Introduction
Thyroid paraganglioma (TP) is an uncommon
neuroendocrine tumor that arises from the inferior laryngeal
paraganglia (1). One hypothesis is
that the inferior laryngeal paraganglia may form within the thyroid
capsule, which could eventually develop into an intrathyroid
paraganglioma (2). Another
hypothesis is that paraganglioma develops from the inferior
laryngeal paraganglia, pulls downward slowly, and eventually rests
lateral to the thyroid gland (3).
The diagnosis of TP is challenging, not due to its
low prevalence (only 60 cases have been reported), but the
cytologic and histopathologic similarities with other thyroid
tumors, such as medullary thyroid carcinoma (MTC) (4,5). The
examination of immunohistochemical staining serves an important
role in the definitive diagnosis; however, to the best of our
knowledge, the malignant potential of TP remains unknown (3,6,7). Since it was described by Van Miert
(8) in 1964, TP has been commonly
considered to have low malignant potential (1,9,10).
In the present study, two cases of TP are presented,
both of which tended to mimic MTC, one was accompanied by lymph
node metastasis, and the other exhibited high malignant potential
of trachea invasion. Furthermore, a clinical strategy of positive
diagnostic and therapeutic management was introduced with the
guidance of a systematic review.
Case report
Case 1
A 44-year-old man presented with a 3-month history
of hoarseness, but without any signs of breathing or swallowing
difficulties. The patient was admitted to The Second Affiliated
