Introduction
Breast cancer (BC) is the most common malignant
tumor affecting women worldwide, and has the highest incidence
among female malignancies. The morbidity and incidence of BC in
China account for ~12.2 and 9.6% of the total cases worldwide,
respectively, and they exhibiting a rapid increasing trend
(1). The development and progression
of BC includes the interaction of tumor cells and the
microenvironment, involving gene mutations, and also the
interaction of microbes and tumor cells, immune cells,
extracellular matrix, and new supporting blood vessels. The complex
evolution of the tumor microenvironment is also closely associated
with the development and progression of BC (2). Tumor immunotherapy is a new treatment
strategy in addition to surgery, radiotherapy and chemotherapy, and
its mechanism of action is based on the stimulation of the body's
own immune system and enhancement of the ability of the
microenvironment to antagonize tumor immunity, thereby acting to
control and eliminate tumor cells. It has high specificity and
minimal damage to normal tissues, and can also stimulate
immunological memory, among other functions. T lymphocytes (αβ and
γδ T cells) play an important role in human immunity, and there is
a relatively constant proportion of cells among the subsets, which
ensures immune system stability. Tumors can disrupt the balance
between αβ and γδ T cells, thereby disrupting immune function. Of
note, the proportion of T-lymphocyte subsets reflect the immune
status of the body (3). The
CD3+, CD4+ and CD8+ T-lymphocyte
subsets are relatively constant, and they coordinate and restrict
each other, which ensures the stability of immune function and
prevents invasion by harmful pathogens. The
CD4+/CD8+ ratio reflects a dynamic balance
under normal conditions (4). The
proportion of CD8+ cells increases, while the
proportions of CD3+, CD4+ and
CD19+ cells decrease in the peripheral blood of tumor
patients (5,6). In recent years, γδ T cells have been
attracting increasing attention due to their unique histological
distribution and immunological characteristics, and their key
anti-infection and antitumor properties. These cells can exert
cytotoxic effects against malignant tumors through participating in
immune surveillance and tumor cell elimination (7). The proportion of αβ and γδ T cells in
the peripheral blood of patients with BC may be a dynamically
changing factor and exhibits a specific association with clinical
pathology. In order to further elucidate the background of the
immune function of αβ and γδ T cells in patients with BC and their
role in the development of BC, the aim of the present study was to
determine and analyze αβ and γδ T-cell counts in the peripheral
blood of patients with BC and their association with clinical
phenotype, so as to provide clinical evidence supporting the use of
immunotherapy for BC in the future.
Materials and methods
Study population
Between January 2017 and December 2018, 138 female
patients with BC, with a mean age of 43.62±3.28 years, were
included in the present study. All the patients had undergone
modified radical mastectomy at the Second Affiliated Hospital of
Shandong First Medical University (Tai'an, China). The patients had
the following pathological types of cancer: Invasive ductal
carcinoma (n=128), medullary carcinoma (n=4), invasive lobular
carcinoma (n=2), cribriform carcinoma (n=2), mucinous carcinoma
(n=2) and ductal carcinoma (n=1). Hematoxylin and eosin staining of
tissue samples was performed. There were 89 cases with lymph node
(LN) metastasis and 49 cases without LN metastasis. According to
the 7th edition of TNM staging described by the Union for
International Cancer Control and the American Joint Committee on
Cancer (5), there were 56 cases with
stage I, 55 cases with stage II and 27 cases with stage III BC. No
patient received neoadjuvant therapy prior to surgery. Patients
with the following conditions were excluded: i) Autoimmune
diseases; ii) recent major infections; iii) use of drugs that
affect the immune function of the body; and iv) serious diseases of
the heart, liver, kidney, lung, or other major organ, and endocrine
disorders. A total of 50 healthy volunteers were selected as the
control group, and they did not use any drugs or foods known to
affect the immune function of the body. The study protocol was
approved by the hospital medical ethics committee, and all
participants provided written informed consent.
Reagents and instruments
Fluorescence-labeled mouse anti-human monoclonal
antibodies: Mouse anti-human TCR-γ/APC (0.2 mg/ml; cat. no.
BD-555718); mouse anti-human CD3/FITC (0.1 mg/ml; cat. no.
BD-561806); mouse anti-human CD45/PerCP-Cy™5.5 (0.1 mg/ml; cat. no.
BD-564105); mouse anti-human CD4/APC (0.2 mg/ml; cat. no.
BD-551980); mouse anti-human CD8/PE (0.2 mg/ml; cat. no.
BD-555367); FACS hemolysin, mouse IgG1-FITC (0.5 mg/ml; cat. no.
BD-550618); and mouse IgG2-PE (0.25 mg/ml; cat. no. BD-560550) were
purchased from BD Biosciences. A flow cytometer (FACSCalibur) and
tissue cell separator were also purchased from BD Biosciences.
Detection of αβ T cells in the
serum
A total of 3 ml of peripheral blood was collected
from all patients on the day prior to surgery and on postoperative
day 15 for anticoagulation in anticoagulant tubes. In addition, 3
ml of venous blood was collected from the 50 healthy volunteers to
use as control. The total number of lymphocytes and proportion of
lymphocyte subsets in the peripheral blood, including
CD3+ and CD4+ helper T lymphocytes,
CD8+ killer T lymphocytes and the
CD4+/CD8+ T-cell ratio, were detected by FACS
flow cytometry. For performing the procedure, 50 µl of
anticoagulated whole blood was collected into each test tube and
mixed gently, followed by the addition of 30 µl of
CD3/FITC-CD8/PE-CD4/APC-CD45/PerCP-Cy™5.5 fluorescent antibodies.
