Introduction
Glioma is the most common primary malignant brain
tumor in 2016 worldwide (1), which
can be classified into grades I–IV according to the World Health
Organization (WHO) 2016 classification system (2). Glioblastoma multiforme (GBM) is also
known as high-grade glioma (HGG), which is the most aggressive form
of glioma, whereas the others are considered low-grade gliomas
(LGG) (3). Although, significant
efforts have been made to find effective treatment approaches for
glioma, the 5 year survival rate of GBM remains poor (~5%)
(1). Studies investigating molecular
biomarkers have demonstrated progress and provided optimism for the
future, compared with the limited success of conventional therapies
(4). Thus, there is an urgent need
for the development of novel and effective biomarkers for molecular
subtyping, predicting prognosis and the design of personalized
treatments for patients with glioma.
F-box proteins are the substrate recognizing
subunits of Skp1-Cullin 1-F-box ubiquitin ligase complex, which
recognizes specific targets and promotes their ubiquitination
(5,6). S phase kinase-associated protein 2
(SKP2) is a typical F-box protein that serves a crucial role in
multiple cellular processes including proliferation, invasion and
metastasis (7). It functions as a
promoter of cell cycle progression via the ubiquitination and
subsequent degradation of cell cycle inhibitors, including p21,
p27, p57 and cyclin E (8). As the
majority of SKP2 substrates are tumor suppressors, it is widely
considered to be an oncoprotein (9).
Previous studies have indicated that SKP2 is upregulated in a
variety of human malignancies, including breast cancer and gastric
cancer, and is associated with carcinogenesis and tumor progression
(10,11). Emerging evidence indicates that SKP2
is also involved in chemoresistance and may be a novel therapeutic
target. For example, the inhibition of SKP2 sensitizes lung cancer
cells to paclitaxel or rapamycin (12,13). In
gliomas, several in vitro experiments have demonstrated that
suppressing the expression of SKP2 significantly blocks cell cycle
progression, induces cell apoptosis and inhibits cell invasion
(14,15). However, the clinical implications of
SKP2 in gliomas remain unclear, despite SKP2 detection being
reported in certain operative specimens (16,17).
Isocitrate dehydrogenase 1 (IDH1) and telomerase
reverse transcriptase promoter (TERTp) mutation have been used in
the clinic for glioma (18). IDH1
mutation was an early event in glioma development, which was
observed in almost 80% of grade II–III gliomas and secondary GBM
(19). TERTp mutation is inversely
correlated with IDH1 mutation in glioblastoma, and is therefore
predictive of poor prognosis (20).
Phosphorylated retinoblastoma protein (p-Rb) is also known as the
cell cycle regulating protein (21).
Rb protein binds and inhibits the transcription factor for the E2F
family, which causes cell cycle arrest at G1 (22). When Rb is phosphorylated, it
dissociates from E2F and allows progression to the S phase
(22). Epidermal growth factor
receptor (EGFR) is a member of the transmembrane tyrosine kinases
and it overexpressed in 60% of glioblastoma (23). Furthermore, inhibiting the expression
of p-Rb and EGFR can result in tumor cell arrest in the
G1 phase and dysregulate the expression of SKP2
(24,25).
The aim of the present study was to investigate the
expression and prognostic value of SKP2 in samples from 395
patients with glioma. The results of the present study demonstrated
that SKP2 is mainly expressed in glioblastomas (GBMs) and high
expression of SKP2 indicates poor survival. The findings of the
present study demonstrate that SKP2 is a novel prognostic biomarker
in GBMs.
Materials and methods
Patients and collection of clinical
samples
A total of 395 specimens were obtained from patients
with glioma who were enrolled in the present study. Of these, 36
were astrocytomas, 21 oligodendrogliomas, 40 anaplastic
astrocytomas, 24 anaplastic oligodendrogliomas, 269 GBMs and 5 were
other types. There were 234 male and 161 female patients. The mean
age was 48 years, ranging from 5–79 years. All patients underwent
tumor resection between September 2011 and January 2018 in the
Department of Neurosurgery at the National Cancer Center
(NCC)/Cancer Hospital of Chinese Academy of Medical Sciences
(Beijing, China). None of the patients had a history of other
tumors and the neurosurgical treatment was performed to treat
glioma alone. All the samples used were residual specimens
collected for diagnosis. A total of 20 non-neoplastic tissues were
acquired using two methods: Normal brain tissues around tumor and
edema tissues surrounding the high-grade gliomas. None of the
patients received neoadjuvant treatment prior to surgery and they
all signed separate informed consent forms for the sample
collection and molecular analyses. Data were recorded for the
clinical/pathological parameters of tumors; including age, sex of
patients and the WHO grade of tumors. Follow-up was performed every
3 months and 25 patients dropped out prior to the last follow-up
call. The present study was approved by the Ethics Committee of the
NCC (approval no. NCC2014G-12; Beijing, China).
Tissue microarray and
immunohistochemistry
Tumor samples were fixed in 10% formalin for 24 h at
room temperature following resection, and embedded in paraffin.
Tissue microarrays were prepared as previously described (26). A total of three tissue cores were
selected from every primary paraffin-embedded samples (n=359). The
microarrays were cut into 4 µm-thick sections. As it is difficult
to acquire normal brain tissues, normal tissues (n=20) from
patients with glioma were used as controls in the current study.
The sections were baked at 72°C for 1 h, then deparaffinized with
xylene and rehydrated in gradient ethanol including 100, 85 and 75%
for 15 min. Citrate buffer (ZSGB-BIO, Ltd.) was diluted to pH 6.0
and heated to boil for antigen retrieval. Subsequently, specimens
were kept in 95–98°C citrate buffer by microwaving for 20 min.
Then, endogenous peroxidase activity was blocked with 3% hydrogen
peroxide at room temperature for 10 min. The primary SKP2 antibody
(1:100; cat. no. 2652S; CST Biological Reagents Co., Ltd.),
anti-isocitrate dehydrogenase 1 (IDH1R132H) antibody
(working solution; cat. no. ZM0447; OriGene Technologies, Inc.),
phosphorylated retinoblastoma protein (p-Rb) antibody (1:100; cat.
no. 8516T, CST Biological Reagents Co., Ltd.), epidermal growth
factor receptor (EGFR) antibody (working solution; cat. no.
