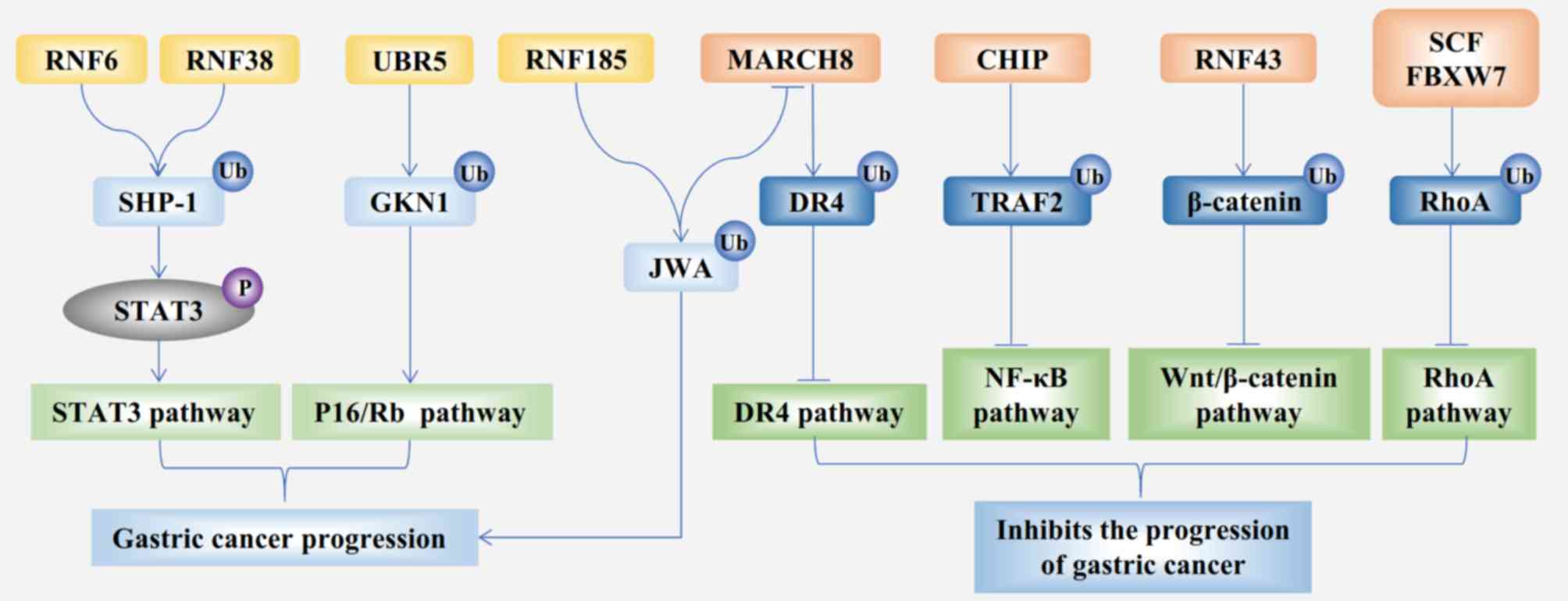
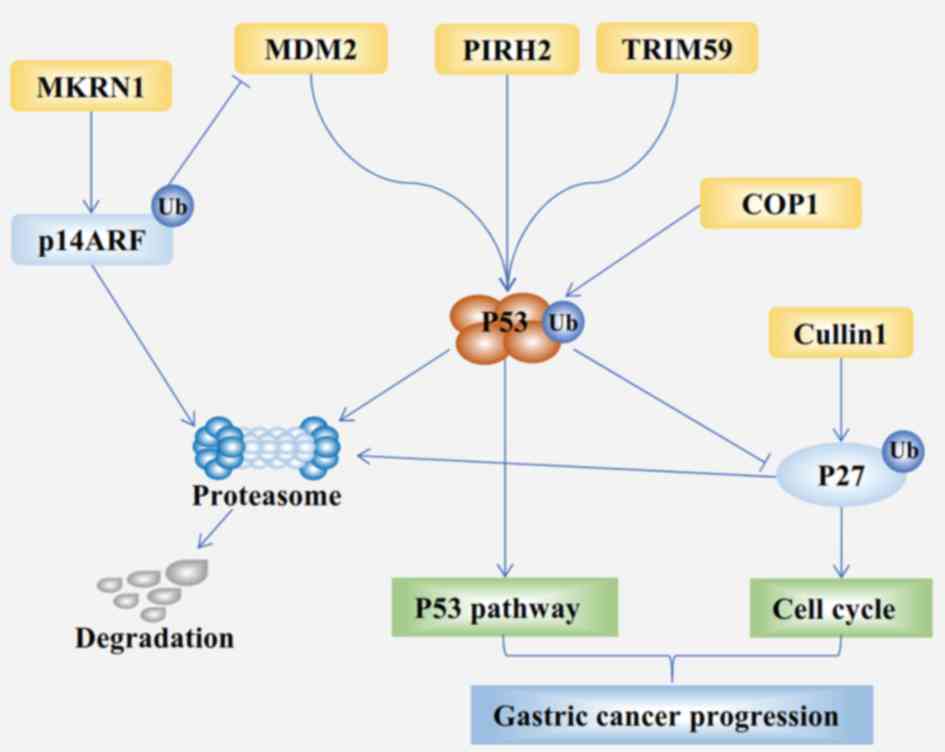
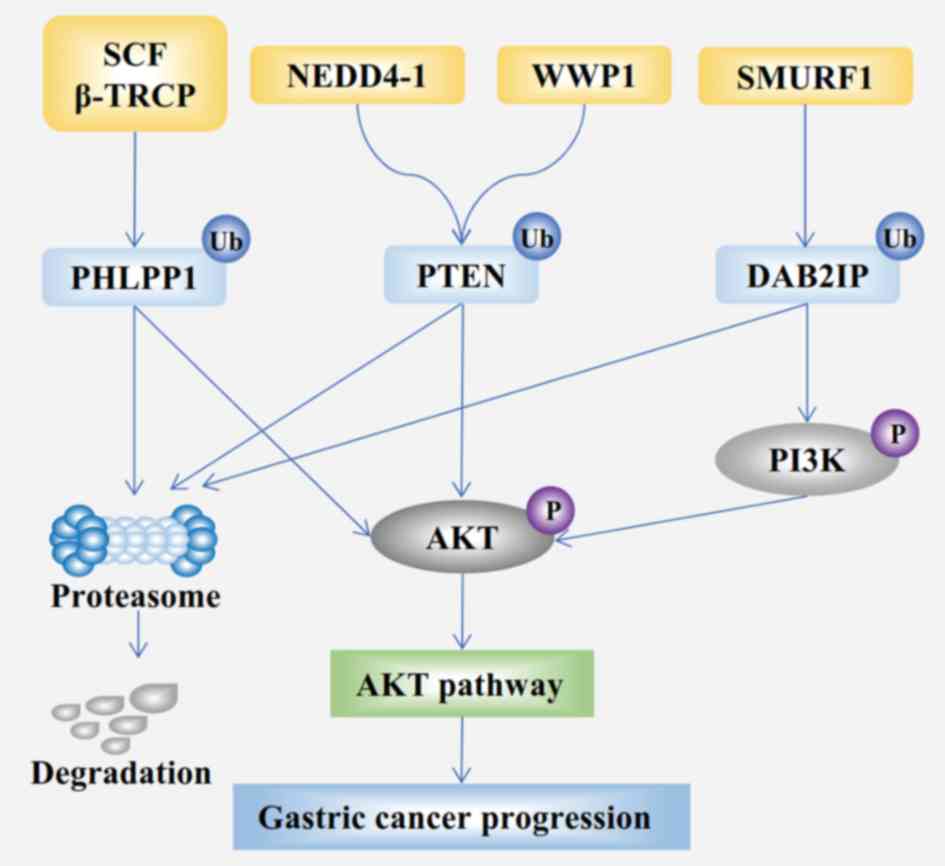
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wu Y, Fan Y, Jiang Y, Wang Y, Liu H and
Wei M: Analysis of risk factors associated with precancerous lesion
of gastric cancer in patients from eastern China: A comparative
study. J Cancer Res Ther. 9:205–209. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zeng H, Zheng R, Guo Y, Zhang S, Zou X,
Wang N, Zhang L, Tang J, Chen J, Wei K, et al: Cancer survival in
China, 2003–2005: A population-based study. Int J Cancer.
136:1921–1930. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Woo Y, Goldner B, Son T, Song K, Noh SH,
Fong Y and Hyung WJ: Western validation of a novel gastric cancer
prognosis prediction model in US gastric cancer patients. J Am Coll
Surg. 226:252–258. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wen T, Wang Z, Li Y, Li Z, Che X, Fan Y,
Wang S, Qu J, Yang X, Hou K, et al: A four-factor immunoscore
system that predicts clinical outcome for stage II/III gastric
cancer. Cancer Immunol Res. 5:524–534. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Abramov IS, Emelyanova MA, Ryabaya OO,
Krasnov GS, Zasedatelev AS and Nasedkina TV: Somatic mutations
associated with metastasis in acral melanoma. Mol Biol (Mosk).
53:648–653. 2019.(In Russian). View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hou YC and Deng JY: Role of E3 ubiquitin
ligases in gastric cancer. World J Gastroenterol. 21:786–793. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hickey CM, Xie Y and Hochstrasser M: DNA
binding by the MATα2 transcription factor controls its access to
alternative ubiquitin-modification pathways. Mol Biol Cell.
29:542–556. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bulatov E, Valiullina A, Sayarova R and
Rizvanov A: Promising new therapeutic targets for regulation of
inflammation and immunity: RING-type E3 ubiquitin ligases. Immunol
Lett. 202:44–51. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lu W, Yang C, He H and Liu H: The
CARM1-p300-c-Myc-Max (CPCM) transcriptional complex regulates the
expression of CUL4A/4B and affects the stability of CRL4 E3 ligases