Following incubation in the dark at room temperature (24°C) for 20
min, 2 ml of 1:10 diluted 1X FACS lysing solution was added to each
tube. The samples were vortexed, and the test tubes were incubated
in the dark for 10 min at room temperature. The supernatant was
discarded after centrifugation at 300 × g at 4°C for 5 min, and 2
ml PBS was added into each tube. The samples were vortexed and
centrifuged 300 × g at 4°C for 5 min. Subsequently, the supernatant
was removed, and 0.5 ml PBS was added to resuspend the cells for
analysis in the flow cytometer.
Detection of γδ T cells in the
peripheral blood by fluorescence staining
Two FACS sample tubes were taken from each sample,
and 100 µl of anticoagulated whole blood was added to each sample
tube. Mouse anti-CD3/FITC (20 µl) and mouse anti-human
CD45/PerCP-Cy™5.5 (5.55 µl) were added into one tube as negative
control. In the other tube, mouse anti-TCR-γδ/APC (5 µl), mouse
anti-human CD3/FITC (20 µl), and mouse anti-human CD45/PerCP-Cy™5.5
(5.55 µl) were added as the testing sample. The mixture was
uniformly mixed with mild oscillation and kept away from light for
15–30 min at room temperature (20–25°C). Then, 2 ml of FACS
hemolysin was added to each tube, the contents were mixed well, and
the tubes were kept away from light at room temperature for 8–12
min. Subsequently, the sample was centrifuged at 300 × g at 4°C for
5 min. The supernatant was discarded, and 2 ml PBS was added to the
precipitate. After shaking and centrifuging at 300 × g at 4°C for 5
min, the supernatant was discarded and the precipitate was mixed
with 1% paraformaldehyde buffer at 0.5 ml. The supernatant was
stored at 2–8°C away from light and analyzed using a Nikon TE-2000S
microscope (Nikon Corp.; magnification, ×40) within 24 h.
Statistical analysis
The results were analyzed using SPSS 19.0 for
Windows (SPSS Inc.) and are expressed as mean ± standard deviation.
Statistical significance was determined with the help of the t-test
used to compare pairwise sample means between groups. P<0.05 was
considered to indicate statistically significant differences.
Results
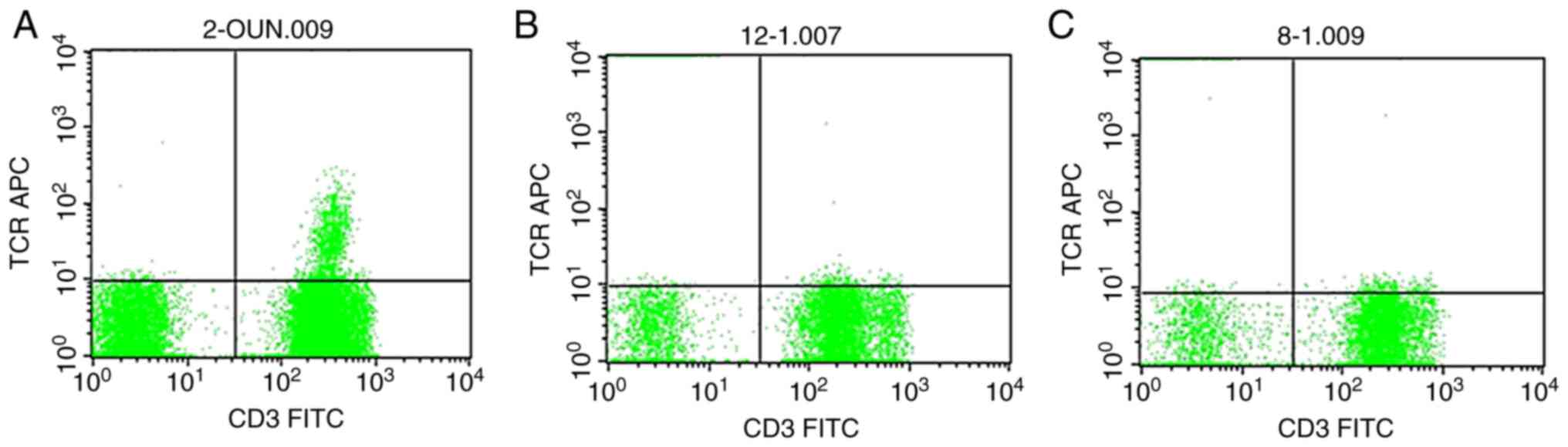
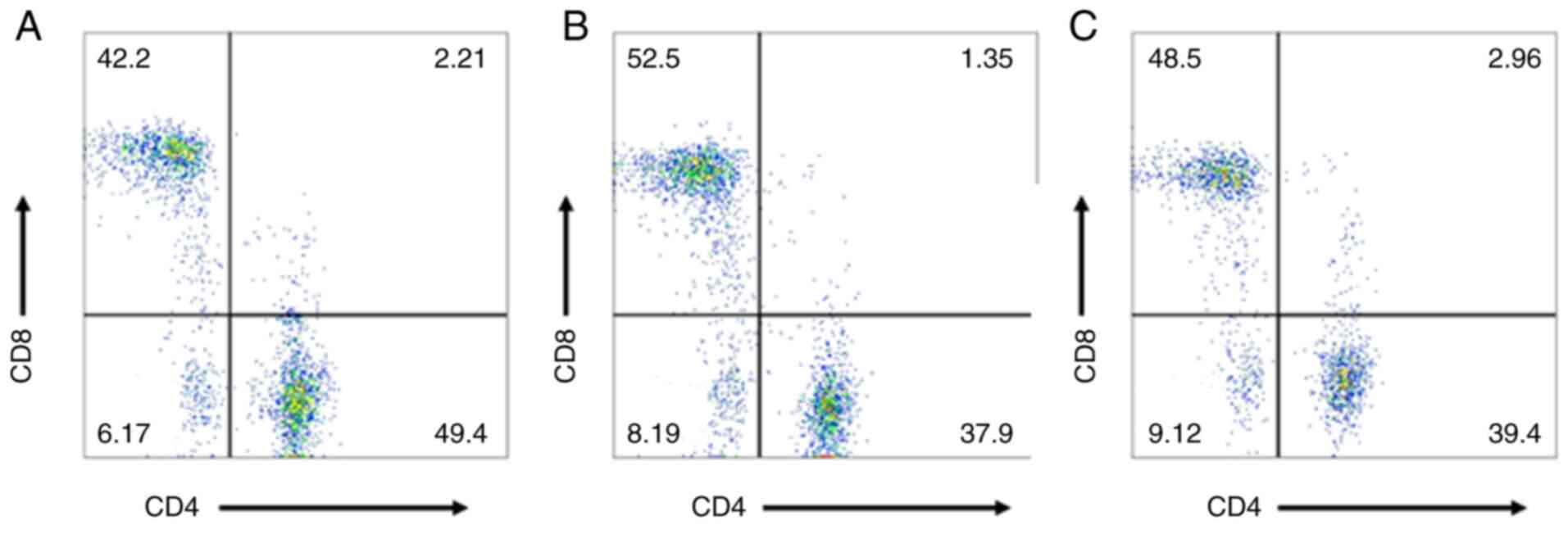
Detection of serum αβ and γδ T cells
in patients with BC
The numbers of CD3+, CD4+ and
γδ T cells in the blood of Patients with BC prior to surgery were
lower compared with those in healthy volunteers (P=0.0077, 0.0116
and 0.0003, respectively), while the ratio of
CD4+/CD8+ cells did not differ significantly
between the two groups (P>0.05). The number of CD8+
cells was also higher in Patients with BC compared with that in
healthy volunteers (P=0.0241). The numbers of CD3+,
CD4+ and γδ T cells and the
CD4+/CD8+ ratio after surgery were
significantly higher compared with those prior to surgery
(P=0.0109, 0.0031, 0.0165 and 0.018, respectively), while the
number of CD8+ cells was not significantly different
from that prior to surgery (P>0.05) and was still higher
compared with that in healthy volunteers (P=0.0053). The results
are summarized in Table I and
Figs. 1–3.
 | Table I.Comparison of T-cell subsets in the
