Introduction
Hepatocellular carcinoma (HCC) is the fifth most
common malignancy, accounting for 600,000-700,000 deaths worldwide
in 2014, and its incidence is increasing in Western countries
(1,2). HCC often invades the portal vein and
hepatic vein or forms a venous tumour thrombus in the inferior vena
cava. However, HCC with bile duct tumour thrombus (BDTT) is
relatively rare and has been identified in 0.99-13% of autopsy and
surgical specimens (2–6). Once BDTT in HCC is considered as
advanced liver cancer, palliative treatment is often performed due
to a misunderstanding of the cause of the obstructive jaundice,
cholestasis and hepatic dysfunction (7–9). The
complexity of the disease causes considerable challenges for
clinical diagnosis and treatment. In recent years, patients with
BDTT have more often been treated with surgery in a number of
medical centres due to advances in diagnosis, surgical techniques
and perioperative management (4,10), and
the outcomes of surgical treatment have been widely accepted to be
significantly improved compared with those of palliative treatment
(11,12). However, due to the rarity of the
disease, the clinicopathological characteristics are not well
recognized and a limited number of studies are available,
especially studies regarding long-term postoperative outcomes when
compared with those of patients without BDTT; the possibility of
selection bias therefore remains. Furthermore, whether extrahepatic
bile duct resection is necessary remains controversial.
The present study aimed to evaluate the
clinicopathological features and long-term oncological outcomes of
patients with HCC and macroscopic BDTT. To overcome selection bias,
propensity score matching (PSM) was applied and prognostic factors
after liver resection were analysed.
Patients and methods
Patients
Between January 2013 and November 2018, 773 patients
with HCC underwent hepatectomy at the Department of
Hepatopancreatobiliary Surgery, The First Affiliated Hospital of
Fujian Medical University (Fuzhou, China). Patients who had
previously received locoregional therapy, such as hepatectomy
(n=63), transarterial chemoembolization (TACE; n=11),
radiofrequency ablation (RFA; n=4), Gamma Knife®
treatment (n=1) or TACE combined with RFA (n=4), were excluded.
Patients were also excluded if the tumour had been found to rupture
(n=25), invade peripheral organs or metastasize (n=2) during the
surgery. Patients whose data were incomplete or those who were lost
to follow-up (n=32) were also excluded from this study. Ultimately,
a total of 631 patients (age range, 20–85 years) were included in
the current study, 25 (3.96%) of whom were allocated to the HCC
with macroscopic BDTT group, and 606 (96.04%) of whom were
allocated to the group without BDTT. The clinicopathological
characteristics and survival rates of the two groups were compared.
To decrease possible confounding and selection biases of the
baseline characteristics in the two groups, 1:1 matching variables
[age, sex, body mass index, presence of comorbidity, α-fetoprotein
(AFP), hepatitis B virus, hepatitis C virus, indocyanine green
retention rate at 15 min, Child-Pugh classification (13), maximum tumour size, number of
tumours, cirrhosis, macrovascular invasion, microvascular invasion,
tumour differentiation, American Joint Committee on Cancer (AJCC)
stage (14) and resection margins]
were applied using PSM. As a result, 50 patients were divided into
two groups: The BDTT group (n=25) and the group without BDTT
(n=25). The clinicopathological characteristics and survival rates
of the two groups were compared once more after PSM and the
prognostic risk factors of HCC patients who underwent liver
resection were assessed by the Cox proportional hazards model after
excluding other influencing factors. In addition, the curative
effects on BDTT in patients with HCC who underwent tumour
thrombectomy and bile duct resection were compared. The current
study was approved by the Institutional Review Board of The First
Affiliated Hospital of Fujian Medical University and performed in
accordance with the ethical guidelines of the institute.
Preoperative evaluation and
management
Primary tumours were evaluated by contrast-enhanced
thin-slice computed tomography (CT) or magnetic resonance imaging
(MRI) and MR cholangiopancreatography. Liver function and whether
another comorbidity was present were also assessed. Liver function
was evaluated using the Child-Pugh classification system. An
attempt was made to convert Child-Pugh class B disease into class A
disease via appropriate preoperative treatment. To improve liver
function and treat cholangitis, it was necessary to perform biliary
drainage by percutaneous transhepatic biliary drainage (PTBD) or to
use an endoscopic retrograde cholangiopancreatography in certain
patients. The serum total bilirubin (TBIL) level of patients with
obstructive jaundice after appropriate preoperative management
needed to decrease to <2.0 mg/dl or by >50% before major
hepatectomy was performed (normal range, 0.1-1.0 mg/dl).
Surgical strategy
The surgical strategy was determined preoperatively
at the department specialist meeting (similar to a
multidisciplinary team conference). Sometimes, adjustments were
necessary during surgery to select a reasonable procedure based on
intraoperative exploration. The basic policy for the surgical
treatment of HCC in The First Affiliated Hospital of Fujian Medical
University is that if the remaining liver function is sufficient,
there is an inclination to perform an anatomical resection;
non-anatomical resection is recommended only when the tumour is
small, the position is superficial or the remaining liver function
is insufficient. Anatomical resection involved resection of the
tumour with its related portal vein branches and corresponding
hepatic region. The surgical treatment strategy was complete
resection of the primary HCC and BDTT, trying not to remove the
extrahepatic bile duct, similar to the ‘peeling off technique’
(10). The extrahepatic bile duct
was resected only when the BDTT could not be completely peeled off
or would have violated the bile duct wall.
Pathological evaluation
After the specimens were stored and fixed in 10%
formalin at room temperature for 12 h, they were embedded in
paraffin and cut into 5-µm-thick sections. Hematoxylin and eosin
staining (performed for 10 min at room temperature) and a light
microscope (Nikon Corporation; magnification, ×100, ×200 and ×400;
analyzed using QImaging MicroPublisher 3.3 with Real-Time Viewing;
Teledyne QImaging) were used. The numbers, sizes (maximum tumour
diameter) and locations of the tumours were recorded during a
macroscopic examination of the resected specimens by a pathologist.
Moreover, the microscopic examination included determination of the
histological differentiation, invasion of the bile duct (the size
and location of BDTT), microvasculature and macrovasculature, the
specimen margin and the presence of cirrhosis. The histological
differentiation of HCC was assigned according to the
Edmondson-Steiner system (15).
Tumour stage was classified in accordance with the criteria of the
AJCC (8th edition). HCC with macroscopic BDTT represents a tumour
thrombus found in the common hepatic duct or the first to second
branches of the intrahepatic bile duct. Classification of HCC with
macroscopic BDTT was in accordance with the criteria of the Ueda
classification (3).