in colorectal cancer. Int J Biol Sci. 16:1071–1085. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu L, Wong CC, Gong B and Yu J:
Functional significance and therapeutic implication of ring-type E3
ligases in colorectal cancer. Oncogene. 37:148–159. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Uchida C and Kitagawa M: RING-, HECT-, and
RBR-type E3 ubiquitin ligases: Involvement in human cancer. Curr
Cancer Drug Targets. 16:157–174. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Johansson H, Isabella Tsai YC, Fantom K,
Chung CW, Kümper S, Martino L, Thomas DA, Eberl HC, Muelbaier M,
House D and Rittinger K: Fragment-based covalent ligand screening
enables rapid discovery of inhibitors for the RBR E3 ubiquitin
ligase HOIP. J Am Chem Soc. 141:2703–2712. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yu JM, Sun W, Wang ZH, Liang X, Hua F, Li
K, Lv XX, Zhang XW, Liu YY, Yu JJ, et al: TRIB3 supports breast
cancer stemness by suppressing FOXO1 degradation and enhancing SOX2
transcription. Nat Commun. 10:57202019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang J, Wu H, Yi B, Zhou J, Wei L, Chen Y
and Zhang L: RING finger protein 38 induces gastric cancer cell
growth by decreasing the stability of the protein tyrosine
phosphatase SHP-1. FEBS Lett. 592:3092–3100. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang Z, Cai Y, Yang C, Chen Z, Sun H, Xu
Y, Chen W, Xu D, Tian W and Wang H: Knockdown of RNF6 inhibits
gastric cancer cell growth by suppressing STAT3 signaling. Onco
Targets Ther. 11:6579–6587. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Berndsen CE and Wolberger C: New insights
into ubiquitin E3 ligase mechanism. Nat Struct Mol Biol.
21:301–307. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ohi MD, Vander Kooi CW, Rosenberg JA,
Chazin WJ and Gould KL: Structural insights into the U-box, a
domain associated with multi-ubiquitination. Nat Struct Biol.
10:250–255. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Leslie PL, Ke H and Zhang Y: The MDM2 RING
domain and central acidic domain play distinct roles in MDM2
protein homodimerization and MDM2-MDMX protein heterodimerization.
