Introduction
According to the World Health Organization (WHO)
classification of the central nervous system (CNS) tumors,
glioblastoma (GBM) is defined as a grade IV astrocytoma (1). GBM represents the most malignant glioma
and it is characterized by necrosis, neovascularization and
histological heterogeneity (2). GBM
represents the most frequent glial tumor, with almost 3 new cases
per 100,000 people per year (3). The
current standard of care for GBM consists of surgical resection,
followed by radiotherapy and chemotherapy with temozolomide
(4). Despite treatment, the
prognosis for GBM patients remains extremely poor, with a median
survival period of 14.6 months, and the 5-year survival is less
than 5% (4).
In recent years, great progress has been made in the
area of immunotherapy and accumulating preclinical and clinical
data seem to suggest potential novel therapeutic avenues for GBM
patients (5,6). It is generally believed that GBM
creates a highly immunosuppressive/immuneregulatory
microenvironment. Several checkpoint molecules capable of
inhibiting the immune responses against neo-antigens, including
CTLA4 and PD1/PDL-1, are expressed on both T cells and cancer
cells. Immune checkpoint inhibitors, such as nivolumab, ipilimumab
and pembrolizumab, have strikingly improved patient survival in
solid tumors, such as non-small lung cancer and melanoma. However,
the trials assessing the efficacy of immune checkpoint inhibitors
in GBM are still disappointing (7).
A retrospective study of the use of pembrolizumab in the treatment
of recurrent CNS tumors, including GBM, demonstrated that patients
treated with Pembrolizumab did not have improved survival (7). Another Phase III randomized trial
comparing radiation and concomitant temozolomide with or without
nivolumab showed that no progression-free survival benefits were
obtained by the addition of nivolumab. However, in a Phase II
trial, preoperative administration of nivolumab increased chemokine
expression and T-cell receptor clonal diversity, which likely
promotes immune-cell infiltration and antitumor immune response
(7).
It is reasonable that targeting multiple immune
checkpoints in combination with cytotoxic drugs could represent a
promising strategy for GBM. The present study characterized the
expression levels of several inhibitory immune checkpoints in GBM
(i.e., CD276, VTCN1, CD47, PVR, TNFRSF14, CD200, LGALS9, NECTIN2
and CD48) in order to evaluate their prognostic value. Moreover,
their potential effects in regulating immune-cell infiltration was
investigated.
Materials and methods
Profiling of inhibitory immune
checkpoints in GBM
In order to evaluate the expression levels of
inhibitory immune checkpoints in GBM as compared to lower grade
astrocytomas and normal brain samples, RSEM-normalized RNA Seq data
were downloaded from the The Cancer Genome Atlas (TCGA) databank.
Selected genes were CD276, VTCN1, CD47, PVR, TNFRSF14, CD200,
LGALS9, NECTIN2 and CD48. Complete clinical data of the patients
were retrieved and only data from primary tumors, with no
neoadjuvant therapy prior to excision, were selected. Data were
subjected to logarithmic transformation and Linear Model for
Microarray Analysis (LIMMA) was used to assess statistical
significance for the differences among cancer types. Overall, this
study comprised 153 GBM samples, 130 anaplastic astrocytoma (grade
III) samples, 63 astrocytoma (grade II) samples and 5 normal brain
samples. The results shown here are based upon data generated by
the TCGA Research Network (https://www.cancer.gov/tcga). TCGA Ethics &
Policies were originally published by the National Cancer
Institute.
Survival analysis
Samples were stratified in quartiles based on the
expression of the genes of interest and samples in the upper and
lower quartiles were selected for comparison. Kaplan-Meier curves
were constructed for overall survival and disease-free survival and
its significance analyzed by log-rank (Mantel-Cox) test.
Computational deconvolution of
infiltrating immune cells
In order to evaluate the relative proportions of the
infiltrating immune cell subsets in GBM samples diverging for the
expression of the selected immune checkpoints and stratified in
accordance to survival analysis, we performed a computational
deconvolution analysis. The web-based utility, xCell, was used. It
is a computational tool that is able, by using gene signatures, to
infer the presence in a sample of various cell types, including
immature dendritic cells (iDCs), conventional DCs (cDCs), active
DCs (aDCs), plasmacytoid DCs (pDCs), B cells, CD4+ naive
T cells, memory B cells, plasma cells, Th1 cells, Th2 and Treg
cells and macrophages (8).
Statistical analysis
Gene expression differences were evaluated using
LIMMA on log-transformed RSEM-normalized expression values. FDR
<0.05 was considered for statistical significance. Gene
expression was visualized as heatmap, using the group mean value.
Clustering was performed for both sample groups and genes of
interest, using Pearson correlation as distance metrics.
Correlation analysis was performed using the Pearson's correlation
test. Survival analysis was performed using Kaplan-Meier and its
significance analyzed by the log-rank (Mantel-Cox) test. For the
analysis, P<0.05 was considered to indicate a statistically
significant difference. Statistical analysis was performed with
GraphPad Prism 8 (GraphPad Software, Inc.) and SPSS 24 (IBM
Corp.).
