Cancer is one of the leading causes of mortality
worldwide, it was estimated that 14.1 million new cancer cases and
8.2 million cancer mortalities occurred in 2012, worldwide
(1,2). Metastasis is the most dangerous stage
in the occurrence and development of cancer (3). Clinically, numerous patients with
malignant tumors present with metastases at the time of diagnosis
(4). Therefore, the prevention and
suppression of tumor metastasis is a critical issue that requires
attention.
Invadopodia is an important structure formed in
cancer metastasis, therefore, it is considered promising to
investigate the suppression of cancer metastasis from the
perspective of inhibiting invadopodia. In addition, the topic
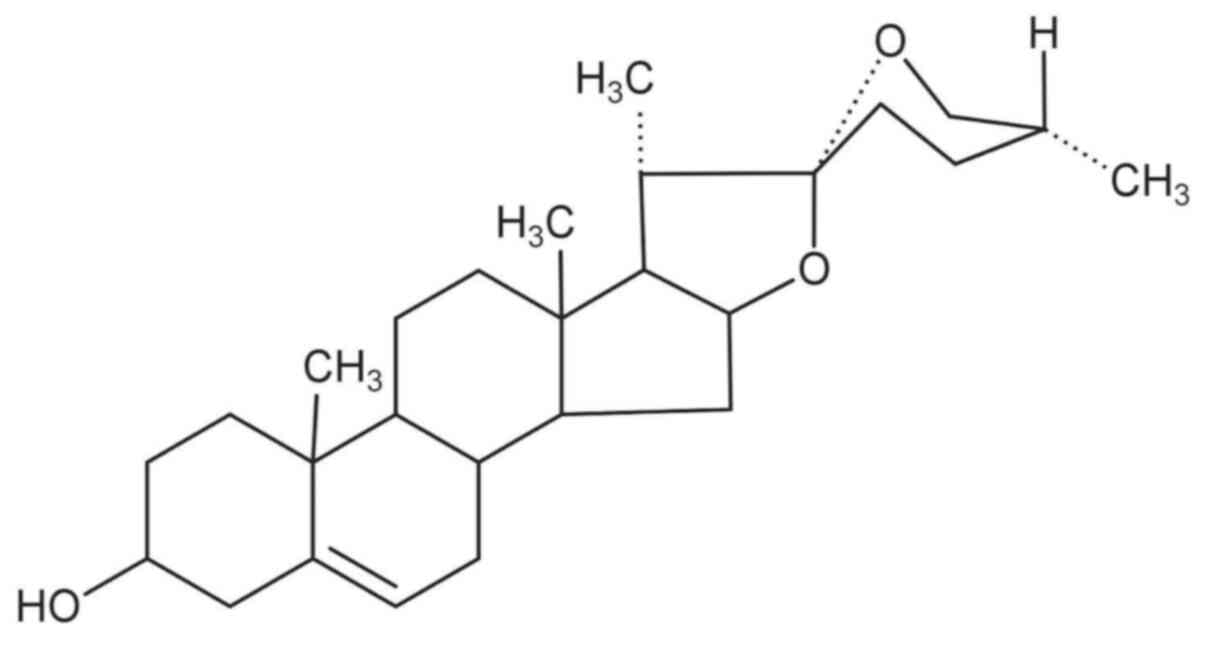
concerning traditional Chinese medicine, including diosgenin
suppressing cancer metastasis through inhibiting invadopodia has
been paid more attention. The present review will discuss and
summarize the potential molecular mechanism of diosgenin inhibiting
the formation and function of invadopodia.
Several studies have demonstrated that invadopodia
are formed in the early stages of invasion and metastasis of tumor
cells (7,8). The invadopodium is an essential
structure that is involved in the invasion and metastasis of cancer
cells (9). The invadopodium is a
type of special membrane structure process that is rich in actin
and involved in the degradation and remodeling of the extracellular
matrix (10). Electron microscopy
has revealed that invadopodia are slender, protruding structures
(11).
In other words, to break through the barrier of the
extracellular matrix, tumor cells need to extend cellular
protrusions, which reconstruct and degrade the extracellular matrix
(13). These types of cell
protrusions are essential for the ability of tumor cells to break
through the basement membrane and vascular wall (10,13). The
protruding structures (protrusions) formed by invasive tumor cells
on one side of the basement membrane are the invadopodia, which are
rich in actin regulatory proteins, adhesion molecules, signaling or
receptor proteins, cell membrane recombinant proteins, and matrix
proteolytic enzymes (10,13,19–23).
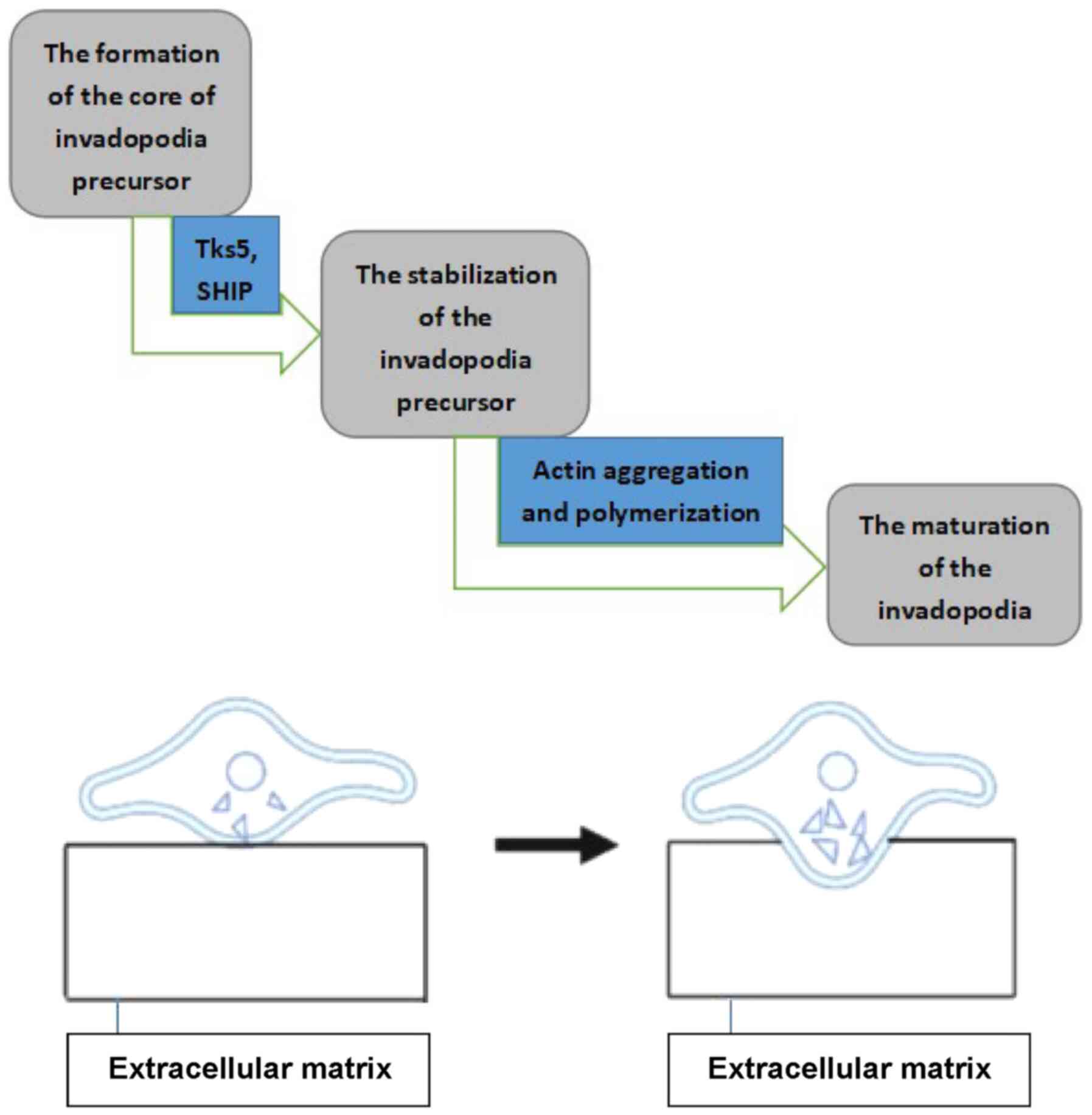
Invadopodia are involved in the process of tumor cell invasion
through the basement membrane as follows: i) The structure forms
first, and then the invadopodia perforate the basement membrane;
ii) the invadopodia then elongate and extend through and beyond the
basement membrane; and iii) finally, the invadopodia lead to the
migration of tumor cells (19).
