Introduction
Ovarian cancer is a common malignancy of the female
reproductive system, with the second highest incidence and the
highest mortality among cancers worldwide (1–3). Ovarian
cancer is asymptomatic in its early stages, and the rapid growth of
ovarian cancer makes its diagnosis and treatment challenging
(4,5). Serous ovarian cancer (SOC) is the main
type of ovarian cancer, which progresses quickly, lacks effective
treatment and is prone to bladder metastasis (6). At present, surgery supplemented by
chemotherapy remains the main treatment option for advanced stages
of SOC, and treatment outcomes are unsatisfactory (7). In recent years, targeted therapy has
shown advantages for SOC treatment (8,9).
However, in order to treat SOC effectively, more effective
therapeutic targets need to be developed (10).
Adenylate kinase 4 (AK4) is a ubiquitous enzyme and
a member of adenylate kinases with multiple biological functions on
cellular metabolism, such as maintaining the homeostasis of
cellular nucleotides and controlling cellular ATP levels by
regulating phosphorylation and activation of the energy sensor
protein kinase AMPK (11,12). Human AK4 was initially named AK3, due
to its 58% homology with the bovine AK3, and was subsequently
renamed as AK4 when identified in the mammalian central nervous
system (13). Additionally, AK4
contains an N-terminal mitochondrial import sequence which mediates
localization to the mitochondrial matrix (14). AK4 is widely expressed in multiple
types of tissues such as kidney, heart and liver (15). AK4 was also reported to participate
in protection from oxidative stress (16).
The involvement of AK4 in the progression of
multiple types of cancer has been investigated (17–21). AK4
has been associated with the clinical features of patients with
lung cancer and promoted the metastasis of lung cancer by
downregulating ATF3 (17,18). Another study on lung cancer found
that AK4 could modulate oxidative stress and stabilize HIF-1α to
promote the metastasis of lung adenocarcinoma (19). Similarly, AK4 contributed to bladder
cancer cell proliferation and invasion in vitro and in
vivo (20). In esophageal
cancer, microRNA (miR)-199a-3p, regulated the radioresistance of
cancer cells by targeting AK4 (21).
However, the possible role of AK4 in the progression and metastasis
of SOC remains unclear.
In the present study, the expression levels of AK4
were investigated in human SOC tissues to investigate the role of
AK4 in the progression of SOC and to evaluate the potential of AK4
as a therapeutic target for SOC treatment.
Materials and methods
Antibodies, primers and short hairpin
(sh)RNA plasmids
Rabbit anti-AK4 [cat. no. ab131327;
immunohistochemical (IHC) assay dilution, 1:100; western blotting
assay dilution, 1:1,000; Abcam] and mouse anti-β-actin (dilution,
1:2,000; cat. no. ab8226; Abcam) antibodies were used.
The following primers were used for reverse
transcription-quantitative PCR (RT-qPCR): AK4 forward,
5′-AKATGGACCGTGTGCTGCTGAAGT-3′ and reverse,
5′-TCCGAAACTTCTCTCCTGGCTC-3′; and GAPDH forward,
5′-GAGTCAACGGATTTGGTCGT-3′ and reverse,
5′-TTGATTTTGGAGGGATCTCG-3′.
AK4 shRNA plasmids (cat. no. sc-38908-SH) were
purchased from the Santa Cruz Biotechnology, Inc. The shRNA
sequence specifically targeting AK4 was as follows: Sense,
5′-AACTTTGGTCTCCAGCATCTCTC-3′. The negative control (NC) shRNA
plasmids used in the present contained a non-coding shRNA fragment.
The NC shRNA sequence was as follows: Sense
5′-UUCUCCGAGCGUGUCACGUTT-3′.
Bioinformatic analysis
Bioinformatic analysis was conducted via Gene
Expression Profiling Interactive Analysis (GEPIA; http://gepia.cancer-pku.cn/detail.php?gene=AK4/) to
analyze The Cancer Genome Atlas (TCGA; http://www.cancer.gov/about-nci/organization/ccg/research/structural-genomics/tcga)
data with a threshold of P<0.05 and Log(fold-change) >1 or
<-1 for differential genes. The median of the survival rates was
used as the basis for dividing patients into two groups (low and
high AK4 expression) for Kaplan-Meier survival analysis.