J Biol Chem. 290:12941–12950. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Genschik P, Sumara I and Lechner E: The
emerging family of CULLIN3-RING ubiquitin ligases (CRL3s): Cellular
functions and disease implications. EMBO J. 32:2307–2320. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kelsall IR, Kristariyanto YA, Knebel A,
Wood NT, Kulathu Y and Alpi AF: Coupled monoubiquitylation of the
co-E3 ligase DCNL1 by Ariadne-RBR E3 ubiquitin ligases promotes
cullin-RING ligase complex remodeling. J Biol Chem. 294:2651–2664.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Weber J, Polo S and Maspero E: HECT E3
ligases: A tale with multiple facets. Front Physiol. 10:370–377.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lorenz S: Structural mechanisms of
HECT-type ubiquitin ligases. Biol Chem. 399:127–145. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Geng R, Tan X, Wu J, Pan Z, Yi M, Shi W,
Liu R, Yao C, Wang G, Lin J, et al: RNF183 promotes proliferation
and metastasis of colorectal cancer cells via activation of
NF-κB-IL-8 axis. Cell Death Dis. 8:e29942017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Peng R, Zhang PF, Yang X, Wei CY, Huang
XY, Cai JB, Lu JC, Gao C, Sun HX, Gao Q, et al: Overexpression of
RNF38 facilitates TGF-β signaling by Ubiquitinating and degrading
AHNAK in hepatocellular carcinoma. J Exp Clin Cancer Res.
38:1132019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Macdonald DH, Lahiri D, Sampath A, Chase
A, Sohal J and Cross NC: Cloning and characterization of RNF6, a
novel RING finger gene mapping to 13q12. Genomics. 58:94–97. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Eisenberg I, Hochner H, Levi T, Yelin R,
Kahan T and Mitrani-Rosenbaum S: Cloning and characterization of a
novel human gene RNF38 encoding a conserved putative protein with a
RING finger domain. Biochem Biophys Res Commun. 294:1169–1176.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Katoh M: Molecular cloning and
characterization of RNF26 on human chromosome 11q23 region,
encoding a novel RING finger protein with leucine zipper. Biochem
Biophys Res Commun. 282:1038–1044. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jongsma ML, Berlin I, Wijdeven RH, Janssen
L, Janssen GM, Garstka MA, Janssen H, Mensink M, van Veelen PA,
Spaapen RM and Neefjes J: An ER-associated pathway defines
endosomal architecture for controlled cargo transport. Cell.
166:152–166. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Qin Y, Zhou MT, Hu MM, Hu YH, Zhang J, Guo
L, Zhong B and Shu HB: RNF26 temporally regulates virus-triggered
type I interferon induction by two distinct mechanisms. PLoS
Pathog. 10:e10043582014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang R, Zhao X, Xu J, Wen Y, Li A, Lu M
and Zhou J: Astrocytic JWA deletion exacerbates dopaminergic
neurodegeneration by decreasing glutamate transporters in mice.
Cell Death Dis. 9:3522018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhu T, Chen R, Li A, Liu J, Gu D, Liu Q, C
Chang H and Zhou J: JWA as a novel molecule involved in oxidative
stress-associated signal pathway in myelogenous leukemia cells. J
Toxicol Environ Health A. 69:1399–1411. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Qiu D, Wang Q, Wang Z, Chen J, Yan D, Zhou
Y, Li A, Zhang R, Wang S and Zhou J: RNF185 modulates JWA
ubiquitination and promotes gastric cancer metastasis. Biochim
Biophys Acta Mol Basis Dis. 1864:1552–1561. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gao Y, Cai A, Xi H, Li J, Xu W, Zhang Y,
Zhang K, Cui J, Wu X, Wei B and Chen L: Ring finger protein 43
associates with gastric cancer progression and attenuates the
stemness of gastric cancer stem-like cells via the Wnt-β/catenin
signaling pathway. Stem Cell Res Ther. 8:982017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xi HQ, Cai AZ, Wu XS, Cui JX, Shen WS,
Bian SB, Wang N, Li JY, Lu CR, Song Z, et al: Leucine-rich
repeat-containing G-protein-coupled receptor 5 is associated with
invasion, metastasis, and could be a potential therapeutic target
in human gastric cancer. Br J Cancer. 110:2011–2020. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li XB, Yang G, Zhu L, Tang YL, Zhang C, Ju
Z, Yang X and Teng Y: Gastric Lgr5(+) stem cells are the cellular
origin of invasive intestinal-type gastric cancer in mice. Cell
Res. 26:838–849. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Barker N, Huch M, Kujala P, van de
Wetering M, Snippert HJ, van Es JH, Sato T, Stange DE, Begthel H,
van den Born M, et al: Lgr5(+ve) stem cells drive self-renewal in
the stomach and build long-lived gastric units in vitro. Cell Stem
Cell. 6:25–36. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhou Y, Lan J, Wang W, Shi Q, Lan Y, Cheng
Z and Guan H: ZNRF3 acts as a tumour suppressor by the Wnt
signalling pathway in human gastric adenocarcinoma. J Mol Histol.