Results
Expression of inhibitory immune
checkpoints in GBM
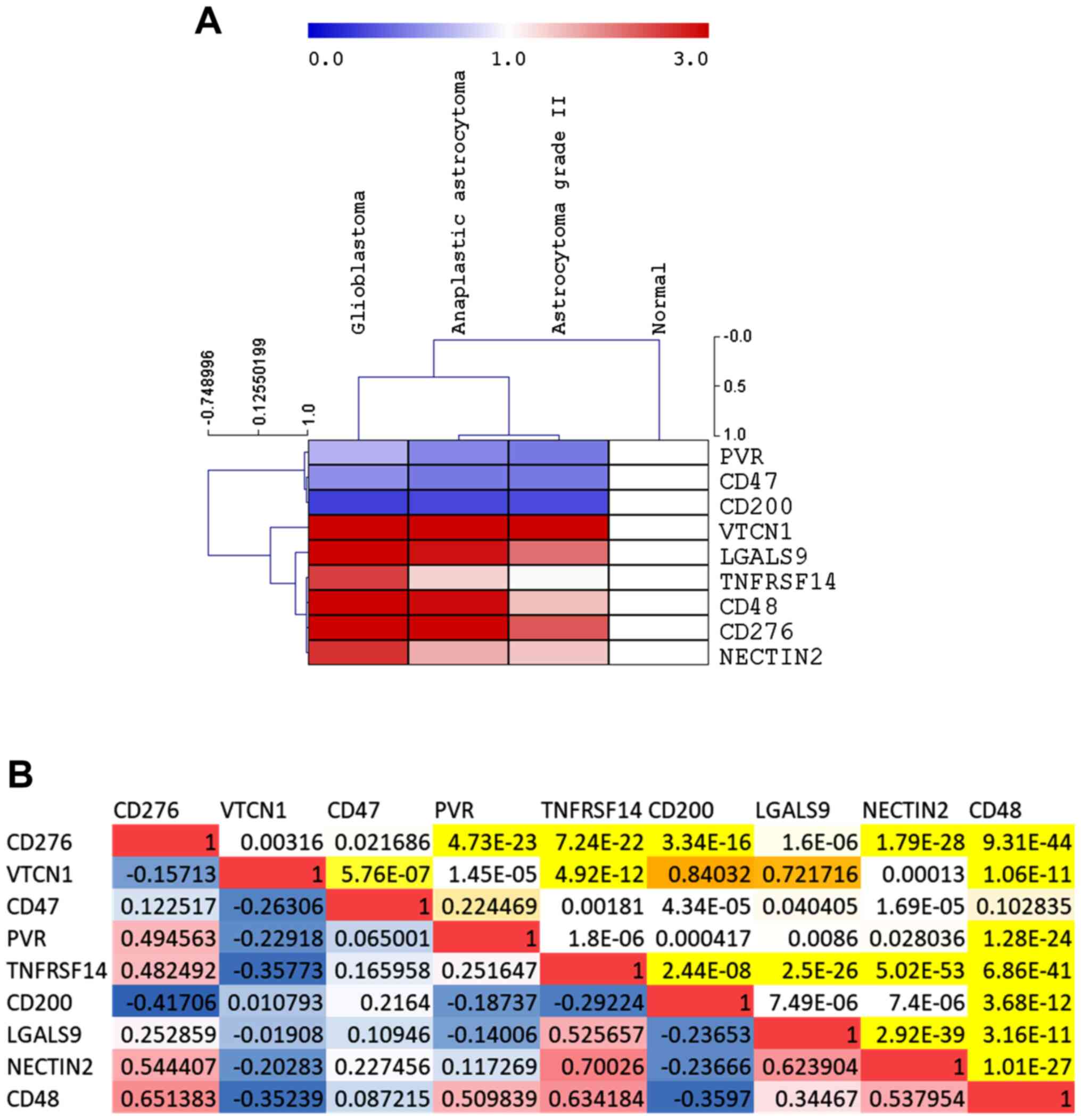
A significant upregulation in the expression levels
of CD276, VTCN1, TNFRSF14, LGALS9, NECTIN2 and CD48 was observed in
GBM as compared to normal brain samples (Fig. 1A, Table
I). On the contrary, a significant downregulation of CD47 and
CD200 was observed in GBM as compared to normal brain samples,
while a trend of downregulation was observed for PVR (Fig. 1A, Table
I). Along the same lines, with the exception of LGALS9 and
CD200, a significant modulation in the expression levels of the
investigated immune checkpoints was observed between the GBM and
anaplastic astrocytoma groups of samples (Fig. 1A, Table
I). Moreover, CD276, TNFRSF14, LGALS9 and CD48 resulted
significantly upregulated in anaplastic astrocytoma samples as
compared to grade II astrocytomas (Fig.
1A, Table I). A significant
direct correlation was observed for CD276, PVR, TNFRSF14, NECTIN2
and CD48 (Fig. 1B). Among the GBM
samples, a significant negative correlation was instead observed
between VTCN1 and PVR, NECTIN2 and CD48 (Fig. 1B).
 | Table I.Expression of selected immune
checkpoints in gliomas. |
Table I.
Expression of selected immune
checkpoints in gliomas.
|
| CD276 | VTCN1 | CD47 | PVR | TNFRSF14 | CD200 | LGALS9 | CD48 | NECTIN2 |
|---|
| Glioblastoma |
| Log
(mean ± SD) | 11.47±0.61 | 2.09±1.61 | 11.3±0.45 | 9.17±0.55 | 9.09±0.84 | 8.9±0.83 | 10.2±0.89 | 6.77±1.43 | 10.37±0.61 |
| Anaplastic
astrocytoma |
| Log
(mean ± SD) | 10.46±0.70 | 2.78±1.54 | 11.10±0.44 | 8.77±0.55 | 8.19±1.14 | 9.03±0.71 | 10.12±1 | 4.77±2.10 | 9.7±0.69 |
| Astrocytoma grade
II |
| Log
(mean ± SD) | 9.99±0.63 | 2.98±1.36 | 11.074±0.52 | 8.62±0.52 | 7.81±0.78 | 9.17±0.75 | 9.69±0.94 | 3.81±1.97 | 9.53±0.49 |
| Normal |
| Log
(mean ± SD) | 8.79±0.36 | 0.15±0.83 | 12.17±0.13 | 9.69±0.51 | 7.75±0.46 | 10.91±0.46 | 8.59±0.49 | 3.21±0.93 | 8.98±0.33 |
| Glioblastoma vs.
anaplastic astrocytoma |
|
Adjusted P-value | 1.38896E-30 | 0.000373262 | 3.08282E-05 | 6.10399E-09 | 2.15289E-13 | 0.20064689 | 0.41158742 | 1.06584E-17 | 8.13945E-17 |
| Glioblastoma vs.
astrocytoma grade II |
|
Adjusted P-value | 9.64928E-39 | 0.000241293 | 0.000199704 | 1.78558E-10 | 1.31977E-16 | 0.031546623 | 0.00035424 | 2.96428E-23 | 8.10789E-17 |
| Glioblastoma vs.