Hospital of Zhejiang University (Hangzhou, China) on September 26,
2016. A 2.6-cm hypoechoic, noncalcific neoplasm in the left thyroid
lobe was identified in the ultrasound examination. Serum thyroid
stimulating hormone, T3 and T4 were consistent with euthyroid state
and serum calcitonin was normal. A fine needle aspiration biopsy
(FNAB) was performed, and cytological results were regarded as
suspicious for malignancy according to the Bethesda classification
system (11,12). During the surgical procedure, a
2.5-cm gray nodule was detected in the thyroid capsule and was
presumptively diagnosed as MTC by frozen section analysis. The
patient underwent total thyroidectomy and bilateral central neck
dissection. In the examination of definitive histopathology, TP was
diagnosed by histopathologic and immunohistochemistry features.
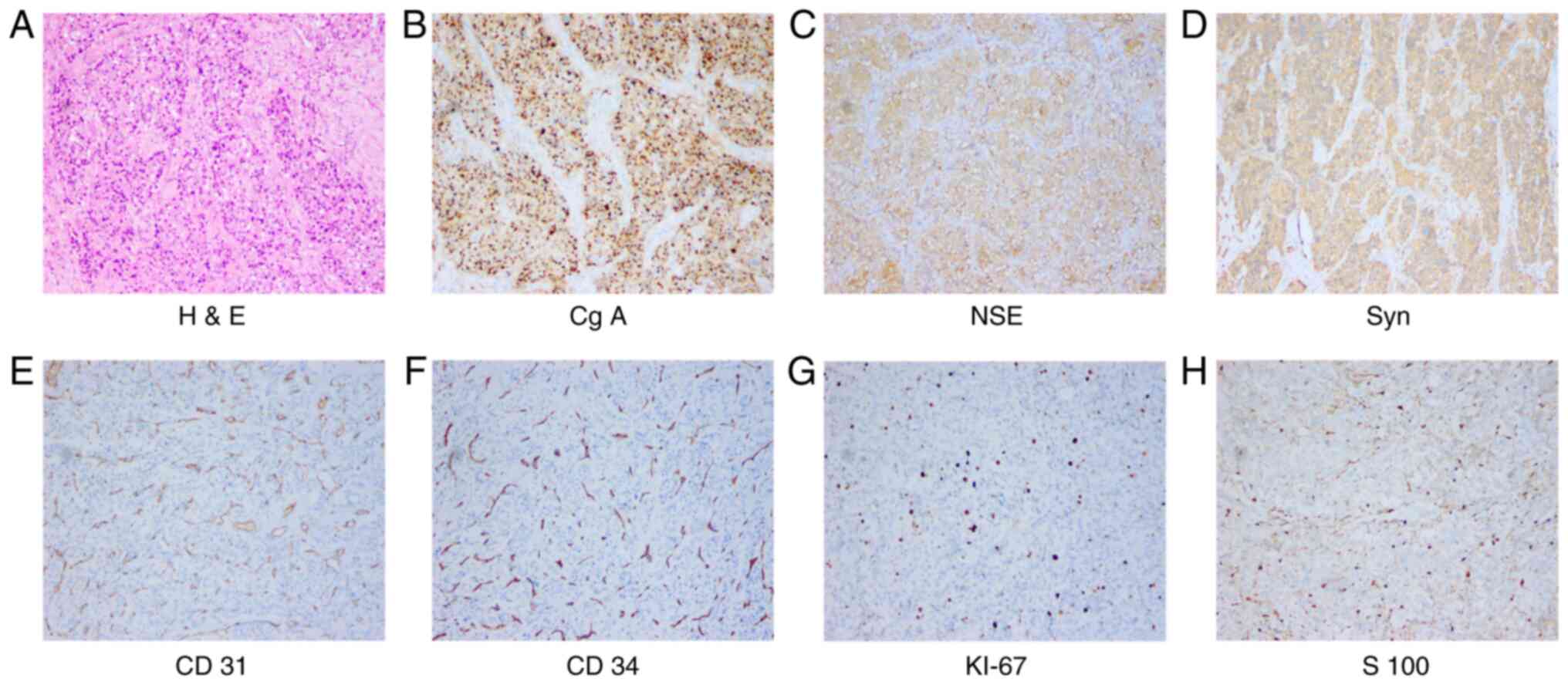
Tumor cells appeared to be nesting and exhibited a ‘zellballen’
growth pattern in H&E staining, and were positive for Cg A,
Syn, neuronal specific enolase (NSE), Ki67, S-100, CD34, CD31 and
p53, and negative for thyroglobulin, thyroid transcription factor 1
(TTF-1), calcitonin, CEA, cytokeratin (CK) (AE1/3), CD45, Bcl2,
inhibin and galectin-3. Among them, the most representative
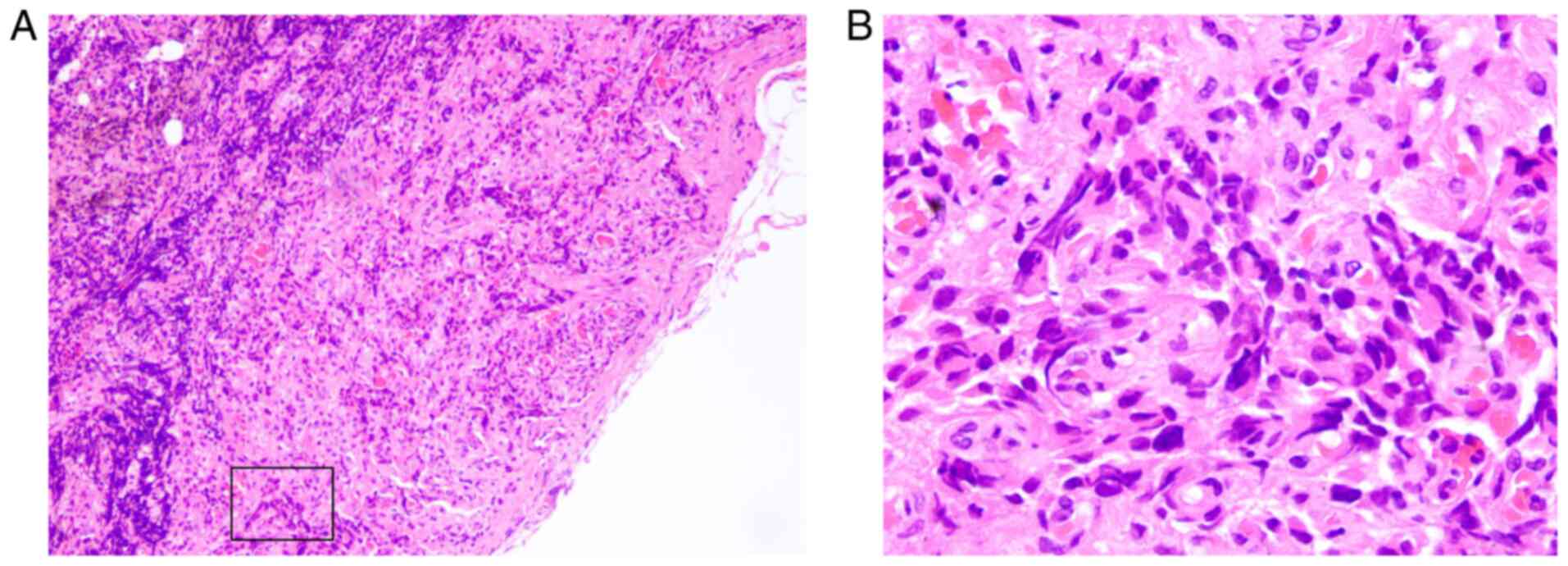
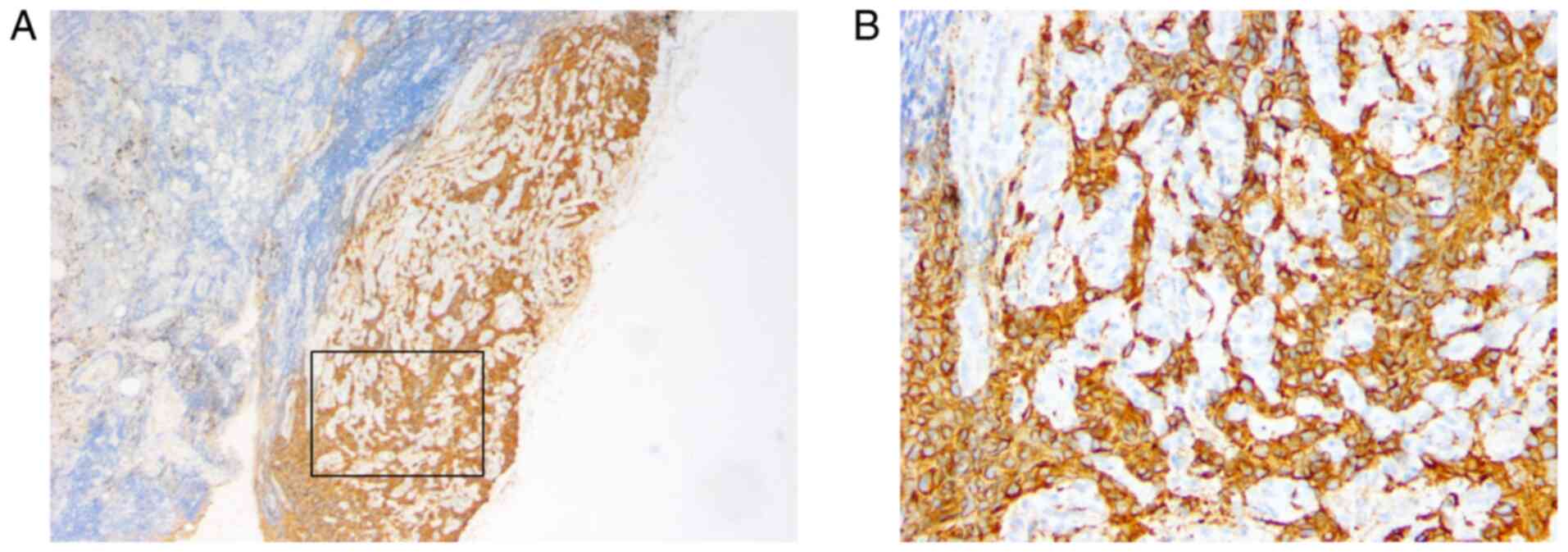
characteristics were analyzed and exhibited in Fig. 1. In addition, the dissected lymph
nodes were assessed and metastasis was confirmed by H&E
staining and immunohistochemistry (Figs.
