Introduction
During inflammatory conditions, nitric oxide
synthases (NOSs), which include endothelial, neuronal and the
inducible isoform of NOS, use oxygen and nitrogen to catalyze the
production of nitric oxide (NO) (1).
NO reacts with another free radical, superoxide (•OO-) to form
peroxynitrite (ONOO-) (2) and ONOO-
can nitrate tyrosine or protein tyrosine residues to form
nitrotyrosine (3). Nitrotyrosine is
similar to tyrosine in structure and is incorporated into tubulin
by the enzyme tubulin-tyrosine ligase to produce the
nitrotyrosination of tubulin, a process which is irreversible
(4). Nitrotyrosination of tubulin
can induce cell death, which is a way for the human body to remove
apoptotic and abnormal cells (5).
Long-term chronic inflammation, such as ulcers, gingivitis and
periodontitis, may be associated with the malignant transformation
of cells, resulting in the occurrence of oral squamous cell
carcinoma (OSCC) as the malignant transformation of cells is not
effectively eliminated (6,7). Thus, nitrotyrosine is considered a
biochemical marker for oral inflammation (8) and oral cancer prognosis (9).
OSCC is one of the most common malignant tumors of
the head and neck region (10). The
5-year survival rate of OSCC remains ~50% and has not significantly
improved in the past decades (11).
The incidence of OSCC was >300,000 cases worldwide in 2017
(12) and is increasing annually
(13). Previous studies have focused
on OSCC due to its high risk of malignancy, invasion and metastasis
(14,15). As it invades adjacent tissues, the
tumor has been demonstrated to affect speech, eating, swallowing
(16) and breathing (17), which causes the quality of life of
patients to decrease substantially (18). Thus, there is an urgent need to
identify and develop novel effective treatment for OSCC. The
occurrence and development of OSCC is a complex biological process
(19), in which the overexpression
of oncogenes (for example Notch1, fibroblast growth factor receptor
4 and c-myc plays an important role (20,21).
Recently, a novel gene called tubulin tyrosine ligase like 12
(TTLL12) has demonstrated great interest. TTLL12 expression is
significantly upregulated in lung adenocarcinoma, colorectal cancer
and prostate cancer, and is closely associated with poor prognosis
of these patients (22–25). Previous studies have reported that
the TTL domain of TTLL12 may be involved in microtubule
modification (26,27). The aim of the present study was to
investigate the function of TTLL12 and develop an experiment with a
novel target (tubulin tyrosine nitration).
Materials and methods
Cell culture
The human OSCC cell line, SCC-25 was purchased from
the Center Laboratory of Chongqing Medical University and
maintained in DMEM (Sigma-Aldrich; Merck KGaA) supplemented with
10% fetal calf serum, 1 mM sodium pyruvate and 40 µg/ml gentamycin
(all purchased from Gibco; Thermo Fisher Scientific, Inc.), at 37°C
in 5% CO2.
Lentiviral transfection
hTTLL12 complementary DNA was cloned into pTY
linkers (Sigma-Aldrich; Merck KGaA), which was transiently
transfected into 293T cells (Shanghai Institute of Life Sciences;
http://life.fudan.edu.cn/) using calcium
phosphate-mediated transient transfection reagent. A lentivirus
(Sigma-Aldrich; Merck KGaA) was transfected into SCC-25 cells. The
TTLL12-overexpressing cell lines, TTLL12_A and TTLL12_B, and the
control clones, control_A and control_B were constructed.
siRNA targeted against TTLL12
A total of two small interfering (si)RNA sequences
targeting TTLL12 (siTTLL12-1; 5′-GAGUUCAUCCCCGAGUUUG-3′ and
siTTLL12-2; 5′-GGAACGAGCUGUGCUACAA-3′), and two negative control
sequences [siControl (CONTROL® Non-Targeting siRNA #1)
and siLuciferase (GL2 luciferase siRNA)] were purchased from GE
Healthcare Dharmacon, Inc. siRNA transfections were performed using
Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol.
Briefly, 100,000 cells were seeded into a 12-well plate and
incubated in modified Eagle's medium (Shanghai Institute of Life
Sciences; http://life.fudan.edu.cn/)
supplemented with 10% fetal calf serum (Shanghai Institute of Life
Sciences), 1 mM sodium pyruvate, 40 µg/ml gentamycin (Shanghai
Institute of Life Sciences) at a temperature of 37°C in an
environment containing 5% CO2 for 18 h. Subsequently,
cells were further incubated in OPTIMEM (Sigma-Aldrich; Merck KGaA)
at a temperature of 37°C for 3 h, prior to transfection. A total of
10 nM siRNA was transfected per well. After 6 h, the medium was
replaced with normal growth medium and cells were incubated until
cell lysis. After 24 h, subsequent experimentation was
performed.
Cell proliferation assay
In total, 10,000 cells were seeded into 96-well
plates. Cell proliferation was assessed via the MTT-based
colorimetric assay (Chemicon International), according to the
manufacturer's instructions. Dimethyl sulfoxide was used to
dissolve the purple formazan crystals. The optical density (OD) of
the MTT reaction was measured at 450 nm.
Western blotting
Cells were lysed using 50 µl lysis buffer (Shanghai
Institute of Life Sciences) (1 mM DTT, 0.125 mM EDTA, 5% glycerol,
1 mM PMSF, 1 lg/ml leupeptin, 1 lg/ml pepstatin, 1 lg/ml aprotinin
and 1% Triton X-100 in 12.5 mM Tris-HCl buffer, pH 7.0) (Shanghai
Institute of Life Sciences). Protein concentration was quantified
using a BCA Protein Assay kit. The protein samples (20 µg/lane)
were separated via SDS-PAGE on a 10% gel and subsequently
transferred onto nitrocellulose membranes. The membranes were
blocked with 5% dry milk at a temperature of 37°C for 45 min prior
to incubation with primary antibodies against: TTLL12 (1:2,000;
cat. no. ab154086; Abcam), tributyl phosphate (TBP) (1:800; cat.
no. ab220788; Abcam) and nitrotyrosine (1:1,000; cat. no. ab110282;
Abcam) at a temperature of 37°C for 3 h. Following the primary
incubation, membranes were incubated with a horseradish
peroxidase-conjugated secondary antibody (1:2,000; cat. no. ab6271;
Abcam) at a temperature of 37°C for 1 h. Protein bands were
visualized using the enhanced chemiluminescence kit (cat. no.
15159; Thermo Fisher Scientific, Inc.). The target bands were
quantified using Image-Pro Plus J software (version 6.0; National
Institutes of Health) with TBP as internal control.
Immunofluorescence
In total, 100,000 cells were cultured on glass
coverslips with gelatin (Shanghai Institute of Life Sciences) at
37°C for 12 h, fixed with 4% cold paraformaldehyde at room
temperature for 20 min and incubated with 2.5% bovine serum albumin
in phosphate buffered saline (PBS) for 1 h. After washing with PBS
twice, cells were incubated with primary antibodies against TTLL12
(1:2,000; cat. no. ab154086; Abcam), overnight at 4°C. Following
the primary incubation, cells were incubated with a
peroxidase-conjugated IgG secondary antibody (1:1,000; cat. no.
A0545; Sigma-Aldrich; Merck KGaA) for 1 h at room temperature.
Nuclei were counterstained with 1 µM DAPI at 37°C for 5 min and
cells were observed under a Leica Sp8 confocal scanning laser
microscope (magnification, ×400; Leica Microsystems, Inc.).
Determining negative and positive
controls
Paclitaxel is known to exert a therapeutic effect on
OSCC and increases tubulin tyrosine nitration (28,29);
thus, it was used as a positive control in the present study.
