Introduction
Primary effusion lymphoma (PEL) is a rare and
distinct type of non-Hodgkin's lymphoma typically characterized by
serous effusions without any detectable tumor masses (1). It accounts for ~4% of all human
immunodeficiency virus (HIV)-related non-Hodgkin lymphoma (NHL)
cases and <1% of all non-HIV-related NHL cases in the United
States (between 2001 and 2012) (1).
According to the 2017 World Health Organization (WHO)
Classification of Tumors of Hematopoietic and Lymphoid Tissues
(2), human herpesvirus 8 (HHV8)
infection is commonly detected in neoplastic lymphoid cells. Most
cases of PEL are associated with an immunocompromised status,
particularly human immunodeficiency virus (HIV) infection, and
frequently, there is co-infection with Epstein-Barr virus (EBV)
(3). Neoplastic tumor cells exhibit
a wide range of cytological appearances, from immunoblastic to
anaplastic morphology (3). Although
HHV8-negative effusion-based lymphoma, a rare and underappreciated
subgroup of non-Hodgkin's lymphoma, is cytomorphologically similar
to PEL, it exhibits several distinct clinicopathological
characteristics since it generally develops in older patients with
better outcomes; unlike PEL, the neoplastic cells in HHV8-negative
effusion-based lymphoma express pan-B-cell markers (4).
In the present report, a 75-year-old male with an
indolent variant of HHV8-negative effusion-based lymphoma is
described. In addition, a brief review of similar cases reported in
the literature is presented.
Case report
A 75-year-old male patient presented with cough and
shortness of breath, which lasted for 1 month. The patient was
admitted to Severance Hospital (Seoul, South Korea) in December
2018. Written informed consent for participation in the present
study was obtained from the patient. The patient had a medical
history of hypertension and tuberculosis, which was treated with
anti-tuberculous drugs 50 years ago. The patient was a former
smoker (1 pack/day for 10 years) but stopped smoking 45 years ago.
Chest X-ray revealed cardiomegaly and mild pleural effusion.
Electrocardiogram revealed atrial fibrillation with a right bundle
branch block. A complete blood count provided results within the
