Introduction
Cancer is a leading cause of mortality worldwide. In
2015, 4.292 million new cancer cases and 2.814 million
cancer-related mortalities were reported in China (1). Cancer immunotherapy is one of several
treatments for various types of cancer, and has yielded great
success when applied to the treatment of certain hematological and
solid malignancies (2–4). Currently available immune-targeted
cancer therapies include Toll-like receptor (TLR) agonists,
vaccines, immune checkpoint inhibitors (ICIs) and adoptive T-cell
therapy.
ICIs serve a key role in the treatment of several
cancer types. This class of therapy is exemplified by monoclonal
antibodies that block the programmed death protein 1/programmed
death ligand 1 (PD-1/PD-L1) axis and the cytotoxic T-lymphocyte
antigen-4 (CTLA-4) cell-surface receptor (5,6). ICIs
restore the immune system's ability to target and kill cancer cells
by inhibiting suppressive interactions between T-cell receptors and
homologous ligands on cancer cells (7). The present review investigated the
increasing volume of research regarding gut microbiota as a
regulator of the effects of immunotherapy in patients with
cancer.
Gut microbiota and cancer
The gut microbiota is closely associated with cancer
via its ability to influence malignancy through a variety of direct
and indirect mechanisms (8). For
example, certain products of the gut microbiota may directly
promote cancer growth, including metabolites produced by intestinal
microorganisms that directly induce oncogenic mutations in the host
(9). Furthermore, Escherichia
coli strains that harbor the pks virulence gene island
may produce the toxin colibactin, which has been shown to cause
genetic damage and subsequent colorectal cell malignancy when
injected into cultured human intestinal stem cells in vitro
(9).
Intestinal bacteria may not directly promote
tumorigenesis and cancer development, but instead may interact with
the immune system to indirectly promote malignancy (8). A defective immune response may also
lead to an increase in the abundance of certain bacterial genera,
and the resulting immune response triggers signaling pathways that
lead to the transcription of oncogenes (8). Furthermore, the gut microbiota may
indirectly promote cancer by inducing inflammation or
immunosuppression (10,11). Finally, changes in the composition of
the gut microbiota are closely associated with the development of
various malignancies, including gastric cancer, colorectal cancer,
liver cancer, pancreatic cancer, breast cancer and melanoma
(12).
Gut microbiota and immunity
The gut microbiota affects various aspects of host
immunity (Fig. 1). In particular,
the interaction between the gut microbiota and the intestinal
mucosal immune system is considered a key factor in the maintenance
of mucosal homeostasis. The intestinal mucosal immune system has
unique structures and functions that are relatively independent of
systemic immunity (11). For
example, it can effectively inhibit bacterial adhesion and
colonization in intestinal epithelial cells and can sequester
bacteria and harmful antigens within the body (6). The gut microbiota exerts a wide range
of effects on the intestinal mucosal immune system of the host
(13). For example, Bacteroides
fragilis may induce naïve CD4+ naive T-cells to
differentiate into regulatory T-cells (Treg cells) that secrete
large quantities of anti-inflammatory cytokines (e.g., IL-10)
(14). Furthermore, Cebula et
al (15) reported that Treg
cells in the colon may recognize antigenic substances associated
with the bacterial genera Clostridium and Bacteroides
(15,16).
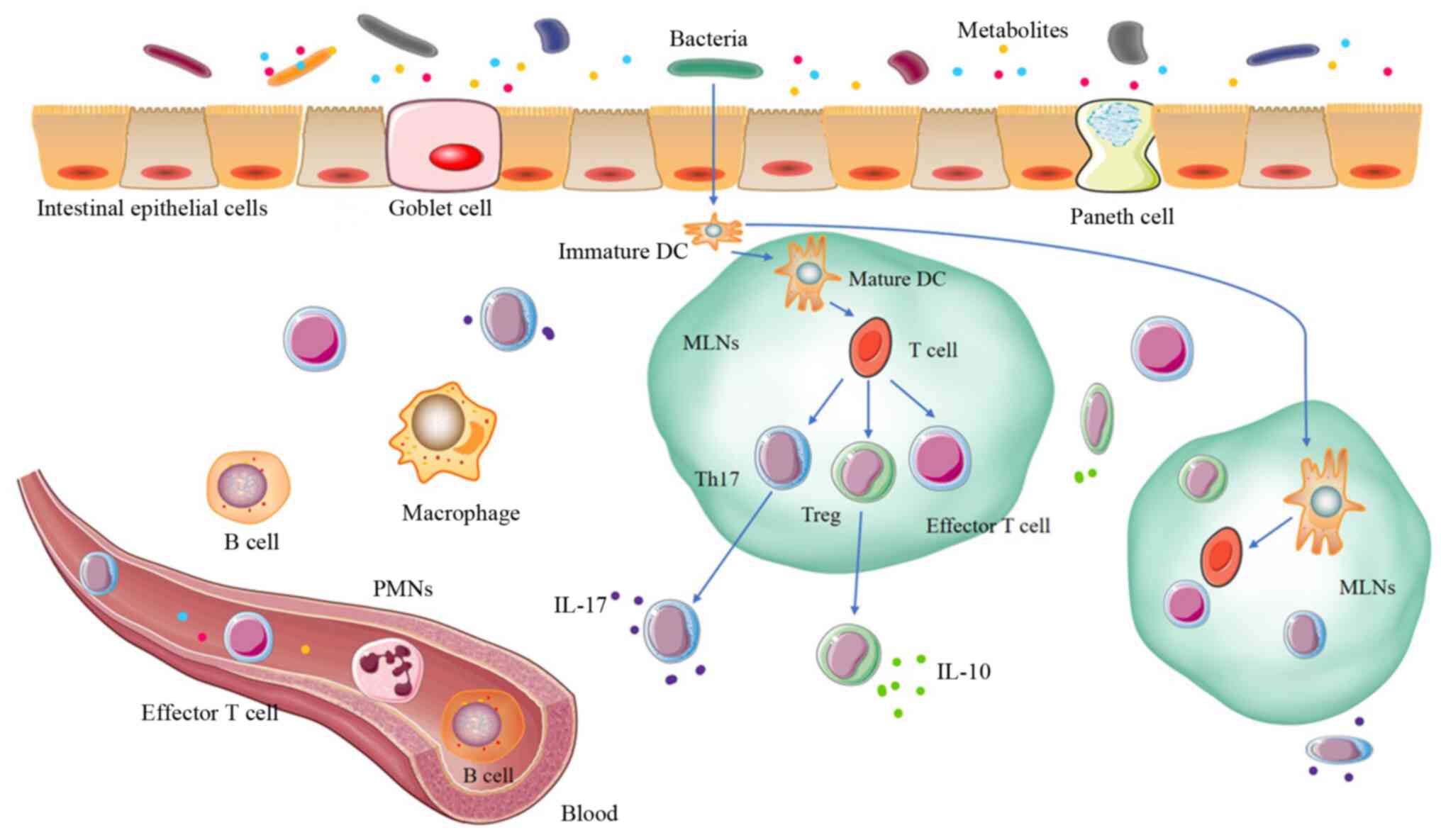 | Figure 1.Gut microbiota and immunity. The
intestinal mucosa is a single epithelial cell layer, composed of
IECs and intraepithelial lymphocytes; the goblet and pane cells are
located among the IECs. The bacteria and bacterial metabolites can
activate DCs to transfer to MLNs. Mature DCs activate T cells to
differentiate into effector T cells, Tregs or Th17 cells, which can
transfer them back to the intestinal mucosa and systemic
circulation. In the local immune response, Tregs secrete IL-10 and
create a local anti-inflammatory cytokine environment. Th17 cells
secrete IL-17 cytokines, and IL-17 can induce paneth cells to
produce antimicrobial peptides and can recruit PMNs from the
bloodstream. Certain bacterial metabolites can enter the blood
directly, further changing the immune system. B cells and T cells
can enter the systemic circulation to promote the immune response
to the same antigen at a distance (11,79).
