Introduction
Granulosa cell tumors (GCTs) comprise granulosa
cells and stromal components (1).
GCTs are generally low-grade malignancies, manifested by indolent
growth and a low risk of metastasis (1). However, the prognosis of GCTs is
stage-dependent, and patients at advanced tumor stages tend to have
a higher risk of recurrence (2),
making long-term surveillance necessary. The recurrence also
increases the mortality rate and the economic/emotional burden of
the patients. Thus, it is critical to understand the molecular
mechanism of GCT development and identify predictors for tumor
recurrence and optimal regimen for tumor treatment.
Ovarian GCTs are the major type of malignant sex
cord-stromal tumors (3). There are
two subtypes of ovarian GCTs, namely the adult type and the
juvenile type (4). It has been
reported that >80% of girls <8 years of age with
juvenile-type GCTs demonstrate precocious pseudopuberty (5). By contrast, adult-type GCTs often occur
in perimenopausal women, with an unpredictable outcome of relapse.
The development of adult-type GCTs is often accompanied by symptoms
of hormone dysregulation (e.g., amenorrhea, uterine bleeding and
endometrial hyperplasia) (6,7). The clinical symptoms, diagnostic
imaging, histology of surgery-obtained tumor samples and presence
of tumor markers [e.g., inhibins and anti-Mullerian hormone (AMH)]
provide useful information for the diagnosis of GCTs (8,9).
GCTs can also occur in the testis. Similar to
ovarian GCTs, testicular GCTs (TGCTs) contain the adult and the
juvenile subtypes. While ovarian GCTs account for ~90% of ovarian
sex cord-stromal tumors (reported in 2012) (4), the adult or juvenile type of TGCTs
accounts for <0.5% of testicular sex cord-stromal tumors
(reported in 2017) (10). Although
similarities exist between GCTs in the testis and the ovary
(11,12), mechanisms underlying the development
of these tumors remain poorly characterized, partially owing to the
rarity of this type of testicular malignancy. In the present
review, the subtypes and pathology of TGCTs and important signaling
pathways associated with tumorigenesis are discussed. The study
delves into forkhead box L2 (FOXL2)-related signaling,
wingless-related MMTV integration site (WNT)/β-Catenin (CTNNB1)
signaling, the phosphoinositide 3-kinase (PI3K) pathway and the
transforming growth factor β (TGFβ) pathway in the development of
TGCTs. With the development of new mouse models that focus on
TGCTs, it is anticipated that the pace of investigation into the
molecular and genetic basis of these tumors will be
accelerated.
Tumors in the testes
Testicular tumors occur mostly in males of 14–44
years old (13). Based on the 2016
classification by the World Health Organization, testicular tumors
contain germ cell tumors of two groups [i.e., tumors derived from
germ cells neoplasia in situ (GCNIS) and those unrelated to GCNIS],
as well as sex cord-stromal tumors and several other types
(14). Germ cell tumors account for
the majority of testicular tumors. Sex cord-stromal tumors make up
4% of tumors in the testis (15) and
consist of Leydig cell tumors, Sertoli cell tumors, GCTs, fibroma
and thecoma group tumors, mixed-type tumors and unclassified tumors
(14). Leydig cell tumors are the
most common type of sex cord-stromal tumors. These tumors are often
well circumscribed and appear brown, yellow or gray-white in color
on the cut surface (16). The cell
types in a given Leydig cell tumor may be variable. Histologically,
the cells are often medium to large in size and polygonal in shape,
with eosinophilic granular cytoplasm (16,17). Due
to the histological and immunohistochemical similarities between
GCTs in females and males (11,12), a
comparative approach is likely to be valuable in gaining
mechanistic insights into tumorigenesis and discovering common
regulatory pathways. As the causes and pathogenesis of these rare
testicular tumors are poorly defined, clinically relevant mouse
models are particularly useful in this research field to determine
the oncogenic insult and potential therapeutic targets (12,18,19).
TGCTs: Subtypes and histopathology
TGCTs can be divided into the juvenile type and the
adult type (Table I). Juvenile-type
TGCT is a more common form compared with adult-type TGCT. The
juvenile type represents the most common tumors in the male gonad
in patients <6 months of age and can even be diagnosed shortly
after birth due to the increased size of the testis (20). Histologically, follicular components
are present in juvenile-type TGCTs (10,20).
Tumor cells have round dense nuclei with infrequent nuclear
grooves, and abundant mitosis can be found (21). The juvenile-type tumors are generally
benign, with rarely observed metastasis. In a report of 70 cases,
only 2 cases showed lymphovascular invasion and 4 cases exhibited
rete testis involvement (21). The
juvenile-type TGCTs were reported to be positively stained for
FOXL2, steroidogenic factor-1 and vimentin (21). Some tumors also express inhibin,
calretinin, Wilms tumor 1 and SRY-box transcription factor 9 (SOX9)
(21). As inhibin is expressed by
both granulosa cells and Sertoli cells, it is unclear whether the
variable expression of the inhibin observed in juvenile TGCTs is
stage-dependent or merely reflects the individual variation of
these tumors.
 | Table I.Differences between the TGCT
subtypes. |
Table I.
Differences between the TGCT
subtypes.
| TGCT-related
features | Juvenile-type
TGCTs | Adult-type
TGCTs | (Refs.) |
|---|
| Age | Most common tumors
in the testis at <6 months of age | Median age, 44
years (range, 12–87 years) | (10,25) |
| Metastasis | Rare | Metastatic
potential | (21,27) |
| Macroscopic
feature | Yellow to tan-white
cut surface; cystic or solid structures | Yellow-tan cut
surface; solid and/or cystic structures | (10,21) |
| Microscopic
feature | Round dense nuclei;
infrequent nuclear grooves; abundant mitosis | Vague cell borders;
pale nuclei with nuclear grooves; Call-Exner bodies | (10,21,26) |
|
Genomics/genetics | Abnormal sex
chromosome and gonadal development | Some tumors contain
the FOXL2 mutation | (22,23,39) |
Some studies have suggested that the formation of
granulosa cell tumors is associated with sex chromosome
abnormalities and aberrant gonadal development (22,23). It
has been shown that infants with mixed gonadal dysgenesis or
intersexual disorder develop juvenile-type GCTs (23). Another example of this link was found
in the case of a newborn baby with the X/XY karyotype who developed
congenital juvenile-type TGCT (22).