normal ranges [white blood cell (WBC), 6,540/µl (normal range,
4,000-10,800/µl); red blood cell (RBC), 5.01×106/µl
(normal range, 4.4–6.1×106/µl); hemoglobin, 15.4 g/dl
(normal range, 13.0–17.4 g/dl); hematocrit, 46.1% (normal range,
40–52%)]. The amounts of lactate hydrogenase and total protein in
the blood serum were 205 IU/l (normal, 119–247 IU/l) and 7.0 g/dl
(normal, 6.0–8.0 g/dl), respectively. Immunoassays for the
detection of HIV antibody, hepatitis B surface antigen and
hepatitis C virus (HCV) antigen were performed following routine
hospital procedures and the results were all negative (data not
shown). Transthoracic echocardiogram (TTE) revealed enlarged left
and right atria, mild pulmonary hypertension (right ventricular
systolic pressure, 43 mmHg) with dilated inferior vena cava, and a
moderate amount of pericardial effusion. The patient refused
hospital admission for a further diagnostic work-up. Diuretics were
administered to control the pericardial effusion. After 6 months,
the patient presented with aggravated dyspnea and a continuous
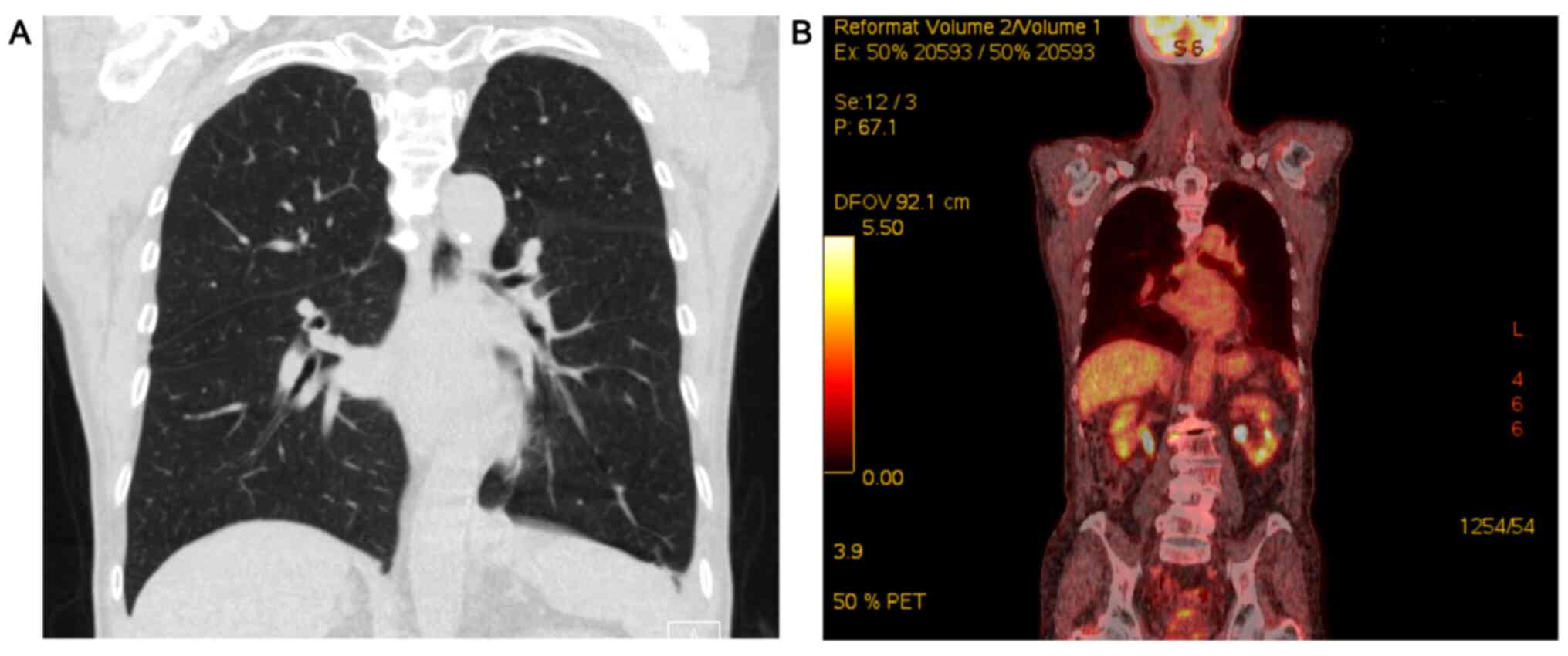
low-grade fever. Chest computed tomography (CT) demonstrated
pleural thickening and effusion (Fig.
1A), but neither hilar lymphadenopathy nor a mass lesion was
detected. A positron emission-CT (PET-CT) scan showed mildly
increased 18F-fluorodeoxyglucose uptake in the left pleura
(Fig. 1B). A follow-up TTE revealed
constrictive pericarditis, as well as persistent left atrial
enlargement and pleural effusion. A thoracentesis was performed
under a tentative diagnosis of tuberculous pericarditis and
pleurisy. Pleural fluid analysis revealed the following counts:
RBC, 30,000/µl (normal range, 0–100,000/µl); WBC, 10,446/µl (normal
range, 0–10,000/µl); protein, 4,800 mg/dl (normal range, 0–2000
mg/dl); glucose, 16 mg/dl (normal range, 0–60 mg/dl); lactate
dehydrogenase, 4,355 IU/l (normal range, 100–300 IU/l); and
adenosine deaminase, 698.4 IU/l (normal range, 0–40 IU/l).
Microbiological tests for tuberculosis, including an acid-fast
bacilli smear, quantitative polymerase chain reaction and cultures,
were performed according to routine procedures, and the results
