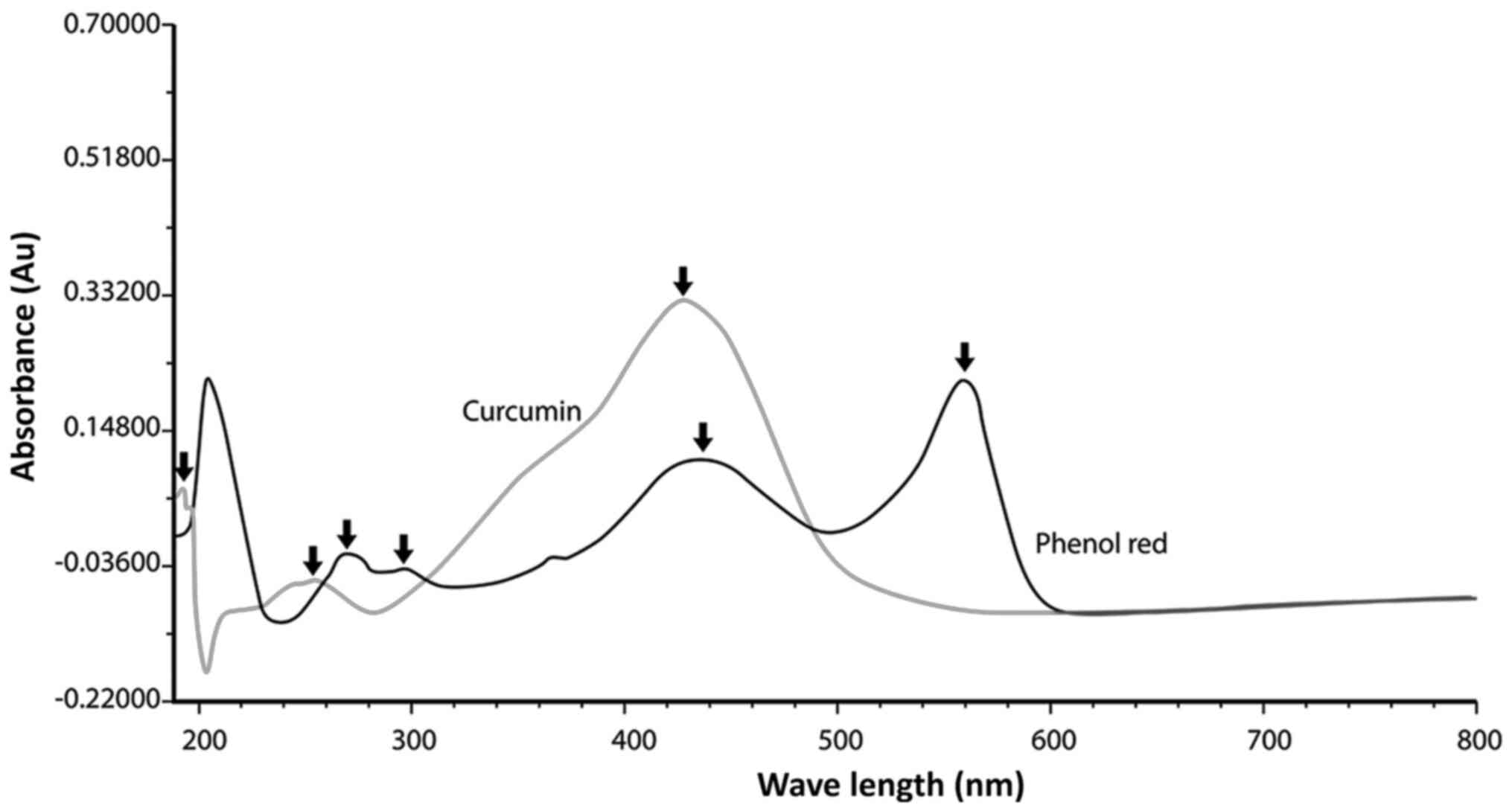
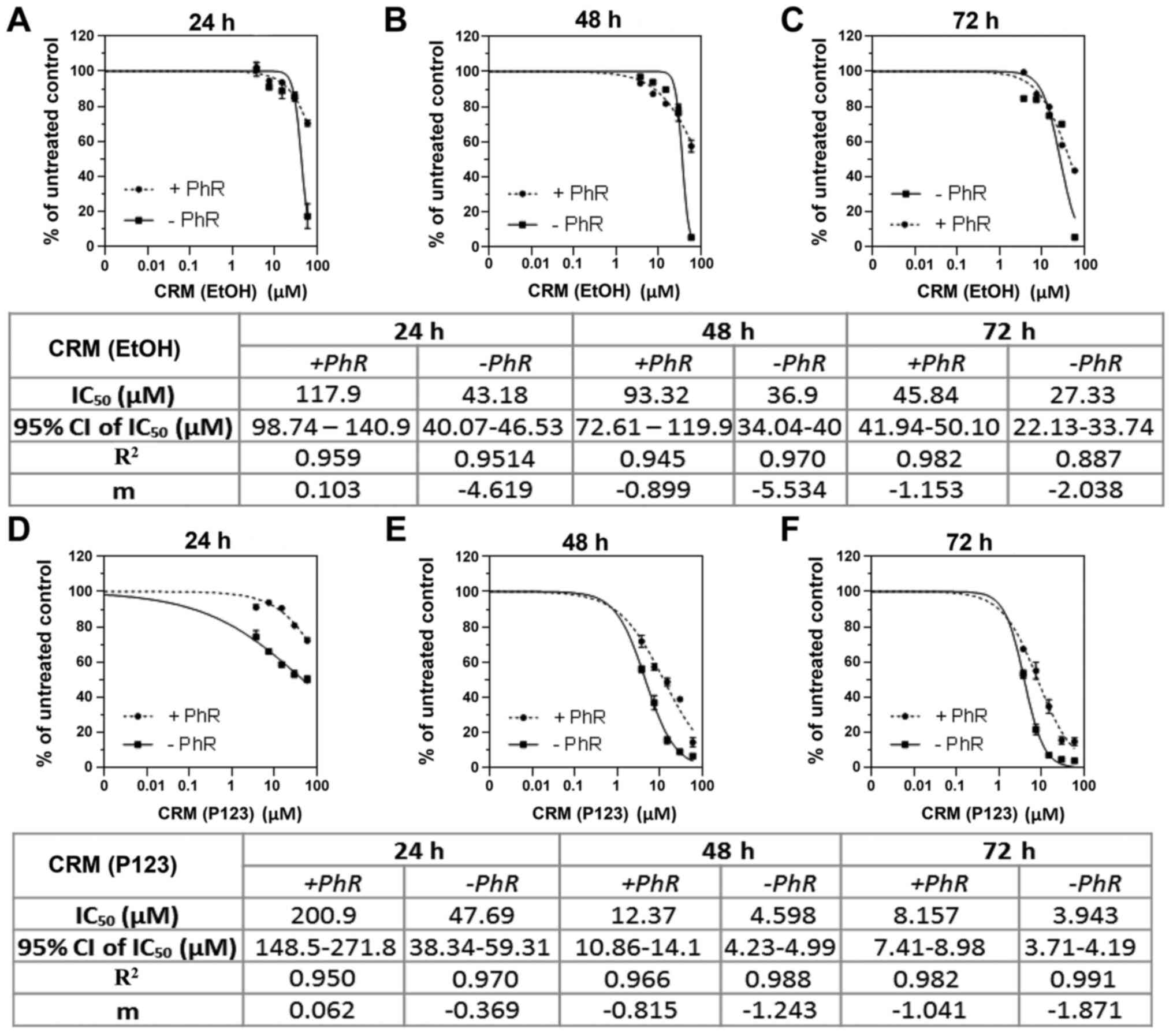
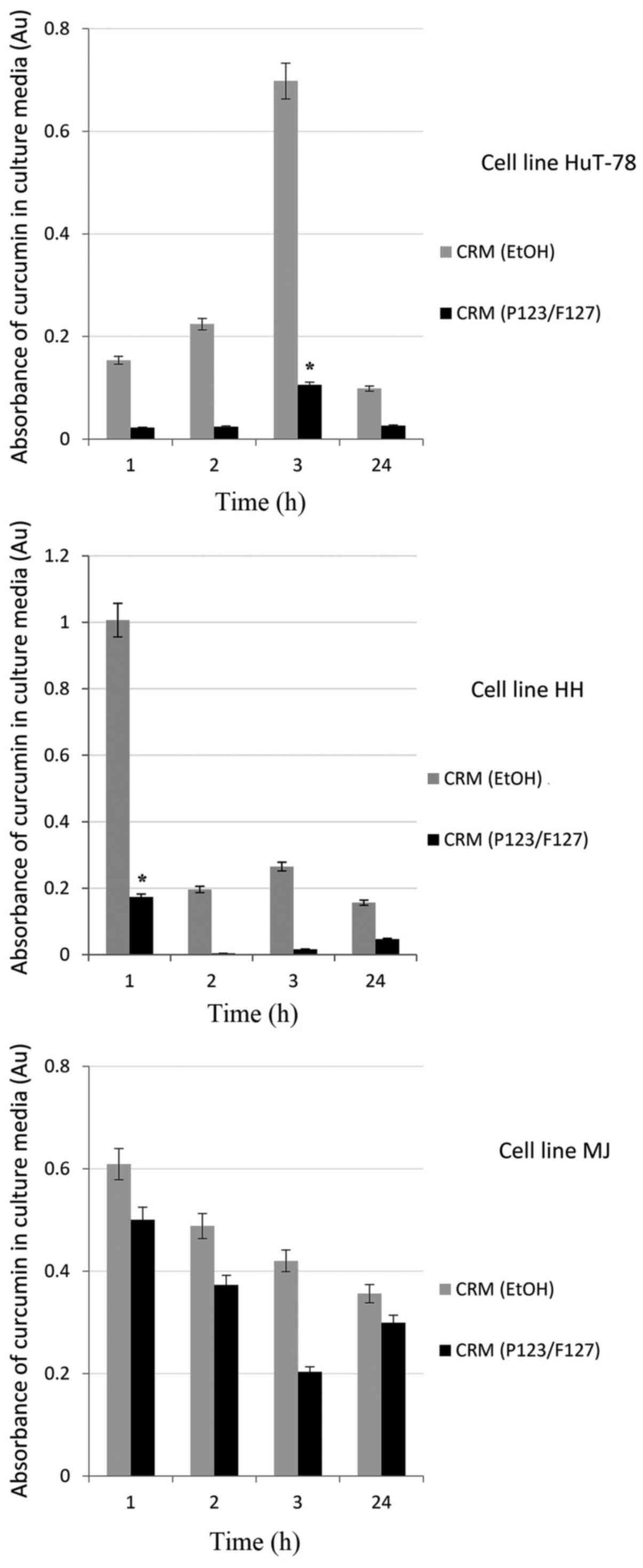
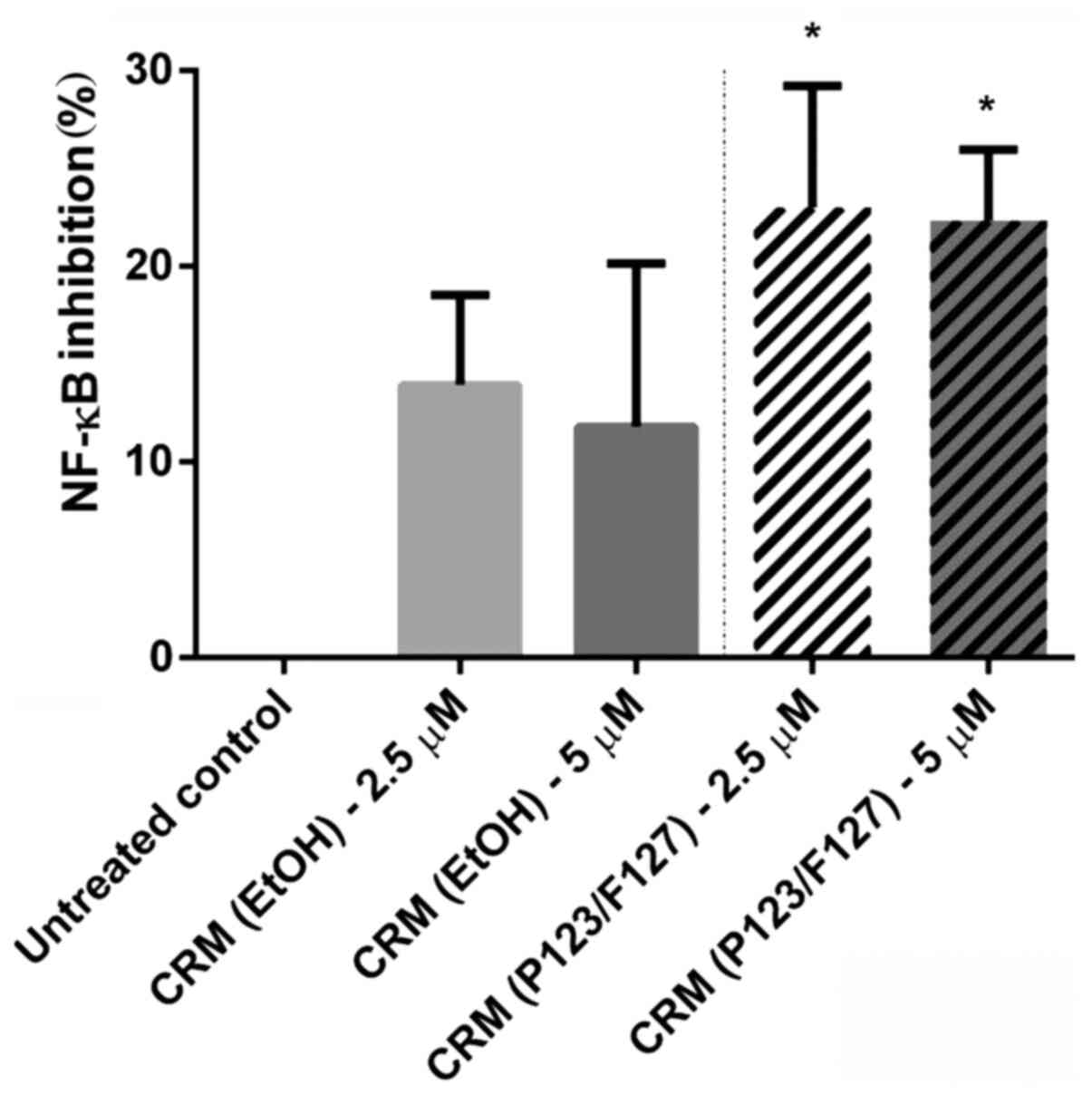
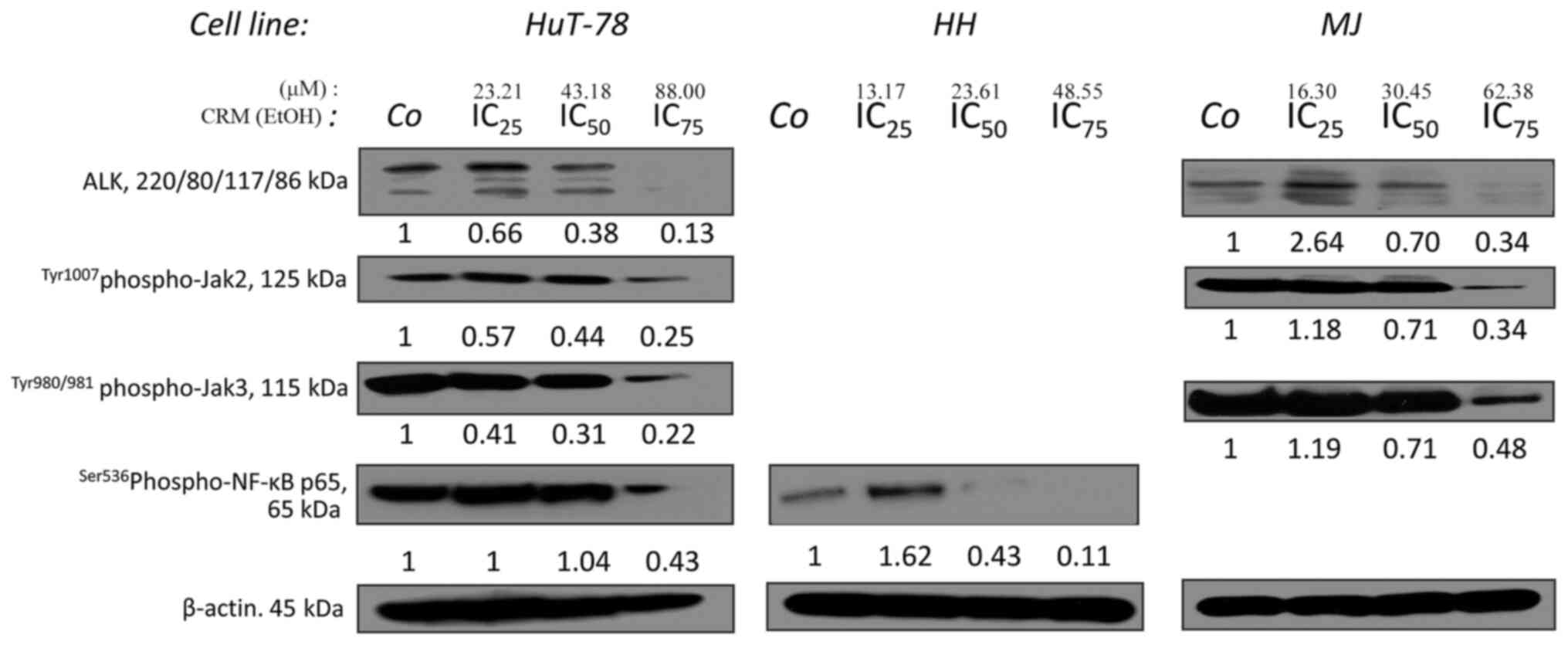