Human tissue sample collection and
analysis
A total of 98 SOC tissues and the corresponding
adjacent normal tissues (5 mm from the tumor tissues) in the
current study were collected from patients receiving surgical
therapy at the Second Hospital of Lianyungang (Lianyungang, China)
between July 2017 and June 2019. The clinical-pathological
characteristics, such as patient age, gender, tumor stage and
grade, lymph node metastasis, recurrence, and vascular invasion are
listed in Table I.
 | Table I.Association between AK4 expression
level and clinicopathological characteristics in 98 patients with
serous ovarian cancer. |
Table I.
Association between AK4 expression
level and clinicopathological characteristics in 98 patients with
serous ovarian cancer.
|
|
| AK4 expression |
|
|
|---|
|
|
|
|
|
|
|---|
| Characteristic | Number of
patients | Low (n=50) | High (n=48) | χ2 | P-value |
|---|
| Age, years |
|
|
| 0.428 | 0.513 |
|
<55 | 58 | 28 | 30 |
|
|
|
≥55 | 40 | 22 | 18 |
|
|
| Tumor size, cm |
|
|
| 6.862 | 0.009 |
|
<10 | 46 | 17 | 29 |
|
|
|
≥10 | 52 | 33 | 19 |
|
|
| Preoperative
chemotherapy |
|
|
| 2.081 | 0.149 |
|
Yes | 44 | 26 | 18 |
|
|
| No | 54 | 24 | 30 |
|
|
|
Differentiation |
|
|
| 2.506 | 0.113 |
|
Low | 32 | 20 | 12 |
|
|
|
High | 66 | 30 | 36 |
|
|
| FIGO stage |
|
|
| 5.176 | 0.023 |
|
I–II | 42 | 27 | 15 |
|
|
|
III–IV | 56 | 23 | 33 |
|
|
| Lymph node
metastasis |
|
|
| 1.440 | 0.230 |
|
Yes | 53 | 30 | 23 |
|
|
| No | 45 | 20 | 25 |
|
|
To explore the possible association between the
expression level of AK4 and SOC progression, IHC assays were
performed. Briefly, sections were fixed with 4% paraformaldehyde
(PFA) at room temperature for 20 min and embedded into paraffin.
Subsequently, human tissue sample sections (5-µm-thick) were
deparaffinized at 60°C for 60 min and washed with xylene.
Rehydration was performed in a descending alcohol series.
Subsequently, sections were blocked with 2% BSA (Sigma-Aldrich;
Merck KGaA) for 30 min at room temperature. Slides were incubated
with the aforementioned AK4 antibody (cat. no. ab131327; 1:100;
Abcam) at room temperature for 2 h. Subsequently the sections were
incubated with a biotinylated secondary antibody (cat. no. ab99807;
1:100; Abcam) for another 1.5 h at room temperature, and
3,3′-diaminobenzidine was used as a chromogen substrate. A light
microscope (IX71; Zeiss AG) was used for imaging at ×100 and ×200
magnification.
In brief, the percentage of cells with positive
staining was scored as follows: 0, 0% stained cells; 1, 1–20%
stained cells; 2, 21–60% stained cells; and 3, 61–100% stained
cells. The following scoring was used for staining intensity: 0 (no
staining), 1 (low staining), 2 (moderate staining) and 3 (high
staining). The expression levels of AK4 were classified based on
the following staining index: Score of staining intensity + score
of staining cell percentage. Staining index <3 was considered as
low expression, while staining index ≥3 was considered as high
expression.