normal |
|
Adjusted P-value | 7.41774E-16 | 0.013900759 | 0.00035894 | 0.068411075 | 0.006012107 | 1.76232E-07 | 0.000529205 | 7.99978E-05 | 7.29563E-06 |
| Anaplastic
astrocytoma vs. astrocytoma grade II |
|
Adjusted P-value | 0.000115669 | 0.58144605 | 0.81457853 | 0.18047059 | 0.042150598 | 0.43026862 | 0.018488223 | 0.005033033 | 0.19248255 |
| Anaplastic
astrocytoma vs. normal |
|
Adjusted P-value | 6.24305E-07 | 0.001064924 | 7.15003E-06 | 0.001204611 | 0.46713257 | 2.27282E-06 | 0.001895901 | 0.12621635 | 0.032178745 |
| Astrocytoma grade
II vs. normal |
|
Adjusted P-value | 0.000601994 | 0.000684009 | 1.00213E-05 | 0.000269684 | 0.95525837 | 2.65911E-05 | 0.039250672 | 0.649572 | 0.1347119 |
Survival analysis
Samples were stratified in quartiles based on the
expression of the genes of interest, and samples in the upper and
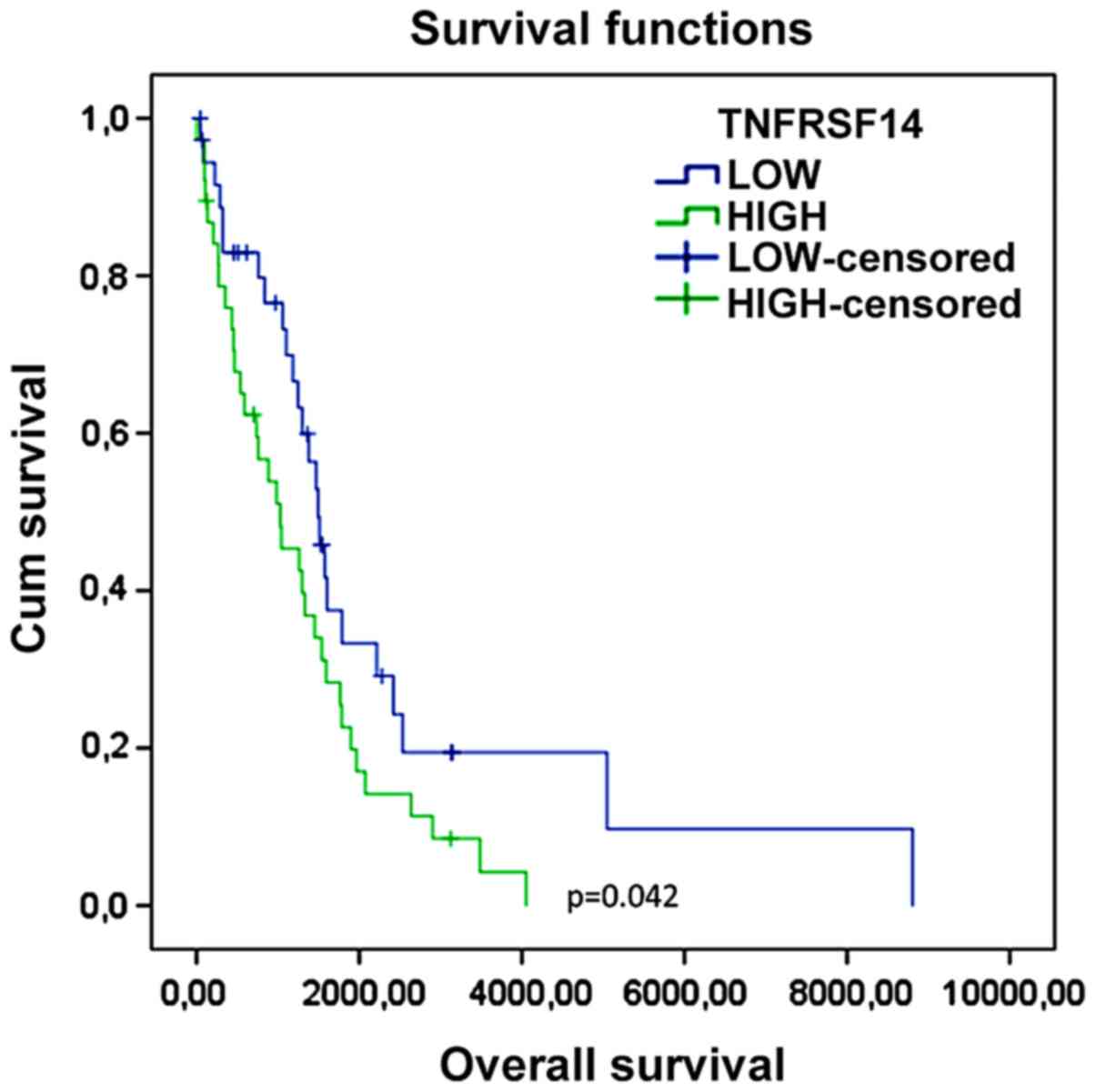
lower quartiles were selected for comparison. As shown in Table II and Fig. 2, higher expression levels of TNFRSF14
in GBM were associated to a significantly lower overall survival.
No significance was observed for any of the other immune
checkpoints. Accordingly, higher TNFRSF14 levels were associated to
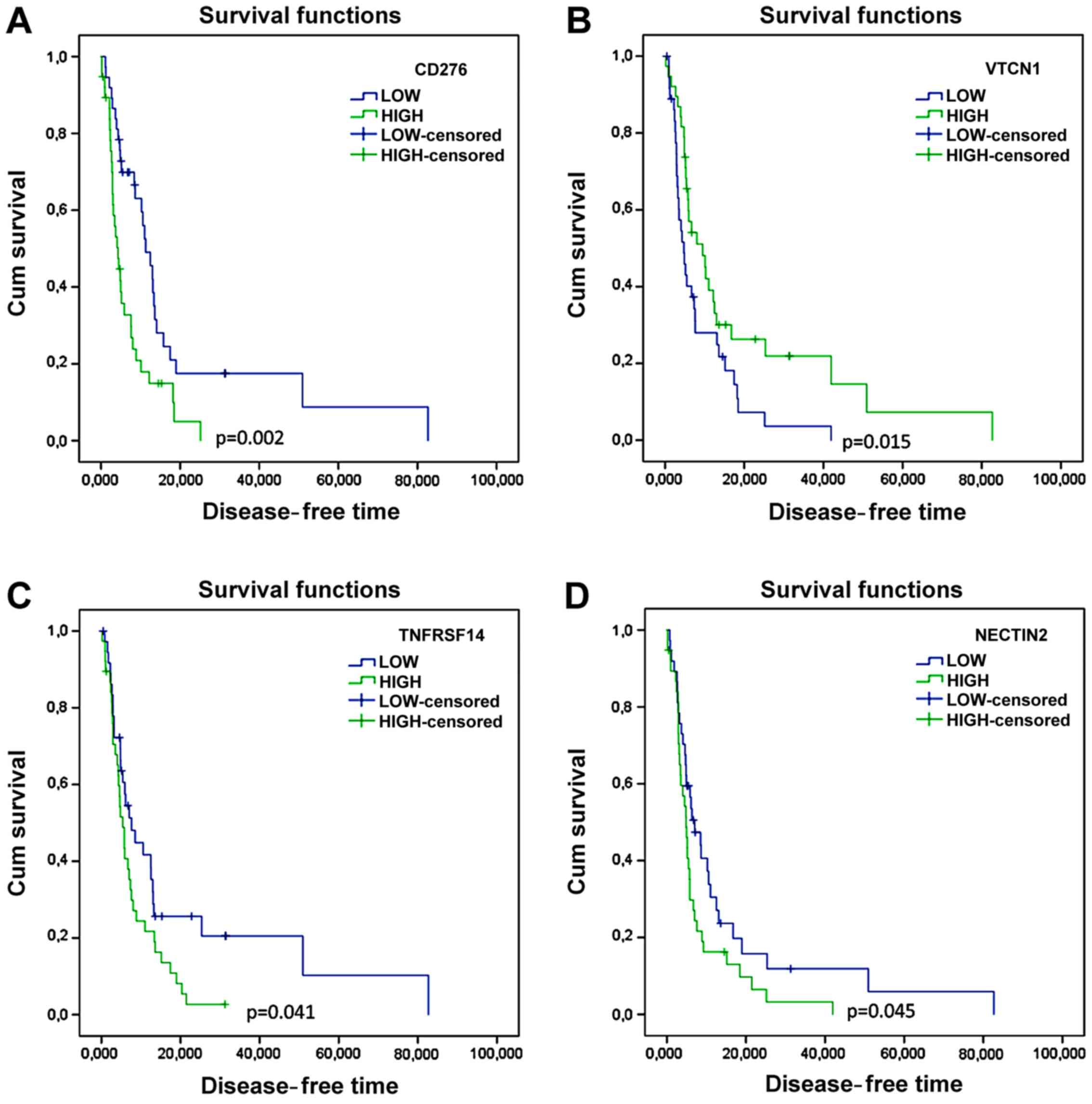
a shorter disease-free time (Fig. 3
and Table III). Lower levels of
CD276 and NECTIN2 were also significantly associated to better
disease-free time (Fig. 3 and
Table III). Unexpectedly, higher
levels of VTCN1 were associated to a longer disease-free time
(Fig. 3 and Table III).
 | Table II.Overall survival for the selected
immune checkpoints in glioblastoma. |
Table II.