ZA-0505; OriGene Technologies, Inc.) were added and the sections
were incubated at 4°C overnight. Following, 4 washes with PBS, the
PV-9000 kit (working solution; cat. no. PV-9000; OriGene
Technologies, Inc.) was used for color development according to the
manufacturer's protocols. Next, DAB and hematoxylin were used for
counterstaining at room temperature for 3 min and 8 sec,
respectively. Finally, the slides were dehydrated and mounted with
coverslips. Slides were scanned using a NanoZoomer (Japan SLC,
Inc.) high-resolution scanner with maximum magnification, ×400. NDP
analysis software (Visiopharm) was used to identify immunostaining
and calculate the proportion of positive cells. The cutoff values
were 10% for IDH1R132H and p-Rb. The immune scores of
EGFR protein expression were evaluated as described previously
(27).
DNA extraction and sanger
sequencing
Genomic DNA was extracted from consecutive formalin
fixation and paraffin embedding glioma sections of 10 µm using the
QIAmp DNA mini kit (Qiagen GmbH). The telomerase reverse
transcriptase (TERT) promoter region was amplified with the forward
primer, 5′-TTCCAGCTCCGCCTCCT-3′ and the reverse primer,
5′-GCGCTGCCTGAAACTCG-3′. PCR was performed with an initial
denaturing step at 95°C for 5 min; then 30 cycles of 96°C for 15
sec, 60°C for 30 sec and 72°C for 30 sec; followed by a final
extension at 72°C for 10 min. The PCR products were sequenced by
Beijing Tianyi Huiyuan Bioscience and Technology Inc.
Statistical analysis
Significant differences between two groups were
determined by the Mann-Whitney U test. X-tile software v3.6.1 (Rimm
Lab, Yale School of Medicine) was used to ascertain the optimal
cut-off points for survival analysis. Data were represented as
percentages. There were no biological replicates. The χ2
test was used to assess the associations between SKP2 expression
and clinicopathological parameters. Overall survival (OS) curves
were plotted according to the Kaplan-Meier method, with the
log-rank test applied for comparison. Multiple Cox regression
analysis was used to predict independent prognostic factors. All
statistical analyses were performed using both SPSS Statistics
v21.0 (IBM Corp.) and GraphPad Prism v5.0 (GraphPad Software,
Inc.). P<0.05 was considered to indicate a statistically
significant difference. All the tests performed were two-sided.
Results
Association between SKP2 expression
and clinicopathological parameters in glioma
SKP2 expression was investigated using
immunohistochemistry in a total of 395 glioma tissues. These
specimens included grade II (60), III (66) and IV (269) tumors
(Table I). A total of 20 paired
non-neoplastic brain tissues were used as the control. Positive
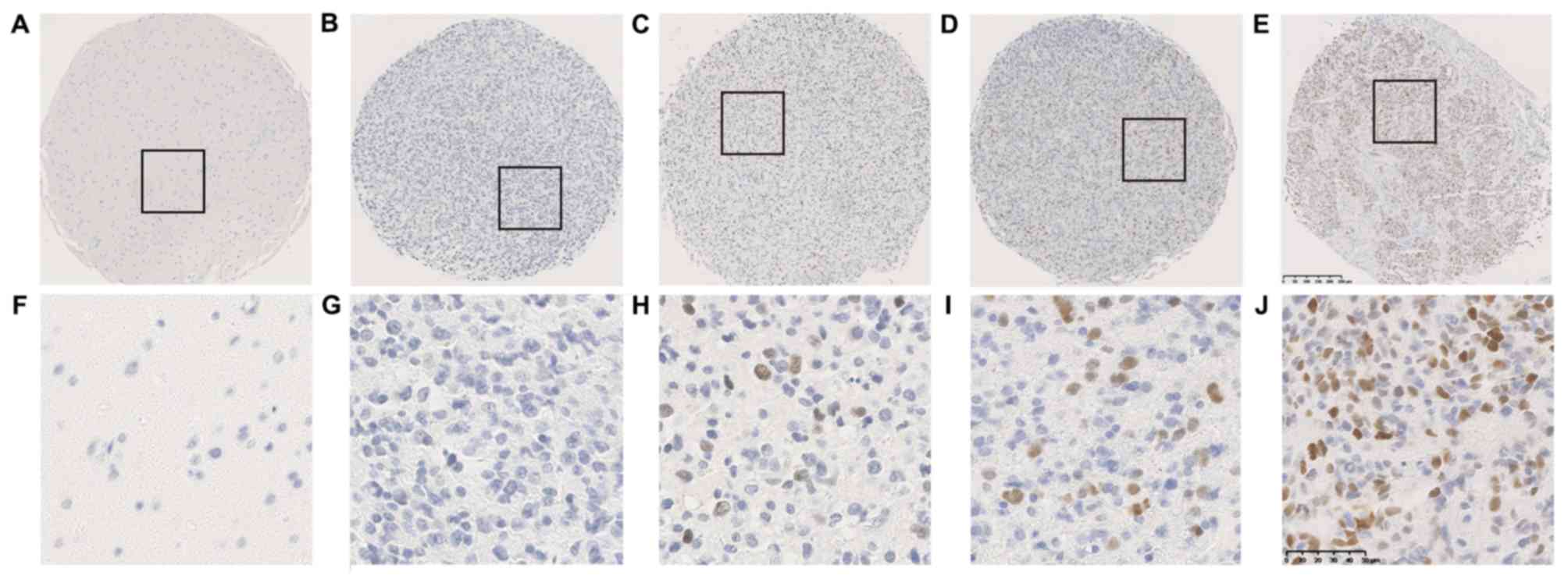
immunostaining of SKP2 was observed in the nuclei of glioma cells,
whereas no SKP2 signal was presented in non-neoplastic tissues
(Fig. 1). Glioma tissues with
different degrees of immunostaining for SKP2, including negative,
low, moderate and high expression (Fig.