were all negative (data not shown).
Morphological analysis of the pleural fluid revealed
the presence of numerous apoptotic figures and large atypical cells
with cytomorphological features of centroblasts in the pleural
fluid (Fig. 2A).
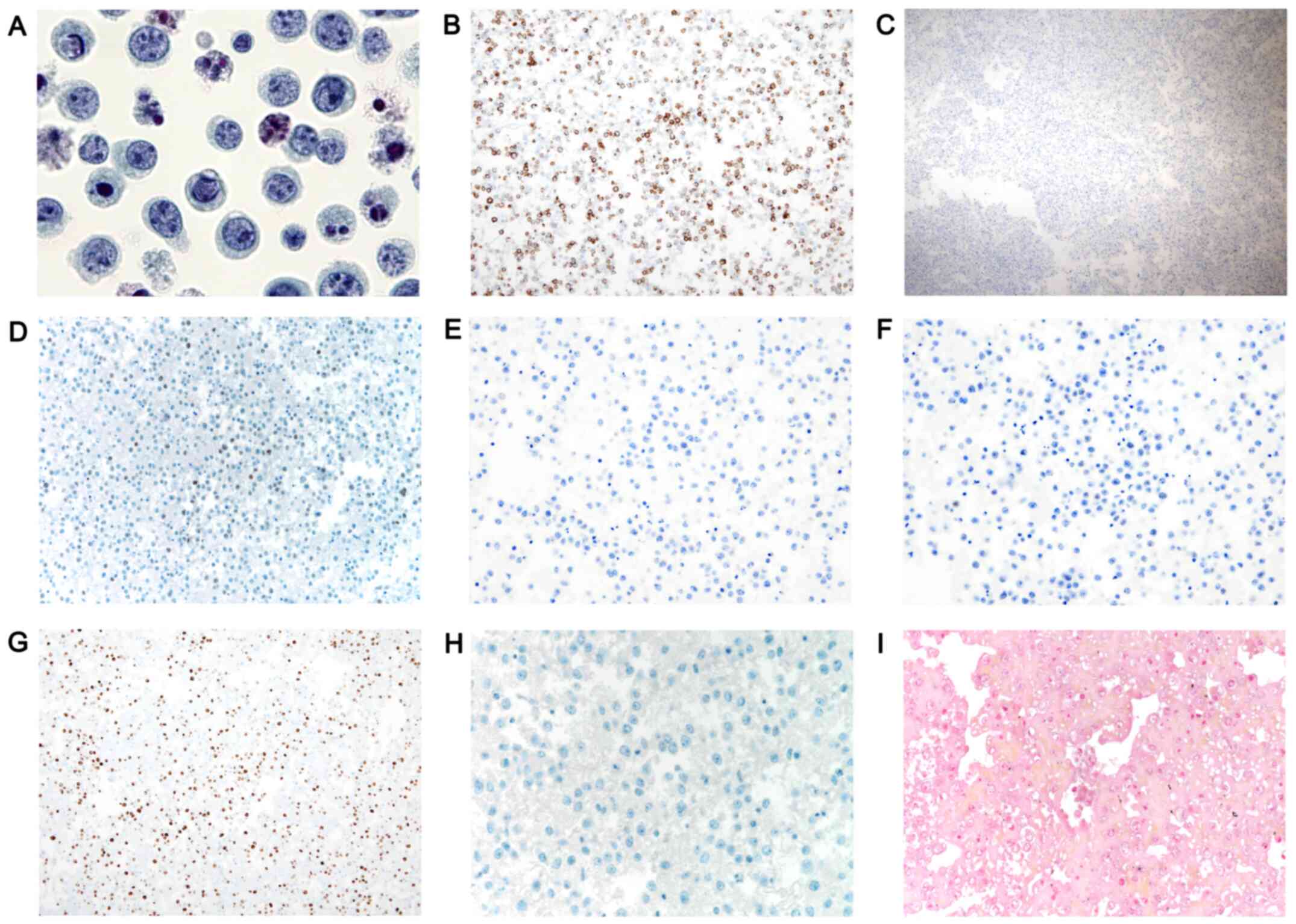 | Figure 2.Histopathological and
immunohistochemical findings in HHV8-negative effusion-based
lymphoma. (A) Cytomorphological appearance of malignant lymphoid
cells in the pleural fluid (magnification, ×1,000). Malignant cells
showing (B) membranous CD20 staining (magnification, ×100) and (C)
no staining for CD138 (magnification, ×40). Tumor cells showing (D)
focal positivity (10% of tumor cells) for c-MYC (magnification,
×200) and no staining for (E) BCL-2 (magnification, ×200) and (F)
BCL-6 (magnification, ×200). (G) Immunohistochemical staining for
Ki-67 revealed a labeling index of 73.5% (magnification, ×100). (H)
Immunohistochemical staining for HHV8 (magnification, ×400) and (I)
Epstein-Barr virus-encoded RNA in situ hybridization
(magnification, ×400) of the pleural fluid was negative. HHV8,
human herpesvirus 8. |
For immunohistochemistry, the cytology cell block
from the pleural fluid aspiration was fixed with 10% buffered
neutral formalin at room temperature overnight and embedded in
paraffin. Tissue sections (4-µm-thick) from paraffin-embedded cell
blocks were deparaffinized and rehydrated with xylene and a
descending alcohol series. Immunostaining was performed using
automatic immunostaining instruments, namely Ventana Benchmark XT
automated staining system (Ventana Medical Systems) or Dako Omnis
(Dako; Agilent Technologies, Inc.), according to the manufacturer's
recommendations. Antigen retrieval was performed using Cell
Conditioning Solution (Ventana Medical Systems) or EnVision FLEX
Target Retrieval Solution, High pH (Dako; Agilent Technologies,
Inc.). Endogenous peroxidase activity was blocked using 3% hydrogen
peroxide at room temperature for 15 min. The sections were then
incubated with primary antibodies at 37°C for 32 min against CD45
(mouse monoclonal; clone 2B11; 1:50; cat. no. M0701; Agilent
Technologies, Inc.), CD20 (mouse monoclonal; clone L26; 1:50; cat.