2 and 3). The patient was
treated with radiation after the surgery and was in a good
condition without any evidence of other metastases during the
follow-up (twice a year) at 3 years postoperatively.
Case 2
A 39-year-old woman was suspected to have TP
recurrence following hemi-thyroidectomy surgery. The patient was
admitted to The Second Affiliated Hospital of Zhejiang University
on April 17, 2016. The patient was diagnosed with TP during the
last hemithyroidectomy surgery performed 8 years ago (2008), based
on the typical histopathologic and immunohistochemical
characteristics as reported in a previous article (5). Postoperatively, the patient was doing
well without any sign of recurrence or metastasis, and no other
relevant finding was found during the follow-up (twice a year) for
7 years. However, a 2.5-cm hypoechoic nodule was identified by
physical and ultrasound examination in the ‘thyroid area’ 1 year
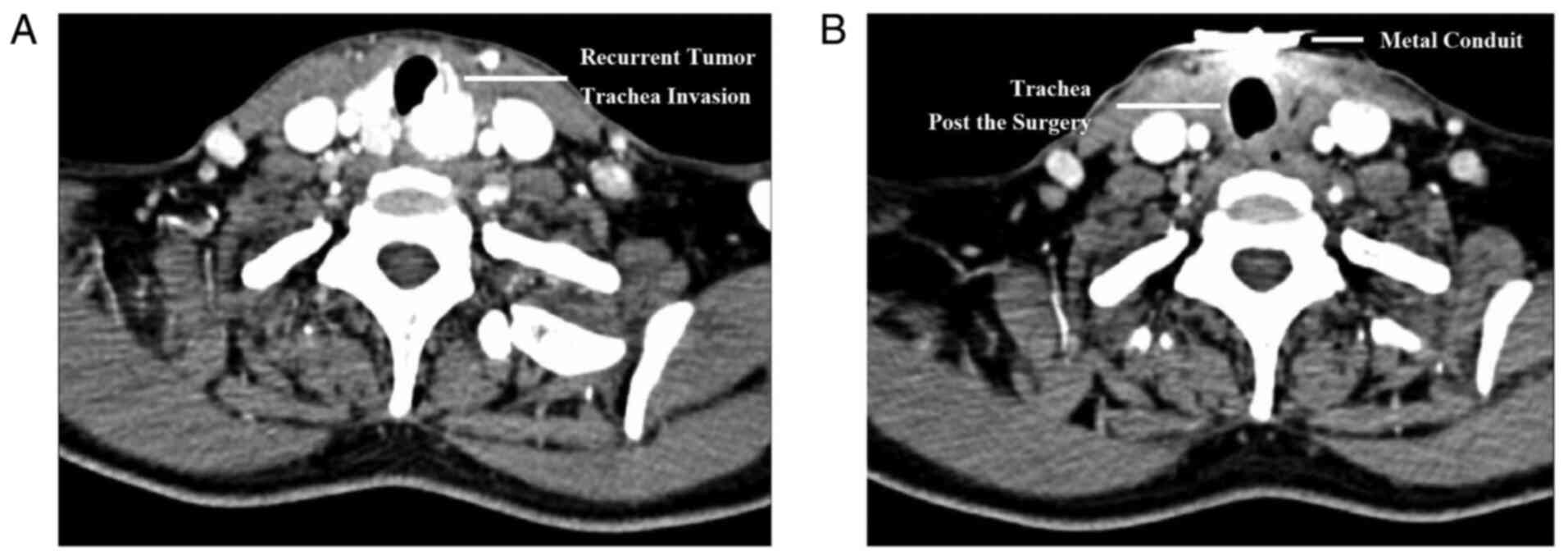
ago (2015). According to the computed tomography examination, the
tumor exhibited aggressive potential with trachea invasion
(Fig. 4A). For radical resection,
thyroidectomy and trachea incision were performed. All tumor
tissues were completely dissected, and a temporary cannula was
indwelling in the channel of trachea (Fig. 4B). Immunohistochemical staining was
positive for Cg A, Syn, NSE and CD56 in tumor cells, negative for
thyroglobulin, TTF-1, calcitonin, CEA, CK (AE1/3) and P53 in tumor
cells, and 20% positive for S-100 in sustentacular cells.
