Introduction
Endometrial cancer is the fourth most common cancer
in women in the United States (1,2), and the
most common gynecological malignancy in developed countries from
Cancer Statistics in 2018 (3,4).
Statistics obtained from the American Cancer Society suggest that
there were 382,069 new cases and ~89,929 deaths associated with
corpus uteri worldwide in 2018 (5,6).
Endometrioid adenocarcinomas represent 80% of all endometrial
cancers, and are viewed as estrogen-dependent endometrioid type I
endometrial cancer and serous endometrial cancers are often
referred to estrogen-independent carcinoma type II (2). The majority of newly diagnosed patients
have a favorable prognosis with a 5-year survival rate of 81.8%,
but patients with recurrent/metastatic disease have poor survival,
with a 5-year survival rate of 55 and 42% after radiotherapy and
chemotherapy, respectively (1,2).
Clinical stage (7), histological
subtype (8), grade (9), depth of invasive disease (10) and positive lymph node (11) have been reported as important
prognostic factors. Elevated serum cancer antigen 125 level is
observed during recurrence and tumor relapse (12). Surgery is the initial management for
early-stage patients (2). However,
due to the limits of effective biomarkers, developing novel
treatment options, such as immunotherapy/targeted therapy for
patients with recurrent/metastatic disease, remains as a challenge
(13).
According to molecular features, The Cancer Genome
Atlas (TCGA) divides endometrial cancer into four subgroups: POLE
ultra-mutated, hypermutated/microsatellite unstable, copy number
low/microsatellite stable and copy number high (serous-like)
(1,14). Some studies have reported that 83% of
endometrioid adenocarcinomas harbor PTEN mutations (13), which is the initial event occurring
with co-mutations of PIK3CA and PIK3R1 (1). In serous endometrial cancers, TP53
mutations are the most common, with an incidence of 90% (13). Furthermore, F-box/WD
repeat-containing protein 7, PIK3CA and serine/threonine-protein
phosphatase 2A 65 kDa regulatory subunit A α isoform somatic
mutations and G1/S-specific cyclin-E1 amplifications are observed
in this type (1,15). Despite these findings for the
diagnosis and differential diagnosis, the evaluation of novel
biomarkers for targeted therapy/immunotherapy in endometrial cancer
is needed to improve the treatment options.
Secretoglobin, family 2A, member 1 (SCGB2A1),
which is also known as mammaglobin-B, is a member of the
uteroglobin gene family and was first identified by Becker et
al (16) in 1998. SCGB2A1 is
highly expressed in human tears, breast and uterus tissue, and is
involved in regulating androgen biosynthesis and the androgen
receptor/steroid signaling pathway in prostate cancer (17). SCGB2A1 is the top
differentially expressed gene in primary epithelial ovarian cancer
with a 905-fold upregulation (18–20). In
invasive and borderline ovarian carcinoma, presentation of SCGB2A1
expression was correlated to less aggressive behavior and a more
favorable outcome (21). However,
overexpression of SCGB2A2 is positively correlated with
carcinogenesis, especially in advanced the Federation of Gynecology
and Obsetrics stage for primary ovarian cancer, increased
Silverberg tumor grade and the elevated mitotic index (22). Reference to ovarian cancer, SCGB2A1
maybe a novel prognostic biomarker for UCEC. Tassi et al
(23) evaluated 70 patients to
analyze SCGB2A1 gene expression by quantitative PCR and
immunohistochemistry (IHC), and reported that SCGB2A1 is
upregulated in endometrioid endometrial cancer, particularly in
well- and moderately-differentiated tumors. However, the
association between SCGB2A1 and endometrial cancer and its
prognostic value for UCEC are not clear at present. Endometrial
cancer cases are primarily estrogen-dependent endometroid types,
which are known as type I, and these respond to
estrogen/progesterone antagonists (10). SCGB2A1 may participate in
estrogen/progesterone synthesis and the estrogen
receptor/progesterone receptor signaling pathway, and maybe a
potentially novel therapeutic target for endometrial cancer.
The present study aimed to determine the role of
SCGB2A1 expression in UCEC using TCGA dataset, and to investigate
the associated downstream signaling pathways. In addition, the
study aimed to determine a novel candidate biomarker to inform
targeted advanced therapeutics in endometrial cancer.
Materials and methods
Case identification and bioinformatics
analysis
In total, data of 561 endometrial cancer cases were
included in the present study, including 23 adjacent normal
endometrium tissues and 552 tumor tissues. Clinicopathological
characteristics and gene expression data were downloaded from TCGA
official website for the UCEC project (https://portal.gdc.cancer.gov). After cases with
incomplete gene expression and incomplete follow-up information
were excluded, 528 tumor cases and 23 normal cases were finally
identified. The staging system used in the present study was
American Joint Committee on Cancer (AJCC) TNM
(tumor-node-metastasis) staging system for endometrial cancer
(24). For validation, surgical
resected tissues were obtained from 47 patients with a median age
of 52 years (age range, 42–69 years) who were diagnosed with
endometrial cancer at Fudan University Shanghai Cancer Center
(FUSCC) from January 2015 to June 2015. The inclusion criteria were
as follows: Patients could accept surgical resection without
distant disease and resected tumor tissues were available and
sufficient for further research. Patients with incomplete follow-up
information or unavailable tumor samples were excluded from the
present study. All patients provided informed written consent.
Functional analysis
Gene Set Enrichment Analysis (GSEA) is a
computational method that determines whether a defined set of genes
exhibits differential expression, and the concordant differences
between two biological states (25).
There were 186 pathways for KEGG analysis and a total 10,192
biological processes for GO analysis, including 7,530 items for
biological process (BP), 999 items for cellular component (CC), and
1,663 items for molecular functions (MF). In the present study,
GSEA was performed to elucidate the overall survival difference
between the high and low expression groups. Gene set permutations
were performed for 1,000 times for each analysis. The most
significant SCGB2A1-assocaited biological pathway (P<0.05) was
selected for further analysis. The significant results of the GSEA
were identified using the nominal P<0.05 and a false discovery
rate <0.25. Gene Ontology (GO) (https://golang.org) analysis of these DEGs between
UCEC and normal tissues was performed using blast2GO with a P≤0.05,
and the pathway enrichment analysis was carried out against the
Kyoto Encyclopedia of Genes and Genomes (KEGG) database with a
Q-value of ≤1 (26). Genotype-Tissue
Expression (GTEx) database is a powerful tool to unravel the
complex patterns of genetic variation and gene regulation across
diverse human tissue types (27). A
total of 78 normal endometrium samples were collected from the GTEx
database and combined with normal samples from the GTEx database
and 23 normal tissues from TCGA database. Finally, differentially
expressed genes (DEGs) in 101 normal endometrium tissues and 552
endometrial tumor tissues were analyzed.
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was isolated from 47 endometrial cancer
tissues using TRIzol® reagent (cat. no. 15596026; Thermo
Fisher Scientific, Inc.). RNA was converted into cDNA using a
PrimeScript™ RT Master Mix kit (cat. no. RR036A, Takara
Biotechnology Co., Ltd.). Then, qPCR was performed using TB
Green® Premix Ex Taq™ II (Tli RNaseH Plus kit, cat. no.
RR820A, Takara Biotechnology Co., Ltd.). RT-qPCR analysis was
performed according to the manufacturer's protocol: The procedure
of RT reaction was 37°C for 15 min, followed by 85°C for 5 sec. The
following thermocycling conditions were used for qPCR: 95°C for 30
sec, followed by 40 cycles at 95°C for 5 sec and 60°C for 30 sec,
with a final extension at 70°C for 10 sec. For measurement, the
fluorescence level of TB Green (TB Green Premix Ex Taq II, Takara
Bio, Inc.) was detected to measure the concentration of PCR
production. The forward primer for SCGB2A1 mRNA was
5′-AAACTCCTGGAGGACATGGTT-3′. The reverse primer for SCGB2A1
mRNA was 5′-ACTGCTTGAATTTCCCCATAGC-3′. GAPDH mRNA was used
as the internal reference. The forward primer for GAPDH mRNA
was 5′-GGAGCGAGATCCCTCCAAAAT-3′. The reverse primer for
GAPDH mRNA was 5′-GGCTGTTGTCATACTTCTCATGG-3′. Relative
SCGB2A1 gene expression levels were calculated using the
2−ΔΔCq method (28).