IECs, intestinal epithelial cells; DCs, dendritic cells; MLNs,
mesenteric lymph nodes; PMNs, polymorphonuclear leukocytes; IL,
interleukin; Treg, regulatory T cells; Th17, T-helper cell 17. |
The gut microbiota and systemic immunity are also
closely associated through several mechanisms. To begin with, small
molecular substances produced by the gut microbiota may enter the
blood circulation and thereby affect immune responses in distant
organs. Furthermore, the gut microbiota shares a mucosal network
and therefore a common mucosal immune system with mucosal tissues
throughout the body. Additionally, extra-intestinal diseases may be
caused by changes in the immune response induced by signals
produced by the gut microbiota and recognized by TLRs on host
immune cells. Furthermore, the gut microbiota regulates the
development of systemic immune cells (6). Therefore, a gut microbiota that
contains a higher proportion of beneficial bacteria is associated
with a more completely developed immune system that is better
adapted to the external environment (6). In summary, the gut microbial community
has profound effects on the local and systemic immune systems.
Furthermore, the immune system may also effect change in the gut
microbiome (11).
Gut microbiota and immunotherapy
Antitumor mechanism of ICIs
Immunotherapy targets regulatory T-cell pathways and
thus enhances the anticancer immune response (17). To date, applications of this novel
cancer therapeutic modality have been proven effective in a range
of clinical contexts. Certain patients can obtain long-lasting
clinical effects from immunotherapy and may even achieve good
long-term outcomes in the absence of a cancer burden. Furthermore,
immunotherapy has afforded researchers an improved understanding of
the human immune response in the cancer microenvironment (18).
Host recognition and killing of cancer cells relies
on T-cell-mediated cellular immunity. T-cells bind via their T-cell
receptors (TCRs) to specific antigens associated with major
histocompatibility complex (MHC) molecules expressed on the
surfaces of cancer cells. These interactions of TCRs with MHC
molecules are controlled by a series of immune checkpoints,
co-stimulatory or co-suppressive signals that lead to the
activation or suppression of T-cells. CTLA-4, PD-1 and PD-L1 are
synergistic inhibitory molecules that suppress immune responses and
thus prevent the pathological targeting of self-antigens (i.e.,
autoimmune disease). The PD-1/PD-L1 axis serves an important role
in immune tolerance via the transmission of co-suppressive signals
that may suppress the immune activity of T-cells and enable cancer
cells to escape host immunity (18).
To date, monoclonal antibodies targeting the checkpoints CTLA-4 and
the PD-1/PD-L1 axis have yielded significant successes in the field
of clinical immunotherapy.
Gut microbiota and CTLA-4
The effects of the gut microbiota on the efficacy
and toxicity of anti-CTLA-4 therapy have been previously
investigated (Table I). In a study
on patients with metastatic melanoma who were treated with a
CTLA-4-targeting antibody, those whose gut microbiota profiles were
rich in species of the Enterobacteriaceae family, including
Enterobacter faecalis, and other Firmicutes spp., achieved
longer progression-free survival (PFS) and overall survival (OS)
times. However, similar outcomes were not observed in patients with
a microbiota rich in Bacteroides spp. (19). Another study demonstrated that
germ-free (GF) or antibiotic-treated mice do not respond as well to
CTLA-4-inhibiting antibodies as do specific pathogen-free (SPF)
mice (20). An analysis of bacterial
isolates revealed that the presence of the species B.
polymorpha, B. fragilis and Burkholderia cepacia was
closely associated with the efficacy of CTLA-4-targeting therapy
and associated with fewer adverse treatment effects (21). Another study demonstrated that oral
supplementation with Bacteroides spp. could restore the
efficacy of immunotherapy by increasing the number of mature
dendritic cells in a tumor and enhancing the Th1 response in the
draining lymph nodes. Taken together, these findings indicated that
the effects and toxicities associated with anti-CTLA-4 treatment
may be influenced by the profile and concentration of intestinal
bacteria (21,22).
 | Table I.Summary of featured microorganisms in
cancer immunotherapy studies. |
Table I.
Summary of featured microorganisms in
cancer immunotherapy studies.
| Immunotherapy | Tumor | Model | Bacteria | Main findings | (Refs.) |
|---|
| CTLA-4 mAb | Metastatic
melanoma | Human |
Faecalibacterium, other
Firmicutes | Enriched with
Faecalibacterium and other Firmicutes is associated
with ICI responders | (19) |
| CTLA-4 mAb | Melanoma | Human, mouse | Bacteroides
thetaiotaomicron, B. fragilis | Increased level of
Bacteroides thetaiotaomicron or B. fragilis was
associated with the efficacy of CTLA-4 blockade | (20) |
| PD-1 mAb | Metastatic
melanoma | Human | Bifidobacterium
longum, Collinsella aerofaciens, Enterococcus faecium | Bacterial species
more abundant in responders included Bifidobacterium longum,
Collinsella aerofaciens and Enterococcus faecium | (23) |
| CTLA-4 mAb, PD-1
mAb | Metastatic
melanoma | Human | Bacteroides
caccae | ICI responders were
enriched for Bacteroides caccae | (26) |
| PD-L1 mAb | Melanoma | Mouse |
Bifidobacterium | Combination of
Bifidobacterium and PD-L1 abolished tumor outgrowth | (28) |
| PD-1 mAb | Melanoma | Human |
Ruminococcaceae | Increased the level
of Ruminococcaceae family in responding patients | (29) |
| PD-1 mAb | Melanoma | Mouse | Akkermansia
muciniphila | Relative abundance
of Akkermansia muciniphila in ICI clinical responders | (40) |
| PD-1 mAb | NSCLC, GC | Human |
Ruminococcaceae | Higher alpha
diversity and Ruminococcaceae levels in ICI responders | (80) |
| PD-1 mAb | RCC | Human | Roseburia spp,
Faecalibacterium spp | Increased the level
of Roseburia and Faecalibacterium spp in ICI
responders | (27) |
Notably, antibiotic treatment was demonstrated to
decrease the effectiveness of anti-IL-10/CpG oligonucleotide
immunotherapy in mouse models of MC38 cell line-induced colon
cancer and subcutaneous B16 cell line-induced melanoma. It is
possible that this antibiotic therapy decreased the gut microbiota
load and the population of monocytes that produce pro-inflammatory
cytokines (23,24). The results of a recent study also
suggested that patients who used antibiotics 30 days before
immunotherapy had a significantly shorter PFS time than those who
did not receive antibiotic therapy (25). Regarding toxicity, studies of the gut
microbiota in patients receiving anti-CTLA-4 treatment revealed an
increased abundance of Faecalibacterium spp. and decreased
abundance of Bacteroides spp., as well as an increased risk
of colitis (11,21,26).