Furthermore, gene analysis was performed, and there was no mutation
in the genes of Kras exon 2/3/4, NRAS proto-oncogene, GTPase exon
2/3/4, telomerase reverse transcriptase C228T/T250T,
phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit α
E545K/H1047R and BRAF V600E. In addition, the right thyroid lobe
was confirmed to be normal, and no metastatic lymph node was found
in the neck VI area (0/10 positive). The patient was diagnosed with
recurring TP and treated with radiation (8). The patient was follow-up at the
outpatient twice a year, and no sign of recurrence or metastasis 4
years postoperatively.
H&E and immunohistochemical
staining
Surgical specimens were fixed in 10%
phosphate-buffered, neutral formaldehyde solution at room
temperature for 24 h. Tissue sections (4-µm thick) were
deparaffinized in a descending alcohol series, rehydrated (using 10
mM sodium citrate, PH 8.5, at 80°C for 30 min), and exposed to
heat-induced antigen retrieval for 5 min in an autoclave at 121°C
in pH 7.8 Tris-EDTA-Citrate buffer. 4-µm sections were stained with
H&E and immunohistochemistry, respectively. H&E
staining: Incubate the slides with hematoxylin solution in a
staining jar for 10 min to stain the nuclei at room temperature.
Transfer the slides to a staining jar with running water (tap water
is fine) till the water is clear. Transfer the slides to a staining
jar with Eosin solution for 3 min at room temperature. Then
transfer the slides into staining jars with 70% ethanol for 20 sec,
90% ethanol for 20 sec, 100% ethanol for 1 min and xylene for 3
min. Take out slides from xylene and place the slides in a fume
hood till the slides are dry. Store the slides at room temperature.
H&E images were captured with a 14.0 MP digital microscope
camera which is attached via a c-mount to the side port of a Leica
DM 2500 microscope (for the light, magnifications: ×40, ×100, ×200,
and ×400). Immunohistochemistry (IHC) staining: Place the
slides in a wet chamber (with 0.3% nonfat dry milk) and block the
sections with blocking buffer for at least 30 min at room
temperature. Then incubated at room temperature for 2 h in a humid
chamber. Primary antibody was applied at 37°C for 60 min. The
specific antibodies included TTF-1 (8G7G3/1; 1:100 dilution),
calcitonin (GA51561-2; 1:100 dilution), CEA (IS62230-2; 1:400
dilution), NSE (M0873; 1:100 dilution), Syn (GA660; 1:400
dilution), Cg A (M0869; 1:100 dilution), S-100 (GA504; 1:400
dilution) (4), Ki67 (M7240; 1:100
dilution) (5), CK (AE1/3) (IS053;
1:100 dilution), thyroglobulin (A0251; 1:200 dilution) (7), p53 (GA616; dilution 1:200) (10), galectin-3 (M3/38; 1:100 dilution) and
Bcl2 (M0887; 1:400 dilution) (13)
(Agilent Technologies, Inc.). Subsequently the sections were washed
three times with PBS and anti-mice/rabbit enzyme-labeled secondary
antibodies (P0447 or E0432; 1:200 dilution, Dako; Agilent
Technologies, Inc.) were then applied at room temperature for 15
min. IHC images were captured with a 14.0 MP digital microscope
camera which is attached via a c-mount to the side port of a Leica
DM 2500 microscope (magnifications: ×40, ×100, ×200 and ×400).
Immunohistochemistry was performed according to the DAKO EnVision
method (13,14). The expression levels of TTF-1,
calcitonin, CEA, NSE, Syn, Cg A, S-100, Ki67, CK (AE1/3),
thyroglobulin, p53, galectin-3 and Bcl2 were semi-quantified using
a visual grading system based on the extent of staining as
previously described (14): Grade 0,
virtually no immunoreactivity; grade 1, patchy to diffuse weak
immunoreactivity; grade 2, patchy to diffuse moderate
immunoreactivity; and grade 3, patchy to diffuse strong
immunoreactivity. According to the results of the
immunohistochemistry analysis, grade 0 cases were classified as the
negative group, and grade 1–3 cases as the positive group.