44:555–563. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Nanki K, Toshimitsu K, Takano A, Fujii M,
Shimokawa M, Ohta Y, Matano M, Seino T, Nishikori S, Ishikawa K, et
al: Divergent routes toward Wnt and R-spondin niche independency
during human gastric carcinogenesis. Cell. 174:856–869.e17. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hao HX, Jiang X and Cong F: Control of Wnt
receptor turnover by R-spondin-ZNRF3/RNF43 signaling module and its
dysregulation in cancer. Cancers (Basel). 8(pii): E542016.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang K, Yuen ST, Xu J, Lee SP, Yan HH, Shi
ST, Siu HC, Deng S, Chu KM, Law S, et al: Whole-genome sequencing
and comprehensive molecular profiling identify new driver mutations
in gastric cancer. Nat Genet. 46:573–582. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu H, Mintern JD and Villadangos JA:
MARCH ligases in immunity. Curr Opin Immunol. 58:38–43. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang Q, Chen Q, Zhu L, Chen M, Xu W,
Panday S, Wang Z, Li A, Røe OD, Chen R, et al: JWA regulates
TRAIL-induced apoptosis via MARCH8-mediated DR4 ubiquitination in
cisplatin-resistant gastric cancer cells. Oncogenesis. 6:e3532017.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yin J, Ji Z, Hong Y, Song Z, Hu N, Zhuang
M, Bian B, Liu Y and Wu F: Sh-MARCH8 inhibits tumorigenesis via
PI3K pathway in gastric cancer. Cell Physiol Biochem. 49:306–321.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sun M, Li S, Yu K, Xiang J and Li F: An E3
ubiquitin ligase TRIM9 is involved in WSSV infection via
interaction with β-TrCP. Dev Comp Immunol. 97:57–63. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sun Y, Keown JR, Black MM, Raclot C,
Demarais N, Trono D, Turelli P and Goldstone DC: A dissection of
oligomerization by the TRIM28 tripartite motif and the interaction
with members of the Krab-ZFP family. J Mol Biol. 431:2511–2527.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yokoe T, Toiyama Y, Okugawa Y, Tanaka K,
Ohi M, Inoue Y, Mohri Y, Miki C and Kusunoki M: KAP1 is associated
with peritoneal carcinomatosis in gastric cancer. Ann Surg Oncol.
17:821–828. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kosaka Y, Inoue H, Ohmachi T, Yokoe T,
Matsumoto T, Mimori K, Tanaka F, Watanabe M and Mori M: Tripartite
motif-containing 29 (TRIM29) is a novel marker for lymph node
metastasis in gastric cancer. Ann Surg Oncol. 14:2543–2549. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhou Z, Ji Z, Wang Y, Li J, Cao H, Zhu HH
and Gao WQ: TRIM59 is up-regulated in gastric tumors, promoting
ubiquitination and degradation of p53. Gastroenterology.
147:1043–1054. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ma X, Zhang S, Zhang M, Zhu Y, Ma P, Yang
S, Su L, Li Z, Lv W and Luan W: TRIM28 down-regulation on
methylation imprints in bovine preimplantation embryos. Zygote.
26:449–456. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhang P, Zhang H, Wang Y, Zhang P and Qi
Y: Tripartite motif-containing protein 59 (TRIM59) promotes
epithelial ovarian cancer progression via the focal adhesion
kinase(FAK)/AKT/matrix metalloproteinase (MMP) pathway. Med Sci
Monit. 25:3366–3373. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Shen H, Zhang J, Zhang Y, Feng Q, Wang H,
Li G, Jiang W and Li X: Knockdown of tripartite motif 59 (TRIM59)
inhibits proliferation in cholangiocarcinoma via the PI3K/AKT/mTOR
signaling pathway. Gene. 698:50–60. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Chen G, Chen W, Ye M, Tan W and Jia B:
TRIM59 knockdown inhibits cell proliferation by down-regulating the
Wnt/β-catenin signaling pathway in neuroblastoma. Biosci Rep.
39(pii): BSR201812772019. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Cui Z, Liu Z, Zeng J, Chen L, Wu Q, Mo J,
Zhang G, Song L, Xu W, Zhang S and Guo X: Eugenol inhibits
non-small cell lung cancer by repressing expression of
NF-κB-regulated TRIM59. Phytother Res. 33:1562–1569. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Halaby MJ, Hakem R and Hakem A: Pirh2: An
E3 ligase with central roles in the regulation of cell cycle, DNA
damage response, and differentiation. Cell Cycle. 12:2733–2737.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Bao Y, Wu X, Yuan D, Shi W and Shi J: High
expression of Pirh2 is associated with poor prognosis in glioma.
Cell Mol Neurobiol. 37:1501–1509. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Daks A, Petukhov A, Fedorova O, Shuvalov
O, Merkulov V, Vasileva E, Antonov A and Barlev NA: E3 ubiquitin
ligase Pirh2 enhances tumorigenic properties of human non-small
cell lung carcinoma cells. Genes Cancer. 7:383–393. 2016.PubMed/NCBI
|
|
58
|
Yang S, Chen Y, Sun F, Ni Q, Wang H, Huang
Y, Zhang C, Liu K, Wang S, Qiu J, et al: Downregulated pirh2 can
decrease the proliferation of breast cancer cells. Arch Med Res.
47:186–195. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Yang G, Gong Y, Wang Q, Wang L and Zhang
X: miR-100 antagonism triggers apoptosis by inhibiting
ubiquitination-mediated p53 degradation. Oncogene. 36:1023–1037.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Eichinger L, Pachebat JA, Glöckner G,
Rajandream MA, Sucgang R, Berriman M, Song J, Olsen R, Szafranski
K, Xu Q, et al: The genome of the social amoeba Dictyostelium
discoideum. Nature. 435:43–57. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Langdon WY, Hyland CD, Grumont RJ and
Morse HC III: The c-cbl proto-oncogene is preferentially expressed
in thymus and testis tissue and encodes a nuclear protein. J Virol.
63:5420–5424. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Lee H and Tsygankov AY: Cbl-family
proteins as regulators of cytoskeleton-dependent phenomena. J Cell
Physiol. 228:2285–2293. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Kamei T, Machida K, Nimura Y, Senga T,
Yamada I, Yoshii S, Matsuda S and Hamaguchi M: C-Cbl protein in
human cancer tissues is frequently tyrosine phosphorylated in a
tumor-specific manner. Int J Oncol. 17:335–339. 2000.PubMed/NCBI
|
|
64
|
Dong Q, Liu YP, Qu XJ, Hou KZ and Li LL:
Expression of c-Cbl, Cbl-b, and epidermal growth factor receptor in
gastric carcinoma and their clinical significance. Chin J Cancer.
29:59–64. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Feng D, Ma Y, Liu J, Xu L, Zhang Y, Qu J,
Liu Y and Qu X: Cbl-b enhances sensitivity to 5-fluorouracil via
EGFR- and mitochondria-mediated pathways in gastric cancer cells.
Int J Mol Sci. 14:24399–24411. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Yu P, Fan Y, Qu X, Zhang J, Song N, Liu J
and Liu Y: Cbl-b regulates the sensitivity of cetuximab through
ubiquitin-proteasome system in human gastric cancer cells. J BUON.
