Introduction
Colorectal cancer (CRC) is the third most common
non-cutaneous carcinoma and the third leading cause of
cancer-associated mortality worldwide (1). The Tumor-Node-Metastasis (TNM) staging
system established by the American Joint Committee on Cancer is
widely used to predict prognosis and provide the most pertinent and
effective treatment strategies in patients with CRC (2). According to the TNM staging system, the
presence of positive lymph nodes (LN) determines the need for
adjuvant chemotherapy in patients with colon cancer (3) and is associated with increased use of
adjuvant radiation and chemotherapy in patients with rectal cancer
(4). Although the 5-year-survival
rate of patients with CRC and LN metastasis is only 69.2% (5), preoperative diagnosis of LN metastasis
absence makes endoscopic mucosal resection or minimum bowel
resection some possible options for patients with CRC. The
development of tools for the accurate preoperative detection of LN
metastasis might therefore serve an important role in optimizing
the management of patients with CRC, such as minimizing surgery and
evaluating the need for neoadjuvant chemotherapy and/or intensive
adjuvant chemotherapy.
Non-invasive imaging modalities, including computed
tomography (CT), magnetic resonance imaging (MRI), endorectal
ultrasonography (EUS) and positron emission tomography/CT (PET/CT),
are currently used for the preoperative diagnosis of LN metastasis
in patients with CRC. However, these imaging modalities are
unreliable, with relatively poor accuracy (22–73, 39–95, 62–83 and
63% for CT, MRI, EUS and PET/CT, respectively) (6,7).
The identification of predictive biomarkers of LN
metastasis in CRC has mainly focused on the determination of the
expression of several proteins by immunohistochemistry, of gene
expression level (Carcinoembryonic antigen-related cell adhesion
molecule 5 or Kallikrein-6 mRNAs and FER1 like member 4 non-coding
RNAs) or DNA methylation in colorectal tissues (8–11). A
previous study from our laboratory performed a comprehensive
analysis using a proteomics approach and selected candidate
proteins that might be used to identify LN metastasis in CRC. The
expression level of HSP47 in CRC and the number of HSP47-positive
spindle-shaped stroma cells in CRC stroma evaluated by
immunohistochemistry were significantly associated with LN
metastasis, and the number of HSP47-positive spindle-shaped stroma
cells was identified as a novel predictive biomarker for LN
metastasis and poor prognosis in patients with CRC (12). However, immunohistochemical analysis
depends on tissue processing and staining conditions, properties of
the immunohistochemical reagents, including antibodies, and
visualization system. In addition, it is difficult to evaluate
expression level objectively and/or quantitatively as required for
clinical biomarkers. Furthermore, immunohistochemical analysis
using formalin-fixed, paraffin-embedded samples from resected CRC
tissues cannot be used to identify LN metastasis status
preoperatively.
The present study aimed to evaluate whether HSP47
gene expression level could be used in CRC tissues to identify LN
metastasis status preoperatively. To do so, HSP47 gene expression
level was quantified by reverse transcription quantitative
(RT-q)PCR in frozen CRC and control samples from surgical specimens
and the association between HSP47 gene expression and
clinicopathological characteristics of patients with CRC was
analyzed.
Materials and methods
Patients and sample collection
Patients with primary CRC who underwent surgical
resection at Mie University Medical Hospital, Japan between January
2000 and January 2005 were enrolled in the present study. Mean age
of the patients was 69 years and mean tumor size was 40 mm.
Patients with preoperative treatment, incomplete clinical data,
inadequate follow-up or inadequate tissue samples were excluded.
Patients with inflammatory bowel disease, familial adenomatous
polyposis, hereditary non-polyposis colon cancer or other rare and
complex types of tumors were also excluded from the present study.
All tissue samples were collected from surgically resected
specimens and were immediately frozen in liquid nitrogen and kept
at −80°C until RNA extraction. A total of 175 surgical samples,
comprising 36 normal mucosa samples from patients with benign
colonic disease and 139 cancer tissue samples from patients with
CRC were analyzed. The clinicopathological characteristics of
patients with CRC were based on TNM classification using the
American Joint Committee on Cancer (2). This cohort comprised 79 men and 60
women (average age, 67.7 years), of whom 33, 44, 33 and 29 patients
had stages I, II, III and IV CRC, respectively. The median
follow-up time was 32.9 months (range, 0.4–115 months). The 5-year
survival rate was 80.1%. No patient received chemotherapy or
radiotherapy before surgery and no perioperative mortality was
observed. The research protocol was approved by Mie University
Hospital Ethical Review Committee. All participants provided
written informed consent and agreed to donate their clinical
specimens for research purposes.
RNA extraction and RT-qPCR
Surgical specimens (200 µg) were homogenized using a
Mixer Mill MM 300 homogenizer (Qiagen Sciences, Inc.) and total RNA
was isolated using the RNeasy Mini Kit (Qiagen, Inc.) according to
the manufacturers' instructions. cDNA was synthesized using random
hexamers and Superscript III reverse transcriptase (Invitrogen,
Carlsbad, CA, USA) according to the manufacturers' protocol.
