Introduction
Squamous cell carcinoma of the head and neck (SCCHN)
develops in the mucous membranes of the mouth, nose and throat, and
can include carcinomas of the oral cavity, floor of the mouth,
tongue, tonsils, juxtatonsillar fossae, larynx and pharynx
(1). In India, lip and oral cavity
cancer is the second most common cancer (119,992 cases; 11.54%)
after breast cancer; it is the most common cancer among Indian men
(92,011 cases; 16.1%) and the fourth most common cancer in Indian
women (27,981 cases; 4.8%) as per GLOBOCAN 2018 data (2). In total, 60–80% of SCCHN cases in India
are diagnosed at advanced stages (3).
Early stage (I or II) SCCHN is usually treated with
surgery and/or radiation therapy (RT), whereas multimodality
treatment is generally required for patients with locally advanced
(LA; stage III/IV) or metastatic SCCHN (4). The various treatment modalities include
surgery followed by chemoradiotherapy (CRT), induction chemotherapy
followed by CRT, RT or surgery (with or without adjuvant therapy),
or epidermal growth factor receptor (EGFR) inhibition plus RT or
CRT (5,6).
Docetaxel in combination with cisplatin and
5-fluorouracil (5-FU), the TPF regimen, is approved for the
induction therapy of patients with LA SCCHN (7). The TPF and docetaxel/cisplatin (TP)
regimens are recommended as induction/sequential chemotherapy for
SCCHN (6). Docetaxel plus
cisplatin/carboplatin, docetaxel plus cisplatin/carboplatin plus
cetuximab and docetaxel monotherapy are recommended for the
treatment of recurrent, unresectable or metastatic SCCHN (6).
Polysorbate 80 and ethanol vehicles in the
conventional docetaxel formulation can cause acute hypersensitivity
reactions, peripheral neuropathy, cumulative fluid retention,
reactions at infusion sites, severe anaphylactoid reactions and
alcohol intoxication (8–12). Nanosomal docetaxel lipid suspension
(NDLS; DoceAqualip; Intas Pharmaceuticals Ltd.), a novel
lipid-based formulation, which is free from polysorbate 80 and
ethanol, was developed to overcome these toxicity issues (13).
NDLS is approved in India for the induction
treatment of LA SCCHN (14). Other
approved indications for NDLS include the treatment of patients
with androgen-independent (hormone refractory) metastatic prostate
cancer, advanced gastric adenocarcinoma, locally advanced or
metastatic breast cancer after failure of prior chemotherapy and
non-small cell lung cancer after failure of prior chemotherapy
(14). NDLS was developed using
lipids generally regarded as safe by the US Food and Drug
Administration based on the patented ‘NanoAqualip’ technology
[patent number: Worldwide (WO2008127358), Europe (2076244), Japan
(5917789) and Canada (CA2666322)] (15). The NDLS development process includes
the addition of docetaxel to high-pressure homogenized soy
phosphatidylcholine and sodium cholesteryl sulfate in sodium
citrate buffer under continuous high-pressure homogenization
(13), resulting in nanosomal
(<100 nm) particles of NDLS (13). The delivery of docetaxel to tumor
tissues is increased with these nanosomal particles, helped by the
already damaged tumor vasculature, which results in an enhanced
permeability and retention effect. A greater systemic availability
of docetaxel (13) is seen, hence
leading to improved therapeutic outcomes in terms of response rates
(16).
NDLS has shown efficacy and safety in the treatment
of breast, ovarian, cervical, penile, gastric, hormone refractory
prostate, non-small cell lung, head and neck cancers, and sarcoma
(14,16–22). The
present study reports a real-world, multicenter, retrospective
account of the use of NDLS-based chemotherapy in the treatment of
SCCHN.
Materials and methods
Study design, patient selection and
endpoints
The present study retrospectively reviewed the
medical records of patients with SCCHN who were treated with
NDLS-based chemotherapy as part of their clinical care and followed
up between August 2014 and September 2018 at multiple centers
including All India Institute of Medical Sciences, Bhubaneswar
(n=10), VS Hospital, Madras Cancer Institute, Advanced Cancer Care,
Chennai (n=21), and Sparsh Hospital, Bhubaneswar (n=3), India. The
study inclusion criteria were: i) Patients of all age groups and
ii) both sexes, with iii) histopathologically or cytologically
confirmed tumors, and iv) patients who received NDLS as part of
routine clinical practice, who had at least one measurable lesion
as per the Response Evaluation Criteria in Solid Tumors (RECIST)
1.1 (23). Patients who had cancer
other than SCCHN were excluded from this report. The efficacy
endpoints included: i) Overall response rate (ORR), defined as the
total proportion of patients achieving complete response (CR) plus
those with a partial response (PR); ii) disease control rate (DCR),
defined as the proportion of patients achieving CR + PR + stable
disease (SD); and iii) overall survival, defined as the time from
treatment to death due to any cause. For patients who were still
alive at the time of last follow-up (September 30, 2018) or who
were lost to follow-up, OS was censored at the last recorded date
that the patient was known to be alive. RECIST 1.1 was used for
efficacy evaluation (23). Incidence
of adverse events (AEs) were graded (where available) as per Common
Terminology Criteria for Adverse Events 5.0 (24).