Subsequently the immunohistochemical results were collected and
analyzed (Table I).
 | Table I.Immunohistochemical staining results
of the two cases of thyroid paraganglioma. |
Table I.
Immunohistochemical staining results
of the two cases of thyroid paraganglioma.
|
Immunohistochemistry | Case 1 | Case 2 |
|---|
| Tumor cells,
chromogranin A | Positive | Positive |
| Tumor cells, NSE | Positive | Positive |
| Tumor cells,
synaptophysin | Positive | Positive |
| Tumor cells,
calcitonin | Negative | Negative |
| Tumor cells,
cytokeratin | Negative | Negative |
| Tumor cells,
thyroglobulin | Negative | Negative |
| Tumor cells,
TTF-1 | Negative | Negative |
| Tumor cells,
calcitonin | Negative | Negative |
| Tumor cells, CEA | Negative | Negative |
| Tumor cells, CK
(AE1/3) | Negative | Negative |
| Tumor cells, P53 | Weakly positive | Negative |
| Tumor cells,
CD56 | Not performed | 20% positive |
| Endothelial cells,
CD34 | Positive | Not performed |
| Endothelial cells,
CD31 | Positive | Not performed |
| Tumor cells,
Ki67 | 10% positive | 10% positive |
| Sustentacular cells,
S-100 | Positive | 20% positive |
Discussion
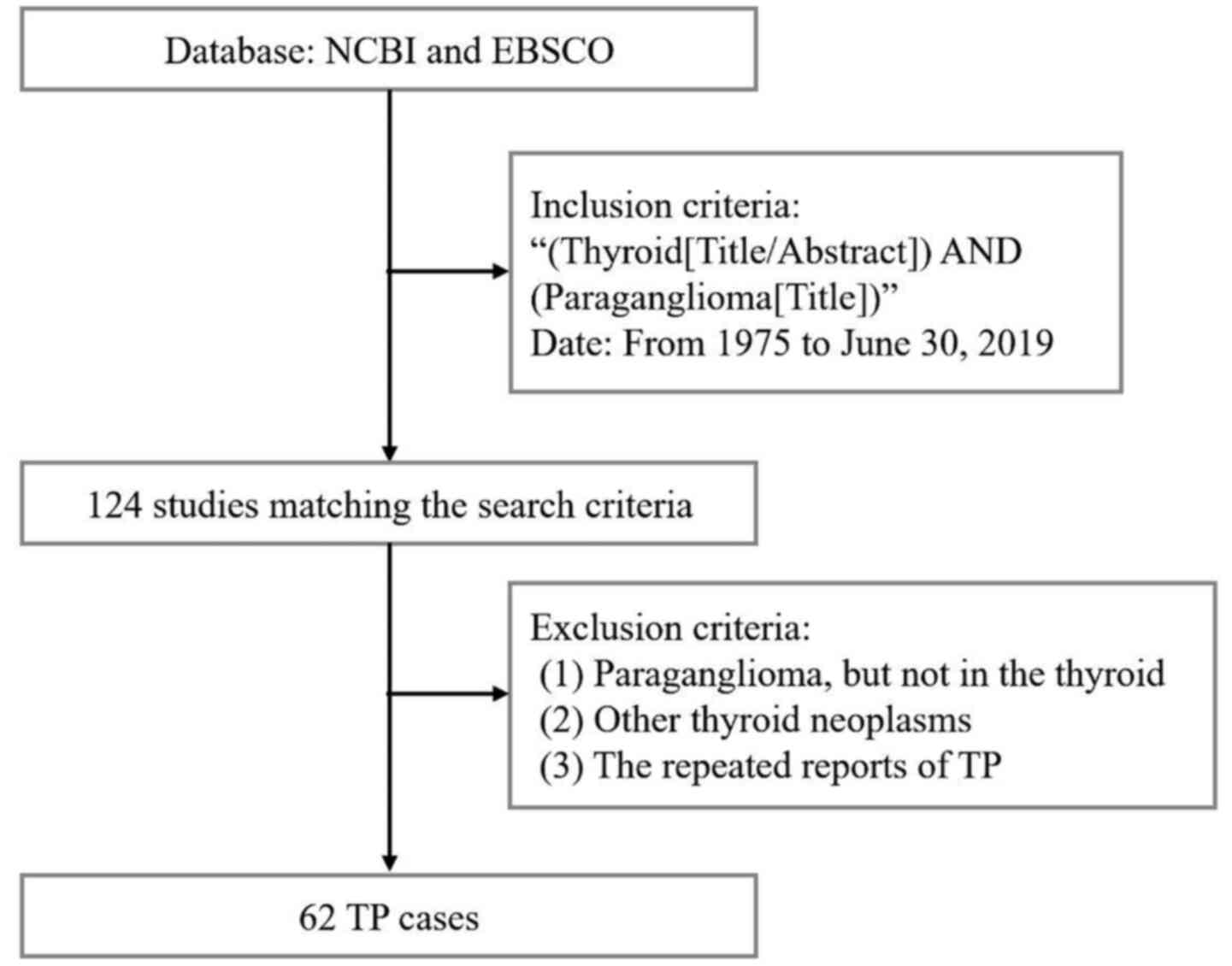
TP is rare. A detailed literature search [inclusion
criteria (Thyroid[Title/Abstract]) AND (Paraganglioma[Title]);
Database NCBI (https://pubmed.ncbi.nlm.nih.gov)] of TPs between 1975
and June 30, 2019 was performed. Excluding the diagnosis of carotid
body paraganglioma, jugular paraganglioma, medullary thyroid
carcinoma, and the repeated reported cases, 62 reported TP cases
were included from 124 related studies (Fig. 5). TP represents a challenge for
surgeons in terms of diagnosis and making appropriate treatment
decisions (15). Among them, the
malignant potential of TPs remains controversial, and more studies
are required to help surgeons make diagnostic and therapeutic
management decisions. Due to the rarity of this disease, samples
from every available case need to be collected to validate the
findings.