peripheral blood of patients with breast cancer before and after
surgery and healthy volunteers. |
Table I.
Comparison of T-cell subsets in the
peripheral blood of patients with breast cancer before and after
surgery and healthy volunteers.
|
|
| T-cell subsets |
|
|---|
|
|
|
|
|
|---|
| Groups | Number of cases | CD3+ | CD4+ | CD8+ |
CD4+/CD8+ | γδT |
|---|
| Healthy
volunteers | 50 | 68.16±7.12 | 36.24±4.68 | 31.56±3.02 | 1.38±0.62 | 3.38±2.16 |
| Patients before
surgery | 138 |
65.24±6.36b |
34.12±5.16a |
32.78±3.33a | 1.27±0.29 |
2.12±2.04b |
| Patients after
surgery | 138 |
67.18±6.22c |
35.81±4.19d |
32.61±3.19a |
1.35±0.26c |
2.74±2.28c |
Association between preoperative
levels of αβ and γδ T cells in the peripheral blood and
clinicopathological characteristics in patients with BC
Following TNM staging, the number of CD3+
cells in stage II/III was higher than that in stage I BC (P=0.187
and 0.022, respectively); the CD4+/CD8+ ratio
and number of γδ T cells were lower in stage III patients compared
with those in stage I patients (P=0.0065 and 0.0176, respectively),
and there was no statistical difference between I vs. II and II vs.
III (P>0.05). Histological classification showed that the
CD4+/CD8+ ratio and number of γδ T cells were
lower in stage III patients compared with those in stage I patients
(P=0.02 and 0.0128, respectively), and there was no statistically
significant difference between the other groups (P>0.05). Among
the different molecular subtypes, the number of γδ T cells was
significantly higher in patients with luminal A and luminal B
subtype compared with that in patients with basal-like subtype
(P=0.004 and 0.0104, respectively), and there was no significant
difference in the number of T-cell subsets among the other subtypes
(P>0.05). The number of CD3+ cells was significantly
lower in patients with LN metastasis compared with that in patients
without LN metastasis. The difference in the number of
CD3+ cells was statistically significant when comparing
patients without LN metastases with those with 1–3 LN metastases
(P=0.0097), 4–9 LN metastases (P=0.0024), or >10 LN metastases
(P=0.0086). The number of γδ T cells was significantly higher in
patients without LN metastases compared with that in patients with
LN metastases. The difference was statistically significant when
comparing patients without LN metastases with those with 1–3 LN
metastases (P<0.0000), 4–9 LN metastases (P=0.0004), or >10
LN metastases (P=0.0000). The number of CD4+ cells was
higher in patients without LN metastasis and patients with 1–3 LN
metastases compared with that in patients with >10 LN metastases
(P=0.00468 and 0.0494, respectively). The number of CD8+
cells was lower in patients without LN metastasis compared with
that in patients with 4–9 or >10 LN metastases (P=0.0435 and
0.0283, respectively). There was no significant difference in the
number of CD8+ cells among the other groups (P>0.05;
Table II).
 | Table II.Comparison of T-cell subsets in the
peripheral blood of patients with breast cancer in different
pathological groups. |
Table II.
