Introduction
The discovery and use of tyrosine kinase inhibitors
(TKI) specifically targeting epidermal growth factor receptor
(EGFR) in the management of non-small-cell lung cancer (NSCLC) in
2004 spearheaded the development of the molecular pathology
classification for lung cancer and targeted therapies (1,2). Soda
et al (3) revealed the
presence of a hybrid gene borne as a result of anaplastic lymphoma
kinase (ALK) gene chromosomal rearrangement. Furthermore, Rikova
et al (4) characterized c-ros
oncogene 1 (ROS1) rearrangement in NSCLC tumors. The molecular
pathology-based classification has significantly increased the
efficacy of targeted tyrosine kinase inhibitors in comparison with
standard chemotherapy.
Crizotinib is an ALK TKI and was successful in
gaining approval as a therapeutic agent for advanced NSCLCs with
ALK and ROS1 rearrangement (5–8). Indeed,
ALK and ROS1 genes share similar properties in terms of their amino
acid sequence of the kinase domain and the adenosine triphosphate
binding site. This similarity provided a basis for the properties
of crizotinib in combating NSCLCs that possess these gene fusions.
The phase I PROFILE 1001 study of crizotinib included 50 patients
with ROS1-rearranged NSCLC who achieved a median progression-free
survival (PFS) of 19.3 months and a median overall survival (OS) of
51.4 months with crizotinib treatment, with an objective response
rate of 72% (8). This efficacy rate
was much better than that for traditional chemotherapy, further
highlighting the importance of genetic mutation detection in
guiding NSCLC treatment. However, limited tissue samples are
usually obtained, given the extremely invasive procedures necessary
to procure them (lung biopsy and bronchoscopy). This reason coupled
with the high cost of genetic testing frequently lead to numerous
patients not undergoing genetic testing at all. Furthermore,
testing for ROS1 rearrangements is frequently not performed, as
they have been detected in only a low proportion of patients with
NSCLC (0.9–2%). Therefore, there is an urgent requirement for a
non-invasive and low-cost method to distinguish ROS1/ALK-rearranged
patients from EGFR-mutated or wild-type (WT) patients to facilitate
the selection of individualized treatment strategies. In this
regard, it has been indicated that the clinical and imaging
characteristics of patients with NSCLC may be able to predict their
genetic status.
ALK rearrangements are typically encountered in
patients with lung adenocarcinoma. These patients frequently have
no history or a history of light smoking and are generally young
(9–12). Solid-pattern growth on CT, an
elevated glucose metabolism on positron-emission tomography
(PET)/CT and relatively more rapid metastasis to lymph nodes or
distant sites on PET/CT have been reported to be the primary
radiological features of ALK-rearranged tumors compared with
non-ALK-rearranged tumors in previous studies (12–14). A
previous study by our group has highlighted the different imaging
features between ALK-rearranged NSCLC, EGFR-mutated NSCLC and WT
NSCLC, and noted that solid patterns were the predominant distinct
features of ALK-rearranged tumors compared with other tumors
(13).
In addition to ALK rearrangement, ROS1 rearrangement
detection and targeted therapies have also been studied. Several
studies have discussed the clinical and pathological features of
NSCLC tumors with or without ROS1 rearrangement (15,16).
There are a number of studies on the radiological characteristics
of tumors with ROS1 rearrangement compared with tumors with other
gene statuses. Digumarthy et al (17) indicated that ROS1-rearranged tumors
more frequently displayed features of lymphangitic carcinomatosis
(ROS1-rearranged, 42%; EGFR-mutated, 12%; P<0.01), had a lower
chance of developing lymphangitic carcinomatosis and were more
likely to have air bronchograms present in the primary tumor
(ROS1-rearranged, 2%; EGFR-mutated, 28%; P<0.01). The above
study qualitatively described the radiographic features, while the
present study performed a quantitative volumetric analysis to
evaluate the imaging features in lung adenocarcinoma with different
gene statuses.
The present retrospective study aimed to assess the
ability of PSV (also in combination with other clinical parameters)
to predict the ROS1/ALK rearrangement status in lung adenocarcinoma
in order to facilitate the specific targeted treatment.
Materials and methods
Patient selection and grouping
All procedures of the present study involving human
participants were performed in strict compliance with the ethical
standards of the institutional research committee and with the 1964
Helsinki declaration and its later amendments or comparable ethical
standards. The ethics committee of the First Affiliated Hospital of
Zhejiang University (Hangzhou, China) approved the study and waived
the requirement for obtaining informed consent.
For all patients included, CT images had been
obtained at the First Affiliated Hospital of Zhejiang University
(Hangzhou, China). Clinical data were obtained from the medical
records of each patient retrospectively. The TNM classification was
also extracted and TNM staging was based on the seventh edition of
the Union for International Cancer Control and American Joint
Committee on Lung Cancer (18).
Confirmation of ROS1 rearrangement
through real-time PCR
A 4-µm thick formalin-fixed (at 25°C for 12–24 h),
paraffin-embedded (FFPE) tissue slice was used to evaluate ROS1 and
ALK gene rearrangement. A total of three 5-µm sections of each
tumor were used for total RNA extraction, which was performed based
on predefined protocols (19)
(RNeasy FFPE kit; Qiagen GmbH). The ROS1 rearrangement status was
determined by multiplex real-time PCR in a Stratagene Mx3000P
real-time PCR system (Stratagene Corp.) with an AmoyDx®
ROS1 fusion gene detection kit (Amoy Diagnostics Co., Ltd.). ROS1
fusion combinations, including SLC34A2-ROS1, EZR-ROS1, CD74-ROS1,
SDC4-ROS1, LRIG3-ROS1, TPM3-ROS1 and GOPC-ROS1, were detected in
this assessment. The internal reference gene (β-actin) was used as
a negative control and a confirmed ROS1-rearranged DNA was used as
a positive control in the AmoyDx® ROS1 Gene Fusions
Detection kit (cat. no. 8.01.23201W012A; Amoy Diagnostics Co.,
Ltd.).
Confirmation of ALK rearrangement
using fluorescence in situ hybridisation (FISH)
ALK gene fusion was evaluated with FISH utilizing an
ALK break-apart probe (Vysis LSI ALK Dual Color, Break Apart
Rearrangement Probe; Abbott Molecular), in accordance with
previously published protocols (13).
EGFR mutation analysis using
pyrosequencing assay
A PCR-based pyrosequencing assay was used to analyze
EGFR mutations located on exons 18–21 (20). The PyroMark system (Qiagen GmbH) was
used to perform sequence analysis.
CT imaging
Each patient received thoracic CT scans with either
the 64-slice Brilliance, 256-slice iCT (Philips Medical Systems,
Inc.) or 64-slice light-speed VCT (GE Healthcare). CT scan systems
had the following parameters: Rotation speed, 0.5/sec; pitch, 1;
120 kV; 120–380 mA; field of view, 300–350 mm; and collimation,
0.625 mm. A high-frequency reconstruction algorithm with a 512×512
matrix was used for image reconstruction. The reconstruction
thicknesses and intervals were 5.0 and 5.0 mm, respectively. CT
imaging information was reviewed in a Philips CT workstation using
the mediastinal window setting [width, 200 Hounsfield Units (HU);
level, 40 HU] and lung window setting (width, 1,500 HU; level, 500
HU), which were obtained prior to contrast administration.
Interpretations of CT images
All CT images were independently reviewed by JL and
JX, two radiologists with 10 years of experience. The
radiologists were not blinded to the patients' diagnoses of lung
adenocarcinoma but were blinded to their genetic statuses. The
presence or absence of multiple lesions, lobulated borders, fine
spiculated margins, bubble-like lucency, cavity, liquefaction
necrosis and air bronchogram were assessed.