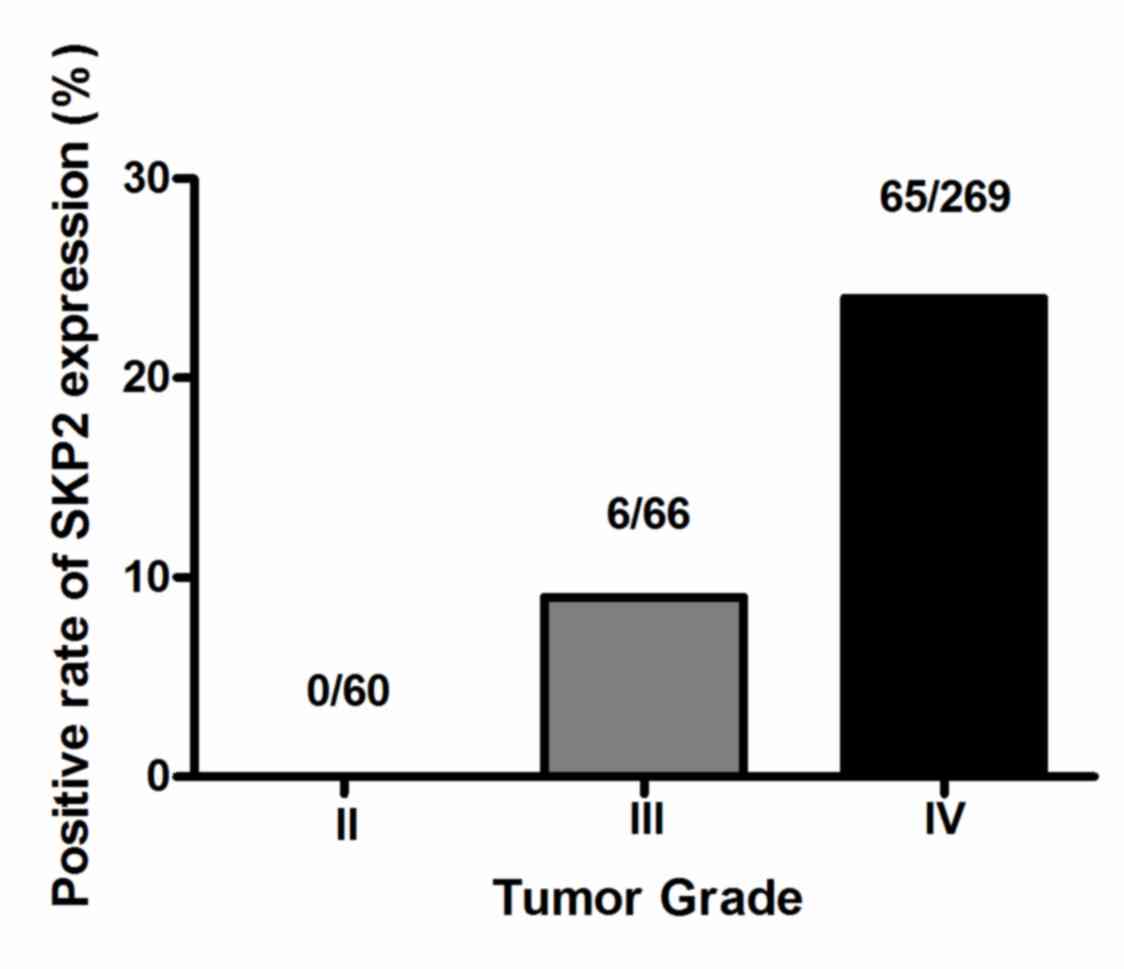
1). SKP2 expression was detected in 18.0% (71/395) of all
glioma tumors, with 0.0% (0/60) in grade II gliomas, 9.1% (6/66) in
grade III gliomas and 24.2% (65/269) in glioblastomas (Fig. 2). SKP2 expression was significantly
associated with tumor grade and histology (P<0.001; Table I). High SKP2 expression levels were
more frequently observed in GBM tissues (Table I). However, SKP2 expression was not
associated with sex, age and Karnofsky Performance Status (KPS)
(Table I).
 | Table I.Clinical and pathological
characteristics of patients with glioma (n=395). |
Table I.
Clinical and pathological
characteristics of patients with glioma (n=395).
|
|
| SKP2
expression |
|
|---|
|
|
|
|
|
|---|
| Variable | Patients, n | Positive, n
(%) | Negative, n
(%) |
P-valuea |
|---|
| Sex |
|
|
| 0.987 |
|
Male | 234 | 42 (17.9) | 192 (82.1) |
|
|
Female | 161 | 29 (18.0) | 132 (82.0) |
|
| Age, years |
|
|
| 0.808 |
|
≤50 | 211 | 37 (17.5) | 174 (82.5) |
|
|
>50 | 184 | 34 (18.5) | 150 (81.5) |
|
| KPS |
|
|
| 0.254 |
|
≤60 | 79 | 15 (19.0) | 64 (81.0) |
|
|
>60 | 205 | 31 (15.1) | 174 (84.9) |
|
| NA | 111 | 25 (22.5) | 86 (77.5) |
|
| Tumor grade |
|
|
| <0.001 |
| II | 60 | 0 (0.0) | 60 (100.0) |
|
|
III | 66 | 6 (9.1) | 60 (90.9) |
|
| IV | 269 | 65 (24.2) | 204 (75.8) |
|
| Histology |
|
|
| <0.001 |
| A | 36 | 0 (0.0) | 36 (100.0) |
|
| O | 21 | 0 (0.0) | 21 (100.0) |
|
| AA | 40 | 4 (10.0) | 36 (90.0) |
|
| AO | 24 | 2 (8.3) | 22 (91.7) |
|
|
GBM | 269 | 65 (24.2) | 204 (75.8) |
|
|
Other | 5 | 0 (0.0) | 5 (100.0) |
|
SKP-2 expression is associated with
p-Rb and EGFR expression, but not with the TERT promoter
mutation
Expression of p-Rb, EGFR and a mutation in the TERT
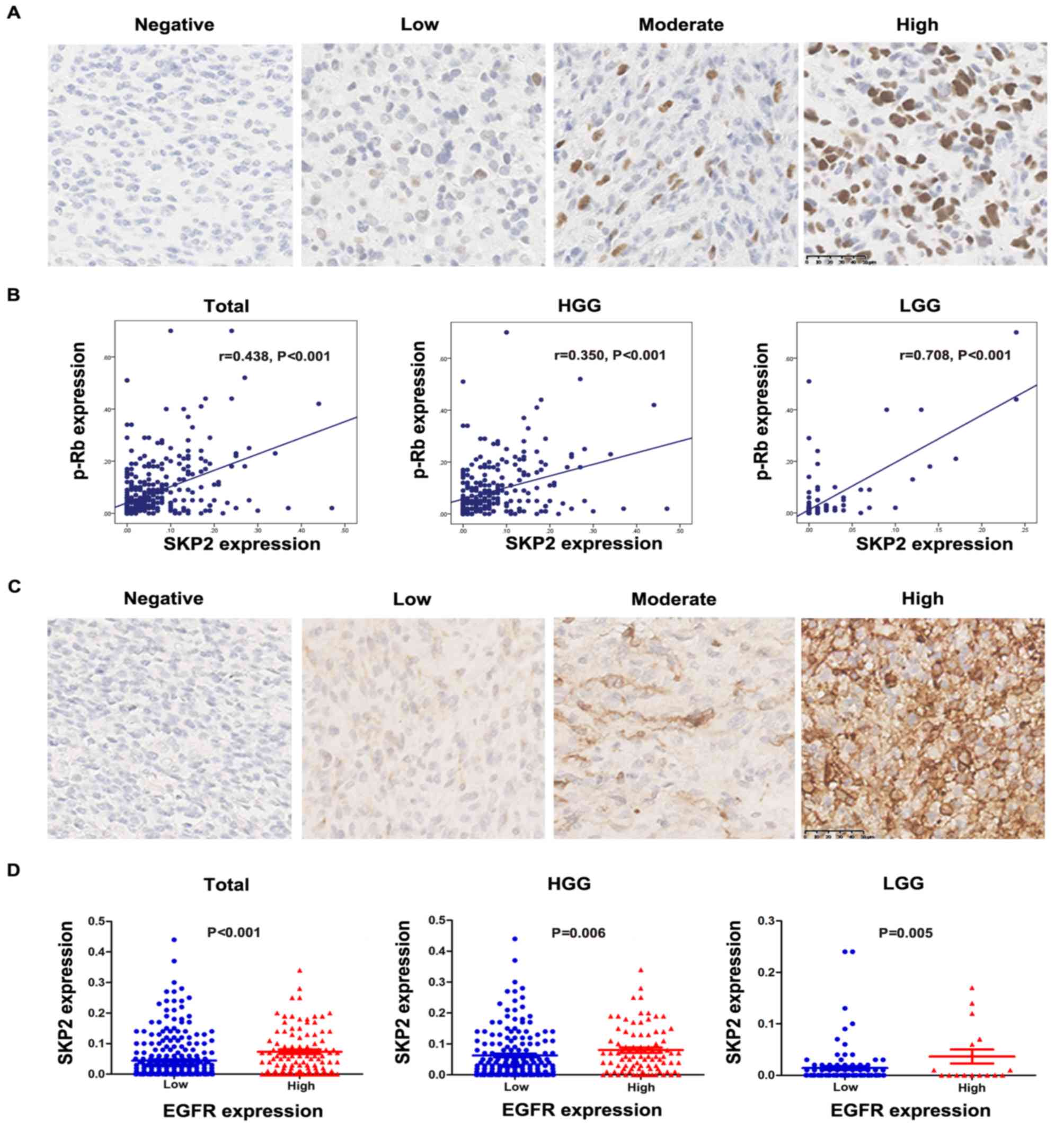
promoter were found in patients with glioma. Positive