Gut microbiota and PD-1/PD-L1
Previous studies have also investigated the effects
of gut microbiota on the therapeutic effects and toxicities
associated with treatments targeting the PD-1/PD-L1 axis (Table I) (19,27).
Sivan et al (28) observed
that different responses to anticancer immunotherapy in a mouse
model of melanoma were associated with differences in commensal gut
microbiota profiles. Furthermore, these differences disappeared
following cohabitation or fecal transplantation between groups
(23). A 16S rRNA sequence analysis
revealed that Bifidobacterium spp. may enhance the
effectiveness of anticancer immunotherapy. Specifically, an orally
administered Bifidobacterium supplement was shown to improve
the level of cancer control afforded by PD-L1-specific antibody
treatment when compared with the probiotic-free antibody treatment.
Furthermore, combined treatment with the PD-L1-targeted therapy and
Bifidobacterium nearly eliminated tumor growth (23). Mechanistically, this combination
therapy appears to promote the functions of dendritic cells, which
leads to an increase and accumulation of CD8+ T-cells in
the cancer microenvironment and an enhanced anticancer effect
(23).
In another study, the response to anti-PD-L1 therapy
was shown to depend on the composition of the gut microbiota, with
Bifidobacterium spp. found to be highly associated with an
effective anti-PD-L1 response (28).
In mouse experiments, supplementation with Bifidobacterium
spp. restored the efficacy of PD-L1-targeted immunotherapy by
promoting an increase in the CD8+/IFN-γ+
T-cell population within tumors (23). Additional studies have demonstrated
that microbiota composition is predictive of the response statuses
of patients receiving anti-PD-1/PD-L1 therapies for solid
epithelial cancers (29–31).
Gopalakrishnan et al (29) reported that Faecalibacterium
were abundant in the intestinal microbiota of patients with
melanoma who responded to anti-PD-L1 treatment (29), while Bacteroides thetaiotaomicron,
Escherichia coli and Anaerotruncus colihominis were
abundant in patients who achieved poor treatment effects. Notably,
transplantation of the fecal microbiota from a human patient who
had responded well to anti-PD-L1 treatment into GF mice improved
the efficacy of this immunotherapy in mice. The efficacy of this
anticancer immunotherapy may have bene positively associated with
the numbers of mature dendritic cells (DCs) and IFN-γ+,
CD8+ and/or CD4+ anticancer T-cells in a
tumor, and negatively associated with the number of
CD4+, FoxP3+ Treg cells in a tumor (29,30).
Notably, a study on the gut microbiota of 25
patients with melanoma who had received anti-PD-1 therapy revealed
significant differences in the diversity and composition of
patients' microbiota, with the stool of patients who responded well
to therapy containing large concentrations of Bacilli
(29). However, although these
strains were positively correlated with PFS, they were also
associated with an increased ultimate risk of cancer recurrence and
growth (29). Other studies have
analyzed baseline stool samples collected from immunotherapy-naïve
patients with metastatic melanoma and identified a significant
association between patient gut microbiota and their clinical
response to immunotherapy (23). In
particular, Bifidobacterium longum, Collinsella aerogenes
and Enterococcus faecium were more abundant in the gut
microbiota of patients who responded well to treatment (23). Regarding toxicity, previous studies
have reported that antibodies targeting PD-1 and PD-L1 may cause
thyroid dysfunction and pneumonia (32,33).
In summary, it is evident that there is an
association between patient gut microbiota and their response to
anticancer immunotherapy. Specifically, the abundance and range of
organisms in the gut microbiota may aid or hinder cancer
immunotherapies, in terms of efficacy and side effects. These
observations strongly support the integration of microbial
therapies into anticancer immunotherapy strategies with the aim of
improving the efficacy of immunotherapy while decreasing toxicity.
Therefore, the regulation of the gut microbiota to ensure more
effective anticancer immunotherapy within a wider therapeutic
window (i.e., with fewer toxic effects) has become a promising
research direction.
Improving immunotherapy by regulating the
gut microbiota
As demonstrated earlier, the gut microbiota
intimately affects the outcomes of cancer immunotherapy.
Consequently, it has been demonstrated that regulation of the gut
microbiota may improve the efficacy and decrease the adverse
effects of cancer immunotherapy (34–36).
Despite this, associated improvements in cancer treatment outcomes
and prognoses remain very limited (37). The methods used to regulate the gut
microbiota and potentially optimize anticancer immunity are
therefore discussed in the following sections.
Fecal microbiota transplantation
(FMT)
FMT is defined as the transplantation of the gut
microbiota from a healthy donor, in the form of diluted fecal
material, into a patient via the upper or lower digestive tract
with the intent to restore intestinal microbial diversity (38,39). FMT
may regulate the effects of anticancer immunotherapy by rebuilding
the gut microbiota and improving bile acid metabolism (12). Studies of FMT in a mouse model of
MCA-205 sarcoma revealed that mice treated with anti-PD-1 therapy
and an effective FMT from patients who responded well to PD-1
therapy exhibited significantly delayed cancer growth, while no
delay in tumor growth was observed in mice that received FMT from
patients who did not respond to PD-1 treatment (40). Wang et al (41) reported the first case of successful
treatment of ICI-associated colitis with FMT (41), using a method that reconstructed the
gut microbiota and led to a relative increase in the proportion of
Treg cells in the intestinal mucosa (6). Although cancer in GF or
antibiotic-treated mice responds poorly to ICI therapy, mice dosed
with FMT from patients who have responded successfully to
immunotherapy responded more positively to the same ICI therapies.