Multiple lesions were defined as more than one tumor
lesion on a CT scan. A lobulated border was defined as a shallow
wavy configuration seen on part of the border of the lesion,
excluding lesions that were abutting the pleura (13,20–22). A
lesion that possessed fine linear strands extending 1–2 mm radially
was defined as having fine spiculated margins. Lesions that
possessed air attenuation spots within them were classified as
having bubble-like lucency (13,20) and
a cavity was defined as a gas-filled space appearing as an area of
lower attenuation or lucency (21).
A hypodense area on non-enhanced and contrast-enhanced CT images
was defined as liquefaction necrosis (23). An air bronchogram was defined as an
air-filled (low-attenuation) bronchus against an opaque background
(high-attenuation) (13,21).
Quantitative volumetric
assessment
Lung nodule reconstruction was performed using the
256-slice iCT (Philips Medical Systems), which allowed for the
quantification of the volume of individual lesions. The software is
able to automatically generate solid tumor boundaries on each
cross-section, while extracting intramodular bronchial and
cavitating structures in order to reconstruct a three-dimensional
structure of the nodule and provide data on the volume (V) and
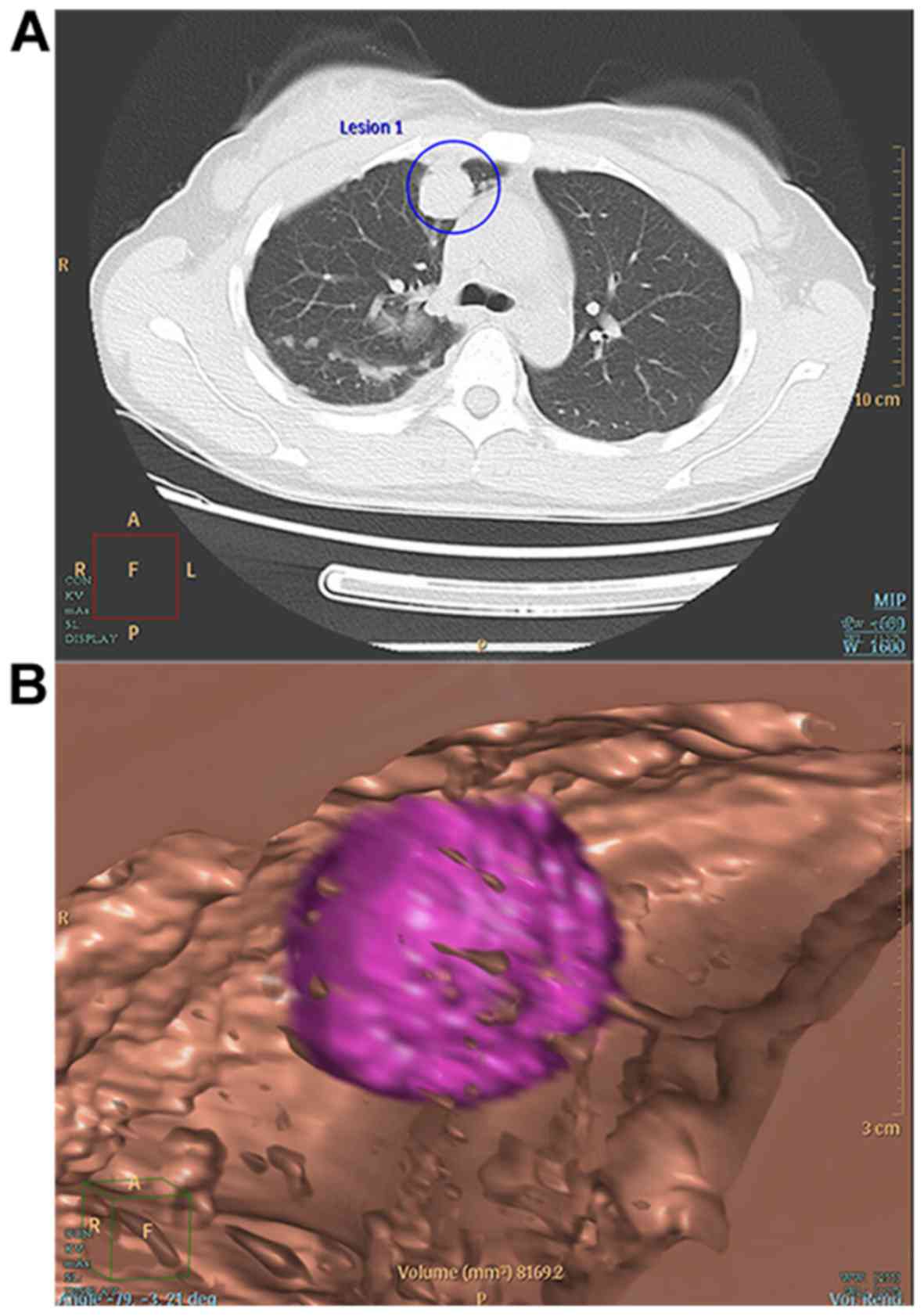
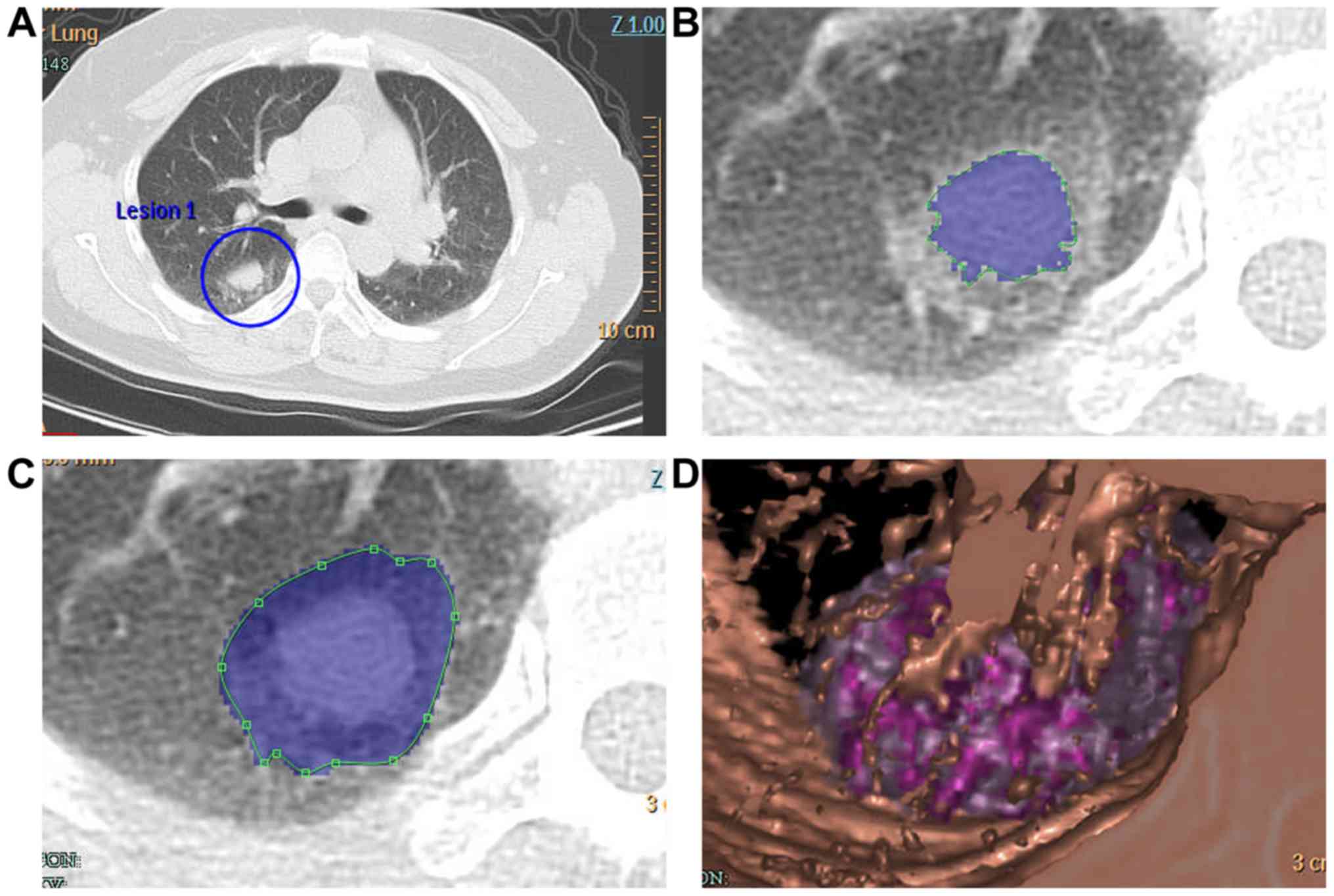
estimated diameter (D) (Fig. 1). For
mixed tumor nodules with solid and ground-glass opacity (GGO)
portions (Fig. 2A and B), as well as
for the entire nodule (Fig. 2C), all
computer-derived boundaries were assessed and adjusted as necessary
by the two experienced radiologists. The software reconstructed the
three-dimensional structure of the nodule and provided data on the
entire nodule and the solid portion (Fig. 2D). The radiologists recorded the
volume and the estimated diameter mentioned above for the total
nodule (tV, tD) and the solid portion (sV, sD). The PSV was
calculated using the formula PSV=sV/tV.
