Introduction
Although advances have been achieved in medical
technology in recent years, esophageal cancer (EC) still has a poor
prognosis. The five-year survival rate of EC patients undergoing
esophagectomy is only 55.5% in Japan (1). EC is the 10th leading cause of all
cancer deaths in Japan (2). Patients
with EC are often diagnosed at an advanced stage with distant
metastasis, and, thus, the timing for surgical intervention may be
missed. Furthermore, although most patients are eligible for
surgery, they often cannot receive chemotherapy or radiotherapy.
Therefore, a safer and less invasive treatment is urgently
needed.
Photodynamic therapy (PDT) is a minimally invasive
therapeutic modality that is used in the management of various
cancers, including lung, early gastric, and skin cancers (3–6), as well
as pre-malignant diseases (7–10). PDT
involves the administration of a non-toxic photosensitizing drug
that accumulates in host and tumor cells, and this followed by the
illumination of the tumor site with visible light corresponding to
an appropriate photosensitizer absorption wavelength (11–13). PDT
with Photofrin®, Lazerphyrin® and an
Excimer-dye laser are the only options commonly available in Japan
for the treatment of early gastric cancer and superficial EC.
However, the adoption of PDT is decreasing due to the widespread
use of endoscopic submucosal dissection (ESD) (14). Furthermore, PDT using
Photofrin® and Lazerphyrin® need long shading
periods to avoid photosensitivity and, thus, have not been widely
adopted.
5-Aminolevulinic acid (ALA) is a second-generation
photosensitizer. Protoporphyrin IX (PpIX) is synthesized from 5-ALA
in mitochondria (15), and
accumulates in several malignant tumors following the
administration of ALA (16–18). Once PpIX has accumulated in cells, it
absorbs energy from light of an appropriate excitation wavelength
in its ground stage, generating excited-state PpIX, which then
transfers energy to oxygen. This, in turn, generates cytotoxic
reactive oxygen species (ROS), mainly singlet reactive oxygen,
which causes cell death (12,19).
5-ALA has the advantage of skin photosensitivity being avoided due
to its elimination within 24 h (20).
ALA therapy is used in dermatology for the treatment
of various superficial diseases, such as actinic keratosis, basal
cell carcinoma, and Bowen's disease, and good outcomes have been
reported (21–23). However, limited information is
currently available for gastrointestinal cancer. We previously
reported the effects of ALA-PDT on human colorectal cancer cells
and gastric cancer cells (24,25). The
aim of the present study was to investigate the effects of ALA-PDT
on EC cells. The efficacy of ALA-PDT in esophageal cancer cell
lines was examined at three wavelengths to identify the optimal
wavelength. ALA-PDT was performed for esophageal cancer using blue
LED, the effectiveness of which remains unclear. The suppressive
effects of ALA-PDT on lymph node metastasis were also investigated.
To the best of our knowledge, this is the first in vivo
study to examine lymph node metastasis of esophageal cancer
cells.
Materials and methods
Cancer cell lines and cultures
We used four types of human EC cell lines. TE5 was
purchased from the Cell Resource Center for the Biomedical Research
Cell Bank. KYSE70, KYSE150 and KYSE170 cells were purchased from
the National Institute of Biomedical Innovation, Health and
Nutrition. Cells were grown in RPMI medium with 10% fetal bovine
serum (FBS), 100 U/ml penicillin, and 100 µg/ml streptomycin at
37°C in a water-saturated atmosphere with 5% CO2/95%
air. TE5 cells were verified by a short tandem repeat analysis and
confirmed to be consistent with each other and with the original
cell source from the Cell Resource Center for the Biomedical
Research Cell Bank. KYSE70, KYSE150 and KYSE170 cells were verified
by a short tandem repeat analysis and confirmed to be consistent
with the original cell source from the National Institute of
Biomedical Innovation, Health and Nutrition.
Animals
Five-week-old female BALB/c nude mice weighing 16–18
g (18–20 g by the end of the study) were used in the present study.
They were housed in groups in plastic cages with stainless-steel
grid tops in an air-conditioned environment with a 12-h light/dark
cycle and were provided food and water ad libitum. Animal
experiments were conducted in accordance with the institutional
guidelines of the Kyoto Prefectural University of Medicine, Kyoto,
Japan. The present study was approved by the Ethics Committee of
Kyoto Prefectural University of Medicine. The permit number was
M28-578. Isoflurane (3%) was used for induction and 1.5% isoflurane
was used for maintenance of anesthesia in the process of ALA-PDT
and measuring small tumors of the mice. During our experiments, all
the mice were alive, and almost all of them showed wasting, but
they were able to eat and drink normally. All the mice were
anesthetized with 3% isoflurane gas inhalation and sacrificed by
cervical dislocation, then the tumors were removed.