Follow-up
Specially trained researchers used outpatient
records and telephone calls to follow-up with the patients. After
discharge from the hospital, AFP tests, ultrasound (US) and
contrast-enhanced CT or MRI were performed at least every 3 months
during the follow-up period. If recurrence or metastasis was
observed, follow-up treatments, such as TACE, RFA, microwave
ablation, reoperation and targeted drug administration, such as
Sorafenib (400 mg bid), Rivatinib (8–12 mg qd) or Regorafenib (160
mg qd), were performed, as determined by the patient's condition.
Survival time was calculated from the date of surgery to the date
of last contact, death or collection of survival information. The
follow-up deadline was May 2019.
Statistical analysis
PSM was performed with a multiple factor logistic
regression model, and a calliper of 0.10 of the standard deviation
of the logit was imposed. Continuous variables are reported as the
mean ± standard deviation, and categorical variables are reported
as the count and percentage. Continuous variables were compared
using Student's t-test or the Mann-Whitney U test as appropriate.
Categorical variables were compared using the χ2 test or
Fisher's exact test if necessary. Patient survival rates were
analysed by the Kaplan-Meier method, and survival curves were
compared between groups using the log-rank test. Patients who
succumbed within 30 days of surgery were excluded from the survival
analysis. Moreover, patients who underwent non-curative surgical
resection were excluded from the recurrence analysis. Univariate
and multivariate analyses were performed using Cox proportional
hazards regression analyses. The variables found to have prognostic
significance in the univariate analysis were entered into the Cox
multivariate proportional hazards regression analysis to identify
factors that independently predicted the HCC prognosis. P<0.05
was considered to indicate a statistically significant difference.
SPSS statistics software (version 24.0; IBM Corp.) was used for all
statistical analyses.
Results
Perioperative outcomes of HCC patients
with BDTT
The clinical features of the 25 HCC patients with
macroscopic BDTT are shown in Table
I. The common bile duct (CBD) was the most frequent location of
BDTT in this study (15 patients; 60%), followed by the left hepatic
duct (5 patients; 20%), the right hepatic duct (3 patients; 12%)
and the second-order branch of the intrahepatic bile duct (2
patients; 8%). According to the Ueda classification, BDTT was
classified as type 1 in 2 patients (8%), type 2 in 8 patients (32%)
and type 3 in 15 patients (60%). No type 4 cases were included in
this study. Among these patients, 11 (44%) were diagnosed with
obstructive jaundice on admission. A subsequent biliary drainage
procedure was performed using PTBD guided by US in 8 patients
(32%), and 2 patients (8%) underwent PTBD of the right hepatic duct
and then PTBD of the left hepatic duct again on postoperative days
19 and 27. Another 3 patients (12%) did not have biliary drainage
as their TBIL increased in the short term and did not increase
markedly before surgery. The median duration from biliary drainage
to surgical treatment was 23 days (range, 13–42 days). A total of
17 patients (68%) had TBIL levels <2.0 mg/dl at the time of
surgery, and TBIL levels decreased by 14.3±6.4 mg/dl at the time of
preoperative biliary drainage. However, preoperative TBIL in the
BDTT group was significantly higher than that in the without BDTT
group (1.98±1.72 vs. 1.00±0.62 mg/dl; P=0.010; data not shown).
 | Table I.Clinical features and operative
procedures of patients with macroscopic BDTT. |
Table I.
Clinical features and operative
procedures of patients with macroscopic BDTT.
| Patient no. | Sex | Age, years | Location of tip of
BDTT | Ueda
typea | Biliary
decompression | Operative
procedure | Bile duct
resection | Tumour
thrombectomy |
| 1 | M | 54 | Left hepatic
duct | 2 | No | Left
hepatectomy | No | No |
| 2 | M | 50 | CBD | 3 | No | Right
hepatectomy | No | Yes |
| 3 | M | 67 | CBD | 3 | Yes | Expand right
hepatectomy | Yes | No |
| 4 | M | 60 | CBD | 3 | Yes | Right
hepatectomy | No | Yes |
| 5 | F | 61 | Right hepatic
duct | 2 | No | Right
hepatectomy | No | No |
| 6 | M | 55 | CBD | 3 | Yes | Central
hepatectomy | No | Yes |
| 7 | F | 64 | CBD | 3 | No | Right
hepatectomy | No | Yes |
| 8 | M | 65 | CBD | 3 | Yes | Right
hepatectomy | No | Yes |
| 9 | M | 39 | CBD | 3 | No | Right
hepatectomy | Yes | No |
| 10 | M | 65 | Right hepatic
duct | 2 | No | Right
hepatectomy | No | No |
| 11 | M | 68 | CBD | 3 | No | Right
hepatectomy | No | Yes |
| 12 | F | 49 | Left hepatic
duct | 2 | No | Left
hepatectomy | No | No |
| 13 | M | 41 | CBD | 3 | Yes | Right
hepatectomy | Yes | No |
| 14 | M | 61 | CBD | 3 | Yes | Central
hepatectomy | No | Yes |
| 15 | M | 64 | CBD | 3 | No | Left
hepatectomy | No | Yes |
| 16 | M | 59 | CBD | 3 | No | Left
hepatectomy | No | Yes |
| 17 | M | 62 | CBD | 3 | Yes | Left
hepatectomy | No | Yes |
| 18 | F | 44 | The second
branch | 1 | No | Right posterior
section | No | No |
| 19 | F | 70 | The second
branch | 1 | No | Right posterior
section | No | No |
| 20 | M | 58 | CBD | 3 | No | Central
hepatectomy | No | Yes |
| 21 | M | 62 | Right hepatic
duct | 2 | No | Right
hepatectomy | No | No |
| 22 | M | 55 | Left hepatic
duct | 2 | No | Left
hepatectomy | No | No |
| 23 | F | 63 | Left hepatic
duct | 2 | No | Left
hepatectomy | No | No |
| 24 | M | 47 | Left hepatic
duct | 2 | No | Left
hepatectomy | No | No |
| 25 | M | 44 | CBD | 3 | Yes | Left
hepatectomy | Yes | No |
The operative procedures used in the BDTT group are
shown in Table I. The primary
tumours were removed by right hemihepatectomy in 11 patients (44%),
including an extended right hemihepatectomy in 1 patient (4%), by
left hemihepatectomy in 9 patients (36%), by central hepatectomy in
3 patients (12%) and by right posterior sectionectomy in 2 patients
(8%). Caudate lobectomy was performed in 8 patients (32%). The BDTT
was removed by thrombectomy either through choledochotomy or the
cut end of the bile duct in 11 patients (44%), and extrahepatic
bile duct resection was performed in 4 patients (16%). In the
remaining 10 patients (40%), BDTTs were resectioned en bloc
together with the primary tumour. Compared with those in the group
without BDTT, the average operative time and intraoperative blood
loss in the BDTT group were significantly greater (249.67±80.54 vs.
