Introduction
Colorectal cancer has become one of the most common
tumors and is the third most common malignancy. Globally, ~1.2
million patients are diagnosed with colorectal cancer and
>600,000 die from the disease in 2008 (1). Neoadjuvant chemoradiotherapy (nCRT)
prior to radical surgery and post-operative adjuvant chemotherapy
is the standard treatment for locally advanced rectal cancer (LARC)
(2,3). Certain complications can arise from
radical surgery that decrease quality of life, including
anastomotic leakage, anastomotic stenosis, urinary and/or sexual
dysfunction, low anterior resection syndrome and increased
probability of enterostomy (4–6).
Response to neoadjuvant therapy is heterogeneous and
~10-30% of patients with colorectal cancer achieve pathological
complete response (pCR) (7). The
‘watch and wait’ strategy and local resection for patients with pCR
following nCRT has become a focus of study (8,9).
Generally, pCR is predicted through digital rectum examination,
imaging examination, endoscopy and tumor-related markers (9–12).
However, the predictions made using these techniques are not always
in line with the pathology results (8,13).
Currently, there are no affordable and reliable markers that can
predict tumor response.
Systemic inflammatory responses have been reported
to be predictors of prognosis for numerous types of solid tumors,
including gastrointestinal (14,15),
pancreatic (16), renal (17) and breast cancer (18). Systemic inflammatory responses can be
reflected by hematologic parameters, including lymphocyte count and
platelet count, or the ratio of different cell types, including the
platelet-to-lymphocyte ratio (PLR) and neutrophil-to-lymphocyte
ratio (NLR) (19,20). The tumor microenvironment is closely
associated with the occurrence and development of cancer (21). In the peripheral circulation,
neutrophils and lymphocytes act as immunocytes, reflecting the
tumor microenvironment, and the number of immunocytes changes
according to patient age and immunoreactivity (22). Changes in the NLR may be associated
with changes in tumor size during regression due to the nCRT for
rectal cancers (23). However, it
remains unclear whether systematic inflammatory responses could be
used as a marker for identifying patients with tumors achieving pCR
following nCRT.
Therefore, the current study investigated pre- and
post-nCRT systemic inflammatory indices and changes in these
indices from pre-nCRT to post-nCRT to determine the association
between these parameters and responsiveness to nCRT in patients
with LARC.
Materials and methods
Patients
Data from patients with rectal cancer who received
nCRT and radical surgery at the Sixth Affiliated Hospital between
January 2013 and October 2018, were retrospectively analyzed. A
total of 321 patients were included in this study. The mean age of
patients was 58.5 years. A total of 218 patients were male (67.9%)
and 103 patients were female (32.1%). The inclusion criteria were
as follows: i) 18–75 years old; ii) diagnosis of rectal
adenocarcinoma confirmed by biopsy pathology; iii) stage II (T3/T4;
N0M0) or stage III (T1-4; N+M0) evaluated by thoracic
and abdominal pelvic CT, pelvic MRI or transluminal ultrasound
prior to nCRT; iv) the distance from the anal verge evaluated by
digital rectum examination or enteroscopy was <12 cm; v) an
Eastern Cooperative Oncology Group score of 0–2; vi) (24) no radiotherapy, chemotherapy or
surgical contraindications; and vii) no past medical history of
malignant tumors. The exclusion criteria included the following: i)
Absence of clinical data; ii) infections prior to new adjuvant
therapy or surgery; iii) use of leukocyte enhancing drugs; and iv)
incomplete new adjuvant therapy or radical surgery. Prior to the
study, all patients provided written informed consent. The current
study was approved by the Institutional Review Board of the Sixth
Affiliated Hospital following rigorous review.
Treatment
All patients were assessed by a multiple
disciplinary team at The Sixth Affiliated Hospital of Sun Yat-sen
University and underwent nCRT and radical surgery. The total dose
of radiotherapy given was 46–50.4, or 1.8–2.0 Gy for 23–28
fractions. Patients received one of the following two chemotherapy
regimens: mFOLFOX6 [85 mg/m2 oxaliplatin, 400
mg/m2 leucovorin and 400 mg/m2 fluorouracil
(5-FU) administered through intravenous drip on day 1, followed by
2,400 mg/m2 fluorouracil continuously administered
through intravenous infusion for 48 h] or DeGramont (400
mg/m2 leucovorin, 400 mg/m2 F-5U administered
through intravenous drip on day 1, followed by 2,400
mg/m2 fluorouracil continuously administered through
intravenous infusion for 48 h). The interval between radiotherapy
and surgery was 4–12 weeks and the interval between chemotherapy
and surgery was 2–4 weeks.
Peripheral blood examinations
Peripheral blood (2 ml) was collected from all
patients within 1 week prior to the first nCRT (pre-nCRT) and 1
week prior to surgery (post-nCRT). Neutrophil, lymphocyte and
platelet counts were obtained from the hospital information system.
The PLR was calculated as the absolute count of
platelets/lymphocytes and NLR was calculated as the absolute count
of neutrophils/lymphocytes. The percentage change in NLR from
pre-nCRT to post-nCRT was defined as [(post-NLR-pre-NLR)/pre-NLR
×100%]. A result of ≥0 indicated that NLR following new adjuvant
treatment was increased, whereas a result of <0 indicated that
NLR after new adjuvant treatment was decreased. The percentage
change in PLR from pre-nCRT to post-nCRT was defined as
[(post-PLR-pre-PLR)/pre-PLR ×100%].