Thiirene was used as a negative control as it does not affect
tubulin tyrosine nitration (30),
and no evidence associating thiirene and tumor progression has been
documented in the following: PubMed (https://pubmed.ncbi.nlm.nih.gov), Web of Science
(http://login.webofknowledge.com), Ovid
(http://ovidsp.ovid.com), Science Direct
(https://www.sciencedirect.com), Wan Fang
(http://www.cquc.net) or the China National
Knowledge Infrastructure databases (https://www.cnki.net).
Stable TTLL12-overexpressing SCC-25 cells
(experimental group) were treated with 400 µM nitrotyrosine and 100
µM paclitaxel or thiirene (all Shanghai Institute of Life Sciences)
at 37°C for 24 h, while cells in the control group were treated
with 400 µM nitrotyrosine at 37°C for 24 h. Cells were harvested in
lysis buffer (Shanghai Institute of Life Sciences) (1 mM DTT, 0.125
mM EDTA, 5% glycerol, 1 mM PMSF, 1 lg/ml leupeptin, 1 lg/ml
pepstatin, 1 lg/ml aprotinin and 1% Triton X-100 in 12.5 mM
Tris-HCl buffer, pH 7.0) (Shanghai Institute of Life Sciences)and
subjected to western blotting. Tubulin tyrosine nitration levels
were quantified using Quantity One version 4.62 software (Bio-Rad
Laboratories, Inc.) and normalized to TBP. Error bars represent the
mean ± standard error of the mean (SEM) of three independent
experiments.
Determining the optimum concentration
of nitrotyrosine and drug
TTLL12-overexpressing cells were seeded into 96-well
plates at a density of 1×104 cells/well and cultured in
modified Eagle's medium supplemented with 10% fetal calf serum, 1
mM sodium pyruvate, 40 µg/ml gentamycin (Shanghai Institute of Life
Sciences) in a 5% CO2 incubator at 37°C for 6 h.
Different concentrations of nitrotyrosine (800, 400, 200, 100 and
50 µM) and paclitaxel or thiirene (40, 20, 10 and 5 µM) were added
into the wells to determine the optimal concentration of
nitrotyrosine and drug by square matrix titrimetry (31). After cells were cultured at 37°C for
24 h, the MTT assay was subsequently performed to assess cell
proliferation.
In vitro experiment with novel target
(tubulin tyrosine nitration) to screen anticancer drugs
Stable TTLL12-overexpressing SCC-25 cells were
seeded into 96-well plates at a density of 10,000 cells/well and
incubated in modified Eagle's medium supplemented with 10% fetal
calf serum, 1 mM sodium pyruvate, 40 µg/ml gentamycin. (Shanghai
Institute of Life Sciences) at 37°C for 6 h. MEM (Shanghai
Institute of Life Sciences) (20 µl) supplemented with nitrotyrosine
(final concentration, 400 µM) and anticancer drugs (final
concentration, 10 µM) was added to the cells and further incubated
at 37°C for 24 h. Paclitaxel and thiirene were used as the positive
and negative controls, respectively. Following incubation, the
upper medium was gently removed and 100 µl buffer [90 mM Mes pH
6.7, 1 mM EGTA, 1 mM MgCl2, 10% (v/v) glycerol and 0.5%
(v/v) Triton X-100; Shanghai Institute of Life Sciences] was added
to the cells and further incubated at 37°C for 3 min. Once again,
the upper medium was gently removed and 100 µl PBS supplemented
with 3.7% paraformaldehyde and 0.05% Tween-20 (Shanghai Institute
of Life Sciences) was added. Following incubation at 37°C for 10
min, the upper medium was gently removed and cells were further
incubated with 100 µl PBS containing anti-nitrotyrosine antibody
(1:1,000; cat. no. 16-207, clone 1A6, HRP conjugate, Sigma-Aldrich;
Merck-KGaA) for 1 h at room temperature. Once again, the upper
medium was gently removed and well was washed once with 200 µl PBS
supplemented with 0.05% Tween-20. Finally, the upper medium was
gently removed and 100 µl OPD-H2O2 (Shanghai
Institute of Life Sciences) was added at 37°C for 3 min, prior to
addition of 50 µl H2SO4 (Shanghai Institute
of Life Sciences) (2 mol/l) at 37°C for 3 min. The absorbance was
detected at a wavelength of 450 nm, using a microplate reader
(Thermo Fisher Scientific, Inc.). The Nitrotyrosine ELISA kit
(K4158-100, Hycult Biotech Inc.) was used for detection in strict
accordance with the manufacturer's instructions and repeated three
times.
Sensitivity, specificity and
repeatability of the experimental method
In vitro experiments must have good
sensitivity, specificity and repeatability, therefore the following
experiments are done.
Sensitivity of the experiment
Stable TTLL12-overexpressing SCC-25 cells were
seeded into 96-well plates at a density of 10,000 cells/well and
incubated in modified Eagle's medium supplemented with 10% fetal
calf serum, 1 mM sodium pyruvate, 40 µg/ml gentamycin (Shanghai
Institute of Life Sciences) at 37°C for 6 h. The following
concentrations of nitrotyrosine (80, 40, 20, 10, 5 and 2.5 µM) were
added to the cells. Paclitaxel and thiirene were used as the
positive and negative controls, respectively. Following incubation,
the upper medium was gently removed and 100 µl buffer [90 mM Mes pH
6.7, 1 mM EGTA, 1 mM MgCl2, 10% (v/v) glycerol and 0.5%
(v/v) Triton X-100; Shanghai Institute of Life Sciences] was added
to the cells and further incubated at 37°C for 3 min. Once again,
the upper medium was gently removed and 100 µl PBS supplemented
with 3.7% paraformaldehyde and 0.05% Tween-20 (Shanghai Institute
of Life Sciences) was added. The Nitrotyrosine ELISA kit (cat. no.
K4158-100; Hycult Biotech Inc.) was used for detection in strict
accordance with the manufacturer's instructions and repeated three
times.
Specificity of the experiment
It is well-known that carboplatin (CDDP),
5-fluorouracil (5-FU), cyclophosphamide (CTX) and methotrexate
(MTX) exert notable effects on cancer development but have no
effects on microtubules (32–35).
Stable TTLL12-overexpressing SCC-25 cells were seeded into 96-well
plates at a density of 10,000 cells/well and incubated in modified
Eagle's medium supplemented with 10% fetal calf serum, 1 mM sodium
pyruvate, 40 µg/ml gentamycin. (Shanghai Institute of Life
Sciences) at 37°C for 6 h. MEM (Shanghai Institute of Life
Sciences) (20 µl) supplemented with nitrotyrosine (final
concentration, 400 µM) and anticancer drugs (CDDP, 5-FU, CTX and
MTX) (Shanghai Institute of Life Sciences) (final concentration, 10
µM) was added to the cells and further incubated at 37°C for 24 h.
The rest of the experiment was performed as aforementioned.
Repeatability of the experiment
Stable TTLL12-overexpressing SCC-25 cells were
seeded into 96-well plates at a density of 10,000 cells/well and
incubated in modified Eagle's medium supplemented with 10% fetal
calf serum, 1 mM sodium pyruvate, 40 µg/ml gentamycin (Shanghai
Institute of Life Sciences) at 37°C for 6 h. MEM (Shanghai
Institute of Life Sciences) (20 µl) supplemented with nitrotyrosine
(final concentration, 400 µM) and samples (Paclitaxel, thiirene,
CDDP, 5-FU and CTX) (final concentration, 10 µM) was added to the
cells and further incubated at 37°C for 24 h. The rest of the
experiment was performed as aforementioned. It was the intra-batch
assay. The same experiment was subsequently done over 4 different
days for the inter-batch assay.