The levels of inhibin B, β-hCG and testosterone appear normal in
some juvenile-type GCT patients (20). High levels of serum α-fetoprotein
(AFP) are observed in some juvenile-type TGCTs (20,21);
however, AFP levels are physiologically high in infants and
newborns (24).
Adult-type TGCTs are extremely rare, with 91 cases
described to date (25).
Microscopically, the tumor cells have vague cell borders and pale
nuclei containing nuclear grooves (10,26). The
tumor cells are less mitotic compared with those of juvenile-type
GCTs (10). It is notable that
juvenile-type TGCTs lack Call-Exner bodies (i.e., small
eosinophilic fluid-filled spaces within microfollicular structures)
that are observed in the adult-type TGCTs (10). Although most adult-type TGCTs are
benign, the metastatic potential of these tumors remains of
concern. For instance, in a previous study, one patient was found
to develop metastases 10 years after the first diagnosis, while
additional metastasis was found in the inguinal lymph node of
another patient 1 year after the diagnosis and detection of
retroperitoneal lymph node metastasis (27). In another case, metastasis was found
in the bone of a patient 6 years after orchidectomy surgery
(28). Thus, long-term
follow-up/monitoring is needed for patients with TGCTs.
Histopathologically, the adult-type GCTs are identified as solid
and/or cystic tumors (10).
Laterality has been reported in most documented adult-type GCT
cases in males (25). The
histological/pathological criteria or clinical features that
predict the malignant/benign disposition of TGCTs are not well
defined. It appears that tumor size (>5 cm), but not mitotic
count, tumor necrosis or other parameters, is positively associated
with the malignancy of adult-type TGCTs (29). Orchidectomy and testis-sparing
surgery have been used to treat TGCTs (25). Currently, it remains unclear with
regard to the genetic or molecular determinants that contribute to
the phenotypic and prognostic outcomes of the juvenile-type versus
the adult-type TGCTs. Answering this question may help develop
tailored treatment options for the two subtypes of TGCTs.
FOXL2 mutation in GCT
development
FOXL2, a granulosa cell-expressed gene,
regulates granulosa cell fate and ovarian function (30). Supporting a critical role of
Foxl2 as a female gene, disruption of FOXL2 in adult ovaries
induces the expression of SOX9 specific to the male gonad (31). FOXL2 is expressed in juvenile-type
TGCTs (32). Notably, the expression
of SOX9 is found in the cytoplasm of FOXL2-positive cells in some
juvenile-type TGCTs (32). As FOXL2
is a granulosa cell lineage marker, this finding suggests potential
Sertoli cell-granulosa cell transdifferentiation during the
formation of TGCTs (32).
A missense mutation of FOXL2 [nt. 402C>G
(C134W)] is vital in the pathogenesis of adult-type ovarian GCTs
(33). With regard to its
contribution to GCT development, studies have shown that this
mutation impairs the capability of growth differentiation factor 9,
an oocyte-produced protein, in promoting follistatin transcription
in the presence of SMAD3 (34). This
may lead to increased cell proliferation due to unopposed activin
signaling (34,35). In addition, FOXL2 mutation
also reduces apoptosis and increases the induction of aromatase
(CYP19), which promotes estrogen synthesis (36–38).
Lima et al (39) identified a
FOXL2 mutation in adult-type TGCTs, with a lower mutation
frequency compared with that in ovarian GCTs. However, this
mutation was not found by the same researchers in other testicular
tumors such as juvenile-type TGCTs and Sertoli-Leydig cell tumors,
likely due to the limited number of cases examined and/or the low
mutation frequency or lack of mutation in those tumors (39). Thus, mutational analysis of
FOXL2 may prove beneficial in the differential diagnosis of
the two subtypes of TGCTs if they demonstrate a different profile
of FOXL2 mutation. Moreover, an in-depth understanding of
the potential pathogenic function of the FOXL2 mutation in
TGCTs will be instrumental for developing tailored treatment
modalities.
Genetically modified mouse models to study
TGCTs
Elegant reviews on molecular pathogenesis, signaling
pathways and mouse models of ovarian GCTs have been published
(4,40,41). The
present review focuses on several mouse models that have been
reported to develop testicular tumors with a sex cord-stromal
origin (12,18,19,42–44).
Inhibins and activins are key regulators of ovarian development and
function. In the ovary, inhibins are mainly synthesized by
granulosa cells and negatively regulate the secretion of
follicle-stimulating hormone (FSH) (45). In the male gonad, Sertoli cells
produce inhibins that regulate the testicular function (46). Inhibin α (Inha)-knockout mice
develop sex cord-stromal tumors in both sexes (42). The neoplasms are mixed or
incompletely differentiated tumors, accompanied by increased serum
FSH levels (42). Deletion of both
Inha and gonadotropin-releasing hormone inhibits tumor
development and reduces the levels of FSH and luteinizing hormone
(47). CDKN1B (also known as p27) is
a cyclin-dependent kinase inhibitor that suppresses G1
phase progression. Compound deletion of Cdkn1b and
Inha accelerates the development of testicular tumors in
males compared with deletion of Inha alone (48). Deletion of another regulator of the
G1/S transition, cyclin D2, inhibits tumor progression
in Inha null mice (49). Loss
of inhibins potentiates the activin signaling. It has been found
that SMAD3 acts as an essential mediator of the unopposed activin
signaling in testicular tumor development induced by Inha
deletion (50). A sexually dimorphic
function has been observed for SMAD3 in gonadal tumor development
induced by the loss of inhibins, where depletion of SMAD3 has a
more pronounced protective effect on tumorigenesis in the male
compared with that in the female (50).
