZZ designed the study. HL, GL and WZZ
acquired the data. GL drafted the initial manuscript. All authors
revised and approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wallace MC, Preen D, Jeffrey GP and Adams
LA: The evolving epidemiology of hepatocellular carcinoma: A global
perspective. Expert Rev Gastroenterol Hepatol. 9:765–779. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hackl C, Schlitt HJ, Kirchner GI, Knoppke
B and Loss M: Liver transplantation for malignancy: Current
treatment strategies and future perspectives. World J
Gastroenterol. 20:5331–5344. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wald C, Russo MW, Heimbach JK, Hussain HK,
Pomfret EA and Bruix J: New OPTN/UNOS policy for liver transplant
allocation: Standardization of liver imaging, diagnosis,
classification, and reporting of hepatocellular carcinoma.
Radiology. 266:376–382. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zamora-Valdes D, Taner T and Nagorney DM:
Surgical treatment of hepatocellular carcinoma. Cancer Control.
24:10732748177292582017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Balogh J, Victor D III, Asham EH,
Burroughs SG, Boktour M, Saharia A, Li X, Ghobrial RM and Monsour
HP Jr: Hepatocellular carcinoma: A review. J Hepatocell Carcinoma.
3:41–53. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Forner A, Vilana R, Ayuso C, Bianchi L,
Solé M, Ayuso JR, Boix L, Sala M, Varela M, Llovet JM, et al:
Diagnosis of hepatic nodules 20 mm or smaller in cirrhosis:
Prospective validation of the noninvasive diagnostic criteria for
hepatocellular carcinoma. Hepatology. 47:97–104. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Le Moigne F, Durieux M, Bancel B, Boublay
N, Boussel L, Ducerf C, Berthezène Y and Rode A: Impact of
diffusion-weighted MR imaging on the characterization of small
hepatocellular carcinoma in the cirrhotic liver. Magn Reson
Imaging. 30:656–665. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xu PJ, Yan FH, Wang JH, Lin J and Ji Y:
Added value of breathhold diffusion-weighted MRI in detection of
small hepatocellular carcinoma lesions compared with dynamic
contrast-enhanced MRI alone using receiver operating characteristic
curve analysis. J Magn Reson Imaging. 29:341–349. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim SS, Kim SH, Song KD, Choi SY and Heo
NH: Value of gadoxetic acid-enhanced MRI and diffusion-weighted
imaging in the differentiation of hypervascular hyperplastic nodule
from small (<3 cm) hypervascular hepatocellular carcinoma in
patients with alcoholic liver cirrhosis: A retrospective
case-control study. J Magn Reson Imaging. 51:70–80. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhong X, Tang H, Lu B, You J, Piao J, Yang
P and Li J: Differentiation of small hepatocellular carcinoma from
dysplastic nodules in cirrhotic liver: Texture analysis based on
MRI improved performance in comparison over gadoxetic acid-enhanced
MR and diffusion-weighted imaging. Front Oncol. 9:13822020.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Whiting PF, Rutjes AW, Westwood ME,
Mallett S, Deeks JJ, Reitsma JB, Leeflang MM, Sterne JA and Bossuyt
PM; QUADAS-2 Group, : QUADAS-2: A revised tool for the quality
assessment of diagnostic accuracy studies. Ann Intern Med.