Determining the validity of the
experiment
In total, 40 compounds were randomly selected by
Shanghai Institute of Life Sciences and assessed in the experiment.
Stable TTLL12-overexpressing SCC-25 cells (experimental group) were
treated with 400 µM nitrotyrosine and 10 µM nocodazole (Shanghai
Institute of Life Sciences) at 37°C for 24 h, while cells in the
control group were treated with 400 µM nitrotyrosine at 37°C for 24
h. Cells were harvested in buffer and subjected to western
blotting. Tubulin tyrosine nitration levels were quantified using
Quantity One software and normalized to TBP. Error bars represent
the mean ± SEM of three independent experiments.
Statistical analysis
Each experiment was repeated three times.
Statistical analysis was performed using SPSS 10.0 software (SPSS,
Inc.). Tubulin tyrosine nitration levels and the ratio of optical
density (OD) 450 nm are presented as the mean ± standard deviation.
One-way analysis of variance followed by Tukey's post hoc test was
used to compare differences between multiple groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Effect of different concentrations of
nitrotyrosine on the proliferation of SCC-25 cells
SCC-25 cells were treated with different
concentrations of nitrotyrosine (0–400 µM) to determine the
concentration of nitrotyrosine that effects tubulin tyrosine
nitration. Western blot analysis demonstrated that the
concentration of tubulin tyrosine nitration increased in relation
to the concentration of nitrotyrosine (Fig. 1A). The proliferation of SCC-25 cells
was assessed for 7 days via the MTT assay every 24 h. The results
demonstrated that the proliferation of SCC-25 cells was inhibited
following treatment with nitrotyrosine, whereby the higher the
concentration of nitrotyrosine, the greater the inhibition of
proliferation of SCC-25 cells (Fig.
1B). The tyrosine residues of tubulin were nitrated to form
tubulin tyrosine nitration, which causes cells to die (36).
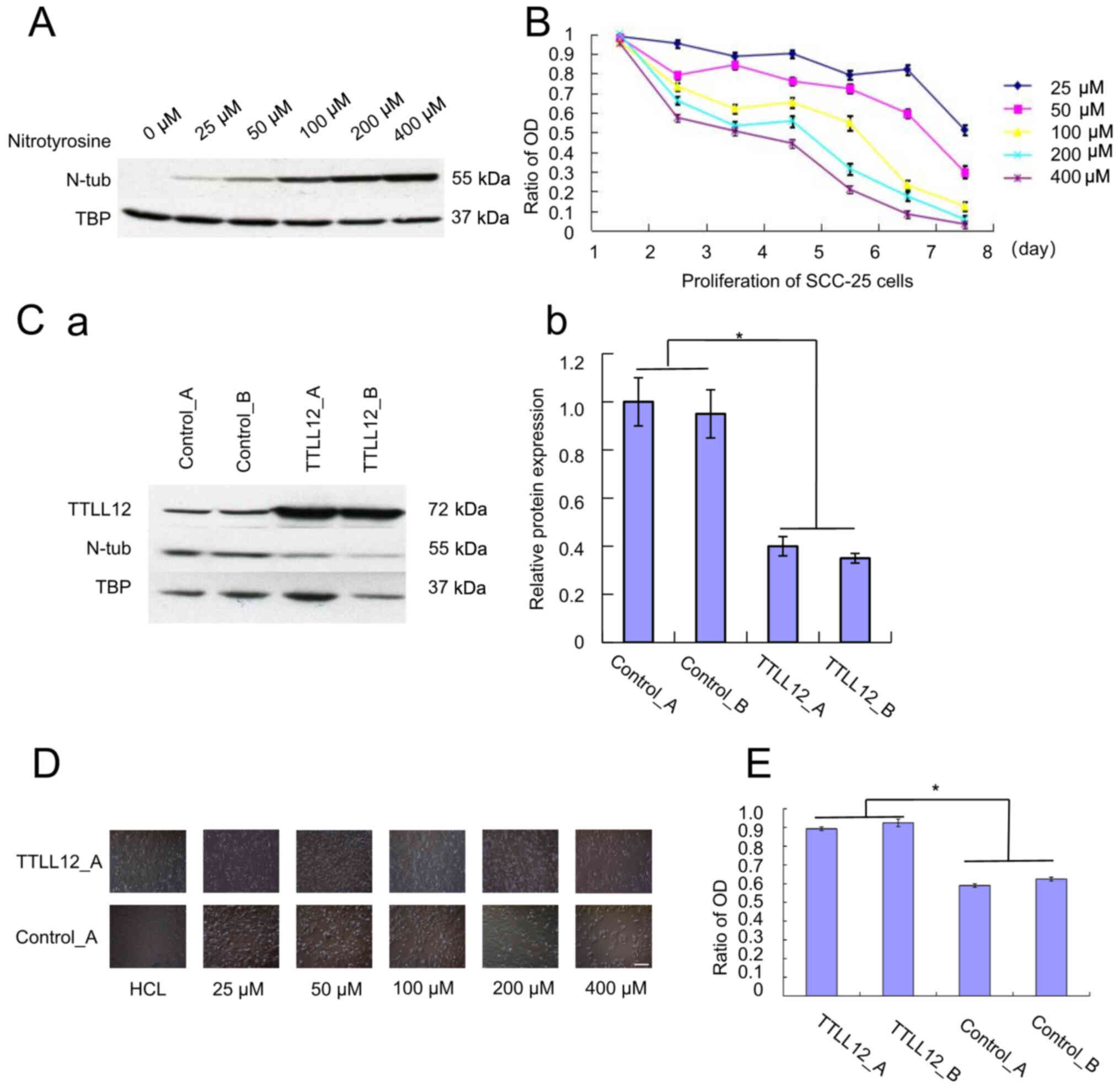 | Figure 1.Effect of overexpressing TTLL12 and
nitrotyrosine on the proliferation of SCC-25 cells. (A) Cells were
subjected to western blot analysis to determine protein expression.
(B) Cell proliferation was assessed via the MTT assay every 24 h.
The ratio of OD 450 nm was calculated as OD assay/OD control. Error
bars represent the mean ± SEM of three independent experiments. (C)
TTLL12-overexpressing clones, TTLL12_A and TTLL12_B, as well as
control_A and control_B cells were treated with 400 µM
nitrotyrosine for 24 h. (Ca) Cells from each group were subjected
to western blot analysis to determine protein expression. (Cb) The
blots were quantified via densitometry and normalized to TBP.
Tubulin tyrosine nitration levels of all groups are presented
relative to the average tubulin tyrosine nitration level of
control_A. Error bars represent the mean ± SEM of three independent
experiments. (D) TTLL12_A and control_A cells were cultured in
different concentrations of nitrotyrosine (0–400 µM) for 24 h.
Scale bar, 100 µm. (E) TTLL12_A, TTLL12_B, control_A and control_B
cells were treated with 20 µl modified Eagle's medium supplemented
with 400 µmol/l nitrotyrosine for 24 h, while the untreated group
received medium supplemented with HCl. Cells proliferation was
assessed via the MTT assay. The ratio of OD 450 nm represents the
OD 450 nm of treated cells normalized to that of untreated cells.