Light sources for PDT
Cultured cell plates and inoculated mice were
exposed to three types of LED lights (peak wavelength blue, 410 nm;
green, 525 nm; red, 635 nm). Light intensity was measured using a
photo-radiometer.
Fluorescence microscopy analysis
Cells (TE2, TE5, KYSE70, KYSE150) were plated at a
density of 1×106 cells in glass-bottomed plates. After
an incubation for 24 h, cells were treated with 1 mM 5-ALA for 3 h,
and PpIX fluorescence (excitation, 440 nm; emission, 575–675 nm)
was examined under an inverted fluorescence microscope (IX81;
Olympus).
PDT in vitro
KYSE170 (5×104 cells/0.1 ml), KYSE70, and
KYSE150 (2.5×104 cells/0.1 ml) cells were seeded on
96-well plates and placed in an incubator at 37°C for 24 h. Medium
was then replaced with that containing 1 mM 5-ALA (Cosmo Bio
International) (26). After three
hours, 5-ALA-containing medium was replaced with PBS. Cells were
irradiated with the three types of LEDs at a light dosage of 3
J/cm2. PBS was replaced with fresh medium immediately
after irradiation. The control group was not administered 5-ALA or
exposed to LED irradiation. After 24 h, cell viability was measured
using the water-soluble tetrazolium (WST) assay (24,25).
Sample absorbance was read on a MAXline microplate reader equipped
with a 550-nm filter.
Apoptosis assay
KYSE70, KYSE150 and KYSE170 cells were incubated in
a 6-well plate and divided into two groups: a control group and
ALA-PDT group. After being cultured for 24 h, cells in the ALA-PDT
group were incubated with 1 mM ALA to the final concentration for 3
h, washed with PBS, transferred to fresh medium, and then
irradiated with a light dosage of 3 J/cm2 under blue
LED. After ALA-PDT, cells were further cultured for 12 h and then
washed twice with ice-cold PBS. Cells were resuspended in 100 µl of
binding buffer and stained with 1 µl of Annexin V-fluorescein
isothiocyanate (Annexin V-FITC) and 5 µl of propidium iodide (PI)
(Beckman Coulter) at 37°C for 15 min in the dark. Cell apoptosis
was analyzed by a flow cytometry analysis in a FACScan system (BD
Accuri C6; BD Biosciences) (26). At
least 30,000 events were collected for each sample.
PDT in vivo (submucosal tumor
model)
KYSE150 cells (1.0×106) were
subcutaneously inoculated in 50 µl of PBS into the left ankle of
nude mice under general anesthesia. Ten days later, the longest
diameter of the xenograft tumor was between 3 and 5 mm (24). Mice were then divided into treatment
and control groups. The treatment group was subdivided into the
blue-LED, green-LED and red-LED subgroups. The control group and
treatment groups comprised 4 or 5 mice each. Nude mice in each
treatment group received an intraperitoneal injection of 250 mg/kg
of 5-ALA. Four hours later, mice were irradiated with LED at 30
J/cm2. The three types of LEDs described above were used
in the present study. The control group was not administered ALA or
exposed to LED irradiation. ALA-PDT was repeated once a week for 4
weeks. Mice were sacrificed 4 weeks after the initial treatment
with isoflurane, and tumors were removed and weighed (27).
PDT in vivo (popliteal lymph node
(PLN) metastasis model)
KYSE150 cells (1.0×106) were inoculated
in 100 µl of PBS into the left footpad of nude mice. After 4 weeks,
mice were divided into treatment and control groups (28). The control group and treatment
subgroups comprised 8 or 9 mice each. Nude mice in each treatment
group received an intraperitoneal injection of 5 mg/body of 5-ALA.
After 4 h, the left footpads were irradiated with blue-LED light at
a measured rate of 30 J/cm2. The control group was not
administered ALA or exposed to LED irradiation. ALA-PDT was
repeated once a week for 4 weeks. Mice were sacrificed with
isoflurane 3 weeks after the initial treatment. PLNs were removed
and then evaluated by hematoxylin and eosin staining.
Statistical analysis
Differences in weight and size of tumors and cell
viability among the groups were analyzed using ANOVA by using
Kruskal-Wallis (non-parametric) H-test. A post hoc Tukey test was
subsequently performed. Differences in the number of PLN metastases
were analyzed using the Chi-squared test. P<0.05 was considered
to be significant.