155:529–536. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Reitsma JB, Glas AS, Rutjes AW, Scholten
RJ, Bossuyt PM and Zwinderman AH: Bivariate analysis of sensitivity
and specificity produces informative summary measures in diagnostic
reviews. J Clin Epidemiol. 58:982–990. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Choo SP, Tan WL, Goh BKP, Tai WM and Zhu
AX: Comparison of hepatocellular carcinoma in Eastern versus
Western populations. Cancer. 122:3430–3446. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Basha MAA, Refaat R, Mohammad FF, Khamis
MEM, El-Maghraby AM, El Sammak AA, Al-Molla RM, Mohamed HAE,
Alnaggar AA, Hassan HA, et al: The utility of diffusion-weighted
imaging in improving the sensitivity of LI-RADS classification of
small hepatic observations suspected of malignancy. Abdom Radiol
(NY). 44:1773–1784. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Di Martino M, Di Miscio R, De Filippis G,
Lombardo CV, Saba L, Geiger D and Catalano C: Detection of small
(≤2 cm) HCC in cirrhotic patients: Added value of diffusion
MR-imaging. Abdom Imaging. 38:1254–1262. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hwang J, Kim YK, Kim JM, Lee WJ, Choi D
and Hong SS: Pretransplant diagnosis of hepatocellular carcinoma by
gadoxetic acid-enhanced and diffusion-weighted magnetic resonance
imaging. Liver Transpl. 20:1436–1446. 2014.PubMed/NCBI
|
|
17
|
Kwon HJ, Byun JH, Kim JY, Hong GS, Won HJ,
Shin YM and Kim PN: Differentiation of small (≤2 cm) hepatocellular
carcinomas from small benign nodules in cirrhotic liver on
gadoxetic acid-enhanced and diffusion-weighted magnetic resonance
images. Abdom Imaging. 40:64–75. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Park MJ, Kim YK, Lee MW, Lee WJ, Kim YS,
Kim SH, Choi D and Rhim H: Small hepatocellular carcinomas:
Improved sensitivity by combining gadoxetic acid-enhanced and
diffusion-weighted MR imaging patterns. Radiology. 264:761–770.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhao XT, Li WX, Chai WM and Chen KM:
Detection of small hepatocellular carcinoma using gadoxetic
acid-enhanced MRI: Is the addition of diffusion-weighted MRI at
3.0T beneficial? J Dig Dis. 15:137–145. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vandecaveye V, De Keyzer F, Verslype C, Op
de Beeck K, Komuta M, Topal B, Roebben I, Bielen D, Roskams T,
Nevens F and Dymarkowski S: Diffusion-weighted MRI provides
additional value to conventional dynamic contrast-enhanced MRI for
detection of hepatocellular carcinoma. Eur Radiol. 19:2456–2466.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rhee H, Kim MJ, Park MS and Kim KA:
Differentiation of early hepatocellular carcinoma from benign
hepatocellular nodules on gadoxetic acid-enhanced MRI. Br J Radiol.
85:e837–e844. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tang A, Bashir MR, Corwin MT, Cruite I,
Dietrich CF, Do RKG, Ehman EC, Fowler KJ, Hussain HK, Jha RC, et
al: Evidence supporting LI-RADS major features for CT- and MR
imaging-based diagnosis of hepatocellular carcinoma: A systematic
review. Radiology. 286:29–48. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ronot M, Purcell Y and Vilgrain V:
Hepatocellular carcinoma: Current imaging modalities for diagnosis
and prognosis. Dig Dis Sci. 64:934–950. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hanna RF, Miloushev VZ, Tang A,
Finklestone LA, Brejt SZ, Sandhu RS, Santillan CS, Wolfson T, Gamst
A and Sirlin CB: Comparative 13-year meta-analysis of the
sensitivity and positive predictive value of ultrasound, CT, and
MRI for detecting hepatocellular carcinoma. Abdom Radiol (NY).
41:71–90. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hsiao CY, Chen PD and Huang KW: A
prospective assessment of the diagnostic value of contrast-enhanced
ultrasound, dynamic computed tomography and magnetic resonance
imaging for patients with small liver tumors. J Clin Med.