Error bars are represent the mean ± SEM of three independent
experiments. *P<0.05. TTLL12, tubulin tyrosine ligase like 12;
OD, optical density; TBP, tributyl phosphate, SEM, standard error
of the mean; N-tub, nitrotyrosine tubulin. |
Effect of overexpressing TTLL12 on
tubulin tyrosine nitration and SCC-25 cell proliferation
The TTLL12-overexpressing cell lines, TTLL12_A and
TTLL12_B, and the control clones, control_A and control_B were
constructed, and maintained in medium supplemented with 400 µM
nitrotyrosine for 24 h. The results demonstrated that tubulin
tyrosine nitration was significantly lower in TTLL12-overexpressing
clones compared with the control clones (P<0.05; Fig. 1C). Subsequently, in order to assess
the effect of overexpressing TTLL12 on cell proliferation, TTLL12_A
and control_A cells were treated with different concentrations of
nitrotyrosine (0–400 µM) for 24 h and observed under an inverted
microscope. The results demonstrated that the proliferation of the
control cells was notably inhibited following treatment with
nitrotyrosine compared with the TTLL12-overexpressing cells
(Fig. 1D). TTLL12-overexpressing and
control clones were treated with 400 µM nitrotyrosine for 24 h. The
results demonstrated a statistically significant increase in the
proliferation of TTLL12-overexpressing clones compared with the
control clones (P<0.05; Fig. 1E).
TTLL12 localization was assessed via immunofluorescence. Nuclei
were stained with DAPI (blue), TTLL12 stained red and α-tubulin
stained green (Fig. 2A).
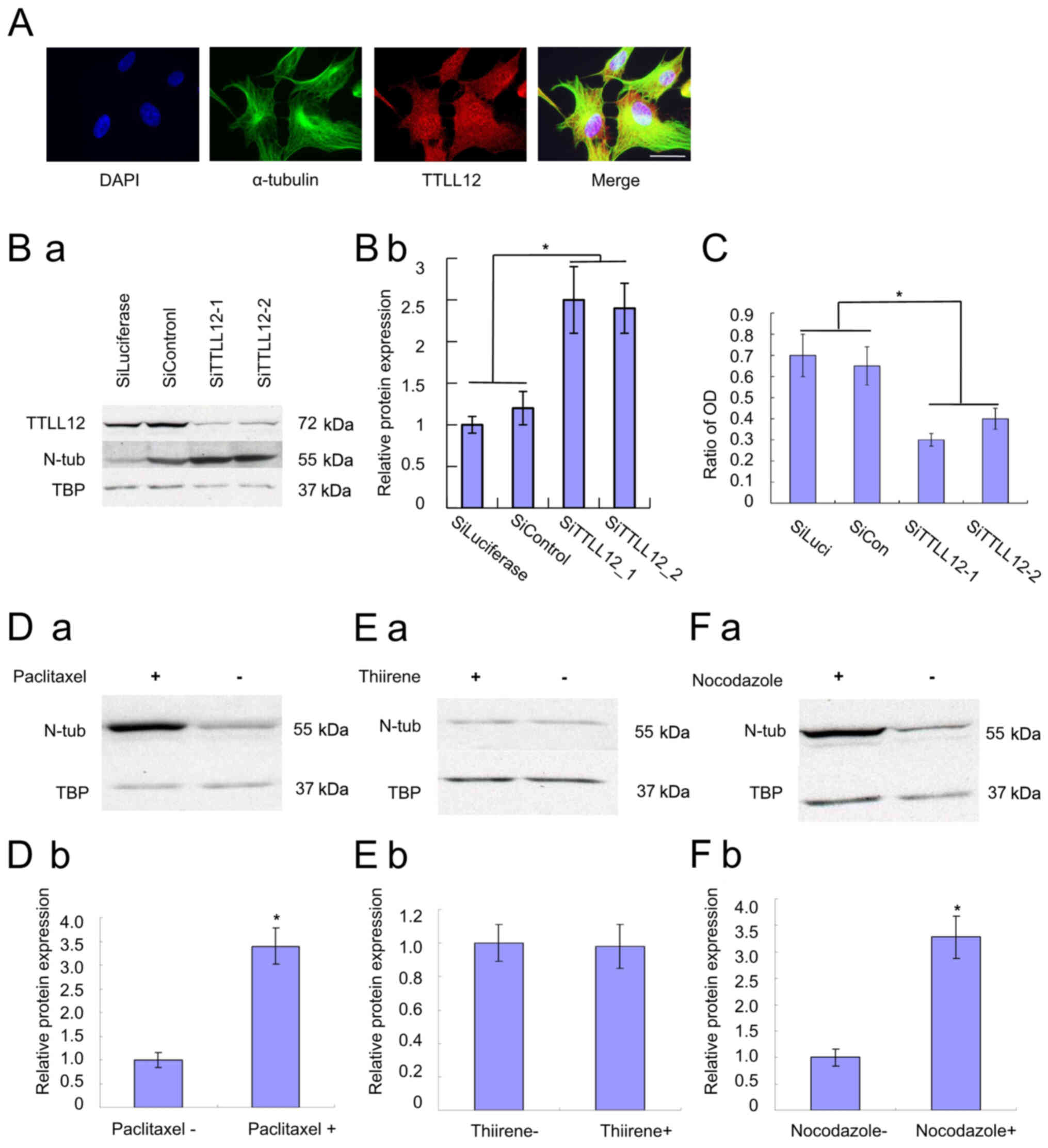 | Figure 2.Immunofluorescence of TTLL12, and the
effects of TTLL12 silencing and drugs on nitrotyrosine tubulin. (A)
Overexpressing cells were used for the immunofluorescence assay to
investigate the localization of TTLL12. Scale bar, 25 µm. (B)
siTTLL12-1, siTTLL12-2, siLuciferase and siControl cells were
treated with 400 µM nitrotyrosine for 24 h. (Ba) Cells from each
group were subjected to western blot analysis to determine protein
expression. (Bb) The blots were quantified via densitometry and
normalized to TBP. Tubulin tyrosine nitration levels of all groups
are presented relative to the average tubulin tyrosine nitration
level of control siRNAs. Error bars represent the mean ± SEM of
three independent experiments. (C) SiTTLL12-1, SiTTLL12-2,
SiLuciferase and SiControl cells were treated with 20 µl modified
Eagle's medium supplemented with 400 µmol/l nitrotyrosine or 400
µmol/l HCl for 24 h. Cell proliferation was assessed via the MTT
assay. The ratio of OD represents the OD 450 nm of treated cells
normalized to that of untreated cells. Error bars represent the
mean ± SEM of three independent experiments. (D) Stable
TTLL12-overexpressing SCC-25 cells were treated with 400 µM
nitrotyrosine. Paclitaxel (10 µM) was added to cells in the
experimental groups, while nothing was added to the control cells.
(a) After 24 h, cells from each group were subjected to western
blot analysis to determine protein expression. (b) The blots were
quantified via densitometry and normalized to TBP. Nitrotyrosine
tubulin levels are presented relative to the average of control.
Error bars represent the mean ± SEM of three independent
experiments. (E) Cells were treated with 10 µM thiirene. (F) Cells
were treated with 10 µM nocodazole. Nitrotyrosine tubulin levels
were compared with the treatment and control groups. *P<0.05.
TTLL12, tubulin tyrosine ligase like 12; si, small interfering;
SEM, standard error of the mean; OD, optical density; TBP, tributyl
phosphate; N-tub, nitrotyrosine tubulin. |
Effect of silencing TTLL12 on tubulin
tyrosine nitration and SCC-25 cell proliferation
TTLL12 expression was downregulated following siRNA
transfection, which was verified via western blotting. The
TTLL12-silenced cell lines, siTTLL12-1 and siTTLL12-2, and control
cells, siLuciferase and siControl, were treated with 400 µM
nitrotyrosine for 24 h. Western blot analysis demonstrated that
tubulin tyrosine nitration was significantly higher in
TTLL12-silenced cells compared with the control cells (P<0.05;
Fig. 2B). Furthermore,
downregulation of TTLL12 led to an increase in tubulin tyrosine
nitration. The results of the MTT assay demonstrated that the
proliferation of TTLL12-silenced cells was significantly inhibited
compared with the control cells (P<0.05; Fig. 2C). In addition, downregulation of
TTLL12 decreased cell proliferation of TTLL12-silenced cells
compared with the control cells (Fig.