Statistical analysis
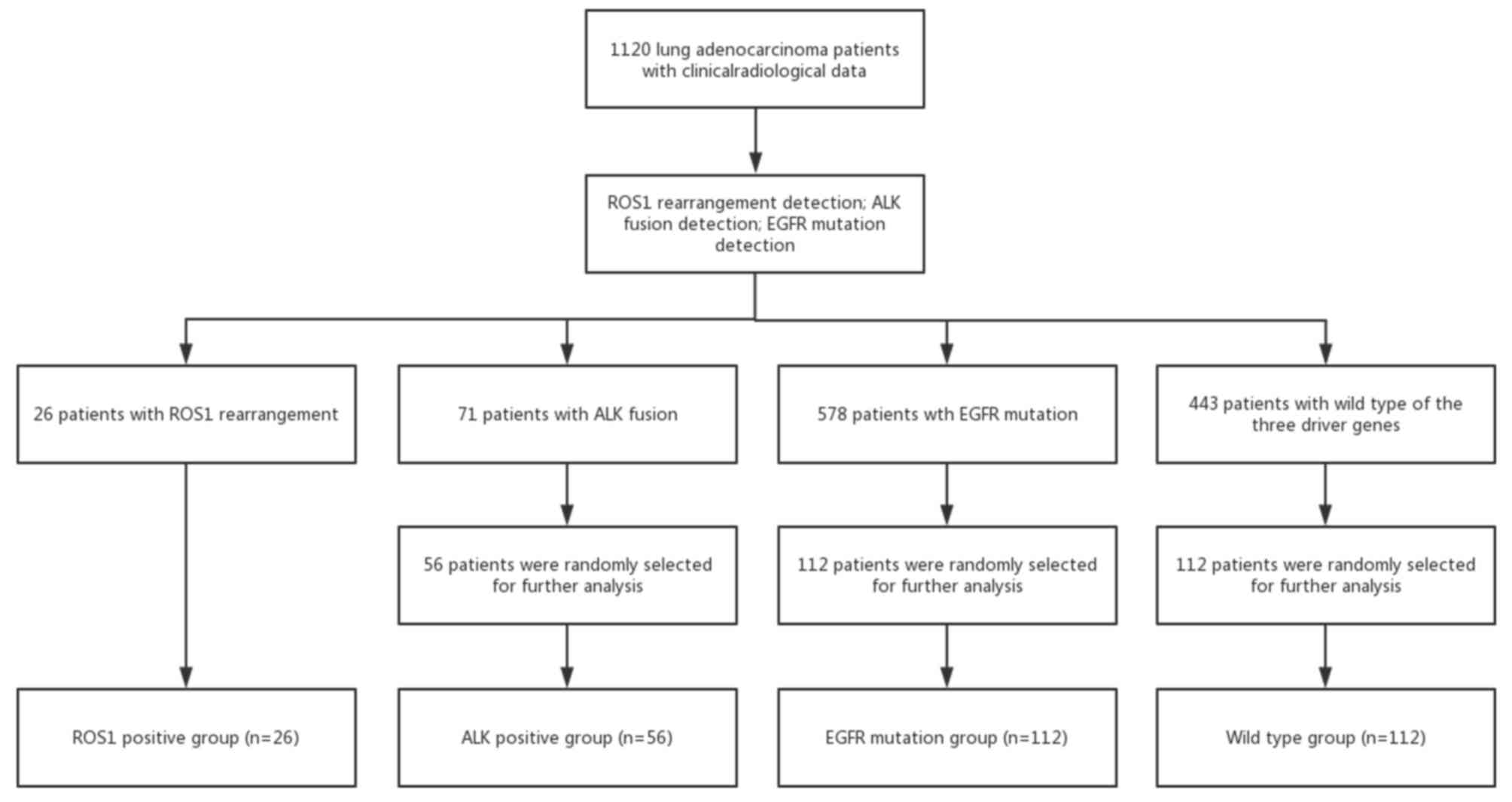
The patients with different gene status' were
included in different groups via a simple randomization method
(Fig. 3). A Chi-squared test was
used to compare categorical variables. For continuous variables,
including age, tV, tD and PSV, differences in median values between
two groups were compared by the Mann-Whitney U-test, while the
Kruskal-Wallis U-test was used for comparisons among four groups.
The univariate and multivariate logistic regression analyses were
performed to predict ROS1/ALK-rearranged. Variates with P≤0.10 in
the univariate analysis were included in a multivariable logistic
regression analysis. A receiver operating characteristic (ROC)
analysis was carried out to evaluate the predictive value of PSV
and the multi-factor (all independent predictive factors selected
by multivariate analysis) predictive model to predict ROS1
rearrangement or ROS1/ALK rearrangement. All statistical analyses
were performed using SPSS version 17.0 (SPSS, Inc.). P<0.05 was
considered to indicate statistical significance.
Results
Clinical characteristics of patients
with lung adenocarcinoma with different gene statuses
In the present study, 28 ROS1-rearranged, 578
EGFR-mutated and 71 ALK-rearranged patients were retrospectively
identified among 1,120 patients with histology-confirmed lung
adenocarcinoma diagnosed at the First Affiliated Hospital of
Zhejiang University (Hangzhou, China) between March 2008 and
October 2015. The 28 ROS1-rearranged patients were assigned to the
ROS1 rearrangement group (ROS1 rearrangement group or
ROS1+ group; n=28). Furthermore, 56 ALK-rearranged
patients were randomly selected from the 71 ALK-rearranged patients
(ALK rearrangement group or ALK+ group; n=56), as well
as 112 patients with EGFR mutations from the 578 EGFR-mutated
patients (EGFR mutation group or EGFR+ group; n=112) and
112 patients from those who exhibited the WT for all three genes
(WT group; n=112). The patient selection procedure is presented in
Fig. 3. The EGFR mutation group
included 65 patients with EGFR 19 exon mutation, 44 patients with
EGFR 21 exon mutation, 1 patient with EGFR 20 exon mutation, 1
patient with both EGFR 19 and 20 exon mutation and 1 patient with
both EGFR 20 and 21 exon mutation.