HSP47 expression level was determined by RT-qPCR
using Power SYBR Green PCR Master Mix (Applied Biosystems; Thermo
Fisher Scientific, Inc.) and a Step One Plus Real-Time PCR system
(Applied Biosystems; Thermo Fisher Scientific, Inc.). The sequences
of the primers were as follows: HSP47, forward
5′-ATGCAGAAGAAGGCTGTTGC-3′, reverse 5′-GGCCTTGTTCTTGTCAATGG-3′; and
β-actin, forward 5′-ACAGAGCCTCGCCTTTGC-3′ and reverse
5′-GCGGCGATATCATCATCC-3′. The amplification conditions were as
follows: 95°C for 10 min, 40 cycles of 95°C for 15 sec, and 60°C
for 1 min. After amplification, the products were subjected to an
increasing temperature gradient from 60°C to 95°C at a rate of
0.3°C/sec with continuous fluorescence monitoring, to produce a
melting curve. The HSP47 expression level in each sample was
normalized to β-actin expression level using the 2−ΔΔCt
method (13).
Statistical analysis
All statistical analyses were performed using JMP
version 10 (SAS Institute, Inc.). The results were expressed as the
median values or as the mean ± standard deviation. Comparisons were
made using non-parametric Mann-Whitney U-tests for continuous
variables. Differences between groups were estimated using
Pearson's χ2 or Kruskal-Wallis tests. Dunn's tests were
used as a method for multiple comparisons following Kruskal-Wallis
test. Receiver operating characteristic (ROC) curves were
established to determine cut-off expression values for predicting
LN metastasis and prognosis using Youden's index. The Kaplan-Meier
method was used to determine the cumulative probability of overall
survival (OS) with Stage I–IV and disease-free survival (DFS) with
Stage I–III patients with CRC, and differences were evaluated using
log-rank tests. Prognostic factors were examined by univariate and
multivariate analyses (Cox proportional hazards regression model).
Logistic regression analysis was used to evaluate the factors
influencing LN metastasis. P<0.05 was considered to indicate a
statistically significant difference.
Results
Differential gene expression patterns
of HSP47 in normal colonic mucosa and CRC tissues
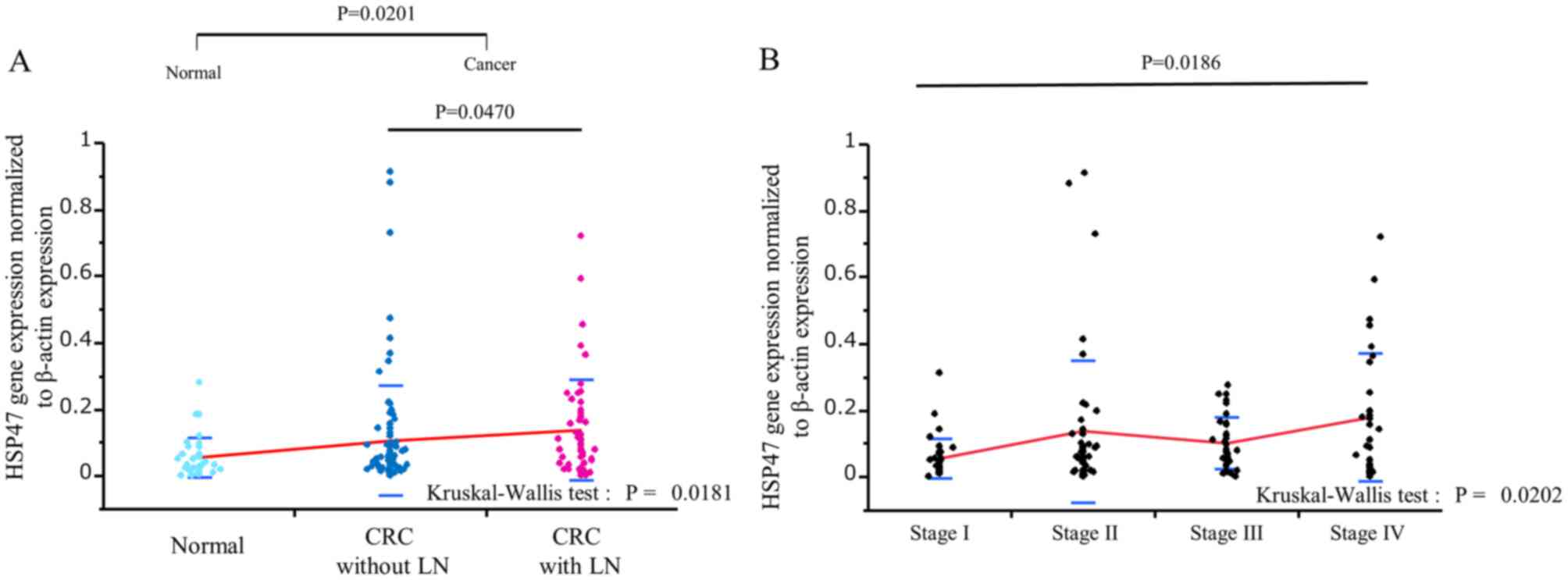
HSP47 expression level was significantly higher in
CRC tissues (n=139) compared with normal colonic mucosa (n=36;
P=0.0201; Fig. 1A), and
significantly higher in CRC with LN metastasis compared with CRC
without LN metastasis (P=0.0470; Fig.
1A). In addition, HSP47 expression level was significantly
higher in stage IV CRC tissues compared with stage I CRC tissues
(P=0.0186; Fig. 1B).