2C).
Effects of paclitaxel and thiirene on
tubulin tyrosine nitration
Western blot analysis demonstrated that tubulin
tyrosine nitration increased in cells treated with paclitaxel,
suggesting a positive association between the two (Fig. 2D). Thus, paclitaxel was used as a
positive control. Conversely, the results demonstrated no
significant differences in tubulin tyrosine nitration in cells
without or with thiirene (Fig. 2E),
suggesting that thiirene does not affect the level of tubulin
tyrosine nitration. Thus, thiirene was used as a negative
control.
Determining the optimum concentration
of nitrotyrosine and drug
The results of the square matrix titrimetry assay
demonstrated that the OD 450 nm value of 400 µM nitrotyrosine and
10 µM paclitaxel was ~1.0, while the OD 450 nm value of thiirene
was lower, and the ratio of the positive to negative control (P/N
value) was the highest. Thus, 400 µM was considered the optimal
concentration of nitrotyrosine, and 10 µM the optimal concentration
of drug (Table I).
 | Table I.Determining the optimum concentration
of nitrotyrosine and drug. |
Table I.
Determining the optimum concentration
of nitrotyrosine and drug.
|
|
| Concentration of
nitrotyrosine |
|---|
|
|
|
|
|---|
| Concentration of
drug | 50 µM | 100 µM | 200 µM | 400 µM | 800 µM |
|---|
| 5 µM | Paclitaxel | 0.427±0.031 | 0.596±0.043 | 0.714±0.052 | 0.846±0.061 | 1.126±0.109 |
|
| Thiirene | 0.187±0.011 | 0.195±0.009 | 0.224±0.019 | 0.253±0.022 | 0.225±0.021 |
| 10 µM | Paclitaxel | 0.647±0.041 | 0.864±0.061 | 1.110±0.129 | 1.097±0.055 | 1.230±0.097 |
|
| Thiirene | 0.110±0.009 | 0.126±0.011 | 0.130±0.014 | 0.122±0.011 | 0.147±0.015 |
| 20 µM | Paclitaxel | 0.801±0.067 | 1.104±0.096 | 1.135±0.109 | 1.116±0.091 | 1.235±0.115 |
|
| Thiirene | 0.108±0.091 | 0.124±0.011 | 0.132±0.012 | 0.126±0.014 | 0.139±0.012 |
| 40 µM | Paclitaxel | 0.810±0.064 | 1.130±0.122 | 1.180±0.096 | 1.281±0.108 | 1.332±0.113 |
|
| Thiirene | 0.110±0.089 | 0.129±0.093 | 0.133±0.101 | 0.147±0.107 | 0.149±0.112 |
Sensitivity, specificity and
repeatability of the experiment
The results of the sensitivity assay demonstrated
that the OD 450 nm value of paclitaxel with a minimum concentration
of 2.5 µM was 0.681, which was higher than the OD 450 nm value of
10 µM thiirene (0.122) (Table II).
Thus, the sensitivity of the novel experiment was considered
positive.
 | Table II.Sensitivity assay results. |
Table II.
Sensitivity assay results.
| Concentration of
nitrotyrosine | 80 µM | 40 µM | 20 µM | 10 µM | 5 µM | 2.5 µM |
|---|
| OD, 450 nm | 1.330±0.119 | 1.291±0.107 | 1.118±0.113 | 1.086±0.064 | 0.846±0.076 | 0.681±0.054 |
The results of the specificity assay demonstrated
that the OD 450 nm value of paclitaxel (1.102) was considerably
higher compared with the other drugs (Thiirene, 0.157; CDDP, 0.108;
5-FU, 0.179; CTX, 0.207; MTX, 0.251; Table III). Thus, the specificity of the
novel experiment was considered positive (Table III).
 | Table III.Specificity assay results. |
Table III.
Specificity assay results.
|
| Paclitaxel | Thiirene | CDDP | 5-FU | CTX | MTX |
|---|
| OD, 450 nm | 1.102±0.042 | 0.157±0.012 | 0.108±0.014 | 0.179±0.011 | 0.207±0.014 | 0.251±0.017 |
The results of the repeatability assay demonstrated
that the coefficient of variation (CV) of the intra-batch assay was
3.50–7.87%, while the CV of the inter-batch assay was 2.86–6.99%.
Given that both values were <10%, the repeatability of the
experiment was considered positive (Table IV).
 | Table IV.Intra-batch and inter-batch
reproducibility assay results. |
Table IV.
Intra-batch and inter-batch
reproducibility assay results.
|
| Intra-batch assay
(OD, 450 nm) | Inter-batch assay
(OD, 450 nm) |
|---|
|
|
|
|
|---|
|
| Mean | SD | CV | Mean | SD | CV |
|---|
| Paclitaxel | 1.107 | 0.047 | 4.25 | 1.084 | 0.031 | 2.86 |
| Thiirene | 0.163 | 0.011 | 6.75 | 0.143 | 0.006 | 4.20 |
| CDDP | 0.114 | 0.004 | 3.50 | 0.121 | 0.007 | 5.79 |
| 5-FU | 0.186 | 0.008 | 4.30 | 0.179 | 0.011 | 6.15 |
| CTX | 0.216 | 0.017 | 7.87 | 0.229 | 0.016 | 6.99 |
Application of the experiment
The results of the last experiment demonstrated that
the OD 450 nm value of most samples was <0.452 (Table V), and only the OD 450 nm value of
sample no. 14 was >1 (1.043; Table
V), showing that shwed nocodazole could have an improved
anticancer effect compared with paclitaxel. Western blot analysis
demonstrated that tubulin tyrosine nitration increased in cells
treated with nocodazole, suggesting a positive association between
the two (Fig. 2F).
 | Table V.Novel experiment results. |
Table V.
Novel experiment results.
| Sample | OD, 450 nm | Sample | OD, 450 nm | Sample | OD, 450 nm | Sample | OD, 450 nm |
|---|
| Paclitaxel | 1.091±0.043 | 1 | 0.132±0.010 | 2 | 0.147±0.013 | 3 | 0.151±0.011 |
| 4 | 0.129±0.012 | 5 | 0.136±0.013 | 6 | 0.158±0.018 | 7 | 0.235±0.019 |
| 8 | 0.321±0.025 | 9 | 0.351±0.027 | 10 | 0.358±0.029 | 11 | 0.247±0.021 |
| 12 | 0.241±0.019 | 13 | 0.411±0.037 | 14 | 1.043±0.042 | 15 | 0.412±0.022 |
| 16 | 0.314±0.019 | 17 | 0.452±0.031 | 18 | 0.189±0.009 | 19 | 0.338±0.026 |
| 20 | 0.371±0.019 | 21 | 0.121±0.011 | 22 | 0.365±0.024 | 23 | 0.146±0.015 |
| 24 | 0.352±0.029 | 25 | 0.347±0.017 | 26 | 0.122±0.009 | 27 | 0.157±0.014 |
| 28 | 0.163±0.013 | 29 | 0.221±0.019 | 30 | 0.289±0.017 | 31 | 0.113±0.008 |
| 32 | 0.167±0.009 | 33 | 0.148±0.011 | 34 | 0.128±0.012 | 35 | 0.168±0.014 |
| 36 | 0.278±0.016 | 37 | 0.263±0.019 | 38 | 0.410±0.031 | 39 | 0.247±0.022 |
| 40 | 0.162±0.011 |
|
|
|
|
|
|
Discussion
Microtubules are the basic units of the
cytoskeleton, which exist in all eukaryotic cells (37). They are polymerized by microtubule
protein and can be assembled into cilia, flagella, axon, neural
tube, basal granule, centriole and spindle (38). Microtubules participate in the
formation of the cytoskeleton, maintenance of cell morphology, cell
contraction, pseudopodia movement, material transport and cell
division (39,40). Prior to functioning, microtubules
need to be coated with tubulin tyrosine ligase (TTL) (41). TTL is involved in
nitrotyrosine-mediated post-translational modification of tubulin
(4,42). In the presence of nitrotyrosine, TTL
induces tubulin tyrosine nitration, which significantly changes the
structure of microtubules, decreases the adhesive ability of cells,
causes cell deformation and intracellular transport obstacle, and
eventually leads to cell apoptosis and organ function loss
(43,44).