Pathological assessment
Two experienced pathologists evaluated the tumor
regression grades (TRG) of patients based on the American Joint
Committee on Cancer (AJCC; 8th edition) (25) as follows: 0, no viable cancer cells
(complete response); 1, single cells or rare small groups of cancer
cells (near-complete response); 2, residual cancer with evident
tumor regression and with single cells or rare small groups of
cancer cells (partial response); and 3, extensive residual cancer
with no evident tumor regression (poor or no response), pCR was
defined as no signs of viable cancer cells in resected specimens
and in the lymph nodes.
Statistical analysis
SPSS software (version 24.0; IBM Corp.) was used to
analyze data. P<0.05 was considered to indicate a statistically
significant difference. The χ2 test was used to compare
the differences between the qualitative variables of the cohorts.
The Shapiro-Wilk test confirmed that the quantitative data did not
conform to a normal distribution. Therefore, quantitative data
between cohorts were compared by the Mann-Whitney U test. Tumor
patients were strictly divided into a pCR group (TRG 0) and a
non-pCR group (TRG 1–3) according to TRG stages. The ‘cut-off’
value of the indices was determined by the receiver operating
characteristic curve (ROC). Parameters were compared using the
χ2 and t-tests between pCR and non-pCR groups. The
association between systemic inflammatory indices and pCR were
analyzed by univariate and multivariate logistic regression
analysis. The multivariate analysis included the variables of
P<0.1 in the univariate analysis.
Results
Patient characteristics
Baseline characteristics of patients in the two
cohorts are presented in Table 1. In
the training cohort, a total of 152 patients were male (67.6%) and
73 patients were female (32.4%). A total of 162 (72%) patients
received a chemotherapy regimen with oxaliplatin (mFOLFOX6).
Furthermore, 69 patients (30.6%) achieved pCR. In the validation
cohort, 66 patients (68.7%) were male and 30 patients (31.3%) were
female. A total of 67 patients (69.8%) received chemotherapy
regimens with oxaliplatin. There were 26 patients (27.1%) who
achieved pCR. No statistical differences were observed in clinical
characteristics, treatment factors and systemic inflammatory
indices between the two cohorts (P>0.05).
 | Table I.Baseline characteristics of the
patients in the training and validation cohorts. |
Table I.
Baseline characteristics of the
patients in the training and validation cohorts.
| Variable | Training
cohort | Validation
cohort | P-value |
|---|
| Age, years |
|
| 0.670 |
|
≤65 | 183 | 80 |
|
|
>65 | 42 | 16 |
|
| Sex |
|
| 0.834 |
|
Male | 152 | 66 |
|
|
Female | 73 | 30 |
|
| CEA prior to
treatment, µg/l |
|
| 0.621 |
| ≤5 | 134 | 60 |
|
|
>5 | 91 | 36 |