Association between HSP47 expression
level and clinicopathological characteristics in CRC
The associations between HSP47 expression level and
various clinicopathological characteristics in patients with CRC
were evaluated after dividing the 139 CRC cases into two groups,
based on HSP47 expression level (high vs. low; Table I). The high/low cut-off values were
determined by ROC analysis for LN metastasis (cut-off 0.0969762).
Of the 139 CRC samples, 49.6% (69/139) cases had high HSP47
expression (>0.0969762) and 50.4% (70/139) had low expression.
Furthermore, high HSP47 expression level was significantly
associated with advanced T stage (P=0.0163), LN metastasis
(P=0.0186), venous invasion (P=0.0328), and advanced TNM stage
(P=0.0115; Table I).
 | Table I.Association between HSP47 expression
level and the clinicopathological characteristics in patients with
colorectal cancer. |
Table I.
Association between HSP47 expression
level and the clinicopathological characteristics in patients with
colorectal cancer.
|
|
| HSP47 expression |
|
|---|
|
|
|
|
|
|---|
| Variable | n | High (n=69) | Low (n=70) | P-value |
|---|
| Age, years |
|
|
| 0.1072 |
|
<69 | 70 | 30 | 40 |
|
|
≥69 | 69 | 39 | 30 |
|
| Sex |
|
|
| 0.4479 |
|
Male | 79 | 37 | 42 |
|
|
Female | 60 | 32 | 28 |
|
| Histology |
|
|
| 0.2908 |
|
Undifferentiated | 9 | 6 | 3 |
|
|
Differentiated | 130 | 63 | 67 |
|
| Tumor size, mm |
|
|
| 0.1247 |
|
<40 | 49 | 20 | 29 |
|
|
≥40 | 90 | 49 | 41 |
|
| T
classification |
|
|
| 0.0163a |
|
T1-T2 | 39 | 13 | 26 |
|
|
T3-T4 | 100 | 56 | 44 |
|
| Lymph node
metastasis |
|
|
| 0.0186a |
|
Present | 88 | 37 | 51 |
|
|
Absent | 51 | 32 | 19 |
|
| Lymphatic
invasion |
|
|
| 0.1811 |
|
Present | 124 | 64 | 60 |
|
|
Absent | 15 | 5 | 10 |
|
| Venous
invasion |
|
|
| 0.0328a |
|
Present | 113 | 61 | 52 |
|
|
Absent | 26 | 8 | 18 |
|
| Hepatic
metastasis |
|
|
| 0.1376 |
|
Present | 20 | 13 | 7 |
|
|
Absent | 119 | 56 | 63 |
|
| Peritoneal
metastasis |
|
|
| 0.9918 |
|
Present | 2 | 1 | 1 |
|
|
Absent | 137 | 68 | 69 |
|
| Distant
metastasis |
|
|
| 0.1041 |
|
Present | 16 | 11 | 5 |
|
|
Absent | 123 | 58 | 65 |
|
| Recurrence |
|
|
| 0.1041 |
|
Present | 24 | 15 | 9 |
|
|
Absent | 84 | 38 | 46 |
|
| Stage |
|
|
|
|
| I | 33 | 9 | 24 | 0.0115a |
| II | 44 | 21 | 23 |
|
|
III | 33 | 21 | 12 |
|
| IV | 29 | 18 | 11 |
|
Differential gene expression patterns
of HSP47 in normal colonic mucosa and CRC tissues
A comparison of HSP47 expression between colon
cancer and rectum cancer samples revealed that there was no
significant difference in expression between the two cancer types
(Fig. S1).
High HSP47 expression level predicts
LN metastasis in patients with CRC
The present study investigated various factors
associated with LN involvement by univariate and multivariate
logistic analyses (Table II).
Advanced T stage (P=0.0007), lymphatic invasion (P=0.0043), venous
invasion (P=0.0002) and high HSP47 expression (P=0.0042) in CRC
were significantly associated with LN metastasis following
univariate analysis. Furthermore, multivariate analysis identified
high HSP47 expression [odds ratio (OR): 2.3946, 95% confidence
interval (CI): 1.116397–5.209946; P=0.0249] as the only independent
predictor of LN metastasis in patients with CRC (Table II).
 | Table II.Univariate and multivariate analyses
of associations with lymph node metastasis in patients with
colorectal cancer. |
Table II.
Univariate and multivariate analyses
of associations with lymph node metastasis in patients with
colorectal cancer.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variable | OR | 95% CI | P-value | OR | 95% CI | P-value |
|---|
| Age (<69 vs. ≥69
years) | 1.2321 |
0.617647–2.4697243 | 0.5534 | – | – | – |
| Sex (female vs.
male) | 1.2840 |
0.6395638–2.5783842 | 0.4810 | – | – | – |
| Tumor size (≥40 vs.
<40 mm) | 2.0237 |
0.9624956–4.4365109 | 0.0632 | – | – | – |
| T classification
(T3-4 vs. T1-2) | 5.0000 |
1.8374783–12.775448 | 0.0007a | 2.1400 |
0.7456599–6.9057309 | 0.1606 |
| Pathology (poor or
mucinous vs. | 1.4128 |
0.335628–5.5889128 | 0.6218 | – | – | – |
| moderate/well
differentiated) |
|
|
|
|
|
|
| Lymphatic
invasion | 9.4595 |
1.8123151–174.04766 | 0.0043a | 1.0124 |
0.0343703–29.441451 | 0.9935 |
| (present vs.
absent) |
|
|
|
|
|
|
| Venous
invasion | 9.1875 |
2.5583968–58.877309 | 0.0002a | 5.2985 |
0.8617945–102.43786 | 0.0752 |
| (present vs.
absent) |
|
|
|
|
|
|
| HSP47 gene
expression | 2.8846 |
1.3963896–6.0536216 | 0.0042a | 2.3946 |
1.116397–5.209946 | 0.0249a |
| (≥0.0969762 vs.