Previous studies have detected nitrotyrosine in
fresh-frozen human tissue samples and have demonstrated that
nitrotyrosine is an important marker for oral cancer prognosis and
survival (9). However, there is a
lack of studies assessing the effect of nitrotyrosine on the
proliferation of oral cancer cells. The results of the present
study demonstrated that the proliferation inhibition of SCC-25
cells was proportional to the content of nitrotyrosine, which
suggest that nitrotyrosine inhibits SCC-25 cell proliferation.
TTLL12-overexpressing clones (TTLL12_A and TTLL12_B) and control
clones (control_A and control_B) were constructed and treated with
nitrotyrosine. The results demonstrated that TTLL12 promoted the
proliferation of SCC-25 cells by inhibiting the formation of
tubulin tyrosine nitration. Furthermore, TTLL12-silenced clones
(siTTLL12-1 and siTTLL12-2) and control clones (siLuciferase and
siControl) were constructed and treated with nitrotyrosine. The
results demonstrated that tubulin tyrosine nitration formation
increased, and the proliferation of SCC-25 cells was inhibited in
TTLL12-silenced cell lines compared with the control cells.
Following overexpression of TTLL12, tubulin tyrosine nitration
formation decreased, and cells continued to proliferate by escaping
the attack of tubulin tyrosine nitration. Conversely, TTLL12
silencing increased tubulin tyrosine nitration and inhibited cell
proliferation. Taken together, these results suggest that TTLL12
may function as a potential oncogene. Although the
immunofluorescence results demonstrated that TTLL12 appeared to
overlap with α-tubulin, the molecular mechanism by which TTLL12
regulates tubulin tyrosine nitration remains unclear and requires
further investigation. It can be hypothesized that TTLL12 and nitro
have the same binding site on α-tubulin, and thus competes with
α-tubulin. It was hypothesized that when TTLL12 is overexpressed,
it becomes difficult to nitrate the tyrosine residues of tubulin,
thus tubulin tyrosine nitration decreases.
Malignant tumors (for example oral, liver and
gastric cancer) seriously threaten the well-being and quality of
life of affected patients (45).
Currently, there are no effective treatments for cancer, and
comprehensive treatment is extensively applied in the clinical
setting (46). Chemotherapy, as the
only effective systemic treatment, began in 1948 (folic acid
antagonists were used as antitumor drugs to treat leukemia), which
plays an irreplaceable role in the comprehensive treatment of
cancer (47). Due to the
heterogeneity of tumors (48),
different individuals have different reactions to the same drug,
which affects the treatment outcome. In response to this problem,
some medical studies have proposed to use a drug sensitivity
experiment to provide individualized treatment to improve the
clinical efficacy (49,50). In vitro drug sensitivity
experiments have been extensively used in the last century due to
their effective and efficient outcomes (51). However, the microenvironment in
vivo may lead to the emergence of drug resistance (52), which decreases the efficacy of drug
sensitivity experiments in vitro to guide clinical
individualized treatment. However, effective methods (drug
sensitivity experiments in vitro and in vivo) to
identify potential effective anticancer reagents remain (53,54).
The majority of drug sensitivity experiments in
vitro aim to assess the anticancer effect of drugs by detecting
the ratio of living cells to dead cells after the tumor cells are
cultured with drugs (55). Novel
effective drugs that can kill tumors are identified by drug
sensitivity experiments; however, the anticancer molecular
mechanism of these drugs remains unknown. This notably disrupts the
clinical application of novel drugs. Thus, additional experiments
are required to identify the anticancer molecular mechanism of
novel drugs, which are time consuming and expensive, and delay the
treatment of patients.
Based on this concept, the present study established
a novel type of drug sensitivity test targeting tubulin tyrosine
nitration in vitro by combining the research results with
the enzyme-linked immunosorbent assay (ELISA). This novel assay can
screen out new effective anticancer drugs and determine the
anticancer molecular mechanism of these drugs. Paclitaxel exerts a
notable therapeutic effect on OSCC and increases tubulin tyrosine
nitration (28,29); thus, it was used as a positive
control in the present study. Conversely, thiirene is not
associated with tumors and does not affect tubulin tyrosine
nitration (30); thus, it was used
as a negative control in the present study. TTLL12-overexpressing
cells were treated with the samples and nitrotyrosine, and tubulin
tyrosine nitration was detected via ELISA. If the OD value of the
samples was greater than that of the positive control, the samples
were considered to have a positive anticancer effect. The results
of the experiment demonstrated that nocodazole increased tubulin
tyrosine nitration, and its OD 450 nm value was high, thus, it may
be considered a promising anticancer drug.
The novel experiment has the following
characteristics: It is simple, sensitive, fast, specific, cheap,
stable and easy to be popularized. This experiment consists of a
few steps and simple technologies, including cell culture and
ELISA, which are both easy to perform. The highly efficient
biocatalysis of enzymes is used in the novel experiment, so that
markedly low contents of tubulin tyrosine nitration can be
detected, and the novel experiment has high sensitivity. The
experiments lasts for ~32 h and detection occurs using a 96-well
plate, thus, the overall time required is relatively short and the
experiment is fast. The combination of anti-nitrotyrosine antibody
and tubulin tyrosine nitration is that of antibody and antigen,
thus, the experiment has high specificity. Special or valuable
materials are not involved, thus the materials are easily attained
and affordable. The experiment comprises only a few steps, few
reagents and few variation factors, which facilitates good
repeatability and stability. Considering the aforementioned
advantages, the experiment can be easily popularized and used for
large-scale screening. The experiment can automatically and
mechanically detect multiple 96-well plates simultaneously, whereby
one 96-well plate can analyze 16 samples at a time. Thus, if
multiple 96-well plates are detected in parallel, hundreds of
samples can be detected within 32 h. This high-throughput test has
another benefit in that the results of the experiment not only
present the anticancer effect of the sample, but also reveal its
anticancer molecular mechanism. If the sample participates in the
process of microtubule modification, whereby it can promote the
formation of tubulin tyrosine nitration, tumor cells will
experience apoptosis. Simultaneously, tubulin tyrosine nitration
will combine with the antibody, which will eventually increase the
OD value (56). If the OD value of
the sample is higher than that of the positive control, this
suggests that the sample is a promising anticancer drug. Such a
drug can kill cancer cells, and its anticancer effect may even be
better than that of paclitaxel; its anticancer molecular mechanism
involves its participation in the process of microtubule
modification, which promotes the formation of tubulin tyrosine
nitration, thus causing tumor cell apoptosis. This information can
greatly speed up the application of the drug in the clinical
setting, and is expected to improve patients survival outcomes. To
the best of our knowledge, the present study was the first to use
TTLL12-overexpressing cell lines to screen anticancer drugs, paving
the way for future research on novel anticancer drugs targeting the
microtubule system. However, our results need to be verified in
vivo in the future.