Comparison of T-cell subsets in the
peripheral blood of patients with breast cancer in different
pathological groups.
|
|
| T-cell subsets |
|
|---|
|
|
|
|
|
|---|
| Groups | Number of
cases |
CD3+ |
CD4+ |
CD8+ |
CD4+/CD8+ | γδT |
|---|
| TNM stage | 138 |
|
|
|
|
|
| I | 56 | 66.86±6.36 | 34.32±4.16 | 31.52±5.82 | 1.39±0.45 | 2.92±1.54 |
| II | 55 |
65.24±5.27a | 33.27±5.02 | 31.63±4.82 | 1.29±0.39 | 2.46±1.39 |
|
III | 27 |
64.35±4.76a | 32.72±5.35 | 32.51±6.05 |
1.12±0.32a |
2.12±1.08a |
| Histological
grade | 138 |
|
|
|
|
|
| I | 48 | 66.29±7.32 | 35.02±5.13 | 32.26±4.86 | 1.48±0.75 | 2.76±1.12 |
| II | 59 | 66.02±6.63 | 34.62±5.08 | 32.56±6.01 | 1.39±0.69 | 2.56±1.18 |
|
III | 31 | 64.74±7.16 | 33.19±4.86 | 33.06±5.18 |
1.09±0.65b |
2.12±1.04b |
| Molecular
subtype | 138 |
|
|
|
|
|
| Luminal
A | 38 | 66.24±5.36 | 35.12±4.12 | 31.87±4.78 | 1.38±0.68 |
2.36±1.12c |
| Luminal
B | 32 | 67.12±6.33 | 35.18±5.08 | 31.76±4.89 | 1.36±1.05 |
2.32±1.18c |
|
ERBB2+ | 28 | 65.15±4.63 | 34.82±5.13 | 32.86±6.12 | 1.37±0.82 | 2.02±1.14 |
|
Basal-like | 31 | 64.29±4.55 | 33.11±4.76 | 33.59±5.82 | 1.28±0.66 | 1.52±1.22 |
| Special
type | 9 |
|
|
|
|
|
| Endocrine
responsive | 5 | 67.06±1.31 | 34.78±5.06 | 31.96±2.16 | 1.35±0.45 | 1.41±1.06 |
| Endocrine
non-responsive | 4 | 66.72±1.36 | 34.56±5.18 | 32.16±3.42 | 1.33±0.65 | 1.39±1.13 |
| No lymph node
metastasis | 49 | 67.36±3.28 | 36.36±5.25 | 31.28±4.39 | 1.37±0.69 | 3.29±1.22 |
| Lymph node
metastases | 89 |
|
|
|
|
|
|
1-3 | 52 |
65.61±3.38e,f |
36.15±5.12f | 31.34±5.38 | 1.42±0.85 |
2.01±1.74e |
|
4-9 | 25 |
64.52±4.35e | 35.19±5.34 |
31.66±5.81d | 1.39±0.76 |
2.12±1.43e |
|
≥10 | 12 |
64.23±4.65e |
33.11±5.67d |
33.52±4.82d | 1.22±1.06 |
1.39±1.75e |
Discussion
BC occurs and progresses via pathological mechanisms
such as immune escape and immunosuppression. The proportion and
distribution of T lymphocytes in the peripheral blood and tumor
microenvironment are closely associated with tumor immune
stability, immune clearance, or immune escape. Moreover, the
relative stability, mutual coordination and mutual restriction of
T-lymphocyte subsets in healthy individuals ensures the stability
of immune function in the body and helps prevent invasion by
pathogens. Changes in the number and ratio of T-lymphocyte subsets
may disrupt the immune system and normal immune function of the
body, thus leading to lesions such as tumor and immune-related
conditions. CD3+ T cells are the main active cells in
cellular immunity, representing the proportion of mature
lymphocytes among the total T cells. CD4+ T cells are
helper T cells, whereas CD8+ T cells exert both
cytotoxic and immunosuppressive effects, and the
CD4+/CD8+ ratio reflects the status of
cellular immunity (8). The results
of the present study demonstrated that, due to the presence of BC
target cells, the CD3+, CD4+ and γδ Τ-cell
counts and the CD4+/CD8+ ratio in the
peripheral blood of the patients prior to surgery were
significantly lower compared with those in healthy volunteers. This
indicates that the tumor cells can elicit immune function-related
changes in T-lymphocyte subsets, recruiting more CD8+
cells to exert cytotoxic effects, and reducing the number of
CD3+, CD4+ and γδ Τ cells in the circulation.
In the present study patients with BC generally exhibit decreased
immune function, as well as changes in the number and function of
LNs that perform cellular immune functions.
Based on the different T-cell receptors (TCRs), T
cells are mainly divided into αβ and γδ T cells; αβ T-cell surface
receptors usually express CD4+ and CD8+. The
recognition of antigens mainly relies on APCs. Based on the
histocompatibility complex (MHC) molecules on the cell surface,
most αβ T cells can differentiate into both cytotoxic T and helper
T cells. CD8+ cells are referred to as cytotoxic T
cells, as their biological function is to directly kill the labeled
target cells, and these cells are the main effectors of the
antitumor immune response. CD4+ cells are characterized
as helper T cells, which mainly regulate or stimulate other
lymphocytes to exert their immune effects. γδ T cells are cells
that often do not express CD4+ or CD8+,
recognize antigens mainly in a non-MHC-restricted manner, and
induce an adaptive immune response by secreting a variety of
cytokines. This cell type is considered to be an unconventional T
cell that serves as a link between innate and adaptive immunity.