|
| Clinical T
stage |
|
| 0.977 |
| T2 | 8 | 3 |
|
| T3 | 167 | 72 |
|
| T4 | 50 | 21 |
|
| Clinical N
stage |
|
| 0.574 |
| N0 | 32 | 16 |
|
|
N1-2 | 193 | 80 |
|
| Tumor size, cm |
|
| 0.126 |
| ≤5 | 173 | 66 |
|
|
>5 | 52 | 30 |
|
| Tumor
circumference, % |
|
| 0.838 |
|
≤50 | 91 | 40 |
|
|
>50 | 134 | 56 |
|
| Mesorectal
fascia |
|
| 0.659 |
|
Positive | 76 | 30 |
|
|
Negative | 149 | 66 |
|
| Distance from anal
verge, cm |
|
| 0.280 |
| ≤5 | 126 | 60 |
|
|
>5 | 99 | 36 |
|
| Tumor
differentiation |
|
| 0.929 |
|
High | 71 | 8 |
|
|
Medium | 133 | 56 |
|
|
Low | 21 | 32 |
|
| Operation interval
of radiotherapy, weeks |
|
| 0.178 |
| ≤7 | 93 | 32 |
|
|
>7 | 132 | 64 |
|
| Chemotherapy
regimens |
|
| 0.689 |
| With
oxaliplatin | 162 | 67 |
|
| Without
oxaliplatin | 63 | 29 |
|
| Chemotherapy
courses |
|
| 0.055 |
| ≤4 | 30 | 21 |
|
|
>4 | 195 | 75 |
|
| Tumor response |
|
| 0.520 |
|
pCR | 69 | 26 |
|
|
Non-pCR | 156 | 70 |
|
| Mean value of
pre-NLR (interval) | 2.39
(0.59–17.04) | 2.52
(0.79–27.46) | 0.626 |
| Mean value of
pre-PLR (interval) | 148.57
(40.31–536.00) | 144.21
(47.92–448.84) | 0.596 |
| Mean value of
post-NLR (interval) | 3.66
(0.81–16.79) | 3.09
(0.77–12.99) | 0.078 |
| Mean value of
post-PLR (interval) | 237.63
(77.25–1007.07) | 220.87
(84.11–595.30) | 0.556 |
| Mean value of
percentage change in NLR, % (interval) | 80 (−81-1066) | 57 (−91-412) | 0.179 |
| Mean value of
percentage change in PLR, % (interval) | 74 (−73-605) | 69 (−60-343) | 0.614 |
Analysis for pCR in the training
cohort
Associations between systemic inflammation indices
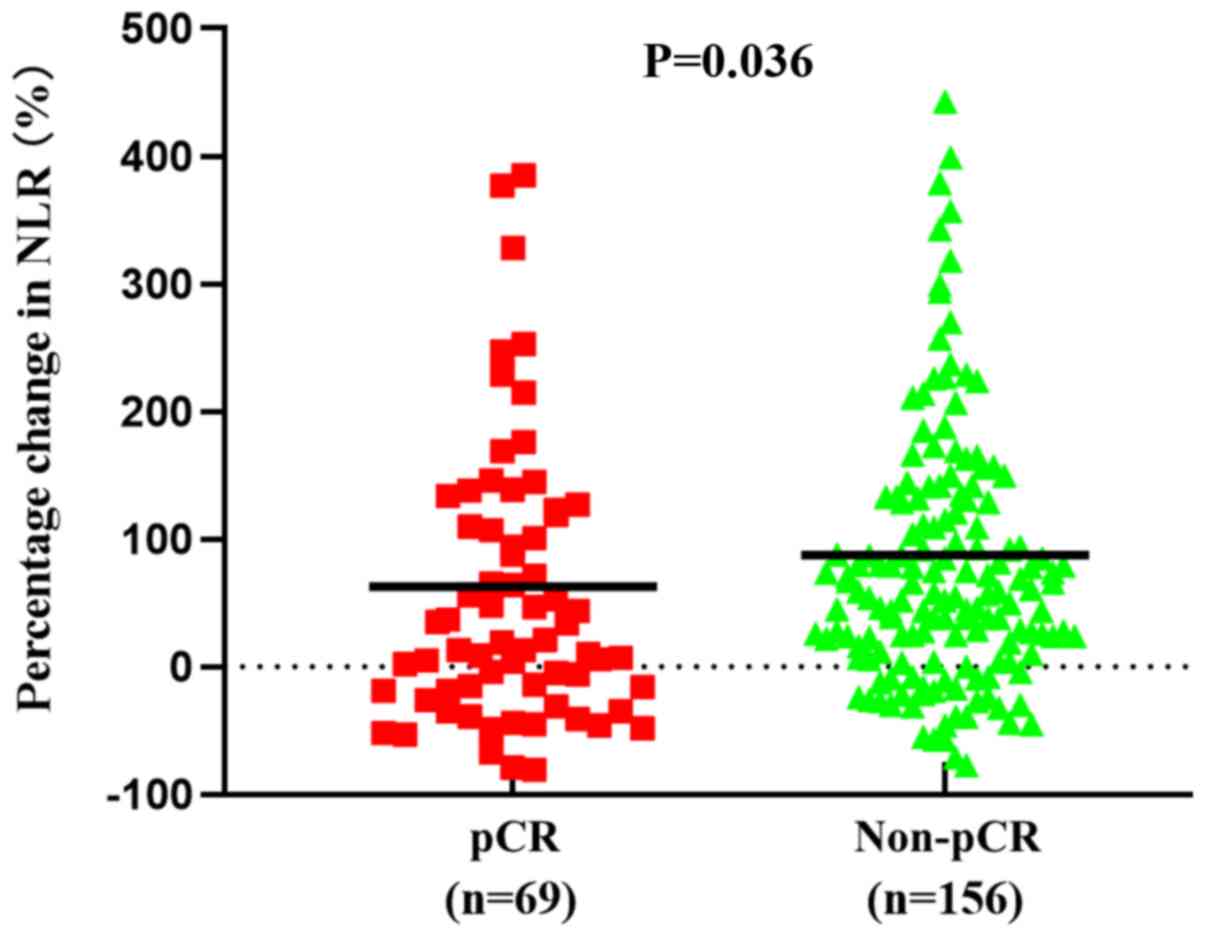
and pCR for the training cohort are presented in Table II. The mean value of the percentage
change in NLR was 63% in the pCR group and 87% in the non-pCR
group. Additionally, the percentage change in NLR was significantly
increased in the pCR group compared with the non-pCR group
(P=0.036; Fig. 1). However, there
were no significant differences in the other pre- and
post-treatment systemic inflammation indices between the pCR and
non-pCR group.
 | Table II.Association between systemic
inflammatory indices and pCR in the training cohort. |
Table II.
Association between systemic
inflammatory indices and pCR in the training cohort.
| Variable
(mean) | pCR Group
(n=69) | Non-pCR Group
(n=156) | P-value |
|---|
| Pre-NLR | 2.55 | 2.32 | 0.234 |
| Pre-PLR | 157.30 | 144.71 | 0.302 |
| Post-NLR | 3.34 | 3.80 | 0.071 |
| Post-PLR | 232.40 | 239.94 | 0.761 |
| % change in
NLR | 63 | 87 | 0.036 |
| % change in
PLR | 61 | 80 | 0.170 |
The area under the curve of the ROC analysis was
0.588 (P=0.036; Fig. 2). Therefore,
we divided the percentage change in NLR into two categories for
subsequent analyses: A percentage change of ≤21.5% NLR and a
percentage change of >21.5% NLR.