166.75±55.73 min, P<0.001; and 966.67±917.16 vs. 352.86±335.72
ml, P=0.012, respectively). The perioperative mortality rate was
not significantly different between the two groups (4.0 vs. 1.16%;
P=0.287) (data not shown).
Clinicopathological characteristics
before and after PSM
The clinicopathological characteristics of the two
groups before and after PSM are shown in Table II. The proportion of females and AFP
levels in the BDTT group seemed to be higher, but these results did
not reach statistical significance. Fewer patients had a Child-Pugh
A classification in the BDTT group than in the group without BDTT
just before surgery (P=0.002), which corresponds to the
aforementioned result that the preoperative TBIL level in the BDTT
group was significantly higher than that in the group without BDTT.
The numbers of tumours were not significantly different between the
two groups, while the tumour diameter in the BDTT group was
significantly larger than that in the group without BDTT (P=0.019).
In the BDTT group, 5 patients (20%) had macrovascular invasion and
15 patients (60%) had microvascular invasion, with both values
being significantly higher than those in the group without BDTT
(P=0.002 and P<0.001, respectively). The Edmondson-Steiner grade
and AJCC stage in the BDTT group were also significantly higher
than those in the group without BDTT (P=0.003 and P=0.001,
respectively). However, the presence of cirrhosis was significantly
lower in the BDTT group than in the group without BDTT
(P=0.001).
 | Table II.Clinicopathological characteristics
before and after propensity score matching. |
Table II.
Clinicopathological characteristics
before and after propensity score matching.
|
| All patients | Propensity-matched
patients |
|---|
|
|
|
|
|---|
| Variables | With BDTT
(n=25) | Without BDTT
(n=606) | P-value | With BDTT
(n=25) | Without BDTT
(n=25) | P-value |
|---|
| Age, years | 57.08±8.98 | 55±11.65 | 0.290 | 57.08±8.98 | 55.24±9.34 | 0.479 |
| Sex, n |
|
| 0.164 |
|
| 0.269a |
|
Male | 19 | 521 |
| 19 | 22 |
|
|
Female | 6 | 85 |
| 6 | 3 |
|
| BMI,
kg/m2 | 22.77±3.29 | 23.06±9.58 | 0.680 | 22.77±3.29 | 22.67±3.55 | 0.920 |
| Presence of
comorbidity, n |
|
| 0.694 |
|
| 0.771 |
|
Yes | 10 | 219 |
| 10 | 9 |
|
| No | 15 | 387 |
| 15 | 16 |
|
| AFP, n |
|
| 0.171 |
|
| 0.777 |
| ≤400
µg/l | 14 | 188 |
| 14 | 13 |
|
| >400
µg/l | 11 | 418 |
| 11 | 12 |
|
| HBV, n |
|
| 0.212 |
|
| 0.480a |
|
Positive | 19 | 516 |
| 19 | 21 |
|
|
Negative | 6 | 90 |
| 6 | 4 |
|
| HCV, n |
|
| 1.000b |
|
| 1.000b |
|
Positive | 0 | 17 |
| 0 | 0 |
|
|
Negative | 25 | 589 |
| 25 | 25 |
|
| ICGR15, % | 7.53±4.36 | 6.96±3.87 | 0.396 | 7.53±4.36 | 7.25±3.66 | 0.810 |
| Child-Pugh
classificationc, n |
|
| 0.002a |
|
| 0.221a |
| A | 20 | 586 |
| 20 | 23 |
|
| B | 5 | 20 |
| 5 | 2 |
|
| Maximum tumour
size, cm | 6.97±3.45 | 5.15±3.58 | 0.019 | 6.97±3.45 | 6.51±5.12 | 0.714 |
| Number of tumours,
n |
|
| 0.461a |
|
| 1.000a |
|
Solitary | 21 | 538 |
| 21 | 21 |
|
|
Multiple | 4 | 68 |
| 4 | 4 |
|
| Cirrhosis, n |
|
| 0.001 |
|
| 0.145 |
|
Yes | 7 | 366 |
| 7 | 12 |
|
| No | 18 | 240 |
| 18 | 13 |
|
| Macrovascular
invasion, n |
|
| 0.002a |
|
| 0.440a |
|
Yes | 5 | 31 |
| 5 | 3 |
|
| No | 20 | 575 |
| 20 | 22 |
|
| Microvascular
invasion, n |
|
| <0.001 |
|
| 0.089 |
|
Yes | 15 | 153 |
| 15 | 9 |
|
| No | 10 | 453 |
| 10 | 16 |
|
| Tumour
differentiation, n |
|
| 0.003a |
|
| 0.172a |
|
Well | 0 | 36 |
| 0 | 2 |
|
|
Moderate | 12 | 429 |
| 12 | 15 |
|
|
Poor | 13 | 141 |
| 13 | 8 |
|
| AJCC
stagec, n |
|
| 0.001 |
|
| 0.505a |
| I | 8 | 403 |
| 8 | 12 |
|
| II | 11 | 148 |
| 11 | 8 |
|
|
III | 6 | 55 |
| 6 | 5 |
|
| Resection margins,
n |
|
| 0.026a |
|
| 0.157a |
| R0 | 21 | 592 |
| 21 | 24 |
|
| R1 | 4 | 14 |
| 4 | 1 |
|
The imbalance in clinicopathological characteristics
between the two groups indicates that the data between the two
groups were incomparable before PSM. To balance this difference,
the clinicopathological indices were set as matching covariates for
PSM. The aforementioned variables were not significantly different
between these two groups after PSM (P>0.05), indicating that the
two sets of data were comparable (Table
II).