21:867–873. 2016.PubMed/NCBI
|
|
67
|
CHe X, Zhang Y, Qu X, Guo T, Ma Y, Li C,
Fan Y, Hou K, Cai Y, Yu R, et al: The E3 ubiquitin ligase Cbl-b
inhibits tumor growth in multidrug-resistant gastric and breast
cancer cells. Neoplasma. 64:887–892. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Xu L, Zhang Y, Qu X, Che X, Guo T, Cai Y,
Li A, Li D, Li C, Wen T, et al: E3 ubiquitin ligase Cbl-b prevents
tumor metastasis by maintaining the epithelial phenotype in
multiple drug-resistant gastric and breast cancer cells. Neoplasia.
19:374–382. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Zhang Y, Qu X, Hu X, Yang X, Hou K, Teng
Y, Zhang J, Sada K and Liu Y: Reversal of P-glycoprotein-mediated
multi-drug resistance by the E3 ubiquitin ligase Cbl-b in human
gastric adenocarcinoma cells. J Pathol. 218:248–255. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Lai AZ, Durrant M, Zuo D, Ratcliffe CD and
Park M: Met kinase-dependent loss of the E3 ligase Cbl in gastric
cancer. J Biol Chem. 287:8048–8059. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Gao YJ, Xin Y, Zhang JJ and Zhou J:
Mechanism and pathobiologic implications of CHFR promoter
methylation in gastric carcinoma. World J Gastroenterol.
14:5000–5007. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Li Y, Yang Y, Lu Y, Herman JG, Brock MV,
Zhao P and Guo M: Predictive value of CHFR and MLH1 methylation in
human gastric cancer. Gastric Cancer. 18:280–287. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Satoh A, Toyota M, Itoh F, Sasaki Y,
Suzuki H, Ogi K, Kikuchi T, Mita H, Yamashita T, Kojima T, et al:
Epigenetic inactivation of CHFR and sensitivity to microtubule
inhibitors in gastric cancer. Cancer Res. 63:8606–8613.
2003.PubMed/NCBI
|
|
74
|
Kashima L, Idogawa M, Mita H, Shitashige
M, Yamada T, Ogi K, Suzuki H, Toyota M, Ariga H, Sasaki Y and
Tokino T: CHFR protein regulates mitotic checkpoint by targeting
PARP-1 protein for ubiquitination and degradation. J Biol Chem.
287:12975–12984. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Kim JH, Park SM, Kang MR, Oh SY, Lee TH,
Muller MT and Chung IK: Ubiquitin ligase MKRN1 modulates telomere
length homeostasis through a proteolysis of hTERT. Genes Dev.
19:776–781. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Ko A, Shin JY, Seo J, Lee KD, Lee EW, Lee
MS, Lee HW, Choi IJ, Jeong JS, Chun KH and Song J: Acceleration of
gastric tumorigenesis through MKRN1-mediated posttranslational
regulation of p14ARF. J Natl Cancer Inst. 104:1660–1672. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Jang JH: FIGC, a novel FGF-induced
ubiquitin-protein ligase in gastric cancers FEBS. Lett. 578:21–25.
2004.
|
|
78
|
Wu CE, Esfandiari A, Ho YH, Wang N, Mahdi
AK, Aptullahoglu E, Lovat P and Lunec J: Targeting negative
regulation of p53 by MDM2 and WIP1 as a therapeutic strategy in
cutaneous melanoma. Br J Cancer. 118:495–508. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Hu C, Ni Z, Li BS, Yong X, Yang X, Zhang
JW, Zhang D, Qin Y, Jie MM, Dong H, et al: hTERT promotes the
invasion of gastric cancer cells by enhancing FOXO3a ubiquitination
and subsequent ITGB1 upregulation. Gut. 66:31–42. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Chi XZ, Kim J, Lee YH, Lee JW, Lee KS, Wee
H, Kim WJ, Park WY, Oh BC, Stein GS, et al: Runt-related
transcription factor RUNX3 is a target of MDM2-mediated
ubiquitination. Cancer Res. 69:8111–8119. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Jung CR, Lim JH, Choi Y, Kim DG, Kang KJ,
Noh SM and Im DS: Enigma negatively regulates p53 through MDM2 and
promotes tumor cell survival in mice. J Clin Invest. 120:4493–4506.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Feng Y, Gao S, Gao Y, Song D, Wang X and
Chen Z: Runx3 expression in rectal cancer cells and its effect on
cell invasion and proliferation. Oncol Lett. 18:3290–3294.
2019.PubMed/NCBI
|
|
83
|
Connell P, Ballinger CA, Jiang J, Wu Y,
Thompson LJ, Höhfeld J and Patterson C: The co-chaperone CHIP
regulates protein triage decisions mediated by heat-shock proteins.
Nat Cell Biol. 3:93–96. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Xiao M, Yan M, Zhang J, Xu Q and Chen W:
Carboxy-terminus Hsc70 interacting protein exerts a tumor
inhibition function in head and neck cancer. Oncol Rep.
38:1629–1636. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Liu F, Zhou J, Zhou P, Chen W and Guo F:
The ubiquitin ligase CHIP inactivates NF-κB signaling and impairs
the ability of migration and invasion in gastric cancer cells. Int
J Oncol. 46:2096–2106. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Zheng N, Schulman BA, Song L, Miller JJ,
Jeffrey PD, Wang P, Chu C, Koepp DM, Elledge SJ, Pagano M, et al:
Structure of the Cul1-Rbx1-Skp1-F boxSkp2 SCF ubiquitin ligase
complex. Nature. 416:703–709. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Lisztwan J, Marti A, Sutterlüty H,
Gstaiger M, Wirbelauer C and Krek W: Association of human CUL-1 and
ubiquitin-conjugating enzyme CDC34 with the F-box protein
p45(SKP2): Evidence for evolutionary conservation in the subunit
composition of the CDC34-SCF pathway. EMBO J. 17:368–383. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Uddin S, Bhat AA, Krishnankutty R, Mir F,
Kulinski M and Mohammad RM: Involvement of F-BOX proteins in
progression and development of human malignancies. Semin Cancer
Biol. 36:18–32. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Jiang ZH, Peng T, Qian HL, Lu CD, Qiu F
and Zhang SZ: DNA damage-induced activation of ATM promotes
β-TRCP-mediated ARID1A ubiquitination and destruction in gastric
cancer cells. Cancer Cell Int. 19:1622019. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Milne AN, Leguit R, Corver WE, Morsink FH,
Polak M, de Leng WW, Carvalho R and Offerhaus GJ: Loss of
CDC4/FBXW7 in gastric carcinoma. Cell Oncol. 32:347–359.