Analysis of these gut microbiota in FMT revealed large abundances
of the genera Bacteroides (42), Burkholderia (42), Akkermansia (43), Faecalis (44) and Clostridium (45). FMT has been used widely and very
successfully to treat refractory Clostridium infections
(46), which is encouraging with
respect to the treatment of other diseases.
There are three important points to note prior to
administering FMT. To begin with, bacterial species must be
accurately isolated and screened to ensure that they may improve
the efficacy of anticancer immunotherapy in the host. Furthermore,
harmful bacteria, viruses and parasites must be removed.
Additionally, attention should be paid to the isolation and
cultivation of less abundant but important microorganisms (11).
The clinical value of FMT has been demonstrated in
certain studies, but data specific to the field of cancer
immunotherapy remain limited and are primarily derived from animal
models (38,47). The fecal bacterial composition and
pathogenicity are unknown, and the safety of FMT remains
controversial. Certain studies have reported certain minor adverse
events, including low-grade fever, constipation and diarrhea
following FMT. Serious side effects are relatively rare, including
infection and/or sepsis, pneumonia and complications of endoscopy
(38,47,48).
Furthermore, FMT is an emerging treatment without a long history of
use, and therefore long-term safety surveys are lacking (12). Further investigation is required in
this area, and several clinical trials are underway (Table II) (49).
 | Table II.Selected registered immunotherapy
studies evaluating therapeutic roles of gut microbiota. |
Table II.
Selected registered immunotherapy
studies evaluating therapeutic roles of gut microbiota.
| Identifier | Study title | Status | Phase | Condition or
disease |
Intervention/treatment |
|---|
| NCT03341143 | Fecal Microbiota
Transplant (FMT) in Melanoma Patients | Recruiting | Phase 2 | Melanoma | Drug: FMT with
Pembrolizumab |
| NCT04130763 | Fecal Microbiota
Transplant (FMT) Capsule for Improving the Efficacy of Anti-
PD-1 | Recruiting | Phase 1 | Gastrointestinal
cancer | Biological: FMT
capsule |
| NCT04116775 | Fecal Microbiota
Transplant and Pembrolizumab for Men with Metastatic
Castration-Resistant Prostate Cancer. | Recruiting | Phase 2 | Prostate cancer;
metastatic prostate cancer | Biological: FMT;
Drug: Pembrolizumab; Drug: Enzalutamide |
| NCT03353402 | Fecal Microbiota
Transplantation (FMT) in Metastatic Melanoma Patients Who Failed
Immunotherapy | Recruiting | Phase 1 | Melanoma stage IV;
unresectable stage III melanoma | Procedure: FMT |
| NCT03772899 | Fecal Microbial
Transplantation in Combination with Immunotherapy in Melanoma
Patients (MIMic) | Recruiting | Phase 1 | Melanoma | Drug: FMT |
Antibiotics
It has been demonstrated that the use of antibiotics
may reduce the benefits of anticancer immunotherapy (50,51). For
example, Elkrief et al (25)
studied patients with advanced melanoma who were treated with
anti-PD-1 or anti-CTLA-4 monoclonal antibody therapies alone or in
combination with chemotherapy and had or had not received
antibiotics within 30 days after the start of immunotherapy.
Notably, the authors observed that antibiotic treatment adversely
affected the patient prognoses (25).
In another study it was determined that the use of
antibiotics, and particularly broad-spectrum antibiotics, affected
the prognosis of patients treated with ICIs (52). Specifically, Ahmed et al
(52) investigated whether the use
of antibiotics during immunotherapy would alter the efficacy of the
latter treatment in a sample of 60 patients with advanced cancer,
including 17 who had received antibiotics for a microbial infection
within 2 weeks before or after the start of immunotherapy; the
results demonstrated that immunotherapy was less effective in
patients who had received systemic antibiotics than in those who
had not received antibiotics, and reduced immunotherapeutic
efficacy and shorter OS duration were observed in patients who used
broad-spectrum antibiotics (those effective against both
Gram-positive and negative bacteria, including aerobic and
anaerobic bacteria) compared with those who used narrow-spectrum
antibiotics (those effective only against Gram-positive bacteria)
(52).
Other researchers retrospectively analyzed 90
patients with non-small-cell lung cancer who were treated with
nivolumab, 13 of whom had also received antibiotic therapy;
although the researchers observed a negative effect of antibiotic
use on the outcome of immunotherapy, they did not find a
statistically significant correlation between survival and
antibiotic use (53). They suggested
that the interval between antibiotic treatment and nivolumab
treatment initiation may play an important role in the
microbiota-influenced response to treatment, as the composition of
a patient's gut microbiota changes dramatically after antibiotic
therapy is stopped (54).
Antibiotic use within 2–3 months before or after the
start of immunotherapy was also reported to be associated with
reduced PFS duration and OS, which may be related to the loss of
homeostasis in the gut microbiota (49). Three factors can explain the gut
microbiota changes caused by antibiotics: The loss of
microorganisms, the direct effects of antibiotics on host tissues,
and the effects of antibiotics on the remaining resistant
microorganisms. Normal microbial depletion leads to the suppression
of all aspects of immunity, whereas the direct effects of
antibiotics on the host tissues and the actions of
antibiotic-resistant microorganisms inhibit mitochondrial gene
expression and reduce the number of active mitochondria, leading to
epithelial cell death (55). These
actions of antibiotics may reduce the effectiveness of anti-cancer
immunotherapy, and, together with the observations detailed above,
highlight that antibiotic therapy should be minimized before and
during cancer immunotherapy.
Probiotics, prebiotics and
symbiotics
Probiotics are active microorganisms that may
maintain health by improving or restoring the intestinal flora
(56). Certain combinations of
probiotics may enhance the immune responses of patients by changing
the gut microbiota (57). It has
been demonstrated that significantly higher proportions of patients
treated with probiotics had normal ratios of health-related blood
biomarkers, namely CD3+, CD4+ and
CD8+ T-cells and total lymphocytes and normal hemoglobin
concentrations compared with the control group (57). Supplementation with Akkermansia
muciniphila increased the efficacy of anti-PD-1 immunotherapy
in antibiotic-treated mice (40). In
a mouse model of melanoma, supplementation with
Bifidobacteria improved the response to anti-PD-L1 treatment
and almost eliminated cancer growth (28,58).
Certain probiotic strains that may be associated with the efficacy
of ICI therapy have been tested in GF or SPF animal models of
cancer (31). At present, the
ability of probiotics to regulate the intestinal microbiota and
thereby improve the efficacy of ICI therapies are being
investigated in clinical trials. One such trial is aiming to
investigate the efficacy of the probiotic strain Clostridium
butyricum CBM588 when combined with nivolumab and ipilimumab
for the treatment of kidney cancer (trial no. NCT 03829111).