The demographic and clinicopathological features of
the patients with lung adenocarcinoma are presented in Table I. The median age (range) of the
patients in the ROS1+, ALK+, EGFR+
and WT groups was 54.5 (27–60), 51.0 (23–79), 60.0 (34–83) and 62.0
(31–83) years, respectively. Individuals possessing ROS1
rearrangements were noted to be markedly younger in comparison with
those with EGFR mutations (P=0.007) and patients who did not test
positive for all three genes (P=0.003). Patients with ROS1
mutations were more likely to be female (P=0.006) in contrast to
the WT group. Those who had no history of smoking were also more
likely to have ROS1 mutations (P=0.005) in comparison with the WT
group. The demographic features (age, sex and smoking history) of
ROS1-rearranged patients were not different compared to those of
ALK-rearranged patients. Patients across the four groups did not
exhibit any notable variability in terms of carcinoembryonic
antigen levels.
 | Table I.Clinical characteristics of the
patients based on genetic alterations in lung adenocarcinomas. |
Table I.
Clinical characteristics of the
patients based on genetic alterations in lung adenocarcinomas.
|
| Genetic
alteration | P-value |
|---|
|
|
|
|
|---|
| Clinical
characteristic | ROS1+
(n=28) | ALK+
(n=56) | EGFR+
(n=112) | WT (n=112) | Among the 4
groups | ROS1+
vs. ALK+ | ROS1+
vs. EGFR+ | ROS1+
vs. WT |
|---|
| Age (years) | 54.5 (27–60) | 51.0 (23–79) | 60.0 (34–83) | 62.0 (31–83) | <0.001 | 0.798 | 0.007 | 0.003 |
| Male sex | 8 (28.6) | 27 (48.2) | 44 (39.3) | 72 (64.3) | <0.001 | 0.057 | 0.099 | 0.006 |
| Smoking | 5 (17.9) | 16 (28.6) | 31 (27.7) | 54 (48.2) | 0.001 | 0.423 | 0.342 | 0.005 |
Comparison of the tD, tV and PSV
between tumors with different gene statuses
Fig. 4 provides
comparisons of the tD, tV and PSV across tumors with various
genetic aberrations. The PSV [median (interquartile range)] in
tumors with ROS1 rearrangements [87.9 (82.7–92.3) %] was markedly
higher compared with those with EGFR mutations [70.4 (51.4–83.4)%;