Overall survival for the selected
immune checkpoints in glioblastoma.
|
| Mean | Median |
|
|
|---|
|
|
|
|
|
|
|---|
|
|
|
| 95% CI |
|
| 95% CI | Log-rank
(Mantel-Cox) |
|---|
|
|
|
|
|
|
|
|
|
|---|
|
| Estimate | SE | Lower bound | Upper bound | Estimate | SE | Lower bound | Upper bound | Chi-square | Significance |
|---|
| CD276 |
|
Low | 2,106.954 | 437.733 | 1,248.998 | 2,964.910 | 1,511.000 | 231.470 | 1,057.320 | 1,964.680 |
|
|
|
High | 1,170.322 | 156.700 |
863.189 | 1,477.455 | 1,124.000 | 247.168 |
639.551 | 1,608.449 |
|
|
|
Overall | 1,750.302 | 291.264 | 1,179.424 | 2,321.180 | 1,275.000 | 67.205 | 1,143.278 | 1,406.722 | 2.771 | 0.096 |
| VTCN1 |
|
Low | 1,501.133 | 334.027 |
846.440 | 2,155.825 | 1,173.000 | 388.775 |
411.000 | 1,935.000 |
|
|
|
High | 2,332.769 | 419.625 | 1,510.304 | 3,155.233 | 1,419.000 | 273.388 |
883.160 | 1,954.840 |
|
|
|
Overall | 1,915.041 | 270.368 | 1,385.119 | 2,444.963 | 1,298.000 | 131.107 | 1,041.029 | 1,554.971 | 3.254 | 0.071 |
| CD47 |
|
Low | 1,550.759 | 265.609 | 1,030.166 | 2,071.352 | 1,275.000 | 165.243 |
951.123 | 1,598.877 |
|
|
|
High | 1,563.020 | 348.405 |
880.146 | 2,245.894 | 1,101.000 | 292.895 |
526.925 | 1,675.075 |
|
|
|
Overall | 1,565.487 | 229.512 | 1,115.643 | 2,015.332 | 1,183.000 | 126.196 |
935.656 | 1,430.344 | 0.018 | 0.893 |
| PVR |
|
Low | 1,812.897 | 344.718 | 1,137.251 | 2,488.544 | 1,491.000 | 227.945 | 1,044.228 | 1,937.772 |
|
|
|
High | 1,316.999 | 227.311 |
871.470 | 1,762.527 | 1,124.000 | 184.382 |
762.612 | 1,485.388 |
|
|
|
Overall | 1,579.214 | 214.791 | 1,158.224 | 2,000.205 | 1,255.000 | 102.776 | 1,053.560 | 1,456.440 | 1.711 | 0.191 |
| TNFRSF14 |
|
Low | 2,376.561 | 534.969 | 1,328.021 | 3,425.100 | 1,495.000 | 123.626 | 1,252.693 | 1,737.307 |
|
|
|
High | 1,249.227 | 179.475 |
897.456 | 1,600.999 | 1,028.000 | 293.848 |
452.057 | 1,603.943 |
|
|
|
Overall | 1,696.854 | 245.369 | 1,215.931 | 2,177.777 | 1,298.000 | 150.294 | 1,003.424 | 1,592.576 | 4.148 | 0.042 |
| CD200 |
|
Low | 1,538.345 | 268.458 | 1,012.168 | 2,064.523 | 1,294.000 | 191.464 |
918.730 | 1,669.270 |
|
|
|
High | 1,960.634 | 491.408 |
997.474 | 2,923.794 | 1,419.000 | 184.815 | 1,056.762 | 1,781.238 |
|
|
|
Overall | 1,647.845 | 242.431 | 1,172.680 | 2,123.010 | 1,298.000 | 157.111 |
990.063 | 1,605.937 | 0.184 | 0.668 |
| LGALS9 |
|
Low | 1,678.101 | 252.097 | 1,183.991 | 2,172.212 | 1,495.000 | 126.246 | 1,247.557 | 1,742.443 |
|
|
|
High | 1,775.014 | 291.699 | 1,203.283 | 2,346.744 | 1,403.000 | 234.376 |
943.623 | 1,862.377 |
|
|
|
Overall | 1,754.181 | 202.451 | 1,357.377 | 2,150.985 | 1,403.000 | 115.366 | 1,176.882 | 1,629.118 | 0.011 | 0.918 |
| NECTIN2 |
|
Low | 1,914.122 | 383.529 | 1,162.406 | 2,665.839 | 1,495.000 | 117.504 | 1,264.692 | 1,725.308 |
|
|
|
High | 1,467.364 | 265.874 |
946.251 | 1,988.477 | 1,124.000 | 146.219 |
837.410 | 1,410.590 |
|
|
|
Overall | 1,693.007 | 232.899 | 1,236.525 | 2,149.490 | 1,275.000 | 187.661 |
907.184 | 1,642.816 | 1.066 | 0.302 |
| CD48 |
|
Low | 1,535.158 | 253.307 | 1,038.675 | 2,031.640 | 1,376.000 | 182.438 | 1,018.422 | 1,733.578 |
|
|
|
High | 1,685.861 | 357.415 |
985.327 | 2,386.395 | 1,275.000 | 260.223 |
764.962 | 1,785.038 |
|
|
|
Overall | 1,630.422 | 240.486 | 1,159.068 | 2,101.775 | 1,298.000 | 156.655 |
990.956 | 1,605.044 | 0.001 | 0.970 |
 | Table III.Disease-free survival for the
selected immune checkpoints in glioblastoma. |
Table III.
