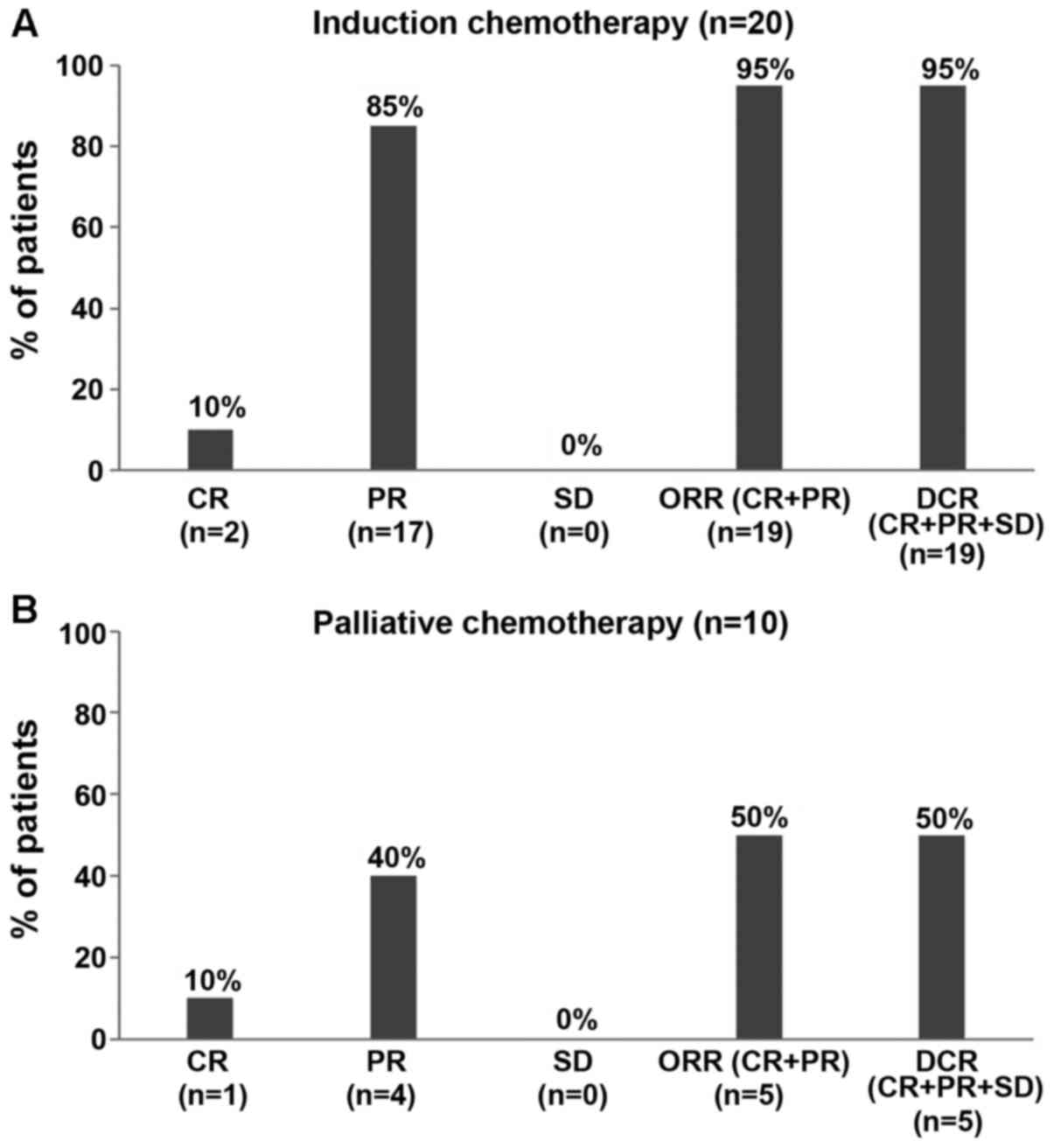
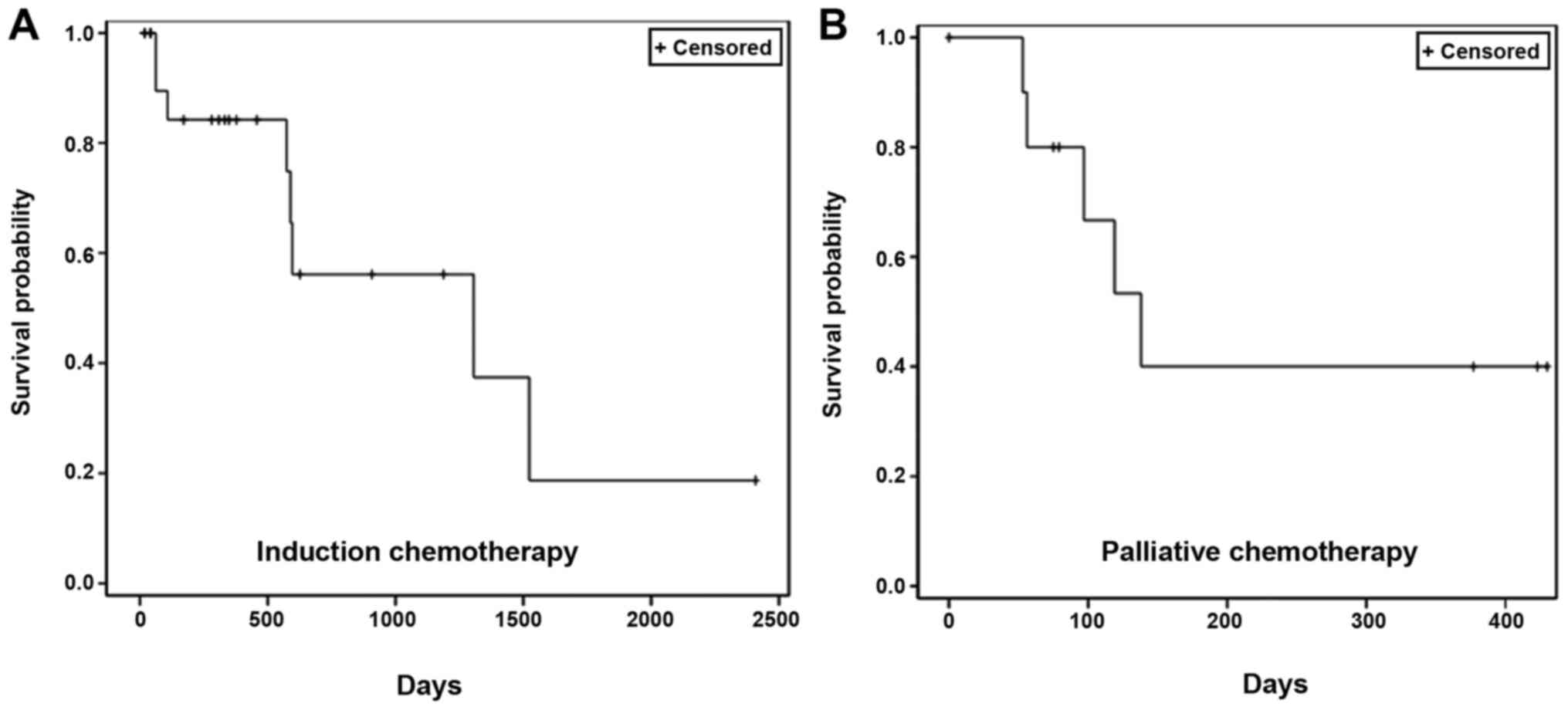
|
1
|
Rapidis A, Sarlis N, Lefebvre JL and Kies
M: Docetaxel in the treatment of squamous cell carcinoma of the
head and neck. Ther Clin Risk Manag. 4:865–886. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kulkarni MR: Head and neck cancer burden
in India. Int J Head Neck Surg. 4:29–35. 2013. View Article : Google Scholar
|
|
4
|
Machiels JP, Lambrecht M, Hanin FX, Duprez
T, Gregoire V, Schmitz S and Hamoir M: Advances in the management
of squamous cell carcinoma of the head and neck. F1000Prime Rep.
6:442014. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Seiwert TY and Cohen EEW: State-of-the-art
management of locally advanced head and neck cancer. Br J Cancer.
92:1341–1348. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
NCCN Clinical Practice Guidelines in
Oncology (NCCN Guidelines®), . Head and Neck Cancer. Version
1.2019. simplehttps://www.nccn.org/March
14–2019
|
|
7
|
Van Cutsem E, Moiseyenko VM, Tjulandin S,
Majlis A, Constenla M, Boni C, Rodrigues A, Fodor M, Chao Y, Voznyi
E, et al: Phase III study of docetaxel and cisplatin plus
fluorouracil compared with cisplatin and fluorouracil as first-line
therapy for advanced gastric cancer: A report of the V325 study
group. J Clin Oncol. 24:4991–4997. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
ten Tije AJ, Verweij J, Loos WJ and
Sparreboom A: Pharmacological effects of formulation vehicles:
Implications for cancer chemotherapy. Clin Pharmacokinet.