External validation in multiple public
databases and the FUSCC dataset
SCGB2A1 expression data were collected from other
two databases to verify the role of SCGB2A1 in endometrial cancer.
SCGB2A1 expression data in endometrial cancer tissues determined by
immunohistochemistry (IHC) was obtained from The Human Protein
Atlas (HPA) database (https://www.proteinatlas.org/). The expression of
SCGB2A1 in cancer cell lines was obtained from Broad Institute
Cancer Cell Line Encyclopedia (CCLE) database (https://portals.broadinstitute.org/).
Furthermore, SCGB2A1 mRNA expression was analyzed using
RT-qPCR and the 47 patients were divided into the high-expression
and low expression group according to the median value (cycle
threshold=24.385) of SCGB2A1 mRNA expression. Then, survival
analysis was performed for the two groups in FUSCC dataset. SCGB2A1
protein expression was analyzed in these tumor tissues via IHC
analysis, as previously described (29). Furthermore, to investigate aberrant
expression of SCGB2A1 in UCEC, DNA methylation analysis was
performed using the Mexpress tool (https://mexpress.be).
Statistical analysis
All statistical analyses were analyzed using R
software version 3.6.1 software (https://www.r-project.org). Kaplan-Meier survival
analysis was performed to compare the overall survival between the
low-SCGB2A1 expression group and the high-SCGB2A1 expression group.
Logistic regression was used to calculate the odds ratio (OR) of
SCGB2A1 expression in groups with different clinical
characteristics. The association between the clinicopathologic
characteristics and SCGB2A1 expression was analyzed using a
Wilcoxon rank sum test. The Cox repression univariate and
multivariate model were used for the survival analysis according to
overall survival. The cut-off value of continuous variables such as
age at diagnosis (median age, 52 years) and SCGB2A1 expression data
were determined according to the median value. P<0.05 was
considered to indicate a statistically significant difference.
P-value was adjusted using the Benjamini and Hochberg (BH) method
to decrease sampling bias.
Results
SCGB2A1 expression between UCEC and
normal tissues
From the TCGA database, 23 normal samples and 528
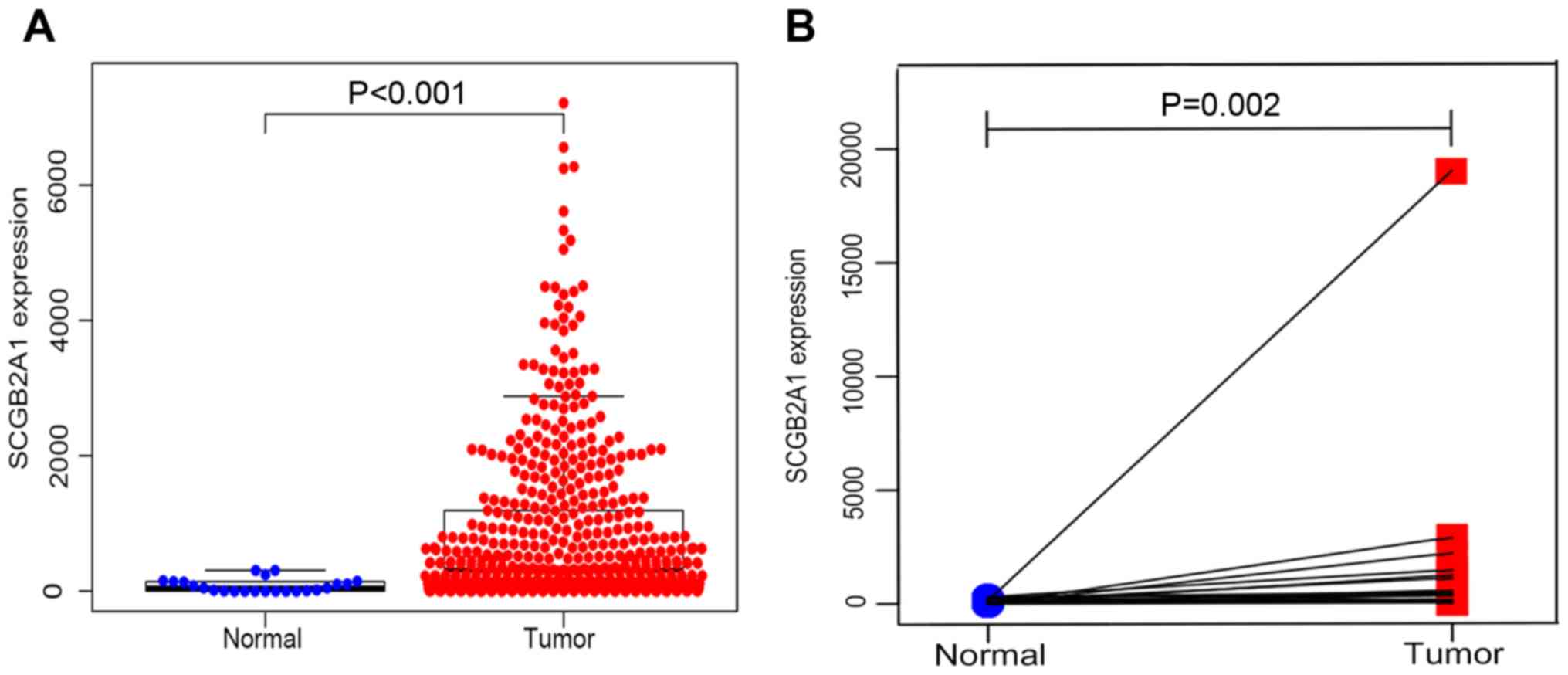
tumor samples were obtained. As shown in Fig. 1A, SCGB2A1 gene expression was
significantly higher in tumor tissues compared with normal tissues
by unpaired t-test (P<0.001). In addition, paired analysis for
the SCGB2A1 expression was performed in tumor tissues and normal
tissues, demonstrating that SCGB2A1 expression was also
elevated in tumor tissues by paired t-test (P=0.002, Fig. 1B). Both unpaired and paired t-test's
confirmed that SCGB2A1 expression was elevated in UCEC compared
with normal tissues.
Clinicopathological characteristics of
patients from the TCGA database and FUSCC dataset
As shown in Table I,
528 patients with UCEC with clinical and gene expression data were
obtained from the TCGA database. The age at diagnosis ranged from
31 to 94 years old, with a median age of 64 years. The majority of
patients were Caucasian, accounting for 72%. Furthermore, 89% of
patients were post-menopausal vs. around 11% of patients were
pre-menopausal. Moreover, about 61% of patients underwent open
surgery, compared with 39% of patients who underwent minimally
invasive surgery. As for the histological type, most of the tumors
(76%, n=403) were endometrioid endometrial adenocarcinoma, 20%
(n=104) were serous endometrial adenocarcinoma and 4% (n=21) were
mixed serous and endometrioid. In TCGA cohort, approximately 19% of
the tumors (n=98) were low grade and well-differentiated tumors,
22.73% (n=120) were moderately differentiated tumors and a large
proportion of the tumors (58.71%, n=310) were high grade tumors
with poor differentiation. Furthermore, 202 (44.30%) tumors had
>50% depth of stromal invasion. The tumor status included 418
(85%) tumor-free and 73 (15%) with tumor. Moreover, 90.52% (n=363)
reached R0 (no residual tumor), while 9.48% (n=38) had a positive
margin (R1/R2). For the peritoneal washing, 343 (86.40%) were
negative, while 13.60% (n=54) were positive. The majority of cases
(83.88%, n=359) had a negative pelvic lymph node. The para-aortic
lymph nodes were usually found to be negative in most cases
(90.17%, n=321). In TCGA cohort, 332 patients (62.88%) were stage
I, 51 patients (9.66%) were stage II, 119 patients (22.54%) were
stage III and 26 patients (4.92%) were stage IV. The median
survival time was 27.7 months (range, 0–228 months). The clinical
characteristics of 47 patients with endometrial cancer from FUSCC
were listed in Table SI.
 | Table I.Clinical characteristics of 528
patients with uteri corpus endometrial carcinoma from the Cancer
Genome Atlas database. |
Table I.