Ethics statement
The study was conducted after due approval from The
OM Ethics Committee (Ahmedabad, India). The study was conducted in
accordance with the ethical principles that have their origin in
the Declaration of Helsinki (25),
and in accordance with the International Conference on
Harmonization's Good Clinical Practice guidelines (26), applicable regulatory requirements and
in compliance with the submitted study protocol.
Statistical analysis
Demographic and baseline characteristics were
summarized descriptively. Frequency and percentage were used for
categorical variables and count, mean, standard deviation, median,
minimum and maximum for continuous variables. The frequency and
percentage of patients were used to present the response rates.
Survival was analyzed using a non-parametric procedure performed
using PROC LIFETEST (version 9.4; SAS Institute, Inc.). OS was
measured using the Kaplan-Meier method and log-rank test. The AEs
were summarized as frequencies and percentages by type of
reaction.
Results
Patient disposition and
demographics
In total, 228 patients with cancer who had received
NDLS for their routine clinical care at different centers were
evaluated. In the present report, the data of patients with SCCHN
who received NDLS-based chemotherapy are presented.
Data of 34 patients with SCCHN, who were treated
with NDLS-based chemotherapy regimens, were retrospectively
analyzed. The baseline characteristics of these patients are
summarized in Table I. The mean (SD)
age of the patients was 54.70 (10.2) years and majority (73.52%) of
the patients were men. Majority (61.76%) of the patients had stage
IV cancer. All the patients had the Eastern Cooperative Oncology
Group (ECOG) scores (27) of either
0 or 1. The most common anatomical sites for SCCHN were the
mandible (n=9), buccal mucosa (n=7), hypopharynx (n=5), tongue
(n=4), pharynx (n=2), oral cavity, oropharynx, parotid (n=1 for
each) and not specified (n=4).
 | Table I.Disposition and baseline
characteristics of patients treated with induction (n=23) and
palliative (n=11) chemotherapy. |
Table I.
Disposition and baseline
characteristics of patients treated with induction (n=23) and
palliative (n=11) chemotherapy.
| Parameters | All patients | Induction
chemotherapy | Palliative
chemotherapy |
|---|
| Mean age ± SD
(range), years | 54.70±10.2
(32–75) | 55.91±10.3
(35–75) | 53.61±9.26
(32–68) |
| Mean BSA ± SD,
kg/m2 | 1.56±0.2 | 1.57±0.2 | 1.55±0.22 |
| Sex, n (%) |
|
|
|
|
Male | 25 (73.52) | 16 (69.6) | 9
(81.12) |
|
Female | 9
(26.47) | 7
(30.4) | 2
(18.18) |
| Cancer stage, n
(%) |
|
|
|
| I | 2 (5.88) | 2
(8.69) | – |
| II | 5
(14.70) | 5
(21.74) | – |
|
III | 6
(17.64) | 6
(26.09) | – |
| IV | 21 (61.76) | 10
(43.48) | 11 (100.0) |
| Metastasis site, n
(%)a |
|
|
|
| Lymph
node | 8
(23.53) | 2
(8.69) | 6
(54.54) |
|
Lungs | 1 (2.94) | – | 1 (9.09) |
|
Liver | 1 (2.94) | – | 1 (9.09) |
|
Others | 1 (2.94) | – | 1 (9.09) |
| ECOG performance
score, n (%) |
|
|
|
| 0 | 4
(11.76) | 3
(13.04) | 1 (9.09) |
| 1 | 30 (88.23) | 20
(86.96) | 10 (90.91) |
| Comorbid disease, n
(%) |
|
|
|
|
Hypertension | 8
(23.52) | 6
(26.09) | 2
(18.18) |
|
Diabetes | 3 (8.82) | 3
(13.04) | – |
|
Othersb | 6
(17.64) | 1
(4.35) | 5
(45.45) |
NDLS was administered as a 1-h infusion in 3-weekly
cycles at 75 mg/m2 (n=29; 85.2%) and 50 mg/m2
(n=5; 14.8%); NDLS was used as first-line therapy in the majority
(91.2%) of the patients. Granulocyte-colony stimulating factor was
used in all the patients as primary prophylaxis.
As induction chemotherapy (n=23), 5 patients
received an NDLS-based TPF (NDLS, platinum and 5-FU) regimen, 6
received NDLS plus cisplatin, 9 received an NDLS-based TPF plus
nimotuzumab regimen and 3 received an NDLS-based TPF plus cetuximab
regimen. An NDLS-based TP regimen (n=5), an NDLS-based TP plus
nimotuzumab (n=3), an NDLS-based TP plus cetuximab regimen (n=2)
and NDLS monotherapy (n=1) were used as palliative chemotherapy
(n=11).