Cell culture and transfection
The human SOC cell lines, CAOV3 and OVCAR3, were
purchased from American Type Culture Collection. CAOV3 and OVCAR3
cells were incubated in DMEM and RPMI-1640 medium, respectively,
supplemented with 10 and 20% FBS (Gibco; Thermo Fisher Scientific,
Inc.), respectively, in a 5% CO2 incubator at 37°C with
100 U/ml penicillin and 0.1 mg/ml streptomycin.
The aforementioned AK4 shRNA plasmids were
transfected into both CAOV3 and OVCAR3 cells using
Lipofectamine® 2000 (cat. no. 11668019; Invitrogen;
Thermo Fisher Scientific, Inc.). AK4 knockdown was confirmed in
both CAOV3 and OVCAR3 cells, two of the most commonly used in
vitro models for ovarian cancer. In 6-well plates, 5 µl
transfection reagent and 1.5 µg shRNA plasmids were mixed in 300 µl
serum-free DMEM, left to stand for 5 min and then mixed. Following
incubation at room temperature for 20 min, the mix was added to
serum-starved cells and incubated at 37°C for 4 h. For the control
group, the shRNA targeting sequence was nonsense and did not target
intracellular RNAs. Only CAOV3 cells were used for the in
vivo assays, since these cells are used more often in
vivo, for which the stable AK4-knockdown cells were used. CAOV3
cell line with stable AK4 depletion were screened through shRNA
plasmid transfection and used for the xenograft and lung metastasis
assays in vivo.
RT-qPCR assay
TRIzol® (cat. no. 15596026; Invitrogen;
Thermo Fisher Scientific, Inc.) was used to extract total RNA from
human CAOV3 and OVCAR3 cells. Total RNA was reverse transcribed
into cDNA using a cDNA synthesis system (cat. no. 6110A; Takara
Bio, Inc.) at 42°C for 1 h. qPCR was performed using a SYBR Ex Taq
kit (cat. no. 638319; Takara Bio, Inc.). The following
thermocycling conditions were used for qPCR: Initial denaturation
at 95°C for 3 min; followed by 30 cycles of denaturation at 95°C
for 30 sec, annealing at 58°C for 30 sec and extension at 72°C for
30 sec. The 2−ΔΔCq method was used to quantify the
results (22). AK4 expression levels
were normalized to the expression of GAPDH.
Western blotting assays
CAOV3 and OVCAR3 cells or tissues from mice were
lysed using RIPA buffer (cat. no. 9800; Cell Signaling Technology,
Inc.). The BCA method was used for protein determination. Proteins
(20 µg/lane) were separated via 8% SDS-PAGE and were transferred
onto PVDF membranes. After blocking with 5% milk in TBS with 0.5%
Tween-20 at room temperature for 2 h, the membranes were incubated
at room temperature for 2 h with the aforementioned primary
antibodies: Rabbit anti-AK4 (cat. no. ab131327; 1:1,000; Abcam) and
mouse anti-β-actin (cat. no. ab8226; 1:3,000; Abcam). Subsequently,
the PVDF membranes were incubated with HRP-conjugated goat
anti-mouse and anti-rabbit secondary antibodies (1:5,000; cat. nos.
ab6789 and ab6721, respectively; Abcam) for 45 min at room
temperature. Signals were detected using an ECL kit (Novex™ ECL
Chemiluminescent Substrate Reagent kit; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. The blots were
semi-quantified using ImageJ v1.8.0 software (National Institutes
of Health).
Colony formation assays
A total of 1,000 CAOV3 and OVCAR3 cells were seeded
into a 6-well culture plate and transfected with NC or AK4 shRNA
plasmids. For six-well plates, 6 µl transfection reagent and 1.5 µg
the plasmids were added into 300 µl of serum-free medium, incubated
for 5 min at room temperature and then mixed. After additional
incubation at room temperature for 15 min, the mix was added to
serum-starved cells for 4 h at 37°C.
The culture medium was replaced with 2 ml fresh
medium every 2 days. After 14 days of incubation at 37°C and 5%
CO2, cells in the six-well plate were fixed with 4% PFA
for 30 min at room temperature and stained with 0.2% crystal violet
at room temperature for 20 min, then washed with PBS. The number of
colonies was manually counted.
MTT assay
CAOV3 and OVCAR3 cells were seeded into 96-well
plates at a density of ~500 cells/well, transfected with NC or AK4
shRNA plasmids, as aforementioned, and incubated for 24 h. Cells
were then incubated with MTT for 4 h at room temperature.
Subsequently, the culture medium was removed and the cells were
washed with PBS. Then 150 µl DMSO was added into each well to
dissolve the purple formazan, and the absorbance value was measured
with a microplate reader at a wavelength of 570 nm.