Clinical characteristics of 528
patients with uteri corpus endometrial carcinoma from the Cancer
Genome Atlas database.
| Clinicopathological
characteristics | Number (%) |
|---|
| Age at
diagnosisa, years | 64 (31–90) |
| Ethnicity |
|
|
Caucasian | 361 (72.34) |
| African
American | 105 (21.04) |
|
Other | 33 (6.61) |
| Menopause
status |
|
|
Pre | 51 (10.56) |
|
Post | 432 (89.44) |
| Surgical
approach |
|
|
Minimally invasive | 199 (39.25) |
|
Open | 308 (60.75) |
| Histological
type |
|
| Serous
endometrial adenocarcinoma | 104 (19.70) |
|
Endometrioid endometrial
adenocarcinoma | 403 (76.33) |
| Mixed
serous and endometrioid | 21 (3.98) |
| Grade |
|
| Low
G1 | 98 (18.56) |
|
Moderate G2 | 120 (22.73) |
| High
G3 | 310 (58.71) |
| Tumor invasion
depth, % |
|
|
<50 | 254 (55.70) |
|
≥50% | 202 (44.30) |
| Tumor status |
|
| Tumor
free | 418 (85.13) |
| With
tumor | 73 (14.87) |
| Residual tumor |
|
|
Without, R0 | 363 (90.52) |
| With,
R1/R2 | 38 (9.48) |
| Peritoneal
washing |
|
|
Negative | 343 (86.40) |
|
Positive | 54 (13.60) |
| Pelvic lymph node
status |
|
|
Negative | 359 (83.88) |
|
Positive | 69 (16.12) |
| Para-aortic lymph
node status |
|
|
Negative | 321 (90.17) |
|
Positive | 35 (9.83) |
| Stage |
|
| I | 332 (62.88) |
| II | 51 (9.66) |
|
III | 119 (22.54) |
| IV | 26 (4.92) |
Association between SCGB2A1 expression
and clinicopathological variables
A total of 528 samples identified from TCGA database
were analyzed for the association with SCGB2A1 expression
and clinicopathological characteristics. As shown in Table II, the decrease in SCGB2A1
expression was significantly associated with the age at diagnosis
(>64 years old vs. <64 years old, P<0.001), grade
(high vs. low/moderate, P<0.001), tumor status (with tumor vs.
tumor free, P<0.001), residual tumor (R1/R2 vs. R0, P=0.046),
peritoneal cytology (positive vs. negative, P=0.004), pelvic lymph
node (positive vs. negative, P<0.001), para-aortic lymph node
(positive vs. negative, P=0.024) and clinical stage (II vs. I,
P<0.001). Elevated SCGB2A1 expression was significantly
associated with the histological type (endometrioid vs. serous,
P<0.001). Therefore, it was hypothesized that patients with UCEC
with a decreased SCGB2A1 expression were more likely to
possess high grade, lymph node metastasis and advanced clinical
stage.
 | Table II.Association between SCGB2A1
expression and clinicopathological variables. |
Table II.
Association between SCGB2A1
expression and clinicopathological variables.
| Characteristics
(group A vs. group B) | OR of SCGB2A1
expression (95% CI) | Median (P25,P75) of
SCGB2A1 expression (group A) | Median (P25,P75) of
S CGB2A1 expression (group B) | P-value |