Univariate analysis demonstrated that
carcino-embryonic antigen (CEA) prior to treatment, tumor
differentiation, chemotherapy regimens and the percentage change in
NLR were associated with pCR (Table
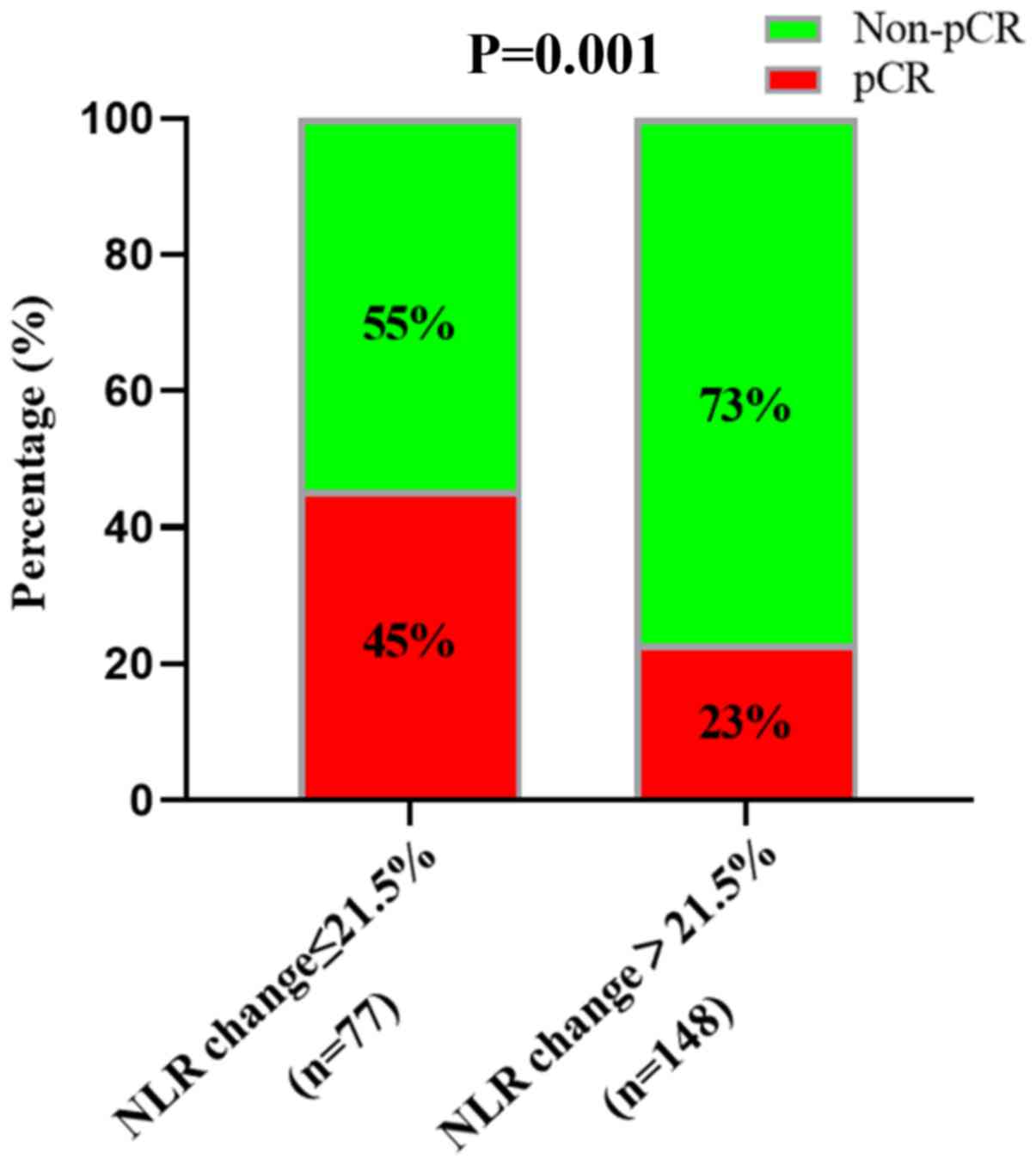
III). A total of 45% of patients with a percentage change of
≤21.5% NLR achieved pCR compared with 23% of patients with a
percentage change of >21.5% NLR (P=0.001; Fig. 3). Multivariate analysis included all
variables in the univariate analysis, including sex, CEA prior to
treatment, tumor scope, tumor differentiation, chemotherapy
regimens and the percentage change in NLR (P<0.1). However, only
the percentage change in NLR (P=0.006; OR=0.413; 95% CI=0.22–0.773)
and chemotherapy regimens (P=0.042; OR=2.257; 95% CI=1.031–4.942)
were significant following multivariate analysis. Sex (P=0.345),
CEA prior to treatment (P=0.052), tumor differentiation (P=0.173)
and tumor circumference (P=0.294) were not significant. Thus, the
percentage change in NLR and chemotherapy regimens were significant
predictors of pCR.
 | Table III.Univariate and multivariate analysis
in the training cohort. |
Table III.
Univariate and multivariate analysis
in the training cohort.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Variable | OR value (95%
CI) | P-value | OR value (95%
CI) | P-value |
|---|
| Age (≤65 vs. >65
years) | 1.14
(0.62–2.11) | 0.668 |
|
|
| Sex (male vs.
female) | 0.47
(0.21–1.08) | 0.070 | 1.38
(0.71–2.68) | 0.345 |
| CEA prior to
treatment (≤5 vs. >5 µg/l) | 0.44
(0.24–0.82) | 0.009 | 0.53
(0.27–1.01) | 0.052 |
| Clinical T stage
(T2 vs. >T3-4) | 1.08
(0.55–2.13) | 0.817 |
|
|
| Clinical N stage
(N0 vs. N1-2) | 0.97
(0.43–2.17) | 0.955 |
|
|
| Tumor size (≤5 vs.