WNT/CTNNB1 and PI3K/AKT signaling pathways play
important roles in regulating the development of multiple types of
cancer (51–54). In the female, dysregulation of CTNNB1
signaling triggers the formation of ovarian GCTs (52). Male mice bearing conditional
expression of a stable CTNNB1 mutant and deletion of phosphatase
and tensin homolog (Pten) using AMH type 2 receptor
(Amhr2)-cyclization recombination (Cre) develop TGCTs at an
early age, with lung metastases in nearly half of the mice by 4
months (18). These tumors express
Wnt4 and FOXL2 (18). The
mechanism underlying tumor development in this mouse model remains
unclear. A loss of PTEN enhances PI3K/AKT signaling activity and
promotes the phosphorylation of FOXO1A (18); however, the role of FOXO1A in
tumorigenesis awaits further elucidation. Notably, it was recently
found that the conditional overactivation of CTNNB1 in mouse
Sertoli cells using Amh-Cre through elimination of a
Ctnnb1 exon required for CTNNB1 protein degradation induces
transdifferentiation of Sertoli cells into granulosa-like cells and
the formation of TGCTs (43).
Mechanistically, activation of WNT signaling increases the
expression of FOXL2 via the binding of CTNNB1 to the FOXL2
promotor at the T-cell factor/lymphoid enhancer factor binding
sites (43). This finding may also
partially explain how overactivation of CTNNB1 promotes the
formation of TGCTs in the aforementioned mouse model containing
simultaneous activation of WNT and PI3K/AKT signaling (18).
Kirsten rat sarcoma viral oncogene homolog
(Kras) is an oncogene that encodes a small GTPase (55). Expression of KRASG12D
inhibits granulosa cell proliferation and differentiation in early
ovarian follicles, but slightly enhances cell proliferation in
large antral follicles, revealing follicular stage-dependent roles
of the KRAS mutant (56). Mouse
models with oncogenic KRASG12D expression or PTEN
ablation in conjunction with CTNNB1 overactivation using
Amhr2-Cre or Cyp19-Cre have been created to determine
interactions between WNT and PI3K/RAS signaling (19). It was found that constitutive
activation of KRAS or loss of PTEN promotes the development of
ovarian GCTs or TGCTs in stable CTNNB1-expressing mice (19). Consistent with the benign feature of
TGCTs, metastasis was not found and the viability of mice was not
compromised up to 8 months. As expected, these mice are infertile
due to tumor development and impaired spermatogenesis (19).
Members of the FOX family are implicated in multiple
developmental processes and diseases (57,58).
FOXL2 and FOXO3 play key roles in ovarian development and function
(58). FOXO1 acts as a tumor
suppressor through inhibiting CYP19 expression via mutant FOXL2
(C134W) and SMAD3 in the human non-luteinized granulosa cell line
(59). In addition, ~20% of
Foxo1/3 double conditional knockout mice in the ovary using
Amhr2-Cre or Cyp19-Cre develop ovarian GCTs by 6–8
months (60). These tumors cause
increased levels of inhibins and estradiol (60). It is yet unclear whether FOXO1/3 is
involved in TGCT development.
TGFβ superfamily signaling is implicated in numerous
physiological and pathological processes (61). TGFβ ligands signal through
membrane-associated type II and I receptors (TGFBR2/TGFBR1) and
activate receptor-regulated SMADs (R-SMADs), including SMAD2/3
(TGFβ/activin-responsive SMADs) and SMAD1/5/8 [bone morphogenetic
protein (BMP)-responsive SMADs]. R-SMADs then complex with SMAD4 to
elicit biological responses via the regulation of gene
transcription (62). TGFβ signaling
plays divergent roles in cancer development (63) and is important for GCT development
(62). A study by Pangas et
al (44) revealed a role of BMP
signaling in GCT development by demonstrating that conditional
deletion of Smad1 and Smad5 promotes the development
of GCTs in the ovary, but not in the testis. Instead,
Sertoli-Leydig tumors form in Smad1/5 conditionally deleted
males (44). In a continuum of
research interrogating the role of TGFβ signaling in reproductive
development and function, a mouse model has been generated with
constitutively activated TGFBR1 (TGFBR1-CA) in the gonad (12,64).
Both male and female TGFBR1-CA mice develop GCTs (12,64).
TGCTs express granulosa cell markers [i.e., INHA, FOXO1 and FOXL2].
In addition, expression of CTNNB1 is increased in the testes of
TGFBR1-CA mice (12), reinforcing a
role of WNT/CTNNB1 signaling in GCT formation. The cellular origin
of TGCTs remains enigmatic. In male TGFBR1-CA mice, constitutive
activation of TGFBR1 is induced by Amhr2-Cre, which is
expressed in both Sertoli cells and Leydig cells (65–67).
Notably, Sertoli cells and granulosa cells appear to arise from the
same progenitor cells (68).
Moreover, Sertoli cells with dysregulated gene expression can
transdifferentiate into granulosa-like cells (43). Thus, it is conceivable that TGCTs in
TGFBR1-CA males are derived from Sertoli cells. To determine the
potential contribution of Sertoli cells to TGCT formation, the
developmental dynamics of TGCTs were assessed by comprehensive
histological and immunohistochemical analyses (12). It was found that tumors arise within
seminiferous tubules, where the only somatic cell type is the
Sertoli cell (12). Moreover, loss
of doublesex and mab-3 related transcription factor 1 (a
testis-determining protein), and gain of FOXL2 were found in
seminiferous tubules enriched for Sertoli cells in TGFBR1-CA males
(12). Studies are ongoing with
regard to identifying the tumorigenic program in the testis that
mediates the overactivation of TGFβ signaling.