|---|
| Age at diagnosis,
>64 vs. <64 years | 0.41
(0.29-0.58) | 561 (122,1696) | 207 (40,799) | <0.001 |
| Ethnicity, African
American vs. Caucasian | 0.87
(0.64-1.16) | 174 (36,1334) | 388 (77,1289) | 0.344 |
| Menopause status,
post vs. pre | 0.89
(0.54-1.44) | 296 (55,1088) | 926 (263,2069) | 0.622 |
| Surgical approach,
open vs. minimally invasive | 0.98
(0.68-1.40) | 334 (62,1169) | 374 (61,1355) | 0.899 |
| Histological type,
endometrioid vs. serous | 4.00
(2.60-6.33) | 555 (142,1710) | 48 (13,141) | <0.001 |
| Grade, high vs.
low/moderate | 0.11
(0.07-0.17) | 113 (26,420) | 1,166
(415,2264) | <0.001 |
| Tumor invasion
depth, ≥50 vs. <50% | 0.73
(0.50-1.05) | 297 (34,1186) | 491 (100,1517) | 0.090 |
| Tumor status, with
vs. tumor free | 0.28
(0.15-0.48) | 81 (16,380) | 448 (102,1466) | <0.001 |
| Residual tumor,
R1/R2 vs. R0 | 0.49
(0.24-0.97) | 82 (15,549) | 350 (68,1355) | 0.046 |
| Peritoneal washing,
positive vs. negative | 0.41
(0.22-0.75) | 99 (26,544) | 388 (68,1335) | 0.004 |
| Pelvic lymph node
metastasis, yes vs. no | 0.27
(0.15-0.47) | 89 (11,349) | 426 (82,1467) | <0.001 |
| Para-aortic lymph
node metastasis, yes vs. no | 0.42
(0.19-0.87) | 141 (8,411) | 362 (66,1205) | 0.024 |
| Stage, II vs.
I | 0.59
(0.49-0.71) | 373 (78,782) | 484 (104,1654) | <0.001 |
Univariate analysis and multivariate
analysis for UCEC
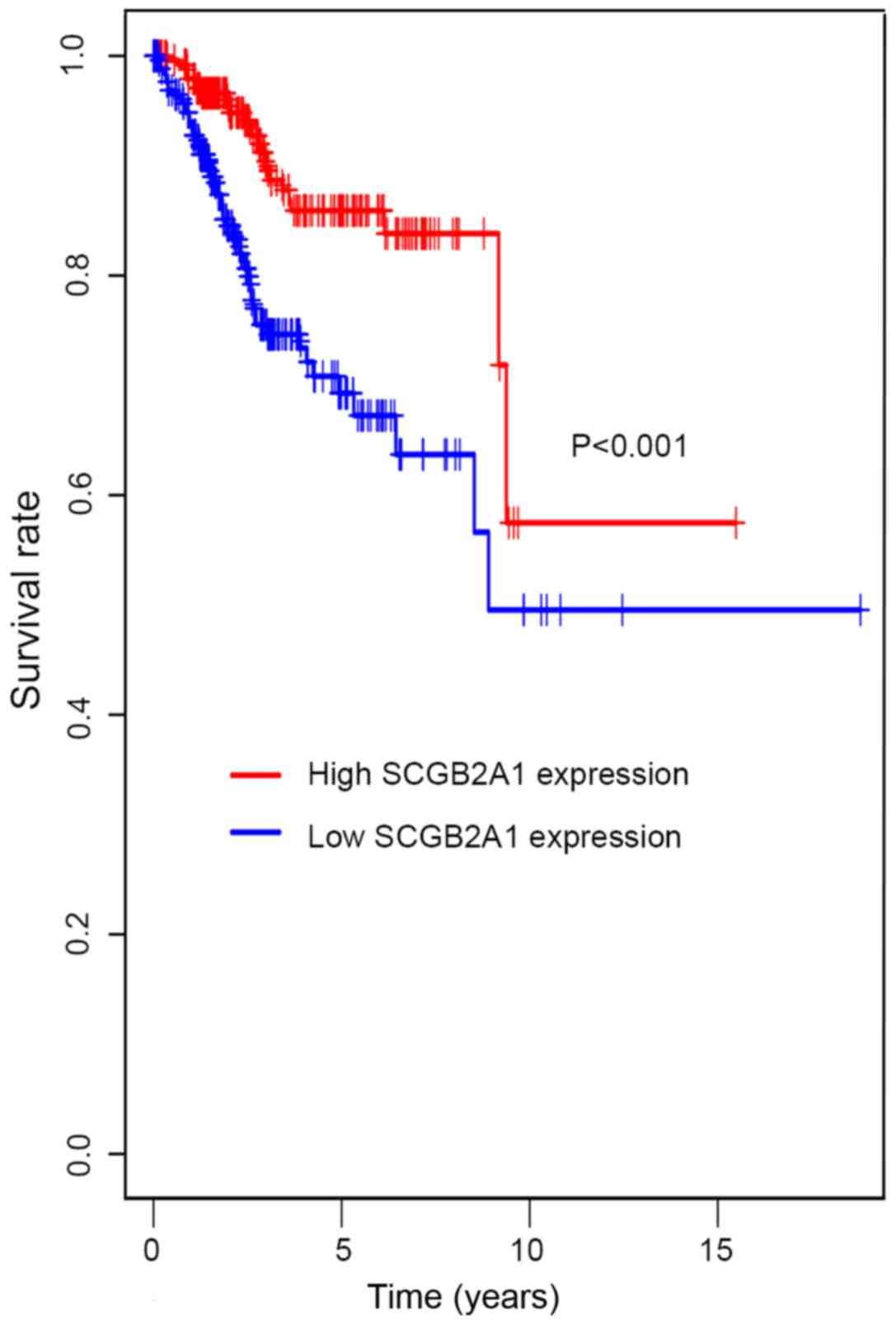
In Fig. 2,
Kaplan-Meier survival analysis was performed for patients with UCEC
with low and high SCGB2A1 gene expression. The results
suggested that low SCGB2A1 expression was associated with
worse survival compared with high expression (P<0.001). In
Table III, it was illustrated that
the decrease in SCGB2A1 expression (P<0.001), age at
diagnosis >64 years old (P=0.007), histological type (P=0.033),
high grade (P<0.001), deep tumor invasion (P<0.001), with
tumor (P<0.001), residual tumor (P<0.001), positive
peritoneal cytology (P<0.001), positive pelvic/para-aortic lymph
node (P<0.001) and clinical stage (P<0.001) predicted poorer
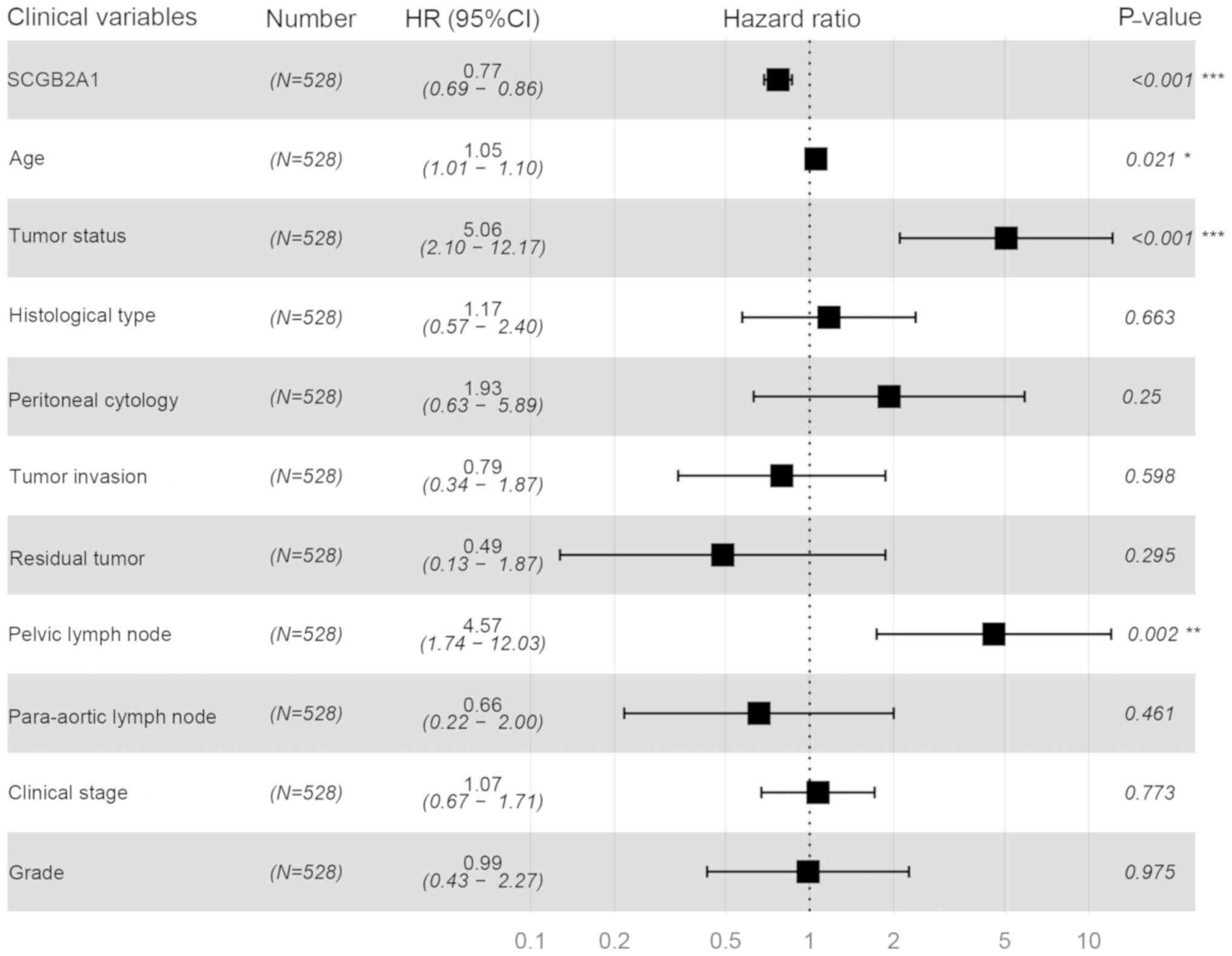
prognosis in the univariate analysis. In the multivariate analysis,
SCGB2A1 expression was significantly associated with the
prognosis (high vs. low, HR=0.77, P<0.001). SCGB2A1
expression (P<0.001), age at diagnosis (P=0.021), tumor status
(P<0.001) and positive lymph node (P=0.002) plotted in the
forest map (Fig. 3) were independent
prognostic factors.
 | Table III.Univariate analysis and multivariate
analysis for uteri corpus endometrial carcinoma according to
overall survival. |
Table III.
Univariate analysis and multivariate
analysis for uteri corpus endometrial carcinoma according to
overall survival.