no. M0755; Dako; Agilent Technologies, Inc.), CD79a (mouse
monoclonal; clone JCB117; 1:50; cat. no. M7050; Dako; Agilent
Technologies, Inc.), paired box 5 (PAX5; rabbit monoclonal; clone
SP34; 1:100; cat. no. 790-4420; Ventana Medical Systems, Inc.),
CD138 (mouse monoclonal; clone MI15; 1:100; cat. no. M7228; Dako;
Agilent Technologies, Inc.), MYC (rabbit monoclonal; clone EP121;
1:50; cat. no. 395R-15; Cell Marque; Merck KGaA), BCL-2 (mouse
monoclonal; clone 124; 1:100; cat. no. M0887; Dako; Agilent
Technologies, Inc.), BCL-6 (mouse monoclonal; clone G1191E/A8;
1:100; cat. no. 227M-96; Cell Marque; Merck KGaA), CD3 (rabbit
polyclonal; 1:100; cat. no. A0452; Dako; Agilent Technologies,
Inc.), CD68 (mouse monoclonal; clone PG-M1; 1:200; cat. no. M0876;
Dako; Agilent Technologies, Inc.), calretinin (mouse monoclonal;
clone DAK-Calret 1; 1:50; cat. no. M7245; Dako; Agilent
Technologies, Inc.), Ki-67 (mouse monoclonal; clone MB-1; 1:150;
cat. no. M7240; Agilent Technologies, Inc.) and HHV8 (mouse
monoclonal; clone 13B10; 1:100; cat. no. 760-4260; Ventana Medical
Systems, Inc.). After chromogenic visualization using an ultraView
Universal DAB Detection kit (Ventana Medical Systems; cat. no.
760-500, including secondary antibody incubation at 37°C for 8 min
using ultraView Universal HRP Multimer), sections were
counterstained with hematoxylin at 37°C for 8 min. Light microscopy
(BX43 System Microscope; Olympus Corporation; magnification,
×40-400) was used to observe the sections. Appropriate positive
controls were stained concurrently to validate the staining method.
Negative controls were prepared by substituting non-immune serum
(mouse monoclonal IgG κ isotype control; clone P3.6.2.8.1; cat. no.
AB_1944423; Invitrogen; Thermo Fisher Scientific, Inc.) for the
primary antibody. No staining was detected in the negative
controls.
Immunohistochemistry analysis revealed that the
atypical cells were uniformly positive for CD45 and that a subset
of these cells exhibited varying degrees of CD20 expression
(Fig. 2B), CD79a and PAX5. The cells
were negative for CD138 (Fig. 2C).
Although 10% of the atypical cells were positive for focal MYC
(Fig. 2D), they were negative for
BCL-2 (Fig. 2E), BCL-6 (Fig. 2F), CD3, CD68 and calretinin. The
Ki-67 labeling index was 73.5%, accounting for the average of four
high-power fields (Fig. 2G). The
atypical cells were negative for HHV8 (Fig. 2H), and EBV-encoded RNA was not
detected in the nuclei of the atypical cells (Fig. 2I) using in situ hybridization,
which was performed as previously described (5). Pathological features in the patient
suggested the possibility of an unusual variant of PEL or diffuse
large B-cell lymphoma associated with chronic inflammation. As the
patient's symptoms remained stable, symptomatic treatment was
provided and the patient was closely followed up. The mild pleural
effusion persisted over a 1-year follow-up period. Repeated pleural
fluid analysis revealed large atypical cells with the same
cytological features of centroblasts and immunophenotypic features.
Some large atypical cells expressed MUM1, but were negative for
HHV8, CD30 and CD138. Polymerase chain reaction analysis of
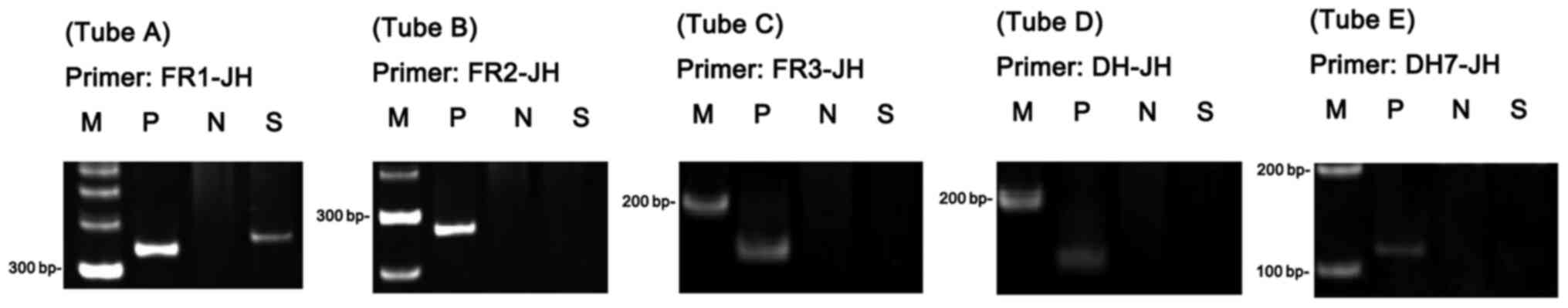
immunoglobulin heavy chain (IgH) gene rearrangement was performed
using BIOMED-2 multiplex primers in accordance with the
manufacturer's protocol (Invivoscribe, Inc.) as previously
described (6). The reaction assays
for the IgH rearrangement tests involved a full set of five
reactions targeting the IGH (IGHA, FR1-JH;
IGHB, FR2-JH; IGHC, FR3-JH; IGHD,
DH1-6-JH; and IGHE, DH7-JH). The results demonstrated
clonal rearrangement of the IgH gene (Fig. 3). The pathological diagnosis of
HHV8-negative effusion-based lymphoma was made on the basis of
clinicopathological findings noted 6 months after the first visit
of the patient. The pleural effusion resolved following the
insertion of a drainage catheter. Systemic imaging including a
PET-CT scan demonstrated no other lesions. Thus, the patient was
closely observed without chemotherapy. Follow-up chest CT, which
was performed at 10 months post-diagnosis, did not reveal an
increase in pleural effusion or suspicious pleural enhancement. At
a regular 2-month follow-up for six visits in total, the patient
was alive without any symptoms for 13 months post-diagnosis.