immunostaining of p-Rb and EGFR was in the nuclei and cytoplasm,
respectively (Fig. 3A and C). The
TERT promoter mutation was observed in 50.5% of patients with
glioma. The exact mutation is presented in Fig. S1. Linear correlation analysis showed
that SKP2 expression was significantly correlated with p-Rb
expression (P<0.001 in all gliomas; Fig. 3B). Mann-Whitney U test revealed that
SKP2 expression was strongly associated with EGFR expression
(P<0.001 in total group; P=0.006 in HGG and P=0.005 in LGG;
Fig. 3D). No correlation was
observed between SKP2 expression and the TERT promoter mutation
(P>0.05, data not shown).
SKP2 expression is associated with a
poor prognosis in patients with glioma
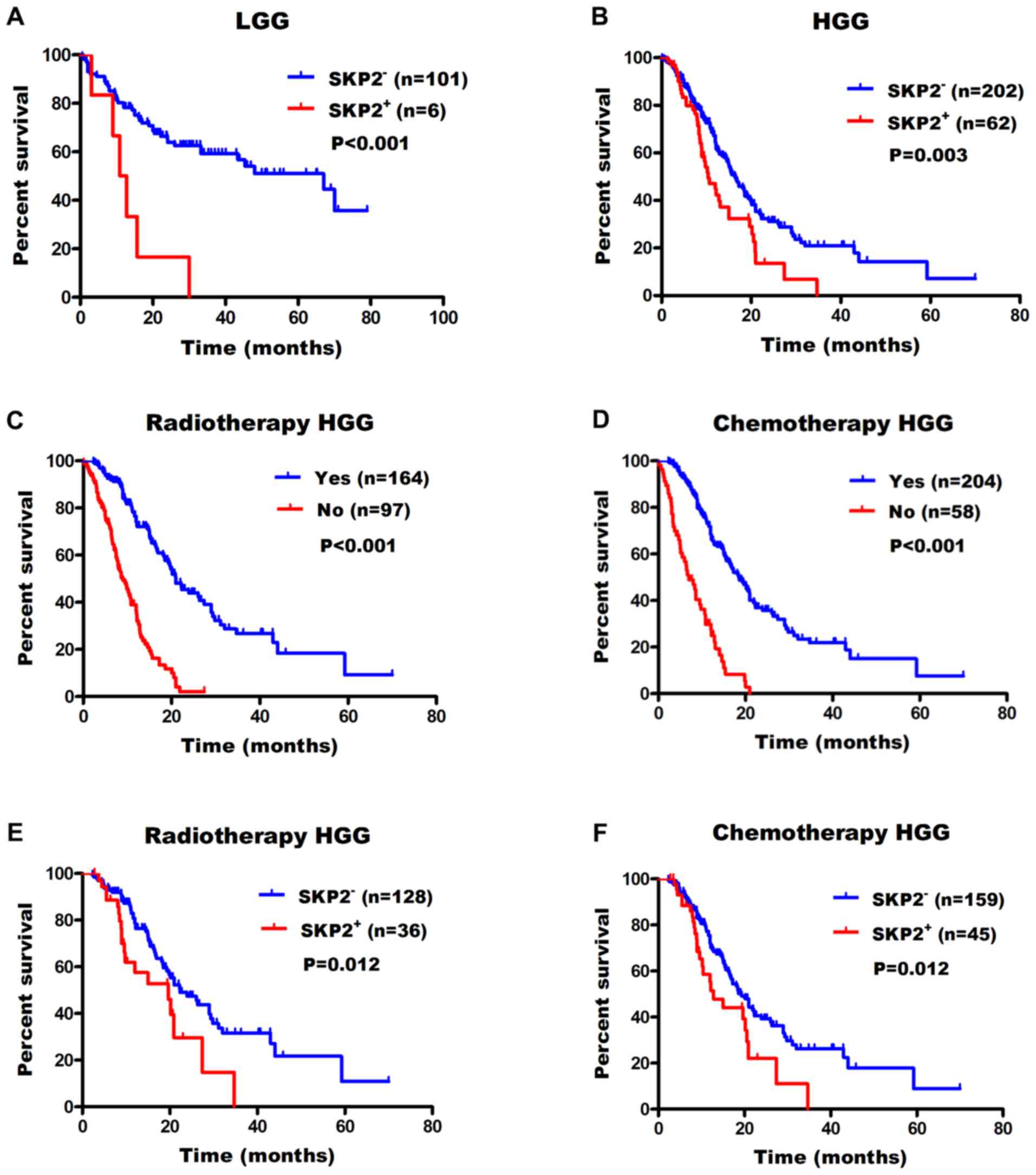
Using X-tile software, the optimum cut-off value for
SKP2 expression was defined as 10%. Subsequently, univariate
analysis was performed to examine the prognostic significance in
LGG and HGG. It revealed that elevated SKP2 expression was
associated with a poor prognosis in patients with glioma compared
with low expression [hazard ratio (HR), 4.2; LGG median survival
times, 67.0 months vs. 11.9 months; P<0.001; Fig. 4A], (HR, 1.7; HGG median survival
times, 16.5 months vs. 10.4 months; P=0.003; Fig. 4B). In HGG, patients with radiotherapy
(median survival times, 22.3 months vs. 19.6 months; P<0.001;
Fig. 4C) or chemotherapy (median
survival times, 19.3 months vs. 12.8 months; P<0.001; Fig. 4D) showed longer survival times. The
associations between SKP2 expression and sensitivity to adjuvant
treatments were also investigated. The results demonstrated that in
HGG patients who received radiotherapy or chemotherapy, patients
with a low SKP2 expression had a longer survival time (radiotherapy
median OS time, 21.0 months vs. 9.0 months; P=0.012; Fig. 4E; chemotherapy median OS time, 18.5
months vs. 7.0 months; P=0.012; Fig.