2010.PubMed/NCBI
|
|
91
|
Li H, Wang Z, Zhang W, Qian K, Xu W and
Zhang S: Fbxw7 regulates tumor apoptosis, growth arrest and the
epithelial-to-mesenchymal transition in part through the RhoA
signaling pathway in gastric cancer. Cancer Lett. 370:39–55. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Huang LY, Zhao J, Chen H, Wan L, Inuzuka
H, Guo J, Fu X, Zhai Y, Lu Z, Wang X, et al:
SCFFBW7-mediated degradation of Brg1 suppresses gastric
cancer metastasis. Nat Commun. 9:35692018. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Kuai X, Li L, Chen R, Wang K, Chen M, Cui
B, Zhang Y, Li J, Zhu H, Zhou H, et al:
SCFFBXW7/GSK3β-Mediated GFI1 Degradation Suppresses
Proliferation of Gastric Cancer Cells. Cancer Res. 79:4387–4398.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Zhou J, Hayakawa Y, Wang TC and Bass AJ:
RhoA mutations identified in diffuse gastric cancer. Cancer Cell.
26:9–11. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Gong J, Cui Z, Li L, Ma Q, Wang Q, Gao Y
and Sun H: MicroRNA-25 promotes gastric cancer proliferation,
invasion, and migration by directly targeting F-box and WD-40
domain protein 7, FBXW7. Tumour Biol. 36:7831–7840. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Lv Z, Zhang Y, Yu X, Lin Y and Ge Y:
RETRACTED: The function of long non-coding RNA MT1JP in the
development and progression of gastric cancer. Pathol Res Pract.
214:1218–1223. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Frescas D and Pagano M: Deregulated
proteolysis by the F-box proteins SKP2 and beta-TrCP: Tipping the
scales of cancer. Nat Rev Cancer. 8:438–449. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Saitoh T and Katoh M: Expression profiles
of betaTRCP1 and betaTRCP2, and mutation analysis of betaTRCP2 in
gastric cancer. Int J Oncol. 18:959–964. 2001.PubMed/NCBI
|
|
99
|
Gao G, Kun T, Sheng Y, Qian M, Kong F, Liu
X, Yu Z, Zhang H, Zhang Q, Gu J and Zhang X: SGT1 regulates Akt
signaling by promoting beta-TrCP-dependent PHLPP1 degradation in
gastric cancer cells. Mol Biol Rep. 40:2947–2953. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Wang S, Zhang Y, Wang Y, Ye P, Li J, Li H,
Ding Q and Xia J: Amphiregulin confers regulatory T cell
suppressive function and tumor invasion via the EGFR/GSK-3β/Foxp3
axis. J Biol Chem. 291:21085–21095. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Li LQ, Pan D, Chen H, Zhang L and Xie WJ:
F-box protein FBXL2 inhibits gastric cancer proliferation by
ubiquitin-mediated degradation of forkhead box M1. FEBS Lett.
590:445–452. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Cen G, Ding HH, Liu B and Wu WD: FBXL5
targets cortactin for ubiquitination-mediated destruction to
regulate gastric cancer cell migration. Tumour Biol. 35:8633–8638.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Wu W, Ding H, Cao J and Zhang W: FBXL5
inhibits metastasis of gastric cancer through suppressing Snail1.
Cell Physiol Biochem. 35:1764–1772. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Zou S, Ma C, Yang F, Xu X, Jia J and Liu
Z: FBXO31 Suppresses gastric cancer EMT by targeting Snail1 for
proteasomal degradation. Mol Cancer Res. 16:286–295. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Petroski MD and Deshaies RJ: Function and
regulation of cullin-RING ubiquitin ligases. Nat Rev Mol Cell Biol.
6:9–20. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Morimoto M, Nishida T, Honda R and Yasuda
H: Modification of cullin-1 by ubiquitin-like protein Nedd8
enhances the activity of SCF(skp2) toward p27(kip1). Biochem
Biophys Res Commun. 270:1093–1096. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Cheng Q and Yin G: Cullin-1 regulates MG63
cell proliferation and metastasis and is a novel prognostic marker
of osteosarcoma. Int J Biol Markers. 32:e202–e209. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Zhou YH, Xia J, Xu WH, Zhu X, Wu XH, Hua D
and Xing C: Cullin-1 promotes cell proliferation in human breast
cancer and is related to diabetes. Int J Biol Markers.
31:e375–e381. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Jiang H, He D, Xu H, Liu J, Qu L and Tong
S: Cullin-1 promotes cell proliferation via cell cycle regulation
and is a novel in prostate cancer. Int J Clin Exp Pathol.
8:1575–1583. 2015.PubMed/NCBI
|
|
110
|
Chen TJ, Gao F, Yang T, Thakur A, Ren H,
Li Y, Zhang S, Wang T and Chen MW: CDK-associated Cullin 1 promotes
cell proliferation with activation of ERK1/2 in human lung cancer
A549 cells. Biochem Biophys Res Commun. 437:108–113. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Michail O, Moris D, Theocharis S and
Griniatsos J: Cullin-1 and −2 protein expression in colorectal
cancer: Correlation with clinicopathological variables. In Vivo.
32:391–396. 2018.PubMed/NCBI
|
|
112
|
Bai J, Zhou Y, Chen G, Zeng J, Ding J, Tan
Y, Zhou J and Li G: Overexpression of Cullin1 is associated with
poor prognosis of patients with gastric cancer. Hum Pathol.