As invadopodia are so important for cancer
metastasis, an improved understanding of the formation of and
regulatory mechanism controlling invadopodia is critical. Research
results in this field are expected to provide new therapeutic
targets and directions for tumor treatment.
Cortical actin-binding protein (Cortactin) has been
demonstrated to be associated with cancer. Previous studies have
demonstrated that Cortactin is upregulated in a variety of tumors,
such as breast cancers and head and neck tumors (54,55). It
is involved in a variety of cell activities, including invadopodia
formation and cell adhesion, invasion, migration and division
(54,56).
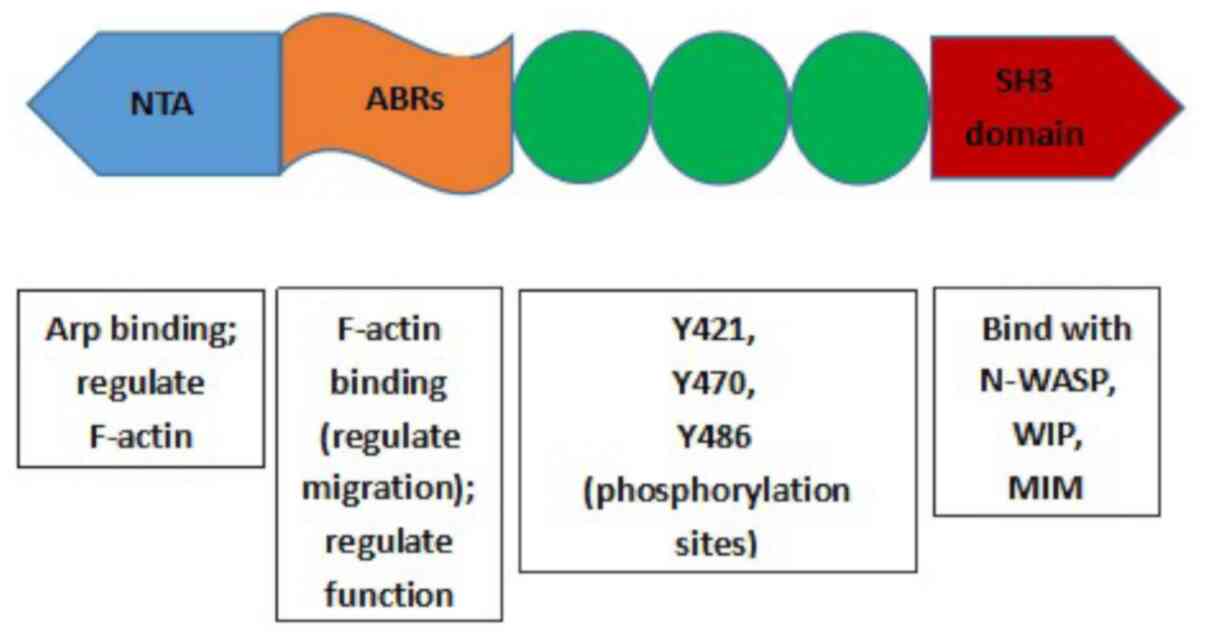
Human Cortactin is encoded by the CTTN gene
(formerly known as the EMSl gene), which is located on chromosome
11q13 (57). Cortactin protein has
three main domains: i) The N-terminal acidic region (NTA); ii)
filamentous actin (F-actin) repetitive domain (ABR) and iii) the
SH3 domain in the C-terminal (54,58)
(Fig. 3). The NTA binds with Arp in
the Arp2/3 complex and can also regulate the polymerization and
shrinkage of F-actin (54,56,58). The
ABR is responsible for the binding of Cortactin to F-actin
(58). The function of Cortactin is
also regulated at the ABR via post-translational modifications
(56,58). A study by Uruno et al revealed
that the number of repeats in the ABRs determines the affinity of
Cortactin to F-actin, as well as its ability to regulate cell
migration (57). The SH3 domain is a
conserved protein module found in various signaling proteins that
mediates the interaction with various other proteins, such as
neural Wiskott-Aldrich syndrome protein (N-WASP) (59), WASP binding protein (WIP) (60) and missing in metastasis (MIM)
(61). The tyrosine phosphorylation
of Cortactin is usually associated with the SH3 domain or
proline-rich domain-binding proteins (58). The molecular structure of Cortactin
changes after phosphorylation, bringing the SH3 domain closer to
the SH3 binding protein, increasing the chances of binding
(54,58). Cortactin is the main substrate of the
Src family tyrosine kinases, and tyrosine phosphorylation serves an
important role in the assembly of cortical microfilament actin
(54). Cortactin phosphorylation via
Src kinase is required for invadopodia formation mediated by
Cortactin (62,63); that is, the Src family tyrosine
kinases may promote cell migration via the phosphorylation of
Cortactin.
Cortactin and its associated proteins perform
functions in the cortical areas associated with cell membrane
deformation and the actin cytoskeleton; in pseudopods and cell
wrinkles, these proteins enhance the formation and/or stability of
dendritic actin networks (64).
Previous studies have reported that Cortactin
phosphorylation is associated with the rate of cell migration in a
number of different types of tumor cell (54,55,74,75). The
upregulation of Cortactin promotes the formation of invadopodia,
the degradation of the extracellular matrix and the invasiveness of
cancer cells (54,61,76).
Cortactin is positively correlated with tumor invasiveness and
metastasis and is closely associated with the synaptic membrane
structure of tumor cells (54,55,61,76).
Other studies have demonstrated that Cortactin binds
the Arp2/3 complex and N-WASP and regulates the formation of
invadopodia via the Nck1-N-WASP/Arp2/3 signaling pathway (76,77).
WASP family proteins can induce the rearrangement of actin
molecules in cells by activating Arp2/3 and thus promote the rapid
formation and maturation of invadopodia (78). Genna et al (79) revealed that the tyrosine kinase Pyk2
activates Abl-related gene (Arg) through the
EGFR-Pyk2-Src-Arg-cortactin signaling pathway, and directly or
indirectly mediates the phosphorylation and polymerization of
Cortactin induced by epidermal growth factor (EGF). This results in
invadopodia actin polymerization, invadopodia maturation and
enhanced invasion of breast cancer cells.
Overall, the aforementioned studies indicate that
Cortactin serves a pivotal role in the formation of invadopodia and
the degradation of the extracellular matrix to promote cancer cell
migration and invasion.