Postoperative pathological
features
Although the most common location of BDTT was the
CBD (60%) in the present study, BDTT rarely invaded the wall of the
CBD histologically. The underlying epithelium of the resected large
bile ducts was well preserved based on the histological examination
of the 4 patients who underwent extrahepatic bile duct resection in
this study. One of the postoperative histopathological findings was
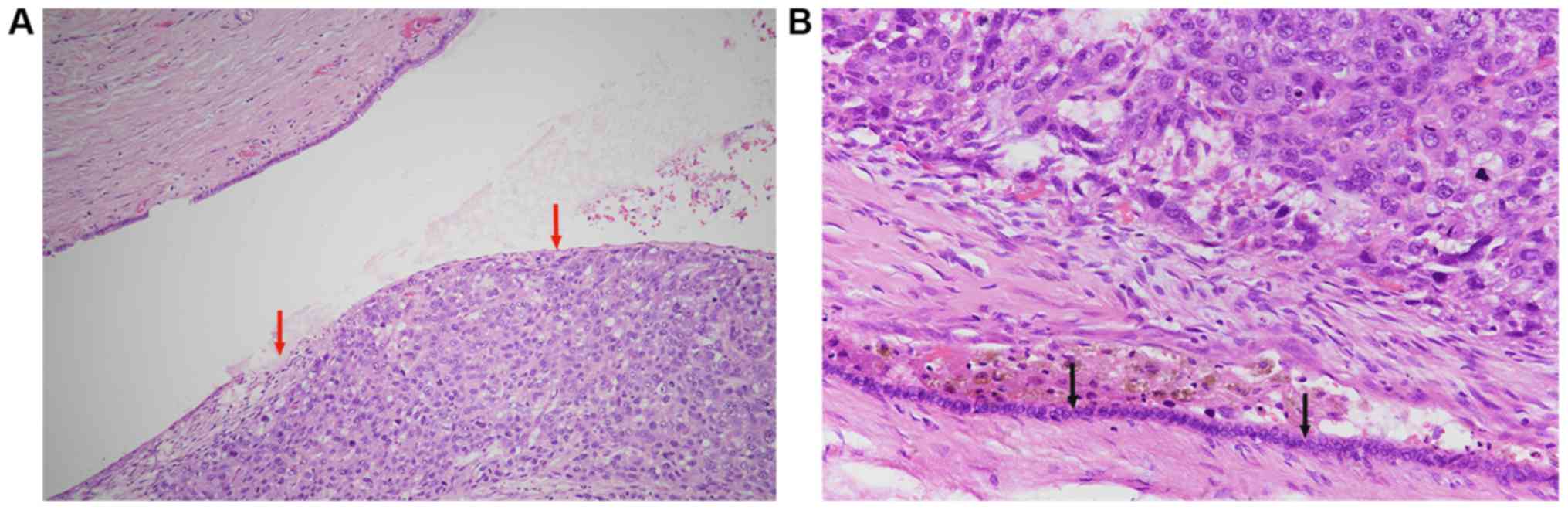
that BDTT did not usually adhere to the CBD. The cancer cells in
BDTT were flaky and solid, the cells had obvious atypia and
pleomorphism, and pathological mitosis was not uncommon (Fig. 1A). Although the tumour cells were
scattered and haemosiderin deposition was evident, suggesting old
bleeding, biliary epithelial cells were still continuous and
intact, and were protected by profibrotic reactions (Fig. 1B).
Postoperative long-term outcomes
Patients who died perioperatively were excluded from
the survival analysis. The median follow-up duration was 32 months
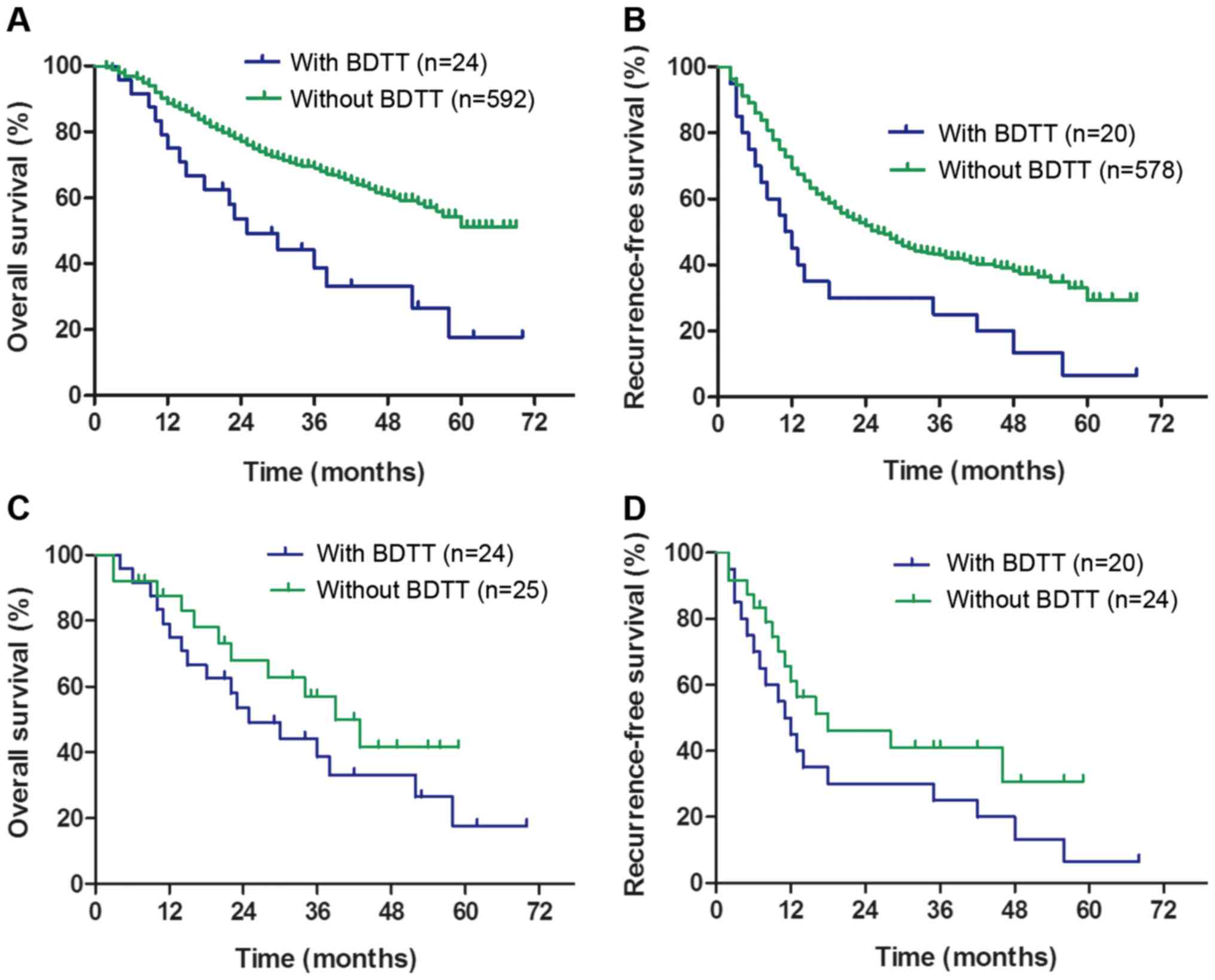
(range, 1–70 months). The cumulative 1-, 3- and 5-year overall
survival (OS) rates in the group without BDTT (88.61, 69.01 and
51.16%, respectively) were significantly higher than those in the
BDTT group (75.00, 38.67 and 17.68%, respectively) (P<0.001;
Fig. 2A). The cumulative 1-, 3- and
5-year recurrence-free survival (RFS) rates in the group without
BDTT (69.32, 43.01 and 29.40%, respectively) were significantly
higher than those in the BDTT group (45.00, 25.00, and 6.67%,
respectively) (P=0.003; Fig. 2B).
However, no significant differences in the cumulative 1-, 3- and
5-year OS or RFS rates were identified between the two groups after
PSM (OS: 87.62, 57.07 and 41.61%, respectively, vs. 75.00, 38.67
and 17.68%, respectively; P=0.249; Fig.