Overall, several key genes and signaling pathways
have been associated with TGCT development (Fig. 1). Although robust genetic evidence
supports the phenotypic relevance of these mouse models to TGCTs,
their potential utility for investigating the etiology and
pathogenesis of TGCTs, as well as testing therapeutic agents,
requires further evaluation.
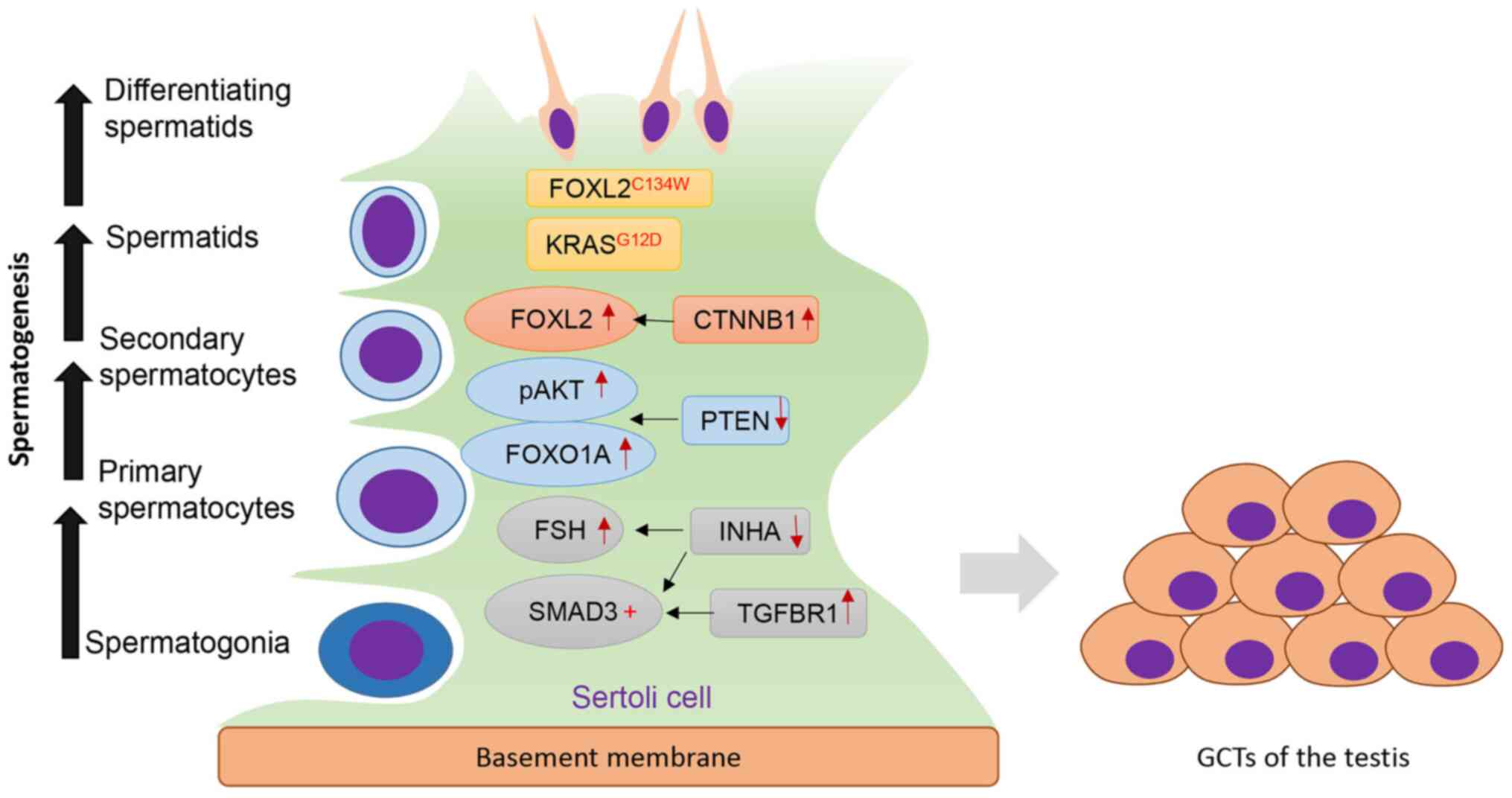 | Figure 1.Key regulators of TGCT development.
Sertoli cells serve an important role in maintaining normal
spermatogenesis. Dysregulation of several genes/signaling pathways
induces the formation of TGCTs. Increased TGFβ signaling via TGFBR1
activates SMAD3, whereas ablation of INHA increases FSH levels and
enhances SMAD3 signaling. Loss of PTEN promotes pAKT and FOXO1A
signaling. Activation of CTNNB1 results in increased expression of
FOXL2. In addition, KRASG12D and FOXL2 mutation
(FOXL2C134W) are also implicated in TGCT development.
TGCT, testicular granulosa cell tumor; FOXL2, forkhead box L2;
KRAS, Kirsten rat sarcoma viral oncogene homolog; CTNNB1,
β-Catenin; PTEN, phosphatase and tensin homolog; FSH,
follicle-stimulating hormone; INHA, inhibin α; TGFBR1, TGF-β
receptor type-1; p, phosphorylated. |
Concluding remarks and future
directions
TGCTs are rare tumors that remain enigmatic in
numerous aspects. To better define tumor etiology and discover
early diagnostic and therapeutic options, it is beneficial to
develop pre-clinical mouse models that recapitulate TGCTs. To
unambiguously define the origin of TGCTs in the TGFBR1-CA mouse
model (12), it is necessary to
specifically activate TGFBR1 using a Cre driver specific to Sertoli
cells (Fig. 2). It is anticipated
that sustained activation of TGFBR1 in Sertoli cells
(TGFBR1-CASC) will induce TGCT development
(Fig. 2). Our future genetic
labeling experiments using a dual fluorescence reporter mouse line,
membrane-targeted tdTomato (mT)/membrane-targeted EGFP (mG)
(69), may elucidate tumor cell
origin. In the mT/mG mouse, Cre-negative cells express tdTomato, a
red fluorescent protein (69)
(Fig. 2A). By contrast, Cre-positive
cells are expected to express GFP that can be tracked by green
fluorescence (69,70) (Fig.