| A, Univariate
analysis |
|---|
|
|---|
| Clinical
characteristics | HR (95% CI) | P-value |
|---|
| SCGB2A1
expression | 0.75
(0.71-0.80) | <0.001 |
| Age at
diagnosis | 1.03
(1.00-1.05) | 0.007 |
| Race | 0.95
(0.66-1.36) | 0.769 |
| Menopause
status | 0.68
(0.37-1.26) | 0.221 |
| Surgical
approach | 0.85
(0.52-1.37) | 0.499 |
| Histological
type | 0.60
(0.37-0.96) | 0.033 |
| Grade | 3.49
(2.12-5.75) | <0.001 |
| Tumor invasion
depth | 2.43
(1.56-3.87) | <0.001 |
| Tumor status | 6.85
(4.57-10.26) | <0.001 |
| Residual tumor | 2.65
(1.54-4.55) | <0.001 |
| Peritoneal
washing | 3.05
(1.85-5.04) | <0.001 |
| Pelvic lymph node
metastasis | 4.50
(2.87-7.05) | <0.001 |
| Para-aortic lymph
node metastasis | 3.70
(2.11-6.49) | <0.001 |
| Stage | 1.80
(1.51-2.15) | <0.001 |
|
| B, Multivariate
analysis |
|
| Clinical
characteristics | HR (95%
CI) | P-value |
|
| SCGB2A1
expression | 0.77
(0.69-0.86) | <0.001 |
| Age at
diagnosis | 1.05
(1.01-1.10) | 0.021 |
| Tumor status | 5.06
(2.10-12.17) | <0.001 |
| Pelvic lymph
node | 4.57
(1.73-12.03) | 0.002 |
GSEA for the SCGB2A1-associated
pathways
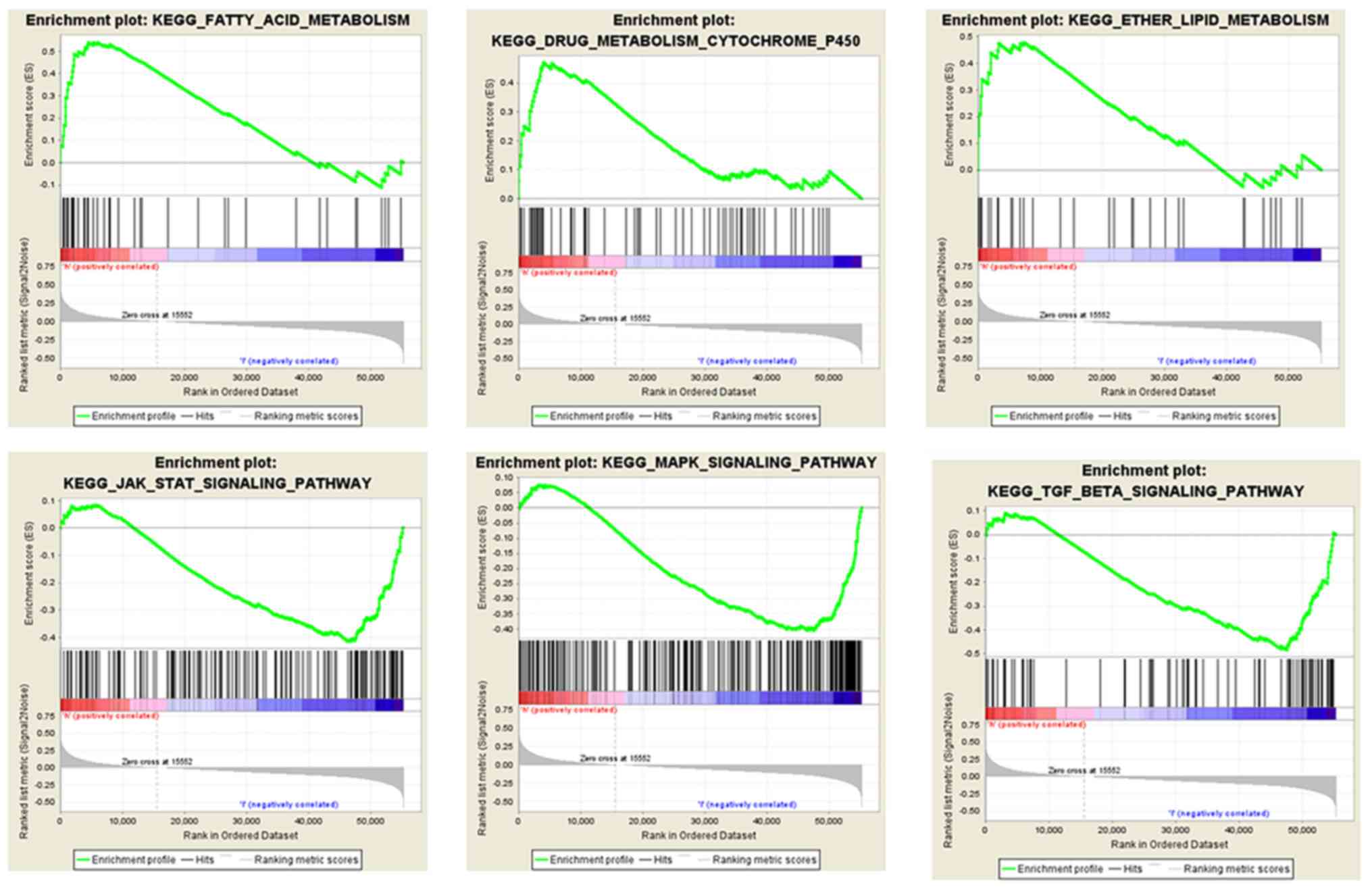
GSEA was conducted for the low and high
SCGB2A1 expression groups in order to identify the
SCGB2A1-related pathways. It was found that the high SCGB2A1
expression was enriched in ‘fatty acid metabolism’, ‘lipid
metabolism’ and ‘cytochrome P450 drug metabolism’. In the low
SCGB2A1 expression group, there were 31 predicted pathways with
significance. Furthermore, it was found that low SCGB2A1
expression was enriched in the ‘transforming growth factor (TGF)-β
signaling pathway’, ‘Janus kinase (JAK)-STAT pathway’ and
‘mitogen-activated protein kinase (MAPK) signaling pathway’
(Fig. 4).
Differentially expressed genes in UCEC
and normal tissues
In total, 78 normal endometrial tissues with gene
expression data were identified from the Genotype-Tissue Expression
(GTEx) database. Then, the expression data of normal and tumor
tissues from the TCGA and GTEx databases were merged. Finally, 101
normal tissues and 552 tumor samples were obtained for further
analysis. These results revealed that 1,192 genes were
differentially expressed in tumors and normal tissues, both with
|Log fold-change |≥2 and P<0.05 (Table SII). Heatmaps were established for
the differential genes, as shown in Fig.
5A. The GO and KEGG analysis were also conducted with the DEGs
(Fig. 5B and C). DEGs were enriched
in focal adhesion, cell cycle, ECM-receptor interaction, both
P<0.05 in KEGG analysis. DEGs were mostly enriched in
extracellular matrix/structure organization for biological process
(BP), extracellular matrix/collagen-containing extracellular matrix
for cell component (CC) and extracellular matrix structural
constituent/cell adhesion molecule binding for molecular function
(MF), with P<0.05 in GO analysis.
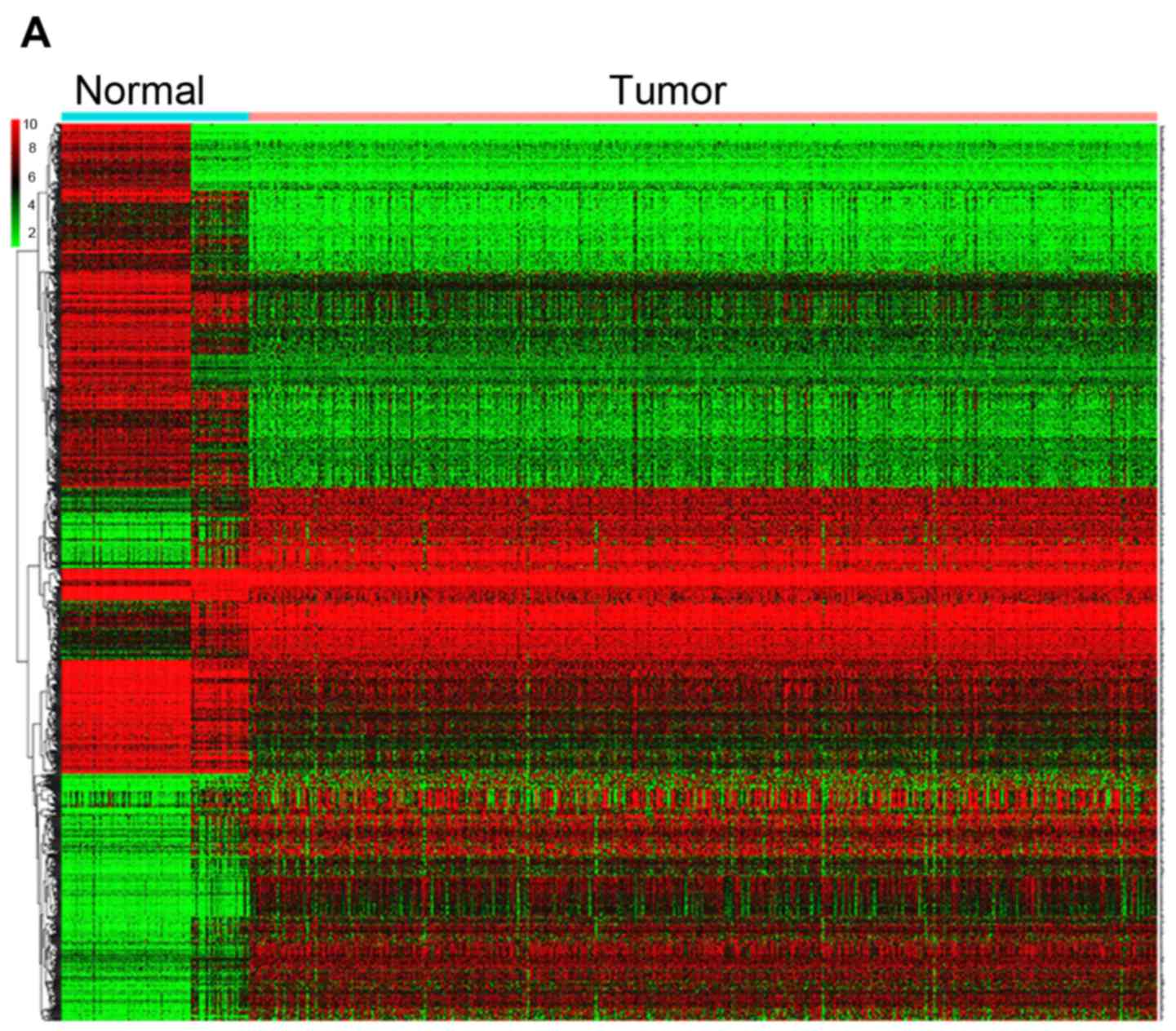 | Figure 5.DEGs between UCEC and normal tissues.