In conclusion, the results of the present study
demonstrated that TTLL12 promoted SCC-25 cell survival in the
presence of nitrotyrosine by decreasing the formation of tubulin
tyrosine nitration. The novel experiment described in the present
study is simple, sensitive, rapid, specific, affordable, stable and
easy to be popularized, and has good application for basic research
on anticancer drugs.
Acknowledgements
Not applicable.
Funding
The present study was partly funded by the Natural
Science Foundation Project of CQ CSTC (grant no.
cstc2018jcyjAX0763) and the Chongqing Municipal Health Bureau
(grant no. 2017HBRC004).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
YL designed the present study. CF and WC
participated in statistical analyses. CF interpreted the western
blot results. WC performed immunofluorescence assessment of the
cells. YL, LX and YZ interpreted the results, and prepared and
drafted the initial manuscript. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Anavi S and Tirosh O: iNOS as a metabolic
enzyme under stress conditions. Free Radic Biol Med. 146:16–35.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Goldstein S and Merényi G: The chemistry
of peroxynitrite: Implications for biological activity. Methods
Enzymol. 436:49–61. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang B, Wang Y, Wang Y, Zhao Y, Yang C,
Zeng Z, Huan S, Song G and Zhang X: Oxygen-embedded pentacene based
near-infrared chemiluminescent nanoprobe for highly selective and
sensitive visualization of peroxynitrite in vivo. Anal Chem.
92:4154–4163. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Eiserich JP, Estévez AG, Bamberg TV, Ye
YZ, Chumley PH, Beckman JS and Freeman BA: Microtubule dysfunction
by posttranslational nitrotyrosination of alpha-tubulin: A nitric
oxide-dependent mechanism of cellular injury. Proc Natl Acad Sci
USA. 96:6365–6370. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bartesaghi S and Radi R: Fundamentals on
the biochemistry of peroxynitrite and protein tyrosine nitration.
Redox Biol. 14:618–625. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tampa M, Mitran MI, Mitran CI, Sarbu MI,
Matei C, Nicolae I, Caruntu A, Tocut SM, Popa MI, Caruntu C and
Georgescu SR: Mediators of inflammation-A potential source of
biomarkers in oral squamous cell carcinoma. J Immunol Res.
2018:10617802018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee BJ, Chan MY, Hsiao HY, Chang CH, Hsu
LP and Lin PT: Relationship of Oxidative Stress, Inflammation, and
the Risk of Metabolic Syndrome in Patients with Oral Cancer. Oxid
Med Cell Longev. 2018:93030942018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chaiyarit P, Ma N, Hiraku Y, Pinlaor S,
Yongvanit P, Jintakanon D, Murata M, Oikawa S and Kawanishi S:
Nitrative and oxidative DNA damage in oral lichen planus in
relation to human oral carcinogenesis. Cancer Sci. 96:553–559.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Silva Servato JP, Ueira Vieira C, de Faria
PR, Cardoso SV and Loyola AM: The importance of inducible nitric
oxide synthase and nitrotyrosine as prognostic markers for oral
squamous cell carcinoma. J Oral Pathol Med. 48:967–975. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Olek M, Kasperski J, Skaba D, Wiench R,
Cieślar G and Kawczyk-Krupka A: Photodynamic therapy for the
treatment of oral squamous carcinoma-clinical implications
resulting from in vitro research. Photodiagnosis Photodyn Ther.
27:255–267. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Irani S: Pre-cancerous lesions in the oral
and maxillofacial region: A literature reviewwith special focus on
etiopathogenesis. Iran J Pathol. 11:303–322. 2016.PubMed/NCBI
|
|
12
|
Vucicevic Boras V, Fucic A, Virag M,
Gabric D, Blivajs I, Tomasovic-Loncaric C, Rakusic Z, Bisof V,
Novere NL and Velimir Vrdoljak D: Significance of stroma in biology
of oral squamous cell carcinoma. Tumori. 104:9–14. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bao X, Liu F, Lin J, Chen Q, Chen L, Chen
F, Wang J, Qiu Y, Shi B, Pan L, et al: Nutritional assessment and
prognosis of oral cancer patients: A large-scale prospective study.
BMC Cancer. 20:1462020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang SS, Zheng M, Pang X, Zhang M, Yu XH,
Wu JB, Gao XL, Wu JS, Yang X, Tang YJ, et al: Macrophage migration
inhibitory factor promotes the invasion and metastasis of oral
squamous cell carcinoma through matrix metalloprotein-2/9. Mol
Carcinog. 58:1809–1821. 2019. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ortiz RC, Lopes NM, Amôr NG, Ponce JB,
Schmerling CK, Lara VS, Moyses RA and Rodini CO: CD44 and ALDH1
immunoexpression as prognostic indicators of invasion and
metastasis in oral squamous cell carcinoma. J Oral Pathol Med.
47:740–747. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Romer CAE, Broglie Daeppen MA, Mueller M,
Huber GF, Guesewell S and Stoeckli SJ: Long-term speech and
swallowing function after primary resection and sentinel node
biopsy for early oral squamous cell carcinoma. Oral Oncol.
89:127–132. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Deng W, Yang L, Xie C, Lu H, Xiao Y, Zeng
B, Liang Y and Liao G: Prediction of postoperative lower
respiratory tract infections in tongue cancer patients based on
pretreatment swallowing function. Oral Dis. 26:537–546. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Goswami S and Gupta SS: How cancer of oral
cavity affects the family caregivers? -A cross-sectional study in
Wardha, India, using the caregiver quality of life index-cancer
questionnaire. South Asian J Cancer. 9:62–65. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sun Y, Liu N, Guan X, Wu H, Sun Z and Zeng
H: Immunosuppression induced by chronic inflammation and the
progression to oral squamous cell carcinoma. Mediators Inflamm.
2016:57157192016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu L, Hou TJ and Yang P: Mechanism of
lncRNA FEZF1-AS1 in promoting the occurrence and development of
oral squamous cell carcinoma through targeting miR-196a. Eur Rev
Med Pharmacol Sci. 23:6505–6515. 2019.PubMed/NCBI
|
|
21
|
Xiao T, Sun J, Xing Z, Xie F, Yang L and
Ding W: MTFP1 overexpression promotes the growth of oral squamous
cell carcinoma by inducing ROS production. Cell Biol Int.
44:821–829. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sanada H, Seki N, Mizuno K, Misono S,
Uchida A, Yamada Y, Moriya S, Kikkawa N, Machida K, Kumamoto T, et
al: Involvement of dual strands of miR-143 (miR-143-5p and
miR-143-3p) and their target oncogenes in the molecular
pathogenesis of lung adenocarcinoma. Int J Mol Sci. 20:44822019.
View Article : Google Scholar
|
|
23
|
Liu J, Li H, Shen S, Sun L, Yuan Y and
Xing C: Alternative splicing events implicated in carcinogenesis
and prognosis of colorectal cancer. J Cancer. 9:1754–1764. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wen R, Xiao Y, Zhang Y, Yang M, Lin Y and
Tang J: Identification of a novel transcript isoform of the TTLL12
gene in human cancers. Oncol Rep. 36:3172–3180. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Massoner P, Lueking A, Goehler H, Höpfner
A, Kowald A, Kugler KG, Amersdorfer P, Horninger W, Bartsch G,
Schulz-Knappe P and Klocker H: Serum-autoantibodies for discovery
of prostate cancer specific biomarkers. Prostate. 72:427–436. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li H, Huang Y, Yu Y, Li G and Karamanos Y:
Self-catalyzed assembly of peptide scaffolded nanozyme as a dynamic
biosensing system. ACS Appl Mater Interfaces. 8:2833–2839. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wasylyk C, Zambrano A, Zhao C, Brants J,
Abecassis J, Schalken JA, Rogatsch H, Schaefer G, Pycha A, Klocker
H and Wasylyk B: Tubulin tyrosine ligase like 12 links to prostate
cancer through tubulin posttranslational modification and
chromosome ploidy. Int J Cancer. 127:2542–2553. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Robert BM, Dakshinamoorthy M,
Ganapathyagraharam Ramamoorthy B, Dhandapani M, Thangaiyan R,
Muthusamy G, Nirmal RM and Prasad NR: Predicting tumor sensitivity
to chemotherapeutic drugs in oral squamous cell carcinoma patients.