42:375–383. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Clifford SC, Cockman ME, Smallwood AC,
Mole DR, Woodward ER, Maxwell PH, Ratcliffe PJ and Maher ER:
Contrasting effects on HIF-1alpha regulation by disease-causing
pVHL mutations correlate with patterns of tumourigenesis in von
Hippel-Lindau disease. Hum Mol Genet. 10:1029–1038. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Yokoe S, Nakagawa T, Kojima Y, Higuchi K
and Asahi M: Indomethacin-induced intestinal epithelial cell damage
is mediated by pVHL activation through the degradation of collagen
I and HIF-1α. Biochem Biophys Res Commun. 468:671–676. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Clifford SC, Astuti D, Hooper L, Maxwell
PH, Ratcliffe PJ and Maher ER: The pVHL-associated SCF ubiquitin
ligase complex: molecular genetic analysis of elongin B and C, Rbx1
and HIF-1alpha in renal cell carcinoma. Oncogene. 20:5067–5074.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Gao L, Wu GJ, Liu B, Shen MZ, Pan TJ, Yu
CG, Wang QH, Ru Y, Liu XP, Niu TS, et al: Up-regulation of pVHL
along with down-regulation of HIF-1α by NDRG2 expression attenuates
proliferation and invasion in renal cancer cells. PLoS One.
8:e841272013. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Bowers AJ and Boylan JF: Nek8, a NIMA
family kinase member, is overexpressed in primary human breast
tumors. Gene. 328:135–142. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Ding XF, Zhou J, Hu QY, Liu SC and Chen G:
The tumor suppressor pVHL down-regulates never-in-mitosis A-related
kinase 8 via hypoxia-inducible factors to maintain cilia in human
renal cancer cells. J Biol Chem. 290:1389–1394. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Ding XF, Chen J, Zhou J, Chen G and Wu YL:
Never-in-mitosis A-related kinase 8, a novel target of
von-Hippel-Lindau tumor suppressor protein, promotes gastric cancer
cell proliferation. Oncol Lett. 16:5900–5906. 2018.PubMed/NCBI
|
|
120
|
Bianchi E, Denti S, Catena R, Rossetti G,
Polo S, Gasparian S, Putignano S, Rogge L and Pardi R:
Characterization of human constitutive photomorphogenesis protein
1, a RING finger ubiquitin ligase that interacts with Jun
transcription factors and modulates their transcriptional activity.
J Biol Chem. 278:19682–19690. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Zhu D, Maier A, Lee JH, Laubinger S, Saijo
Y, Wang H, Qu LJ, Hoecker U and Deng XW: Biochemical
characterization of Arabidopsis complexes containing CONSTITUTIVELY
PHOTOMORPHOGENIC1 and SUPPRESSOR OF PHYA proteins in light control
of plant development. Plant Cell. 20:2307–2323. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Saijo Y, Zhu D, Li J, Rubio V, Zhou Z,
Shen Y, Hoecker U, Wang H and Deng XW: Arabidopsis COP1/SPA1
complex and FHY1/FHY3 associate with distinct phosphorylated forms
of phytochrome A in balancing light signaling. Mol Cell.
31:607–613. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Ka WH, Cho SK, Chun BN, Byun SY and Ahn
JC: The ubiquitin ligase COP1 regulates cell cycle and apoptosis by
affecting p53 function in human breast cancer cell lines. Breast
Cancer. 25:529–538. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Zou S, Zhu Y, Wang B, Qian F, Zhang X,
Wang L, Fu C, Bao H, Xie M, Gao S, et al: The ubiquitin ligase COP1
promotes glioma cell proliferation by preferentially downregulating
tumor suppressor p53. Mol Neurobiol. 54:5008–5016. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Li YF, Wang DD, Zhao BW, Wang W, Huang CY,
Chen YM, Zheng Y, Keshari RP, Xia JC and Zhou ZW: High level of
COP1 expression is associated with poor prognosis in primary
gastric cancer. Int J Biol Sci. 8:1168–1177. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Sawada G, Ueo H, Matsumura T, Uchi R,
Ishibashi M, Mima K, Kurashige J, Takahashi Y, Akiyoshi S, Sudo T,
et al: Loss of COP1 expression determines poor prognosis in
patients with gastric cancer. Oncol Rep. 30:1971–1975. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Rotin D and Kumar S: Physiological
functions of the HECT family of ubiquitin ligases. Nat Rev Mol Cell
Biol. 10:398–409. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Zheng H, Ke X, Li D and Wang Q, Wang J,
Liu X, Deng M, Deng X, Xue Y, Zhu Y and Wang Q: NEDD4 promotes cell
growth and motility in hepatocellular carcinoma. Cell Cycle.
17:728–738. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Shao G, Wang R, Sun A, Wei J, Peng K, Dai
Q, Yang W and Lin Q: The E3 ubiquitin ligase NEDD4 mediates cell
migration signaling of EGFR in lung cancer cells. Mol Cancer.
17:242018. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Kim SS, Yoo NJ, Jeong EG, Kim MS and Lee
SH: Expression of NEDD4-1, a PTEN regulator, in gastric and
colorectal carcinomas. APMIS. 116:779–784. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Zou X, Levy-Cohen G and Blank M: Molecular
functions of NEDD4 E3 ubiquitin ligases in cancer. Biochim Biophys
Acta. 1856:91–106. 2015.PubMed/NCBI
|
|
132
|
Zhang L, Wu Z, Ma Z, Liu H, Wu Y and Zhang
Q: WWP1 as a potential tumor oncogene regulates PTEN-Akt signaling
pathway in human gastric carcinoma. Tumour Biol. 36:787–798. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Ma L, Chen X, Li C, Cheng R, Gao Z, Meng
X, Sun C, Liang C and Liu Y: miR-129-5p and −3p co-target WWP1 to
suppress gastric cancer proliferation and migration. J Cell
Biochem. Nov 11–2018.(Epub ahead of print).
|
|
134
|
Li Q, Li Z, Wei S, Wang W, Chen Z, Zhang
L, Chen L, Li B, Sun G, Xu J, et al: Overexpression of miR-584-5p
inhibits proliferation and induces apoptosis by targeting WW
domain-containing E3 ubiquitin protein ligase 1 in gastric cancer.