(A) Heatmap for 1,192 DEGs in endometrial cancer and normal
tissues. Each column represents one sample and each row represents
one gene. The red color and the blue color represent tumor and
normal tissues. The gradual color ranging from green to red
represent the extent of down to upregulation, respectively. DEGs
between UCEC and normal tissues. (B) KEGG bar plot and circle plot
for DEGs. The upper graph represents the significant biological
process that DEGs were enriched in. The bottom diagram shows the
DEGs and their associated biological processes. DEGs between UCEC
and normal tissues. (C) GO bar plot and circle plot for DEGs. The
upper graph presents the enrichment of DEGs in the three parts of
GO terms as BP, CC and MF. The bottom diagram shows the DEGs and
mostly significant BPs. DEG, differentially expressed gene; KEGG,
Kyoto Encyclopedia of Genes and Genomes; GO, Gene Ontology; BP,
biological process; CC, cellular component; MF, molecular function;
FC, fold-change. |
Validation of the role of SCGB2A1 in
endometrial cancer
To identify the role of SCGB2A1 in endometrial
cancer, SCGB2A1 expression data in endometrial cancer cell
lines and tissues was obtained from the CCLE database and HPA
database. It was demonstrated that SCGB2A1 expression was higher in
endometrial cancer cell lines compared with other cancer cell lines
(in Fig. S1). The results
demonstrated that SCGB2A1 was mostly overexpressed in prostate
cancer cell lines, endometrial cancer cell lines and small cell
lung cancer cell lines. The expression of SCGB2A1 was stronger in
patient tissues with endometrial cancer (in Fig. S2). In the FUSCC validation set, it
was reported that the low-expression group had a less favorable
overall survival compared with the high-expression group
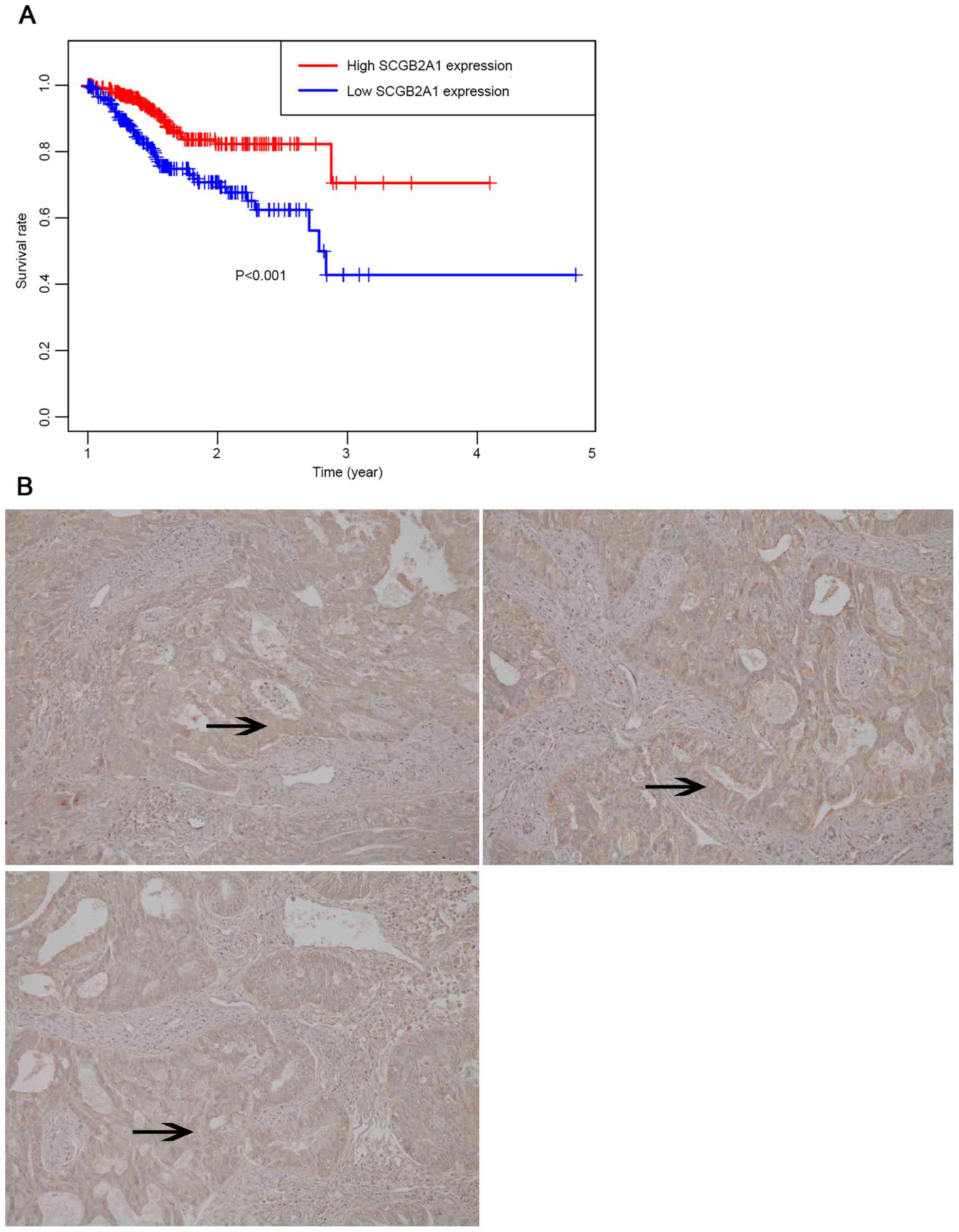
(P<0.001, Fig. 6A). Moreover,
immunohistochemical analysis of SCGB2A1 protein in FUSCC tumor
tissues was used to verify SCGB2A1 protein expression in
endometrial cancer. It was demonstrated that SCGB2A1 was more
highly expressed in patients with endometrial cancer compared with
normal tissues (Fig. 6B), which was
consistent with findings from the HPA database.
To investigate the mechanisms underlying aberrant
SCGB2A1 gene expression in endometrial cancer, DNA
methylation analysis was performed. It was demonstrated that five
methylation sites of the SCGB2A1 CpG island (cg05277881,
cg16986846, cg06334737, cg23206745 and cg14265033) were
significantly correlated with SCGB2A1 gene expression
regulation, but with a weak association in UCEC (P<0.001,
Fig. S3).
Discussion
Endometrial cancer has been reported to have
favorable prognosis (10). However,
over the past few years, the morality rate has rapidly increased
(5) due to its advanced stage and
high risk factors (13).
Furthermore, local treatment such as surgery or radiotherapy is not
sufficient for advanced diseases. Hence, novel biomarkers for novel
therapeutic strategies are needed (13). SCGB2A1, a member of the secretoglobin
family of proteins (17), has been
considered as a candidate diagnostic biomarker for detecting
metastasis in breast cancer. In a study conducted by Mercatali
et al (29), SCGB2A1 was
detected in the peripheral blood in 7% of patients with breast
cancer, while this was not detected in healthy donors. Furthermore,
this was positivity correlated with advanced pathological stage
(P=0.013) and node metastasis. Limited reports have revealed the
role of SCGB2A1 in gynecological cancer types (19,30,31). It
has been reported that SCGB2A1 is significantly elevated in
epithelial ovarian cancer tissue compared with normal ovarian
tissues (32). Elevated SCGB2A1
expression has been reported to present with less aggressive
behavior with a decreased risk of recurrence and disease
progression, and be correlated with a favorable outcome in ovarian
cancer (21). Furthermore,
Dieters-Castator et al (19)
proposed that SCGB2A1 has value as an endometrioid
carcinoma-specific diagnostic biomarker, as well as
1-phosphatidylinositol 4,5-bisphosphate phosphodiesterase β-1 and
UPF0577 protein KIAA1324, which were also significantly associated
with favorable prognosis of patients with endometrioid carcinoma.