2B). Should TGCTs not occur in these mice, efforts will be
undertaken to investigate how interactions between Sertoli cells
and Leydig cells contribute to the formation of TGCTs in the
context of TGFBR1 activation (Fig.
2B).
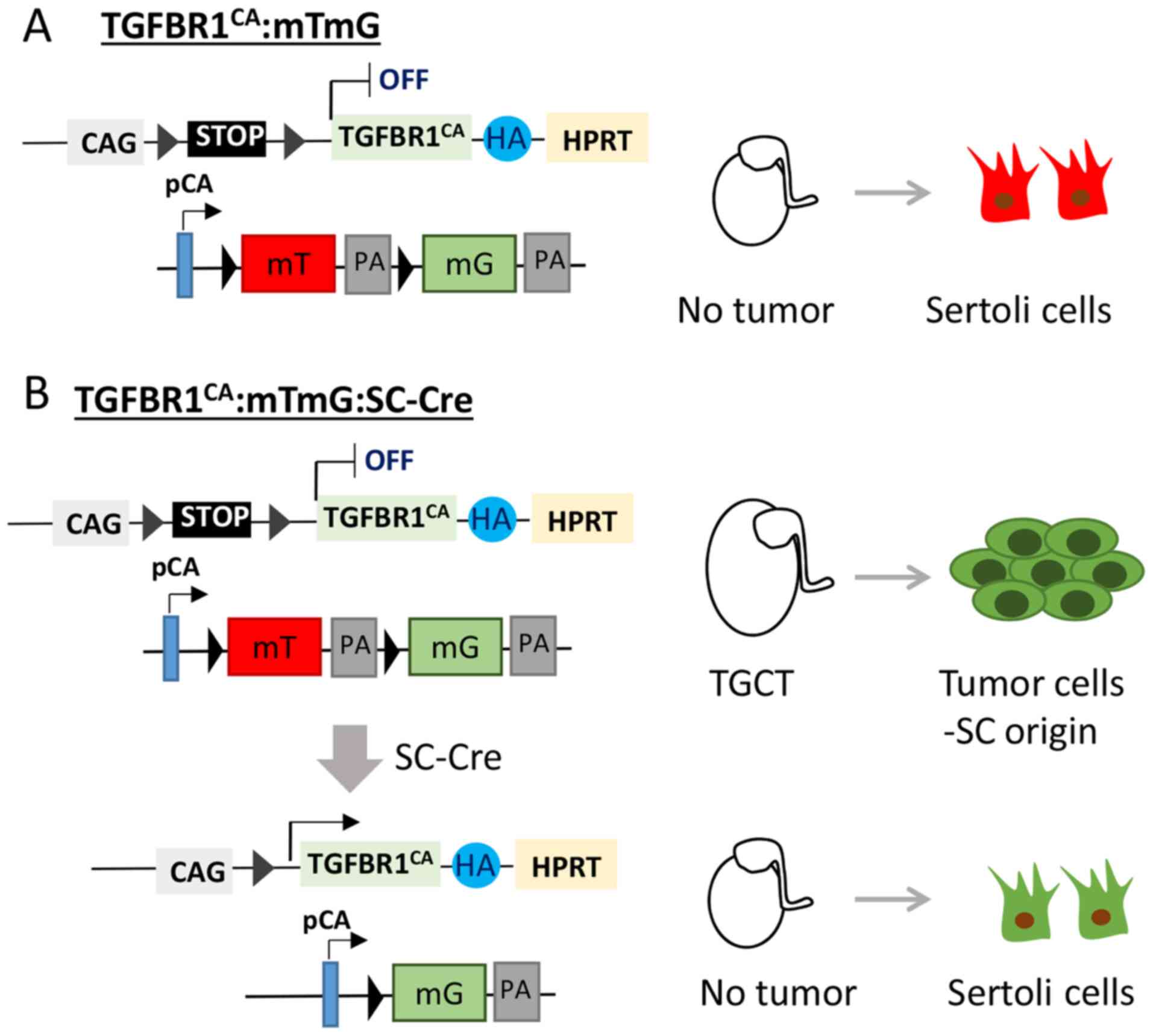 | Figure 2.Proposed genetic labeling to trace
TGCT origin in TGFBR1-CA mice. Mice harboring constitutively active
TGFBR1 will be bred with mTmG mice and Sertoli cell-specific Cre
mice. (A) Sertoli cells in the testes of control mice express
tdTomato (red). (B) In the TGFBR1-CA:mTmG:SC-Cre testes, Sertoli
cells express constitutively active TGFBR1 and EGFP (green). The
experiment is expected to elucidate whether Sertoli cells
contribute to the development of TGCTs and whether activation of
TGFBR1 in Sertoli cells is sufficient to induce TGCTs. mT,
membrane-targeted tdTomato; mG, membrane-targeted EGFP; PA,
polyadenylation sequences; pCA, chicken β-actin promoter with CMV
enhancer; CAG, human cytomegalovirus enhancer and chicken β-actin;
HA, hemagglutinin; SC, Sertoli cell; TGCT, testicular granulosa
cell tumor; TGFBR1, TGF-β receptor type-1; HPRT, hypoxanthine
guanine phosphoribosyl transferase. |
In some genetically modified mouse models, GCTs
occur in both males and females. Since there are both
histopathological and molecular similarities between ovarian and
testicular GCTs, it will be informative to perform comparative
analyses of the tumor transcriptome/proteome between males and
females. Commonly regulated genes are likely to be valuable
candidates for investigating tumor etiology and treatment.