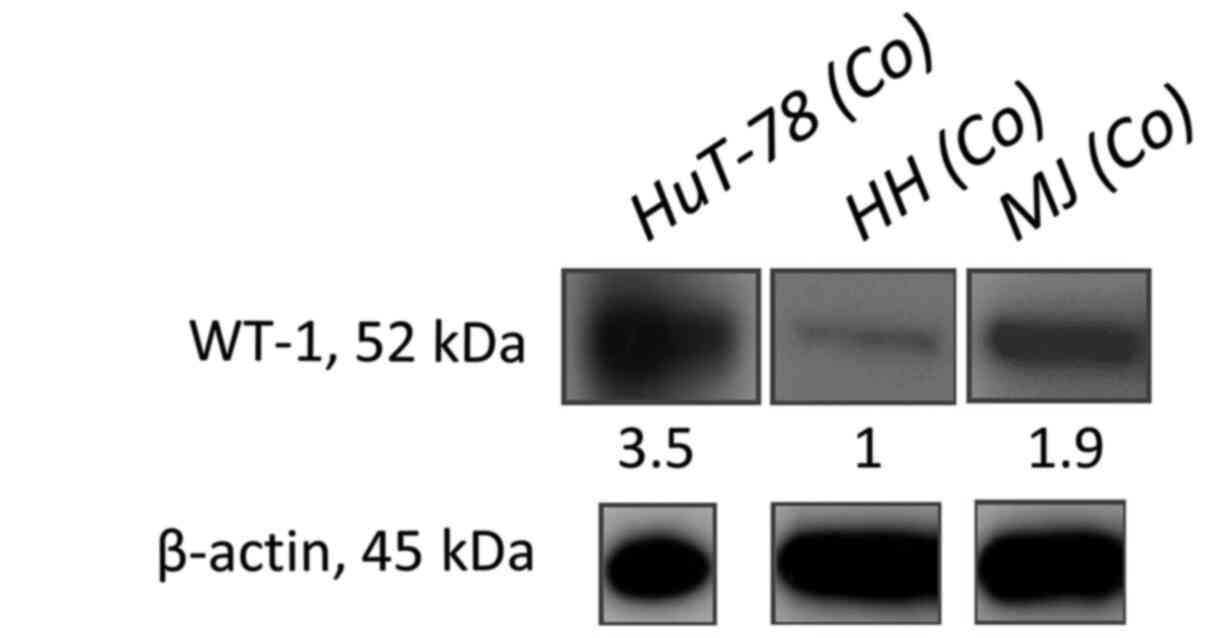
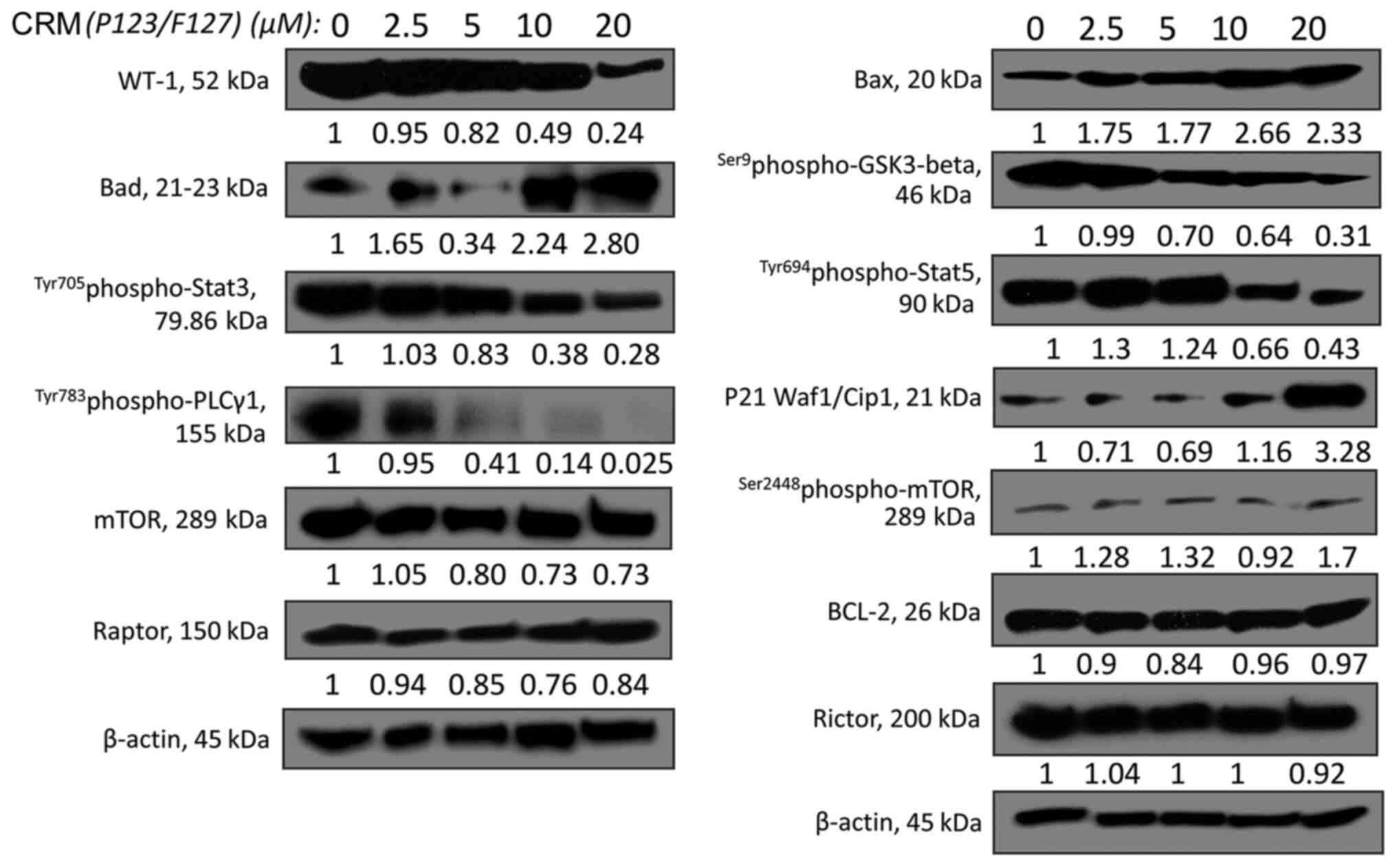
|
1
|
Hwang ST, Janik JE, Jaffe ES and Wilson
WH: Mycosis fungoides and Sézary syndrome. Lancet. 371:945–957.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Willemze R, Kerl H, Sterry W, Berti E,
Cerroni L, Chimenti S, Diaz-Peréz JL, Geerts ML, Goos M, Knobler R,
et al: EORTC classification for primary cutaneous lymphomas: As
proposal from the cutaneous lymphoma study group of the European
organization for research and treatment of cancer. Blood.