4F).
Prognostic value of SKP2 in patients
with glioma
To further validate the prognostic value of SKP2, a
multiple Cox proportional hazards regression analysis was performed
using clinical and genetic variables, including sex, age, KPS,
adjuvant treatments, TERT promoter mutation, IDH1 mutation and SKP2
expression in HGG (Table II). Both
univariate and multivariate analyses revealed that KPS, adjuvant
treatments, IDH1 mutation and SKP2 expression, but not sex, age and
TERT promoter mutation were associated with the OS time of the
patients (Table II).
 | Table II.Univariate and multivariate
regression analyses of overall survival in patients with GBM. |
Table II.
Univariate and multivariate
regression analyses of overall survival in patients with GBM.
|
| GBM |
|---|
|
|
|
|---|
|
| Univariate |
| Multivariate |
|
|---|
|
|
|
|
|
|
|---|
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Sex, male vs.
female | 0.962 | 0.698–1.327 | 0.814 |
|
|
|
| Age, ≤50 vs. >50
years | 1.153 | 0.839–1.583 | 0.381 |
|
|
|
| KPS, ≤60 vs.
>60 | 0.530 | 0.355–0.791 | 0.002 | 0.580 | 0.364–0.923 | 0.021 |
| Chemo/radiotherapy,
no vs. yes | 0.253 | 0.179–0.357 | <0.001 | 0.246 | 0.150–0.403 | <0.001 |
| TERT promoter
mutation, no vs. yes | 1.630 | 1.157–2.297 | 0.005 | 1.440 | 0.931–2.230 | 0.102 |
| IDH1 mutation, no
vs. yes | 0.682 | 0.466–0.999 | 0.049 | 0.502 | 0.292–0.864 | 0.013 |
| SKP2 expression, no
vs. yes | 1.715 | 1.196–2.457 | 0.003 | 1.767 | 1.045–2.988 | 0.034 |
Using SKP2, IDH1 mutation and TERT
promoter mutation data classifies patients with glioma into 3
subgroups
A previous study reported the clinical relevance of
IDH1 mutation in gliomas (28). In
the present study, it was observed that patients with IDH1 mutation
showed favorable prognosis (P=0.048; Fig. S2A). Furthermore, the TERT promoter
mutation is associated with the OS of patients with GBM (P=0.004;
Fig. S2B). When SKP2, IDH1 mutation
and TERT promoter (p) mutation factors are combined, patients
having 3 protective factors
(SKP2−/IDH1+/TERTp−) had the
longest OS time in all enrolled gliomas compared with the patients
having 2 protective factors
(SKP2−/IDH+/TERTp+;
SKP2+/IDH+/TERTp−;
SKP2−/IDH−/TERTp−) (median OS
time, 20.1 months; P=0.010; Fig.
5A). Patients with <=1 protective factor
(SKP2−/IDH−/TERTp+;
SKP2+/IDH+/TERTp+;
SKP2+/IDH−/TERTp−; or
SKP2+/IDH−/TERTp+) had the least
favorable prognosis (median OS time, 12.2 months; P<0.001;
Fig. 5A). Furthermore, patients with
GBM demonstrated similar results with median OS times not attained,
18.5 and 12.2 months (those with 3, 2 or <=1 protective factors,
respectively; P<0.001; Fig.
5B).