2C; RFS: 61.09, 41.01 and 30.76%, respectively, vs. 45.00,
25.00 and 6.67%, respectively; P=0.121; Fig. 2D). These results illustrate that
covariates other than BDTT also affect the RFS and OS rates.
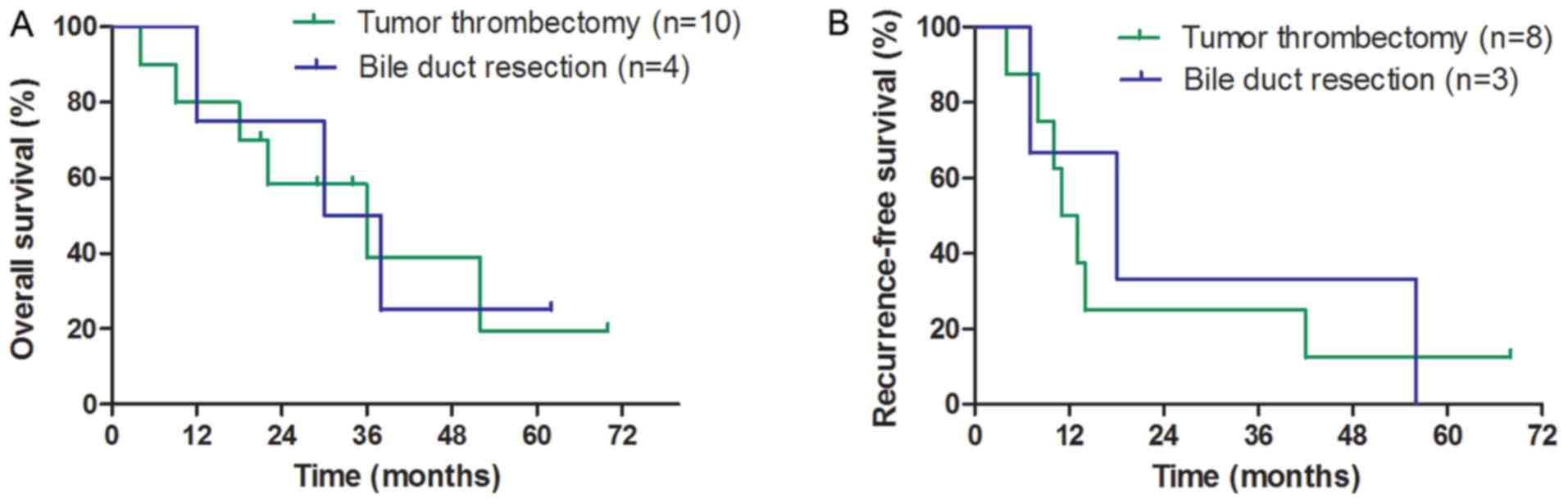
In the BDTT group, the median OS and RFS times of
patients who underwent tumour thrombectomy and bile duct resection
were not significantly different (OS: 36 vs. 30 months,
respectively; P=0.891; Fig. 3A; RFS:
11 vs. 18 months, respectively; P=0.787; Fig. 3B). A total of 17 HCC (68.0%) patients
with BDTT experienced recurrence during the follow-up; intrahepatic
recurrence was the most common type [12/17 (70.59%)], followed by
combined intrahepatic and extrahepatic recurrence [3/17 (17.65%)]
and extrahepatic recurrence [2/17 (11.76%)], both of which were in
the lungs (data not shown). None of the patients who underwent
tumour thrombectomy exhibited peritoneal dissemination, and only
one of these patients experienced bile duct recurrence and
underwent re-resection.
Univariate and multivariate analyses
of OS
Univariate and multivariate analyses were performed
by Cox proportional hazards regression in 49 patients who underwent
PSM. The univariate analysis revealed that macrovascular invasion
(HR, 4.613; P=0.001), microvascular invasion (HR, 2.942; P=0.007),
tumour differentiation (HR, 2.302; P=0.031) and resection margins
(HR, 5.715; P=0.001), rather than BDTT (HR, 1.558; P=0.254),
significantly affected OS (Table
III). These potential risk factors were further examined by
multivariate Cox proportional hazards regression analysis. The
results revealed that macrovascular invasion (HR, 3.220; 95% CI,
1.184-8.756; P=0.022) was the only independent predictor of OS
(Table III).
 | Table III.Univariate and multivariate cox
proportional analysis for overall survival. |
Table III.
Univariate and multivariate cox
proportional analysis for overall survival.
|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|---|
| Variables | n | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Group |
|
|
|
|
|
|
|
| Without
BDTT | 25 | 1.000 |
|
|
|
|
|
| With
BDTT | 24 | 1.558 | 0.727-3.341 | 0.254 |
|
|
|
| Age, years |
|
|
|
|
|
|
|
|
<60 | 31 | 1.000 |
|
|
|
|
|
|
≥60 | 18 | 1.230 | 0.5745-2.633 | 0.593 |
|
|
|
| Gender |
|
|
|
|
|
|
|
|
Male | 40 | 1.000 |
|
|
|
|
|
|
Female | 9 | 1.242 | 0.464-3.323 | 0.666 |
|
|
|
| Presence of
comorbidity |
|
|
|
|
|
|
|
| No | 31 | 1.000 |
|
|
|
|
|
|
Yes | 18 | 1.151 | 0.543-2.441 | 0.715 |
|
|
|
| AFP, µg/l |
|
|
|
|
|
|
|
|
<400 | 26 | 1.000 |
|
|
|
|
|
|
≥400 | 23 | 1.617 | 0.764-3.423 | 0.209 |
|
|
|
| HBV virus
antigen |
|
|
|
|
|
|
|
|
Negative | 9 | 1.000 |
|
|
|
|
|
|
Positive | 40 | 1.005 | 0.405-2.498 | 0.991 |
|
|
|
| ICGR15a, % |
|
|
|
|
|
|
|
|
≤10 | 22 | 1.000 |
|
|
|
|
|
|
>10 | 8 | 2.417 | 0.703-8.307 | 0.161 |
|
|
|
| Child-Pugh
classification |
|
|
|
|
|
|
|
| A | 42 | 1.000 | |
|
|
|
|
| B | 7 | 1.655 | 0.666-4.113 | 0.278 |
|
|
|
| Maximum tumour
size, cm |
|
|
|
|
|
|
|
|
>5 | 24 | 1.000 |
|
|
|
|
|
| ≥5 | 25 | 1.614 | 0.743-3.506 | 0.226 |
|
|
|
| Number of
tumours |
|
|
|
|
|
|
|
|
Solitary | 41 | 1.000 |
|
|
|
|
|
|
Multiple | 8 | 1.717 | 0.725-4.065 | 0.219 |
|
|
|
| Cirrhosis |
|
|
|
|
|
|
|
| No | 31 | 1.000 |
|
|
|
|
|
|
Yes | 18 | 1.27 | 0.587-2.747 | 0.544 |
|
|
|
| Macrovascular
invasion |
|
|
|
|
|
|
|
|
Negative | 41 | 1.000 |
|
| 1.000 |
|
|
|
Positive | 8 | 4.613 | 1.944-10.947 | 0.001 | 3.220 | 1.184-8.756 | 0.022 |
| Microvascular
invasion |
|
|
|
|
|
|
|
|
Negative | 24 | 1.000 |
|
| 1.000 |
|
|
|
Positive | 25 | 2.942 | 1.350-6.413 | 0.007 | 1.728 | 0.737-4.051 | 0.208 |
| Tumour
differentiation |
|
|
|
|
|
|
|
|
Wellb | 2 | – | – | – | – | – | – |
|
Moderate | 27 | 1.000 |
|
| 1.000 |
|
|
|
Poor | 20 | 2.302 | 1.081-4.905 | 0.031 | 2.150 | 0.968-4.775 | 0.06 |
| Resection
margins |
|
|
|
|
|
|
|
| R0 | 44 | 1.000 |
|
| 1.000 |
|
|
| R1 | 5 | 5.715 | 2.065-15.819 | 0.001 | 2.563 | 0.789-8.326 | 0.117 |
Discussion
Manifestations of jaundice in patients with HCC are
thought to be caused by massive tumour infiltration of the liver
parenchyma or advanced underlying liver cirrhosis (16,17).