The examination of immunohistochemistry staining
serves an important role in the definitive diagnosis (5). In the present study, tissues were
obtained from two patients for diagnostic purposes. Several typical
characteristics of the two cases are recorded in Table I, and the results were consistent
with the World Health Organization's classification of endocrine
tumors for paraganglioma (16). The
molecular features of TPs have been presented and summarized in
previous studies (3–5). Based on the studies identified in the
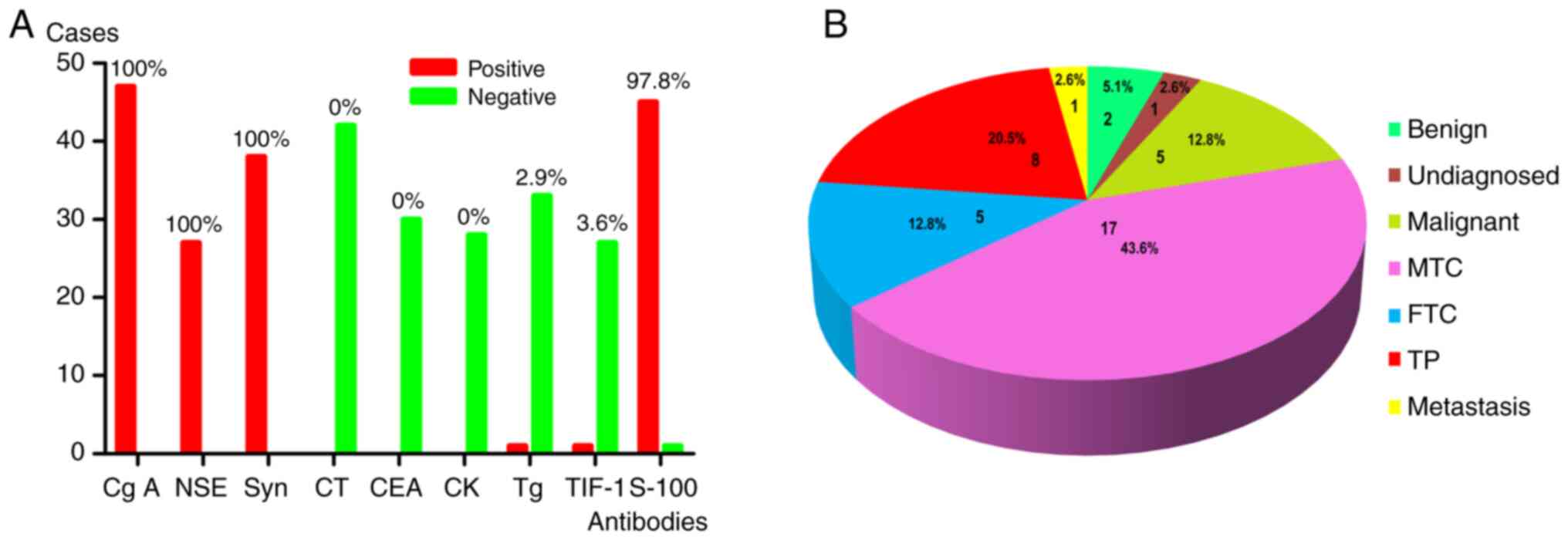
literature analysis, chief cells exhibit strong positivity for the
markers of neuroendocrine tumors, including Cg A (positive rate,
100%), NSE (positive rate, 100%) and Syn (positive rate, 100%). The
negative makers include calcitonin (negative rate, 100%), CK
(AE1/3) (negative rate, 100%) and CEA (negative rate, 100%). The
thyroid originated markers also exhibit negative staining,
including thyroglobulin (negative rate, 97.1%) and TTF-1 (negative
rate, 96.4%). In addition, S-100 staining was positive in
sustentacular cells (positive rate, 97.8%; Fig. 6A). Therefore, a wide range of
antibodies should be examined for the definitive diagnosis of TP,
including at least Cg A, NSE, Syn, calcitonin, CEA, CK (AE1/3),
thyroglobulin and TTF-1 in tumor cells, and S-100 in sustentacular
cells.