Although the FOXL2 mutation is a hallmark of
adult ovarian GCTs (33), this
mutation has only been analyzed in a small population of patients
with TGCTs (39). Thus, the
significance of this mutation in TGCTs remains unclear. Studies
assessing the FOXL2 mutation in TGCTs in more patients,
either retrospectively or prospectively, appear necessary in the
future.
The pathogenesis of TGCTs is complex and involves
multiple signaling pathways, including, but not limited to, WNT,
KRAS and TGFβ. In the TGFBR1-CA mouse model, activation of WNT
signaling (12), PI3K/AKT signaling
and extracellular signal-regulated kinase 1/2 (ERK1/2) singling
pathways in TGCTs (Fang and Li, unpublished data) was found. A
number of questions remain with regard to how these signaling
pathways alter the identity of Sertoli cells and promote oncogenic
transformation, whether there is crosstalk between these signaling
branches, what the convergence points of these pathways are in the
development of TGCTs, and how genetic factors, if any, impact
cellular properties and outputs of signaling pathways in the
process of tumorigenesis. Future studies that address these
questions using new mouse models, as well as mathematical modeling
(71,72), will help our understanding of the
pathogenesis of TGCTs and will guide the design of new therapies
for this type of rare tumor.
Acknowledgements
The authors would like to thank Ms. Nan Ni (Texas
A&M University, College Station, TX, USA) for providing
editorial assistance.
Funding
Research in the Li laboratory at Texas A&M
University (College Station, TX, USA) for granulosa cell tumors is
supported by the National Cancer Institute of the National
Institutes of Health under award number R03CA235001. The funding
agency plays no role in literature analysis and interpretation and
manuscript preparation.
Availability of data and materials
Not applicable.
Author's contributions
XF and QL analyzed the literature and wrote the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Scully RE: Ovarian tumors. A review. Am J
Pathol. 87:686–720. 1977.PubMed/NCBI
|
|
2
|
Sakr S, Abdulfatah E, Thomas S, Al-Wahab
Z, Beydoun R, Morris R, Ali-Fehmi R and Bandyopadhyay S: Granulosa
cell tumors: Novel predictors of recurrence in early-stage
patients. Int J Gynecol Pathol. 36:240–252. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Colombo N, Parma G, Zanagnolo V and
Insinga A: Management of ovarian stromal cell tumors. J Clin Oncol.
25:2944–2951. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jamieson S and Fuller PJ: Molecular
pathogenesis of granulosa cell tumors of the ovary. Endocr Rev.
33:109–144. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vassal G, Flamant F, Caillaud JM, Demeocq
F, Nihoul-Fekete C and Lemerle J: Juvenile granulosa cell tumor of
the ovary in children: A clinical study of 15 cases. J Clin Oncol.
6:990–995. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nasu K, Fukuda J, Yoshimatsu J, Takai N,
Kashima K and Narahara H: Granulosa cell tumor associated with
secondary amenorrhea and serum luteinizing hormone elevation. Int J
Clin Oncol. 12:228–230. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Szewczuk W, Szewczuk O, Czajkowski K,
Grala B and Semczuk A: Ovarian adult-type granulosa cell tumor
concomitant with simple endometrial hyperplasia: A case study with
selected immunohistochemistry. J Int Med Res 300060519886984.
2019.(Epub ahead of print). View Article : Google Scholar
|
|
8
|
Levin G, Zigron R, Haj-Yahya R, Matan LS
and Rottenstreich A: Granulosa cell tumor of ovary: A systematic
review of recent evidence. Eur J Obstet Gynecol Reprod Biol.
225:57–61. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schumer ST and Cannistra SA: Granulosa
cell tumor of the ovary. J Clin Oncol. 21:1180–1189. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Roth LM, Lyu B and Cheng L: Perspectives
on testicular sex cord-stromal tumors and those composed of both
germ cells and sex cord-stromal derivatives with a comparison to
corresponding ovarian neoplasms. Hum Pathol. 65:1–14. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Young RH: Sex cord-stromal tumors of the
ovary and testis: Their similarities and differences with
consideration of selected problems. Mod Pathol. 18 (Suppl
2):S81–S98. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fang X, Ni N, Gao Y, Vincent DF, Bartholin
L and Li Q: A novel mouse model of testicular granulosa cell
tumors. Mol Hum Reprod. 24:343–356. 2018.PubMed/NCBI
|
|
13
|
Cheng L, Albers P, Berney DM, Feldman DR,
Daugaard G, Gilligan T and Looijenga LHJ: Testicular cancer. Nat
Rev Dis Primers. 4:292018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Moch H, Cubilla AL, Humphrey PA, Reuter VE
and Ulbright TM: The 2016 WHO classification of tumours of the
urinary system and male genital organs-Part A: Renal, penile, and
testicular tumours. Eur Urol. 70:93–105. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Idrees MT, Ulbright TM, Oliva E, Young RH,
Montironi R, Egevad L, Berney D, Srigley JR, Epstein JI and Tickoo
SK; Members of the International Society of Urological Pathology
Testicular Tumour Panel, : The World Health Organization 2016
classification of testicular non-germ cell tumours: A review and
update from the International Society of Urological Pathology
Testis Consultation Panel. Histopathology. 70:513–521. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Al-Agha OM and Axiotis CA: An in-depth
look at Leydig cell tumor of the testis. Arch Pathol Lab Med.
131:311–317. 2007.PubMed/NCBI
|
|
17
|
Kim I, Young RH and Scully RE: Leydig cell
tumors of the testis. A clinicopathological analysis of 40 cases
and review of the literature. Am J Surg Pathol. 9:177–192. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Boyer A, Paquet M, Lague MN, Hermo L and
Boerboom D: Dysregulation of WNT/CTNNB1 and PI3K/AKT signaling in
testicular stromal cells causes granulosa cell tumor of the testis.