J Exp Clin Cancer Res. 36:592017. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Zhu H, Kavsak P, Abdollah S, Wrana JL and
Thomsen GH: A SMAD ubiquitin ligase targets the BMP pathway and
affects embryonic pattern formation. Nature. 400:687–693. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Koganti P, Levy-Cohen G and Blank M:
Smurfs in protein homeostasis, signaling, and cancer. Front Oncol.
8:2952018. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Dote H, Toyooka S, Tsukuda K, Yano M, Ota
T, Murakami M, Naito M, Toyota M, Gazdar AF and Shimizu N: Aberrant
promoter methylation in human DAB2 interactive protein (hDAB2IP)
gene in gastrointestinal tumour. Br J Cancer. 92:1117–1125. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Li X, Dai X, Wan L, Inuzuka H, Sun L and
North BJ: Smurf1 regulation of DAB2IP controls cell proliferation
and migration. Oncotarget. 7:26057–26069. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Tao Y, Sun C, Zhang T and Song Y: SMURF1
promotes the proliferation, migration and invasion of gastric
cancer cells. Oncol Rep. 38:1806–1814. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Yang M, Jiang N, Cao QW, Ma MQ and Sun Q:
The E3 ligase UBR5 regulates gastric cancer cell growth by
destabilizing the tumor suppressor GKN1. Biochem Biophys Res
Commun. 478:1624–1629. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Kozlov G, Nguyen L, Lin T, De Crescenzo G,
Park M and Gehring K: Structural basis of ubiquitin recognition by
the ubiquitin-associated (UBA) domain of the ubiquitin ligase EDD.
J Biol Chem. 282:35787–35795. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Kim MS, Oh JE, Eom HS, Yoo NJ and Lee SH:
Mutational analysis of UBR5 gene encoding an E3 ubiquitin ligase in
common human cancers. Pathology. 42:93–94. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Richardson PG, Hideshima T and Anderson
KC: Bortezomib (PS-341): A novel, first-in-class proteasome
inhibitor for the treatment of multiple myeloma and other cancers.
Cancer Control. 10:361–369. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Zhang B and Gu Y: Bortezomib inhibits
gastric carcinoma HGC-27 cells through the phospho-Jun N-terminal
kinase (p-JNK) pathway in vitro. Gene. 559:164–171. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Yi H, Yan X, Luo Q, Yuan L, Li B, Pan W,
Zhang L, Chen H, Wang J, Zhang Y, et al: A novel small molecule
inhibitor of MDM2-p53 (APG-115) enhances radiosensitivity of
gastric adenocarcinoma. J Exp Clin Cancer Res. 37:972018.
View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Vassilev LT, Vu BT, Graves B, Carvajal D,
Podlaski F, Filipovic Z, Kong N, Kammlott U, Lukacs C, Klein C, et
al: In vivo activation of the p53 pathway by small-molecule
antagonists of MDM2. Science. 303:844–848. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Impicciatore G, Sancilio S, Miscia S and
Di Pietro R: Nutlins and ionizing radiation in cancer therapy. Curr
Pharm Des. 16:1427–1442. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Yee-Lin V, Pooi-Fong W and Soo-Beng AK:
Nutlin-3, A p53-Mdm2 antagonist for nasopharyngeal carcinoma
treatment. Mini Rev Med Chem. 18:173–183. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Meijer A, Kruyt FA, van der Zee AG,
Hollema H, Le P, ten Hoor KA, Groothuis GM, Quax WJ, de Vries EG
and de Jong S: Nutlin-3 preferentially sensitises wild-type
p53-expressing cancer cells to DR5-selective TRAIL over rhTRAIL. Br
J Cancer. 109:2685–2695. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Lee DM, Kim IY, Seo MJ, Kwon MR and Choi
KS: Nutlin-3 enhances the bortezomib sensitivity of p53-defective
cancer cells by inducing paraptosis. Exp Mol Med. 49:e3652017.
View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Endo S, Yamato K, Hirai S, Moriwaki T,
Fukuda K, Suzuki H, Abei M, Nakagawa I and Hyodo I: Potent in vitro
and in vivo antitumor effects of MDM2 inhibitor nutlin-3 in gastric
cancer cells. Cancer Sci. 102:605–613. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Wei YS and Adachi I: Inhibitory effect of
triptolide on colony formation of breast and stomach cancer cell
lines. Zhongguo Yao Li Xue Bao. 12:406–410. 1991.PubMed/NCBI
|
|
153
|
Jiang XH, Wong BC, Lin MC, Zhu GH, Kung
HF, Jiang SH, Yang D and Lam SK: Functional p53 is required for
triptolide-induced apoptosis and AP-1 and nuclear factor-kappaB
activation in gastric cancer cells. Oncogene. 20:8009–8018. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Wang BY, Cao J, Chen JW and Liu QY:
Triptolide induces apoptosis of gastric cancer cells via inhibiting
the overexpression of MDM2. Med Oncol. 31:2702014. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Choi HS, Seo HS, Kim JH, Um JY, Shin YC
and Ko SG: Ethanol extract of paeonia suffruticosa Andrews (PSE)
induced AGS human gastric cancer cell apoptosis via fas-dependent
apoptosis and MDM2-p53 pathways. J Biomed Sci. 19:822012.
View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Lan H, Tang Z, Jin H and Sun Y:
Neddylation inhibitor MLN4924 suppresses growth and migration of
human gastric cancer cells. Sci Rep. 6:242182016. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Eto K, Iwatsuki M, Watanabe M, Ishimoto T,
Ida S, Imamura Y, Iwagami S, Baba Y, Sakamoto Y, Miyamoto Y, et al:
The sensitivity of gastric cancer to trastuzumab is regulated by
the miR-223/FBXW7 pathway. Int J Cancer. 136:1537–1545. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Schneekloth JS Jr and Crews CM: Natural
product inhibitors of the ubiquitin-proteasome pathway. Curr Drug
Targets. 12:1581–1594. 2011. View Article : Google Scholar : PubMed/NCBI
|