Due to its immune characteristics, it plays a key role in
anti-infection and antitumor responses, can produce effective
cytotoxicity against malignant tumors, and has the functions of
immune surveillance and tumor cell elimination (9). In the peripheral blood, αβ T cells
account for ~95%, while γδ T cells account for only 5% of the total
CD3+ cells. Based on the differences between the γ and δ
chains, γδ T cells are also divided into several subgroups, and
different subsets have specific tissue distribution among different
species. As shown in Fig. 4, the
majority of γδ T cells are distributed in human epithelial tissues,
while Vγ9Vδ2 TCR subsets are mainly expressed in peripheral blood
lymphocyte repository. Generally, 50–75% of γδ T lymphocytes in the
peripheral blood express the Vδ2 chain and co-express the Vγ9
chain. These cells are referred to as Vγ9Vδ2T cells, Vγ9Vδ2T cells
are only found in human and non-human primates, and 1–10% of T
cells are found in the peripheral blood of healthy individuals. It
was previously reported that early-stage BC is associated with low
expression of Vg9Vd2+ T lymphocytes in the circulation
(10). Human γδ T cells can mediate
antitumor immunity through different pathways, such as secretion of
pro-apoptotic molecules and pro-inflammatory cytokines, and
cell-cell contact-dependent cleavage through the NK transduction
pathway or TCR-dependent pathways. Activated γδ T cells can secrete
several cytokines that act on tumor cells or their
microenvironment, such as interferon-γ, tumor necrosis factor-α,
interleukin (IL)-2, perforin, granzyme B and Fas/FasL. As shown in
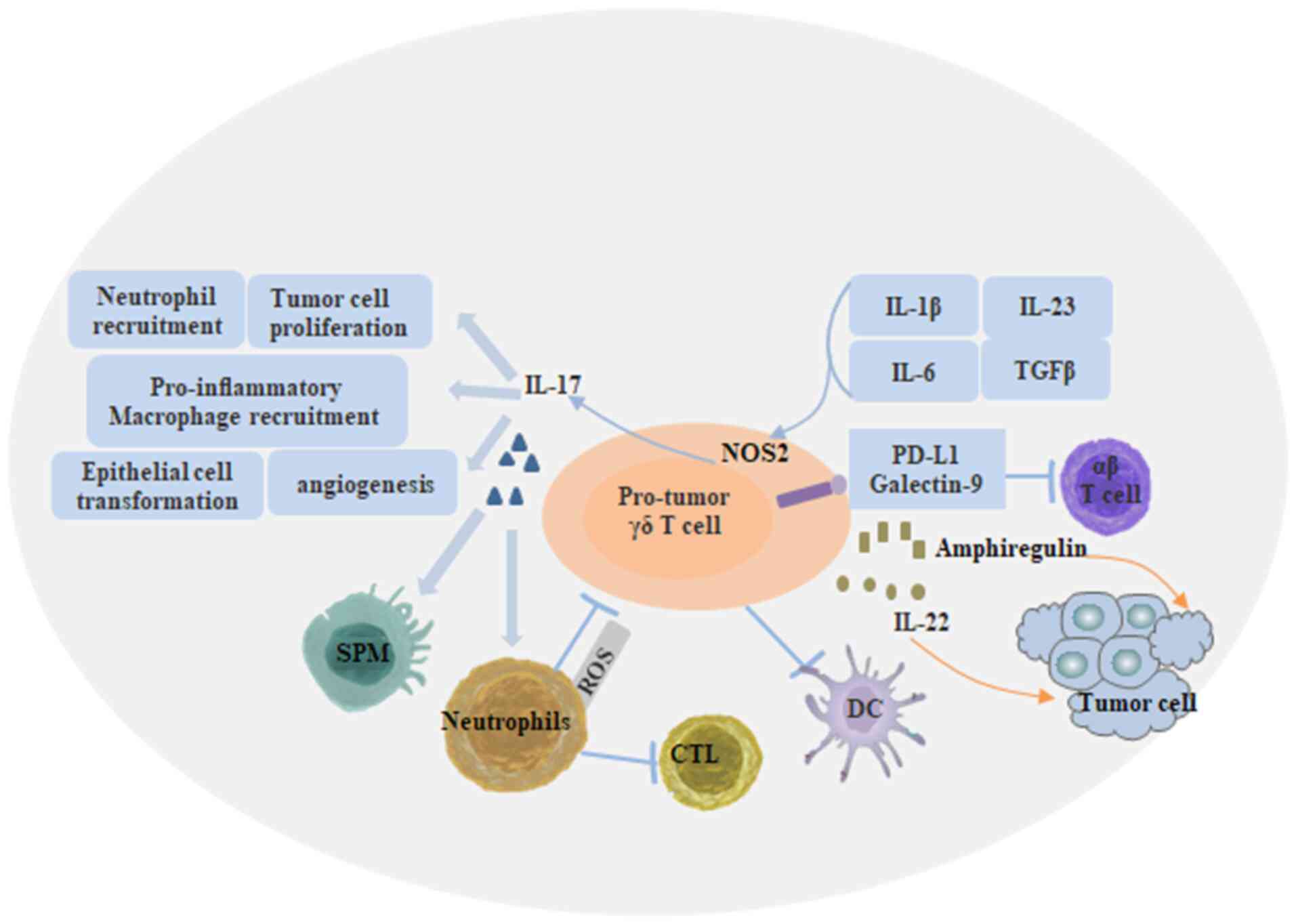
Fig. 5, γδ T cells also negatively
regulate the function of tumor cell killing. This is mainly
associated with the production of IL-17, which can stimulate tumor
cell proliferation and induce angiogenesis. Other negative
regulatory IL-7-mediated effects of γδ T cells on tumor cell
killing include inhibition of maturation of dendritic cells,
inhibition of T-cell response through PDL1 expression, and
inhibition of IL-17-producing γδ T cells through reactive oxygen
species generation by neutrophils (11). As shown in Fig. 6, although the number of γδ T cells is
small in vivo, the γδ T cell population may markedly enlarge
under phosphate stimulation both in vitro and in
vivo, which plays an important role in antitumor immunity
(12).