Results
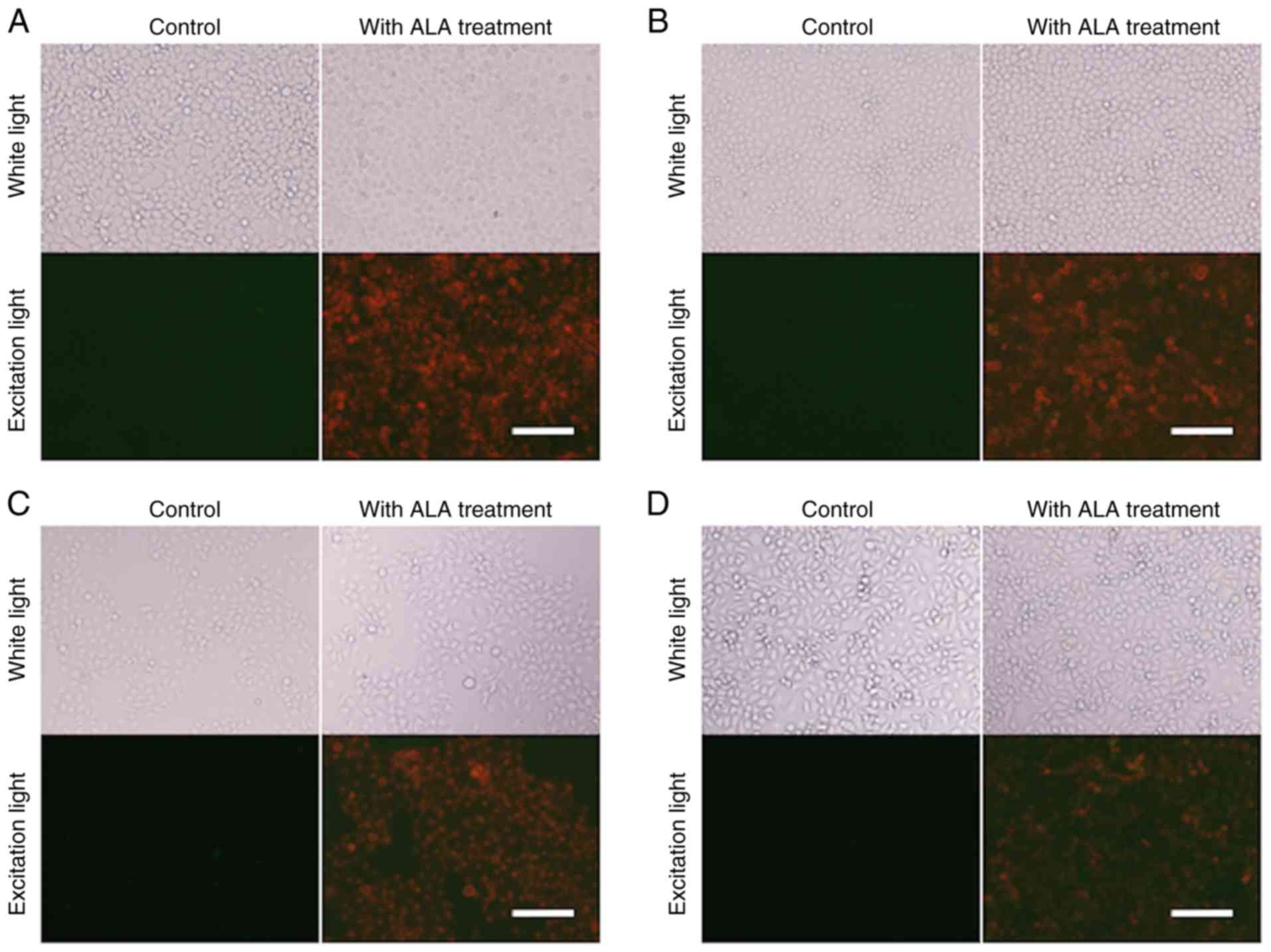
Fluorescence microscopy analysis
All fluorescence images were obtained under
identical cell conditions, including the photomultiplier voltage,
acquisition time, and excitation light intensity. Although there
were some variations, the red fluorescence of PpIX was observed in
all EC cell lines (TE2, TE5, KYSE70 and KYSE150) treated with 5-ALA
(Fig. 1).