Disease-free survival for the
selected immune checkpoints in glioblastoma.
|
| Mean | Median |
|
|
|---|
|
|
|
|
|
|
|---|
|
|
|
| 95% CI |
|
| 95% CI | Log-rank
(Mantel-Cox) |
|---|
|
|
|
|
|
|
|
|
|
|---|
|
| Estimate | SE | Lower bound | Upper bound | Estimate | SE | Lower bound | Upper bound | Chi-square | Significance |
|---|
| CD276 |
|
Low | 19.417 | 4.607 | 10.386 | 28.447 | 11.270 | 1.527 | 8.277 | 14.263 |
|
|
|
High |
6.726 | 1.132 |
4.507 |
8.945 |
4.300 | 0.760 | 2.810 |
5.790 |
|
|
|
Overall | 13.590 | 2.671 |
8.354 | 18.825 |
7.590 | 1.724 | 4.211 | 10.969 | 10.000 | 0.002 |
| VTCN1 |
|
Low |
8.510 | 1.615 |
5.346 | 11.675 |
4.730 | 0.966 | 2.836 |
6.624 |
|
|
|
High | 19.102 | 4.450 | 10.380 | 27.824 |
9.460 | 2.380 | 4.795 | 14.125 |
|
|
|
Overall | 13.618 | 2.366 |
8.981 | 18.255 |
5.980 | 1.070 | 3.883 |
8.077 |
5.944 | 0.015 |
| CD47 |
|
Low | 11.583 | 2.051 |
7.562 | 15.603 |
8.510 | 1.797 | 4.987 | 12.033 |
|
|
|
High | 11.562 | 2.939 |
5.801 | 17.323 |
5.390 | 0.833 | 3.757 |
7.023 |
|
|
|
Overall | 11.544 | 1.811 |
7.994 | 15.094 |
7.030 | 1.056 | 4.960 |
9.100 |
0.182 | 0.670 |
| PVR |
|
Low | 13.275 | 3.278 |
6.851 | 19.699 |
5.910 | 2.202 | 1.593 | 10.227 |
|
|
|
High |
6.936 | 1.188 |
4.608 |
9.264 |
4.860 | 0.715 | 3.458 |
6.262 |
|
|
|
Overall | 10.343 | 1.906 |
6.608 | 14.078 |
5.190 | 0.506 | 4.199 |
6.181 |
2.563 | 0.109 |
| TNFRSF14 |
|
Low | 19.741 | 5.110 |
9.725 | 29.756 |
7.620 | 1.766 | 4.158 | 11.082 |
|
|
|
High |
7.659 | 1.124 |
5.455 |
9.862 |
5.390 | 0.715 | 3.988 |
6.792 |
|
|
|
Overall | 13.405 | 2.628 |
8.253 | 18.557 |
5.910 | 0.974 | 4.000 |
7.820 |
4.168 | 0.041 |
| CD200 |
|
Low |
8.966 | 1.925 |
5.194 | 12.738 |
5.160 | 0.873 | 3.448 |
6.872 |
|
|
|
High | 17.597 | 4.920 |
7.954 | 27.239 |
8.410 | 1.419 | 5.628 | 11.192 |
|
|
|
Overall | 12.064 | 2.308 |
7.539 | 16.588 |
6.670 | 0.761 | 5.179 |
8.161 |
2.805 | 0.094 |
| LGALS9 |
|
Low | 14.228 | 2.693 |
8.950 | 19.507 | 10.580 | 2.960 | 4.779 | 16.381 |
|
|
|
High | 10.808 | 2.001 |
6.887 | 14.729 |
6.340 | 1.336 | 3.722 |
8.958 |
|
|
|
Overall | 12.388 | 1.641 |
9.171 | 15.604 |
7.620 | 1.210 | 5.248 |
9.992 |
1.283 | 0.257 |
| NECTIN2 |
|
Low | 14.843 | 3.950 |
7.101 | 22.584 |
7.030 | 1.789 | 3.524 | 10.536 |
|
|
|
High |
7.493 | 1.462 |
4.627 | 10.359 |
4.860 | 0.698 | 3.491 |
6.229 |
|
|
|
Overall | 10.843 | 1.991 |
6.942 | 14.745 |
5.190 | 0.480 | 4.249 |
6.131 |
4.010 | 0.045 |
| CD48 |
|
Low | 13.008 | 2.508 |
8.092 | 17.924 |
8.510 | 3.055 | 2.522 | 14.498 |
|
|
|
High | 12.505 | 3.101 |
6.426 | 18.584 |
6.410 | 1.560 | 3.352 |
9.468 |
|
|
|
Overall | 13.026 | 2.283 |
8.552 | 17.501 |
7.360 | 1.459 | 4.501 | 10.219 |
0.392 | 0.531 |
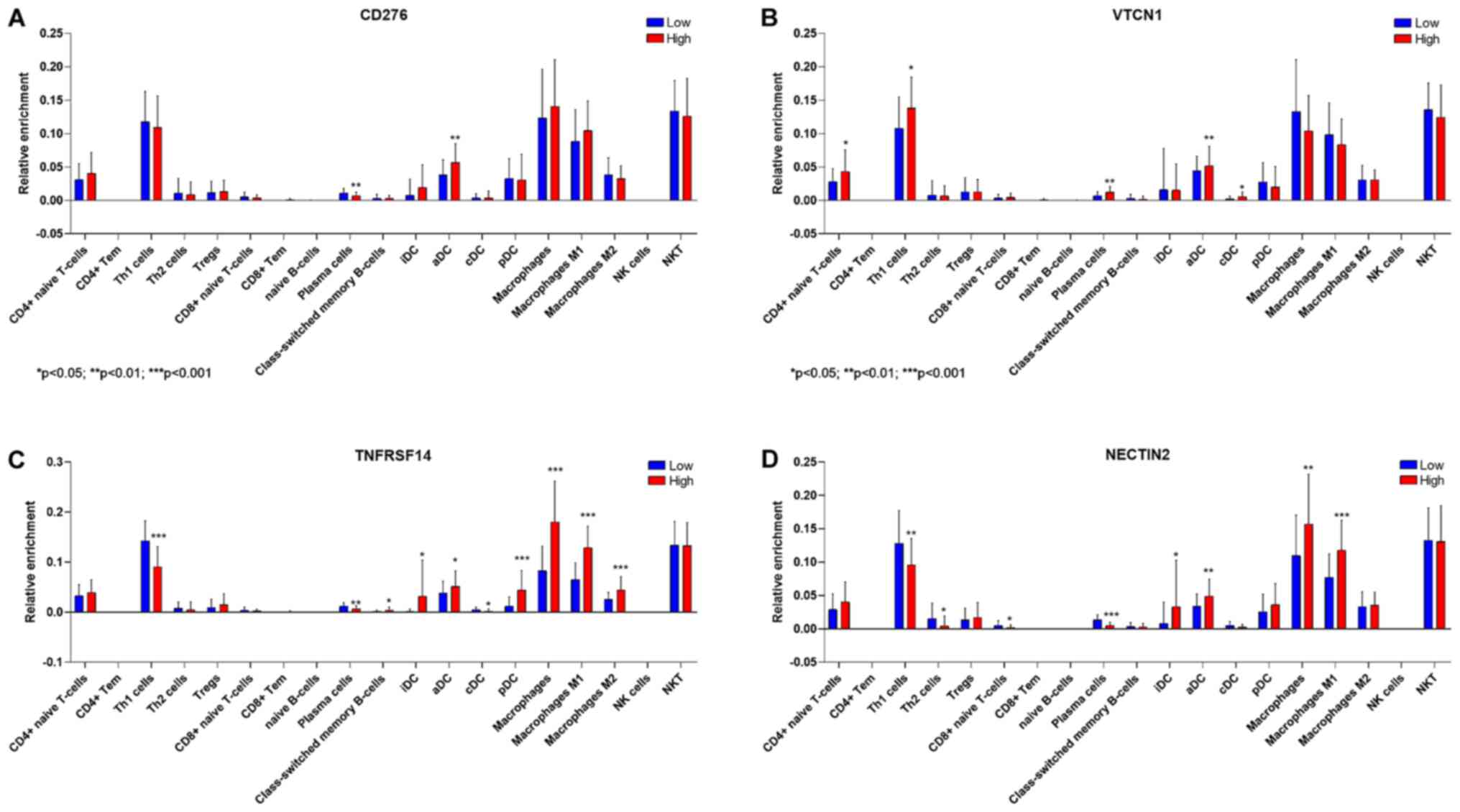
Deconvolution analysis
Deconvolution analysis of cell infiltration in GBM
was performed on samples dichotomized on the expression levels of
the immune checkpoints associated to a significant modulation of
survival, i.e., CD276, VTCN1, TNFRSF14 and NECTIN2. As shonw in
Fig. 4, higher levels of CD276,
TNFRSF14 and NECTIN2 were associated with a significant lower
proportion of infiltrating plasma cells. Higher VTCN1 levels were
associated to higher proportions of infiltrating plasma cells,
along with higher infiltration of Th1, aDCs and cDCs (Fig. 4B). Samples with high expression
levels of TNFRSF14 were characterized by a significant lower
infiltration of Th1 cells and cDC, and higher proportions of iDCs,
aDCs, pDCs and of macrophages (both M1 and M2) (Fig. 4C). A significantly higher
infiltration of iDCs, aDCs and M1 macrophages, along with reduced
proportions of Th1, Th2 and CD8 T cells, were observed in GBM
samples with high NECTIN2 expression levels (Fig. 4D).