90:354–371. 1997.PubMed/NCBI
|
|
3
|
Willemze R, Jaffe ES, Burg G, Cerroni L,
Berti E, Swerdlow SH, Ralfkiaer E, Chimenti S, Diaz-Perez JL,
Duncan LM, et al: WHO-EORTC classification for cutaneous lymphomas.
Blood. 105:3768–3785. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Burg G, Kempf W, Cozzio A, Feit J,
Willemze R, S Jaffe E, Dummer R, Berti E, Cerroni L, Chimenti S, et
al: WHO/EORTC classification of cutaneous lymphomas 2005:
Histological and molecular aspects. J Cutan Pathol. 32:647–674.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Girardi M, Heald PW and Wilson LD: The
pathogenesis of mycosis fungoides. N Engl J Med. 350:1978–1988.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Manso R, Martínez-Magunacelaya N,
Eraña-Tomás I, Monsalvez V, Rodríguez-Peralto JL, Ortiz-Romero PL,
Santonja C, Cristóbal I, Piris MA and Rodríguez-Pinilla SM: Mycosis
fungoides progression could be regulated by microRNAs. PLoS One.
13:e01984772018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Criscione VD and Weinstock MA: Incidence
of cutaneous T-cell lymphoma in the United States, 1973–2002. Arch
Dermatol. 143:854–859. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hutchinson CB, Stoecker M, Wang FF,
Papalas J, Sebastian S, Burchette J, Datto M and Wang E: Molecular
detection of circulating Sezary cells in patients with mycosis
fungoides: Could it predict future development of secondary Sezary
syndrome? A single-institution experience. Leuk Lymphoma.
53:868–877. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu XS, Lonsdorf AS and Hwang ST: Cutaneous
T-cell lymphoma: Roles for chemokines and chemokine receptors. J
Invest Dermatol. 129:1115–1119. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yamashita T, Abbade LP, Marques ME and
Marques SA: Mycosis fungoides and Sezary syndrome: Clinical,
histopathological and immunohistochemical review and update. An
Bras Dermatol. 87:817–830. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yumeen S and Girardi M: Insights into the
molecular and cellular underpinnings of cutaneous T cell lymphoma.
Yale J Biol Med. 93:111–121. 2020.PubMed/NCBI
|
|
12
|
Prince HM, Whittaker S and Hoppe RT: How I
treat mycosis fungoides and Sézary syndrome. Blood. 114:4337–4353.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wollina U: Cutaneous T cell lymphoma:
Update on treatment. Int J Dermatol. 51:1019–1036. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Esche BA and Fitzpatrick PJ: Cutaneous
malignant lymphoma. Int J Radiat Oncol Biol Phys. 12:2111–2115.
1986. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zucca E and Cavalli F: Extranodal
lymphomas. Ann Oncol. 11 (Suppl 3):S219–S222. 2000. View Article : Google Scholar
|
|
16
|
Sokołowska-Wojdyło M, Olek-Hrab K and
Ruckemann-Dziurdzińska K: Primary cutaneous lymphomas: Diagnosis
and treatment. Postepy Dermatol Alergol. 32:368–383. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Richter T, Nestler-Parr S, Babela R, Khan
ZM, Tesoro T, Molsen E and Hughes DA; International Society for
Pharmacoeconomics and Outcomes Research Rare Disease Special
Interest Group, : Rare disease terminology and definitions-A
systematic global review: Report of the ISPOR rare disease special
interest group. Value Health. 18:906–914. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Orphanet, . Orphanet: An online rare
disease and orphan drug data base. © INSERM 1999. simplehttp://www.orpha.net2019
|
|
19
|
Okoye AA and Picker LJ: CD4(+) T-cell
depletion in HIV infection: Mechanisms of immunological failure.
Immunol Rev. 254:54–64. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vidya Vijayan KK, Karthigeyan KP, Tripathi
SP and Hanna LE: Pathophysiology of CD4+ T-Cell
Depletion in HIV-1 and HIV-2 Infections. Front Immunol. 8:5802017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Krejsgaard T, Odum N, Geisler C, Wasik MA
and Woetmann A: Regulatory T cells and immunodeficiency in mycosis
fungoides and Sézary syndrome. Leukemia. 26:424–432. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Heald P, Yan SL and Edelson R: Profound
deficiency in normal circulating T cells in erythrodermic cutaneous
T-cell lymphoma. Arch Dermatol. 130:198–203. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Coondoo A, Phiske M, Verma S and Lahiri K:
Side-effects of topical steroids: A long overdue revisit. Indian
Dermatol Online J. 5:416–425. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Farber EM, Abel EA and Cox AJ: Long-term
risks of psoralen and UV-A therapy for psoriasis. Arch Dermatol.