<0.0969762) |
|
|
|
|
|
|
High HSP47 gene expression affects
prognosis in patients with CRC
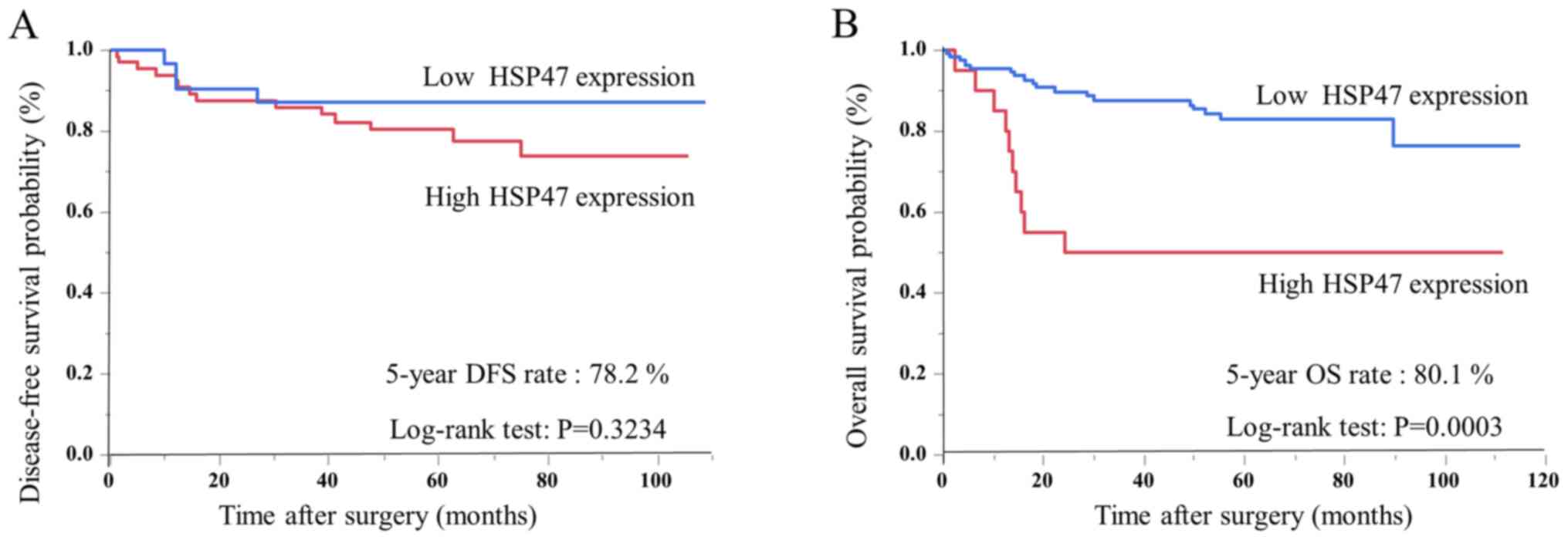
Patients with CRC were divided into two groups
according to HSP47 expression level determined by ROC analysis for
prognosis (cut-off 0.0924641). Kaplan-Meier analysis indicated that
disease-free survival tended to be poorer in the high-expression
group, although the difference was not significant (Fig. 2A); however, OS was also significantly
poorer in the high-expression group compared with the
low-expression group (P=0.0003, log-rank test; Fig. 2B). Cox's univariate and multivariate
analyses of clinicopathological factors related to OS in patients
with CRC are presented in Table
III. The results from univariate analysis indicated that poor
OS was significantly associated with advanced T stage
(P<0.0001), LN metastasis (P=0.0040), hepatic metastasis
(P<0.0001), distant metastasis (P<0.0001), lymphatic invasion
(P=0.0056), venous invasion (P=0.0051) and high HSP47 expression in
CRC (P=0.0021). In addition, multivariate analysis identified
hepatic metastasis [hazard ratio (HR): 9.1250, 95% CI:
3.7270458–22.288004; P<0.0001] and high HSP47 expression (HR:
2.7407, 95% CI: 1.1623509–6.1627421; P=0.0224) as independent
prognostic factors in patients with CRC (Table III).
 | Table III.Univariate and multivariate analyses
of associations with overall survival in patients with colorectal
cancer. |
Table III.
Univariate and multivariate analyses
of associations with overall survival in patients with colorectal
cancer.
| (Cohort 1: Protein
analysis) | Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|---|
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age (<69 vs. ≥69
years) | 1.7043 |
0.8012998–3.8397503 | 0.1682 | – | – | – |
| Sex (male vs.
female) | 1.3229 |
0.6220246–2.9802979 | 0.4731 | – | – | – |
| Tumor size (≥40 vs.