Discussion
Conventional immune checkpoint inhibitors,
Nivolumab/ Pembrolizumab for PD-1/PDL1 blockade or Ipilimumab for
CTLA4, have proven beneficial effects on the clinical course of
different cancer types, including metastatic melanoma, non-small
cell lung cancer, renal cell carcinoma, and Hodgkin lymphoma
(9–11). However, these treatments have often
failed in gliomas (12–14). A possible explanation for this
outcome seems to be due to two main glioma features: the low tumor
mutational burden (TMB) and a highly immunosuppressive
microenvironment. Identifying genomic markers of response to immune
checkpoint may benefit cancer patients by providing predictive
biomarkers for patient stratification and identifying resistance
mechanisms for therapeutic targeting.
The present investigation evaluated the potential
role of a series of inhibitory immune checkpoints not previously
studied or only marginally characterized in GBM, i.e., CD276,
VTCN1, CD47, PVR, TNFRSF14, CD200, LGALS9, NECTIN2 and CD48. To
this aim, a computational analysis of RNA-seq data obtained from
the TCGA (The Cancer Genome Atlas) database was performed.
Whole-genome expression data was largely used (15) to identify pathogenic pathways and
therapeutic targets for several disorders, including autoimmune
diseases (16–23) and cancer (24–29).
We found that VTCN1 and CD200 are highly
over-expressed in GBM, anaplastic astrocytoma and astrocytoma grade
II compared to normal brain. Previously, Yao et al (30) showed that VTCN1 has a crucial role in
the creation and maintenance of the immunosuppressive
microenvironment in gliomas, correlating with prognosis and
malignant grades. Furthermore, lower levels of VTCN1 are associated
with a higher survival in a clinical trial of DC based vaccination
(31). This is in contrast with our
observations, which appears to show a protective role for VTCN1 in
GBM. The reasons for this counterintuitive data is currently object
of further exploration.
On the contrary, CD200 expression levels resulted in
significantly reduced astrocytomas in comparison to normal brain.
CD200 is a type I transmembrane glycoprotein that plays an
inhibitory role in the activation of microglia. For this reason,
many studies have shown that its expression is enhanced in brain
tumors (32), and especially in
higher grade tumors (33). However,
its role is still controversial, indeed in the same study Wang
et al (33) found that CD200
down-expression can lead to a particular microglia tumor
microenvironment that promotes tumor progression, in agreement with
our results. Recent studies in dogs also showed that targeting
CD200, enhanced the capacity of antigen-presenting cells to prime
T-cells to mediate an anti-glioma response (34).
PVR and CD47 were also found down-expressed in
astrocytomas when compared to normal brain, while higher levels of
expression were found for LGALS9, TNFRSF14, CD48, CD276 and
NECTIN2. PVR has been described as regulator of cell adhesion in a
rat model of GBM (35) and a recent
study in mice proved that the combination of anti-PD-1 and anti-PVR
leads to a better survival (36).
CD47 is a member of the immunoglobulin superfamily
that activates the signal regulatory protein-α (SIRP-α) expressed
on macrophages, preventing phagocytosis. In contrast with previous
studies (37,38), we found decreased levels in gliomas
compared to normal brain. We consider that this down-expression can
represent an attempt to maintain homeostasis. Recent studies have
associated CD47 with the tumor-associated macrophages (TAMs) in the
GBM microenvironment. Zhang et al (39) have also proven that anti-CD47
treatment leads to enhanced tumor cell phagocytosis by both M1 and
M2 macrophage subtypes with a higher phagocytosis rate by M1
macrophages. A combination of anti-CD47 treatment and temozolamide
has also been reported (40).
TNFRSF14 was found to be elevated in aggressive
gliomas and its expression seemed to be associated with
amplification of EGFR and loss of PTEN (41). TNFRSF14 plays an important role in
the recruitment and activation of immune system in the tumor
microenvironment. We showed that TNFRSF14 seems to have a
significant impact on both the overall survival and the
disease-free time. Interestingly, in metastatic melanoma, TNFRSF14
shows a similar behavior (42),
further reinforcing our observations and suggesting that similar
mechanisms can be shared also in glioma and that a combinatory
blocking strategy can improve patients outcome.
Finally, we performed a deconvolution analysis
showing that higher levels of CD276, TNFRSF14 and NECTIN2 are
associated with a significant lower proportion of infiltrating
plasma cells, while higher levels of VTCN1 were associated to
higher proportions of infiltrating plasma cells, Th1, aDCs and
cDCs. Higher levels of TNFRSF14 were associated with a major
infiltration of iDCs, aDCs, pDCs and macrophages, but lower levels
of Th1 cells and cDCs. Higher expression of NECTIN2, associated
with shorter survival, is associated with reduced proportions of
Th1, Th2 and CD8 T cells. Together these findings suggest that the
main immune cell types that help to reduce the tumor mass and
improve the survival are Th1 and cDCs, and that their expression is
strictly dependent on these immune checkpoints. In agreement with
our hypothesis, previous studies have shown that in gliomas, there
is a prevalent Th2 response and that switching from Th2 to Th1 can
help to block glioma growth (43).
Additionally, recent studies have proven that combinational therapy
that blocks more immune checkpoints is a possibility to create a
more vigorous Th1 antitumor response (44,45) and
its association with better outcome (46). Future preclinical and clinical
studies are necessary to ascertain whether, in addition to the
prognostic value we have highlighted, the dysregulated expression
of the inhibitory immune checkpoint presently studied may translate
into clinical applications, as novel immunotherapeutic approaches
for the treatment of gliomas and possibly other types of
cancers.