119:426–431. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Farol LT and Hymes KB: Bexarotene: A
clinical review. Expert Rev Anticancer Ther. 4:180–188. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nurgali K, Jagoe RT and Abalo R:
Editorial: Adverse effects of cancer chemotherapy: Anything new to
improve tolerance and reduce sequelae? Front Pharmacol. 9:2452018.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sleijfer S, Bannink M, Van Gool AR, Kruit
WH and Stoter G: Side effects of interferon-alpha therapy. Pharm
World Sci. 27:423–431. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Subramanian S, Bates SE, Wright JJ,
Espinoza-Delgado I and Piekarz RL: Clinical toxicities of histone
deacetylase inhibitors. Pharmaceuticals (Basel). 3:2751–2767. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Vidula N, Villa M, Helenowski IB,
Jovanovic BD, Meagher R, Mehta J, Singhal S, Winter JN, Frankfurt
O, Altman JK, et al: Adverse events during hematopoietic stem cell
infusion: Analysis of the infusion product. Clin Lymphoma Myeloma
Leuk. 15:e157–e162. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tsambiras PE, Patel S, Greene JN, Sandin
RL and Vincent AL: Infectious complications of cutaneous T-cell
lymphoma. Cancer Control. 8:185–188. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sharma RA, Gescher AJ and Steward WP:
Curcumin: The story so far. Eur J Cancer. 41:1955–1968. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hsu CH and Cheng AL: Clinical studies with
curcumin. Adv Exp Med Biol. 595:471–480. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang C, Li B, Zhang X, Hazarika P,
Aggarwal BB and Duvic M: Curcumin selectively induces apoptosis in
cutaneous T-cell lymphoma cell lines and patients' PBMCs: Potential
role for STAT-3 and NF-kappaB signaling. J Invest Dermatol.
130:2110–2119. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Khan MA, Gahlot S and Majumdar S:
Oxidative stress induced by curcumin promotes the death of
cutaneous T-cell lymphoma (HuT-78) by disrupting the function of
several molecular targets. Mol Cancer Ther. 11:1873–1883. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yosifov DY, Kaloyanov KA, Guenova ML,
Prisadashka K, Balabanova MB, Berger MR and Konstantinov SM:
Alkylphosphocholines and curcumin induce programmed cell deathin
cutaneous T-cell lymphoma cell lines. Leuk Res. 38:49–56. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Devaraj S, Jagannathan N and Neelakantan
P: Antibiofilm efficacy of photoactivated curcumin, triple and
double antibiotic paste, 2% chlorhexidine and calcium hydroxide
against Enterococcus fecalis in vitro. Sci Rep. 6:247972016.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Trochopoulos A, Ivanov E, Yakub G, Rashkov
I, Manolova N, Momekova D, Zaharieva MM, Najdenski H, Berger MR and
Konstantinov S: Antineoplastic potential of curcumin loaded
polymeric formulations against human malignant cells. Humboldt
Union in Bulgaria; Sofia, Bulgaria: pp. 44–57. 2018
|
|
38
|
Zaharieva MM, Kroumov AD, Dimitrova L,
Tsvetkova I, Trochopoulos A, Konstantinov SM, Berger MR, Momchilova
M, Yoncheva K and Najdenski HM: Micellar curcumin improves the
antibacterial activity of the alkylphosphocholines erufosine and
miltefosine against pathogenic Staphyloccocus aureus.
Biotechnol Biotechnological Equip. 33:38–53. 2019. View Article : Google Scholar
|
|
39
|
Axelrod PI, Lorber B and Vonderheid EC:
Infections complicating mycosis fungoide and sézary syndrome. JAMA.
267:1354–1358. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bonin S, Tothova SM, Barbazza R, Brunetti
D, Stanta G and Trevisan G: Evidence of multiple infectious agents
in mycosis fungoides lesions. Exp Mol Pathol. 89:46–50. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Willerslev-Olsen A, Krejsgaard T, Lindahl
LM, Bonefeld CM, Wasik MA, Koralov SB, Geisler C, Kilian M, Iversen
L, Woetmann A and Odum N: Bacterial toxins fuel disease progression
in cutaneous T-cell lymphoma. Toxins (Basel). 5:1402–1421. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kunwar A, Barik A, Pandey R and
Priyadarsini KI: Transport of liposomal and albumin loaded curcumin
to living cells: An absorption and fluorescence spectroscopic
study. Biochim Biophys Acta. 1760:1513–1520. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Anand P, Kunnumakkara AB, Newman RA and
Aggarwal BB: Bioavailability of curcumin: Problems and promises.
Mol Pharm. 4:807–818. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Aggarwal BB, Sundaram C, Malani N and
Ichikawa H: Curcumin: The Indian solid gold. Adv Exp Med Biol.
595:1–75. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Goel A, Kunnumakkara AB and Aggarwal BB:
Curcumin as ‘Curecumin’: From kitchen to clinic. Biochem Pharmacol.
75:787–809. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Mohanty C and Sanjeeb K: The in vitro
stability and in vivo pharmacokinetics of curcumin prepared as an
aqueous nanoparticulate formulation. Biomaterials. 31:6597–6611.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Song Z, Feng R, Sun M, Guo C, Gao Y, Li L
and Zhai G: Curcumin-loaded PLGA-PEG-PLGA triblock copolymeric
micelles: Preparation, pharmacokinetics and distribution in vivo. J
Colloid Interface Sci. 354:116–123. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Li R, Qiao X, Li Q, He R, Ye M, Xiang C,
Lin X and Guo D: Metabolic and pharmacokinetic studies of curcumin,
demethoxycurcumin and bisdemethoxycurcumin in mice tumor after
intragastric administration of nanoparticle formulations by liquid
chromatography coupled with tandem mass spectrometry. J Chromatogr
B Analyt Technol Biomed Life Sci. 879:2751–2758. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yallapu MM, Jaggi M and Chauhan SC:
Curcumin nanoformulations: A future nanomedicine for cancer. Drug
Discov Today. 17:71–80. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Jelezova I, Drakalska E, Momekova D,
Shalimova N, Momekov G, Konstantinov S, Rangelov S and Pispas S:
Curcumin loaded pH-sensitive hybrid lipid/block copolymer nanosized
drug delivery systems. Eur J Pharm Sci. 78:67–78. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Mehanny M, Hathout RM, Geneidi AS and
Mansour S: Exploring the use of nanocarrier systems to deliver the
magical molecule; Curcumin and its derivatives. J Control Release.