8:13532019. View Article : Google Scholar
|
|
26
|
Liu X, Jiang H, Chen J, Zhou Y, Huang Z
and Song B: Gadoxetic acid disodium-enhanced magnetic resonance
imaging outperformed multidetector computed tomography in
diagnosing small hepatocellular carcinoma: A meta-analysis. Liver
Transpl. 23:1505–1518. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li J, Li X, Weng J, Lei L, Gong J, Wang J,
Li Z, Zhang L and He S: Gd-EOB-DTPA dynamic contrast-enhanced
magnetic resonance imaging is more effective than enhanced 64-slice
CT for the detection of small lesions in patients with
hepatocellular carcinoma. Medicine (Baltimore). 97:e139642018.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kierans AS, Kang SK and Rosenkrantz AB:
The diagnostic performance of dynamic contrast-enhanced MR imaging
for detection of small hepatocellular carcinoma measuring up to 2
cm: A meta-analysis. Radiology. 278:82–94. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wu LM, Hu J, Gu HY, Hua J and Xu JR: Can
diffusion-weighted magnetic resonance imaging (DW-MRI) alone be
used as a reliable sequence for the preoperative detection and
characterisation of hepatic metastases? A meta-analysis. Eur J
Cancer. 49:572–584. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mannelli L, Bhargava P, Osman SF, Raz E,
Moshiri M, Laffi G, Wilson GJ and Maki JH: Diffusion-weighted
imaging of the liver: A comprehensive review. Curr Probl Diagn
Radiol. 42:77–83. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li X, Li C, Wang R, Ren J, Yang J and
Zhang Y: Combined application of gadoxetic acid disodium-enhanced
magnetic resonance imaging (MRI) and diffusion-weighted imaging
(DWI) in the diagnosis of chronic liver disease-induced
hepatocellular carcinoma: A meta-analysis. PLoS One.
10:e01442472015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
van den Bos IC, Hussain SM, Dwarkasing RS,
Hop WC, Zondervan PE, de Man RA, IJzermans JN, Walker CW and
Krestin GP: MR imaging of hepatocellular carcinoma: Relationship
between lesion size and imaging findings, including signal
intensity and dynamic enhancement patterns. J Magn Reson Imaging.
26:1548–1555. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Parikh T, Drew SJ, Lee VS, Wong S, Hecht
EM, Babb JS and Taouli B: Focal liver lesion detection and
characterization with diffusion-weighted MR imaging: Comparison
with standard breath-hold T2-weighted imaging. Radiology.
246:812–822. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pankaj Jain T, Kan WT, Edward S, Fernon H
and Kansan Naider R: Evaluation of ADCratio on liver MRI
diffusion to discriminate benign versus malignant solid liver
lesions. Eur J Radiol Open. 5:209–214. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Goshima S, Kanematsu M, Kondo H, Yokoyama
R, Kajita K, Tsuge Y, Watanabe H, Shiratori Y, Onozuka M and
Moriyama N: Diffusion-weighted imaging of the liver: Optimizing b
value for the detection and characterization of benign and
malignant hepatic lesions. J Magn Reson Imaging. 28:691–697. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Miller FH, Hammond N, Siddiqi AJ, Shroff
S, Khatri G, Wang Y, Merrick LB and Nikolaidis P: Utility of
diffusion-weighted MRI in distinguishing benign and malignant
hepatic lesions. J Magn Reson Imaging. 32:138–147. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Le Moigne F, Boussel L, Haquin A, Bancel
B, Ducerf C, Berthezène Y and Rode A: Grading of small
hepatocellular carcinomas (≤2 cm): Correlation between histology,
T2 and diffusion-weighted imaging. Br J Radiol. 87:201307632014.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Okamura S, Sumie S, Tonan T, Nakano M,
Satani M, Shimose S, Shirono T, Iwamoto H, Aino H, Niizeki T, et
al: Diffusion-weighted magnetic resonance imaging predicts
malignant potential in small hepatocellular carcinoma. Dig Liver
Dis. 48:945–952. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mori Y, Tamai H, Shingaki N, Moribata K,
Deguchi H, Ueda K, Inoue I, Maekita T, Iguchi M, Kato J, et al:
Signal intensity of small hepatocellular carcinoma on apparent
diffusion coefficient mapping and outcome after radiofrequency
ablation. Hepatol Res. 45:75–87. 2015. View Article : Google Scholar : PubMed/NCBI
|