Collectively, in this study, we evaluated the
expression of several inhibitory immune checkpoints that can play a
role in glioma progression. Among the investigated immune
checkpoints, TNFRSF14 and NECTIN2 were identified as the most
promising targets in GBM. In particular, TNFRSF14 expression is
associated with worse overall survival and disease-free survival,
correlating with a lower Th1 response and suggesting that it could
become an interesting biomarker or therapeutic target.
Acknowledgements
Not applicable.
Funding
This study was supported by current research funds
2020 of IRCCS ‘Centro Neurolesi Bonino-Pulejo’, Messina, Italy.
Availability of data and materials
All the data in this study are available for
download from TCGA (The Cancer Genome Atlas) databank.
Authors' contributions
Conceptualization: FN and PF; data curation: SDL,
RB, KM and PF; formal analysis: MP and KM; funding acquisition: AB,
PB and FN; investigation: RC; project administration: PB;
supervision: FN; visualization: MSB; writing-original draft: SDL,
RC, MSB, MP and RB; writing-review and editing: AB, KM, PB, FN and
PF.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Louis DN, Ohgaki H, Wiestler OD, Cavenee
WK, Burger PC, Jouvet A, Scheithauer BW and Kleihues P: The 2007
WHO classification of tumours of the central nervous system. Acta
Neuropathol. 114:97–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bacher M, Schrader J, Thompson N, Kuschela
K, Gemsa D, Waeber G and Schlegel J: Up-regulation of macrophage
migration inhibitory factor gene and protein expression in glial
tumor cells during hypoxic and hypoglycemic stress indicates a
critical role for angiogenesis in glioblastoma multiforme. Am J
Pathol. 162:11–17. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fritz L, Dirven L, Reijneveld JC, Koekkoek
JA, Stiggelbout AM, Pasman HR and Taphoorn MJ: Advance care
planning in glioblastoma patients. Cancers (Basel). 8:1022016.
View Article : Google Scholar
|
|
4
|
Huang B, Zhang H, Gu L, Ye B, Jian Z,
Stary C and Xiong X: Advances in immunotherapy for glioblastoma
multiforme. J Immunol Res. 2017:35976132017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Reardon DA, Wen PY, Wucherpfennig KW and
Sampson JH: Immunomodulation for glioblastoma. Curr Opin Neurol.
30:361–369. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Srinivasan VM, Ferguson SD, Lee S,
Weathers S-P, Kerrigan BCP and Heimberger AB: Tumor vaccines for
malignant gliomas. Neurotherapeutics. 14:345–357. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sanders S and Debinski W: Challenges to
successful implementation of the immune checkpoint inhibitors for
treatment of glioblastoma. Int J Mol Sci. 21:212020. View Article : Google Scholar
|
|
8
|
Aran D, Hu Z and Butte AJ: xCell:
Digitally portraying the tissue cellular heterogeneity landscape.
Genome Biol. 18:2202017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Larkin J, Chiarion-Sileni V, González R,
Grob J, Cowey C and Lao C: Combined nivolumab and ipilimumab or
monotherapy in previously untreated melanoma. N Engl J Med.
373:23–34. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ritprajak P and Azuma M: Intrinsic and
extrinsic control of expression of the immunoregulatory molecule
PD-L1 in epithelial cells and squamous cell carcinoma. Oral Oncol.
51:221–228. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Massari F, Santoni M, Ciccarese C, Santini
D, Alfieri S, Martignoni G, Brunelli M, Piva F, Berardi R,
Montironi R, et al: PD-1 blockade therapy in renal cell carcinoma:
Current studies and future promises. Cancer Treat Rev. 41:114–121.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tan AC, Heimberger AB and Khasraw M:
Immune checkpoint inhibitors in gliomas. Curr Oncol Rep. 19:232017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Desai K, Hubben A and Ahluwalia M: The
role of checkpoint inhibitors in glioblastoma. Target Oncol.
14:375–394. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Caccese M, Indraccolo S, Zagonel V and
Lombardi G: PD-1/PD-L1 immune-checkpoint inhibitors in
glioblastoma: A concise review. Crit Rev Oncol Hematol.
135:128–134. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gustafsson M, Edström M, Gawel D, Nestor
CE, Wang H, Zhang H, Barrenäs F, Tojo J, Kockum I, Olsson T, et al:
Integrated genomic and prospective clinical studies show the
importance of modular pleiotropy for disease susceptibility,
diagnosis and treatment. Genome Med. 6:172014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fagone P, Mazzon E, Cavalli E, Bramanti A,
Petralia MC, Mangano K, Al-Abed Y, Bramati P and Nicoletti F:
Contribution of the macrophage migration inhibitory factor
superfamily of cytokines in the pathogenesis of preclinical and
human multiple sclerosis: In silico and in vivo evidences. J
Neuroimmunol. 322:46–56. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mangano K, Cavalli E, Mammana S, Basile
MS, Caltabiano R, Pesce A, Puleo S, Atanasov AG, Magro G, Nicoletti
F, et al: Involvement of the Nrf2/HO-1/CO axis and therapeutic
intervention with the CO-releasing molecule CORM-A1, in a murine
model of autoimmune hepatitis. J Cell Physiol. 233:4156–4165. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mammana S, Bramanti P, Mazzon E, Cavalli
E, Basile MS, Fagone P, Petralia MC, McCubrey JA, Nicoletti F and
Mangano K: Preclinical evaluation of the PI3K/Akt/mTOR pathway in
animal models of multiple sclerosis. Oncotarget. 9:8263–8277. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fagone P, Muthumani K, Mangano K, Magro G,
Meroni PL, Kim JJ, Sardesai NY, Weiner DB and Nicoletti F: VGX-1027
modulates genes involved in lipopolysaccharide-induced Toll-like
receptor 4 activation and in a murine model of systemic lupus
erythematosus. Immunology. 142:594–602. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nicoletti F, Mazzon E, Fagone P, Mangano
K, Mammana S, Cavalli E, Basile MS, Bramanti P, Scalabrino G, Lange
A, et al: Prevention of clinical and histological signs of
MOG-induced experimental allergic encephalomyelitis by prolonged
treatment with recombinant human EGF. J Neuroimmunol. 332:224–232.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fagone P, Mazzon E, Mammana S, Di Marco R,
Spinasanta F, Basile MS, Petralia MC, Bramanti P, Nicoletti F and
Mangano K: Identification of CD4+ T cell biomarkers for
predicting the response of patients with relapsing-remitting
multiple sclerosis to natalizumab treatment. Mol Med Rep.
20:678–684. 2019.PubMed/NCBI
|
|
22
|
Fagone P, Mangano K, Coco M, Perciavalle
V, Garotta G, Romao CC and Nicoletti F: Therapeutic potential of
carbon monoxide in multiple sclerosis. Clin Exp Immunol.
167:179–187. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Patti F, Cataldi ML, Nicoletti F, Reggio
E, Nicoletti A and Reggio A: Combination of cyclophosphamide and
interferon-β halts progression in patients with rapidly
transitional multiple sclerosis. J Neurol Neurosurg Psychiatry.