Sci Rep. 8:155452018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wood SC, Tang X and Tesfamariam B:
Paclitaxel potentiates inflammatory cytokine-induced prothrombotic
molecules in endothelial cells. J Cardiovasc Pharmacol. 55:276–285.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhou X, Wu J, Hao Y, Zhu C, Zhuo Q, Xia H
and Zhu J: Rational design and synthesis of unsaturated
se-containing osmacycles with σ-aromaticity. Chemistry.
24:2389–2395. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ensafi AA, Dadkhah-Tehrani S and
Karimi-Maleh H: Voltammetric determination of glutathione in
haemolysed erythrocyte and tablet samples using modified-multiwall
carbon nanotubes paste electrode. Drug Test Anal. 4:978–985. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Miyawaki A, Hijioka H, Ikeda R, Ishida T,
Nozoe E and Nakamura N: Analysis of the outcome of concurrent
neoadjuvant chemoradiotherapy with S-1 compared to super-selective
intra-arterial infusion for oral squamous cell carcinoma. Oncol
Lett. 3:995–1001. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Feng X, Luo Q, Zhang H, Wang H, Chen W,
Meng G and Chen F: The role of NLRP3 inflammasome in 5-fluorouracil
resistance of oral squamous cell carcinoma. J Exp Clin Cancer Res.
36:812017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kim J and Chan JJ: Cyclophosphamide in
dermatology. Australas J Dermatol. 58:5–17. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Olasz L, Orsi E, Markó T and Szalma J:
Induction chemotherapy response and recurrence rates in correlation
with N0 or N+ stage in oral squamous cell cancer (OSCC). Cancer
Metastasis Rev. 29:607–611. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Blanchard-Fillion B, Prou D, Polydoro M,
Spielberg D, Tsika E, Wang Z, Hazen SL, Koval M, Przedborski S and
Ischiropoulos H: Metabolism of 3-nitrotyrosine induces apoptotic
death in dopaminergic cells. J Neurosci. 26:6124–6130. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Goodson HV and Jonasson EM: Microtubules
and Microtubule-Associated Proteins. Cold Spring Harb Perspect
Biol. 10:a0226082018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Akhmanova A and Steinmetz MO: Control of
microtubule organization and dynamics: Two ends in the limelight.
Nat Rev Mol Cell Biol. 16:711–726. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Magiera MM, Singh P, Gadadhar S and Janke
C: Tubulin posttranslational modifications and emerging links to
human disease. Cell. 173:1323–1327. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Janke C and Montagnac G: Causes and
consequences of microtubule acetylation. Curr Biol. 27:R1287–R1292.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Nieuwenhuis J and Brummelkamp TR: The
tubulin detyrosination cycle: Function and enzymes. Trends Cell
Biol. 29:80–92. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kalisz HM, Erck C, Plessmann U and Wehland
J: Incorporation of nitrotyrosine into alpha-tubulin by recombinant
mammalian tubulin-tyrosine ligase. Biochim Biophys Acta.
1481:131–138. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Song W, Cho Y, Watt D and Cavalli V:
Tubulin-tyrosine ligase (TTL)-mediated increase in tyrosinated
α-tubulin in injured axons is required for retrograde injury
signaling and axon regeneration. J Biol Chem. 290:14765–14775.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Rivelli JF, Ochoa AL, Santander VS, Nigra
A, Previtali G and Casale CH: Regulation of aldose reductase
activity by tubulin and phenolic acid derivates. Arch Biochem
Biophys. 654:19–26. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bajaj S, Roy PP and Singh J:
1,3,4-oxadiazoles as telomerase inhibitor: Potential anticancer
agents. Anticancer Agents Med Chem. 17:1869–1883. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zugazagoitia J, Guedes C, Ponce S, Ferrer
I, Molina-Pinelo S and Paz-Ares L: Current challenges in cancer
treatment. Clin Ther. 38:1551–1566. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
De Marchi P, Melendez ME, Laus AC,
Kuhlmann PA, de Carvalho AC, Arantes LMRB, Evangelista AF, Andrade
ES, de Castro G Junior, Reis RM, et al: The role of
single-nucleotide polymorphism (SNPs) in toxicity of induction
chemotherapy based on cisplatin and paclitaxel in patients with
advanced head and neck cancer. Oral Oncol. 98:48–52. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Fugazzola L, Muzza M, Pogliaghi G and
Vitale M: Intratumoral genetic heterogeneity in papillary thyroid
cancer: Occurrence and clinical significance. Cancers (Basel).
12:3832020. View Article : Google Scholar
|
|
49
|
Mimoto R, Yogosawa S, Saijo H, Fushimi A,
Nogi H, Asakura T, Yoshida K and Takeyama H: Clinical implications
of drug-screening assay for recurrent metastatic hormone
receptor-positive, human epidermal receptor 2-negative breast
cancer using conditionally reprogrammed cells. Sci Rep.
9:134052019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Hoffman RM and Tanino H: Clinical
usefulness of the histoculture drug response assay for breast
cancer. Methods Mol Biol. 1760:93–100. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Franck S, Fuhrmann-Selter T, Joseph JF,
Michelet R, Casilag F, Sirard JC, Wicha SG and Kloft C: A rapid,
simple and sensitive liquid chromatography tandem mass spectrometry
assay to determine amoxicillin concentrations in biological matrix
of little volume. Talanta. 201:253–258. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wu CP, Lusvarghi S, Tseng PJ, Hsiao SH,
Huang YH, Hung TH and Ambudkar SV: MY-5445, a phosphodiesterase
type 5 inhibitor, resensitizes ABCG2-overexpressing
multidrug-resistant cancer cells to cytotoxic anticancer drugs. Am
J Cancer Res. 10:164–178. 2020.PubMed/NCBI
|
|
53
|
Dhiman N, Kingshott P, Sumer H, Sharma CS
and Rath SN: On-chip anticancer drug screening-recent progress in
microfluidic platforms to address challenges in chemotherapy.
Biosens Bioelectron. 137:236–254. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Mourelatos D: Sister chromatid exchange
assay as a predictor of tumor chemoresponse. Mutat Res Genet
Toxicol Environ Mutagen. 803:1–12. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zhang L, Li B, Zhang B, Zhang H and Suo J:
miR-361 enhances sensitivity to 5-fluorouracil by targeting the
FOXM1-ABCC5/10 signaling pathway in colorectal cancer. Oncol Lett.
18:4064–4073. 2019.PubMed/NCBI
|
|
56
|
Sticozzi C, Aiello F, Andreasi RB, Muresan
XM, Belmonte G, Cervellati F, Maellaro E, Maioli E and Valacchi G:
Antitubulinic effect of new fluorazone derivatives on melanoma
cells. Anticancer Agents Med Chem. 16:601–608. 2016. View Article : Google Scholar : PubMed/NCBI
|
