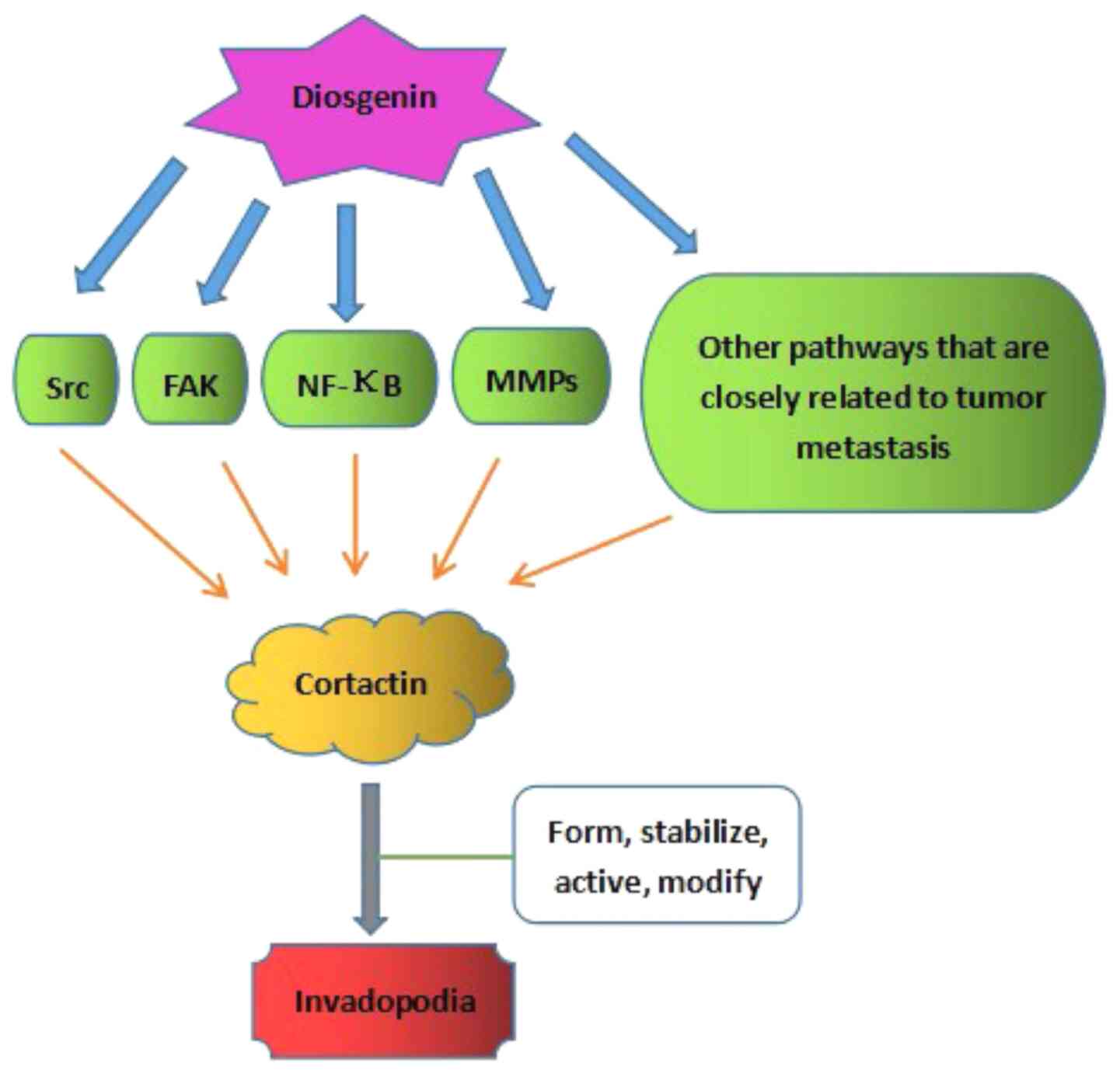
Invadopodium is a convergence point for a number of
signals that regulate tumor cell behaviors, particularly systemic
dissemination and metastasis (13).
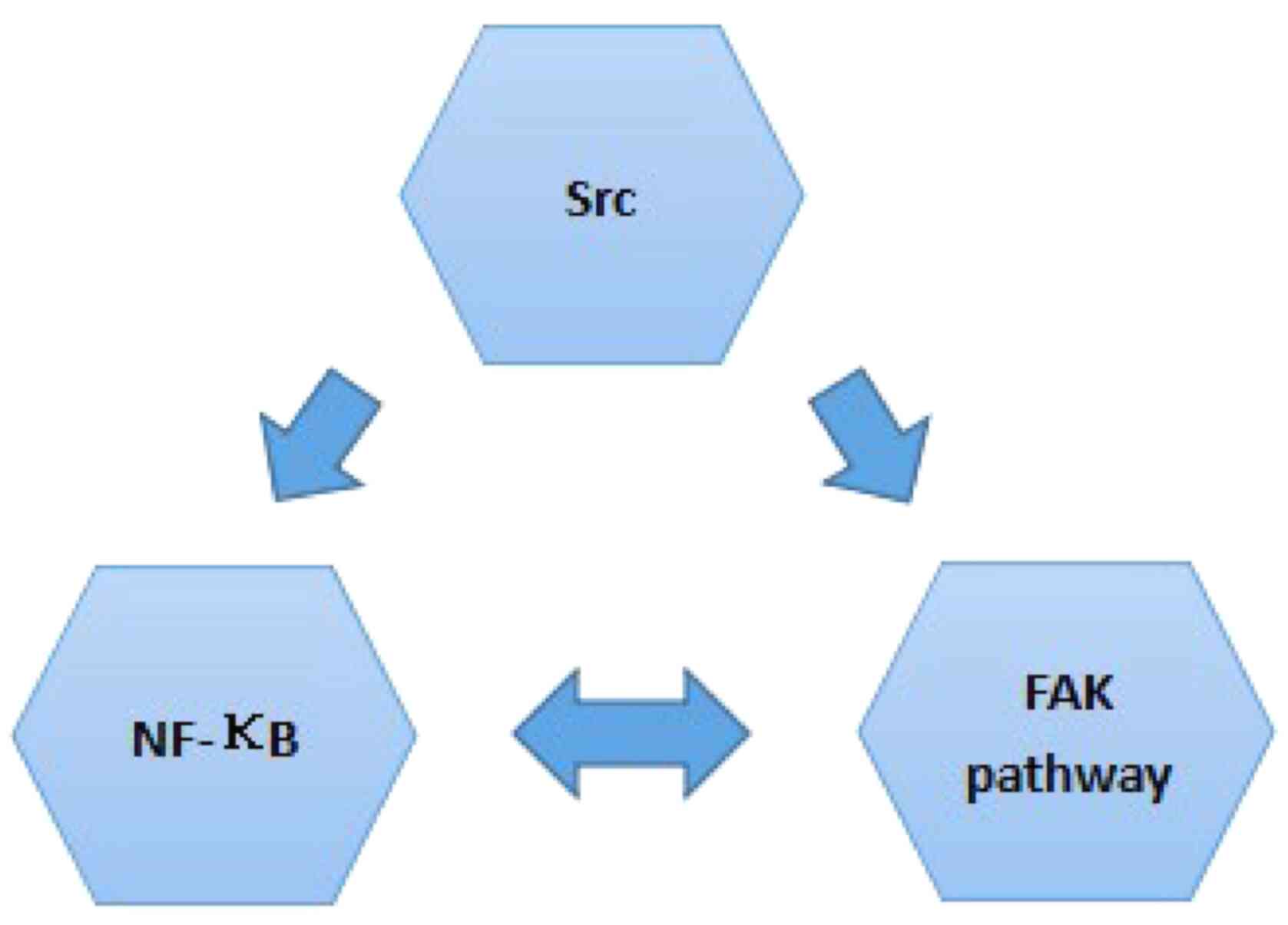
Cortactin is the switch that mediates invadopodia formation
(54,55,67,74,75,80). It
is regulated by numerous signaling pathways and factors, including
the FAK pathway (81), Src (82), NF-κB (54,55), and
other pathways that are closely associated with tumor metastasis
(54,55,74,75,80).