>5 cm) | 1.42
(0.74–2.72) | 0.628 |
|
|
| Tumor circumference
(≤50 vs. >50%) | 0.59
(0.33–1.05) | 0.073 | 0.72
(0.39–1.33) | 0.294 |
| Mesorectal fascia
(negative vs. positive) | 1.07
(0.59–1.94) | 0.832 |
|
|
| Distance from anal
verge (≤5 vs. >5 cm) | 0.69
(0.39–1.23) | 0.204 |
|
|
| Tumor
differentiation (low vs. medium, high) | 0.36
(0.17–0.90) | 0.023 | 0.51
(0.19–1.34) | 0.173 |
| Operation interval
of radiotherapy (≤7 vs. >7 weeks) | 1.36
(0.76–2.44) | 0.301 |
|
|
| Chemotherapy
regimens (with oxaliplatin vs. without oxaliplatin) | 3.04
(1.44–6.41) | 0.003 | 2.26
(1.03–4.94) | 0.042 |
| Chemotherapy
courses (≤4 vs. >4) | 1.91
(0.74–4.91) | 0.174 |
|
|
| % change in NLR
(≤21.5 vs. >21.5) | 0.36
(0.20–0.67) | 0.001 | 0.41
(0.22–0.77) | 0.006 |
Analysis for pCR in the validation
cohort
Percentage change of 21.5% NLR was considered to be
optimal to predict pCR events in the training cohort (Fig. 2). To verify the association between
the percentage change in NLR and pCR, univariate and multivariate
analysis for pCR were performed for the validation cohort (Table IV). Univariate analysis demonstrated
that tumor differentiation, chemotherapy regimens and the
percentage change in NLR were associated with pCR. However, only
the percentage change in NLR (P=0.03; OR=0.337; 95% CI=0.126–0.9)
was significant following multivariate analysis. In summary, the
results verified that the percentage change in NLR was a
significant predictor of pCR.
 | Table IV.Univariate and multivariate analysis
in the validation cohort. |
Table IV.
Univariate and multivariate analysis
in the validation cohort.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Variable | OR value (95%
CI) | P-value | OR value (95%
CI) | P-value |
|---|
| Age (≤65 vs. >65
years) | 1.80
(0.58–5.58) | 0.304 |
|
|
| Sex (male vs.
female) | 1.03
(0.39–2.73) | 0.951 |
|
|
| CEA prior to
treatment (≤5 vs. >5 µg/l) | 1.64
(0.66–4.10) | 0.286 |
|
|
| Clinical T stage
(T2 vs. >T3-4) | 1.10
(0.36–3.23) | 0.862 |
|
|
| Clinical N stage
(N0 vs. N1-2) | 1.75
(0.46–6.72) | 0.411 |
|
|
| Tumor size (≤5 vs.
>5 cm) | 0.80
(0.31–2.10) | 0.650 |
|
|
| Tumor circumference
(≤50 vs. >50%) | 0.51
(0.20–1.26) | 0.140 |
|
|
| Mesorectal fascia
(negative vs. positive) | 1.24
(0.48–3.21) | 0.665 |
|
|
| Distance from anal
verge (≤5 vs. >5 cm) | 0.84
(0.33–2.16) | 0.722 |
|
|
| Tumor
differentiation (low vs. medium, high) | 0.33
(0.08–1.45) | 0.023 | 0.32
(0.07–1.55) | 0.157 |
| Operation interval
of radiotherapy (≤7 vs. >7 weeks) | 1.97
(0.70–5.54) | 0.194 |
|
|
| Chemotherapy
regimens (with oxaliplatin vs. without oxaliplatin) | 3.06
(0.95–9.87) | 0.054 | 2.18
(0.64–7.47) | 0.213 |
| Chemotherapy course
(≤4 vs. >4 courses) | 1.76
(0.53–5.84) | 0.348 |
|
|
| Percentage change
in NLR (≤21.5 vs. >21.5%) | 0.34
(0.13–0.85) | 0.019 | 0.34
(0.13–0.90) | 0.030 |
Discussion
Systemic inflammatory responses have become a focus
of interest for physicians, and cancer-related inflammatory
responses have been recognized as a ‘hallmark’ of cancer
development and progression (26).
PLR and NLR are important indicators of systemic inflammatory
responses (27,28) and the increase in these indicators
has been confirmed as adverse factors for the prognosis of multiple
malignant tumors, including colon cancer and rectal cancer
(29,30). However, the association between
systemic inflammatory responses and tumor regression following nCRT
in patients with LARC remains unclear. In the current study, pre-
and post-nCRT NLR and PLR, and changes in these indices from
pre-nCRT to post-nCRT were investigated. Through logistic
regression analysis, the results demonstrated that the percentage
change in NLR was associated with pCR. These findings were
validated by the validation cohort. To the best of our knowledge,
the current study is the first to assess the predictive impact of
NLR change on tumor treatment outcomes of nCRT in patients with
LARC. The results demonstrated that the percentage change in NLR
from pre-nCRT to post-nCRT could be used to identify patients
achieving pCR following nCRT.