Discussion
The present study reports a case of HHV8- and
EBV-negative pleural effusion-based lymphoma with indolent clinical
behavior in an elderly patient. Typical PEL occurs in
immune-compromised patients, particularly in those with HIV
infection (7). HHV8 universally
infects malignant lymphoid cells and encodes proteins that are
essential for the proliferation and survival of tumor cells
(8). Although EBV co-infection is
found in most cases of HHV8 infection, it is not considered to play
an important role in the pathogenesis (9). The etiology of HHV8-negative
effusion-based lymphoma is still unclear. EBV infection is seen in
28.9% of patients with HHV8-negative effusion-based lymphoma and
65.6% of patients with PEL (10).
Paner et al (11) suggested a
possible association between HHV8-negative effusion-based lymphoma
and HCV; however, a subsequent study demonstrated HCV infection in
only 10% of patients with HHV8-negative effusion-based lymphoma
(3). Ohshima et al (12) proposed that the genetic alteration of
MYC may be involved in the pathogenesis of HHV8-negative
effusion-based lymphoma, and Ichinohasama et al (13) suggested that PAX5 aberration may play
a role in the lymphomagenesis of HHV8-negative effusion-based
lymphoma. Effusion or chronic serositis itself could create ideal
body conditions for lymphomagenesis, similar to fibrin-associated
diffuse large B-cell lymphoma and diffuse large B-cell lymphoma
associated with chronic inflammation (10). A large proportion of HHV8-negative
effusion-based lymphoma is associated with fluid overload caused by
different oedematous disorders, such as liver cirrhosis or heart
failure. A study of 55 HHV8-negative effusion-based lymphoma cases
reported cirrhosis and heart disease in 10 (18.2%) and 9 (16.4%)
cases, respectively (3). The present
case was also associated with localized fluid overload caused by
heart disease and tuberculous pleurisy that occurred several years
previously.
As was observed in the present case, HHV8-negative
effusion-based lymphoma typically occurs in elderly patients with a
median age of 70 years (10).
Studies consistently report a higher incidence of HHV8-negative
effusion-based lymphoma in males compared with that in females
(3,10). In a previous study, the median age of
patients at presentation with PEL was 42 years in an HIV-infected
group, compared with 73 years in an immune-competent group
(2). The immunocompromised state is
likely to be associated with the earlier onset of PEL as there is
no substantial difference in median age between patients with
HHV8-positive and HIV-negative PEL and HHV8-negative effusion-based
lymphoma.
Tumor cells in HHV8-negative effusion-based lymphoma
exhibit cytological features ranging from immunoblasts to highly
pleomorphic cells, which sometimes resemble Hodgkin cells commonly
found in PEL (14). However, the
tumor cells in HHV8-negative effusion-based lymphoma more commonly
demonstrate either centroblast-like or Burkitt-like cytomorphology
compared with those in PEL (3,14). In
the present case, most tumor cells exhibited the cytomorphological
features of centroblasts.
Unlike in PEL, in which the tumor cells usually lack
pan B-cell markers, the majority of tumor cells in HHV8-negative
effusion-based indolent lymphoma express pan B-cell markers and do
not express an activation marker or plasma cell-associated antigen
(15). The tumor cells in the
present case also expressed pan B-cell markers, although not all
cells demonstrated uniform and strong expression of pan B-cell
marker; no tumor cells expressed activation markers or plasma
cell-associated antigens except MUM1.