However, a definitive diagnosis of TP always
requires the examination of immunohistochemistry postoperatively,
while it is rarely diagnosed preoperatively or intraoperatively
(15). The diagnosis of TP is
difficult due to its overlapping cytologic features, and it is
often misdiagnosed as benign or malignant thyroid tumors (16). As shown in the present two cases, the
unique molecular markers of TP compared with other thyroid
neoplasms include positive expression of Cg A, NSE, Syn and S-100,
and negative expression of calcitonin, CK (AE1/3), CEA,
thyroglobulin and TTF-1. To determine the mechanisms of TP, the
embryological origin needs to be investigated more thoroughly.
TP is supposed to be derived from the inferior
laryngeal paraganglion and then migrate into the thyroid lobe with
embryologic development (17). This
embryologic origin explains that the cytologic characteristics of
TP are similar to other neuroendocrine lesions of the thyroid,
including C-cell tumors (C-cell hyperplasia and MTC) and
follicular-derived tumors (follicular and papillary thyroid
carcinoma) (5,16). Therefore, it is difficult to diagnose
TP only by distinguishing cytologic features, such as FNAB, frozen
section analysis or general pathology.
Through the literature review, 39 TP cases were
performed with frozen section analysis intraoperatively, and the
results were collected and summarized. Only eight patients were
diagnosed correctly; however, most patients were misdiagnosed as
having MTC, FTC or secondary metastatic tumors from neuroendocrine
carcinoma (Fig. 6B). A comprehensive
examination of CT, MRI and serum proteins needs to be performed
prior to biopsy. The biomarkers thyroglobulin, calcitonin and
catecholamine are specific for other thyroid neoplasms, and can
help to distinguish TP. Elevated thyroglobulin levels indicate a
diagnosis of papillary thyroid carcinoma or follicular thyroid
carcinoma (18). Furthermore,
elevated calcitonin levels are a specific characteristic of MTC
(19). Catecholamine hypersecretion
can be occasionally detected in malignant paraganglioma, defined by
distant metastases or extensive local invasion into adjacent tissue
(20). However, as reported in the
present study and previous literature (4–6), the
levels of catecholamine were not increased in TP, which can be
differentiated from other paragangliomas. Similarly, a correct
diagnosis could not be established preoperatively by FNAB, except
for by immunohistochemistry, until the introduction of the novel
method using cell block material (21). The cell block method made it possible
to investigate the pathological characteristic of the ‘zellballen’
pattern in FNAB specimen preoperatively. Additionally, it could be
evaluated by immunohistochemistry, which was consistent with the
characteristics of paraganglioma described previously (16,21). The
diagnosis of TP can be successfully achieved, and these
observations suggest that the method comprising FNAB and
immunohistochemistry is promising for the differentiation between
TP and other thyroid neoplasms.
Different options that range between subtotal
thyroidectomy and total thyroidectomy are recommended depending on
the malignancy of neoplasms, which is based on the presence of
metastasis to lymph nodes or other organs (4,5).
Compared with other thyroid neoplasms, adjuvant therapies for TPs
are controversial due to most of them presenting as mild and
limited germination (15). However,
the malignant type of TPs with local infiltration, and lymph node
or distant metastasis, still requires attention (3). In the present study, a patient (case 1)
was diagnosed with TP accompanied by lymph node metastasis. Another
implication is recurrence of TP that probably represented high
malignant potential. As shown in case 2, the patient underwent
hemithyroidectomy and was followed-up for 7 years until recurrence.
The tumor exhibited malignant behaviors of rapid growth and trachea
invasion in the first recurring year. For these patients,
radiotherapy was helpful, and was performed after total
thyroidectomy and bilateral central node dissection (8). Both patients in the present study
underwent standard treatment for TP with favorable outcomes
(2–5,8).
According to these experiences, thyroidectomy is appropriate in the
treatment of TP (4,5). Since some cases of TP exhibit highly
malignant behavior, radiotherapy may be considered as a second-line
treatment (8). The duration of
follow-up should be >3 years due to the possibility of
recurrence.
In conclusion, these two rare cases are helpful to
recognize the malignant potential of TP. The present study is
helpful to create a standardized set of immunohistochemical
staining markers to completely distinguish TP from other thyroid
neoplasms. Besides local infiltration, distant metastasis,
manifestations of recurrence and lymph node metastasis may be other
symptoms representing high malignant potential. The appropriate
therapeutic management requires verification in further clinical
trials, and methods of surgical dissection or radiotherapy depend
on the malignant potential of the tumor.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Public
Welfare Projects of Zhejiang Province (grant no. LGH18H160002).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
PW and JFL assessed the clinical findings of the
cases and were responsible for the conception of the present study.