Previous studies have focused on the predictive
value of baseline systemic inflammatory responses. Dudani et
al (31) reviewed 1,527 patients
with rectal cancer receiving nCRT and surgery, and revealed that
NLR and PLR were not predictors for disease-free survival or
overall survival, and could not predict pCR. Additionally, Shen
et al (29) reported that
there was no significant association between tumor response and NLR
to nCRT. It has previously been reported that baseline systemic
inflammatory responses could not predict tumor responses in
colorectal cancer (32,33). In the present study, there was no
significant association between pCR and NLR or PLR during nCRT.
Furthermore, the results also revealed that adding
oxaliplatin to the chemotherapy regimens improved the rate of pCR.
Currently, it is controversial whether combining Oxaliplatin with
nCRT could improve prognosis and the rate of pCR. The results from
the FOWARC study demonstrated that the application of oxaliplatin
to nCRT for the treatment of middle and lower rectal cancer
exhibited higher rates of pCR (34).
Allegra et al (35) compared
the rates of pCR in patients receiving neoadjuvant
chemoradiotherapy with or without oxaliplatin, and the results did
not indicate any significant differences (17.8 vs. 19.5%; P=0.42),
which were inconsistent with the results obtained in the current
study. This may be due to the higher dose used in the current study
and the FOWARC study compared with that used in other studies (85
vs. 50–60 mg/m2) (34,35).
In numerous studies involving rectal cancer, CEA
levels were investigated as potential predictors of rectal cancer.
Moureau-Zabotto et al (36)
demonstrated that a pre-nCRT serum CEA level of <5 ng/ml was
significantly associated with pCR and tumor downstaging (36). However, other studies did not support
this conclusion (37). In the
current study, a pre-nCRT serum CEA level of ≤5 ng/ml was
significantly associated with pCR according to univariate analysis;
however, the results of multivariate analysis (P=0.052) were not
significant. This result may be due to the small simple size used
in the current study.
The present study had several limitations. Since
data was obtained from a single center, the results could not be
validated with those from another institute. Furthermore, the
retrospective observational design of the current study may cause
bias. Further prospective studies are required to validate the
results of the present study. The results demonstrated an
association between the percentage change in NLR and responsiveness
to nCRT among patients with rectal cancer. However, the results
failed to determine the cause of this association. Furthermore, a
reduction in circulating immunocytes may be due to cytotoxic
neoadjuvant chemotherapy. However, neoadjuvant radiation and
chemotherapy have been reported to release neoantigens by killing
tumor cells, and the immune reaction may cause an increase in
circulating immunocytes (38).
However, the ratios of different immunocytes as an independent
prognostic factor, or parameters associated to therapeutic activity
were not examined. Therefore, additional prognostic data is
required.
In conclusion, the results of the current study
investigated the pre- and post-nCRT systemic inflammatory indices
and changes in these indices from pre-nCRT to post-nCRT. The
association between the percentage change in NLR and responsiveness
to nCRT among patients with rectal cancer was determined, and the
results demonstrated that this association could identify patients
who achieved pCR following neoadjuvant chemoradiotherapy. These
results may provide a novel strategy for colorectal cancer
treatment.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Fundamental
Research Funds for the Central Universities (grant no. 16ykjc25),
the Sun Yat-Sen University Clinical Research 5010 Program (grant
no. 2016005), the National Health and Medical Research Council
Grant (grant no. 1158402) and the Natural Science Foundation of
Guangdong Province (grant no. 2018A030313621).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
SCL, LW, JD and LK designed the study. SLL and LH
were responsible for data collection. ZL and SCL analyzed data.
SLL, LH, JD, LW and LK drafted the manuscript. SCL, JD and LW
revised the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study protocol was reviewed and approved by the
Institutional Review Board of the Sixth Affiliated Hospital, Sun
Yat-sen University, China. The present study was carried out in
accordance with the recommendations of the Declaration of Helsinki
for biomedical research involving human patients. Written informed
consent was obtained from patients prior to enrollment.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
nCRT
|
neoadjuvant chemoradiotherapy
|
|
LARC
|
locally advanced rectal cancer
|
|
NLR
|
neutrophil-to-lymphocyte ratio
|
|
PLR
|
platelet-to-lymphocyte ratio
|
|
pCR
|
pathological complete response
|
|
TRG
|
tumor regression grade
|
|
CEA
|
carcino-embryonic antigen
|
References
|
1
|
Brenner H, Kloor M and Pox CP: Colorectal
cancer. Lancet. 383:1490–1502. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Benson AB, Venook AP, Al-Hawary MM,
Cederquist L, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D,
Engstrom PF, et al: Rectal cancer, version 2.2018, NCCN Clinical
Practice Guidelines in Oncology. J Natl Compr Canc Netw.
16:874–901. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rödel C, Liersch T, Becker H, Fietkau R,
Hohenberger W, Hothorn T, Graeven U, Arnold D, Lang-Welzenbach M,
Raab HR, et al: Preoperative chemoradiotherapy and postoperative
chemotherapy with fluorouracil and oxaliplatin versus fluorouracil
alone in locally advanced rectal cancer: Initial results of the
German CAO/ARO/AIO-04 randomised phase 3 trial. Lancet Oncol.