42:665–685. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Coors EA, Seybold H, Merk HF and Mahler V:
Polysorbate 80 in medical products and nonimmunologic anaphylactoid
reactions. Ann Allergy Asthma Immunol. 95:593–599. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schwartzberg LS and Navari RM: Safety of
polysorbate 80 in the oncology setting. Adv Ther. 35:754–767. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Food and Drug Administration (FDA), . FDA
Drug Safety Communication: FDA warns that cancer drug docetaxel may
cause symptoms of alcohol intoxication after treatment. simplehttps://www.fda.gov/Drugs/DrugSafety/ucm401752.htmJune
20–2014
|
|
12
|
Mirza A and Mithal N: Alcohol intoxication
with the new formulation of docetaxel. Clin Oncol (R Coll Radiol).
23:560–561. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ahmad A, Sheikh S, Ali SM, Ahmad MU,
Paithankar M, Saptarishi D, Maheshwari K, Kumar K, Singh J, Patel
G, et al: Development of aqueous based formulation of docetaxel:
Safety and pharmacokinetics in patients with advanced solid tumors.
J Nanomed Nanotechnol. 6:12015.
|
|
14
|
Narayanan P, Dattatreya PS, Prasanna R,
Subramanian S, Jain K, Somanath NS, Joshi N, Bunger D, Khan MA,
Chaturvedi A and Ahmad I: Efficacy and safety of nanosomal
docetaxel lipid suspension-based chemotherapy in sarcoma: A
multicenter, retrospective study. Sarcoma. 2019:31585902019.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
World Intellectual Property Organization,
. Aqueous systems for the preparation of lipid-based pharmaceutical
compounds; compositions, methods, and uses thereof. Publication No.
WO/2008/127358. simplehttps://patentscope.wipo.int/search/en/detail.jsf?docId=WO2008127358&redirectedID=trueOctober
23–2008
|
|
16
|
Ahmad A, Sheikh S, Taran R, Srivastav SP,
Prasad K, Rajappa SJ, Kumar V, Gopichand M, Paithankar M, Sharma M,
et al: Therapeutic efficacy of a novel nanosomal docetaxel lipid
suspension compared with taxotere in locally advanced or metastatic
breast cancer patients. Clin Breast Cancer. 14:177–181. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ashraf M, Sajjad R, Khan M, Shah M, Bhat Y
and Wani Z: 156P Efficacy and safety of a novel nanosomal docetaxel
lipid suspension (NDLS) as an anti cancer agent-a retrospective
study. Ann Oncol. 27 (Suppl 9):Six46–Six51. 2016. View Article : Google Scholar
|
|
18
|
Naik R and Khan MA: Doceaqualip in a
patient with prostate cancer who had an allergic reaction to
conventional docetaxel: A case report. Mol Clin Oncol. 6:341–343.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Prasanna R, Bunger D and Khan MA: Efficacy
and safety of doceaqualip in a patient with locally advanced
cervical cancer: A case report. Mol Clin Oncol. 8:296–299.
2018.PubMed/NCBI
|
|
20
|
Vyas V, Joshi N and Khan M: Novel
docetaxel formulation (NDLS) in low cardiac reserve ovarian cancer.
Open Access J Cancer Oncol. 2:0001222018.
|
|
21
|
Gupta S, Pawar SS and Bunger D: Successful
downstaging of locally recurrent penile squamous cell carcinoma
with neoadjuvant nanosomal docetaxel lipid suspension (NDLS) based
regimen followed by curative surgery. BMJ Case Rep.
2017:bcr20172206862017. View Article : Google Scholar
|
|
22
|
Murali A, Gupta S and Pendharkar D:
Efficacy and tolerability of nanoparticle docetaxel lipid
suspension. J Clin Oncol. 36 (Suppl 15):e145422018. View Article : Google Scholar
|
|
23
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
U.S. Department of Health and Human
Services, . Common Terminology Criteria for Adverse Events (CTCAE).
Version 5.0. simplehttps://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5×7.pdfNovember
27–2017
|
|
25
|
Dixon JR Jr: The international conference
on harmonization good clinical practice guideline. Qual Assur.