Several studies have reported that diosgenin is closely associated
with the FAK pathway, Src, NF-κB and MMPs (43,83). A
potential mechanism of action underlying
diosgenin-Cortactin-invadopodia is presented in Fig. 4.
In prostate cancer, diosgenin inhibits MMP
expression and, therefore, cancer metastasis (33), while MMP expression promotes
cortactin, and both MMPs and cortactin are required for form and
function of invadopodia (84). This
suggests that the potential mechanism of diosgenin involves the
downregulation of MMPs, and inhibition of Cortactin and
invadopodia, ultimately inhibiting prostate cancer metastasis. It
has also been demonstrated that diosgenin downregulates the NF-κB
signaling pathway, thus inhibiting the metastasis of prostate
cancer, suggesting another mechanism: Downregulation of the NF-κB
signaling pathway results in inhibition of Cortactin, and hence,
inhibition of invadopodia (33). In
addition, diosgenin can inhibit colon cancer metastasis via
regulating the Akt/MAPK signaling pathway (85), while Akt can activate Cortactin
(86,87), thus suggesting that diosgenin
downregulates the Akt/MAPK signaling pathway, which inhibits
Cortactin and hence, inhibits invadopodia, resulting in inhibition
of colon cancer metastasis. It was also reported that diosgenin can
activate the p38 and JNK pathways and thus inhibit Cortactin in
colon cancer (88), suggesting that
diosgenin inhibits the formation and function of invadopodia via
the downregulation of Cortactin via activating the p38 pathway
(89). In breast cancer, it was
revealed that diosgenin downregulates Akt, thus inhibiting the
metastasis of breast cancer (40,90),
similar to the mechanism in colon cancer. Diosgenin can serve as a
dual inhibitor of the MEK/ERK and PI3K/Akt signaling pathways to
overcome tyrosine kinase inhibitor resistance, resulting in
clinical benefits for lung cancer treatment (91). Furthermore, diosgenin downregulates
the NF-κB-p65/p50 and p38-MAPK pathways and attenuates acute lung
injury in mice (92). In human
erythroleukemia, diosgenin inhibits the NF-κB signaling pathway and
thus suppresses metastasis (43).
On the basis of its various functions, diosgenin has
been used medicinally to treat a number of diseases and improve
several physiological functions. Diosgenin has been applied in many
cases, such as treating inflammation (27,29,30),
improving cardiovascular function (27), lowering blood lipid levels (29,30) and
regulating immunity (27,29,30).
Traditionally, diosgenin was used for the treatment
of various symptoms such as cold hands and feet (by its function of
activating blood), loss of appetite caused by diseases including
cancer, and frequent urination (by its function of protecting
kidney) (27,29,30).
Currently, diosgenin is widely used for the treatment of
cardiovascular diseases (110,111).
Several extensive clinical cases (particularly for cardiovascular
diseases) have validated diosgenin as a drug for treating diseases
(110). In addition, certain
studies have demonstrated that psychobehavioral interventions of
traditional Chinese medicine can benefit patients with cancer by
multiple roles, such as decreasing functional impairments, leading
to pain relief, easing depression, decreasing time to flatulence
following surgery and improving sleep quality (112).
At present, the incidence rate of some kinds of
cancers in certain areas is increasing, such as colorectal cancer
in Latin America, Asia, Eastern Europe, breast cancer in low-income
countries, gastric cardia cancer in the United States and many
European countries (119–121), besides, cancer remains a serious
threat to human health and mortality (1). Cancer is often discovered in the late
stages, and in the majority of cases, the primary cancer has
metastasized into adjacent lymph nodes and other sites (3). Diosgenin, as a main component of a
traditional Chinese medicine, has a suppressing effect on tumor
metastasis. Numerous anticancer drugs that are currently used have
toxic side effects, and certain types of cancer develop resistance
to the drugs to a certain extent (30). Diosgenin may have the capability of
avoiding these shortcomings (30,110).
The clinical use of diosgenin as an anticancer
treatment requires further study and testing. Given the multiple
pathways and various targets of diosgenin, future research should
investigate its potential function in cancer inhibition.
Diosgenin may act on: i) Src by inhibiting its
phosphorylation ii) the FAK pathway by inhibiting the expression of
associated molecules and activation of the pathway; and iii) NF-κB
by inhibiting its level and activity, in addition to other
pathways. Furthermore, Src, the FAK pathway and NF-κB have
inter-relationships. The inhibition of diosgenin on Src, the FAK
pathway and NF-κB has a negative effect on the main switch
Cortactin, thus inhibiting invadopodia formation in various cancer
cells.
Future studies should examine the mechanism of
diosgenin inhibition of invadopodia formation to suppress the
metastasis of primary tumors. These findings will aid subsequent
clinical applications, particularly pharmaceutical use.
Not applicable.
The present study was supported by Jilin
University, (Changchun, China; grant nos. 2017QNYB016 and
201910183200) and the Department of Education of Jilin Province
(Changchun, China; grant no. 2016456).
The data used and/or analyzed during the present
study are available from the corresponding author upon reasonable
request.
YL completed the collection and analysis of
relevant literature and wrote the first draft of the manuscript.
DW, XM and XW participated in the analysis and collation of the
literature. HL, LH and HX critically analyzed the relevant
literature and the manuscript structure. JZ revised the manuscript.
All authors read and approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Torre LA, Siegel RL, Ward EM and Jemal A:
Global cancer incidence and mortality rates and trends-an update.
Cancer Epidemiol Biomarkers Prev. 25:16–27. 2016.PubMed/NCBI
|
|
2
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386.
2015.PubMed/NCBI
|
|
3
|
Zeeshan R and Mutahir Z: Cancer
metastasis-tricks of the trade. Bosn J Basic Med Sci. 17:172–182.
2017.PubMed/NCBI
|
|
4
|
Dudjak LA: Cancer metastasis. Semin Oncol
Nurs. 8:40–50. 1992.PubMed/NCBI
|
|
5
|
Yang J and Weinberg RA:
Epithelial-mesenchymal transition: At the crossroads of development
and tumor metastasis. Dev Cell. 14:818–829. 2008.PubMed/NCBI
|
|
6
|
Valastyan S and Weinberg R: Tumor
metastasis: Molecular insights and evolving paradigms. Cell.
147:275–292. 2011.PubMed/NCBI
|
|
7
|
Linder S and Wiesner C: Tools of the
trade: Podosomes as multipurpose organelles of monocytic cells.
Cell Mol Life Sci. 72:121–135. 2015.PubMed/NCBI
|
|
8
|
Popow-Woźniak A, Mazur AJ, Mannherz HG,
Malicka-Błaszkiewicz M and Nowak D: Cofilin overexpression affects
actin cytoskeleton organization and migration of human colon
adenocarcinoma cells. Histochem Cell Biol. 138:725–736.
2012.PubMed/NCBI
|
|
9
|
Leong H, Robertson A, Stoletov K, Leith
SJ, Chin CA, Chien AE, Hague MN, Ablack A, Carmine-Simmen K,
McPherson VA, et al: Invadopodia are required for cancer cell
extravasation and are a therapeutic target for metastasis. Cell
Rep. 8:1558–1570. 2014.PubMed/NCBI
|
|
10
|
Parekh A and Weaver AM: Regulation of
invadopodia by mechanical signaling. Exp Cell Res. 343:89–95.