Carcinogenesis. 30:869–878. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Richards JS, Fan HY, Liu Z, Tsoi M, Lague
MN, Boyer A and Boerboom D: Either Kras activation or Pten loss
similarly enhance the dominant-stable CTNNB1-induced genetic
program to promote granulosa cell tumor development in the ovary
and testis. Oncogene. 31:1504–1520. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zugor V, Labanaris AP, Witt J, Seidler A,
Weingartner K and Schott GE: Congenital juvenile granulosa cell
tumor of the testis in newborns. Anticancer Res. 30:1731–1734.
2010.PubMed/NCBI
|
|
21
|
Kao CS, Cornejo KM, Ulbright TM and Young
RH: Juvenile granulosa cell tumors of the testis: A
clinicopathologic study of 70 cases with emphasis on its wide
morphologic spectrum. Am J Surg Pathol. 39:1159–1169. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Raju U, Fine G, Warrier R, Kini R and
Weiss L: Congenital testicular juvenile granulosa cell tumor in a
neonate with X/XY mosaicism. Am J Surg Pathol. 10:577–583. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Young RH, Lawrence WD and Scully RE:
Juvenile granulosa cell tumor-another neoplasm associated with
abnormal chromosomes and ambiguous genitalia. A report of three
cases. Am J Surg Pathol. 9:737–743. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu JT, Book L and Sudar K: Serum alpha
fetoprotein (AFP) levels in normal infants. Pediatr Res. 15:50–52.
1981. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dieckmann KP, Bertolini J and Wulfing C:
Adult granulosa cell tumor of the testis: A case report with a
review of the literature. Case Rep Urol.
2019:71561542019.PubMed/NCBI
|
|
26
|
Cornejo KM and Young RH: Adult granulosa
cell tumors of the testis: A report of 32 cases. Am J Surg Pathol.
38:1242–1250. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jimenez-Quintero LP, Ro JY, Zavala-Pompa
A, Amin MB, Tetu B, Ordonez NG and Ayala AG: Granulosa cell tumor
of the adult testis: A clinicopathologic study of seven cases and a
review of the literature. Hum Pathol. 24:1120–1125. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Suppiah A, Musa MM, Morgan DR and North
AD: Adult granulosa cell tumour of the testis and bony metastasis.
A report of the first case of granulosa cell tumour of the testicle
metastasising to bone. Urol Int. 75:91–93. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hanson JA and Ambaye AB: Adult testicular
granulosa cell tumor: A review of the literature for
clinicopathologic predictors of malignancy. Arch Pathol Lab Med.
135:143–146. 2011.PubMed/NCBI
|
|
30
|
Pisarska MD, Barlow G and Kuo FT:
Minireview: Roles of the forkhead transcription factor FOXL2 in
granulosa cell biology and pathology. Endocrinology. 152:1199–1208.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Uhlenhaut NH, Jakob S, Anlag K,
Eisenberger T, Sekido R, Kress J, Treier AC, Klugmann C, Klasen C,
Holter NI, et al: Somatic sex reprogramming of adult ovaries to
testes by FOXL2 ablation. Cell. 139:1130–1142. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kalfa N, Fellous M, Boizet-Bonhoure B,
Patte C, Duvillard P, Pienkowski C, Jaubert F, Ecochard A and
Sultan C: Aberrant expression of ovary determining gene FOXL2 in
the testis and juvenile granulosa cell tumor in children. J Urol.
180 (Suppl 4):S1810–S1813. 2008. View Article : Google Scholar
|
|
33
|
Shah SP, Kobel M, Senz J, Morin RD, Clarke
BA, Wiegand KC, Leung G, Zayed A, Mehl E, Kalloger SE, et al:
Mutation of FOXL2 in granulosa-cell tumors of the ovary. N Engl J
Med. 360:2719–2729. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nonis D, McTavish KJ and Shimasaki S:
Essential but differential role of FOXL2wt and FOXL2C134W in GDF-9
stimulation of follistatin transcription in co-operation with Smad3
in the human granulosa cell line COV434. Mol Cell Endocrinol.
372:42–48. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cheng JC, Chang HM, Qiu X, Fang L and
Leung PC: FOXL2-induced follistatin attenuates activin A-stimulated
cell proliferation in human granulosa cell tumors. Biochem Biophys
Res Commun. 443:537–542. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Leung DTH, Fuller PJ and Chu S: Impact of
FOXL2 mutations on signaling in ovarian granulosa cell tumors. Int
J Biochem Cell Biol. 72:51–54. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kim JH, Yoon S, Park M, Park HO, Ko JJ,
Lee K and Bae J: Differential apoptotic activities of wild-type
FOXL2 and the adult-type granulosa cell tumor-associated mutant
FOXL2 (C134W). Oncogene. 30:1653–1663. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fleming NI, Knower KC, Lazarus KA, Fuller
PJ, Simpson ER and Clyne CD: Aromatase is a direct target of FOXL2:
C134W in granulosa cell tumors via a single highly conserved
binding site in the ovarian specific promoter. PLoS One.
5:e143892010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lima JF, Jin L, de Araujo AR,
Erikson-Johnson MR, Oliveira AM, Sebo TJ, Keeney GL and Medeiros F:
FOXL2 mutations in granulosa cell tumors occurring in males. Arch
Pathol Lab Med. 136:825–828. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fuller PJ, Chu S, Fikret S and Burger HG:
Molecular pathogenesis of granulosa cell tumours. Mol Cell
Endocrinol. 191:89–96. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kim SY: Insights into granulosa cell
tumors using spontaneous or genetically engineered mouse models.