<40 mm) | 1.8287 |
0.8144726–4.6473787 | 0.1486 | – | – | – |
| T classification
(T3-4 vs. T1-2) | 13.3454 |
2.8403789–238.09155 | <0.0001 | 3.8129 |
0.733633–70.061812 | 0.1260 |
| Lymph node
metastasis | 3.0116 |
1.4279703–6.5249787 | 0.0040a | 1.3758 |
0.5677664–3.3313589 | 0.4761 |
| Peritoneal
metastasis | 6.5208 |
0.3625655–31.578247 | 0.1555 | – | – | – |
| Hepatic
metastasis | 16.5158 |
7.466573–36.758521 | <0.0001 | 9.1250 |
3.7270458–22.288004 | <0.0001 |
| Distant
metastasis | 7.2862 |
3.2929176–15.473565 | <0.0001 | 2.0538 |
0.7842089–5.1884429 | 0.1403 |
| Pathology (poor or
mucinous vs. | 1.6081 |
0.3825837–4.5888813 | 0.4652 | – | – | – |
| moderate/well
differentiated) |
|
|
|
|
|
|
| Lymphatic
invasion) | 1.6488 |
0.4826371–2.07195 | 0.0056a | 2.1415 |
0.0606119–16.498407 | 0.4061 |
| (present vs.
absent |
|
|
|
|
|
|
| Venous
invasion | 7.6486 |
1.628946–136.4308 | 0.0051a | 1.3460 |
0.2462182–25.080995 | 0.7719 |
| (present vs.
absent) |
|
|
|
|
|
|
| HSP47 gene
expression | 3.7958 |
1.6753552–8.1191931 | 0.0021a | 2.7407 |
1.1623509–6.1627421 | 0.0224a |
| (≥0.0924641 vs.
<0.0924641) |
|
|
|
|
|
|
Association between HSP47 expression
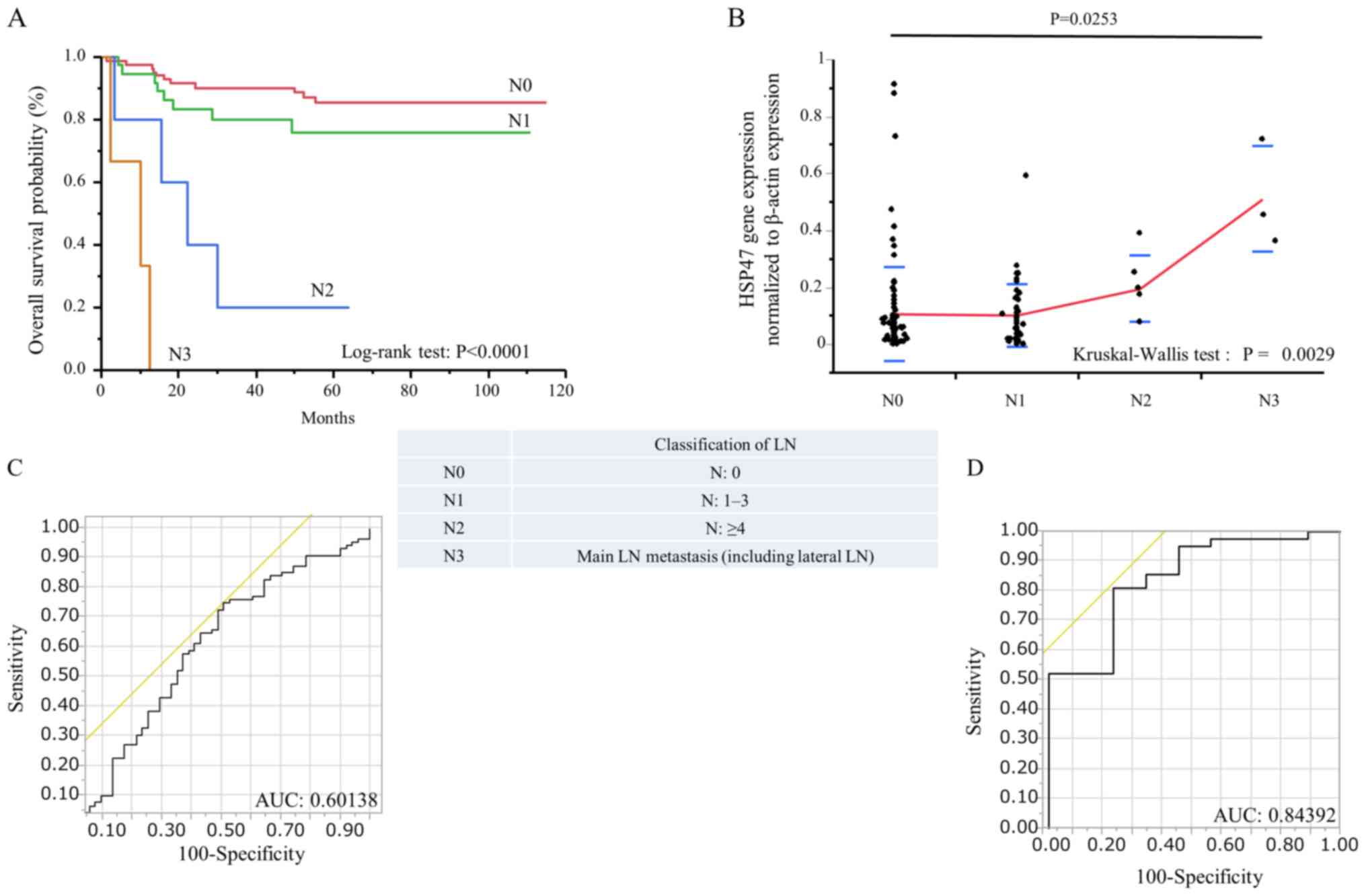
and LN counts in patients with CRC
The association between LN metastasis status and
HSP47 expression level in patients with CRC was evaluated. Patients
were classified according to the stage of LN metastasis based on
the Japanese Society for Cancer of the Colon and Rectum 7th edition
(N0, no metastasis; N1, 1–3 pericolic/perirectal; intermediate LN
metastases; N2, ≥4 pericolic/perirectal, intermediate LN
metastases; N3, main LN metastasis including lateral LN metastasis)
(14). Kaplan-Meier analysis
demonstrated that patients with N3 had significantly poorer OS than
the other groups (P<0.0001; Fig.