Sometimes, precisely distinguishing the cause of jaundice is
difficult in clinical practice (18); therefore, patients with BDTT and
jaundice are often forced to receive conservative treatment, thus
missing the opportunity for suitable treatment with a potential
chance of a complete response. This is mainly due to insufficient
understanding of the clinicopathological characteristics of the
tumour. Although a general consensus indicates that the oncological
prognosis of surgical treatment is significantly improved compared
with that of palliative treatment (11,12),
studies on the surgical treatment of HCC with BDTT are uncommon,
especially studies regarding postoperative long-term outcomes.
Whether the postoperative survival of patients with HCC and BDTT is
equal to that of patients with HCC and no BDTT (2,8,18–24) and
whether the extrahepatic bile duct should be removed remain
controversial (9,25,26).
As reported in previous studies (24,27,28),
compared with HCC without BDTT, HCC with BDTT demonstrates
clinicopathological features such as a longer tumour diameter, a
higher incidence of vascular invasion and a higher
Edmondson-Steiner grade, AJCC stage and Child-Pugh classification;
however, cirrhosis is less common (28), which may be associated with the
biological behaviour of HCC with BDTT. The current results
(Table II) before PSM are
consistent with these earlier findings, which decreases the
comparability of these studies. PSM could have balanced the
confounding variables and reduced the impact of selection biases,
and the results would have been similar to those of randomized
controlled studies (29,30). The clinicopathological features
between the two groups after PSM were not significantly different,
which increased the comparability between groups. Therefore, PSM
was used to control for selection bias in the current study,
increasing the credibility of the research results.
Another notable area for comparison is whether
postoperative survival among HCC patients with BDTT is equal to
that among HCC patients without BDTT. Yang et al (21) reported that the median OS time in the
BDTT group (16.6 months) was significantly worse than that in the
group without BDTT (84.0 months). Other studies (8,22,23) have
also illustrated that, compared with the OS in the group without
BDTT, the OS of the BDTT group was significantly poorer. However,
Shiomi et al (16) reported
that the 3- and 5-year OS rates (47 and 28%, respectively) in the
BDTT group were not significantly different from those in the group
without BDTT (63 and 48%, respectively; P=0.190). The reason for
this difference may be that the potential impact of other variables
on the postoperative survival of patients with HCC and BDTT was
neglected (2,24). In the present study, the 3- and
5-year OS rates in the BDTT group were worse than those in the
group without BDTT before PSM. However, no significant difference
was observed between the two groups after PSM. These results show
that when matching other variables and balancing selection bias,
BDTT does not affect the long-term survival of patients with HCC
who receive liver resection.
The RFS rates in the BDTT group were significantly
worse than those in the group without BDTT before PSM in the
present study, which is consistent with the results of previous
studies (8,21). However, Kim et al (22) reported that RFS rates between the two
groups were not significantly different. Similarly, in the current
study, no significant differences in RFS rates were found between
the two groups after PSM. These results indicate that BDTT does not
affect postoperative RFS in patients with HCC. Tumour recurrence is
generally accepted to affect survival in patients with HCC who
undergo surgery with curative intent. Zeng et al (7) also reported that tumour recurrence was
an unfavourable prognostic factor for OS. BDTT did not affect
postoperative RFS in patients with HCC, which indirectly
illustrates that it is not an unfavourable prognostic factor for OS
after surgery. All the results indicate that BDTT in HCC has low
indolent malignant potential and is not a contraindication for
liver resection.
HCC with portal vein tumour thrombus (PVTT) is
recognized as a relatively advanced-stage disease regardless of the
criteria of the Barcelona Clinic for Liver Cancer Staging System
(31) or the AJCC 8th Edition. HCC
patients with PVTT have a highly unfavourable prognosis with regard
to macrovascular invasion, often causing the widespread
dissemination of cancer cells and leading to intrahepatic
metastasis, which is considered an important mechanism of
intrahepatic recurrence (32,33). In
the present study, the results of the multivariate analysis
revealed that macrovascular invasion was one of the independent
prognostic risk factors for the long-term survival of HCC patients
after resection, which is similar to the results of previous
studies (28). These findings may be
associated with the highly aggressive biological behaviour of HCC
with macrovascular invasion. Non-curative liver resection was
deemed another independent unfavourable risk factor for
postoperative long-term survival in HCC patients in the univariate
analysis, but not in the multivariate analysis, in the present
study. The reason for this finding may be that the number of
patients who underwent non-curative resection was insufficient, and
its statistical weight was inadequate. Therefore, we hypothesize
that when the number of non-curative surgical resections increases,
non-curative resection may increase the risk of impaired long-term
postoperative survival, which is a theory that is widely accepted
by some surgeons (34,35). Therefore, if associated conditions
are permitted, curative resection is recommended when the liver
function of these patients is sufficient after appropriate
preoperative management.
Whether extrahepatic bile duct resection for HCC
with BDTT is necessary remains controversial. Moon et al
(25) claimed that if BDTT could be
successfully removed, bile duct resection may not be necessary.