2016.PubMed/NCBI
|
|
11
|
Artym VV, Yamada KM and Mueller SC: ECM
Degradation assays for analyzing local cell invasion. Methods Mol
Biol. 522:211–219. 2009.PubMed/NCBI
|
|
12
|
Beaty BT and Condeelis J: Digging a little
deeper: The stages of invadopodium formation and maturation. Eur J
Cell Biol. 93:438–444. 2014.PubMed/NCBI
|
|
13
|
Eddy RJ, Weidmann MD, Sharma VP and
Condeelis JS: Tumor cell invadopodia: Invasive protrusions that
orchestrate metastasis. Trends Cell Biol. 27:595–607.
2017.PubMed/NCBI
|
|
14
|
Sharma V, Eddy R, Entenberg D, Kai M,
Gertler FB and Condeelis J: Tks5 and SHIP2 regulate invadopodium
maturation, but not initiation, in breast carcinoma cells. Curr
Biol. 23:2079–2089. 2013.PubMed/NCBI
|
|
15
|
Hughes SK, Oudin MJ, Tadros J, Neil J, Del
Rosario A, Joughin BA, Ritsma L, Wyckoff J, Vasile E, Eddy R, et
al: PTP1B-dependent regulation of receptor tyrosine kinase
signaling by the actin-binding protein Mena. Mol Biol Cell.
26:3867–3878. 2015.PubMed/NCBI
|
|
16
|
Zervantonakis I, Sudo R, Rimchala T, Chung
S and Kamm R: Abstract #2269: A physiological relevant 3D in vitro
model of cancer cell migration and interactions with endothelium.
Cancer Res. 69:2269. 2009.PubMed/NCBI
|
|
17
|
Wang DD, Chen YB, Zhao JJ, Zhang XF, Zhu
GC, Weng DS, Pan K, Lv L, Pan QZ, Jiang SS, et al: TES functions as
a Mena-dependent tumor suppressor in gastric cancer carcinogenesis
and metastasis. Cancer Commun (Lond). 39:32019.PubMed/NCBI
|
|
18
|
Bravo-Cordero JJ, Magalhaes MA, Eddy RJ,
Hodgson L and Condeelis J: Functions of cofilin in cell locomotion
and invasion. Nat Rev Mol Cell Biol. 14:405–415. 2013.PubMed/NCBI
|
|
19
|
Murphy DA and Courtneidge SA: The ‘ins’
and ‘outs’ of podosomes and invadopodia: Characteristics, formation
and function. Nat Rev Mol Cell Biol. 12:413–426. 2011.PubMed/NCBI
|
|
20
|
Linder S: The matrix corroded: Podosomes
and invadopodia in extracellular matrix degradation. Trends Cell
Biol. 17:107–117. 2007.PubMed/NCBI
|
|
21
|
Baldassarre M, Pompeo A, Beznoussenko G,
Castaldi C, Cortellino S, McNiven MA, Luini A and Buccione R:
Dynamin participates in focal extracellular matrix degradation by
invasive cells. Mol Biol Cell. 14:1074–1084. 2003.PubMed/NCBI
|
|
22
|
Buccione R, Orth JD and McNiven MA: Foot
and mouth: Podosomes, invadopodia and circular dorsal Ruffles. Nat
Rev Mol Cell Biol. 5:647–657. 2004.PubMed/NCBI
|
|
23
|
Chuang YY: Role of synaptojanin 2 in
glioma cell migration and invasion. Cancer Res. 64:8271–8275.
2004.PubMed/NCBI
|
|
24
|
Tu Y: The discovery of artemisinin
(qinghaosu) and gifts from Chinese medicine. Nat Med. 17:1217–1220.
2011.PubMed/NCBI
|
|
25
|
Wang YJ, Pan KL, Hsieh TC, Chang TY, Lin
WH and Hsu JT: Diosgenin, a plant-derived sapogenin, exhibits
antiviral activity in vitro against hepatitis c virus. J Nat Prod.
74:580–584. 2011.PubMed/NCBI
|
|
26
|
Cayen MN and Dvornik D: Effect of
diosgenin on lipid metabolism in rats. J Lipid Res.
20:1621979.PubMed/NCBI
|
|
27
|
Chen Y, Tang YM, Yu SL, Han YW, Kou JP,
Liu BL and Yu BY: Advances in the pharmacological activities and
mechanisms of diosgenin. Chin J Nat Med. 13:578–587.
2015.PubMed/NCBI
|
|
28
|
Li F, Fernandez PP, Rajendran P, Hui KM
and Sethi G: Diosgenin, a steroidal saponin, inhibits STAT3
signaling pathway leading to suppression of proliferation and
chemosensitization of human hepatocellular carcinoma cells. Cancer
Lett. 292:197–207. 2010.PubMed/NCBI
|
|
29
|
Tao X, Yin L, Xu L and Peng J: Dioscin: A
diverse acting natural compound with therapeutic potential in
metabolic diseases, cancer, inflammation and infections. Pharmacol
Res. 137:259–269. 2018.PubMed/NCBI
|
|
30
|
Sethi G, Shanmugam MK, Warrier S, Merarchi
M, Arfuso F, Kumar AP and Bishayee A: Pro-Apoptotic and Anti-cancer
properties of diosgenin: A comprehensive and critical review.
Nutrients. 10:6452018.
|
|
31
|
Ma HY, Zhou LL and Wang BX: Antagonistic
effect of DX and diosgenin on hyperlipidemia induced by cholesterol
in vivo and on blood platelet aggregation in vitro. Chin J Hos
Pharmacy. 22:323–325. 2002.
|
|
32
|
Raju J and Mehta R: Cancer chemopreventive
and therapeutic effects of diosgenin, a food saponin. Nutr Cancer.
61:27–35. 2009.PubMed/NCBI
|
|
33
|
Chen PS, Shih YW, Huang HC and Cheng HW:
Diosgenin, a steroidal saponin, inhibits migration and invasion of
human prostate cancer pc-3 cells by reducing matrix
metalloproteinases expression. PLoS One. 6:e201642011.PubMed/NCBI
|
|
34
|
Nie C, Zhou J, Qin X, Shi X, Zeng Q, Liu
J, Yan S and Zhang L: Diosgenin-induced autophagy and apoptosis in
a human prostate cancer cell line. Mol Med Rep. 14:4349–4359.
2016.PubMed/NCBI
|
|
35
|
Sun GC, Jan CR and Liang WZ: Exploring the
impact of a naturally occurring sapogenin diosgenin on underlying
mechanisms of Ca2+ movement and cytotoxicity in human
prostate cancer cells. Environ Toxicol. 35:395–403. 2020.PubMed/NCBI
|
|
36
|
Hu M, Xu L, Yin L, Qi Y, Li H, Xu Y, Han
X, Peng J and Wan X: Cytotoxicity of dioscin in human gastric
carcinoma cells through death receptor and mitochondrial pathways.
J Appl Toxicol. 33:712–722. 2013.PubMed/NCBI
|
|
37
|
Zhao X, Xu L, Zheng L, Yin L, Qi Y, Han X,
Xu Y and Peng J: Potent effects of dioscin against gastric cancer
in vitro and in vivo. Phytomedicine. 23:274–282. 2016.PubMed/NCBI
|
|
38
|
Rahmati-Yamchi M, Ghareghomi S, Haddadchi
G, Milani M, Aghazadeh M and Daroushnejad H: Fenugreek extract
diosgenin and pure diosgenin inhibit the htert gene expression in
a549 lung cancer cell line. Mol Biol Rep. 41:6247–6252.
2014.PubMed/NCBI
|
|
39
|
Xu L, Xu D, Li Z, Gao Y and Chen H:
Synthesis and potent cytotoxic activity of a novel diosgenin
derivative and its phytosomes against lung cancer cells. Beilstein
J Nanotechnol. 10:1933–1942. 2019.PubMed/NCBI
|
|
40
|
Srinivasan S, Koduru S, Kumar R,
Venguswamy G, Kyprianou N and Damodaran C: Diosgenin targets
Akt-mediated prosurvival signaling in human breast cancer cells.