Prebiotics are defined as inactive food ingredients
that selectively promote the growth and activity of one or several
microorganisms in the colon, and are thus beneficial to the health
of the host (59,60). Prebiotics mainly comprise dietary
fiber, and the short-chain fatty acids produced by the metabolism
of these ingredients may decrease intestinal pH and maintain the
growth of beneficial bacteria, including lactic acid bacteria and
bifidobacteria, in the gut (61).
Resistant starch produced by prebiotics promotes the growth of
strains that produce butyric acid, which has anticancer and
anti-inflammatory activities (62).
These effects may be the mechanism by which prebiotics regulate the
outcomes of immunotherapy (58).
Symbiotics are combinations of prebiotics and
specific probiotic bacteria (11)
that have synergistic effects and may potentially improve the
efficacy of immunotherapy. To date, the application of symbiotics
in anticancer immunotherapy has been less thoroughly studied.
However, these approaches combine the advantages of predominant
probiotic bacteria and prebiotics and therefore may be
promising.
In summary, probiotics, prebiotics and symbiotics
may improve the outcomes of cancer immunotherapy, and they require
further investigation.
Lifestyle
The lifestyle of the whole human organism, including
exercise habits, dietary intake and sleep patterns, has significant
effects on the gut microbiota, and the resulting changes may
influence the efficacy of cancer immunotherapy. For example, it has
been demonstrated in exercise oncology studies that exercise may
regulate the cancer microenvironment and enhance the response to
cancer immunotherapies (63). Clarke
et al (64) studied the
effects of exercise on the gut microbiota of athletes and found
that highly active rugby players had significantly more diverse gut
microbiota and lower levels of inflammatory and metabolic
biomarkers, compared with the control group. It has been
demonstrated in other studies that lactic acid may regulate the
expression of PD-L1 in cancer cells (65), while the decrease in lactic acid
concentrations in cancer may increase the number of
tumor-infiltrating immune cells (66). Exercise may decrease lactic acid
concentrations and may thus contribute toward the efficacy of
cancer immunotherapy.
It was determined in dietary studies that the
profiles of the gut microbiota of research subjects who consumed
high-fat diets were significantly different to those of research
subjects who consumed fiber-rich diets (67). The diet serves an important role in
shaping the composition and activity of the complex gut microbiota
(68) and may therefore contribute
toward the efficacy of immunotherapy. Regarding sleep, a late
bedtime may disrupt the balance of the gut microbiota and regulate
the host's metabolism (69). Studies
have identified a potentially close association between sleep
quality and the gut microbiome composition (70). Furthermore, mouse experiments have
confirmed that chronic sleep disruption alters the gut microbiota
composition (71). In summary,
lifestyle factors, including exercise, diet and sleep may regulate
the gut microbiota and may impact the efficacy of cancer
immunotherapy.
Traditional chinese medicine
A number of previous studies have demonstrated the
effects of components of Traditional Chinese Medicine on the gut
microbiota. For example, a combination of the Chinese herbal
compound Gegen Qinlian decoction (GQD) with anti-PD-1 immunotherapy
may be a novel strategy for the treatment of microsatellite stable
(MSS) colorectal cancer (CRC). In a mouse xenograft tumor model,
combined treatment with GQD and an anti-mouse PD-1 antibody
significantly inhibited the growth of CT26 tumors. An analysis of
the gut microbiota in these mice revealed that the combination
therapy led to the significant enrichment of Bacteroides
acidophilus and members of the S24-7 family (72). Shaoyao Ruangan mixture was
demonstrated to increase the abundance of Bacteroides spp.
and effectively inhibit the progression of primary liver cancer
(73).
In terms of small molecules, it has been
demonstrated that curcumin may increase the abundance of bacteria,
particularly Lactobacillus spp (74). Furthermore, ginsenosides may restore
the structure of the intestinal mucosa and improve immunity in this
tissue, thereby promoting the growth of beneficial bacteria and
decreasing the growth of bacteria that results from
cancer-associated cachexia (75).
Berberine may increase the relative abundance of the thick-walled
Mycoplasma spp., decrease the relative abundance of
proteobacteria and decrease ileal inflammatory responses and
intestinal mucosal damage (76).
In summary, Traditional Chinese Medicinal mixtures
and small molecules may regulate the gut microbiota and thus affect
the outcomes of immunotherapy. The mechanisms by which Traditional
Chinese Medicines regulate the gut microbiota in this context
remain unclear, its prospective applications are broad and require
further investigation.
Conclusions
There is increasing evidence of an association
between the gut microbiota and the outcomes of cancer
immunotherapy. In addition, it has been demonstrated that the
efficacy of cancer immunotherapy may be optimized by adjusting the
gut microbiota. Broadly, the evidence has suggested that methods
and tools should be developed to enable adjustment of the
composition and concentration of intestinal microbiota and thereby
improve the prognosis of cancer immunotherapy.
Numerous methods are available for regulating the
gut microbiota, but their mechanisms of action are unclear, the
clinical sample sizes are small and risk-assessment data are
lacking. Therefore, these methods are not widely used, and further
investigation is required to ensure their uptake.
The gut microbiota is a huge and complex system that
is influenced by numerous factors, and is associated with host
immunity. It has been reported that gut microbiota and host
immunity are associated via diet, intestinal absorption and
enterohepatic circulation (77,78).
Therefore, it may be a promising research direction to determine
the association between gut microbiota and immunotherapy. At the
same time, the use of recently introduced scientific and
technological methods, including next-generation sequencing,
big-data analysis and artificial intelligence, will enable further
investigation into the relationship between the gut microbiota and
immunotherapy. In particular, the identification of specific gut
microbes that yield clear benefits during immunotherapy and the
determination of the composition and proportion of the gut
microbiota will facilitate screening to determine individual
patients' suitability for immunotherapy. The use of specific and
effective methods to regulate the gut microbiota, and the careful
and comprehensive observation of its role in immunotherapy
regulation, are required to develop consensus-based guidelines for
clinical application of this promising adjunctive cancer
treatment.
Acknowledgements
Not applicable.
Funding
The present study was supported by Teaching Method
Reform Research Project of Southwest Medical University (grant no.
JG201914) and Health Commission of Luzhou City Program (grant no.
2018-36).
Availability of data and materials
Not applicable.