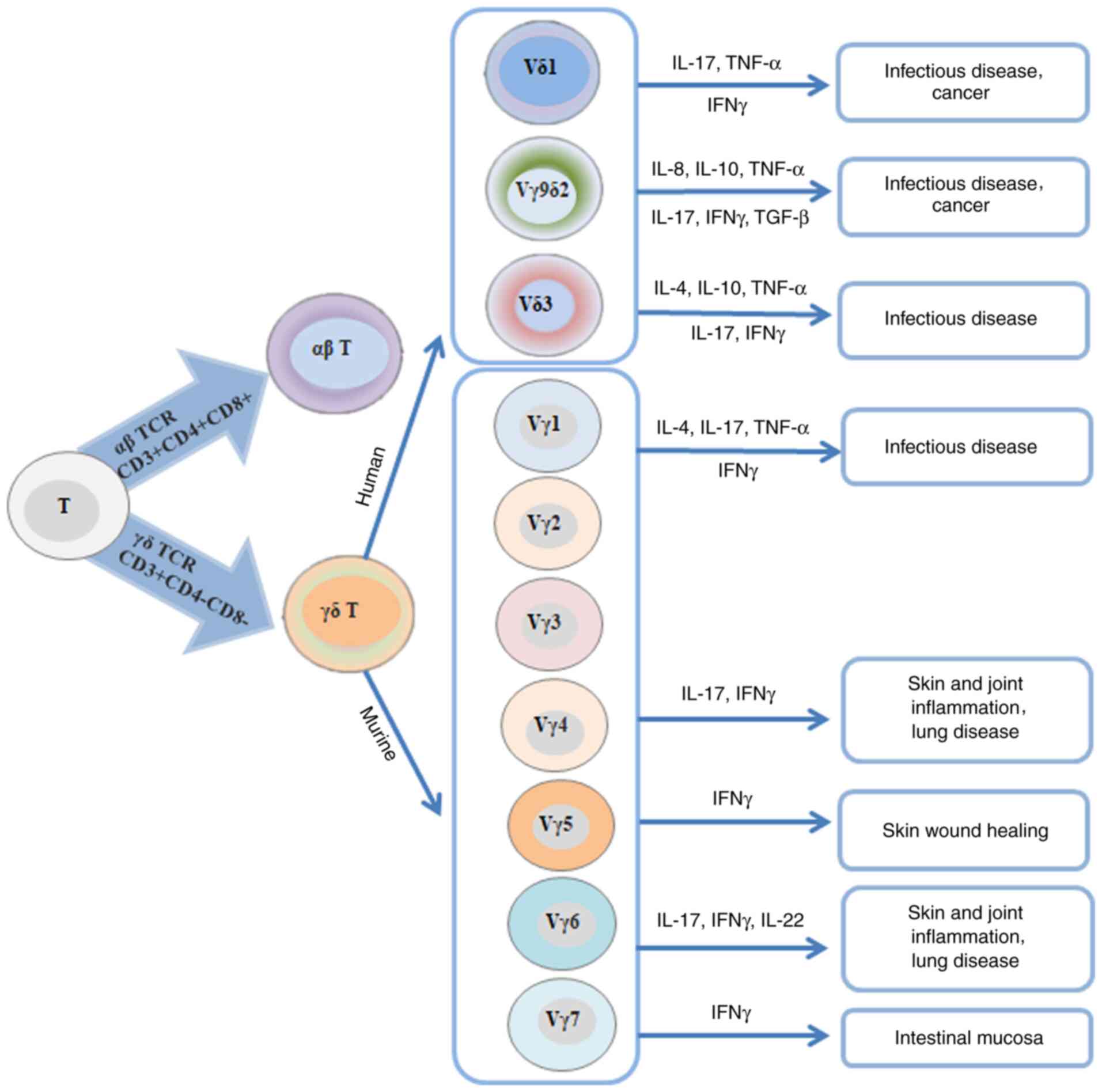 | Figure 4.Classification and characteristics of
human and mouse γδ T-cell subsets. Human γδ T cells are
differentiated into three subtypes, namely Vδ1, Vγ9δ2 and Vδ3, and
they mainly secrete TNF-α, IFN-γ, TGF-β, IL-4, IL-8, IL-10 and
IL-17. In mice, Vγ1δ, Vγ4δ, Vγ5δ, Vγ6δ and Vγ7δ T cells mainly
produce IL-4, IL-17, IL-22 and IFN-γ. TCR, T-cell receptor; IFN,
interferon; IL, interleukin; TNF, tumor necrosis factor; TGF,
transforming growth factor. |
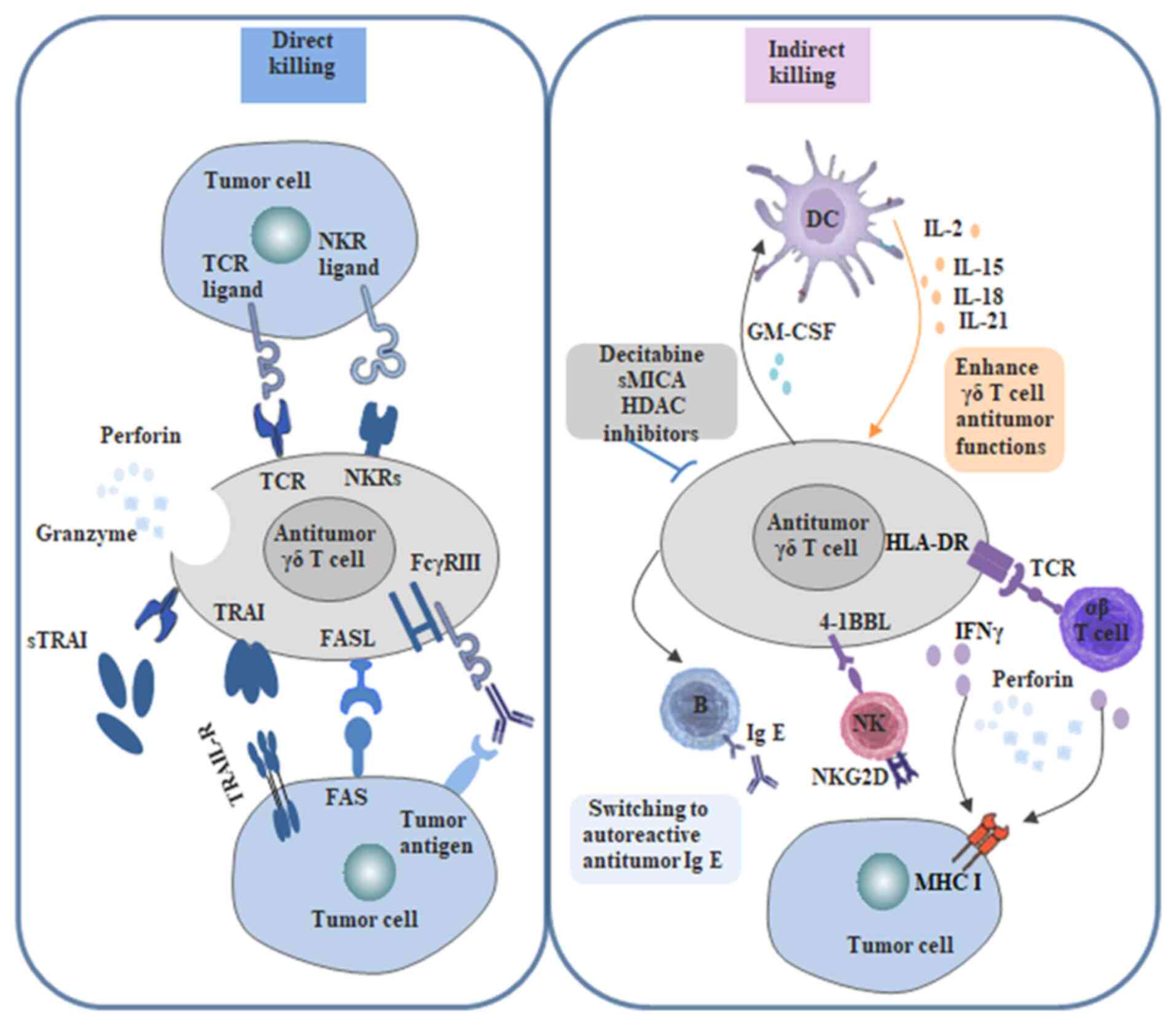 | Figure 5.Regulatory mechanism of tumor cell
killing by γδ T cells. γδ T cells directly recognize tumor-killing
cells through the TCR and NKR. γδ T cells can mediate tumor cell
killing by expressing TRAIL, FASL or release of cytolytic granules.
γδ T cells induce antitumor immunity through IFN-γ production and
antigen-presenting cell function to induce αβ T-cell activation,
whereas expression of 4-1BBL can stimulate NK cells. In addition,
γδ T cells induce antibody class switching in B cells, which
contributes to the protective humoral response. γδ T cells can also
modulate DC infiltration through GM-CSF. TCR, T-cell receptor; NK,
natural killer; NKR, natural killer cell receptor; IFN, interferon;
DC, dendritic cell; 4-1BBL, 4-1BB ligand; GM-CSF,
granulocyte-macrophage colony-stimulating factor. |
Flow cytometry was used to detect αβ and γδ T-cell
subsets in the peripheral blood of Patients with BC and healthy
controls. It has been established that the normal population size
of cell subsets is crucial for clinical diagnosis and disease
determination. The reference values for detecting normal human αβ
and γδ Τ-cell subsets are as follows: CD3+, 61–85%;
CD4+, 28–58%; CD8+, 19–48%;
CD4+/CD8+ ratio, 1–2; and γδ, 1–5%. The
results revealed that the CD3+, CD4+ and γδ
T-cell counts and the CD4+/CD8+ ratio were
lower in Patients with BC prior to surgery compared with those in
healthy volunteers, while the CD8+ cell number was
higher compared with that in healthy subjects. It may be suggested
that, upon the occurrence of BC, the body may recruit more
CD8+ cells and trigger an immune response. BC cell
antigens lead to depletion of CD4+ and γδ T cells, as
well as to a decrease in the number of CD3+ cells among
total lymphocytes. The γδ, CD3+ and CD4+
T-cell counts and the CD4+/CD8+ ratio in the
peripheral blood of patients with BC were higher after surgery
compared with preoperative levels, but were still lower compared
with those in healthy controls. The number of CD8+ cells
was lower compared with that before surgery, but slightly higher
compared with that of controls, although the difference was not
statistically significant. It is hypothesized that BC cells may
secrete substances that inhibit the proliferation of γδ T cells,
and the load of tumor cells is reduced after surgical tumor
resection, which can improve the cellular immune status of the body