Efficacy
Of the 34 patients who received NDLS-based
chemotherapy for the treatment of SCCHN as induction and palliative
chemotherapy, an efficacy evaluation was possible for 30 patients
(induction, 20/23 patients; palliative, 10/11 patients). The ORR
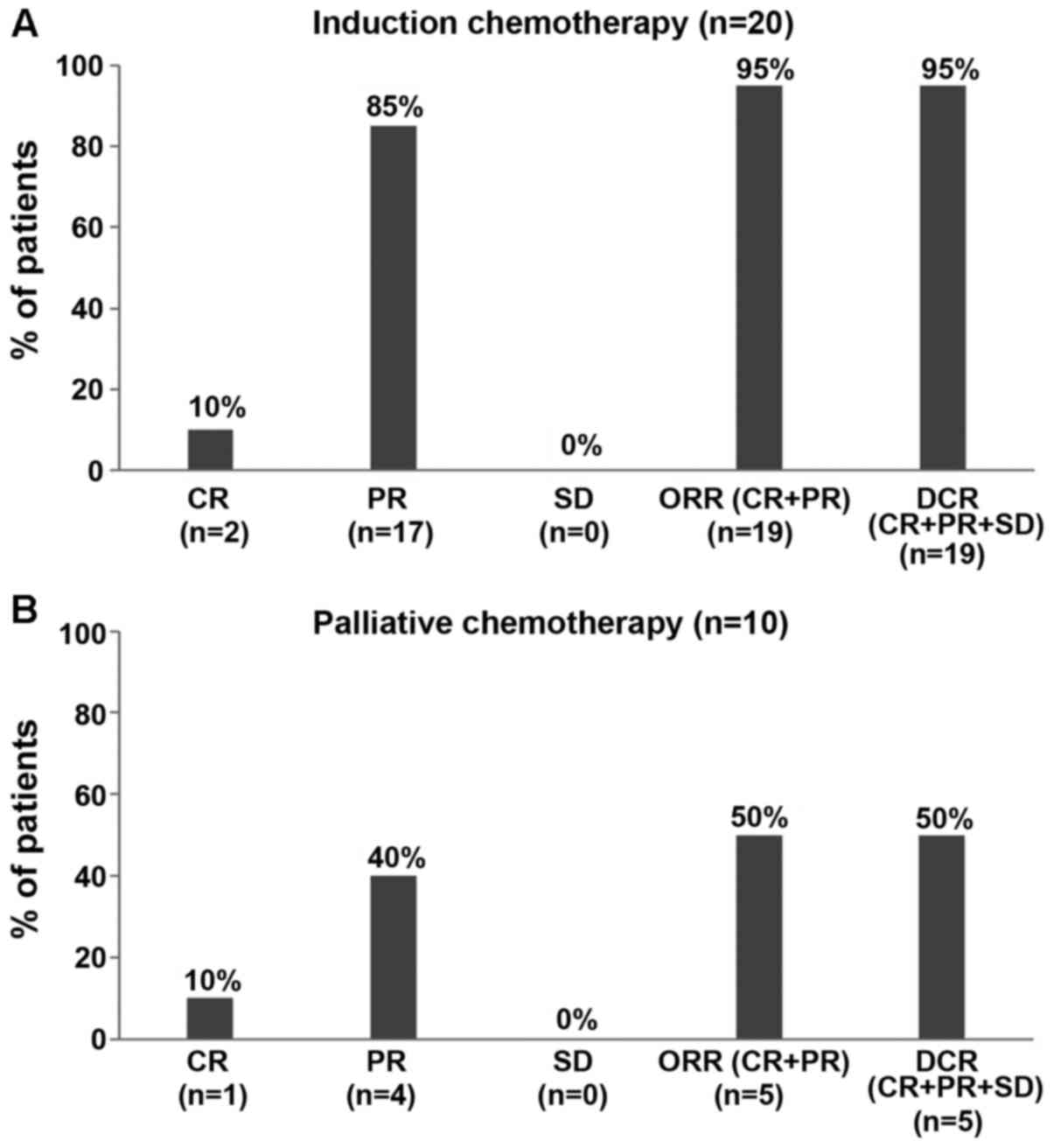
and DCR were 95% each for NDLS-based induction chemotherapy
(CR=10%, n=2; PR=85%, n=17; Fig. 1A;
Table II) and 50% each for
NDLS-based palliative chemotherapy (CR=10%, n=1; PR=40%, n=4;
Fig. 1B; Table II).
 | Table II.Response rates based on NDLS based
chemotherapy regimens. |
Table II.
Response rates based on NDLS based
chemotherapy regimens.