P<0.001] and those of the WT [63.0 (50.9–83.2)%; P<0.001].
However, the difference in PSV between the ROS1+ group
and the ALK+ group [84.0 (70.3–90.0)%; P=0.251] was not
significant. There was no difference in the median value of the tD
and tV among the four groups.
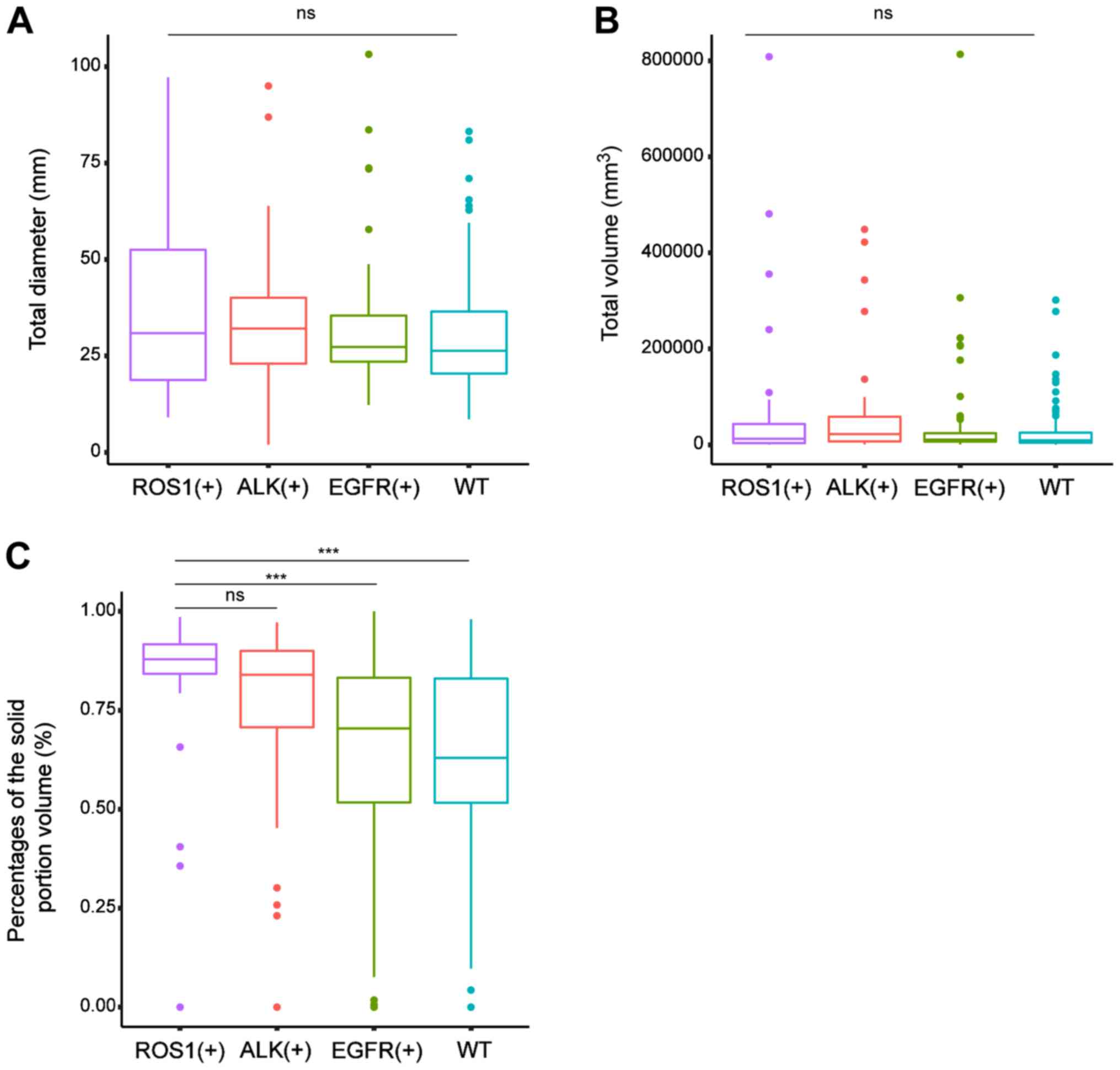 | Figure 4.Comparisons of the estimated volume
and diameter of the total nodule (tV, tD) and the PSV across tumors
with various genetic aberrations. (A and B) levels across tumors
with various genetic aberrations. There was no difference in the
median value of (A) the total diameter and (B) total volume among
the four groups. (C) The median PSV was markedly higher in tumors
with ROS1 mutations (87.9%; IQR, 82.7–92.3%) compared with those
with EGFR changes (70.4%; IQR 51.4–83.4%; P<0.001) and those of
the WT (63.0%; IQR, 50.9–83.2%; P<0.001). However, the
difference in the median PSV between the ROS1+ group and
the ALK+ group (84.0%; IQR, 70.3–90.0%; P=0.251) was not
significant. In the boxplots, the horizontal lines indicate the
median value, the boxes are the IQR, bars are the standard
deviation and dots are the outliers. ***P<0.001. ns, no
significance; IQR, interquartile range; PSV, proportion of solid
volume; ROS1, c-ros oncogene 1; ALK, anaplastic lymphoma kinase;
EGFR, epidermal growth factor receptor; WT, wild-type. |
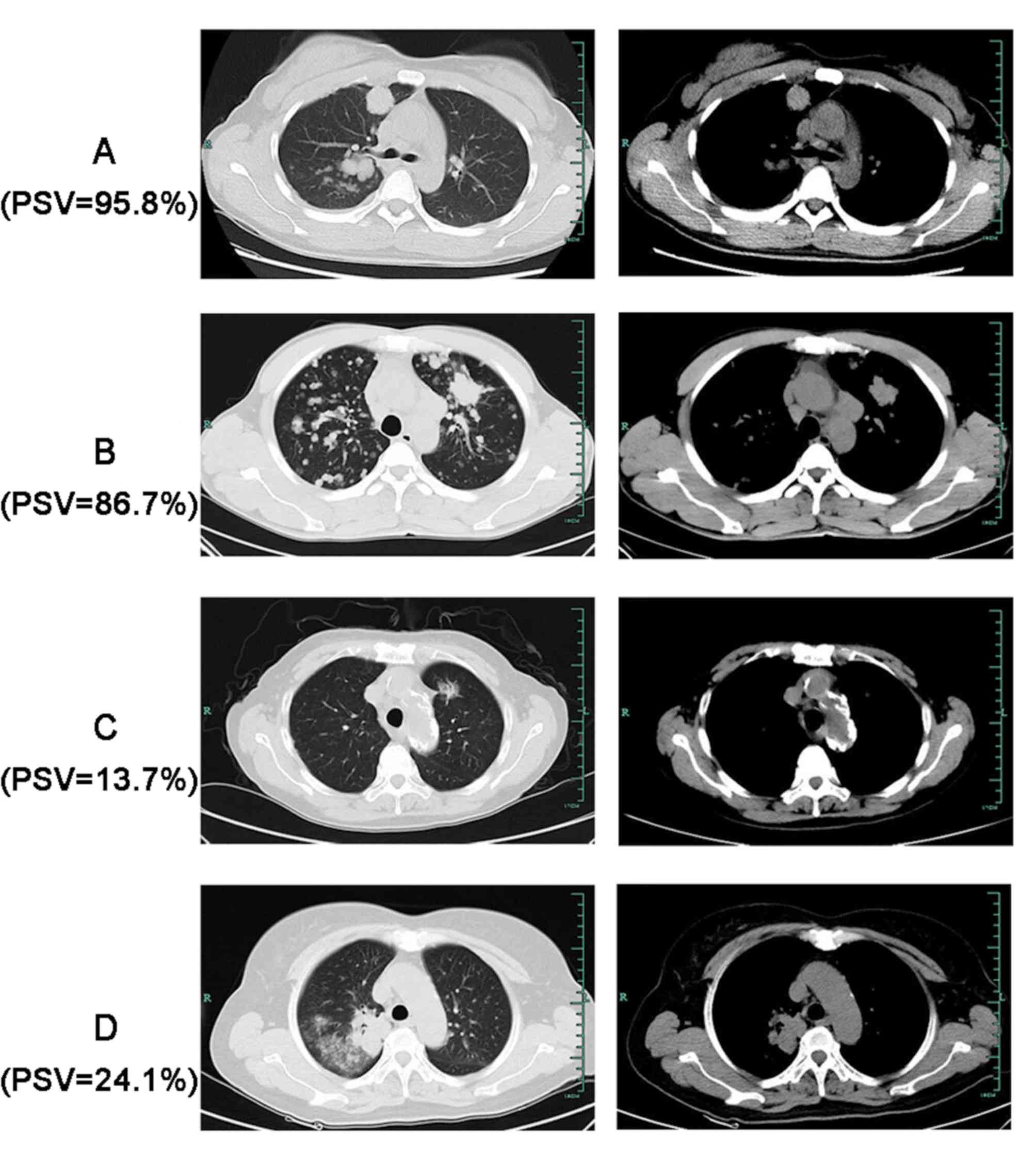
CT images of four representative patients with
different genetic status are presented in Fig. 5. The four tumors exhibited different
PSV on CT.
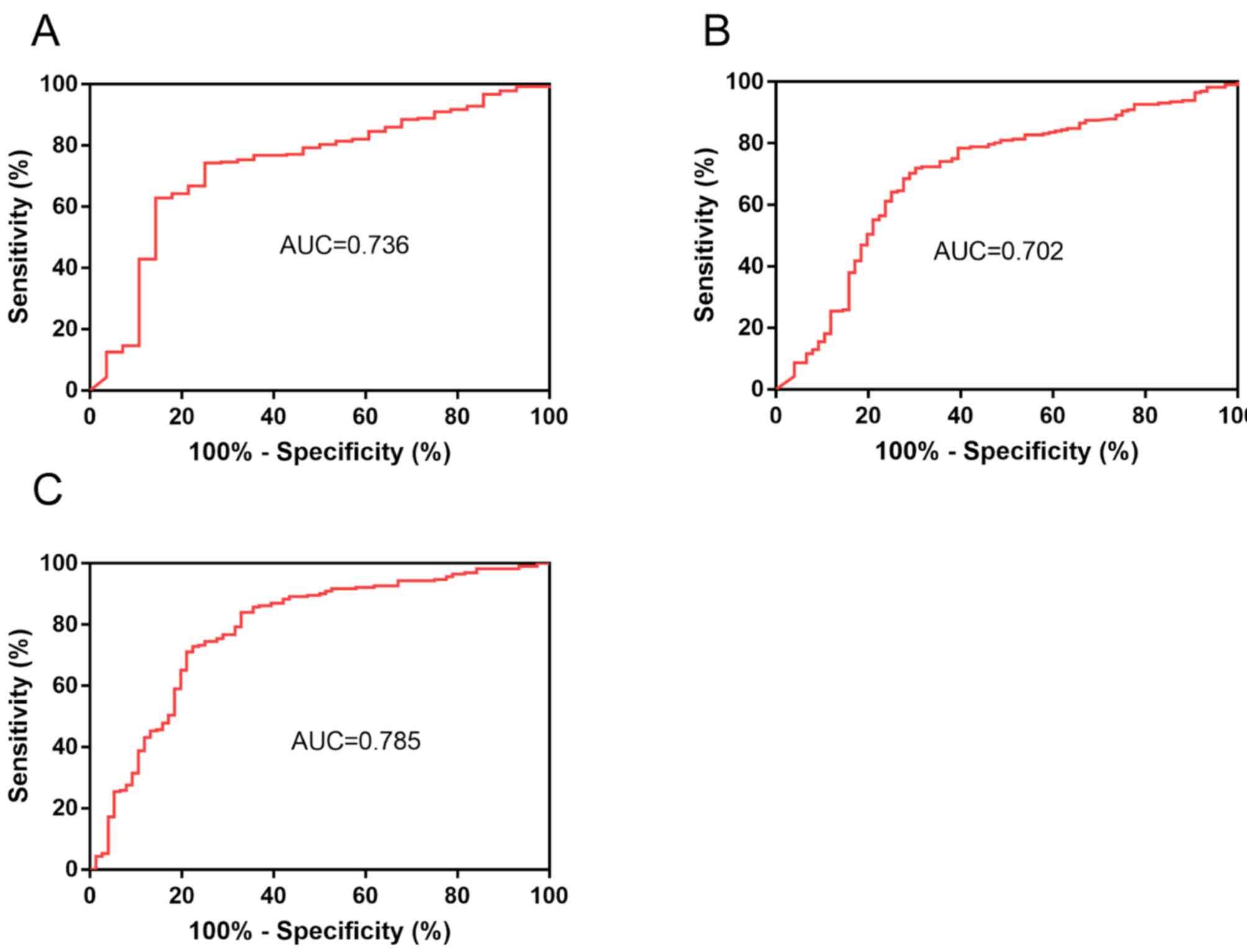
The ROC curves for the predictive value of PSV for
the gene rearrangements are provided in Fig. 6. The area under the ROC curve (AUC)
of PSV to predict ROS1 rearrangement was 0.736 (95% CI,
0.637–0.835; P<0.001; Fig. 6A) at
a cut-off value of 0.849. When the Youden index was maximal, the
sensitivity was 0.750 and the specificity was 0.743. Given the
similar PSV values in the ROS1+ group and the
ALK+ group, a ROC curve to predict ROS1 or ALK
rearrangement was drawn (Fig. 6B).