XY, YW and QX designed the research and were responsible for
quality control of the data. HY, QZ, CX and MZ made substantial
contributions to acquisition, analysis and interpretation of the
data. XY and YW drafted the initial manuscript. PW and JFL provided
constructive discussions and revised the manuscript critically for
important intellectual content. All authors have read and approved
the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the 2nd Affiliated Hospital, School of Medicine,
Zhejiang University (Hangzhou, China; approval no. 2019-393), and
written informed consent was provided by all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lee SM and Policarpio-Nicolas ML: Thyroid
paraganglioma. Arch Pathol Lab Med. 139:1062–1067. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Phitayakorn R, Faquin W, Wei N, Barbesino
G and Stephen AE: Thyroid-associated paragangliomas. Thyroid.
21:725–733. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Navaratne L, Mathew RG, Kousparos G and
McCombe A: The management of locally invasive primary thyroid
paraganglioma: A case report and review of the literature. Head
Neck Pathol. 11:139–145. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pelizzo MR, Conti C, Pennelli G, Bellan E,
Cook GJ, Wong KK, Colletti PM, Boschin IM and Rubello D: Thyroid
paraganglioma: Our experience and systematic review of the
literature on a rare tumor. Am J Clin Oncol. 41:416–423. 2016.
|
|
5
|
Yu X, Wang Y, Wang P, Ji CH, Miao CD and
Zheng S: Primary thyroid paraganglioma mimicking medullary thyroid
carcinoma: A case report. Oncol Lett. 10:1000–1002. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bugalho MJ, Silva AL and Domingues R:
Coexistence of paraganglioma/pheochromocytoma and papillary thyroid
carcinoma: A four-case series analysis. Fam Cancer. 14:603–607.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Armstrong MJ, Chiosea SI, Carty SE, Hodak
SP and Yip L: Thyroid paragangliomas are locally aggressive.
Thyroid. 22:88–93. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vanmiert PJ: The treatment of
chemodectomas by radiotherapy. Proc R Soc Med. 57:946–951.
1964.PubMed/NCBI
|
|
9
|
Kieu V, Yuen A, Tassone P and Hobbs CG:
Cervical paraganglioma presenting as thyroid neoplasia. Otolaryngol
Head Neck Surg. 146:516–518. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Castelblanco E, Gallel P, Ros S, Gatius S,
Valls J, De-Cubas AA, Maliszewska A, Yebra-Pimentel MT, Menarguez
J, Gamallo C, et al: Thyroid paraganglioma. Report of 3 cases and
description of an immunohistochemical profile useful in the
differential diagnosis with medullary thyroid carcinoma, based on
complementary DNA array results. Hum Pathol. 43:1103–1112. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lim JXY, Nga ME, Chan DKH, Tan WB,
Parameswaran R and Ngiam KY: Subclassification of Bethesda atypical
and follicular neoplasm categories according to nuclear and
architectural atypia improves discrimination of thyroid malignancy
risk. Thyroid. 28:511–521. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim M, Jeon MJ, Han M, Lee JH, Song DE,
Baek JH, Kim TY, Kim WB, Shong YK and Kim WG: Tumor growth rate
does not predict malignancy in surgically resected thyroid nodules
classified as Bethesda category III with architectural atypia.
Thyroid. 29:216–221. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bockhorn M, Sheu SY, Frilling A, Molmenti
E, Schmid KW and Broelsch CE: Paraganglioma-like medullary thyroid
carcinoma: A rare entity. Thyroid. 15:1363–1367. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
He Y, Zhou Y, Zhang J, Yuan F, Wang J, Du
L, Zhou Q, Liang J and Ding X: Tumor immunohistochemistry and
preoperative magnetic resonance imaging features predict local
recurrence of giant cell tumor of bone following intralesional
curettage. Oncol Lett. 17:1425–1434. 2019.PubMed/NCBI
|
|
15
|
von Dobschuetz E, Leijon H, Schalin-Jäntti
C, Schiavi F, Brauckhoff M, Peczkowska M, Spiazzi G, Demattè S,
Cecchini ME, Sartorato P, et al: A registry-based study of thyroid
paraganglioma: Histological and genetic characteristics. Endocr
Relat Cancer. 22:191–204. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Williams MD and Tischler AS: Update from
the 4th edition of the world health organization classification of
head and neck tumours: Paragangliomas. Head Neck Pathol. 11:88–95.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Taweevisit M, Bunyayothin W and Thorner
PS: Thyroid paraganglioma: ‘Naked’ nuclei as a clue to diagnosis on
imprint cytology. Endocr Pathol. 26:232–238. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lin JD: Thyroglobulin and human thyroid
cancer. Clin Chim Acta. 388:15–21. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Emmertsen K: Medullary thyroid carcinoma
and calcitonin. Dan Med Bull. 32:1–28. 1985.PubMed/NCBI
|
|
20
|
Fishbein L: Pheochromocytoma and
paraganglioma: Genetics, diagnosis, and treatment. Hematol Oncol
Clin North Am. 30:135–150. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Çetin S, Kir G and Yilmaz M: Thyroid
paraganglioma diagnosed by fine-needle aspiration biopsy,
correlated with histopathological findings: Report of a case. Diagn
Cytopathol. 44:643–647. 2016. View
Article : Google Scholar : PubMed/NCBI
|