3A). HSP47 expression level in N3 patients was significantly
higher compared with N0 patients (P=0.0253; Fig. 3B). ROC curves were also generated to
assess the potential significance of HSP47 expression level for the
detection of LN metastasis status in patients with CRC. The results
demonstrated that HSP47 expression level could discriminate between
N1-3 and N0 CRC patients, with an AUC value of 0.60138 (Fig. 3C). In addition, HSP47 level could
also discriminate between N2-3 and N0-1 CRC patients, with an AUC
of 0.84392 (Fig. 3D).
Discussion
The present study investigated the gene expression
pattern of HSP47 in CRC tissues by RT-qPCR. HSP47 expression level
was significantly higher in CRC tissues compared with normal tissue
from patients with benign colonic disease and was significantly
associated with tumor progression indicated by high T stage, LN
metastasis and venous invasion and high TNM stage. High HSP47
expression level also served as a novel predictive biomarker for
patients with CRC and LN metastasis, and as an independent
prognostic factor in patients with CRC.
HSP47 is a 47-kDa member of a group of heat shock
proteins with unique collagen-specific binding characteristics
(15), which was first characterized
by Kurkinen et al (16) in
murine parietal endoderm cells. Heat shock proteins play an
important role as molecular chaperones in protein folding, by
assembling newly synthesized proteins or reassembling misfolded
proteins (17). HSP47 binds to
nascent single polypeptide chains of procollagen immediately after
entry into the endoplasmic reticulum and dissociates from
procollagen chains in the Golgi apparatus (18). When aberrant procollagen accumulates
in the endoplasmic reticulum due to heat shock, depletion of
ascorbic acid or after treatment with α, α′-dipyridyl, which
inhibits procollagen secretion and causes accumulation of
procollagen in the endoplasmic reticulum (19). Some studies have suggested that
procollagen gene expression level and rates of procollagen
synthesis were regulated with HSP47 (20), and that HSP47 expression was
increased in fibrotic human lung, kidney and skin (21–24).
HSP47 has been mapped to human chromosome 11q13.5,
which is a known ‘hot spot’ in a number of human cancers (25). Interestingly, altered HSP47
expression has been identified in several cancers, including
sarcoma, esophageal squamous cell carcinoma and ulcerative
colitis-associated carcinoma (26–28).
Maitra et al (29) reported
HSP47 immunolabeling in the tumor-associated stroma and suggested
that it may act as a novel diagnostic and therapeutic marker in
pancreatic carcinoma. Furthermore, we previously reported that the
number of HSP47-positive spindle-shaped stroma cells in the cancer
stroma of patients with CRC could serve as a novel predictive
biomarker of LN metastasis, early recurrence and poor prognosis
(8). In addition, HSP47 silencing
has been shown to inhibit cell proliferation, migration and
invasion in cervical squamous cell carcinoma (30).
Regarding HSP47 gene expression, Morino et al
(31) demonstrated that tumor cell
lines derived from metastatic carcinomas that remained metastatic
in animals can synthesize higher levels of HSP47. Naitoh et
al (24) also reported that
HSP47 expression level was upregulated by 8-fold in keloid lesions.
However, the association between HSP47 expression level and tumor
progression, prognosis and metastasis in cancer patients remains
unclear. To the best of our knowledge, the present study was the
first to suggest that high HSP47 expression level in CRC primary
tumors may be considered as an independent marker for the
prediction of LN metastasis and poor prognosis in patients with
CRC.
Neoadjuvant chemotherapy or chemoradiotherapy are
currently used as treatments for patients with advanced rectal
cancer and have been shown to decrease local recurrence and
increase survival (32). Correct
preoperative staging plays therefore a critical role in determining
whether patients with rectal cancer should undergo neoadjuvant
therapy. Similarly, preoperative neoadjuvant treatments and
decisions regarding the range of LN dissection depend on accurate
LN staging in patients with colon cancer (33). In the present study, HSP47 expression
value in the group with LN was significantly higher than in the
group without LN (0.137848±0.149551 vs. 0.106957±0.166488). In
addition, patients with N2 or N3 tumors had significantly poorer OS
than other patients, and HSP47 gene expression level was also
significantly higher in N2 and N3 patients compared with N0 and N1
patients. This was supported by markedly higher AUC values for
comparisons between N2-3 and N0-1 CRC patients (AUC=0.84392).