Authors' contributions
JW and XXW wrote and edited the manuscript. HRY and
DJW contributed toward the conception and design of the study and
critically revised the manuscript. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CTLA-4
|
cytotoxic T-lymphocyte antigen-4
|
|
FMT
|
fecal microbiota transplantation
|
|
GQD
|
Gegen Qinlian decoction
|
|
ICIs
|
immune checkpoint inhibitors
|
|
NSCLC
|
non-small-cell lung cancer
|
|
OS
|
overall survival
|
|
PD-1
|
programmed death protein 1
|
|
PD-L1
|
programmed death ligand 1
|
|
PFS
|
progression-free survival
|
|
SPF
|
specific pathogen-free
|
|
TCRs
|
T-cell receptors
|
|
TLR
|
toll-like receptor
|
References
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Robert C, Thomas L, Bondarenko I, O'Day S,
Weber J, Garbe C, Lebbe C, Baurain JF, Testori A, Grob JJ, et al:
Ipilimumab plus dacarbazine for previously untreated metastatic
melanoma. N Engl J Med. 364:2517–2526. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Borghaei H, Paz-Ares L, Horn L, Spigel DR,
Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al:
Nivolumab versus docetaxel in advanced nonsquamous non-small-cell
lung cancer. N Engl J Med. 373:1627–1639. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ribas A, Hamid O, Daud A, Hodi FS, Wolchok
JD, Kefford R, Joshua AM, Patnaik A, Hwu WJ, Weber JS, et al:
Association of pembrolizumab with tumor response and survival among
patients with advanced melanoma. JAMA. 315:1600–1609. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee L, Gupta M and Sahasranaman S: Immune
Checkpoint inhibitors: An introduction to the next-generation
cancer immunotherapy. J Clin Pharmacol. 56:157–169. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shui L, Yang X, Li J, Yi C, Sun Q and Zhu
H: Gut microbiome as a potential factor for modulating resistance
to cancer immunotherapy. Front Immunol. 10:29892020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pardoll D: Cancer and the immune system:
Basic concepts and targets for intervention. Semin Oncol.
42:523–538. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gagliani N, Hu B, Huber S, Elinav E and
Flavell RA: The fire within: Microbes inflame tumors. Cell.
157:776–783. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pleguezuelos-Manzano C, Puschhof J,
Rosendahl Huber A, van Hoeck A, Wood HM, Nomburg J, Gurjao C,
Manders F, Dalmasso G, Stege PB, et al: Mutational signature in
colorectal cancer caused by genotoxic pks+ E.
coli. Nature. 580:269–273. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yu LX and Schwabe RF: The gut microbiome
and liver cancer: Mechanisms and clinical translation. Nat Rev
Gastroenterol Hepatol. 14:527–539. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li W, Deng Y, Chu Q and Zhang P: Gut
microbiome and cancer immunotherapy. Cancer Lett. 447:41–47. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen D, Wu J, Jin D, Wang B and Cao H:
Fecal microbiota transplantation in cancer management: Current
status and perspectives. Int J Cancer. 145:2021–2031. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pickard JM, Zeng MY, Caruso R and Núñez G:
Gut microbiota: Role in pathogen colonization, immune responses,
and inflammatory disease. Immunol Rev. 279:70–89. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Round JL and Mazmanian SK: Inducible
Foxp3+ regulatory T-cell development by a commensal
bacterium of the intestinal microbiota. Proc Natl Acad Sci USA.
107:12204–12209. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cebula A, Seweryn M, Rempala GA, Pabla SS,
McIndoe RA, Denning TL, Bry L, Kraj P, Kisielow P and Ignatowicz L:
Thymus-derived regulatory T cells contribute to tolerance to
commensal microbiota. Nature. 497:258–262. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yi M, Jiao D, Qin S, Chu Q, Li A and Wu K:
Manipulating gut microbiota composition to enhance the therapeutic
effect of cancer immunotherapy. Integr Cancer Ther.
18:15347354198763512019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang HY, Lee DA, Peng G, Guo Z, Li Y,
Kiniwa Y, Shevach EM and Wang RF: Tumor-specific human
CD4+ regulatory T cells and their ligands: Implications
for immunotherapy. Immunity. 20:107–118. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sharma P and Allison JP: The future of
immune checkpoint therapy. Science. 348:56–61. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Picardo SL, Coburn B and Hansen AR: The
microbiome and cancer for clinicians. Crit Rev Oncol Hematol.
141:1–12. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vétizou M, Pitt JM, Daillère R, Lepage P,
Waldschmitt N, Flament C, Rusakiewicz S, Routy B, Roberti MP, Duong
CP, et al: Anticancer immunotherapy by CTLA-4 blockade relies on
the gut microbiota. Science. 350:1079–1084. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chaput N, Lepage P, Coutzac C, Soularue E,
Le Roux K, Monot C, Boselli L, Routier E, Cassard L, Collins M, et
al: Baseline gut microbiota predicts clinical response and colitis
in metastatic melanoma patients treated with ipilimumab. Ann Oncol.
28:1368–1379. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dubin K, Callahan MK, Ren B, Khanin R,
Viale A, Ling L, No D, Gobourne A, Littmann E, Huttenhower C, et
al: Intestinal microbiome analyses identify melanoma patients at
risk for checkpoint-blockade-induced colitis. Nat Commun.
7:103912016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Matson V, Fessler J, Bao R, Chongsuwat T,
Zha Y, Alegre ML, Luke JJ and Gajewski TF: The commensal microbiome
is associated with anti-PD-1 efficacy in metastatic melanoma
patients. Science. 359:104–108. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Iida N, Dzutsev A, Stewart CA, Smith L,
Bouladoux N, Weingarten RA, Molina DA, Salcedo R, Back T, Cramer S,
et al: Commensal bacteria control cancer response to therapy by
modulating the tumor microenvironment. Science. 342:967–970. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Elkrief A, El Raichani L, Richard C,
Messaoudene M, Belkaid W, Malo J, Belanger K, Miller W, Jamal R,
Letarte N, et al: Antibiotics are associated with decreased
progression-free survival of advanced melanoma patients treated
with immune checkpoint inhibitors. Oncoimmunology. 8:e15688122019.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Frankel AE, Coughlin LA, Kim J, Froehlich
TW, Xie Y, Frenkel EP and Koh AY: Metagenomic shotgun sequencing
and unbiased metabolomic profiling identify specific human gut
microbiota and metabolites associated with immune checkpoint
therapy efficacy in melanoma patients. Neoplasia. 19:848–855. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Maia MC, Poroyko V, Won H, Almeida L,
Bergerot PG, Dizman N, Hsu J, Jones J, Salgia R and Pal SK:
Association of microbiome and plasma cytokine dynamics to nivolumab
response in metastatic renal cell carcinoma (mRCC). J Clin Oncol.
36 (6_suppl):S6562018. View Article : Google Scholar
|
|
28
|
Sivan A, Corrales L, Hubert N, Williams
JB, Aquino-Michaels K, Earley ZM, Benyamin FW, Lei YM, Jabri B,
Alegre M, et al: Commensal Bifidobacterium promotes antitumor
immunity and facilitates anti-PD-L1 efficacy. Science.