The AUC was 0.702 (95% CI, 0.631–0.773; P<0.001) at a cut-off
value of 0.805. When the Youden index was maximal, the sensitivity
was 0.697 and the specificity was 0.702.
Impact of genetic aberrations on CT
imaging
A total of two radiologists independently reviewed
the CT imaging and recorded the interpretations. The differences in
interpretation between the two radiologists were as follows:
Multiple lesions (3/76; 3.9%), lobulated borders (18/236; 7.6%),
fine spiculated margins (19/179; 10.6%), pleural retraction (8/145;
5.5%), bubble-like lucency or cavities (4/42; 9.5%), air
bronchograms (7/62; 11.3%) and liquefaction necrosis (1/17; 5.9%).
Disagreements were resolved by discussions to reduce the
interobserver variability.
Table II summarises
the final different features on CT imaging based on the various
genetic aberrations. There were no differences in the frequency of
multiple lesions, pleural retraction, fine spiculated margins,
lobulated borders, bubble-like lucency or cavities, air
bronchograms or liquefaction necrosis between tumors in the
ROS1+ group and tumors in the ALK+,
EGFR+ or WT groups.
 | Table II.Genetic alterations and visual CT
signs (morphological CT features). |
Table II.
Genetic alterations and visual CT
signs (morphological CT features).
|
| Genetic
alteration | P-value |
|---|
|
|
|
|
|---|
| Morphological CT
feature | ROS1+
(n=28) | ALK+
(n=56) | EGFR+
(n=112) | WT (n=112) | Among the 4
groups | ROS1+
vs. ALK+ | ROS1+
vs. EGFR+ | ROS1+
vs. WT |
|---|
| Multiple
lesions | 5 (17.9) | 20 (35.7) | 26 (23.2) | 25 (22.3) | 0.184 | 0.129 | 0.620 | 0.798 |
| Lobulated
border | 21 (75.0) | 35 (62.5) | 88 (78.6) | 92 (82.1) | 0.038 | 0.329 | 0.799 | 0.425 |
| Fine spiculated
margin | 16 (57.1) | 23 (41.1) | 74 (66.1) | 66 (58.9) | 0.022 | 0.174 | 0.387 | 1 |
| Pleural
retraction | 11 (39.3) | 14 (25.0) | 60 (53.6) | 60 (53.6) | 0.001 | 0.210 | 0.208 | 0.208 |
| Bubble-like lucency
or cavities | 4 (14.3) | 3 (5.4) | 22 (19.6) | 13 (11.6) | 0.069 | 0.215 | 0.599 | 0.747 |
| Air
bronchogram | 3 (10.7) | 9 (16.1) | 29 (25.9) | 21 (18.8) | 0.207 | 0.743 | 0.130 | 0.408 |
| Liquefaction
necrosis | 1 (3.6) | 2 (3.6) | 9 (8.0) | 5 (4.5) | 0.530 | 1 | 0.687 | 1 |
Univariate and multivariate analyses
of clinical radiological parameters to predict
ROS1/ALK-rearrangement by logistic regression
Given the similar clinical radiological
characteristics between ROS1+ tumors and ALK+
lncRNAs, and the same first line recommended targeted treatment,
univariate and multivariate analyses of clinical radiological
parameters were performed to predict ROS1/ALK-rearrangement. All
clinical parameters, including age, sex and smoking status, the
quantitative volumetric parameters, including the total nodule
diameter and PSV and all the aberrations on CT imaging were
assessed via univariate logistic regression analysis. The results
presented in Table III
demonstrated that age, smoking history, total nodule diameter, PSV
and existence of lobulated border, fine spiculated margin and
pleural retraction were different (P<0.1) between
ROS1/ALK-rearranged and non-ROS1/ALK-rearranged patients. These
different parameters were subjected to multivariate logistic
regression analysis, the results of which are also presented in
Table III. Younger age and higher
PSV values were independent predictors of ROS1/ALK rearrangements
in the multivariate analysis. The correlation analysis between PSV
and age suggested that the two factors were not correlated
(Fig. S1). The ROC curve of the
predictive model combined with age and PSV is presented in Fig. 6C, with an AUC of 0.785.
 | Table III.Univariate and multivariate analyses
of clinical-radiological parameters to predict ROS1/ALK
rearrangement. |
Table III.
Univariate and multivariate analyses
of clinical-radiological parameters to predict ROS1/ALK
rearrangement.
|
| Univariate
analysisa | Multivariate
analysisa |
|---|
|
|
|
|
|---|
|
Clinical-radiological factors | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Sex | 0.741 | 0.440–1.249 | 0.261 | – | – | – |
| Age | 0.939 | 0.916–0.962 | <0.001 | 0.946 | 0.921–0.971 | <0.001 |
| Smoking history
(smoker) | 0.606 | 0.341–1.078 | 0.096 | 0.691 | 0.366–1.303 | 0.253 |
| Total nodule
diameter | 1.018 | 1.002–1.034 | 0.023 | 0.998 | 0.980–1.017 | 0.841 |
| PSV | 17.084 | 4.181–69.812 | <0.001 | 16.545 | 3.449–79.374 | <0.001 |
| Multiple
lesions | 1.598 | 0.900–2.836 | 0.109 | – | – | – |
| Lobulated
border | 0.476 | 0.268–0.844 | 0.011 | 0.676 | 0.334–1.372 | 0.278 |
| Fine spiculated
margin | 0.486 | 0.287–0.821 | 0.007 | 0.805 | 0.429–1.509 | 0.498 |
| Pleural
retraction | 0.361 | 0.206–0.631 | <0.001 | 0.537 | 0.278–1.036 | 0.064 |
| Bubble-like lucency
or cavities | 0.571 | 0.242–1.345 | 0.249 | – | – | – |
| Air
bronchogram | 0.683 | 0.342–1.363 | 0.325 | – | – | – |
| Liquefaction
necrosis | 0.640 | 0.179–2.290 | 0.492 | – | – | – |
Discussion
In the present study, the clinical-radiological
features of lung adenocarcinoma with certain genetic alterations
were investigated. As compared with the WT group, the group of
patients with ROS1 aberrations had a younger age, higher proportion
of females and non-smokers and had a higher PSV. In the
ROS1+ group, the PSV was significantly higher than that
in the EGFR+ group. However, the clinical-radiological
features of the ROS1+ group and ALK+ group
were similar.
The clinical characteristics of patients with ROS1
rearrangement have been discussed in several previous studies
(15-17,24-27). The age of ROS1-rearranged positive and
non-ROS1-rearranged patients did not significantly differ in these
previous reports (15,16,24).
However, the analysis of the present cohort revealed that in the
ROS1+ group, the mean age was lower compared with that
in the EGFR+ and WT groups, a result consistent with
those of Bergethon et al (25) and Chen et al (26). Female sex appears to be a clinical
feature associated with an increased prevalence of ROS1 fusion.
Most studies reported that ROS1 fusion was more frequently
encountered in female patients compared with that in their male
counterparts (24,27,28).