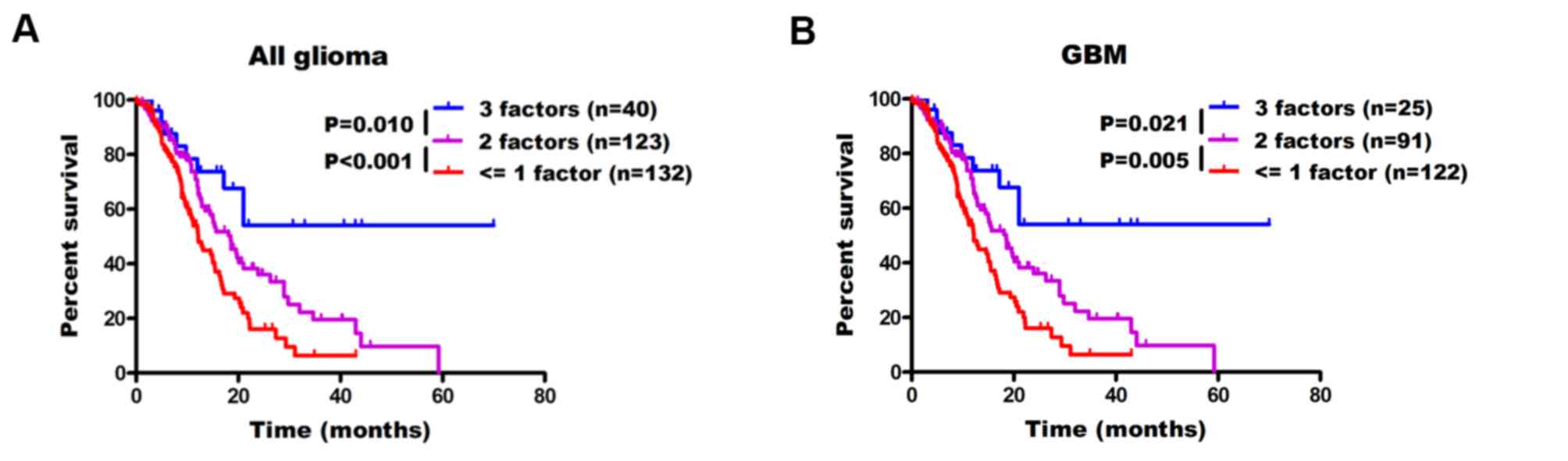 | Figure 5.Using SKP2 expression, IDH1 mutation
and TERT promoter status for subgroup stratification of patients
with (A) all and (B) GBM. Patients with glioma were split into 3
groups according to the number of protective factors they had,
including low SKP2 expression, IDH1 mutation and TERT promoter wild
type (3 factors,
SKP2−/IDH1+/TERTp−; 2 factors,
SKP2−/IDH+/TERTp+,
SKP2−/IDH−/TERTp−,
SKP2+/IDH+/TERTp−; <=1 factor,
SKP2−/IDH−/TERTp+,
SKP2+/IDH+/TERTp+,
SKP2+/IDH−/TERTp−,
SKP2+/IDH−/TERTp+. GBM,
glioblastoma; SKP2, S phase kinase-associated protein 2; IDH 1,
isocitrate dehydrogenase 1; TERTp, telomerase reverse transcriptase
promoter. |
Discussion
SKP2 plays an essential role in cellular biological
processes in vitro (7). The
present study, demonstrated that SKP2 protein is upregulated in
glioma tissues and its expression is strongly associated with tumor
grade and poor prognosis. In addition, SKP2 expression was
associated with p-Rb and EGFR expression, which suggests that SKP2
may be involved in promoting growth and proliferation in GBM cells.
Patients with GBMs were stratified into 3 groups using a
combination of SKP2 expression, TERT promoter and IDH1 status, with
the longest survival time observed in the patients who were
SKP2−/IDH1+/TERTp−.
Cell cycle-associated proteins have become
increasingly important in the understanding of the pathogenesis and
prognosis of gliomas (29). SKP2
serves an oncogenic role in cancer cells, primarily through
regulating the cell cycle (9). It
promotes the transition of the cell cycle from G1 to S
phase and subsequently enhances cell proliferation and tumor growth
(30,31). An in vitro study reported that
SKP2 protein is selectively expressed in a subset of proliferating
human breast cancer cells (32). Lu
et al (33) demonstrated that
SKP2 expression is associated with the histological grade and tumor
size in human hepatocellular carcinoma. Furthermore, Elsherif et
al (34) also demonstrated that
SKP2 proteins serve as a predictor of grade and stage in non-muscle
invasive urothelial bladder carcinoma. Similar to the previous
studies on other tumor types, the present study observed that SKP2
was upregulated in gliomas and was an independent prognostic factor
in GBM. This coincides with the high proliferation activity profile
of glioma cells, particularly in GBM (35).
Certain chemical agents including curcumin,
paeoniflorin, physcion 8-O-β-glucopyranoside, escitalopram oxalate
and butylidenephthalide have also been revealed to regulate the
cell cycle in glioma cells through modulating SKP2 expression
(14,15,36–38).
Therefore, these agents may have potential for clinical use in the
treatment of gliomas with high SKP2 expression. Davidovich et
al (39) reported that patients
with breast cancer that had high SKP2 expression exhibited a poor
response towards preoperative doxorubicin-based chemotherapy. High
SKP2 expression in human lung cancer cells caused paclitaxel
resistance by downregulating p27 expression (12). Treatment with a combination of small
molecule SKP2 inhibitors and paclitaxel conferred a favorable
prognosis in patients with lung cancer (12). A combination of SKP2 downregulation
and these chemotherapeutic agents may have synergistic implications
on tumor control (5). In the present
study, high expression of SKP2 resulted in insensitivity to radio-
and chemotherapy in glioblastoma. Therefore, SKP2 targeted therapy
is required for patients with glioblastoma with high SKP2
expression in addition to standard therapy.
As an important cell cycle regulating protein, p-Rb
serves pivotal roles in tumorigenesis (24). Xu et al (40) used egg antigen p40 of schistosoma
japonicum (Sjp40) to trigger the expression of p27 in LX-2 cells
and observed that both SKP2 and p-Rb were downregulated.
Overexpression of SKP2 reversed p27 protein levels and partially
reversed the inhibition of p-Rb expression in Sjp40 treated LX2
cells (40). In the present study,
consistent alteration of SKP2 with p-Rb was observed in glioma
tissues.
EGFR is the target of antineoplastic drugs, such as
gefitinib (25). Agents targeting
EGFR may promote the downregulation of SKP2 (25). Tan et al (41) also revealed that upregulated EGFR
expression activates the AKT/SKP2 pathway and increases SKP2
expression. In the present study, 38.5% of patients with HGG who
had high SKP2 expression also had EGFR expression. Further studies
should investigate the association between these molecules in
larger samples and explore whether and how they could be used for
the classification of human gliomas.
An increasing number of studies have demonstrated
the clinical value of the TERT promoter and IDH1 mutations for
classifying patients into molecular subtypes in glioma (42,43). The
present study used SKP2 expression combined with TERT promoter and
IDH1 mutations to classify patients into subgroups. The results
revealed that patients with low SKP2 expression, having an IDH1
mutation and wild-type TERT promoter
(SKP2−/IDH1+/TERTp−) had the best
prognosis in all gliomas and GBMs, compared with the other
subtypes. A previous studies have demonstrated that IDH1 mutation
causes cell cycle arrest in G1 via downregulation of
cyclin-dependent kinase 1 expression (44). IDH1 mutation upregulates p21
expression via sterol regulatory element-binding protein 1,
inhibits phosphorylation of Rb and promotes the progression of the
cell cycle from G1 to S phase (45). The involvement of telomerase in
regulating the cell cycle is primarily dependent on the progression
of S phase (46). TERT promoter
mutation can increase the telomerase reverse transcriptase
expression and telomerase activity (47). Thus,
SKP2−/IDH1+/TERTp− glioma cells in
the present study tend to stay in G1 phase and grow
slowly. This helps the patient survive longer compared with the
other subtypes.
The present study has some limitations. As a
retrospective study, the number of cases varied significantly among
subgroups, and it was a single-center study. Additionally, the
detection method used to determine SKP2 expression was restricted
to IHC. New techniques may be required to confirm the results.