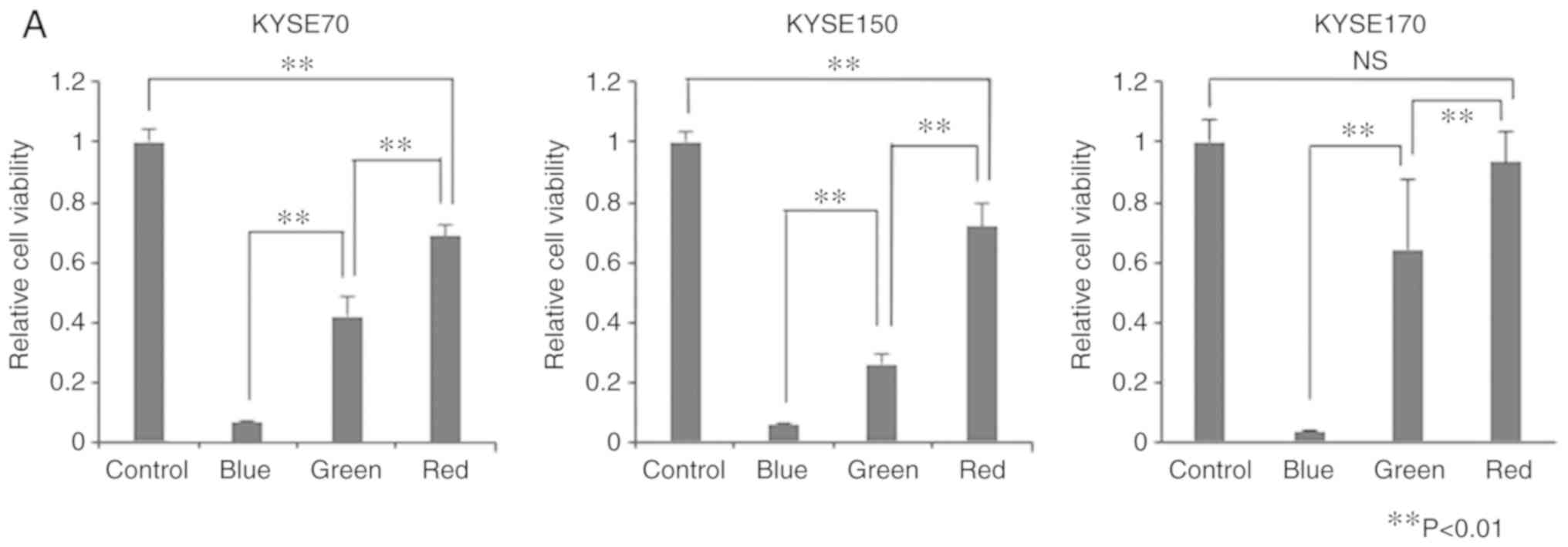
PDT in vitro
We used the three types of LEDs (blue, green, and
red) that are the most suitable for ALA-PDT in EC. Cell viability
was significantly lower in the treatment groups than in the control
group (Fig. 2A). Exceptionally,
there was no significant difference between the control group and
the red-LED group in cell viability of KYSE170. ALA-PDT using blue
LED exerted the strongest antitumor effects, followed by that using
green and red LEDs (P<0.01). The same trend was observed for all
cell lines investigated. The experiment involved the administration
of PDT three times, as previously reported (24). However, this protocol did not result
in any significant differences in the sizes of tumors. The
administration of PDT four times led to significant differences in
LED at each wavelength.
Apoptosis assay
KYSE150 cell apoptosis was assessed by the Annexin
V-FITC/PI binding assay followed by flow cytometry. The apoptosis
rate of cells 12 h after the administration of 1 mM ALA-PDT was
85.8%, which was significantly higher than that in the control
group (28.6%) (Fig. 2B). This result
demonstrated that apoptosis was induced by ALA-PDT. Similar results
were observed in KYSE70 and KYSE170. However, apoptotic effects
were the strongest in KYSE150, suggesting that the induction of
apoptosis varies in a cell line-dependent manner.
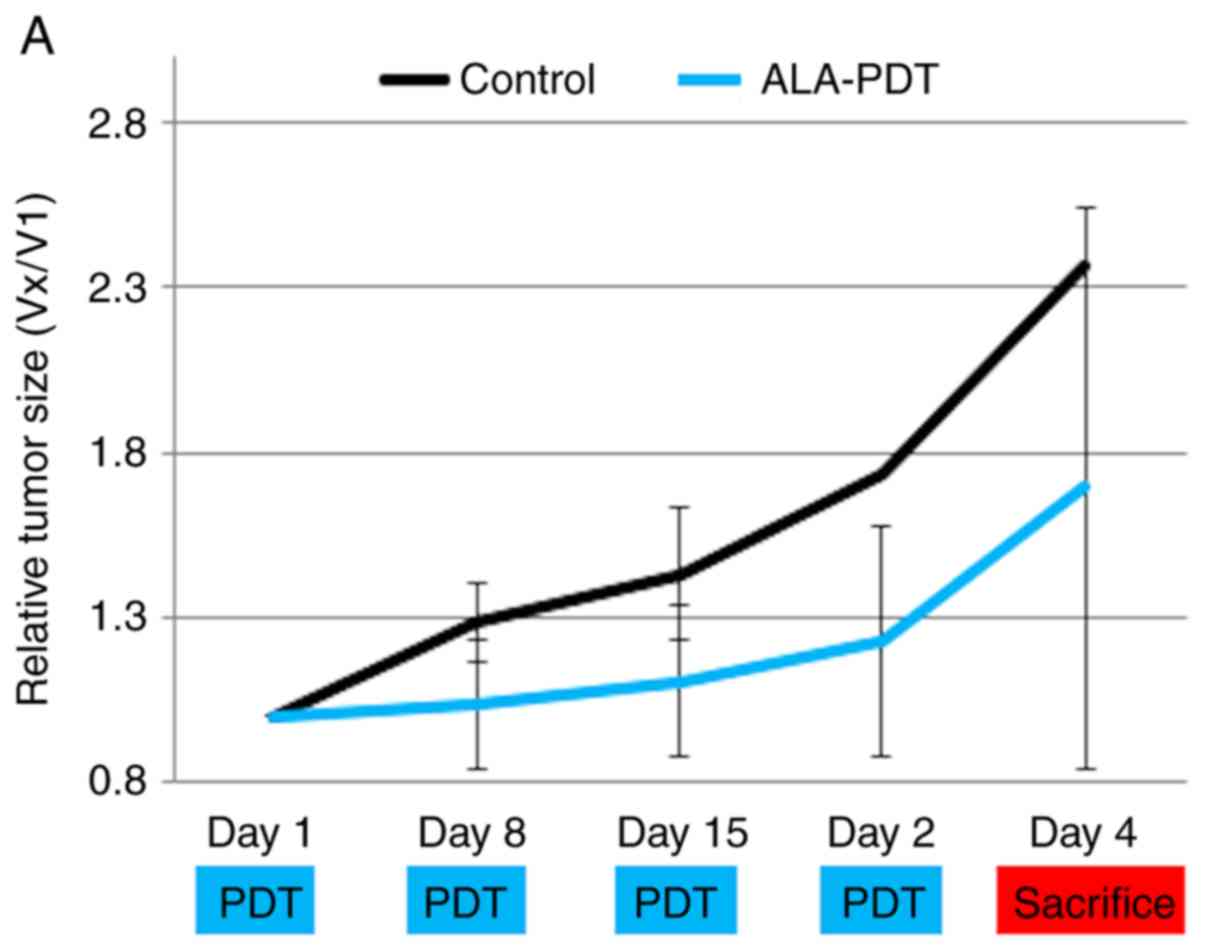
PDT in vivo (submucosal tumor mouse
model): We used three types of LEDs (Fig. 3A)
Tumor size and weights at day 29 were significantly
lower in the blue-LED groups than in the control group (Fig. 3B-D) (P<0.05). No significant
differences were observed in tumor weights and size between the
control group and red-LED group; however, they were slightly lower
in the red-LED group. Tumor weights were significantly lower in the
blue- and green-LED groups than in the red-LED group (P<0.05).
There was significant difference between green-LED and control
group in weight of tumors, but there was no difference in size of
tumors at day 29. As in vitro results, In vivo
results showed that the effects of PDT were the strongest in the
order of blue > green > red LED.
PDT in vivo (PLN model)
Metastatic PLNs were significantly smaller in the
ALA-PDT group than in the control group (P<0.05) (Fig. 4A and B). PLNs were removed 3 weeks
after the initial treatment (Fig.