Regarding genetics, HHV8-negative effusion-based
lymphoma demonstrates complex genomic aberrations (12). Although cytogenetic analysis could
not be performed in the present study, Ohshima et al
(12) reported gain in 19 out of 24
chromosomes in a comparative genomic hybridization study. They also
reported complex chromosomal abnormalities in both number and
structure, including t(8;22) or +8 chromosome and c-myc
amplification. Terasaki et al (16) also reported cases of HHV8-negative
effusion-based lymphoma with complex karyotype abnormalities in a
G-band study.
HHV8-negative effusion-based lymphoma exhibits a
less aggressive clinical course compared with PEL (17). Zaimoku et al (17) reported a markedly higher survival
rate in patients with HHV8-negative effusion-based lymphoma (1-year
survival rate, 60.1%) compared with those with PEL (1-year survival
rate, 39.3%). Notably, the present case demonstrated no progression
of disease over 1 year without chemotherapy. To the best of our
knowledge, 12 patients with indolent HHV8-negative effusion-based
lymphoma who survived longer than 1 year without treatment have
been previously described, as identified by searching Pubmed
(https://pubmed.ncbi.nlm.nih.gov/) using
the key words ‘HHV-8’, ‘non-Hodgkin lymphoma’, ‘effusion’, ‘immune
compromised’ and ‘primary effusion lymphoma’ (7,15,18–24)
(Table I). Among the 13 cases
(including the present case), there were 7 males and 6 females,
with an age range of 60–99 years (mean age, 80.1 years). The cases
were either determined to be negative for HIV infection, hepatitis
B virus infection and HCV infection, or the data were unavailable.
Heart disease, including coronary artery disease and arrythmia, and
chronic heart failure, were observed in 8 (61.6%) and 2 (15.4%)
patients, respectively. EBV co-infection in lymphoma cells was
detected in 2 cases (15.4%). c-MYC amplification was observed in 1
case (7.7%). Cytomorphologically, centroblast-like or small- to
medium-sized Burkitt-like malignant lymphoid cells were observed
more frequently in the cases summarized in Table I (3/13; 23.1%) than in a previous
study on HHV8-negative effusion-based lymphoma (5/55; 9%) (3). Taken together, HHV8-negative
effusion-based lymphoma with an indolent clinical course tends to
occur in older patients with fewer comorbidities, a lower rate of
related virus infections (such as HCV) and fewer aggressive
cytomorphological features compared with conventional HHV8-negative
effusion-based lymphoma. This suggests that HHV8-negative
effusion-based lymphoma can be categorized into subgroups according
to prognostic factors.
 | Table I.Clinicopathological characteristics of
human herpesvirus 8-negative effusion-based lymphoma with an
indolent clinical course. |
Table I.
Clinicopathological characteristics of
human herpesvirus 8-negative effusion-based lymphoma with an
indolent clinical course.
| First author,
year | Sex | Age, years | Other disease(s) | Site | Histology | HIV | EBV | HCV | HBV | C-MYC | Therapy | Survival (at date of
last follow-up) | Duration of
follow-up, months | Refs. |
|---|
| Ashihara et
al, 2001 | F | 60 | Cholesteatoma | Peritoneum | Large,
pleomorphic | – | + | – | – | None | None | Yes | 24 | (18) |
| Adiguzel et
al, 2009 | M | 89 | DM, HTN, CAD | Pleura | Large,
pleomorphic | – | – | – | N/A | N/A | None | Yes | 40 | (19) |
| Terasaki et
al, 2011 | F | 99 | N/A | Pleura,
Pericardium | Medium to large
sized | – | – | – | N/A | A | Drainage | Yes | 16 | (16) |
| Wang et al,
2011 | M | 79 | HTN, arthritis,
aortic dissection | Pleura | Large,
centroblast-like | – | – | – | – | N/A | Pleurodesis with
doxycycline instillation | Yes | 55 | (20) |
| Kim et al,
2012 | F | 80 | HTN,
tuberculosis | Pleura | Medium,
centroblast-like | – | – | – | N/A | N/A | None | Yes | 18 | (21) |
| Saini et al,
2013 | F | 87 | CHF, HTN, AF,
Dyslipidemia, degenerative joint disease | Pleura,
pericardium | Large,
pleomorphic | – | – | N/A | N/A | N/A | talc
pleurodesis | Yes | 29 | (7) |
| Mohammad et
al, 2014 | M | 76 | HTN, AF | Pleura,
pericardium | Large,
pleomorphic | – | – | – | – | N/A | None | Yes | 14 | (22) |
| Nakatsuka et
al, 2013 | M | 70 | Prostate
cancer | Pleura,
pericardium | Large,
pleomorphic | – | – | N/A | N/A | N/A | N/A | Yes | 14 | (23) |
| Nakatsuka et
al, 2013 | M | 70 | N/A | Pleura,
pericardium | Large,
pleomorphic | – | – | N/A | N/A | N/A | N/A | Yes | 25 | (23) |
| Nakamura et
al, 2015 | M | 85 | PVC | Pleura,
pericardium | Medium to large,
anaplastic | – | + | – | – | N/A | Drainage | Yes | 24 | (24) |