and enhance the cellular immune function of the patients. The
occurrence and development of tumors will also cause changes in the
T-lymphocyte subsets in the body. The present study demonstrated
that the number of CD3+ cells in patients with TNM stage
II and III was higher compared with that in patients with stage I
disease, whereas the CD4+/CD8+ ratio and
T-cell counts in stage III patients were lower compared with those
in stage I patients. The histological grading revealed that the
CD4+/CD8+ ratio and the number of T cells in
patients with grade III tumors were lower compared with those in
patients with grade I tumors. Among different molecular subtypes,
the number of T cells in patients with luminal A and luminal B
subtypes was significantly higher compared with that in patients
with basal-like subtype. The numbers of CD3+,
CD4+ and γδ T cells were significantly lower in patients
with LN metastasis compared with those in patients without LN
metastasis. This indicates that there is an overall decrease in
immune function in Patients with BC, which is manifested by a
change in the number and function of lymphocytes performing
cellular immune functions, which is one of the important causes of
BC development and progression. There is a need for more in-depth
basic and clinical research on expanding the peripheral blood γδ
T-cell population and enhancing its tumor cell killing ability, in
order to improve the immune function and quality of life of
Patients with BC. Therefore, measurement of peripheral blood
lymphocyte subsets may help elucidate the immune function status of
Patients with BC with different molecular subtypes, TNM stage and
histological types, and may prove to be of great value for the
diagnosis of BC, as well as for monitoring therapeutic efficacy and
prognosis.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81473687), the
Academic Promotion Program of Shandong First Medical University
(grant no. 2019QL017), the High-level Project Cultivation Program
of Shandong First Medical University (grant no. 2018GCC14), the
Natural Science Foundation of Shandong Province (grant no.
ZR2013HM038) and the Shandong Province Chinese Medicine Science and
Technology Development Plan (grant no. 2017-260).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
Conceptualization, XQL and MZ; methodology, XQL, MZ,
XLL and CW; writing-original draft preparation, XQL, MZ and XLL;
writing-review and editing, XQL and MZ; supervision, XQL. All the
authors have read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Research
Ethics Committee of the Second Affiliated Hospital of Shandong
First Medical University (Tai'an, China). The procedures performed
were in accordance with the ethical standards of the institutional
and/or national research committee and with the 1964 Helsinki
Declaration and its later amendments or comparable ethical
standards.