Numerous studies have also confirmed an association of female sex
with ALK rearrangement and EGFR mutation (11–13). The
present study demonstrated an association between female sex and
ROS1 fusion, with the proportion of female patients in the
ROS1+ group being larger than that in the WT group
(71.4% vs. 35.7%, respectively). ROS1 fusions were observed in
smokers and never smokers, but a number of studies have
demonstrated an association between this genetic aberration and
light or never smokers (24,25). However, other studies have revealed
no association between smoking status and ROS1 fusion (15,26,29).
Most of the ROS1-rearranged individuals in the present study were
never smokers (23/28; 82.1%), with the number of never smokers
being markedly higher than that in the WT group (58/112;
51.8%).
Fukui et al (12) first reported solid radiological
features in lung adenocarcinoma with ALK rearrangement. The mean
tumor shadow disappearance rate (TDR) in their ALK-rearranged group
was markedly less than that in non- ALK-rearranged groups
(P=0.0006) (12). In their and other
previous studies, the TDR was defined as the ratio of the tumor
area of the mediastinal window to that of the lung window (12,22,30). TDR
provides a limited representation of the proportion of the GGO
area, as it is calculated from the GGO proportion at the maximal
section of the tumor (31,32) instead of the whole tumor. In the
present study, data of the whole tumor nodule were first obtained
to exactly calculate the PSV, which improved the accuracy of the
calculation of the solid proportion. To the best of our knowledge,
the present study also provided the first comparison of the volume
of the solid component among the four primary genetic aberrations
of lung adenocarcinomas.
The high PSV in ROS1 rearrangement tumors was
consistent with that of ALK rearrangement tumors to a certain
extent. It may be hypothesized that the similar radiological
characteristics in these two groups reflect similar pathological
features. A solid growth pattern and mucinous cribriform pattern
were the two recognizable pathological findings in ALK-rearranged
cancers (33,34). Yoshida et al (24) reported similar phenotypical features
in tumors possessing either ALK and ROS1 genetic alterations,
specifically, the presence of focal solid growth including
signet-ring cells or cribriform architecture with abundant
extracellular mucus in 53% of ROS1 rearrangement cases. Go et
al (15) also frequently
observed solid and micropapillary patterns in at least the focal
areas of tumors in 78.5% (11 of 14) of ROS1-rearranged cases.
Given the similar clinical-radiological features
between ROS1+ tumors and ALK+ tumors,
multivariate analysis was also performed on the PSV and clinical
features to predict ROS1 or ALK genetic changes in the present
study. The results indicated a good predictive value of higher PSV
and younger age for ROS1/ALK rearrangement in patients with lung
adenocarcinoma. In addition to the excellent efficacy of crizotinib
in ALK rearrangement lung adenocarcinomas, Shaw et al
(8) demonstrated that
ROS1-rearranged tumors were also sensitive to crizotinib treatment.
Their study demonstrated that the median OS with crizotinib was
51.4 months (95% CI 29.3 months to not reached), the PFS was 19.3
months (95% CI 15.2–39.1 months) and the survival probability at
12, 24, 36 and 48 months was 79, 67, 53 and 51%, respectively. As
both ROS1 rearrangement NSCLCs and ALK rearrangement NSCLCs benefit
from treatment with crizotinib, it is important to distinguish
ROS1/ALK rearrangement from EGFR mutation or WT NSCLCs.
Furthermore, given the similar treatment and the clinical-imaging
characteristics, it may be strongly recommended that a young
patient with a high-PSV tumor should be tested preferentially for
ROS1 and ALK rearrangements in the event of limited tissue samples
or for financial sources.
The present study provides clinical data on a
relatively large number of ROS1-rearranged patients and is the
first demonstration of different imaging findings between
ROS1-rearranged, ALK-rearranged, EGFR-mutated and WT lung
adenocarcinomas. One limitation of the present study was that the
CT images were obtained by two different systems, and most of the
CT images were reconstructed with 5.0 mm and not 1.0 mm thickness.
Another limitation was that the ALK-rearranged, EGFR-mutated and WT
groups did not include all patients available but they were
selected from them by a randomization procedure. This may have led
to a slight selection bias for not including all the patients in
the present study.
In conclusion, a solid pattern with a higher PSV on
CT images was determined as the primary characteristic of tumors
with ROS1-rearrangement in contrast to those with EGFR mutation and
WT tumors. However, the solid CT characteristics between the
ROS1+ group and ALK+ group were similar. PSV
and age were independent predictors for ROS1/ALK rearrangement.
Future prospective studies with unified methods and larger sample
sizes are necessary to confirm the present results.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by grants from the Key
Research and Development Program of Zhejiang Province, China (grant
no. 2019C03042 to JYiZ), the Zhejiang Provincial Natural Science
Foundation (grant no. LGF19H160031 to JZ) and the National Natural
Science Foundation of China (grant no. 81972179 to JYaZ). The
funders had no role in the study design, participant recruitment,
data collection, data analysis, data interpretation, manuscript
preparation or the decision to submit the paper for
publication.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JZ, JYaZ and JYiZ were responsible for the
conception and design of the study. JL and JX did the quantitative
volumetric assessments. KS, BW and WD did the genetic analysis. HC
was responsible for data collection and analysis. All authors
contributed significantly to data collection, data analysis,
interpretation of the data and manuscript writing. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the First Affiliated Hospital of Zhejiang University
(Hangzhou, China; approval no. IIT20200137A) who waived the
requirement for informed consent, as it was a retrospective
study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lynch TJ, Bell DW, Sordella R,
Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat
SM, Supko JG, Haluska FG, et al: Activating mutations in the
epidermal growth factor receptor underlying responsiveness of
non-small-cell lung cancer to gefitinib. N Engl J Med.
350:2129–2139. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Paez JG, Janne PA, Lee JC, Tracy S,
Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, et
al: EGFR mutations in lung cancer: Correlation with clinical
response to gefitinib therapy. Science. 304:1497–1500. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Soda M, Choi YL, Enomoto M, Takada S,
Yamashita Y, Ishikawa S, Fujiwara S, Watanabe H, Kurashina K,
Hatanaka H, et al: Identification of the transforming EML4-ALK
fusion gene in non-small-cell lung cancer. Nature. 448:561–566.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rikova K, Guo A, Zeng Q, Possemato A, Yu
J, Haack H, Nardone J, Lee K, Reeves C, Li Y, et al: Global survey
of phosphotyrosine signaling identifies oncogenic kinases in lung
cancer. Cell. 131:1190–1203. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rodig SJ and Shapiro GI: Crizotinib, a
small-molecule dual inhibitor of the c-Met and ALK receptor
tyrosine kinases. Curr Opin Investig Drugs. 11:1477–1490.
2010.PubMed/NCBI
|
|
6
|
Cui JJ, Tran-Dube M, Shen H, Nambu M, Kung
PP, Pairish M, Jia L, Meng J, Funk L, Botrous I, et al: Structure
based drug design of crizotinib (PF-02341066), a potent and
selective dual inhibitor of mesenchymal-epithelial transition
factor (c-MET) kinase and anaplastic lymphoma kinase (ALK). J Med
Chem. 54:6342–6363. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shaw AT, Kim DW, Nakagawa K, Seto T, Crinó
L, Ahn MJ, De Pas T, Besse B, Solomon BJ, Blackhall F, et al:
Crizotinib versus chemotherapy in advanced ALK-positive lung
cancer. N Engl J Med. 368:2385–2394. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shaw AT, Riely GJ, Bang YJ, Kim DW,
Camidge DR, Solomon BJ, Varella-Garcia M, Iafrate AJ, Shapiro GI,
Usari T, et al: Crizotinib in ROS1-rearranged advanced
non-small-cell lung cancer (NSCLC): Updated results, including
overall survival, from PROFILE 1001. Ann Oncol. 30:1121–1126. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Inamura K, Takeuchi K, Togashi Y, Nomura
K, Ninomiya H, Okui M, Satoh Y, Okumura S, Nakagawa K, Soda M, et
al: EML4-ALK fusion is linked to histological characteristics in a
subset of lung cancers. J Thorac Oncol. 3:13–17. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wong DW, Leung EL, So KK, Tam IY, Sihoe
AD, Cheng LC, Ho KK, Au JS, Chung LP, Pik Wong M, et al: The
EML4-ALK fusion gene is involved in various histologic types of
lung cancers from nonsmokers with wild-type EGFR and KRAS. Cancer.