350:1084–1089. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gopalakrishnan V, Spencer CN, Nezi L,
Reuben A, Andrews MC, Karpinets TV, Prieto PA, Vicente D, Hoffman
K, Wei SC, et al: Gut microbiome modulates response to anti-PD-1
immunotherapy in melanoma patients. Science. 359:97–103. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tanoue T, Morita S, Plichta DR, Skelly AN,
Suda W, Sugiura Y, Narushima S, Vlamakis H, Motoo I, Sugita K, et
al: A defined commensal consortium elicits CD8 T cells and
anti-cancer immunity. Nature. 565:600–605. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Frankel AE, Deshmukh S, Reddy A, Lightcap
J, Hayes M, McClellan S, Singh S, Rabideau B, Glover TG, Roberts B
and Koh AY: Cancer immune checkpoint inhibitor therapy and the Gut
microbiota. Integr Cancer Ther. 18:15347354198463792019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Roy S and Trinchieri G: Microbiota: A key
orchestrator of cancer therapy. Nat Rev Cancer. 17:271–285. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Teply BA and Lipson EJ: Identification and
management of toxicities from immune checkpoint-blocking drugs.
Oncology (Williston Park). 28 (Suppl 3):S30–S38. 2014.
|
|
34
|
Bashiardes S, Tuganbaev T, Federici S and
Elinav E: The microbiome in anti-cancer therapy. Semin Immunol.
32:74–81. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Helmink BA, Khan MAW, Hermann A,
Gopalakrishnan V and Wargo JA: The microbiome, cancer, and cancer
therapy. Nat Med. 25:377–388. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Vivarelli S, Salemi R, Candido S, Falzone
L, Santagati M, Stefani S, Torino F, Banna GL, Tonini G and Libra
M: Gut microbiota and cancer: From pathogenesis to therapy. Cancers
(Basel). 11:382019. View Article : Google Scholar
|
|
37
|
Nagano T, Otoshi T, Hazama D, Kiriu T,
Umezawa K, Katsurada N and Nishimura Y: Novel cancer therapy
targeting microbiome. Onco Targets Ther. 12:3619–3624. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Van Nood E, Vrieze A, Nieuwdorp M, Fuentes
S, Zoetendal EG, de Vos WM, Visser CE, Kuijper EJ, Bartelsman JF,
Tijssen JG, et al: Duodenal infusion of donor feces for recurrent
Clostridium difficile. N Engl J Med. 368:407–415. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bakken JS, Borody T, Brandt LJ, Brill JV,
Demarco DC, Franzos MA, Kelly C, Khoruts A, Louie T, Martinelli LP,
et al: Treating Clostridium difficile infection with fecal
microbiota transplantation. Clin Gastroenterol Hepatol.
9:1044–1049. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Routy B, Le Chatelier E, Derosa L, Duong
CPM, Alou MT, Daillère R, Fluckiger A, Messaoudene M, Rauber C and
Roberti MP: Gut microbiome influences efficacy of PD-1-based
immunotherapy against epithelial tumors. Science. 359:91–97. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang Y, Wiesnoski DH, Helmink BA,
Gopalakrishnan V, Choi K, DuPont HL, Jiang ZD, Abu-Sbeih H, Sanchez
CA and Chang CC: Fecal microbiota transplantation for refractory
immune checkpoint inhibitor-associated colitis. Nat Med.
24:1804–1808. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hekmatshoar Y, Rahbar Saadat Y,
Hosseiniyan Khatibi SM, Ozkan T, Zununi Vahed F, Nariman-Saleh-Fam
Z, Pourghassem Gargari B, Sunguroglu A and Zununi Vahed S: The
impact of tumor and gut microbiotas on cancer therapy: Beneficial
or detrimental? Life Sci. 233:1166802019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Olivas AD, Shogan BD, Valuckaite V,
Zaborin A, Belogortseva N, Much M, Meyer F, Trimble WL, An G,
Gilbert J, et al: Intestinal tissues induce an SNP mutation in
Pseudomonas aeruginosa that enhances its virulence: Possible role
in anastomotic leak. PLoS One. 7:e443262012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ashraf SQ, Burns EM, Jani A, Altman S,
Young JD, Cunningham C, Faiz O and Mortensen NJ: The economic
impact of anastomotic leakage after anterior resections in E nglish
NHS hospitals: Are we adequately remunerating them? Colorectal Dis.
15:e190–e198. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bose M and Mukherjee P: Role of microbiome
in modulating immune responses in cancer. Mediators Inflamm.
2019:41079172019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gough E, Shaikh H and Manges AR:
Systematic review of intestinal microbiota transplantation (fecal
bacteriotherapy) for recurrent Clostridium difficile
infection. Clin Infect Dis. 53:994–1002. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang JW, Kuo CH, Kuo FC, Wang YK, Hsu WH,
Yu FJ, Hu HM, Hsu PI, Wang JY and Wu DC: Fecal microbiota
transplantation: Review and update. J Formos Med Assoc. 118 (Suppl
1):S23–S31. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Rossen NG, MacDonald JK, de Vries EM,
D'Haens GR, de Vos WM, Zoetendal EG and Ponsioen CY: Fecal
microbiota transplantation as novel therapy in gastroenterology: A
systematic review. World J Gastroenterol. 21:5359–5371. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Guven DC, Aktas BY, Simsek C and Aksoy S:
Gut microbiota and cancer immunotherapy: Prognostic and therapeutic
implications. Future Oncol. 16:497–506. 2020.PubMed/NCBI
|
|
50
|
Huemer F, Rinnerthaler G, Westphal T,
Hackl H, Hutarew G, Gampenrieder SP, Weiss L and Greil R: Impact of
antibiotic treatment on immune-checkpoint blockade efficacy in
advanced non-squamous non-small cell lung cancer. Oncotarget.
9:16512–16520. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Elkrief A, Derosa L, Kroemer G, Zitvogel L
and Routy B: The negative impact of antibiotics on outcomes in
cancer patients treated with immunotherapy: A new independent
prognostic factor? Ann Oncol. 30:1572–1579. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ahmed J, Kumar A, Parikh K, Anwar A, Knoll
BM, Puccio C, Chun H, Fanucchi M and Lim SH: Use of broad-spectrum
antibiotics impacts outcome in patients treated with immune
checkpoint inhibitors. Oncoimmunology. 7:e15076702018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Hakozaki T, Okuma Y, Omori M and Hosomi Y:
Impact of prior antibiotic use on the efficacy of nivolumab for
non-small cell lung cancer. Oncol Lett. 17:2946–2952.