Patient consent for publication
Not applicable.
Competing interests
All the authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD, Fedewa SA, Ahnen DJ,
Meester RG, Barzi A and Jemal A: Colorectal cancer statistics,
2017. CA Cancer J Clin. 67:177–193. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Markman JL and Shiao SL: Impact of the
immune system and immunotherapy in colorectal cancer. J
Gastrointest Oncol. 6:208–223. 2015.PubMed/NCBI
|
|
3
|
Riazi Rad FR, Ajdary S, Omranipour R,
Alimohammadian MH and Hassan ZM: Comparative analysis of
CD4+ and CD8+ T cells in tumor tissues, lymph
nodes and the peripheral blood from patients with breast cancer.
Iran Biomed J. 19:35–44. 2015.PubMed/NCBI
|
|
4
|
Xu J, Jiang L, Cao H, Jia Y, Wu S, Jiang C
and Sun T: Predictive value of CD4+/CD8+
ratio in patients with breast cancer receiving recombinant human
thrombopoietin. J Interferon Cytokine Res. 38:213–220. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lin KR, Pang DM, Jin YB, Hu Q, Pan YM, Cui
JH, Chen XP, Lin YX, Mao XF, Duan HB and Luo W: Circulating
CD8+ T-cell repertoires reveal the biological
characteristics of tumors and clinical responses to chemotherapy in
breast cancer patients. Cancer Immunol Immunother. 67:1743–1752.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Janssen N, Fortis SP, Speigl L, Haritos C,
Sotiriadou NN, Sofopoulos M, Arnogiannaki N, Stavropoulos-Giokas C,
Dinou A, Perez S, et al: Peripheral T cell responses to tumour
antigens are associated with molecular, immunogenetic and cellular
features of breast cancer patients. Breast Cancer Res Treat.
161:51–62. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Silva-Santos B, Serre K and Norell HK: γδ
T cells in cancer. Nat Rev Immunol. 15:683–691. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kassardjian A, Shintaku PI and Moatamed
NA: Expression of immune checkpoint regulators, cytotoxic T
lymphocyte antigen 4 (CTLA-4) and programmed death-ligand 1
(PD-L1), in female breast carcinomas. PLoS One. 13:e01959582018.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Morrow ES, Roseweir A and Edwards J: The
role of gamma delta T lymphocytes in breast cancer: A review.
Transl Res. 203:88–96. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sugie T, Murata-Hirai K, Iwasaki M, Morita
CT, Li W, Okamura H, Minato N, Toi M and Tanaka Y: Zoledronic
acid-induced expansion of γδ T cells from early-stage breast cancer
patients: Effect of IL-18 on helper NK cells. Cancer Immunol
Immunother. 62:677–687. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Silva-Santos B, Mensurado S and Coffelt
SB: γδ T cells: Pleiotropic immune effectors with therapeutic
potential in cancer. Nat Rev Cancer. 19:392–404. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kobayashi H and Tanaka Y: γδ T cell
immunotherapy-a review. Pharmaceuticals (Basel). 8:40–61. 2015.
View Article : Google Scholar : PubMed/NCBI
|