Clin Exp Reprod Med. 43:1–8. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Matzuk MM, Finegold MJ, Su JG, Hsueh AJ
and Bradley A: Alpha-inhibin is a tumour-suppressor gene with
gonadal specificity in mice. Nature. 360:313–319. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li Y, Zhang L, Hu Y, Chen M, Han F, Qin Y,
Chen M, Cui X, Duo S, Tang F and Gao F: β-Catenin directs the
transformation of testis Sertoli cells to ovarian granulosa-like
cells by inducing Foxl2 expression. J Biol Chem. 292:17577–17586.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Pangas SA, Li X, Umans L, Zwijsen A,
Huylebroeck D, Gutierrez C, Wang D, Martin JF, Jamin SP, Behringer
RR, et al: Conditional deletion of Smad1 and Smad5 in somatic cells
of male and female gonads leads to metastatic tumor development in
mice. Mol Cell Biol. 28:248–257. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ying SY: Inhibins, activins, and
follistatins: Gonadal proteins modulating the secretion of
follicle-stimulating hormone. Endocr Rev. 9:267–293. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
O'Connor AE and De Kretser DM: Inhibins in
normal male physiology. Semin Reprod Med. 22:177–185. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kumar TR, Wang Y and Matzuk MM:
Gonadotropins are essential modifier factors for gonadal tumor
development in inhibin-deficient mice. Endocrinology.
137:4210–4216. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Cipriano SC, Chen L, Burns KH, Koff A and
Matzuk MM: Inhibin and p27 interact to regulate gonadal
tumorigenesis. Mol Endocrinol. 15:985–996. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Burns KH, Agno JE, Sicinski P and Matzuk
MM: Cyclin D2 and p27 are tissue-specific regulators of
tumorigenesis in inhibin alpha knockout mice. Mol Endocrinol.
17:2053–2069. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Li Q, Graff JM, O'Connor AE, Loveland KL
and Matzuk MM: SMAD3 regulates gonadal tumorigenesis. Mol
Endocrinol. 21:2472–2486. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Klaus A and Birchmeier W: Wnt signalling
and its impact on development and cancer. Nat Rev Cancer.
8:387–398. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Boerboom D, Paquet M, Hsieh M, Liu J,
Jamin SP, Behringer RR, Sirois J, Taketo MM and Richards JS:
Misregulated Wnt/beta-catenin signaling leads to ovarian granulosa
cell tumor development. Cancer Res. 65:9206–9215. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Mitsiades CS, Mitsiades N and Koutsilieris
M: The Akt pathway: Molecular targets for anti-cancer drug
development. Curr Cancer Drug Targets. 4:235–256. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Hay N: The Akt-mTOR tango and its
relevance to cancer. Cancer Cell. 8:179–183. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Haigis KM: KRAS alleles: The devil is in
the detail. Trends Cancer. 3:686–697. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Fan HY, Shimada M, Liu Z, Cahill N, Noma
N, Wu Y, Gossen J and Richards JS: Selective expression of KrasG12D
in granulosa cells of the mouse ovary causes defects in follicle
development and ovulation. Development. 135:2127–2137. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Hannenhalli S and Kaestner KH: The
evolution of Fox genes and their role in development and disease.
Nat Rev Genet. 10:233–240. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Uhlenhaut NH and Treier M: Forkhead
transcription factors in ovarian function. Reproduction.
142:489–495. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Belli M, Secchi C, Stupack D and Shimasaki
S: FOXO1 negates the cooperative action of FOXL2C134W
and SMAD3 in CYP19 expression in HGrC1 cells by sequestering SMAD3.
J Endocr Soc. 3:2064–2081. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Liu Z, Ren YA, Pangas SA, Adams J, Zhou W,
Castrillon DH, Wilhelm D and Richards JS: FOXO1/3 and PTEN
depletion in granulosa cells promotes ovarian granulosa cell tumor
development. Mol Endocrinol. 29:1006–1024. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Massague J: TGF-beta signal transduction.
Annu Rev Biochem. 67:753–791. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Fang X, Gao Y and Li Q: SMAD3 activation:
A converging point of dysregulated TGF-Beta superfamily signaling
and genetic aberrations in granulosa cell tumor development? Biol
Reprod. 95:1052016. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Massague J: TGFbeta in cancer. Cell.
134:215–230. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Gao Y, Vincent DF, Davis AJ, Sansom OJ,
Bartholin L and Li Q: Constitutively active transforming growth
factor β receptor 1 in the mouse ovary promotes tumorigenesis.
Oncotarget. 7:40904–40918. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Boyer A, Hermo L, Paquet M, Robaire B and
Boerboom D: Seminiferous tubule degeneration and infertility in
mice with sustained activation of WNT/CTNNB1 signaling in sertoli
cells. Biol Reprod. 79:475–485. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Jamin SP, Arango NA, Mishina Y, Hanks MC
and Behringer RR: Requirement of Bmpr1a for Mullerian duct
regression during male sexual development. Nat Genet. 32:408–410.
2002. View
Article : Google Scholar : PubMed/NCBI
|
|
67
|
Tanwar PS, Kaneko-Tarui T, Zhang L, Rani
P, Taketo MM and Teixeira J: Constitutive WNT/beta-catenin
signaling in murine Sertoli cells disrupts their differentiation
and ability to support spermatogenesis. Biol Reprod. 82:422–432.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Albrecht KH and Eicher EM: Evidence that
Sry is expressed in pre-Sertoli cells and Sertoli and granulosa
cells have a common precursor. Dev Biol. 240:92–107. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Muzumdar MD, Tasic B, Miyamichi K, Li L
and Luo L: A global double-fluorescent Cre reporter mouse. Genesis.
45:593–605. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Snyder CS, Harrington AR, Kaushal S, Mose
E, Lowy AM, Hoffman RM and Bouvet M: A dual-color genetically
engineered mouse model for multispectral imaging of the pancreatic
microenvironment. Pancreas. 42:952–958. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Gammon K: Mathematical modelling:
Forecasting cancer. Nature. 491:S66–S67. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Beerenwinkel N, Schwarz RF, Gerstung M and
Markowetz F: Cancer evolution: Mathematical models and
computational inference. Syst Biol. 64:e1–e25. 2015. View Article : Google Scholar : PubMed/NCBI
|
