Furthermore, HSP47 expression was identified as the only
independent predictive factor for LN metastasis in patients with
CRC. Evaluation of HSP47 expression level may therefore help
deciding the appropriate neoadjuvant chemotherapy to be used, and
determining the extent of LN dissection (local or wide excision).
HSP47 expression level may therefore help decreasing the size of
surgery and evaluate the requirement of neoadjuvant chemotherapy in
patients with CRC before surgery.
Previously, we demonstrated that the number of
HSP47-positive spindle cells in the stroma of CRC may serve as a
novel predictive biomarker of LN metastasis, early recurrence and
poor prognosis via immunostaining (12). In the present study, we used mRNA to
examine LN metastasis and prognostic factors of CRC patients.
Although it is easy to evaluate the prognostic factor of CRC using
protein staining, the objectivity can be poor and the evaluation
can be dependent of the observer. Evaluating mRNA expression may be
a more useful method because it allows quantification, reducing
human bias and leading therefore to a more accurate evaluation of
LN metastasis and prognosis in patients with CRC. Although HSP47
expression level was quantified in frozen surgical specimens rather
than in preoperative biopsy samples, the results from the present
study suggested that evaluation of HSP47 expression level in biopsy
samples may represent a promising clinical tool for the
preoperative prediction of LN metastasis, allowing therefore the
determination of appropriate treatment strategies in patients with
CRC. It may be worth mentioning that the current study did not
actually use preoperative biopsies, although it aimed to determine
if it could be possible to determine the LN metastasis in patients
with CRC.
In summary, the results from the present study
indicated that HSP47 expression level in CRC tissues may be
considered as a potential biomarker for predicting LN metastasis
status and prognosis in patients with CRC. These results suggested
that assessing LN metastasis may lead to a reduction in surgery in
CRC and may help deciding about preoperative chemotherapy in rectal
cancer. By examining the OS via evaluation of HSP47 expression, it
may be possible to predict before surgery that postoperative
chemotherapy may be required for patients with both colon and
rectal cancers.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported in part by a Grant in Aid
for Scientific Research (grant no. 21791280) from the Ministry of
Education, Culture, Sports, Science and Technology, Japan.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
KM, YT, MaK and YO designed the study. TI, YN, SO,
TS, HF, JH, MiK, TA, YI and YM performed the experiments. KM, YT
and YO acquired, analyzed and interpreted data. KM and YT drafted
the manuscript. MaK supervised and coordinated the study. All the
authors were involved in writing the manuscript. All authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
The research protocol was approved by Mie University
Hospital (Mie, Japan) Ethical Review Committee (approval no. 2632).
All participants provided written informed consent and agreed to
donate their clinical specimens for research purposes.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CRC
|
colorectal cancer
|
|
CT
|
computed tomography
|
|
EUS
|
endorectal ultrasonography
|
|
HSP47
|
heat shock protein 47
|
|
LN
|
lymph nodes
|
|
MRI
|
magnetic resonance imaging
|
|
OS
|
overall survival
|
|
PET/CT
|
positron emission tomography/CT
|
|
ROC
|
Receiver operating characteristic
|
|
TNM
|
tumor node metastasis
|
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Edge S, Compton C, Fritz A, Greene F and
Trotti A: AJCC Cancer Staging Manual. 7th. Springer; New York, NY:
pp. 237–46. 2010
|
|
3
|
Chau I and Cunningham D: Adjuvant therapy
in colon cancer: Current status and future directions. Cancer Treat
Rev. 28:223–236. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schrag D, Gelfand SE, Bach PB, Guillem J,
Minsky BD and Begg CB: Who gets adjuvant treatment for stage II and
III rectal cancer? Insight from surveillance, epidemiology, and end
results-Medicare. J Clin Oncol. 19:3712–3718. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kwak JY, Kim JS, Kim HJ, Ha HK, Yu CS and
Kim JC: Diagnostic value of FDG-PET/CT for lymph node metastasis of
colorectal cancer. World J Surg. 36:1898–1905. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Low G, Tho LM, Leen E, Wiebe E, Kakumanu
S, McDonald AC and Poon FW: The role of imaging in the
pre-operative staging and post-operative follow-up of rectal
cancer. Surgeon. 6:222–231. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Imaoka H, Toiyama Y, Saigusa S, Kawamura
M, Kawamoto A, Okugawa Y, Hiro J, Tanaka K, Inoue Y, Mohri Y and
Kusunoki M: RacGAP1 expression, increasing tumor malignant
potential, as a predictive biomarker for lymph node metastasis and
poor prognosis in colorectal cancer. Carcinogenesis. 36:346–354.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Imaoka H, Toiyama Y, Fujikawa H, Hiro J,
Saigusa S, Tanaka K, Inoue Y, Mohri Y, Mori T, Kato T, et al:
Circulating microRNA-1290 as a novel diagnostic and prognostic
biomarker in human colorectal cancer. Ann Oncol. 27:1879–1886.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lina O, Marie-Louise H, Anne I, Gudrun L
and Sten H: Allocating colorectal cancer patients to different risk
categories by using a five-biomarker mRNA combination in lymph node
analysis. PLoS One. 15:e02290072020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li H, Ma SQ, Huang J, Chen XP and Zhou HH:
Roles of long noncoding RNAs in colorectal cancer metastasis.