115:1723–1733. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Takahashi T, Sonobe M, Kobayashi M,
Yoshizawa A, Menju T, Nakayama E, Mino N, Iwakiri S, Sato K,
Miyahara R, et al: Clinicopathologic features of non-small-cell
lung cancer with EML4-ALK fusion gene. Ann Surg Oncol. 17:889–897.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fukui T, Yatabe Y, Kobayashi Y, Tomizawa
K, Ito S, Hatooka S, Matsuo K and Mitsudomi T: Clinicoradiologic
characteristics of patients with lung adenocarcinoma harboring
EML4-ALK fusion oncogene. Lung Cancer. 77:319–325. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou JY, Zheng J, Yu ZF, Xiao WB, Zhao J,
Sun K, Wang B, Chen X, Jiang LN, Ding W and Zhou JY: Comparative
analysis of clinicoradiologic characteristics of lung
adenocarcinomas with ALK rearrangements or EGFR mutations. Eur
Radiol. 25:1257–1266. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Choi H, Paeng JC, Kim DW, Lee JK, Park CM,
Kang KW, Chung JK and Lee DS: Metabolic and metastatic
characteristics of ALK-rearranged lung adenocarcinoma on FDG
PET/CT. Lung Cancer. 79:242–247. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Go H, Kim DW, Kim D, Keam B, Kim TM, Lee
SH, Heo DS, Bang YJ and Chung DH: Clinicopathologic analysis of
ROS1-rearranged non-small-cell lung cancer and proposal of a
diagnostic algorithm. J Thorac Oncol. 8:1445–1450. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Warth A, Muley T, Dienemann H, Goeppert B,
Stenzinger A, Schnabel PA, Schirmacher P, Penzel R and Weichert W:
ROS1 expression and translocations in non-small-cell lung cancer:
Clinicopathological analysis of 1478 cases. Histopathology.
65:187–194. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Digumarthy SR, Mendoza DP, Lin JJ, Chen T,
Rooney MM, Chin E, Sequist LV, Lennerz JK, Gainor JF and Shaw AT:
Computed tomography imaging features and distribution of metastases
in ROS1-rearranged non-small-cell lung cancer. Clin Lung Cancer.
21:153–159.e3. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Detterbeck FC, Boffa DJ and Tanoue LT: The
new lung cancer staging system. Chest. 136:260–271. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shan L, Lian F, Guo L, Qiu T, Ling Y, Ying
J and Lin D: Detection of ROS1 gene rearrangement in lung
adenocarcinoma: Comparison of IHC, FISH and real-time RT-PCR. PLoS
One. 10:e01204222015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee HJ, Kim YT, Kang CH, Zhao B, Tan Y,
Schwartz LH, Persigehl T, Jeon YK and Chung DH: Epidermal growth
factor receptor mutation in lung adenocarcinomas: Relationship with
CT characteristics and histologic subtypes. Radiology. 268:254–264.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hansell DM, Bankier AA, MacMahon H, McLoud
TC, Muller NL and Remy J: Fleischner Society: Glossary of terms for
thoracic imaging. Radiology. 246:697–722. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ikehara M, Saito H, Kondo T, Murakami S,
Ito H, Tsuboi M, Oshita F, Noda K, Nakayama H, Yokose T, et al:
Comparison of thin-section CT and pathological findings in small
solid-density type pulmonary adenocarcinoma: Prognostic factors
from CT findings. Eur J Radiol. 81:189–194. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Quaia E, Baratella E, Pizzolato R, Bussani
R and Cova MA: Radiological-pathological correlation in
intratumoural tissue components of solid lung tumours. Radiol Med.
114:173–189. 2009.(In Italian). View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yoshida A, Kohno T, Tsuta K, Wakai S, Arai
Y, Shimada Y, Asamura H, Furuta K, Shibata T and Tsuda H:
ROS1-rearranged lung cancer: A clinicopathologic and molecular
study of 15 surgical cases. Am J Surg Pathol. 37:554–562. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bergethon K, Shaw AT, Ou SH, Katayama R,
Lovly CM, McDonald NT, Massion PP, Siwak-Tapp C, Gonzalez A, Fang
R, et al: ROS1 rearrangements define a unique molecular class of
lung cancers. J Clin Oncol. 30:863–870. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen YF, Hsieh MS, Wu SG, Chang YL, Shih
JY, Liu YN, Tsai MF, Tsai TH, Yu CJ, Yang JC and Yang PC: Clinical
and the prognostic characteristics of lung adenocarcinoma patients
with ROS1 fusion in comparison with other driver mutations in East
Asian populations. J Thorac Oncol. 9:1171–1179. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhu Q, Zhan P, Zhang X, Lv T and Song Y:
Clinicopathologic characteristics of patients with ROS1 fusion gene
in non-small cell lung cancer: A meta-analysis. Transl Lung Cancer
Res. 4:300–309. 2015.PubMed/NCBI
|
|
28
|
Zhu YC, Zhang XG, Lin XP, Wang WX, Li XF,
Wu LX, Chen HF, Xu CW and Du KQ: Clinicopathological features and
clinical efficacy of crizotinib in Chinese patients with
ROS1-positive non-small cell lung cancer. Oncol Lett. 17:3466–3474.
2019.PubMed/NCBI
|
|
29
|
Cai W, Li X, Su C, Fan L, Zheng L, Fei K,
Zhou C, Manegold C and Schmid-Bindert G: ROS1 fusions in Chinese
patients with non-small-cell lung cancer. Ann Oncol. 24:1822–1827.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shimizu K, Yamada K, Saito H, Noda K,
Nakayama H, Kameda Y and Nakata K: Surgically curable peripheral
lung carcinoma: Correlation of thin-section CT findings with
histologic prognostic factors and survival. Chest. 127:871–878.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Okada M, Nishio W, Sakamoto T, Uchino K
and Tsubota N: Discrepancy of computed tomographic image between
lung and mediastinal windows as a prognostic implication in small
lung adenocarcinoma. Ann Thorac Surg. 76:1828–1832. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Takamochi K, Nagai K, Yoshida J, Suzuki K,
Ohde Y, Nishimura M, Sasaki S and Nishiwaki Y: Pathologic N0 status
in pulmonary adenocarcinoma is predictable by combining serum
carcinoembryonic antigen level and computed tomographic findings. J
Thorac Cardiovasc Surg. 122:325–330. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yoshida A, Tsuta K, Nakamura H, Kohno T,
Takahashi F, Asamura H, Sekine I, Fukayama M, Shibata T, Furuta K
and Tsuda H: Comprehensive histologic analysis of ALK-rearranged
lung carcinomas. Am J Surg Pathol. 35:1226–1234. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rodig SJ, Mino-Kenudson M, Dacic S, Yeap
BY, Shaw A, Barletta JA, Stubbs H, Law K, Lindeman N, Mark E, et
al: Unique clinicopathologic features characterize ALK-rearranged
lung adenocarcinoma in the western population. Clin Cancer Res.
15:5216–5223. 2009. View Article : Google Scholar : PubMed/NCBI
|