| Treatment
regimen | No of patients
treated | Response, n |
|---|
| Induction
chemotherapy (n=23) |
|
|
|
NDLS-based TPF (NDLS, platinum
and 5-FU) | 5 | PR=3, PD=1,
NE=1 |
|
NDLS-based TP (NDLS plus
cisplatin) | 6 | PR=6 |
|
NDLS-based TPF plus
nimotuzumab | 9 | CR=2, PR=6,
NE=1 |
|
NDLS-based TPF plus
cetuximab | 3 | PR=2, NE=1 |
| Palliative
chemotherapy (n=11) |
|
|
|
NDLS-based TP (NDLS plus
cisplatin) | 5 | CR=1, PR=1,
PD=3 |
|
NDLS-based TP plus
nimotuzumab | 3 | PR=2. PD=1 |
|
NDLS-based TP plus cetuximab
regimen | 2 | PR=1, PD=1 |
| NDLS
monotherapy | 1 | NE=1 |
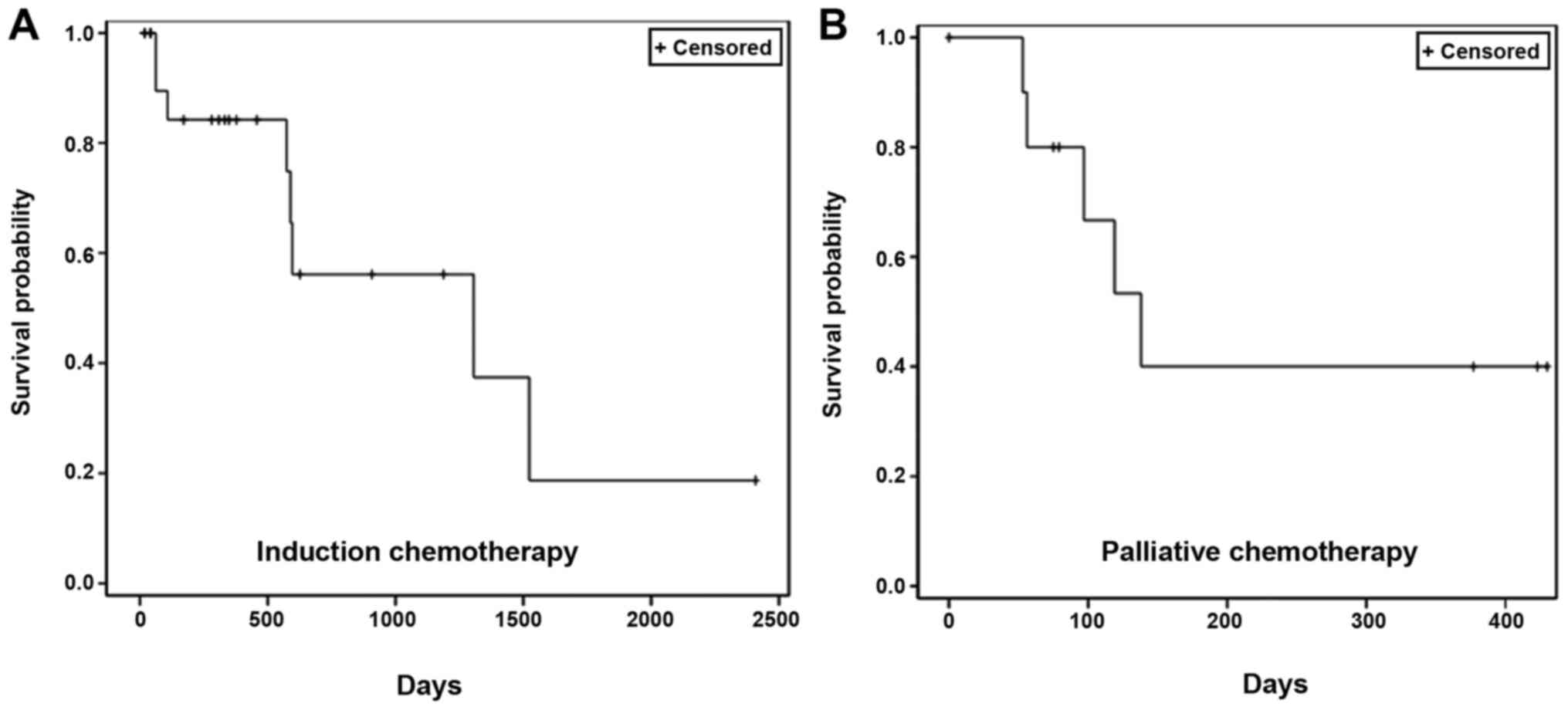
OS
The patient survival data were collected from the
administration of the first dose of NDLS-based therapy until the
date of last follow-up (September 30, 2018) for patients who were
alive and until the date of death for patients who died. The
proportion of patients who were alive at the last follow-up was
65.2% (15/23 patients) in the induction setting and 54.5% (6/11
patients) in the palliative setting. In the induction setting, the
median OS was 43.5 months (follow-up duration, 0.6–80.3 months;
Fig. 2A). In the palliative setting,
the median OS was 4.6 months (follow-up duration, 1.8–14.3 months;
Fig. 2B).
Safety
The data on AEs was available for 23 patients, and
at least one AE was reported in 19 (82.6%) of the patients. Grade 1
AEs were reported in 73.9% (17/23) patients, grade 2 in 13.0%
(3/23) patients, grade 3 in 17.4% (4/23) patients and grade 4 in
4.3% (1/23) patients, respectively. Anemia, lymphopenia,
thrombocytopenia and neutropenia were the reported hematological
AEs, while hyperglycemia, constipation, nausea, vomiting and
weakness were the most frequently reported non-hematological AEs
(Table III). The grade 3/4
hematological AEs were neutropenia (8.7%), lymphopenia (8.7%) and
thrombocytopenia (4.3%). One (4.34%) patient reported grade IV
neutropenia.
 | Table III.Safety profile of nanosomal docetaxel
lipid suspension-based chemotherapy in squamous cell carcinoma of
the head and neck (n=23). |
Table III.