225:1–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Klement JF, Rice NR, Car BD, Abbondanzo
SJ, Powers GD, Bhatt PH, Chen CH, Rosen CA and Stewart CL:
IkappaBalpha deficiency results in a sustained NF-kappaB response
and severe widespread dermatitis in mice. Mol Cell Biol.
16:2341–2349. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Mosmann T: Rapid colorimetric assay for
cellular growth and survival: Application to proliferation and
cytotoxicity assays. J Immunol Methods. 65:55–63. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Konstantinov SM, Eibl H and Berger MR:
BCR-ABL influences the antileukaemic efficacy of
alkylphosphocholines. Br J Haematol. 107:365–374. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Van Meerloo J, Kaspers GJ and Cloos J:
Cell sensitivity assays: The MTT assay. Methods Mol Biol.
731:237–245. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Bradford MM: A rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Schneider CA, Rasband WS and Eliceiri KW:
NIH image to imageJ: 25 years of image analysis. Nat Methods.
9:671–675. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Abramoff MD, Magalhães PJ and Ram SJ:
Image processing with ImageJ. Biophot Int. 11:36–42. 2004.
|
|
59
|
Swinehart DF: The Beer-Lambert Law. J Chem
Educ. 39:3331962. View Article : Google Scholar
|
|
60
|
Hayden MS, West AP and Ghosh S: NF-kappaB
and the immune response. Oncogene. 25:6758–6780. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Liu T, Zhang L, Joo D and Sun SC: NF-κB
signaling in inflammation. Signal Transduct Target Ther.
2:170232017. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Xia Y, Shen S and Verma IM: NF-κB, an
active player in human cancers. Cancer Immunol Res. 2:823–830.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Baldwin AS: Regulation of cell death and
autophagy by IKK and NF-κB: Critical mechanisms in immune function
and cancer. Immunol Rev. 246:327–345. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Juvekar A, Manna S, Ramaswami S, Chang TP,
Vu HY, Ghosh CC, Celiker MY and Vancurova I: Bortezomib induces
nuclear translocation of IκBα resulting in gene-specific
suppression of NF-κB-dependent transcription and induction of
apoptosis in CTCL. Mol Cancer Res. 9:183–194. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Kiessling MK, Klemke CD, Kaminski MM,
Galani IE, Krammer PH and Gulow K: Inhibition of constitutively
activated nuclear factor-kappaB induces reactive oxygen species-
and iron-dependent cell death in cutaneous T-cell lymphoma. Cancer
Res. 69:2365–2374. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Sors A, Jean-Louis F, Pellet C, Laroche L,
Dubertret L, Courtois G, Bachelez H and Michel L: Down-regulating
constitutive activation of the NF-kappaB canonical pathway
overcomes the resistance of cutaneous T-cell lymphoma to apoptosis.
Blood. 107:2354–2363. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Call KM, Glaser T, Ito CY, Buckler AJ,
Pelletier J, Haber DA, Rose EA, Kral A, Yeger H, Lewis WH, et al:
Isolation and characterization of a zinc finger polypeptide gene at
the human chromosome 11 Wilms' tumor locus. Cell. 60:509–520. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Gessler M, Poustka A, Cavenee W, Neve RL,
Orkin SH and Bruns GA: Homozygous deletion in Wilms tumours of a
zinc-finger gene identified by chromosome jumping. Nature.
343:774–778. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Huang A, Campbell CE, Bonetta L,
McAndrews-Hill MS, Chilton-MacNeill S, Coppes MJ, Law DJ, Feinberg
AP, Yeger H and Williams BR: Tissue, developmental, and
tumor-specific expression of divergent transcripts in Wilms tumor.
Science. 250:991–994. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Ujj Z, Buglyo G, Udvardy M, Vargha G, Biro
S and Rejto L: WT1 overexpression affecting clinical outcome in
non-hodgkin lymphomas and adult acute lymphoblastic leukemia.
Pathol Oncol Res. 20:565–570. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Casalegno-Garduno R, Schmitt A, Spitschak
A, Greiner J, Wang L, Hilgendorf I, Hirt C, Ho AD, Freund M and
Schmitt M: Immune responses to WT1 in patients with AML or MDS
after chemotherapy and allogeneic stem cell transplantation. Int J
Cancer. 138:1792–1801. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Baudino TA: Targeted cancer therapy: The
next generation of cancer treatment. Curr Drug Discov Technol.
12:3–20. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Kumar M, Nagpal R, Hemalatha R, Verma V,
Kumar A, Singh S, Marotta F, Jain S and Yadav H: Targeted cancer
therapies: The future of cancer treatment. Acta Biomed. 83:220–233.
2012.PubMed/NCBI
|
|
74
|
Wraith DC: The future of immunotherapy: A
20-year perspective. Front Immunol. 8:16682017. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Yu Y and Cui J: Present and future of
cancer immunotherapy: A tumor microenvironmental perspective. Oncol
Lett. 16:4105–4113. 2018.PubMed/NCBI
|
|
76
|
Zhang H and Chen J: Current status and
future directions of cancer immunotherapy. J Cancer. 9:1773–1781.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
DiDonato JA, Mercurio F and Karin M: NF-κB
and the link between inflammation and cancer. Immunol Rev.