Int J Cancer. 125:961–967. 2009.PubMed/NCBI
|
|
41
|
Swamy MV, Patlolla JM, Jayadev R, Marcus
LA, Choi CI and Rao CV: Chemoprevention of colon cancer by
diosgenin, a steroidal saponin constituent of fenugreek. Cancer
Res. 652005.
|
|
42
|
Shishodia S and Aggarwal BB: Diosgenin
inhibits osteoclastogenesis, invasion, and proliferation through
the downregulation of Akt, I kappa B kinase activation and NF-kappa
B-regulated gene expression. Oncogene. 25:1463–1473.
2006.PubMed/NCBI
|
|
43
|
Liagre B, Bertrand J, Leger DY and
Beneytout JL: Diosgenin, a plant steroid, induces apoptosis in
COX-2 deficient K562 cells with activation of the p38 MAP kinase
signalling and inhibition of NF-kappaB binding. Int J Mol Med.
16:1095–1101. 2005.PubMed/NCBI
|
|
44
|
Cai H, Gong L, Liu J, Zhou Q and Zheng Z:
Diosgenin inhibits tumor angiogenesis through regulating
GRP78-mediated HIF-1α and VEGF/VEGFR signaling pathways. Pharmazie.
74:680–684. 2019.PubMed/NCBI
|
|
45
|
Lepage C, Léger DY, Bertrand J, Martin F,
Beneytout JL and Liagre B: Diosgenin induces death receptor-5
through activation of p38 pathway and promotes TRAIL-induced
apoptosis in colon cancer cells. Cancer Lett. 301:193–202.
2011.PubMed/NCBI
|
|
46
|
Fang K, Dong H, Jiang S, Li F, Wang D,
Yang D, Gong J, Huang W and Lu F: Diosgenin and 5-Methoxypsoralen
ameliorate insulin resistance through ER-α/PI3K/Akt-signaling
pathways in HepG2 cells. Evid Based Complement Alternat Med.
2016:74936942016.PubMed/NCBI
|
|
47
|
Corbiere C, Liagre B, Bianchi A, Bordji K,
Dauça M, Netter P and Beneytout JL: Different contribution of
apoptosis to the antiproliferative effects of diosgenin and other
plant steroids, hecogenin and tigogenin, on human 1547 osteosarcoma
cells. Int J Oncol. 22:899–905. 2003.PubMed/NCBI
|
|
48
|
Corbiere C, Liagre B, Terro F and
Beneytout JL: Induction of antiproliferative effect by diosgenin
through activation of p53, release of apoptosis-inducing factor
(AIF) and modulation of caspase-3 activity in different human
cancer cells. Cell Res. 14:188–196. 2004.PubMed/NCBI
|
|
49
|
Wang WC, Liu SF, Chang WT, Shiue YL, Hsieh
PF, Hung TJ, Hung CY, Hung YJ, Chen MF and Yang YL: The effects of
diosgenin in the Regulation of renal proximal tubular fibrosis. Exp
Cell Res. 323:255–262. 2014.PubMed/NCBI
|
|
50
|
He Z, Chen H, Li G, Zhu H, Gao Y, Zhang L
and Sun J: Diosgenin inhibits the migration of human breast cancer
MDA-MB-231 cells by suppressing Vav2 activity. Phytomedicine.
21:871–876. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wani SA and Kumar P. Fenugreek: A review
on its nutraceutical properties and utilization in various food
products. J Saudi Soc Agricultural Sci. 17:97–106. 2018.
|
|
52
|
Wang C, Huo X, Wang L, Meng Q, Liu Z, Liu
Q, Sun H, Sun P, Peng J and Liu K: Dioscin strengthens the
efficiency of Adriamycin in MCF-7 and MCF-7/ADR cells through
autophagy induction: More than just down-regulation of MDR1. Sci
Rep. 6:284032016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Belsches AP, Haskell MD and Parsons SJ:
Role of c-Src tyrosine kinase in EGF-induced mitogenesis. Front
Biosci. 2:d501–d518. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
54
|
Yin M, Ma W and An L: Cortactin in cancer
cell migration and invasion. Oncotarget. 8:88232–88243. 2015.
View Article : Google Scholar
|
|
55
|
Chien HT, Cheng SD, Chuang WY, Liao CT,
Wang HM and Huang SF: Clinical implications of fadd gene
amplification and protein overexpression in taiwanese oral cavity
squamous cell carcinomas. PLoS One. 11:e01648702016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Bryce NS, Clark ES, Leysath JL, Currie JD,
Webb DJ and Weaver AM: Cortactin promotes cell motility by
enhancing lamellipodial persistence. Curr Biol. 15:1276–1285. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Uruno T, Liu J, Li Y, Smith N and Zhan X:
Sequential interaction of actin-related proteins 2 and 3 (Arp2/3)
complex with neural Wiscott-Aldrich syndrome protein (N-WASP) and
cortactin during branched actin filament network formation. J Biol
Chem. 278:26086–26093. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Cosenbinker LI and Kapus A: Cortactin: The
gray eminence of the cytoskeleton. Physiology (Bethesda).
21:352–361. 2006.PubMed/NCBI
|
|
59
|
Mizutani K, Miki H, He H, Maruta H and
Takenawa T: Essential role of neural Wiskott-Aldrich syndrome
protein in podosome formation and degradation of extracellular
matrix in src-transformed fibroblasts. Cancer Res. 62:669–674.
2002.PubMed/NCBI
|
|
60
|
Kinley AW, Weed SA, Weaver AM, Karginov
AV, Bissonette E, Cooper JA and Parsons JT: Cortactin Interacts
with WIP in regulating Arp2/3 activation and membrane protrusion.
Curr Biol. 13:384–393. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Lin J, Liu J, Wang Y, Zhu J, Zhou K, Smith
N and Zhan X: Differential regulation of cortactin and
N-WASP-mediated actin polymerization by missing in metastasis (MIM)
protein. Oncogene. 24:2059–2066. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Buday L and Downward J: Roles of cortactin
in tumor pathogenesis. Biochim Biophys Acta. 1775:263–273.
2007.PubMed/NCBI
|
|
63
|
He Y, Ren Y, Wu B, Decourt B, Lee AC,
Taylor A and Suter DM: Src and cortactin promote lamellipodia
protrusion and filopodia formation and stability in growth cones.
Mol Biol Cell. 26:3229–3244. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Krueger EW, Orth JD, Cao H and McNiven MA:
A Dynamin-Cortactin-Arp2/3 complex mediates actin reorganization in
growth factor-stimulated cells. Mol Biol Cell. 14:1085–1096. 2013.
View Article : Google Scholar
|
|
65
|
Oser M, Yamaguchi H, Mader CC,
Bravo-Cordero JJ, Arias M, Chen X, Desmarais V, van Rheenen J,
Koleske AJ and Condeelis J: Cortactin regulates cofilin and N-WASp
activities to control the stages of invadopodium assembly and
maturation. J Cell Biol. 186:571–587. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Ayala I, Baldassarre M, Giacchetti G,
Caldieri G, Tetè S, Luini A and Buccione R: Multiple regulatory
inputs converge on cortactin to control invadopodia biogenesis and
extracellular matrix degradation. J Cell Sci. 121:369–378. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Ren XL, Qiao YD, Li JY, Li XM, Zhang D,
Zhang XJ, Zhu XH, Zhou WJ, Shi J, Wang W, et al: Cortactin recruits
FMNL2 to promote actin polymerization and endosome motility in
invadopodia formation. Cancer Lett. 419:245–256. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Desmarais V, Yamaguchi H, Oser M, Soon L,
Mouneimne G, Sarmiento C, Eddy R and Condeelis J: N-WASP and
cortactin are involved in invadopodium-dependent chemotaxis to EGF
In breast tumor cells. Cell Motil Cytoskeleton. 66:303–316. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Uruno T, Liu J, Zhang P, Fan YX, Egile C,
Li R, Mueller SC and Zhan X: Activation of Arp2/3 complex-mediated
actin polymerization by cortactin. Nat Cell Biol. 3:259–266. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Weaver AM, Karginov AV, Kinley AW, Weed
SA, Li Y, Parsons JT and Cooper JA: Cortactin promotes and
stabilizes Arp2/3-induced actin filament network formation. Curr
Biol. 11:370–374. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Artym VV, Zhang Y, Seillier-Moiseiwitsch
F, Yamada KM and Mueller SC: dynamic interactions of cortactin and
membrane type 1 matrix metalloproteinase at invadopodia: Defining
the stages of invadopodia formation and function. Cancer Res.