In addition, Aihara et al (30) reported that the MGB2 gene is a
novel biomarker for lymph node micrometastasis in patients with
abdominal cancer types. However, research investigating the
association between SCGB2A1 and endometrial cancer is limited.
Previous research has reported that the SCGB2A1
gene, which is mediated by the Sp family transcription factors, is
essential for androgen synthesis in prostate cancer (17). Androgen is a precursor to the
synthesis of estrogen and progesterone, and SCGB2A1 is a crucial
regulatory factor for androgen/steroid hormone synthesis, which is
associated with the biological process in endometrial cancer
(17). Therefore, it was
hypothesized that SCGB2A1 may function in regulating female hormone
biosynthesis, and that it may be a novel target in UCEC. Tassi
et al (23) detected the
expression levels of the SCGB2A1 gene in UCEC, and reported
that SCGB2A1 expression is higher in endometrial cancer
tissue when compared with normal tissues, and that higher
SCGB2A1 expression is correlated with good differentiation
in tumor tissues. The present study demonstrated that
SCGB2A1 expression was higher in endometrial cancer compared
with normal tissues (P<0.001). To investigate the
mechanisms underlying aberrant SCGB2A1 gene expression in
UCEC, methylation analysis was performed. The data revealed that
SCGB2A1 CpG island methylation was significantly correlated
with SCGB2A1 gene expression regulation. CpG island
hypermethylation generally decreases gene expression; however, the
results of the present study failed to demonstrate that SCGB2A1
expression was hypermethylated or low-methylated in endometrial
cancer. Epigenetic alterations, such as DNA methylation, have been
associated with cancer development, and CpG islands methylation is
a key contributor to carcinogenesis by silencing tumor-suppressor
genes and activating oncogenes in endometrial cancer (31). Based on the aforementioned results,
it was hypothesized that SCGB2A1 DNA methylation maybe one
of mechanisms underpinning abnormal SCGB2A1 gene expression
in UCEC. Further experiments on DNA methylation, histone
modification and chromatin remodeling should be performed to
investigate the potential mechanism of abnormal SCGB2A1 expression
in epigenetic perspective.
In present findings, SCGB2A1 was highly expressed in
tumors, and the decrease in SCGB2A1 expression was correlated with
higher grade (P<0.001), advanced stage (P<0.001) and
worse overall survival (P<0.001). Huang et al (33) established a five gene biomarker model
(consisting of ASRGL1, RHEX, SCGB2A1, SOX17 and
STX18) to predict the lymph node metastasis in early-stage
endometrial cancer. The group reported that low SCGB2A1
expression is associated with the lymph node metastasis. Meanwhile,
in the present findings, SCGB2A1 expression was
significantly associated with histological grade, clinical stage
and overall survival for patients with UCEC. It was revealed that
SCGB2A1 expression was an independent prognostic factor for
endometrial cancer (high vs. low, HR=0.77, P<0.001) and it was
hypothesized that high SCGB2A1 expression was a protective
factor in UCEC.
To investigate the involvement of SCGB2A1 in
endometrial cancer, functional analysis was conducted to determine
the associated signaling pathways. GSEA enrichment analysis was
performed, and it was revealed that the high SCGB2A1
expression was correlated with lipid metabolism. Furthermore, it
was reported that SCGB2A1 may participate in cell proliferation by
regulating the MAPK and JAK-STAT signaling pathways, which may have
an effect on tumor growth and progression. Similar to the study
conducted by Bignotti et al (32), it was revealed that lipophilin B has
a significant correlation with mammaglobin B in ovarian carcinoma.
Consistent with GSEA that suggested that SCGB2A1 may participate in
lipid metabolism process, lipophilin B, as a homolog to SCGB2A1,
was confirmed to be involved in lipid metabolism. Furthermore,
Bellone et al (18) reported
that SCGB2A1 may be a novel target of immunotherapy in patients
with recurrent disease resistant to chemotherapy in ovarian cancer.
The group illustrated that CD8+ cytotoxic T lymphocytes populations
could decrease SCGB2A1 expression and were able to consistently
induce the lysis of autologous primary (chemo-naive) and
metastatic/recurrent (chemo-resistant) target tumor cells
expressing SCGB2A1. Previous studies have revealed that SCGB2A1
might have a potential as a new biomarker for predicting metastasis
(27), and a new target for novel
therapy in ovarian cancer (28).
However, the role of SCGB2A1 in endometrial cancer has not been
elucidated at present. Based on these present results, it was
hypothesized that SCGB2A1 may be involved in the
estrogen-progestogen signaling pathway and subsequently associated
with tumor proliferation and progression in UCEC.
In general, the present study is the first to
illustrate the prognostic value of SCGB2A1 for UCEC, to the best of
our knowledge, and analyzed the association between
clinicopathological factors and SCGB2A1 expression obtained
from a large-scale public databases. In the present study, it was
demonstrated that low SCGB2A1 expression is indeed
correlated with clinical characteristics, such as advanced clinical
stage, higher tumor grade, histological type and lymph node
metastasis. Furthermore, SCGB2A1 was significantly
associated with overall survival, and is an independent prognostic
factor. Then, it was reported that SCGB2A1 is associated
with tumor growth and progression through the TGF-β, JAK and MAPK
signaling pathways, but this needs further research. In the present
study, the potential molecular mechanism of SCGB2A1 participating
in tumorigenesis and tumor progression of endometrial cancer was
not fully elucidated. In vitro
(SCGB2A1-silenced/overexpressed cancer cell lines) and in
vivo (SCGB2A1 knockdown) xenograft mouse models should be
established to provide more evidence.
In conclusion, SCGB2A1 has potential as a new target
for predicting the prognosis in UCEC, and the associated downstream
pathways of TGF-β, JAK and MAPK signaling may be helpful for
further research. The present study highlights the potential of
SCGB2A1 as a novel prognostic biomarker for advanced therapy
target in UCEC. However, further experimental research is needed to
verify the prognostic value of SCGB2A1 in UCEC.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
The authors of the present study would like to thank
Professor Xueke Zhou from the Department of Pathology, Fudan
University Shanghai Cancer Center (Shanghai, China) and Professor
Bin Chang from the Department of Pathology, Fudan University
Shanghai Cancer Center (Shanghai, China) for their support in
providing immunohistochemistry analyses.
Funding
The present study was supported by the Natural
Science Foundation of Shanghai (grant no. 17ZR1406000). The funding
agency had no role in study design, data collection and analysis,
decision to publish, or preparation of the manuscript.
Authors' contributions
XC and HZ conceived the study. HZ and HL designed
the study, analyzed the data and drafted the initial manuscript. XZ
helped revised the manuscript critically for important intellectual
content and interpreted the data with constructive suggestion. TL
and LC helped collect the data and performed statistical analysis.
All authors read and approved the final manuscript.
Availability of data and materials
The datasets generated and/or analyzed during the
current study are available in the The Cancer Genome Atlas
(https://cancergenome.nih.gov/), the
Broad Institute Cancer Cell Line Encyclopedia (https://portals.broadinstitute.org/) and The
Human Protein Atlas (https://www.proteinatlas.org/) repositories.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Fudan University Shanghai Cancer Center (approval no.
050432-4-1212B). All patients provided informed written
consent.
Patient consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing
interest.