246:379–400. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Godwin P, Baird AM, Heavey S, Barr MP,
O'Byrne KJ and Gately K: Targeting nuclear factor-kappa B to
overcome resistance to chemotherapy. Front Oncol. 3:1202013.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Moore MM, Chua W, Charles KA and Clarke
SJ: Inflammation and cancer: Causes and consequences. Clin
Pharmacol Ther. 87:504–508. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Hoesel B and Schmid JA: The complexity of
NF-κB signaling in inflammation and cancer. Mol Cancer. 12:862013.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Candoni A, Toffoletti E, Gallina R,
Simeone E, Chiozzotto M, Volpetti S and Fanin R: Monitoring of
minimal residual disease by quantitative WT1 gene expression
following reduced intensity conditioning allogeneic stem cell
transplantation in acute myeloid leukemia. Clin Transplant.
25:308–316. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Nowakowska-Kopera A, Sacha T, Florek I,
Zawada M, Czekalska S and Skotnicki AB: Wilms' tumor gene 1
expression analysis by real-time quantitative polymerase chain
reaction for monitoring of minimal residual disease in acute
leukemia. Leuk Lymphoma. 50:1326–1332. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Spassov BV, Stoimenov AS, Balatzenko GN,
Genova ML, Peichev DB and Konstantinov SM: Wilms' tumor protein and
FLT3-internal tandem duplication expression in patients with de
novo acute myeloid leukemia. Hematology. 16:37–42. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Hallberg B and Palmer RH: Mechanistic
insight into ALK receptor tyrosine kinase in human cancer biology.
Nat Rev Cancer. 13:685–700. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Wehkamp U, Oschlies I, Nagel I, Brasch J,
Kneba M, Günther A, Klapper W and Weichenthal M: ALK-positive
primary cutaneous T-cell-lymphoma (CTCL) with unusual clinical
presentation and aggressive course. J Cutan Pathol. 42:870–877.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Stoica GE, Kuo A, Aigner A, Sunitha I,
Souttou B, Malerczyk C, Caughey DJ, Wen D, Karavanov A, Riegel AT
and Wellstein A: Identification of anaplastic lymphoma kinase as a
receptor for the growth factor pleiotrophin. J Biol Chem.
276:16772–16779. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Patel VM, Flanagan CE, Martins M, Jones
CL, Butler RM, Woollard WJ, Bakr FS, Yoxall A, Begum N, Katan M, et
al: Frequent and persistent PLCG1 mutations in sezary cells
directly enhance PLCү1 activity and stimulate NFKB, AP-1, and NFAT
signaling. J Invest Dermatol. 140:380–389.e4. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Smith-Garvin JE, Koretzky GA and Jordan
MS: T cell activation. Annu Rev Immunol. 27:591–619. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Vaque JP, Gomez-Lopez G, Monsalvez V,
Varela I, Martínez N, Pérez C, Domínguez O, Graña O,
Rodríguez-Peralto JL, Rodríguez-Pinilla SM, et al: PLCG1 mutations
in cutaneous T-cell lymphomas. Blood. 123:2034–2043. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Kiessling MK, Oberholzer PA, Mondal C,
Karpova MB, Zipser MC, Lin WM, Girardi M, Macconaill LE, Kehoe SM,
Hatton C, et al: High-throughput mutation profiling of CTCL samples
reveals KRAS and NRAS mutations sensitizing tumors toward
inhibition of the RAS/RAF/MEK signaling cascade. Blood.
117:2433–2440. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Pérez C, González-Rincón J, Onaindia A,
Almaráz C, García-Díaz N, Pisonero H, Curiel-Olmo S, Gómez S,
Cereceda L, Madureira R, et al: Mutated JAK kinases and deregulated
STAT activity are potential therapeutic targets in cutaneous T-cell
lymphoma. Haematologica. 100:e450–e453. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Rovedo MA, Krett NL and Rosen ST:
Inhibition of glycogen synthase kinase-3 increases the cytotoxicity
of enzastaurin. J Invest Dermatol. 131:1442–1449. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Balaji S, Ahmed M, Lorence E, Yan F, Nomie
K and Wang M: NF-κB signaling and its relevance to the treatment of
mantle cell lymphoma. J Hematol Oncol. 11:832018. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Merolle MI, Ahmed M, Nomie K and Wang ML:
The B cell receptor signaling pathway in mantle cell lymphoma.
Oncotarget. 9:25332–25341. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Rosenthal A: Small molecule inhibitors in
chronic lymphocytic lymphoma and B cell non-hodgkin lymphoma. Curr
Hematol Malig Rep. 12:207–216. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Valla K, Flowers CR and Koff JL: Targeting
the B cell receptor pathway in non-Hodgkin lymphoma. Expert Opin
Investig Drugs. 27:513–522. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Kim BS, Howell MD, Sun K, Papp K, Nasir A
and Kuligowski ME; INCB 18424-206 Study Investigators, : Treatment
of atopic dermatitis with ruxolitinib cream (JAK1/JAK2 inhibitor)
or triamcinolone cream. J Allergy Clin Immunol. 145:572–582. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Owens S and Howell MD: Ruxolitinib cream
suppresses Th2 inflammation in adult patients with atopic
dermatitis. J Allergy Clin Immunol. 143:AB1282019. View Article : Google Scholar
|
|
99
|
Rosmarin D, Pandya AG, Lebwohl M, Grimes
P, Hamzavi I, Gottlieb AB, Butler K, Kuo F, Sun K, Ji T, et al:
Ruxolitinib cream for treatment of vitiligo: A randomised,
controlled, phase 2 trial. Lancet. 396:110–120. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Rothstein B, Joshipura D, Saraiya A, Abdat
R, Ashkar H, Turkowski Y, Sheth V, Huang V, Au SC, Kachuk C, et al:
Treatment of vitiligo with the topical Janus kinase inhibitor
ruxolitinib. J Am Acad Dermatol. 76:1054–1060.e1. 2017. View Article : Google Scholar : PubMed/NCBI
|