13:679–687. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bryant CL, Lunniss PJ, Knowles CH, Thaha
MA and Chan CL: Anterior resection syndrome. Lancet Oncol.
13:e403–e408. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fazio VW, Zutshi M, Remzi FH, Parc Y,
Ruppert R, Fürst A, Celebrezze J Jr, Galanduik S, Orangio G, Hyman
N, et al: A randomized multicenter trial to compare long-term
functional outcome, quality of life, and complications of surgical
procedures for low rectal cancers. Ann Surg. 246:481–490. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Paun BC, Cassie S, MacLean AR, Dixon E and
Buie WD: Postoperative complications following surgery for rectal
cancer. Ann Surg. 251:807–818. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nahas SC, Rizkallah Nahas CS, Sparapan
Marques CF, Ribeiro U Jr, Cotti GC, Imperiale AR, Capareli FC, Chih
Chen AT, Hoff PM and Cecconello I: Pathologic complete response in
rectal cancer: Can we detect it? Lessons learned from a proposed
randomized trial of watch-and-wait treatment of rectal cancer. Dis
Colon Rectum. 59:255–263. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Habr-Gama A, Perez RO, Nadalin W, Sabbaga
J, Ribeiro U Jr, Silva e Sousa AH Jr, Campos FG, Kiss DR and
Gama-Rodrigues J: Operative versus nonoperative treatment for stage
0 distal rectal cancer following chemoradiation therapy: Long-term
results. Ann Surg. 240:711–717; discussion 717–718. 2004.PubMed/NCBI
|
|
9
|
van der Valk MJM, Hilling DE, Bastiaannet
E, Meershoek-Klein Kranenbarg E, Beets GL, Figueiredo NL, Habr-Gama
A, Perez RO, Renehan AG and van de Velde CJH; IWWD Consortium, :
Long-term outcomes of clinical complete responders after
neoadjuvant treatment for rectal cancer in the International Watch
& Wait Database (IWWD): An international multicentre registry
study. Lancet. 391:2537–2545. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Habr-Gama A, São Julião GP, Fernandez LM,
Vailati BB, Andrade A, Araújo SEA, Gama-Rodrigues J and Perez RO:
Achieving a complete clinical response After neoadjuvant
chemoradiation that does not require surgical resection: It may
take longer than you think! Dis Colon Rectum. 62:802–808. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yeom SS, Lee SY, Kim CH, Kim YJ, Nam TK
and Kim HR: The outcome of non-operative treatment for rectal
cancer patients who had been cCR after neoadjuvant
chemoradiotherapy. Asian J Surg. 42:2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Renehan AG, Malcomson L, Emsley R, Scott N
and O'Dwyer ST; OnCoRe project investigators, : Watch-and-wait
versus surgical resection for patients with rectal cancer-Authors'
reply. Lancet Oncol. 17:e134–e135. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kong JC, Guerra GR, Warrier SK, Ramsay RG
and Heriot AG: Outcome and salvage surgery following ‘watch and
wait’ for rectal cancer after neoadjuvant therapy: A systematic
review. Dis Colon Rectum. 60:335–345. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Malietzis G, Giacometti M, Askari A,
Nachiappan S, Kennedy RH, Faiz OD, Aziz O and Jenkins JT: A
preoperative neutrophil to lymphocyte ratio of 3 predicts
disease-free survival after curative elective colorectal cancer
surgery. Ann Surg. 260:287–292. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee DY, Hong SW, Chang YG, Lee WY and Lee
B: Clinical significance of preoperative inflammatory parameters in
gastric cancer patients. J Gastric Cancer. 13:111–116. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Stotz M, Gerger A, Eisner F, Szkandera J,
Loibner H, Ress AL, Kornprat P, AlZoughbi W, Seggewies FS, Lackner
C, et al: Increased neutrophil-lymphocyte ratio is a poor
prognostic factor in patients with primary operable and inoperable
pancreatic cancer. Br J Cancer. 109:416–421. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pichler M, Hutterer GC, Stoeckigt C,
Chromecki TF, Stojakovic T, Golbeck S, Eberhard K, Gerger A,
Mannweiler S, Pummer K and Zigeuner R: Validation of the
pre-treatment neutrophil-lymphocyte ratio as a prognostic factor in
a large European cohort of renal cell carcinoma patients. Br J
Cancer. 108:901–907. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Azab B, Shah N, Radbel J, Tan P, Bhatt V,
Vonfrolio S, Habeshy A, Picon A and Bloom S: Pretreatment
neutrophil/lymphocyte ratio is superior to platelet/lymphocyte
ratio as a predictor of long-term mortality in breast cancer
patients. Med Oncol. 30:4322013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yamada S, Fujii T, Yabusaki N, Murotani K,
Iwata N, Kanda M, Tanaka C, Nakayama G, Sugimoto H, Koike M, et al:
Clinical implication of inflammation-based prognostic score in
pancreatic cancer: Glasgow prognostic score is the most reliable
parameter. Medicine (Baltimore). 95:e35822016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li MX, Liu XM, Zhang XF, Zhang JF, Wang
WL, Zhu Y, Dong J, Cheng JW, Liu ZW, Ma L and Lv Y: Prognostic role
of neutrophil-to-lymphocyte ratio in colorectal cancer: A
systematic review and meta-analysis. Int J Cancer. 134:2403–2413.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Roxburgh CS, Salmond JM, Horgan PG, Oien
KA and McMillan DC: The relationship between the local and systemic
inflammatory responses and survival in patients undergoing curative
surgery for colon and rectal cancers. J Gastrointest Surg.