Safety profile of nanosomal docetaxel
lipid suspension-based chemotherapy in squamous cell carcinoma of
the head and neck (n=23).
| AEs | All grades, n
(%) |
|---|
| Hematological |
|
|
Anemia | 14 (60.9) |
|
Lymphopenia | 7 (30.4) |
|
Thrombocytopenia | 6 (26.1) |
|
Neutropenia | 3 (13.04) |
|
Non-hematological |
|
|
Hyperglycemia | 5 (21.7) |
|
Constipation | 2 (8.7) |
|
Nausea | 2 (8.7) |
|
Vomiting | 2 (8.7) |
|
Weakness | 2 (8.7) |
|
Anorexia | 1 (4.3) |
|
Diarrhea | 1 (4.3) |
|
Dyspnea | 1 (4.3) |
|
Hypotension | 1 (4.3) |
| Mouth
ulcer | 1 (4.3) |
|
Mucositis | 1 (4.3) |
|
Rash | 1 (4.3) |
Discussion
The treatment modality for LA SCCHN includes
induction chemotherapy or CRT with a cisplatin and 5-FU combination
as the standard induction regimen (28). The addition of docetaxel to the
standard TPF treatment as induction chemotherapy has shown
significant survival benefits (1,29). The
TPF regimen is recommended for the induction/sequential
chemotherapy of SCCHN by the European Head and Neck Society (EHNC),
European Society for Medical Oncology (ESMO), and European SocieTy
for Radiotherapy and Oncology (ESTRO) guidelines (30,31).
Furthermore, patients who achieve a CR or pathological CR after
induction chemotherapy are likely to have a good prognosis
(30).
An ORR of 68–87% has been reported with
docetaxel-based induction chemotherapy for the treatment of SCCHN
(32,33), whereas in the present study,
NDLS-based induction chemotherapy demonstrated an ORR of 95%. The
median OS with NDLS-based induction chemotherapy was 43.5 months
(follow-up duration, 0.6–80.3 months). Docetaxel-based induction
chemotherapy was previously evaluated in two phase III trials, TAX
323 (32) and TAX 324 (29). In the TAX 323 study, TPF induction
chemotherapy (n=177) resulted in an ORR of 68% and a median OS of
18.8 months (32). Similarly, TPF
induction chemotherapy in the TAX 324 study (n=255) resulted in an
ORR of 72% and a median OS of 71 months (29). In a study by Pointreau et al
(34), the TPF regimen (n=110)
resulted in an ORR of 80%. In the present study, the NDLS-based TPF
regimen was used in 5 patients and resulted in PR in 3 (75%, 3 out
of 4 evaluated, NE=1 excluded) of these patients. Previously,
neoadjuvant chemotherapy with docetaxel and cisplatin (n=34)
demonstrated an ORR of 76.5% and a 3-year OS rate of 94.1%
(35), compared with the 100% PR
recorded in 6 patients (NDLS plus cisplatin) in the present study.
Wang et al (33) used
nimotuzumab, an anti-EGFR humanized monoclonal IgG1 antibody, in
induction chemotherapy with the TPF regimen for LA SCCHN (n=31).
This resulted in an ORR of 87.1% (30), compared with the 100% (CR, 2
patients; PR, 6 patients; 8 out of 8 evaluated, NE=1 excluded)
recorded in the present study. In a phase III study, TPF induction
chemotherapy followed by cetuximab showed a response rate of 78%
(36), while the same regimen was
used in 3 patients in the present study with 2 patients achieving a
PR (100%, 2 out of 2 evaluated, NE=1 excluded).
Docetaxel is recommended as a first-line therapy for
recurrent, unresectable or metastatic SCCHN as a single agent or in
combination with cisplatin/carboplatin with/without 5-FU/cetuximab
(6). In this setting,
docetaxel-based chemotherapy has reported an ORR of 33–97% in the
treatment of SCCHN (37,38). Patients receiving NDLS-based
palliative chemotherapy demonstrated an ORR of 50% and a median OS
of 4.6 months (follow-up duration, 1.8–14.3 months) in the present
study. The Southwest Oncology Group evaluated the combination of
docetaxel with carboplatin for advanced SCCHN (n=68) and reported a
response of 25% and a median OS of 7.4 months (39), while the NDLS-based TP regimen (n=5)
in the present study resulted in a CR and PR in 1 patient each.
In the conventional docetaxel formulation,
polysorbate 80 and ethanol function as formulation vehicles, and
have been implicated in AEs such as acute hypersensitivity
reactions, cumulative fluid retention, peripheral neuropathy
(8), severe non-immunological
anaphylactoid reactions (9),
reactions at injection sites (10)
and alcohol intoxication (11,12). In
the present study, neurotoxicity, fluid retention and acute
hypersensitivity reactions were not reported with NDLS-based
chemotherapy.