66:3034–3043. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Khaitlina SY: Intracellular transport
based on actin polymerization. Biochemistry (Mosc). 79:917–927.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Ren G, Crampton MS and Yap AS: Cortactin:
Coordinating adhesion and the actin cytoskeleton at cellular
protrusions. Cell Motil Cytoskeleton. 66:865–873. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Tehrani S, Faccio R, Chandrasekar I, Ross
FP and Cooper JA: Cortactin has an essential and specific role in
osteoclast actin assembly. Mol Biol Cell. 17:2882–2895. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
van Rossum AG, Moolenaar WH and Schuuring
E: Cortactin affects cell migration by regulating intercellular
adhesion and cell spreading. Exp Cell Res. 312:1658–1670. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Steven M: Markwell; Amanda Gatesman Ammer,
Interval ET, Schafer DA Hames RA and Weed SA: Abstract 5067: Casein
kinase 2 alpha phosphorylation of cortactin governs actin
cytoskeletal regulation of invadopodia function. Cancer Res. 76 (14
Suppl):S5067. 2016.
|
|
77
|
Jeannot P and Besson A: Cortactin function
in invadopodia. Small GTPases. 11:256–270. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Meng DF, Xie P, Peng LX, Sun R, Luo DH,
Chen QY, Lv X, Wang L, Chen MY, Mai HQ, et al: CDC42-interacting
protein 4 promotes metastasis of nasopharyngeal carcinoma by
mediating invadopodia formation and activating EGFR signaling. J
Exp Clin Cancer Res. 36:212017. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Genna A, Lapetina S, Lukic N, Twafra S,
Meirson T, Sharma VP, Condeelis JS and Gil-Henn H: Pyk2 and FAK
differentially regulate invadopodia formation and function in
breast cancer cells. J Cell Biol. 217:375–395. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Kempiak SJ, Yamaguchi H, Sarmiento C,
Sidani M, Ghosh M, Eddy RJ, Desmarais V, Way M, Condeelis J and
Segall JE: A Neural Wiskott-aldrich syndrome protein-mediated
pathway for localized activation of actin polymerization that is
regulated by cortactin. J Biol Chem. 280:5836–5842. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Wang W, Liu Y and Liao K: Tyrosine
phosphorylation of cortactin by the FAK-Src complex at focal
adhesions regulates cell motility. BMC Cell Biol. 12:492011.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Huang C, Ni Y, Wang T, Gao Y, Haudenschild
CC and Zhan X: Down-regulation of the filamentous actin
cross-linking activity of cortactin by Src-mediated tyrosine
phosphorylation. J Biol Chem. 272:13911–13915. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Leger DY, Liagre B and Beneytout JL: Role
of MAPKs and NF-kappaB in diosgenin-induced megakaryocytic
differentiation and subsequent apoptosis in HEL cells. Int J Oncol.
28:201–207. 2006.PubMed/NCBI
|
|
84
|
Siar CH, Rahman ZA, Tsujigiwa H, Mohamed
OM, Alblazi K, Nagatsuka H and Ng KH: Invadopodia proteins,
cortactin, N-WASP and WIP differentially promote local invasiveness
in ameloblastoma. J Oral Pathol Med. 45:591–598. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Tong Q, Qing Y, Wu Y, Hu X, Jiang L and Wu
X: Dioscin inhibits colon tumor growth and tumor angiogenesis
through regulating VEGFR2 and AKT/MAPK signaling pathways. Toxicol
Appl Pharmacol. 281:166–173. 2014.PubMed/NCBI
|
|
86
|
Farhan MA, Azad AK, Touret N and Murray
AG: FGD5 Regulates VEGF Receptor-2 Coupling to PI3 kinase and
receptor recycling. Arterioscler Thromb Vasc Biol. 37:2301–2310.
2017.PubMed/NCBI
|
|
87
|
Choi KW, Park HJ, Jung DH, Kim TW, Park
YM, Kim BO, Sohn EH, Moon EY, Um SH, Rhee DK and Pyo S: Inhibition
of TNF-α-induced adhesion molecule expression by diosgenin in mouse
vascular smooth muscle cells via downregulation of the MAPK, Akt
and NF-κB signaling pathways. Vascul Pharmacol. 53:273–280.
2010.PubMed/NCBI
|
|
88
|
Li S, Cheng B, Hou L, Huang L, Cui Y, Xu
D, Shen X and Li S: Dioscin inhibits colon cancer cells' growth by
reactive oxygen species-mediated mitochondrial dysfunction and p38
and JNK pathways. Anticancer Drugs. 29:234–242. 2018.PubMed/NCBI
|
|
89
|
Lin SC, Gou GH, Hsia CW, Ho CW, Huang KL,
Wu YF, Lee SY and Chen YH: Simulated microgravity disrupts
cytoskeleton organization and increases apoptosis of rat neural
crest stem cells via upregulating CXCR4 expression and
RhoA-ROCK1-p38 MAPK-p53 signaling. Stem Cells Dev. 25:1172–1193.
2016.PubMed/NCBI
|
|
90
|
Chiang CT, Way TD, Tsai SJ and Lin JK:
Diosgenin, a naturally occurring steroid, suppresses fatty acid
synthase expression in HER2-overexpressing breast cancer cells
through modulating Akt, mTOR and JNK phosphorylation. FEBS Lett.
581:5735–5742. 2007.PubMed/NCBI
|
|
91
|
Wang YC, Wu DW, Wu TC, Wang L, Chen CY and
Lee H: Dioscin overcome TKI resistance in EGFR-mutated lung
adenocarcinoma cells via Down-regulation of tyrosine phosphatase
SHP2 expression. Int J Biol Sci. 14:47–56. 2018.PubMed/NCBI
|
|
92
|
Gao M, Chen L, Yu H, Sun Q, Kou J and Yu
B: Diosgenin down-regulates NF-κB p65/p50 and p38MAPK pathways and
attenuates acute lung injury induced by lipopolysaccharide in mice.
Int Immunopharmacol. 15:240–245. 2013.PubMed/NCBI
|
|
93
|
Song JS, Ma L, Kou J and Yu BY: Diosgenin
reduces leukocytes adhesion and migration linked with inhibition of
intercellular adhesion molecule-1 expression and NF-kB p65
activation in endothelial cells. Chin J Nat Med. 10:142–149.
2012.
|
|
94
|
Tavora B, Reynolds LE, Batista S,
Demircioglu F, Fernandez I, Lechertier T, Lees DM, Wong PP,
Alexopoulou A, Elia G, et al: Endothelial-cell FAK targeting
sensitizes tumours to DNA-damaging therapy. Nature. 514:112–116.