Moreover, Noda et al (28)
and Shibata et al (36)
illustrated that the potential for serious complications, such as
liver abscesses, may not be avoided when intrahepatic recurrence is
treated locally after bile duct resection. Neither the median
survival time nor the RFS time between the bile duct resection and
tumour thrombectomy groups was significantly different in the
present study, which is consistent with the results of previous
studies (16,19). The surgical treatment strategy in the
current study was the complete resection of primary HCC and BDTT,
and it aimed not to remove the extrahepatic bile duct; this is
similar to the ‘peeling off technique’ (10). Tumour thrombectomy is believed to
have potential risks of peritoneal dissemination and recurrence in
the preserved bile duct. However, Kim et al (37) proclaimed that thrombectomy does not
significantly increase the risk of peritoneal metastasis. None of
the patients who underwent tumour thrombectomy presented with
peritoneal dissemination in the present study. Although bile duct
recurrence was observed in one of these patients, the risks are
considered minimal as the first recurrence most frequently occurs
in the remnant liver and not in the bile duct, and the opportunity
to undergo curative re-resection is still available.
The absence of bile duct resection for patients with
HCC and BDTT may cause recurrence due to direct invasion of the
tumour into the bile duct, periductal capillary plexus and
lymphatic system (27,38). Recently, a multicentre study reported
that bile duct resection was a significant favourable prognostic
factor for recurrence, OS and survival after recurrence (37). Based on the aforementioned studies,
the selection criteria for resecting the bile duct could not be too
absolute, and an individualized treatment plan was required
according to the condition of each patient. Whether extrahepatic
bile duct resection is necessary for patients with HCC with BDTT
should be further explored. Regardless of the surgical approach to
remove or retain the bile duct, curative resection of the tumour is
the most important aim.
The present study also has some limitations. As a
retrospective case-control study, although a control group was
established for direct comparisons, the number of patients with
BDTT was insufficient. The reasons may be as follows: i) The
incidence of HCC with BDTT is rare (2–6); ii) the
biological and clinicopathological characteristics of the tumours
were inadequately understood, therefore, many patients did not
receive surgical treatment in the early period; and iii) the
clinicopathological data of these patients were incomplete in the
early period and were thus excluded from the study. Considering the
rarity of HCC with BDTT, the present study is of great clinical
significance, as the number of included patients exceeds that in
numerous other studies (2,10,16,28,34).
Although PSM was used to reduce the imbalance caused by selection
bias, some unavoidable confounding variables remain. For instance,
the preoperative TBIL level in the BDTT group was significantly
higher than that in the group without BDTT before and after PSM.
However, the impact of the preoperative TBIL level on survival was
reflected in the Child-Pugh classification system, and it was not
identified as an important prognostic factor for patients with
BDTT. Large, multicentre, prospective, randomized, controlled
trials are still needed for further validation.
Compared with those of patients without BDTT, the
clinicopathological features of patients with HCC and BDTT are more
advanced, and the long-term postoperative outcome of these patients
is worse. Macrovascular invasion, but not BDTT, is a significantly
unfavourable risk factor for the survival of patients with HCC who
undergo liver resection. Curative resection is recommended for
patients with HCC and BDTT, even for those with poor liver function
after proper perioperative management, in order to achieve good
long-term survival.
Acknowledgements
Not applicable.
Funding
This study was funded by Construction Projects of
State Key Clinical Specialty of Health Department (grant no.
2013-GJLCZD).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
QC analyzed and interpreted the patient data, and
was a major contributor in writing the manuscript. SW was involved
in study design, study supervision and critical revision of the
manuscript. ZS, ZZ and XZ analyzed and interpreted the patient
data. LZ performed the histological examination of the HCC samples.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from all
individuals included in the study at the time of initial data
collection. The study was approved by the Institutional Review
Board of The First Affiliated Hospital of Fujian Medical University
and performed in accordance with the ethical guidelines of the
institute.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Shehta A, Han HS, Yoon YS, Cho JY and Choi
Y: Laparoscopic liver resection for hepatocellular carcinoma in
cirrhotic patients: 10-year single-center experience. Surg Endosc.
30:638–648. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Oba A, Takahashi S, Kato Y, Gotohda N,
Kinoshita T, Shibasaki H, Ikeda M and Konishi M: Usefulness of
resection for hepatocellular carcinoma with macroscopic bile duct
tumor thrombus. Anticancer Res. 34:4367–4372. 2014.PubMed/NCBI
|
|
3
|
Ueda M, Takeuchi T, Takayasu T, Takahashi
K, Okamoto S, Tanaka A, Morimoto T, Mori K and Yamaoka Y:
Classification and surgical treatment of hepatocellular carcinoma
(HCC) with bile duct thrombi. Hepatogastroenterology. 41:349–354.
1994.PubMed/NCBI
|
|
4
|
Kudo M, Izumi N, Ichida T, Ku Y, Kokudo N,
Sakamoto M, Takayama T, Nakashima O, Matsui O and Matsuyama Y:
Report of the 19th follow-up survey of primary liver cancer in
Japan. Hepatol Res. 46:372–390. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nakashima T, Okuda K, Kojiro M, Jimi A,
Yamaguchi R, Sakamoto K and Ikari T: Pathology of hepatocellular
carcinoma in Japan. 232 consecutive cases autopsied in ten years.
Cancer. 51:863–877. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Meng KW, Dong M, Zhang WG and Huang QX:
Clinical characteristics and surgical prognosis of hepatocellular
carcinoma with bile duct invasion. Gastroenterol Res Pract.
2014:6049712014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zeng H, Xu LB, Wen JM, Zhang R, Zhu MS,
Shi XD and Liu C: Hepatocellular carcinoma with bile duct tumor
thrombus: A clinicopathological analysis of factors predictive of
recurrence and outcome after surgery. Medicine (Baltimore).
94:e3642015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shao W, Sui C, Liu Z, Yang J and Zhou Y:
Surgical outcome of hepatocellular carcinoma patients with biliary
tumor thrombi. World J Surg Oncol. 9:22011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ikenaga N, Chijiiwa K, Otani K, Ohuchida
J, Uchiyama S and Kondo K: Clinicopathologic characteristics of
hepatocellular carcinoma with bile duct invasion. J Gastrointest
Surg. 13:492–497. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yamamoto S, Hasegawa K, Inoue Y, Shindoh
J, Aoki T, Sakamoto Y, Sugawara Y, Makuuchi M and Kokudo N: Bile
duct preserving surgery for hepatocellular carcinoma with bile duct
tumor thrombus. Ann Surg. 261:e123–e125. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xiangji L, Weifeng T, Bin Y, Chen L,
Xiaoqing J, Baihe Z, Feng S and Mengchao W: Surgery of
hepatocellular carcinoma complicated with cancer thrombi in bile
duct: Efficacy for criteria for different therapy modalities.