Oncotarget. 8:39859–39876. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mori K, Toiyama Y, Otake K, Fujikawa H,
Saigusa S, Hiro J, Kobayashi M, Ohi M, Tanaka K, Inoue Y, et al:
Proteomics analysis of differential protein expression identifies
heat shock protein 47 as a predictive marker for lymph node
metastasis in patients with colorectal cancer. Int J Cancer.
140:1425–1435. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Japanese Society for Cancer of the Colon
and Rectum General Rules for Clinical and Pathological Studies on
Cancer of the Colon RaARV. (7th). (in Japanese). (Tokyo). 36–37.
2009.
|
|
15
|
Nagata K, Saga S and Yamada KM: A major
collagen-binding protein of chick embryo fibroblasts is a novel
heat shock protein. J Cell Biol. 103:223–229. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kurkinen M, Taylor A, Garrels JI and Hogan
BL: Cell surface-associated proteins which bind native type IV
collagen or gelatin. J Biol Chem. 259:5915–5922. 1984.PubMed/NCBI
|
|
17
|
Schlesinger MJ: Heat shock proteins. J
Biol Chem. 265:12111–12114. 1990.PubMed/NCBI
|
|
18
|
Satoh M, Hirayoshi K, Yokota S, Hosokawa N
and Nagata K: Intracellular interaction of collagen-specific stress
protein HSP47 with newly synthesized procollagen. J Cell Biol.
133:469–483. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nakai A, Satoh M, Hirayoshi K and Nagata
K: Involvement of the stress protein HSP47 in procollagen
processing in the endoplasmic reticulum. J Cell Biol. 117:903–914.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kawada N, Kuroki T, Kobayashi K, Inoue M,
Nakatani K, Kaneda K and Nagata K: Expression of heat-shock protein
47 in mouse liver. Cell Tissue Res. 284:341–346. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Razzaque MS, Nazneen A and Taguchi T:
Immunolocalization of collagen and collagen-binding heat shock
protein 47 in fibrotic lung diseases. Mod Pathol. 11:1183–1188.
1998.PubMed/NCBI
|
|
22
|
Razzaque MS, Kumatori A, Harada T and
Taguchi T: Coexpression of collagens and collagen-binding heat
shock protein 47 in human diabetic nephropathy and IgA nephropathy.
Nephron. 80:434–443. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kuroda K, Tsukifuji R and Shinkai H:
Increased expression of heat-shock protein 47 is associated with
overproduction of type I procollagen in systemic sclerosis skin
fibroblasts. J Invest Dermatol. 111:1023–1028. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Naitoh M, Hosokawa N, Kubota H, Tanaka T,
Shirane H, Sawada M, Nishimura Y and Nagata K: Upregulation of
HSP47 and collagen type III in the dermal fibrotic disease, keloid.
Biochem Biophys Res Commun. 280:1316–1322. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sauk JJ, Nikitakis N and Siavash H: Hsp47
a novel collagen binding serpin chaperone, autoantigen and
therapeutic target. Front Biosci. 10:107–118. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Morino M, Tsuzuki T, Iijima H, Shirakami
T, Kiyosuke YI, Ishikawa Y, Yoshimura M and Yoshikumi C: Marked
induction of HSP47, a collagen-binding stress protein, during solid
tumor formation of ascitic Sarcoma 180 in vivo. In Vivo. 9:503–508.
1995.PubMed/NCBI
|
|
27
|
Lee HW, Kwon J, Kang MC, Noh MK, Koh JS,
Kim JH and Park JH: Overexpression of HSP47 in esophageal squamous
cell carcinoma: Clinical implications and functional analysis. Dis
Esophagus. 29:848–855. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Araki K, Mikami T, Yoshida T, Kikuchi M,
Sato Y, Oh-ishi M, Kodera Y, Maeda T and Okayasu I: High expression
of HSP47 in ulcerative colitis-associated carcinomas: Proteomic
approach. Br J Cancer. 101:492–497. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Maitra A, Iacobuzio-Donahue C, Rahman A,
Sohn TA, Argani P, Meyer R, Yeo CJ, Cameron JL, Goggins M, Kern SE,
et al: Immunohistochemical validation of a novel epithelial and a
novel stromal marker of pancreatic ductal adenocarcinoma identified
by global expression microarrays: Sea urchin fascin homolog and
heat shock protein 47. Am J Clin Pathol. 118:52–59. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yamamoto N, Kinoshita T, Nohata N, Yoshino
H, Itesako T, Fujimura L, Mitsuhashi A, Usui H, Enokida H, Nakagawa
M, et al: Tumor-suppressive microRNA-29a inhibits cancer cell
migration and invasion via targeting HSP47 in cervical squamous
cell carcinoma. Int J Oncol. 43:1855–1863. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Morino M, Tsuzuki T, Ishikawa Y, Shirakami
T, Yoshimura M, Kiyosuke Y, Matsunaga K, Yoshikumi C and Saijo N:
Specific expression of HSP47 in human tumor cell lines in vitro. In
Vivo. 11:17–21. 1997.PubMed/NCBI
|
|
32
|
Krishnamurthi SS, Seo Y and Kinsella TJ:
Adjuvant therapy for rectal cancer. Clin Colon Rectal Surg.
20:167–181. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Greene FL PD, Fleming I and Fritz A: AJCC
Cancer Staging Manual. 6th. Springer-Verlag; Berlin: 2002,
View Article : Google Scholar
|