2014.PubMed/NCBI
|
|
95
|
Liu Z, Zhang HM, Yuan J, Lim T, Sall A,
Taylor GA and Yang D: Focal adhesion kinase mediates the
interferon-gamma-inducible GTPase-induced phosphatidylinositol
3-kinase/Akt survival pathway and further initiates a positive
feedback loop of NF-kappaB activation. Cell Microbiol.
10:1787–1800. 2010.
|
|
96
|
Chen J, Zhang W, Wang Y, Zhao D, Wu M, Fan
J, Li J, Gong Y, Dan N, Yang D, et al: The diacylglycerol kinase α
(DGKα)/Akt/NF-κB feedforward loop promotes esophageal squamous cell
carcinoma (ESCC) progression via FAK-dependent and FAK-independent
manner. Oncogene. 38:2533–2550. 2019.PubMed/NCBI
|
|
97
|
Irby RB and Yeatman TJ: Role of Src
expression and activation in human cancer. Oncogene. 19:5636–5642.
2000.PubMed/NCBI
|
|
98
|
Zhou X, Yang F, Zhang Q, Miao Y, Hu X, Li
A, Hou G, Wang Q and Kang J: FAM129B promoted tumor invasion and
proliferation via facilitating the phosphorylation of FAK signaling
and associated with adverse clinical outcome of non-small cell lung
cancer patients. Onco Targets Ther. 11:7493–7501. 2018.PubMed/NCBI
|
|
99
|
Avizienyte E and Frame MC: Src and FAK
signalling controls adhesion fate and the epithelial-to-mesenchymal
transition. Curr Opin Cell Biol. 17:542–547. 2005.PubMed/NCBI
|
|
100
|
Mitra SK and Schlaepfer DD:
Integrin-regulated FAK-Src signaling in normal and cancer cells.
Curr Opin Cell Biol. 18:516–523. 2006.PubMed/NCBI
|
|
101
|
Liang Y, Yi L, Liu P, Jiang L, Wang H, Hu
A, Sun C and Dong J: CX3CL1 involves in breast cancer metastasizing
to the spine via the Src/FAK signaling pathway. J Cancer.
9:3603–3612. 2018.PubMed/NCBI
|
|
102
|
Saijo K, Schmedt C, Su IH, Karasuyama H,
Lowell CA, Reth M, Adachi T, Patke A, Santana A and Tarakhovsky A:
Essential role of Src-family protein tyrosine kinases in NF-kappaB
activation during B cell development. Nat Immunol. 4:274–279.
2003.PubMed/NCBI
|
|
103
|
Chen L, Chen H and Liu F: RACK1 to
modulate expression of MMP10 via Src/NF-κB pathway in gastric
cancer. J Clin Oncol. 35 (15_suppl):e155292017.
|
|
104
|
Lai SW, Bamodu OA, Tsai WC, Chang YM, Lee
WH, Yeh CT and Chao TY: The therapeutic targeting of the
FGFR1/Src/NF-κB signaling axis inhibits pancreatic ductal
adenocarcinoma stemness and oncogenicity. Clin Exp Metastasis.
35:663–677. 2018.PubMed/NCBI
|
|
105
|
Peng X, Zhang Q, Zeng Y, Li J, Wang L and
Ai P: Evodiamine inhibits the migration and invasion of
nasopharyngeal carcinoma cells in vitro via repressing MMP-2
expression. Cancer Chemother Pharmacol. 76:1173–1184.
2015.PubMed/NCBI
|
|
106
|
Lian S, Xia Y, Khoi PN, Ung TT, Yoon HJ,
Kim NH, Kim KK and Jung YD: Cadmium induces matrix
metalloproteinase-9 expression via ROS-dependent EGFR, NF-кB, and
AP-1 pathways in human endothelial cells. Toxicology. 338:104–116.
2015.PubMed/NCBI
|
|
107
|
Hung CY, Lee CH, Chiou HL, Lin CL, Chen
PN, Lin MT, Hsieh YH and Chou MC: Praeruptorin-B Inhibits
12-O-tetradecanoylphorbol-13-Acetate-induced cell invasion by
targeting AKT/NF-κB via matrix metalloproteinase-2/-9 expression in
human cervical cancer cells. Cell Physiol Biochem. 52:1255–1266.
2019.PubMed/NCBI
|
|
108
|
Sabir N, Hussain T, Mangi MH, Zhao D and
Zhou X: Matrix metalloproteinases: Expression, regulation and role
in the immunopathology of tuberculosis. Cell Prolif.
52:e126492019.PubMed/NCBI
|
|
109
|
Liu LP, Liang HF, Chen XP, Zhang WG, Yang
SL, Xu T and Ren L: The role of NF-kappaB in Hepatitis B virus X
protein-mediated upregulation of VEGF and MMPs. Cancer Invest.
28:443–451. 2010.PubMed/NCBI
|
|
110
|
Zhang X, Jin M, Tadesse N, Dang J, Zhou T,
Zhang H, Wang S, Guo Z and Ito Y: Dioscorea zingiberensis C.
H. Wright: An overview on its traditional use, phytochemistry,
pharmacology, clinical applications, quality control, and toxicity.
J Ethnopharmacol. 220:283–293. 2018.PubMed/NCBI
|
|
111
|
Wu FC and Jiang JG: Effects of diosgenin
and its derivatives on atherosclerosis. Food Funct. 10:7022–7036..
2019.PubMed/NCBI
|
|
112
|
Tao W, Luo X, Cui B, Liang D, Wang C, Duan
Y, Li X, Zhou S, Zhao M, Li Y, et al: Practice of traditional
Chinese medicine for psycho-behavioral intervention improves
quality of life in cancer patients: A systematic review and
meta-analysis. Oncotarget. 6:39725–39739. 2015.PubMed/NCBI
|
|
113
|
Wang J, Wong YK and Liao F: What has
traditional Chinese medicine delivered for modern medicine? Expert
Rev Mol Med. 20:e42018.PubMed/NCBI
|
|
114
|
White NJ: Qinghaosu (Artemisinin): The
price of success. Science. 320:330–334. 2008.PubMed/NCBI
|
|
115
|
Zhou L, Zuo Z and Chow MS: Danshen: An
overview of its chemistry, pharmacology, pharmacokinetics, and
clinical use. J Clin Pharmacol. 45:1345–1349. 2005.PubMed/NCBI
|
|
116
|
Yi-Lan LI, Shan-Shan Q and Guo-Xing LI:
Effect of glossy ganoderma on antitumor and immune function in
mice. Chin J Prevention Control Chronic Non-Communicable Dis.
2004.
|
|
117
|
Liu P, Zhao H and Luo Y: Anti-aging
implications of astragalus membranaceus (Huangqi): A Well-known
Chinese tonic. Aging Dis. 8:868–886. 2017.PubMed/NCBI
|
|
118
|
Wang K, Wu J, Duan X, Wu J, Zhang D, Zhang
X and Zhang B: Huangqi injection in the treatment of chronic heart
failure: A systematic review and meta-analysis. Medicine
(Baltimore). 96:e81672017.PubMed/NCBI
|
|
119
|
de Martel C, Forman D and Plummer M:
Gastric cancer: Epidemiology and risk factors. Gastroenterol Clin
North Am. 42:219–240. 2013.PubMed/NCBI
|
|
120
|
Camargo MC, Anderson WF, King JB, Correa
P, Thomas CC, Rosenberg PS, Eheman CR and Rabkin CS: Divergent
trends for gastric cancer incidence by anatomical subsite in US
adults. Gut. 60:1644–169. 2011.PubMed/NCBI
|
|
121
|
Steevens J, Botterweck AAM, Dirx MJ, van
den Brandt PA and Schouten LJ: Trends in incidence of oesophageal
and stomach cancer subtypes in Europe. Eur J Gastroenterol Hepatol.
22:669–678. 2010.PubMed/NCBI
|