In the landmark TAX 323 study, neutropenia (76.9%),
anemia (9.2%), thrombocytopenia (5.2%), febrile neutropenia (5.2%)
and leucopenia (41.6%) were the grade 3/4 hematological AEs,
whereas alopecia (11.6%), infections (6.9%), stomatitis (4.6%),
lethargy (2.9%), diarrhea (2.9%), nausea, vomiting, neurotoxicity,
anorexia and dysphagia (each 0.6%) were the grade 3/4
non-hematological AEs following the TPF regimen. In the TAX 324
study, neutropenia (55%), febrile neutropenia (4.8%), anemia
(4.8%), thrombocytopenia (1.6%) and neutropenic infections (4.8%)
were the grade 3/4 hematological AEs. Meanwhile, stomatitis (8.4%),
nausea (5.6%), dysphagia (5.2%), anorexia (4.8%), vomiting (3.2%),
diarrhea (2.8%), infection (2.4%) and lethargy (2%) were the grade
3/4 non-hematological AEs (29,32). TPF
was the most common regimen used in the present study, and
neutropenia (8.7%), lymphopenia (8.7%) and thrombocytopenia (4.3%)
were the grade 3/4 hematological AEs observed. Grade 4 neutropenia
was reported in 1 (4.34%) patient. Vomiting and weakness were the
most frequently reported non-hematological AEs. Overall, NDLS was
found to be well tolerated in patients with SCCHN.
Corticosteroids are routinely administered as a
premedication to mitigate the toxicity issues of conventional
docetaxel, such as hypersensitivity and the retention of fluid
(40). In a recent study, Obradović
et al (41) used
transcriptional profiling of tumors and matched metastases in
patient-derived xenograft mouse models and indicated the potential
function of glucocorticoid receptor activation in the progression
and metastasis of breast cancer. Corticosteroid premedication is
not warranted with the NDLS formulation, especially when used as
monotherapy, therefore avoidance of corticosteroids may help
circumvent the risk of disease progression.
There are several limitations to the present study.
Due to the retrospective design, the data for safety and survival
are incomplete. The information pertaining to the history of
tobacco use was not available in the medical records of all
patients and hence could not be presented. Progression-free
survival data could not be obtained, as these data and serial scans
were not available for the majority of patients at most of the
follow-up time points.
Overall, the NDLS-based therapy was effective and
well tolerated in the management of SCCHN either as induction or
palliative chemotherapy. The present data provides valuable
insights into the effectiveness and safety of NDLS in the
management of SCCHN. A clinical study is currently underway to
validate the present findings.
Acknowledgements
The authors would like to thank Mr. Shreekant
Sharma, ISMPP CMPP™ for providing assistance in writing the
original manuscript and Dr Venugopal Madhusudhana, ISMPP CMPP™ for
additional editorial assistance in the development of this
manuscript (both Intas Pharmaceuticals Ltd., Ahmedabad, Gujarat,
India).
Funding
This study was funded by an unrestricted research
grant by Intas Pharmaceutical Ltd., Ahmedabad, Gujarat, India,
towards data collection and statistical analysis.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SKDM, SS and GB performed the research, were
involved in the acquisition of data and critically revised the
manuscript for important intellectual content. SKDM, NJ, MAK and IA
conceptualized and designed the study, and were involved in the
data interpretation and critical revision of the manuscript for
important intellectual content. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The study protocol was reviewed and approved by The
OM Ethics Committee (Ahmedabad, India). Dr Nisarg Joshi, who is an
investigator and designed the concept of this multicenter
retrospective study, is not attached to any institute having an
ethics committee; hence, permission was sought from an independent
ethics committee. The study was conducted in accordance with the
ethical principles that have their origin in the Declaration of
Helsinki, and in accordance with the International Conference on
Harmonization's Good Clinical Practice guidelines, applicable
regulatory requirements, and in compliance with the submitted study
protocol. Patient consent to review their medical records was not
required by the ethics committee as NDLS is already approved in
India and patient confidentiality was completely maintained. In
this retrospective study, no patient identifiers were used and
patient data were anonymized.
Patient consent for publication
Not applicable.
Competing interests
Dr Mujtaba A. Khan and Dr Nisarg Joshi are employees
of Intas Pharmaceutical Ltd (Ahmedabad, Gujarat, India). Dr Imran
Ahmad is an employee of Jina Pharmaceutical Inc. (Libertyville, IL,
USA). The NDLS (DoceAqualip) used in the present study was
developed by Intas Pharmaceuticals Limited.
References
|
1
|
Rapidis A, Sarlis N, Lefebvre JL and Kies
M: Docetaxel in the treatment of squamous cell carcinoma of the
head and neck. Ther Clin Risk Manag. 4:865–886. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kulkarni MR: Head and neck cancer burden
in India. Int J Head Neck Surg. 4:29–35. 2013. View Article : Google Scholar
|
|
4
|
Machiels JP, Lambrecht M, Hanin FX, Duprez
T, Gregoire V, Schmitz S and Hamoir M: Advances in the management
of squamous cell carcinoma of the head and neck. F1000Prime Rep.
6:442014. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Seiwert TY and Cohen EEW: State-of-the-art
management of locally advanced head and neck cancer. Br J Cancer.