4C). In the control group, the formation of metastatic lesions
was observed in 3 out of 8 mice (Fig.
4D). Otherwise, no mice had metastatic PLNs in the ALA-PDT
group. The number of metastatic PLNs was high in the control group
(Fig. 4E).
Discussion
PDT using Photofrin® and an Excimer-dye
laser is not widely adopted because it has a long shading time of 6
weeks and a high frequency of hypersensitivity (29). Laserphyrin®, which was
listed in pharmaceutical affairs in 2015, also has a long shading
time of 2 weeks (30). ALA is
superior to other photosensitizers because of its rapid metabolism
and high selectivity for malignant lesions. The systemic clearance
of ALA-induced PpIX within 24 h prevents prolonged photosensitivity
and allows the treatment to be repeated at regular intervals (as
frequently as every 48 h) without cumulative effects or the risk of
damage to normal tissues (20). In
the present study, the red fluorescence of PpIX was clearly
observed in EC cells in vitro, but not in normal cells. The
present results were consistent with previous findings, which
showed high selectivity for malignant cells for the accumulation of
PpIX with human hepatic cell cancer and colon cancer (31,32).
High cancer selectivity may contribute to reducing post-treatment
complications.
ALA-PDT is based on ROS being produced following
exposure to light and inducing apoptosis in tumor cells (33). With a mitochondrion-associated
photosensitizer, photodamage to membrane-bound Bcl-227-29 may be a
permissive signal for mitochondrial outer membrane permeabilization
and the subsequent release of caspase activators, such as
cytochrome c. Apoptotic cells promptly release signals
required for the clearance of remaining corpses by phagocytic
cells, which may minimize damage to normal cells and tissues
(34). In the present study, we
confirmed that apoptosis was mainly caused by ALA-PDT for human EC
cells.
In the present study, we found that the efficiency
of ALA-PDT using blue LED was higher than that with red LED. The
results obtained are consistent with our previous findings, which
showed the efficacy of ALA-PDT using blue LED for human gastric
cancer and colon cancer (24,25). Red
light is generally used in ALA-PDT (35). The longer the wavelength, the greater
the tissue penetration. If the antitumor effects are the same,
light with a longer wavelength will be more advantageous for PDT.