13:2011–2019. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Heo J, Oh YT, Noh OK, Chun M, Park JE and
Cho SR: Nodal tumor response according to the count of peripheral
blood lymphocyte subpopulations during preoperative
chemoradiotherapy in locally advanced rectal cancer. Radiat Oncol
J. 34:305–312. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee IH, Hwang S, Lee SJ, Kang BW, Baek D,
Kim HJ, Park SY, Park JS, Choi GS, Kim JC, et al: Systemic
inflammatory response after preoperative chemoradiotherapy can
affect oncologic outcomes in locally advanced rectal cancer.
Anticancer Res. 37:1459–1465. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Laird BJ, Fallon M, Hjermstad MJ, Tuck S,
Kaasa S, Klepstad P and McMillan DC: Quality of life in patients
with advanced cancer: Differential association with performance
status and systemic inflammatory response. J Clin Oncol.
34:2769–2775. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tong GJ, Zhang GY, Liu J, Zheng ZZ, Chen
Y, Niu PP and Xu X: Comparison of the eighth version of the
American Joint Committee on Cancer manual to the seventh version
for colorectal cancer: A retrospective review of our data. World J
Clin Oncol. 9:148–161. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gomez D, Morris-Stiff G, Toogood GJ, Lodge
JP and Prasad KR: Impact of systemic inflammation on outcome
following resection for intrahepatic cholangiocarcinoma. J Surg
Oncol. 97:513–518. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ku JH, Kang M, Kim HS, Jeong CW, Kwak C
and Kim HH: The prognostic value of pretreatment of systemic
inflammatory responses in patients with urothelial carcinoma
undergoing radical cystectomy. Br J Cancer. 112:461–467. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shen L, Zhang H, Liang L, Li G, Fan M, Wu
Y, Zhu J and Zhang Z: Baseline neutrophil-lymphocyte ratio (≥2.8)
as a prognostic factor for patients with locally advanced rectal
cancer undergoing neoadjuvant chemoradiation. Radiat Oncol.
9:2952014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Braun LH, Baumann D, Zwirner K, Eipper E,
Hauth F, Peter A, Zips D and Gani C: Neutrophil-to-lymphocyte ratio
in rectal cancer-novel biomarker of tumor immunogenicity during
radiotherapy or confounding variable? Int J Mol Sci. 20:24482019.
View Article : Google Scholar
|
|
31
|
Dudani S, Marginean H, Tang PA, Monzon JG,
Raissouni S, Asmis TR, Goodwin RA, Gotfrit J, Cheung WY and Vickers
MM: Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios as
predictive and prognostic markers in patients with locally advanced
rectal cancer treated with neoadjuvant chemoradiation. BMC Cancer.
19:6642019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Picardo SL, Teo M, Jalil KIA, Naqvi SY,
Morris PG, Breathnach OS, Grogan W, Leonard GD and Hennessy B:
Correlation between platelet/lymphocyte ratio,
neutrophil/lymphocyte ratio and response to neoadjuvant
chemoradiation therapy in rectal cancer. J Clin Oncol.
34:e151402016. View Article : Google Scholar
|
|
33
|
Shin US, You YN, Price BA, Rodriguez-Bigas
MA, Skibber JM, Maru DM, Taggart MW, Kopetz S, Eng C, Minsky BD, et
al: Is the neutrophil-lymphocyte ratio (NLR) a predictive and
prognostic factor in rectal cancer patients treated with
neoadjuvant chemoradiation (nCRT)? J Clin Oncol. 34:36052016.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Deng Y, Chi P, Lan P, Wang L, Chen W, Cui
L, Chen D, Cao J, Wei H, Peng X, et al: Modified FOLFOX6 with or
without radiation versus fluorouracil and leucovorin with radiation
in neoadjuvant treatment of locally advanced rectal cancer: Initial
results of the Chinese FOWARC multicenter, open-label, randomized
three-arm phase III trial. J Clin Oncol. 34:3300–3307. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Allegra CJ, Yothers G, O'Connell MJ, Beart
RW, Wozniak TF, Pitot HC, Shields AF, Landry JC, Ryan DP, Arora A,
et al: Neoadjuvant 5-FU or capecitabine plus radiation with or
without oxaliplatin in rectal cancer patients: A phase III
randomized clinical trial. J Natl Cancer Inst. 107:djv2482015.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Moureau-Zabotto L, Farnault B, de
Chaisemartin C, Esterni B, Lelong B, Viret F, Giovannini M, Monges
G, Delpero JR, Bories E, et al: Predictive factors of tumor
response after neoadjuvant chemoradiation for locally advanced
rectal cancer. Int J Radiat Oncol Biol Phys. 80:483–491. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kalady MF, de Campos-Lobato LF, Stocchi L,
Geisler DP, Dietz D, Lavery IC and Fazio VW: Predictive factors of
pathologic complete response after neoadjuvant chemoradiation for
rectal cancer. Ann Surg. 250:582–589. 2009.PubMed/NCBI
|
|
38
|
Burnette B, Fu YX and Weichselbaum RR: The
confluence of radiotherapy and immunotherapy. Front Oncol.
2:1432012. View Article : Google Scholar : PubMed/NCBI
|