92:1341–1348. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
NCCN Clinical Practice Guidelines in
Oncology (NCCN Guidelines®), . Head and Neck Cancer. Version
1.2019. simplehttps://www.nccn.org/March
14–2019
|
|
7
|
Van Cutsem E, Moiseyenko VM, Tjulandin S,
Majlis A, Constenla M, Boni C, Rodrigues A, Fodor M, Chao Y, Voznyi
E, et al: Phase III study of docetaxel and cisplatin plus
fluorouracil compared with cisplatin and fluorouracil as first-line
therapy for advanced gastric cancer: A report of the V325 study
group. J Clin Oncol. 24:4991–4997. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
ten Tije AJ, Verweij J, Loos WJ and
Sparreboom A: Pharmacological effects of formulation vehicles:
Implications for cancer chemotherapy. Clin Pharmacokinet.
42:665–685. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Coors EA, Seybold H, Merk HF and Mahler V:
Polysorbate 80 in medical products and nonimmunologic anaphylactoid
reactions. Ann Allergy Asthma Immunol. 95:593–599. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schwartzberg LS and Navari RM: Safety of
polysorbate 80 in the oncology setting. Adv Ther. 35:754–767. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Food and Drug Administration (FDA), . FDA
Drug Safety Communication: FDA warns that cancer drug docetaxel may
cause symptoms of alcohol intoxication after treatment. simplehttps://www.fda.gov/Drugs/DrugSafety/ucm401752.htmJune
20–2014
|
|
12
|
Mirza A and Mithal N: Alcohol intoxication
with the new formulation of docetaxel. Clin Oncol (R Coll Radiol).
23:560–561. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ahmad A, Sheikh S, Ali SM, Ahmad MU,
Paithankar M, Saptarishi D, Maheshwari K, Kumar K, Singh J, Patel
G, et al: Development of aqueous based formulation of docetaxel:
Safety and pharmacokinetics in patients with advanced solid tumors.
J Nanomed Nanotechnol. 6:12015.
|
|
14
|
Narayanan P, Dattatreya PS, Prasanna R,
Subramanian S, Jain K, Somanath NS, Joshi N, Bunger D, Khan MA,
Chaturvedi A and Ahmad I: Efficacy and safety of nanosomal
docetaxel lipid suspension-based chemotherapy in sarcoma: A
multicenter, retrospective study. Sarcoma. 2019:31585902019.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
World Intellectual Property Organization,
. Aqueous systems for the preparation of lipid-based pharmaceutical
compounds; compositions, methods, and uses thereof. Publication No.
WO/2008/127358. simplehttps://patentscope.wipo.int/search/en/detail.jsf?docId=WO2008127358&redirectedID=trueOctober
23–2008
|
|
16
|
Ahmad A, Sheikh S, Taran R, Srivastav SP,
Prasad K, Rajappa SJ, Kumar V, Gopichand M, Paithankar M, Sharma M,
et al: Therapeutic efficacy of a novel nanosomal docetaxel lipid
suspension compared with taxotere in locally advanced or metastatic
breast cancer patients. Clin Breast Cancer. 14:177–181. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ashraf M, Sajjad R, Khan M, Shah M, Bhat Y
and Wani Z: 156P Efficacy and safety of a novel nanosomal docetaxel
lipid suspension (NDLS) as an anti cancer agent-a retrospective
study. Ann Oncol. 27 (Suppl 9):Six46–Six51. 2016. View Article : Google Scholar
|
|
18
|
Naik R and Khan MA: Doceaqualip in a
patient with prostate cancer who had an allergic reaction to
conventional docetaxel: A case report. Mol Clin Oncol. 6:341–343.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Prasanna R, Bunger D and Khan MA: Efficacy
and safety of doceaqualip in a patient with locally advanced
cervical cancer: A case report. Mol Clin Oncol. 8:296–299.
2018.PubMed/NCBI
|
|
20
|
Vyas V, Joshi N and Khan M: Novel
docetaxel formulation (NDLS) in low cardiac reserve ovarian cancer.
Open Access J Cancer Oncol. 2:0001222018.
|
|
21
|
Gupta S, Pawar SS and Bunger D: Successful
downstaging of locally recurrent penile squamous cell carcinoma
with neoadjuvant nanosomal docetaxel lipid suspension (NDLS) based
regimen followed by curative surgery. BMJ Case Rep.
2017:bcr20172206862017. View Article : Google Scholar
|
|
22
|
Murali A, Gupta S and Pendharkar D:
Efficacy and tolerability of nanoparticle docetaxel lipid
suspension. J Clin Oncol. 36 (Suppl 15):e145422018. View Article : Google Scholar
|
|
23
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
U.S. Department of Health and Human
Services, . Common Terminology Criteria for Adverse Events (CTCAE).
Version 5.0. simplehttps://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5×7.pdfNovember
27–2017
|
|
25
|
Dixon JR Jr: The international conference
on harmonization good clinical practice guideline. Qual Assur.