| Usmani et
al, 2015 | F | 89 | CHF | Pleura | NA | N/A | – | N/A | N/A | N/A | None | Yes | 120 | (15) |
| Usmani et
al, 2015 | F | 82 | PAD, CAD, HTN,
metastatic breast cancer | Pericardial | N/A | N/A | – | N/A | N/A | N/A | None | Yes | 14 | (15) |
| Present study | M | 75 | AF,
tuberculosis | Pleura,
pericardium | Large,
centroblast-like | – | – | – | – | – | None | Yes | 22 |
|
In summary, the present study reports a case of
HHV8-negative effusion-based lymphoma with unusual indolent
clinical behavior and presents a review of the characteristics of
13 biologically similar cases reported in the literature. The
results suggest the existence of an indolent variant of
HHV8-negative effusion-based lymphoma. In order to predict indolent
clinical behavior in this rare type of lymphoma, a
clinicopathological analysis of more cases is needed.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
AYL provided the conception and design of the
manuscript. MK and JA drafted the manuscript. JA and SOY analyzed
the previous articles regarding HHV8-negative effusion-based
lymphoma. MK, SOY and WIY interpreted the results of
immunohistochemistry, Epstein-Barr virus-encoded RNA in situ
hybridization and PCR. AYL, SHY and JSK interpreted the patient
clinical data. SOY, AYL and WIY carefully reviewed and revised the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Written informed consent for participation in the
study was obtained from the patient.
Patient consent for publication
The patient provided written informed consent for
the publication of associated data and accompanying images.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CT
|
computed tomography
|
|
EBV
|
Epstein-Barr virus
|
|
HCV
|
hepatitis C virus
|
|
HHV8
|
human herpesvirus 8
|
|
HIV
|
human immunodeficiency virus
|
|
PEL
|
primary effusion lymphoma
|
|
PET-CT
|
positron emission tomography-computed
tomography
|
|
TTE
|
transthoracic echocardiogram
|
|
WHO
|
World Health Organization
|
References
|
1
|
El-Fattah MA: Clinical characteristics and
survival outcome of primary effusion lymphoma: A review of 105
patients. Hematol Oncol. 35:878–883. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Swerdlow SH, Campo E, Harris NL, Jaffe ES,
Pileri SA, Stein H and Thiele J: WHO classification of tumours of
haematopoietic and lymphoid tissues. WHO classification of tumours,
revised. 4th. IARC, 2; 2017, simplehttps://publications.iarc.fr/Book-And-Report-Series/Who-Classification-Of-Tumours/WHO-Classification-Of-Tumours-Of-Haematopoietic-And-Lymphoid-Tissues-2017
|
|
3
|
Wu W, Youm W, Rezk SA and Zhao X: Human
herpesvirus 8-unrelated primary effusion lymphoma-like lymphoma:
Report of a rare case and review of 54 cases in the literature. Am
J Clin Pathol. 140:258–273. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dai H, Cherian R and Mathur S: Primary
body cavity-based large B-cell lymphoma in an HIV and HHV-8
negative, HCV positive patient: A case report and literature
review. Lab Med. 45:136–140. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ko JN, Jung JK, Park YI, Shin HJ, Huh J,
Back S, Kim YJ, Kim JH and Go H: Multistaining optimization for
epstein-barr virus-encoded RNA in situ hybridization and
immunohistochemistry of formalin-fixed paraffin-embedded tissues
using an automated immunostainer. J Pathol Transl Med. 53:317–326.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Abbas F, Yazbek SN, Shammaa D, Hoteit R,
Fermanian P and Mahfouz R: Invivoscribe BIOMED-2 primer mixes in
B-cell immunoglobulin gene rearrangement studies: Experience of a
molecular diagnostics laboratory in a major tertiary care center.
Genet Test Mol Biomarkers. 18:787–790. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Saini N, Hochberg EP, Linden EA, Jha S,
Grohs HK and Sohani AR: HHV8-Negative primary effusion lymphoma of
B-cell lineage: Two cases and a comprehensive review of the
literature. Case Rep Oncol Med. 2013:2923012013.PubMed/NCBI
|
|
8
|
Asou H, Said JW, Yang R, Munker R, Park
DJ, Kamada N and Koeffler HP: Mechanisms of growth control of
Kaposi's sarcoma-associated herpes virus-associated primary
effusion lymphoma cells. Blood. 91:2475–2481. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen YB, Rahemtullah A and Hochberg E:
Primary effusion lymphoma. Oncologist. 12:569–576. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Alexanian S, Said J, Lones M and Pullarkat
ST: KSHV/HHV8-negative effusion-based lymphoma, a distinct entity
associated with fluid overload states. Am J Surg Pathol.