Glossary
Abbreviations
Abbreviations:
|
SCGB2A1
|
secretoglobin family 2A member 1
|
|
UCEC
|
uteri corpus endometrial carcinoma
|
|
TCGA
|
The Cancer Genome Atlas
|
|
GSEA
|
Gene Set Enrichment Analysis
|
|
KEGG
|
Kyoto Encyclopedia of Genes and
Genomes
|
|
DEGs
|
differentially expressed genes
|
References
|
1
|
Urick ME and Bell DW: Clinical
actionability of molecular targets in endometrial cancer. Nat Rev
Cancer. 19:510–521. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Colombo N, Preti E, Landoni F, Carinelli
S, Colombo A, Marini C and Sessa C; ESMO Guidelines Working Group,
: Endometrial cancer: ESMO Clinical Practice Guidelines for
diagnosis, treatment and follow-up. Ann Oncol. 24 (Suppl
6):vi33–vi38. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Amant F, Mirza MR, Koskas M and Creutzberg
CL: Cancer of the corpus uteri. Int J Gynaecol Obstet. 143 (Suppl
2):S37–S50. 2018. View Article : Google Scholar
|
|
4
|
Saso S, Chatterjee J, Georgiou E, Ditri
AM, Smith JR and Ghaem-Maghami S: Endometrial cancer. BMJ.
343:d39542011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tangjitgamol S, Anderson BO, See HT,
Lertbutsayanukul C, Sirisabya N, Manchana T, Ilancheran A, Lee KM,
Lim SE, Chia YN, et al: Management of endometrial cancer in Asia:
Consensus statement from the Asian Oncology Summit 2009. Lancet
Oncol. 10:1119–1127. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Morice P, Leary A, Creutzberg C,
Abu-Rustum N and Darai E: Endometrial cancer. Lancet.
387:1094–1108. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tanaka K, Kobayashi Y, Sugiyama J,
Yamazaki T, Dozono K, Watanabe M, Shibuya H, Nishigaya Y, Momomura
M, Matsumoto H, et al: Histologic grade and peritoneal cytology as
prognostic factors in type 1 endometrial cancer. Int J Clin Oncol.
22:533–540. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sorosky JI: Endometrial cancer. Obstet
Gynecol. 120:383–397. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Odagiri T, Watari H, Kato T, Mitamura T,
Hosaka M, Sudo S, Takeda M, Kobayashi N, Dong P, Todo Y, et al:
Distribution of lymph node metastasis sites in endometrial cancer
undergoing systematic pelvic and para-aortic lymphadenectomy: A
proposal of optimal lymphadenectomy for future clinical trials. Ann
Surg Oncol. 21:2755–2761. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Reijnen C, IntHout J, Massuger LFAG,
Strobbe F, Küsters-Vandevelde HVN, Haldorsen IS, Snijders MPLM and
Pijnenborg JMA: Diagnostic accuracy of clinical biomarkers for
preoperative prediction of lymph node metastasis in endometrial
carcinoma: A systematic review and meta-analysis. Oncologist.
24:e880–e890. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Arend RC, Jones BA, Martinez A and
Goodfellow P: Endometrial cancer: Molecular markers and management
of advanced stage disease. Gynecol Oncol. 150:569–580. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Le Gallo M and Bell DW: The emerging
genomic landscape of endometrial cancer. Clin Chem. 60:98–110.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Murali R, Delair DF, Bean SM, Abu-Rustum
NR and Soslow RA: Evolving roles of histologic evaluation and
molecular/genomic profiling in the management of endometrial
cancer. J Natl Compr Canc Netw. 16:201–209. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Becker RM, Darrow C, Zimonjic DB, Popescu
NC, Watson MA and Fleming TP: Identification of mammaglobin B, a
novel member of the uteroglobin gene family. Genomics. 54:70–78.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xiao F, Mirwald A, Papaioannou M,
Baniahmad A and Klug J: Secretoglobin 2A1 is under selective
androgen control mediated by a peculiar binding site for Sp family
transcription factors. Mol Endocrinol. 19:2964–2978. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bellone S, Tassi R, Betti M, English D,
Cocco E, Gasparrini S, Bortolomai I, Black JD, Todeschini P, Romani
C, et al: Mammaglobin B (SCGB2A1) is a novel tumour antigen highly
differentially expressed in all major histological types of ovarian
cancer: Implications for ovarian cancer immunotherapy. Br J Cancer.
109:462–471. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dieters-Castator DZ, Rambau PF, Kelemen
LE, Siegers GM, Lajoie GA, Postovit LM and Köbel M:
Proteomics-derived biomarker panel improves diagnostic precision to
classify endometrioid and high-grade serous ovarian carcinoma. Clin
Cancer Res. 25:4309–4319. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tassi RA, Bignotti E, Rossi E, Falchetti
M, Donzelli C, Calza S, Ravaggi A, Bandiera E, Pecorelli S and
Santin AD: Overexpression of mammaglobin B in epithelial ovarian
carcinomas. Gynecol Oncol. 105:578–585. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tassi RA, Calza S, Ravaggi A, Bignotti E,
Odicino FE, Tognon G, Donzelli C, Falchetti M, Rossi E, Todeschini
P, et al: Mammaglobin B is an independent prognostic marker in
epithelial ovarian cancer and its expression is associated with
reduced risk of disease recurrence. BMC Cancer. 9:2532009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fischer K, von Brünneck AC, Hornung D,
Denkert C, Ufer C, Schiebel H, Kuhn H and Borchert A: Differential
expression of secretoglobins in normal ovary and in ovarian
carcinoma-overexpression of mammaglobin-1 is linked to tumor
progression. Arch Biochem Biophys. 547:27–36. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tassi RA, Bignotti E, Falchetti M, Calza
S, Ravaggi A, Rossi E, Martinelli F, Bandiera E, Pecorelli S and
Santin AD: Mammaglobin B expression in human endometrial cancer.
Int J Gynecol Cancer. 18:1090–1096. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
McCluggage WG: Pathologic staging of
endometrial carcinomas: Selected areas of difficulty. Adv Anat
Pathol. 25:71–84. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Subramanian A, Tamayo P, Mootha VK,
Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub
TR, Lander ES and Mesirov JP: Gene set enrichment analysis: A
knowledge-based approach for interpreting genome-wide expression
profiles. Proc Natl Acad Sci USA. 102:15545–15550. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang BL, Li X, Liu P, Ma L, Wu W, Zhang
X, Li Z and Huang B: Transcriptomic analysis of Eruca vesicaria
subs. sativa lines with contrasting tolerance to polyethylene
glycol-simulated drought stress. BMC Plant Biol. 19:4192019.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hua J, Shi S, Xu J, Wei M, Zhang Y, Liu J,
Zhang B and Yu X: Expression patterns and prognostic value of DNA
damage repair proteins in resected pancreatic neuroendocrine
neoplasms. Ann Surg. Mar 20–2020.(Epub ahead of print). View Article : Google Scholar
|
|
29
|
Mercatali L, Valenti V, Calistri D,
Calpona S, Rosti G, Folli S, Gaudio M, Frassineti GL, Amadori D and
Flamini E: RT-PCR determination of maspin and mammaglobin B in
peripheral blood of healthy donors and breast cancer patients. Ann
Oncol. 17:424–428. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Aihara T, Fujiwara Y, Miyake Y, Okami J,
Okada Y, Iwao K, Sugita Y, Tomita N, Sakon M, Shiozaki H and Monden
M: Mammaglobin B gene as a novel marker for lymph node
micrometastasis in patients with abdominal cancers. Cancer Lett.
150:79–84. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bartosch C, Lopes JM and Jeronimo C:
Epigenetics in endometrial carcinogenesis-part 1: DNA methylation.
Epigenomics. 9:737–755. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bignotti E, Tassi RA, Calza S, Ravaggi A,
Rossi E, Donzelli C, Todeschini P, Romani C, Bandiera E, Zanotti L,
et al: Secretoglobin expression in ovarian carcinoma: Lipophilin B
gene upregulation as an independent marker of better prognosis. J
Transl Med. 11:1622013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Huang CY, Liao KW, Chou CH, Shrestha S,
Yang CD, Chiew MY, Huang HT, Hong HC, Huang SH, Chang TH and Huang
HD: Pilot study to establish a novel five-gene biomarker panel for
predicting lymph node metastasis in patients with early stage
endometrial cancer. Front Oncol. 9:15082019. View Article : Google Scholar : PubMed/NCBI
|