6:65–74. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
World Medical Association, . World Medical
Association Declaration of Helsinki: Ethical principles for medical
research involving human subjects. JAMA. 310:2191–2194. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Datta SS, Ghosal N, Daruvala R,
Chakraborty S, Shrimali RK, van Zanten C, Parry J, Agrawal S,
Atreya S, Sinha S, et al: How do clinicians rate patient's
performance status using the ECOG performance scale? A
mixed-methods exploration of variability in decision-making in
oncology. Ecancermedicalscience. 13:9132019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Posner MR and Lefebvre JL: Docetaxel
induction therapy in locally advanced squamous cell carcinoma of
the head and neck. Br J Cancer. 88:11–17. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Posner MR, Hershock DM, Blajman CR,
Mickiewicz E, Winquist E, Gorbounova V, Tjulandin S, Shin DM,
Cullen K, Ervin TJ, et al: Cisplatin and fluorouracil alone or with
docetaxel in head and neck cancer. N Engl J Med. 357:1705–1715.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Haddad R, Tishler R, Wirth L, Norris CM,
Goguen L, Sullivan C, O'Donnell L, Li Y and Posner M: Rate of
pathologic complete responses to docetaxel, cisplatin, and
fluorouracil induction chemotherapy in patients with squamous cell
carcinoma of the head and neck. Arch Otolaryngol Head Neck Surg.
132:678–681. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Grégoire V, Lefebvre JL, Licitra L and
Felip E; EHNS-ESMO-ESTRO Guidelines Working Group, : Squamous cell
carcinoma of the head and neck: EHNS-ESMO-ESTRO clinical practice
guidelines for diagnosis, treatment and follow-up. Ann Oncol. 21
(Suppl 5):Sv184–Sv186. 2010. View Article : Google Scholar
|
|
32
|
Vermorken JB, Remenar E, van Herpen C,
Gorlia T, Mesia R, Degardin M, Stewart JS, Jelic S, Betka J, Preiss
JH, et al: Cisplatin, fluorouracil, and docetaxel in unresectable
head and neck cancer. N Engl J Med. 357:1695–1704. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang X, Gu J, Shao C, Han K and Meng J:
Nimotuzumab plus chemotherapy with docetaxel, cisplatin,
5-fluorouracil for locally advanced head and neck squamous cell
carcinoma: A clinical study. J Cancer Res Ther. 15:312–316.
2019.PubMed/NCBI
|
|
34
|
Pointreau Y, Garaud P, Chapet S, Sire C,
Tuchais C, Tortochaux J, Faivre S, Guerrif S, Alfonsi M and Calais
G: Randomized trial of induction chemotherapy with cisplatin and
5-fluorouracil with or without docetaxel for larynx preservation. J
Natl Cancer Inst. 101:498–506. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hui EP, Ma BB, Leung SF, King AD, Mo F,
Kam MK, Yu BK, Chiu SK, Kwan WH, Ho R, et al: Randomized phase II
trial of concurrent cisplatin-radiotherapy with or without
neoadjuvant docetaxel and cisplatin in advanced nasopharyngeal
carcinoma. J Clin Oncol. 27:242–249. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hitt R, Mesia R, Grau JJ, Iglesias L,
Barco ED, Lozano A, Trufero JM, Giron CG, Martin AL and hernandez
JJC: Randomized phase III trial of induction chemotherapy (ICT)
with docetaxel-cisplatin-5fluorouracil (DCF) followed by
cisplatin-radiotherapy (CRT) or cetuximab-radiotherapy (CetRT) in
patients (pts) with locally advanced unresectable head and neck
cancer (LAUHNC). J Clin Oncol. 34 (Suppl 15):60012016. View Article : Google Scholar
|
|
37
|
Specht L, Larsen SK and Hansen HS: Phase
II study of docetaxel and cisplatin in patients with recurrent or
disseminated squamous-cell carcinoma of the head and neck. Ann
Oncol. 11:845–849. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Rawat S, Tandan H, Patel S and Chaudhari
S: Safety and efficacy of nimotuzumab with concurrent
chemoradiotherapy in unresectable locally advanced squamous cell
carcinoma of head and neck: An Indian rural hospital experience.
South Asian J Cancer. 8:52–56. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Samlowski WE, Moon J, Kuebler JP, Nichols
CR, Gandara DR, Ozer H, Williamson SK, Atkins JN, Schuller DE and
Ensley JF: Evaluation of the combination of docetaxel/carboplatin
in patients with metastatic or recurrent squamous cell carcinoma of
the head and neck (SCCHN): A Southwest oncology group phase II
study. Cancer Invest. 25:182–188. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Weiss RB, Donehower RC, Wiernik PH, Ohnuma
T, Gralla RJ, Trump DL, Baker JR Jr, Van Echo DA, Von Hoff DD and
Leyland-Jones B: Hypersensitivity reactions from taxol. J Clin
Oncol. 8:1263–1268. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Obradović MMS, Hamelin B, Manevski N,
Couto JP, Sethi A, Coissieux MM, Münst S, Okamoto R, Kohler H,
Schmidt A and Bentires-Alj M: Glucocorticoids promote breast cancer
metastasis. Nature. 567:540–544. 2019. View Article : Google Scholar : PubMed/NCBI
|