37:241–249. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Paner GP, Jensen J, Foreman KE and Reyes
CV: HIV and HHV-8 negative primary effusion lymphoma in a patient
with hepatitis C virus-related liver cirrhosis. Leuk Lymphoma.
44:1811–1814. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ohshima K, Ishiguro M, Yamasaki S, Miyagi
J, Okamura S, Sugio Y, Muta T, Sasaki H, Tuchiya T, Kawasaki C and
Kikuchi M: Chromosomal and comparative genomic analyses of
HHV-8-negative primary effusion lymphoma in five HIV-negative
Japanese patients. Leuk Lymphoma. 43:595–601. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ichinohasama R, Miura I, Kobayashi N,
Saitoh Y, DeCoteau JF, Saiki Y, Mori S, Kadin ME and Ooya K: Herpes
virus type 8-negative primary effusion lymphoma associated with
PAX-5 gene rearrangement and hepatitis C virus: A case report and
review of the literature. Am J Surg Pathol. 22:1528–1537. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Carbone A, Cilia AM, Gloghini A,
Canzonieri V, Pastore C, Todesco M, Cozzi M, Perin T, Volpe R,
Pinto A and Gaidano G: Establishment of HHV-8-positive and
HHV-8-negative lymphoma cell lines from primary lymphomatous
effusions. Int J Cancer. 73:562–569. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Usmani A, Walts AE, Patel S, Alkan S and
Kitahara S: HHV8-negative effusion based lymphoma: A series of 17
cases at a single institution. J Am Soc Cytopathol. 4:37–43. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Terasaki Y, Yamamoto H, Kiyokawa H,
Okumura H, Saito K, Ichinohasama R and Ishida Y: Disappearance of
malignant cells by effusion drainage alone in two patients with
HHV-8-unrelated HIV-negative primary effusion lymphoma-like
lymphoma. Int J Hematol. 94:279–284. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zaimoku Y, Takahashi W, Iwaki N, Saito C,
Yoshida A, Aoki G, Yamaguchi M and Nakao S: Human
herpesvirus-8-unrelated primary effusion lymphoma of the elderly
not associated with an increased serum lactate dehydrogenase level:
A benign sub-group of effusion lymphoma without chemotherapy. Leuk
Lymphoma. 57:1625–1632. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ashihara E, Shimazaki C, Hirai H, Inaba T,
Hasegawa G, Mori S and Nakagawa M: Human herpes virus 8-negative
primary effusion lymphoma in a patient with a ventriculoperitoneal
shunt tube. Int J Hematol. 74:327–332. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Adiguzel C, Bozkurt SU, Kaygusuz I, Uzay
A, Tecimer T and Bayik M: Human herpes virus 8-unrelated primary
effusion lymphoma-like lymphoma: Report of a rare case and review
of the literature. APMIS. 117:222–229. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang T, Nava VE, Schechter GP, Lichy JH
and Liu ML: Human herpes virus 8-unrelated primary effusion
lymphoma-like lymphoma: A patient successfully treated with
pleurodesis. J Clin Oncol. 29:e747–e750. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim KH, Lee JH, Jeong HC, Kim GW, Song SH,
Jung SY, Kim GI and Kim EK: A case of human herpes virus-8
unrelated primary effusion lymphoma-like lymphoma presented as
pleural effusion. Tuberc Respir Dis (Seoul). 73:336–341. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mohammad F, Siddique MN, Siddiqui F,
Popalzai M, Asgari M and Odaimi M: A unique case of malignant
pleuropericardial effusion: HHV-8-unrelated PEL-like lymphoma-a
case report and review of the literature. Case Rep Oncol Med.
2014:4368212014.PubMed/NCBI
|
|
23
|
Nakatsuka S, Kimura H, Nagano T, Fujita M,
Kanda T, Iwata T and Hashimoto K: Self-limited effusion large
B-cell lymphoma: Two cases of effusion lymphoma maintaining
remission after drainage alone. Acta Haematol. 130:217–221. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nakamura H, Tsuta K, Nakagawa T, Hirai R
and Ota Y: Human herpes virus 8-unrelated primary effusion
lymphoma-like lymphoma in the pericardium: A case with latency type
III Epstein-Barr virus infection showing good prognosis without
chemotherapy. Pathol Res Pract. 211:1010–1013. 2015. View Article : Google Scholar : PubMed/NCBI
|