However, when the wavelength is short, the intensity of light
generally becomes stronger. Moreover, absorption by PpIX at 410 nm
is ~30-fold greater than that at 635 nm. We previously reported
that ROS generation was significantly higher in PDT-treated cells
with blue LED than with red LED using human gastric cancer cells
(25). ROS generated during PDT are
responsible for the cytotoxicity of cancer cells. These results
suggest that ALA-PDT using blue LED is superior to conventional red
LED for the treatment of EC cells. Since the targeted nodules in
the present study were only ~3–5 mm in diameter, tissue penetration
by light may not have been as critical. The effects of ALA-PDT may
be weakened by the depth of tumors. The depth of the esophageal
wall is ~4 mm, while that of early stage EC lesions to the
submucosal layer is ~2–3 mm. In the case of PDT for early stage EC,
blue LED may be more effective than red LED.
ESD for early EC has recently become more widely
adopted because it is a minimally invasive and curative treatment.
However, esophageal stenosis sometimes occurs when ESD is performed
on lesions of more than 3/4 circumference (36,37). In
that case, ESD is not recommended. When we perform ESD, we excise
the mucosa to the muscle layer and make a deformation in the
esophageal wall. ALA-PDT acts at the mucosa, while not affecting
the deeper muscle layers. It may be possible to lower the frequency
of stenosis even with circumferential lesions by ALA-PDT. Dunn
et al reported the frequency of stenosis with ALA-PDT
against high-grade dysplasia arising in Barrett's esophagus, which
is a precancerous lesion of EC. ALA-PDT has a more acceptable
safety profile than Photofrin-PDT, with a significantly lower
incidence of stricture (5.8 vs. 43%, P<0.01) (38).
In patients diagnosed with EC, one of the important
prognostic indicators for survival after the primary treatment is
metastasis to the regional or distal lymph nodes. In the present
study, ALA-PDT using blue LED resulted in the partial remission of
EC and prevented the occurrence of regional lymph node metastasis.
In vivo experiments using PLNs models, tumors in the
footpads were only slightly larger in the group with than in that
without PLN metastases. The prevention of regional metastasis was
confirmed by a histological analysis. None of the mice in the
ALA-PDT group had positive nodes, in contrast to 3/8 mice in the
control group. The mechanism underlying this additional PDT effect
currently remains unclear, but may be due to a direct effect on
lymphatic vessels in the illuminated area. Tammela et al
previously reported that PDT-treated skin melanoma model mice did
not have metastases due to the selective destruction of draining
lymphatic vessels (39). We
evaluated the efficacy of PDT based on the sizes of the primary
lesions (footpads) and incidence of lymph node metastasis. However,
the underlying mechanisms were not elucidated. This is a limitation
of the present study. Although it was not possible to completely
remove the tumor, ALA-PDT showed the potential to inhibit tumor
growth and lymph node metastasis similar to ESD. Since ALA-PDT may
be performed in a shorter time than ESD, it represents a better
alternative for elderly patients.
In summary, for superficial early stage EC with 3/4
circumference or more, ALA-PDT may be an alternative treatment to
other PDTs and ESD, particularly in elderly patients.
In conclusion, ALA-PDT using LEDs induced tumor cell
death in the EC cell line in vitro and in vivo and
prevented regional lymph node metastasis. ALA-PDT using blue LED
exerted stronger effects than conventional red light for small and
shallow nodules. The prevention of regional metastasis observed in
the present study is of fundamental importance for reducing the
negative impact on the quality of life of EC patients and improving
overall survival. The present results provide insights into a novel
treatment modality for EC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YM designed the current study. YT contributed to
data collection and analysis, manuscript writing and editing. TM
and HK were involved in project development, data analysis and
revision of the manuscript. KH advised on the use of 5-ALA within
the study. HM performed the experiments and analyzed the data. TK,
KO and EO analyzed and interpreted the data. All authors read and
approved the final manuscript and agree to be accountable for all
aspects of the study in ensuring that question related to accuracy
or integrity of any part of the work are appropriately investigated
and resolved.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Kyoto Prefectural University of Medicine. Animal
experimentation within this study was approved by the Institutional
Animal Care and Use Committee and performed according to the Animal
Experimentation Regulation of Kyoto Prefectural University of
Medicine.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tachimori Y, Ozawa S, Numasaki H, Ishihara
R, Musubara H, Muro K, Oyama T, Toh Y, Udagawa H9 and Uno T;
Registration Committee for Esophageal Cancer of the Japan
Esophageal Society, : Comprehensive Registry of Esophageal Cancer
in Japan, 2010. Eshophagus. 14:189–214. 2017. View Article : Google Scholar
|
|
2
|
Cancer Registry and Statistics, . Cancer
Information Service, National Cancer Center. Japan: October
12–2018
|
|
3
|
Dougherty TJ, Kaufman JE, Goldfarb A,
Weishaupt KR, Boyle D and Mittleman A: Photoradiation therapy for
the treatment of malignant tumors. Cancer Res. 38:2628–2635.