Finally, the molecular mechanisms of SKP2 have not been explored in
the present study.
In conclusion, the present study demonstrated a high
expression of SKP2 protein in glioma tissues, and particularly in
GBMs. High SKP2 expression is associated with a shorter survival
time of patients with GBM and is an independent prognostic factor.
Gliomas (including GBMs) with low SKP2 expression, wild-type TERT
promoter and IDH1 mutation have the longest OS time compared with
the other subtypes.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
CAMS Innovation Fund for Medical Sciences (grant no.
2017-I2M-1-005) and the China Postdoctoral Science Foundation
(grant no. 2019M650570).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
JHW, HQC and MRW designed the experiments and
revised the manuscript. ZJC and HQC analyzed the data and wrote the
manuscript. MJZ, YZ, JH and QY contributed to the acquisition,
analysis and interpretation of data. JJH designed the experiments
and analyzed the data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the National Cancer Center/Cancer Hospital, Chinese
Academy of Medical Sciences and Peking Union Medical College
(Beijing, China; approval no. NCC2014G-12). Patients who
participated in this research had complete clinical data. Signed
informed consent was obtained from patients or their guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ostrom QT, Gittleman H, Xu J, Kromer C,
Wolinsky Y, Kruchko C and Barnholtz-Sloan JS: CBTRUS statistical
report: Primary brain and other central nervous system tumors
diagnosed in the United states in 2009–2013. Neuro Oncol. 18 (Suppl
5):v1–v75. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Louis DN, Perry A, Reifenberger G, von
Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD,
Kleihues P and Ellison DW: The 2016 world health organization
classification of tumors of the central nervous system: A summary.
Acta Neuropathol. 131:803–820. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jan CI, Tsai WC, Harn HJ, Shyu WC, Liu MC,
Lu HM, Chiu SC and Cho DY: Predictors of response to autologous
dendritic cell therapy in glioblastoma multiforme. Front Immunol.
9:7272018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
López GY, Van Ziffle J, Onodera C, Grenert
JP, Yeh I, Bastian BC, Clarke J, Oberheim Bush NA, Taylor J, Chang
S, et al: The genetic landscape of gliomas arising after
therapeutic radiation. Acta Neuropathol. 137:139–150. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gong J, Zhou Y, Liu D and Huo J: F-box
proteins involved in cancer-associated drug resistance. Oncol Lett.
15:8891–8900. 2018.PubMed/NCBI
|
|
6
|
Wang Z, Liu P, Inuzuka H and Wei W: Roles
of F-box proteins in cancer. Nat Rev Cancer. 14:233–247. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang Y, Zvi YS, Batko B, Zaphiros N,
O'Donnell EF, Wang J, Sato K, Yang R, Geller DS, Koirala P, et al:
Down-regulation of Skp2 expression inhibits invasion and lung
metastasis in osteosarcoma. Sci Rep. 8:142942018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee SH and McCormick F: Downregulation of
Skp2 and p27/Kip1 synergistically induces apoptosis in T98G
glioblastoma cells. J Mol Med (Berl). 83:296–307. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ding L, Li R, Sun R, Zhou Y, Zhou Y, Han
X, Cui Y, Wang W, Lv Q and Bai J: S-phase kinase-associated protein
2 promotes cell growth and motility in osteosarcoma cells. Cell
Cycle. 16:1547–1555. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sonoda H, Inoue H, Ogawa K, Utsunomiya T,
Masuda TA and Mori M: Significance of skp2 expression in primary
breast cancer. Clin Cancer Res. 12:1215–1220. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Geng Q, Liu J, Gong Z, Chen S, Chen S, Li
X, Lu Y, Zhu X, Lin HK and Xu D: Phosphorylation by mTORC1
stablizes Skp2 and regulates its oncogenic function in gastric
cancer. Mol Cancer. 16:832017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huang T, Yang L, Wang G, Ding G, Peng B,
Wen Y and Wang Z: Inhibition of Skp2 sensitizes lung cancer cells
to paclitaxel. Onco Targets Ther. 10:439–446. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Totary-Jain H, Sanoudou D, Dautriche CN,
Schneller H, Zambrana L and Marks AR: Rapamycin resistance is
linked to defective regulation of Skp2. Cancer Res. 72:1836–1843.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li W, Li F, Zhu Y and Song D: Physcion
8-O-β- glucopyranosideregulates cell cycle, apoptosis, and invasion
in glioblastoma cells through modulating Skp2. Biomed Pharmacother.
95:1129–1138. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ouyang J, Xu H, Li M, Dai X, Fu F, Zhang X
and Lan Q: Paeoniflorin exerts antitumor effects by inactivating S
phase kinase-associated protein 2 in glioma cells. Oncol Rep.
39:1052–1062. 2018.PubMed/NCBI
|
|
16
|
Schiffer D, Cavalla P, Fiano V, Ghimenti C
and Piva R: Inverse relationship between p27/Kip.1 and the F-box
protein Skp2 in human astrocytic gliomas by immunohistochemistry
and Western blot. Neurosci Lett. 328:125–128. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Saigusa K, Hashimoto N, Tsuda H, Yokoi S,
Maruno M, Yoshimine T, Aoyagi M, Ohno K, Imoto I and Inazawa J:
Overexpressed Skp2 within 5p amplification detected by array-based
comparative genomic hybridization is associated with poor prognosis
of glioblastomas. Cancer Sci. 96:676–683. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Reifenberger G, Wirsching HG,
Knobbe-Thomsen CB and Weller M: Advances in the molecular genetics
of gliomas-implications for classification and therapy. Nat Rev
Clin Oncol. 14:434–452. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cohen AL, Holmen SL and Colman H: IDH1 and
IDH2 mutations in gliomas. Curr Neurol Neurosci Rep. 13:3452013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nonoguchi N, Ohta T, Oh JE, Kim YH,
Kleihues P and Ohgaki H: TERT promoter mutations in primary and
secondary glioblastomas. Acta Neuropathol. 126:931–937. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Stone JG, Siedlak SL, Tabaton M, Hirano A,
Castellani RJ, Santocanale C, Perry G, Smith MA, Zhu X and Lee HG:
The cell cycle regulator phosphorylated retinoblastoma protein is
associated with tau pathology in several tauopathies. J Neuropathol
Exp Neurol. 70:578–587. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Thwaites MJ, Cecchini MJ and Dick FA:
Analyzing RB and E2F during the G1-S transition. Methods Mol Biol.