71:404–407. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Presti M, Mazzon E, Basile MS, Petralia
MC, Bramanti A, Colletti G, Bramanti P, Nicoletti F and Fagone P:
Overexpression of macrophage migration inhibitory factor and
functionally-related genes, D-DT, CD74, CD44, CXCR2 and CXCR4, in
glioblastoma. Oncol Lett. 16:2881–2886. 2018.PubMed/NCBI
|
|
25
|
Fagone P, Caltabiano R, Russo A, Lupo G,
Anfuso CD, Basile MS, Longo A, Nicoletti F, De Pasquale R, Libra M,
et al: Identification of novel chemotherapeutic strategies for
metastatic uveal melanoma. Sci Rep. 7:445642017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Basile MS, Mazzon E, Russo A, Mammana S,
Longo A, Bonfiglio V, Fallico M, Caltabiano R, Fagone P, Nicoletti
F, et al: Differential modulation and prognostic values of
immune-escape genes in uveal melanoma. PLoS One. 14:e02102762019.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mangano K, Mazzon E, Basile MS, Di Marco
R, Bramanti P, Mammana S, Petralia MC, Fagone P and Nicoletti F:
Pathogenic role for macrophage migration inhibitory factor in
glioblastoma and its targeting with specific inhibitors as novel
tailored therapeutic approach. Oncotarget. 9:17951–17970. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nicoletti F, Fagone P, Meroni P, McCubrey
J and Bendtzen K: mTOR as a multifunctional therapeutic target in
HIV infection. Drug Discov Today. 16:715–721. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rothweiler F, Michaelis M, Brauer P, Otte
J, Weber K, Fehse B, Doerr HW, Wiese M, Kreuter J, Al-Abed Y, et
al: Anticancer effects of the nitric oxide-modified saquinavir
derivative saquinavir-NO against multidrug-resistant cancer cells.
Neoplasia. 12:1023–1030. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yao Y, Ye H, Qi Z, Mo L, Yue Q, Baral A,
Hoon DSB, Vera JC, Heiss JD, Chen CC, et al: B7-H4(B7×)-mediated
cross-talk between glioma-initiating cells and macrophages via the
IL6/JAK/STAT3 pathway lead to poor prognosis in glioma patients.
Clin Cancer Res. 22:2778–2790. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yao Y, Luo F, Tang C, Chen D, Qin Z, Hua
W, Xu M, Zhong P, Yu S, Chen D, et al: Molecular subgroups and
B7-H4 expression levels predict responses to dendritic cell
vaccines in glioblastoma: An exploratory randomized phase II
clinical trial. Cancer Immunol Immunother. 67:1777–1788. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Moertel CL, Xia J, LaRue R, Waldron NN,
Andersen BM, Prins RM, Okada H, Donson AM, Foreman NK, Hunt MA, et
al: CD200 in CNS tumor-induced immunosuppression: The role for
CD200 pathway blockade in targeted immunotherapy. J Immunother
Cancer. 2:462014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang CY, Hsieh YT, Fang KM, Yang CS and
Tzeng SF: Reduction of CD200 expression in glioma cells enhances
microglia activation and tumor growth. J Neurosci Res.
94:1460–1471. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Olin MR, Ampudia-Mesias E, Pennell CA,
Sarver A, Chen CC, Moertel CL, Hunt MA and Pluhar GE: Treatment
combining CD200 immune checkpoint inhibitor and tumor-lysate
vaccination after surgery for pet dogs with high-grade glioma.
Cancers (Basel). 11:1372019. View Article : Google Scholar
|
|
35
|
Sloan KE, Stewart JK, Treloar AF, Matthews
RT and Jay DG: CD155/PVR enhances glioma cell dispersal by
regulating adhesion signaling and focal adhesion dynamics. Cancer
Res. 65:10930–10937. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hung AL, Maxwell R, Theodros D, Belcaid Z,
Mathios D, Luksik AS, Kim E, Wu A, Xia Y, Garzon-Muvdi T, et al:
TIGIT and PD-1 dual checkpoint blockade enhances antitumor immunity
and survival in GBM. OncoImmunology. 7:e14667692018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li F, Lv B, Liu Y, Hua T, Han J, Sun C, Xu
L, Zhang Z, Feng Z, Cai Y, et al: Blocking the CD47-SIRPα axis by
delivery of anti-CD47 antibody induces antitumor effects in glioma
and glioma stem cells. OncoImmunology. 7:e13919732018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu X, Wu X, Wang Y, Li Y, Chen X, Yang W
and Jiang L: CD47 promotes human glioblastoma invasion through
activation of the PI3K/Akt pathway. Oncol Res. 27:415–422. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang M, Hutter G, Kahn SA, Azad TD,
Gholamin S, Xu CY, Liu J, Achrol AS, Richard C, Sommerkamp P, et
al: Anti-CD47 treatment stimulates phagocytosis of glioblastoma by
M1 and M2 polarized macrophages and promotes M1 polarized
macrophages in vivo. PLoS One. 11:e01535502016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
von Roemeling CA, Wang Y, Qie Y, Yuan H,
Zhao H, Liu X, Yang Z, Yang M, Deng W, Bruno KA, et al: Therapeutic
modulation of phagocytosis in glioblastoma can activate both innate
and adaptive antitumour immunity. Nat Commun. 11:15082020.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Han MZ, Wang S, Zhao WB, Ni SL, Yang N,
Kong Y, Huang B, Chen AJ, Li XG, Wang J, et al: Immune checkpoint
molecule herpes virus entry mediator is overexpressed and
associated with poor prognosis in human glioblastoma. EBioMedicine.
43:159–170. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Malissen N, Macagno N, Granjeaud S,
Granier C, Moutardier V, Gaudy-Marqueste C, Habel N, Mandavit M,
Guillot B, Pasero C, et al: HVEM has a broader expression than
PD-L1 and constitutes a negative prognostic marker and potential
treatment target for melanoma. OncoImmunology. 8:e16659762019.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li G, Hu YS, Li XG, Zhang QL, Wang DH and
Gong SF: Expression and switching of TH1/TH2 type cytokines gene in
human gliomas. Chin Med Sci J. 20:268–272. 2005.PubMed/NCBI
|
|
44
|
Jahan N, Talat H, Alonso A, Saha D and
Curry WT: Triple combination immunotherapy with GVAX, anti-PD-1
monoclonal antibody, and agonist anti-OX40 monoclonal antibody is
highly effective against murine intracranial glioma.
OncoImmunology. 8:e15771082019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Jahan N, Talat H and Curry WT: Agonist
OX40 immunotherapy improves survival in glioma-bearing mice and is
complementary with vaccination with irradiated GM-CSF-expressing
tumor cells. Neuro-oncol. 20:44–54. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Taube JM, Galon J, Sholl LM, Rodig SJ,
Cottrell TR, Giraldo NA, Baras AS, Patel SS, Anders RA, Rimm DL, et
al: Implications of the tumor immune microenvironment for staging
and therapeutics. Mod Pathol. 31:214–234. 2018. View Article : Google Scholar : PubMed/NCBI
|