Langenbecks Arch Surg. 394:1033–1039. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lau W, Leung K, Leung TW, Liew CT, Chan
MS, Yu SC and Li AK: A logical approach to hepatocellular carcinoma
presenting with jaundice. Ann Surg. 225:281–285. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bae SH, Park HC, Yoon WS, Yoon SM, Jung
IH, Lee IJ, Kim JW, Seong J, Kim TH, Nam TK, et al: Treatment
outcome after fractionated conformal radiotherapy for
hepatocellular carcinoma in patients with child-pugh classification
B in Korea (KROG 16-05). Cancer Res Treat. 51:1589–1599. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Abdel-Rahman O: Assessment of the
discriminating value of the 8th AJCC stage grouping for
hepatocellular carcinoma. HPB (Oxford). 20:41–48. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Edmondson HA and Steiner PE: Primary
carcinoma of the liver: A study of 100 cases among 48,900
necropsies. Cancer. 7:462–503. 1954. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shiomi M, Kamiya J, Nagino M, Uesaka K,
Sano T, Hayakawa N, Kanai M, Yamamoto H and Nimura Y:
Hepatocellular carcinoma with biliary tumor thrombi: Aggressive
operative approach after appropriate preoperative management.
Surgery. 129:692–698. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee NW, Wong KP, Siu KF and Wong J:
Cholangiography in hepatocellular carcinoma with obstructive
jaundice. Clin Radiol. 35:119–123. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mok KT, Chang HT, Liu SI, Jou NW, Tsai CC
and Wang BW: Surgical treatment of hepatocellular carcinoma with
biliary tumor thrombi. Int Surg. 81:284–288. 1996.PubMed/NCBI
|
|
19
|
Satoh S, Ikai I, Honda G, Okabe H,
Takeyama O, Yamamoto Y, Yamamoto N, Iimuro Y, Shimahara Y and
Yamaoka Y: Clinicopathologic evaluation of hepatocellular carcinoma
with bile duct thrombi. Surgery. 128:779–783. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yeh CN, Jan YY, Lee WC and Chen MF:
Hepatic resection for hepatocellular carcinoma with obstructive
jaundice due to biliary tumor thrombi. World J Surg. 28:471–475.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang X, Qiu Z, Ran R, Cui L, Luo X, Wu M,
Tan WF and Jiang X: Prognostic importance of bile duct invasion in
surgical resection with curative intent for hepatocellular
carcinoma using PSM analysis. Oncol Lett. 16:3593–3602.
2018.PubMed/NCBI
|
|
22
|
Kim JM, Kwon CH, Joh JW, Sinn DH, Park JB,
Lee JH, Kim SJ, Paik SW, Park CK and Yoo BC: Incidental microscopic
bile duct tumor thrombi in hepatocellular carcinoma after curative
hepatectomy: A matched study. Medicine (Baltimore). 94:e4502015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rammohan A, Sathyanesan J, Rajendran K,
Pitchaimuthu A, Perumal SK, Balaraman K, Ramasamy R, Palaniappan R
and Govindan M: Bile duct thrombi in hepatocellular carcinoma: Is
aggressive surgery worthwhile? HPB (Oxford). 17:508–513. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wong TC, Cheung TT, Chok KS, Chan AC, Dai
WC, Chan SC, Poon RT, Fan ST and Lo CM: Outcomes of hepatectomy for
hepatocellular carcinoma with bile duct tumour thrombus. HPB
(Oxford). 17:401–408. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Moon DB, Hwang S, Wang HJ, Yun SS, Kim KS,
Lee YJ, Kim KH, Park YK, Xu W, Kim BW, et al: Surgical outcomes of
hepatocellular carcinoma with bile duct tumor thrombus: A Korean
multicenter study. World J Surg. 37:443–451. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yasunori M and Masatoshi K: Hepatocellular
carcinoma with obstructive jaundice: Endoscopic and percutaneous
biliary drainage. Dig Dis. 30:592–597. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Orimo T, Kamiyama T, Yokoo H, Wakayama K,
Shimada S, Tsuruga Y, Kamachi H and Taketomi A: Hepatectomy for
hepatocellular carcinoma with bile duct tumor thrombus, including
cases with obstructive Jaundice. Ann Surg Oncol. 23:2627–2634.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Noda T, Nagano H, Tomimaru Y, Murakami M,
Wada H, Kobayashi S, Marubashi S, Eguchi H, Takeda Y, Tanemura M,
et al: Prognosis of hepatocellular carcinoma with biliary tumor
thrombi after liver surgery. Surgery. 149:371–377. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rubin DB: Estimating causal effects from
large data sets using propensity scores. Ann Intern Med.
127:757–263. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Austin PC: An introduction to propensity
score methods for reducing the effects of confounding in
observational studies. Multivariate Behav Res. 46:399–424. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cho HJ, Kim SS, Kang SY, Yang MJ, Noh CK,
Hwang JC, Lim SG, Shin SJ, Lee KM, Yoo BM, et al: A proposal for
modification of the barcelona clinic liver cancer staging system
considering the prognostic implication of performance status. Gut
Liver. 13:557–568. 2019. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Vauthey JN, Klimstra D, Franceschi D, Tao
Y, Fortner J, Blumgart L and Brennan M: Factors affecting long-term
outcome after hepatic resection for hepatocellular carcinoma. Am J
Surg. 169:28–35. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yamamoto J, Kosuge T, Takayama T, Shimada
K, Yamasaki S, Ozaki H, Yamaguchi N and Makuuchi M: Recurrence of
hepatocellular carcinoma after surgery. Br J Surg. 83:1219–1222.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Esaki M, Shimada K, Sano T, Sakamoto Y,
Kosuge T and Ojima H: Surgical results for hepatocellular carcinoma
with bile duct invasion: A clinicopathologic comparison between
macroscopic and microscopic tumor thrombus. J Surg Oncol.
90:226–232. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Han J, Li ZL, Xing H, Wu H, Zhu P, Lau WY,
Zhou YH, Gu WM, Wang H, Chen TH, et al: The impact of resection
margin and microvascular invasion on long-term prognosis after
curative resection of hepatocellular carcinoma: A
multi-institutional study. HPB (Oxford). 21:962–971. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shibata T, Yamamoto N, Ikai I, Shimahara
Y, Yamaoka Y, Itoh K and Konishi J: Choledochojejunostomy: Possible
risk factor for septic complications after percutaneous hepatic
tumor ablation. AJR Am J Roentgenol. 174:985–986. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kim DS, Kim BW, Hatano E, Hwang S,
Hasegawa K, Kudo A, Ariizumi S, Kaibori M, Fukumoto T, Baba H, et
al: Surgical outcomes of hepatocellular carcinoma with bile duct
tumor thrombus: A Korea-Japan multicenter study. Ann Surg.
271:913–921. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kojiro M, Kawabata K, Kawano Y, Shirai F,
Takemoto N and Nakashima T: Hepatocellular carcinoma presenting as
intrabile duct tumor growth: A clinicopathologic study of 24 cases.
Cancer. 49:2144–2147. 1982. View Article : Google Scholar : PubMed/NCBI
|