1170:449–461. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Saadeh FS, Mahfouz R and Assi HI: EGFR as
a clinical marker in glioblastomas and other gliomas. Int J Biol
Markers. 33:22–32. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dick FA and Rubin SM: Molecular mechanisms
underlying RB protein function. Nat Rev Mol Cell Biol. 14:297–306.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shintani S, Li C, Mihara M, Yano J,
Terakado N, Nakashiro K and Hamakawa H: Gefitinib (‘Iressa’,
ZD1839), an epidermal growth factor receptor tyrosine kinase
inhibitor, up-regulates p27KIP1 and induces G1 arrest in oral
squamous cell carcinoma cell lines. Oral Oncol. 40:43–51. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Feng YB, Lin DC, Shi ZZ, Wang XC, Shen XM,
Zhang Y, Du XL, Luo ML, Xu X, Han YL, et al: Overexpression of PLK1
is associated with poor survival by inhibiting apoptosis via
enhancement of survivin level in esophageal squamous cell
carcinoma. Int J Cancer. 124:578–588. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Faulkner C, Palmer A, Williams H, Wragg C,
Haynes HR, White P, DeSouza RM, Williams M, Hopkins K and Kurian
KM: EGFR and EGFRvIII analysis in glioblastoma as therapeutic
biomarkers. Br J Neurosurg. 29:23–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cai HQ, Wang PF, Zhang HP, Cheng ZJ, Li
SW, He J, Zhang Y, Hao JJ, Wang MR, Yan CX and Wan JH:
Phosphorylated Hsp27 is mutually exclusive with ATRX loss and the
IDH1(R132H) mutation and may predict better prognosis among
glioblastomas without the IDH1 mutation and ATRX loss. J Clin
Pathol. 71:702–707. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Soni D, King JA, Kaye AH and Hovens CM:
Genetics of glioblastoma multiforme: Mitogenic signaling and cell
cycle pathways converge. J Clin Neurosci. 12:1–5. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nakayama KI, Hatakeyama S and Nakayama K:
Regulation of the cell cycle at the G1-S transition by proteolysis
of cyclin E and p27Kip1. Biochem Biophys Res Commun. 282:853–860.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Signoretti S, Di Marcotullio L, Richardson
A, Ramaswamy S, Isaac B, Rue M, Monti F, Loda M and Pagano M:
Oncogenic role of the ubiquitin ligase subunit Skp2 in human breast
cancer. J Clin Invest. 110:633–641. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lu M, Ma J, Xue W, Cheng C, Wang Y, Zhao
Y, Ke Q, Liu H, Liu Y, Li P, et al: The expression and prognosis of
FOXO3a and Skp2 in human hepatocellular carcinoma. Pathol Oncol
Res. 15:679–687. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Elsherif E, Elbaky TA, Elserafy F, Elkady
N, Dawood M, Gaber MA, Badawy A and Gharabawy ME: β-catenin and
SKP2 proteins as predictors of grade and stage of non-muscle
invasive urothelial bladder carcinoma. Chin Clin Oncol.
5:62016.PubMed/NCBI
|
|
35
|
Raucher D, Dragojevic S and Ryu J:
Macromolecular drug carriers for targeted glioblastoma therapy:
Preclinical studies, challenges, and future perspectives. Front
Oncol. 8:6242018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang L, Ye X, Cai X, Su J, Ma R, Yin X,
Zhou X, Li H and Wang Z: Curcumin suppresses cell growth and
invasion and induces apoptosis by down-regulation of Skp2 pathway
in glioma cells. Oncotarget. 6:18027–18037. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen VC, Hsieh YH, Chen LJ, Hsu TC and
Tzang BS: Escitalopram oxalate induces apoptosis in U-87MG cells
and autophagy in GBM8401 cells. J Cell Mol Med. 22:1167–1178.
2018.PubMed/NCBI
|
|
38
|
Huang MH, Lin SZ, Lin PC, Chiou TW, Harn
YW, Ho LI, Chan TM, Chou CW, Chuang CH, Su HL and Harn HJ: Brain
tumor senescence might be mediated by downregulation of S-phase
kinase-associated protein 2 via butylidenephthalide leading to
decreased cell viability. Tumour Biol. 35:4875–4884. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Davidovich S, Ben-Izhak O, Shapira M,
Futerman B and Hershko DD: Over-Expression of Skp2 is associated
with resistance to preoperative doxorubicin-based chemotherapy in
primary breast cancer. Breast Cancer Res. 10:R632008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xu T, Chen J, Zhu D, Chen L, Wang J, Sun
X, Hu B and Duan Y: Egg antigen p40 of schistosoma japonicum
promotes senescence in activated hepatic stellate cells via
SKP2/P27 signaling pathway. Sci Rep. 7:2752017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tan Y, Zhou G, Wang X, Chen W and Gao H:
USP18 promotes breast cancer growth by upregulating EGFR and
activating the AKT/Skp2 pathway. Int J Oncol. 53:371–383.
2018.PubMed/NCBI
|
|
42
|
Eckel-Passow JE, Lachance DH, Molinaro AM,
Walsh KM, Decker PA, Sicotte H, Pekmezci M, Rice T, Kosel ML,
Smirnov IV, et al: Glioma groups based on 1p/19q, IDH, and TERT
promoter mutations in tumors. N Engl J Med. 372:2499–2508. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Diplas BH, He X, Brosnan-Cashman JA, Liu
H, Chen LH, Wang Z, Moure CJ, Killela PJ, Loriaux DB, Lipp ES, et
al: The genomic landscape of TERT promoter wildtype-IDH wildtype
glioblastoma. Nat Commun. 9:20872018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang JB, Dong DF, Wang MD and Gao K: IDH1
overexpression induced chemotherapy resistance and IDH1 mutation
enhanced chemotherapy sensitivity in Glioma cells in vitro and in
vivo. Asian Pac J Cancer Prev. 15:427–432. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Miyata S, Urabe M, Gomi A, Nagai M,
Yamaguchi T, Tsukahara T, Mizukami H, Kume A, Ozawa K and Watanabe
E: An R132H mutation in isocitrate dehydrogenase 1 enhances p21
expression and inhibits phosphorylation of retinoblastoma protein
in glioma cells. Neurol Med Chir (Tokyo). 53:645–654. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chien MN, Yang PS, Hsu YC, Liu TP, Lee JJ
and Cheng SP: Transcriptome analysis of papillary thyroid cancer
harboring telomerase reverse transcriptase promoter mutation. Head
Neck. 40:2528–2537. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Jafri MA, Ansari SA, Alqahtani MH and Shay
JW: Roles of telomeres and telomerase in cancer, and advances in
telomerase-targeted therapies. Genome Med. 8:692016. View Article : Google Scholar : PubMed/NCBI
|