1978.PubMed/NCBI
|
|
4
|
Hayata Y, Kato H, Konaka C, Ono J and
Takizawa N: Hematoporphyrin derivative and laser photoradiation in
the treatment of lung cancer. Chest. 81:269–277. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mimura S, Ichii M and Okuda S:
Photodynamic Therapy for Early Gastric Cancer Using Excimer Dye
Laser. Elsevier Science Publication. (Amsterdam). 272–276.
1992.
|
|
6
|
Lu YG, Wang YY, Yang YD, Zhang XC, Gao Y,
Yang Y, Zhang JB and Li GL: Efficacy of topical ALA-PDT combined
with excision in the treatment of skin malignant tumor. Photodiagn
Photodyn Ther. 11:122–126. 2014. View Article : Google Scholar
|
|
7
|
Mlkvy P, Messmann H, Debinski H, Regula J,
Conio M, MacRobert A, Spigelman A, Phillips R and Bown SG:
Photodynamic therapy for polyps in familial adenomatous polyposis -
a pilot study. Eur J Cancer. 31A:1160–1165. 1995.PubMed/NCBI
|
|
8
|
Kübler A, Haase T, Rheinwald M, Barth T
and Mühling J: Treatment of oral leukoplakia by topical application
of 5-aminolevulinic acid. Int J Oral Maxillofac Surg. 27:466–469.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Loh CS, Bliss P, Bown SG and Krasner N:
Photodynamic therapy for villous adenomas of the colon and rectum.
Endoscopy. 26:243–246. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Smolka J, Mateasik A, Cunderlikova B,
Sanislo L and Mlkvy P: In vivo fluorescence diagnostics and
photodynamic therapy of gastrointestinal superficial polyps with
aminolevulinic acid. A clinical and spectroscopic study. Neoplasma.
53:418–423. 2006.PubMed/NCBI
|
|
11
|
Gomer CJ, Rucker N, Ferrario A and Wong S:
Properties and applications of photodynamic therapy. Radiat Res.
120:1–18. 1989. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dougherty TJ, Gomer CJ, Henderson BW, Jori
G, Kessel D, Korbelik M, Moan J and Peng Q: Photodynamic therapy. J
Natl Cancer Inst. 90:889–905. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dolmans DE, Fukumura D and Jain RK:
Photodynamic therapy for cancer. Nat Rev Cancer. 3:380–387. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yano T, Muto M, Minashi K, Onozawa M,
Nihei K, Ishikura S, Kaneko K and Ohtsu A: Long-term results of
salvage photodynamic therapy for patients with local failure after
chemoradiotherapy for esophageal squamous cell carcinoma.
Endoscopy. 43:657–663. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tsai JC, Wu CL, Chien HF and Chen CT:
Reorganization of cytoskeleton induced by 5-aminolevulinic
acid-mediated photodynamic therapy and its correlation with
mitochondrial dysfunction. Lasers Surg Med. 36:398–408. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Malik Z and Lugaci H: Destruction of
erythroleukaemic cells by photoactivation of endogenous porphyrins.
Br J Cancer. 56:589–595. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kennedy JC, Pottier RH and Pross DC:
Photodynamic therapy with endogenous protoporphyrin IX: Basic
principles and present clinical experience. J Photochem Photobiol
B. 6:143–148. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Peng Q, Moan J, Warloe T, Nesland JM and
Rimington C: Distribution and photosensitizing efficiency of
porphyrins induced by application of exogenous 5-aminolevulinic
acid in mice bearing mammary carcinoma. Int J Cancer. 52:433–443.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Almeida RD, Manadas BJ, Carvalho AP and
Duarte CB: Intracellular signaling mechanisms in photodynamic
therapy. Biochim Biophys Acta. 1704:59–86. 2004.PubMed/NCBI
|
|
20
|
Wachowska M, Muchowicz A, Firczuk M,
Gabrysiak M, Winiarska M, Wańczyk M, Bojarczuk K and Golab J:
Aminolevulinic acid (ALA) as a prodrug in photodynamic therapy of
cancer. Molecules. 16:4140–4164. 2011. View Article : Google Scholar
|
|
21
|
Vegter S and Tolley K: A network
meta-analysis of the relative efficacy of treatments for actinic
keratosis of the face or scalp in Europe. PLoS One. 9:e968292014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fargnoli MC and Peris K: Photodynamic
therapy for basal cell carcinoma. Future Oncol. 11:2991–2996. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wong TW, Sheu HM, Lee JY and Fletcher RJ:
Photodynamic therapy for Bowen's disease (squamous cell carcinoma
in situ) of the digit. Dermatol Surg. 27:452–456. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hatakeyama T, Murayama Y, Komatsu S,
Shiozaki A, Kuriu Y, Ikoma H, Nakanishi M, Ichikawa D, Fujiwara H,
Okamoto K, et al: Efficacy of 5-aminolevulinic acid-mediated
photodynamic therapy using light-emitting diodes in human colon
cancer cells. Oncol Rep. 29:911–916. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hino H, Murayama Y, Nakanishi M, Inoue K,
Nakajima M and Otsuji E: 5-Aminolevulinic acid-mediated
photodynamic therapy using light-emitting diodes of different
wavelengths in a mouse model of peritoneally disseminated gastric
cancer. J Surg Res. 185:119–126. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen X, Zhao P, Chen F, Li L and Luo R:
Effect and mechanism of 5-aminolevulinic acid-mediated photodynamic
therapy in esophageal cancer. Lasers Med Sci. 26:69–78. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wakui M, Yokoyama Y, Wang H, Shigeto T,
Futagami M and Mizunuma H: Efficacy of a methyl ester of
5-aminolevulinic acid in photodynamic therapy for ovarian cancers.