2019.PubMed/NCBI
|
|
54
|
Derosa L, Routy B, Enot D, Baciarello G,
Massard C, Loriot Y, Fizazi K, Escudier BJ, Zitvogel L and Albiges
L: Impact of antibiotics on outcome in patients with metastatic
renal cell carcinoma treated with immune checkpoint inhibitors. J
Clin Oncol. 35 (6_suppl):S4622017. View Article : Google Scholar
|
|
55
|
Morgun A, Dzutsev A, Dong X, Greer RL,
Sexton DJ, Ravel J, Schuster M, Hsiao W, Matzinger P and Shulzhenko
N: Uncovering effects of antibiotics on the host and microbiota
using transkingdom gene networks. Gut. 64:1732–1743. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Doron S and Snydman DR: Risk and safety of
probiotics. Clin Infect Dis. 60 (Suppl 2):S129–S134. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Jiang C, Wang H, Xia C, Dong Q, Chen E,
Qiu Y, Su Y, Xie H, Zeng L, Kuang J, et al: A randomized,
double-blind, placebo-controlled trial of probiotics to reduce the
severity of oral mucositis induced by chemoradiotherapy for
patients with nasopharyngeal carcinoma. Cancer. 125:1081–1090.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Panebianco C, Andriulli A and Pazienza V:
Pharmaco microbiomics: Exploiting the drug-microbiota interactions
in anticancer therapies. Microbiome. 6:922018. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Pandey KR, Naik SR and Vakil BV:
Probiotics, prebiotics and synbiotics-a review. J Food Sci Technol.
52:7577–7587. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Gibson GR and Roberfroid MB: Dietary
modulation of the human colonic microbiota: Introducing the concept
of prebiotics. J Nutr. 125:1401–1412. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
McLoughlin RF, Berthon BS, Jensen ME,
Baines KJ and Wood LG: Short-chain fatty acids, prebiotics,
synbiotics, and systemic inflammation: A systematic review and
meta-analysis. Am J Clin Nutr. 106:930–945. 2017.PubMed/NCBI
|
|
62
|
Brouns F, Kettlitz B and Arrigoni E:
Resistant starch and ‘the butyrate revolution’. Trends Food Sci
Technol. 13:251–261. 2002. View Article : Google Scholar
|
|
63
|
Ashcraft KA, Warner AB, Jones LW and
Dewhirst MW: Exercise as adjunct therapy in cancer. Semin Radiat
Oncol. 29:16–24. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Clarke SF, Murphy EF, O'Sullivan O, Lucey
AJ, Humphreys M, Hogan A, Hayes P, O'Reilly M, Jeffery IB,
Wood-Martin R, et al: Exercise and associated dietary extremes
impact on gut microbial diversity. Gut. 63:1913–1920. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Feng J, Yang H, Zhang Y, Wei H, Zhu Z, Zhu
B, Yang M, Cao W, Wang L and Wu Z: Tumor cell-derived lactate
induces TAZ-dependent upregulation of PD-L1 through GPR81 in human
lung cancer cells. Oncogene. 36:5829–5839. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Seth P, Csizmadia E, Hedblom A, Vuerich M,
Xie H, Li M, Longhi MS and Wegiel B: Deletion of lactate
dehydrogenase-A in myeloid cells triggers antitumor immunity.
Cancer Res. 77:3632–3643. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Bibbò S, Ianiro G, Giorgio V, Scaldaferri
F, Masucci L, Gasbarrini A and Cammarota G: The role of diet on gut
microbiota composition. Eur Rev Med Pharmacol Sci. 20:4742–4749.
2016.PubMed/NCBI
|
|
68
|
Conlon MA and Bird AR: The impact of diet
and lifestyle on gut microbiota and human health. Nutrients.
7:17–44. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Wang Y, Kuang Z, Yu X, Ruhn KA, Kubo M and
Hooper LV: The intestinal microbiota regulates body composition
through NFIL3 and the circadian clock. Science. 357:912–916. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Anderson JR, Carroll I, Azcarate-Peril MA,
Rochette AD, Heinberg LJ, Peat C, Steffen K, Manderino LM, Mitchell
J and Gunstad J: A preliminary examination of gut microbiota,
sleep, and cognitive flexibility in healthy older adults. Sleep
Med. 38:104–107. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Poroyko VA, Carreras A, Khalyfa A, Khalyfa
AA, Leone V, Peris E, Almendros I, Gileles-Hillel A, Qiao Z, Hubert
N, et al: Chronic sleep disruption alters gut microbiota, induces
systemic and adipose tissue inflammation and insulin resistance in
mice. Sci Rep. 6:354052016. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Lv J, Jia Y, Li J, Kuai W, Li Y, Guo F, Xu
X, Zhao Z, Lv J and Li Z: Gegen Qinlian decoction enhances the
effect of PD-1 blockade in colorectal cancer with microsatellite
stability by remodelling the gut microbiota and the tumour
microenvironment. Cell Death Dis. 10:4152019. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Zhen H, Qian X, Fu X, Chen Z, Zhang A and
Shi L: Regulation of shaoyao ruangan mixture on intestinal flora in
mice with primary liver cancer. Integr Cancer Ther.
18:15347354198431782019. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
McFadden RM, Larmonier CB, Shehab KW,
Midura-Kiela M, Ramalingam R, Harrison CA, Besselsen DG, Chase JH,
Caporaso JG, Jobin C, et al: The role of curcumin in modulating
colonic microbiota during colitis and colon cancer prevention.
Inflamm Bowel Dis. 21:2483–2494. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Huang G, Khan I, Li X, Chen L, Leong W, Ho
LT and Hsiao WLW: Ginsenosides Rb3 and Rd reduce polyps formation
while reinstate the dysbiotic gut microbiota and the intestinal
microenvironment in Apc min/+mice. Sci Rep. 7:125522017. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Chen H, Zhang F, Li R, Liu Y, Wang X,
Zhang X, Xu C, Li Y, Guo Y and Yao Q: Berberine regulates fecal
metabolites to ameliorate 5-fluorouracil induced intestinal
mucositis through modulating gut microbiota. Biomed Pharmacother.
124:1098292020. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Chassaing B, Etienne-Mesmin L and Gewirtz
AT: Microbiota-liver axis in hepatic disease. Hepatology.
59:328–339. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Giuffrè M, Campigotto M, Campisciano G,
Comar M and Crocè LS: A story of liver and gut microbes: How does
the intestinal flora affect liver disease? A review of the
literature. Am J Physiol Gastrointest Liver Physiol. 318:G889–G906.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Gopalakrishnan V, Helmink BA, Spencer CN,
Reuben A and Wargo JA: The influence of the gut microbiome on
cancer, immunity, and cancer immunotherapy. Cancer Cell.
33:570–580. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Fukuoka S, Daisuke M, Togashi Y, Sugiyama
E, Udagawa H, Kirita K, Kamada T, Kawazoe A, Goto K, Doi T, et al:
Association of gut microbiome with immune status and clinical
response in solid tumor patients who received on anti-PD-1
therapies. J Clin Oncol. 36 (Suppl 15):S30112018. View Article : Google Scholar
|