6:65–74. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
World Medical Association, . World Medical
Association Declaration of Helsinki: Ethical principles for medical
research involving human subjects. JAMA. 310:2191–2194. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Datta SS, Ghosal N, Daruvala R,
Chakraborty S, Shrimali RK, van Zanten C, Parry J, Agrawal S,
Atreya S, Sinha S, et al: How do clinicians rate patient's
performance status using the ECOG performance scale? A
mixed-methods exploration of variability in decision-making in
oncology. Ecancermedicalscience. 13:9132019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Posner MR and Lefebvre JL: Docetaxel
induction therapy in locally advanced squamous cell carcinoma of
the head and neck. Br J Cancer. 88:11–17. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Posner MR, Hershock DM, Blajman CR,
Mickiewicz E, Winquist E, Gorbounova V, Tjulandin S, Shin DM,
Cullen K, Ervin TJ, et al: Cisplatin and fluorouracil alone or with
docetaxel in head and neck cancer. N Engl J Med. 357:1705–1715.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Haddad R, Tishler R, Wirth L, Norris CM,
Goguen L, Sullivan C, O'Donnell L, Li Y and Posner M: Rate of
pathologic complete responses to docetaxel, cisplatin, and
fluorouracil induction chemotherapy in patients with squamous cell
carcinoma of the head and neck. Arch Otolaryngol Head Neck Surg.
132:678–681. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Grégoire V, Lefebvre JL, Licitra L and
Felip E; EHNS-ESMO-ESTRO Guidelines Working Group, : Squamous cell
carcinoma of the head and neck: EHNS-ESMO-ESTRO clinical practice
guidelines for diagnosis, treatment and follow-up. Ann Oncol. 21
(Suppl 5):Sv184–Sv186. 2010. View Article : Google Scholar
|
|
32
|
Vermorken JB, Remenar E, van Herpen C,
Gorlia T, Mesia R, Degardin M, Stewart JS, Jelic S, Betka J, Preiss
JH, et al: Cisplatin, fluorouracil, and docetaxel in unresectable
head and neck cancer. N Engl J Med. 357:1695–1704. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang X, Gu J, Shao C, Han K and Meng J:
Nimotuzumab plus chemotherapy with docetaxel, cisplatin,
5-fluorouracil for locally advanced head and neck squamous cell
carcinoma: A clinical study. J Cancer Res Ther. 15:312–316.
2019.PubMed/NCBI
|
|
34
|
Pointreau Y, Garaud P, Chapet S, Sire C,
Tuchais C, Tortochaux J, Faivre S, Guerrif S, Alfonsi M and Calais
G: Randomized trial of induction chemotherapy with cisplatin and
5-fluorouracil with or without docetaxel for larynx preservation. J
Natl Cancer Inst. 101:498–506. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hui EP, Ma BB, Leung SF, King AD, Mo F,
Kam MK, Yu BK, Chiu SK, Kwan WH, Ho R, et al: Randomized phase II
trial of concurrent cisplatin-radiotherapy with or without
neoadjuvant docetaxel and cisplatin in advanced nasopharyngeal
carcinoma. J Clin Oncol. 27:242–249. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hitt R, Mesia R, Grau JJ, Iglesias L,
Barco ED, Lozano A, Trufero JM, Giron CG, Martin AL and hernandez
JJC: Randomized phase III trial of induction chemotherapy (ICT)
with docetaxel-cisplatin-5fluorouracil (DCF) followed by
cisplatin-radiotherapy (CRT) or cetuximab-radiotherapy (CetRT) in
patients (pts) with locally advanced unresectable head and neck
cancer (LAUHNC). J Clin Oncol. 34 (Suppl 15):60012016. View Article : Google Scholar
|
|
37
|
Specht L, Larsen SK and Hansen HS: Phase
II study of docetaxel and cisplatin in patients with recurrent or
disseminated squamous-cell carcinoma of the head and neck. Ann
Oncol. 11:845–849. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Rawat S, Tandan H, Patel S and Chaudhari
S: Safety and efficacy of nimotuzumab with concurrent
chemoradiotherapy in unresectable locally advanced squamous cell
carcinoma of head and neck: An Indian rural hospital experience.
South Asian J Cancer. 8:52–56. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Samlowski WE, Moon J, Kuebler JP, Nichols
CR, Gandara DR, Ozer H, Williamson SK, Atkins JN, Schuller DE and
Ensley JF: Evaluation of the combination of docetaxel/carboplatin
in patients with metastatic or recurrent squamous cell carcinoma of
the head and neck (SCCHN): A Southwest oncology group phase II
study. Cancer Invest. 25:182–188. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Weiss RB, Donehower RC, Wiernik PH, Ohnuma
T, Gralla RJ, Trump DL, Baker JR Jr, Van Echo DA, Von Hoff DD and
Leyland-Jones B: Hypersensitivity reactions from taxol. J Clin
Oncol. 8:1263–1268. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Obradović MMS, Hamelin B, Manevski N,
Couto JP, Sethi A, Coissieux MM, Münst S, Okamoto R, Kohler H,
Schmidt A and Bentires-Alj M: Glucocorticoids promote breast cancer
metastasis. Nature. 567:540–544. 2019. View Article : Google Scholar : PubMed/NCBI
|