J Cancer Res Clin Oncol. 136:1143–1150. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ito T, Shimada Y, Kan T, David S, Cheng Y,
Mori Y, Agarwal R, Paun B, Jin Z, Olaru A, et al: Pituitary
tumor-transforming 1 increases cell motility and promotes lymph
node metastasis in esophageal squamous cell carcinoma. Cancer Res.
68:3214–3224. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mimura S, Ito Y, Nagayo T, Ichii M, Kato
H, Sakai H, Goto K, Noguchi Y, Tanimura H, Nagai Y, et al:
Cooperative clinical trial of photodynamic therapy with photofrin
II and excimer dye laser for early gastric cancer. Lasers Surg Med.
19:168–172. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kato H, Furukawa K, Sato M, Okunaka T,
Kusunoki Y, Kawahara M, Fukuoka M, Miyazawa T, Yana T, Matsui K, et
al: Phase II clinical study of photodynamic therapy using
mono-L-aspartyl chlorin e6 and diode laser for early superficial
squamous cell carcinoma of the lung. Lung Cancer. 42:103–111. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Murayama Y, Harada Y, Imaizumi K, Dai P,
Nakano K, Okamoto K, Otsuji E and Takamatsu T: Precise detection of
lymph node metastases in mouse rectal cancer by using
5-aminolevulinic acid. Int J Cancer. 125:2256–2263. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nishimura M, Murayama Y, Harada K, Kamada
Y, Morimura R, Ikoma H, Ichikawa D, Fujiwara H, Okamoto K and
Otsuji E: Photodynamic diagnosis of hepatocellular carcinoma using
5-aminolevulinic acid. Anticancer Res. 36:4569–4574. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ishizuka M, Abe F, Sano Y, Takahashi K,
Inoue K, Nakajima M, Kohda T, Komatsu N, Ogura S and Tanaka T:
Novel development of 5-aminolevurinic acid (ALA) in cancer
diagnoses and therapy. Int Immunopharmacol. 11:358–365. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Qiao L, Mei Z, Yang Z, Li X, Cai H and Liu
W: ALA-PDT inhibits proliferation and promotes apoptosis of SCC
cells through STAT3 signal pathway. Photodiagn Photodyn Ther.
14:66–73. 2016. View Article : Google Scholar
|
|
35
|
Peng Q, Warloe T, Berg K, Moan J,
Kongshaug M, Giercksky KE and Nesland JM: 5-Aminolevulinic
acid-based photodynamic therapy. Clinical research and future
challenges. Cancer. 79:2282–2308. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Araki K, Ohno S, Egashira A, Saeki H,
Kawaguchi H and Sugimachi K: Pathologic features of superficial
esophageal squamous cell carcinoma with lymph node and distal
metastasis. Cancer. 94:570–575. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mizuta H, Nishimori I, Kuratani Y,
Higashidani Y, Kohsaki T and Onishi S: Predictive factors for
esophageal stenosis after endoscopic submucosal dissection for
superficial esophageal cancer. Dis Esophagus. 22:626–631. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Dunn JM, Mackenzie GD, Banks MR, Mosse CA,
Haidry R, Green S, Thorpe S, Rodriguez-Justo M, Winstanley A,
Novelli MR, et al: A randomised controlled trial of ALA vs.
Photofrin photodynamic therapy for high-grade dysplasia arising in
Barrett's oesophagus. Lasers Med Sci. 28:707–715. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tammela T, Saaristo A, Holopainen T,
Ylä-Herttuala S, Andersson LC, Virolainen S, Immonen I and Alitalo
K: Photodynamic ablation of lymphatic vessels and intralymphatic
cancer cells prevents metastasis. Sci Transl Med. 3:69ra112011.
View Article : Google Scholar : PubMed/NCBI
|