Wound healing assays
The wound healing assay was used to determine cell
migration. In brief, CAOV3 and OVCAR3 cells were transfected with
the aforementioned plasmids. Subsequently, the cells were wounded
at 100% confluence by scraping with a 20-µl pipette tip, followed
by washing. Cells were serum-starved prior to and during the assay.
Images were captured at 0 and 24 h to evaluate the migration degree
of cancer cells. A light microscope (magnification, ×20; IX71;
Zeiss AG) was used for imaging, and ImageJ software (v1.8.0) was
used to calculate the wound area.
Cell invasion assays
Cell invasion was measured using Transwell chambers
(8-µm pore size; Corning Inc.) with Matrigel. The chambers were
precoated with 20% Matrigel for 30 min at 37°C. After transfection,
~5×105 CAOV3 and OVCAR3 cells in serum-free DMEM or
RPMI-1640, respectively, were seeded into the upper chamber with
20% Matrigel. The complete medium (DMEM or RPMI-1640 with 10% FBS)
was added into the bottom chamber. After 48 h of incubation at
37°C, the cells that invaded into the bottom chamber were fixed in
4% PFA for 20 min, stained with 0.1% crystal violet for 20 min at
room temperature and counted under a light microscope
(magnification, ×50; IX71; Zeiss AG).
Tumor growth assays
All animal protocols were approved by the
Institutional Animal Care and Use Committee (IACUC) of the Second
People's Hospital of Lianyungang. A total of 22 female BALB/c nude
mice (8-week-old; weight, ~20 g) were supplied by Beijing Vital
River Laboratory Animal Technology Co., Ltd. Mice were fed with
food and water ad libitum, and were kept at a Specific
Pathogen-Free level at 20°C and a humidity of 60%, alternating
between light and dark for 12 h. None of the mice died accidentally
during the study. The mice were sacrificed by cervical dislocation,
and their heartbeat was checked to determine whether they were
dead.
The experimental procedure was performed as
previously described (22). Briefly,
CAOV3 cells were stably transfected with NC or AK4 shRNA
lentiviruses, as aforementioned. Subsequently, ~106
CAOV3 cells in 200 µl Matrigel (Corning, Inc.) were subcutaneously
implanted into the abdomen of 8-week-old BALB/c nude mice. A total
of 12 athymic nude mice were included in control (n=6) and AK4
depletion (n=6) groups. From the 14th day after injection, the
volume of each tumor was measured every 3 days using a Vernier
caliper. After 29 days, all animals were sacrificed and the tumor
growth curves were calculated. Tumor volume was calculated as
follows: Tumor volume (mm3)=Tumor length (mm) × Tumor
width (mm)2/2.
Lung metastasis assays
For lung metastasis assays, a total of 10 athymic
nude mice were included in control (n=5) and AK4 depletion (n=5)
groups. 1×106 CAOV3 cells stably infected with NC or AK4
shRNA plasmids (as aforementioned) were resuspended in 100 µl PBS
buffer and injected into the tail vein of 8-week-old BALB/c nude
mice. The mice were sacrificed after 7 weeks, the lungs of the mice
were surgically opened and complete lung tissues were taken out and
were observed and photographed using a camera (magnification,
×2.5). The tumor lung metastasis degree was measured according to
the volume of metastatic tumor tissue in the lung, which was
measured using ImageJ v1.8.0 software.
Statistical analysis
GraphPad Prism 8.0 (GraphPad Software, Inc.)
software was used for statistical analysis. All the experiments
were repeated 3 times. All results are presented as the mean ±
standard deviation. The differences in expression levels of AK4
were analyzed using an unpaired Student's t-test or Mann-Whitney U
test, as appropriate. Kaplan-Meier analysis and log-rank test was
used to analyze the association between AK4 expression and the
survival rate of patients. The association between clinical
features and AK4 expression levels was analyzed using χ2
test. Unpaired student's t-test was used for statistical
comparisons between two groups in the in vitro and in
vivo assays. P<0.05 was considered to indicate a
statistically significant difference.
Results
AK4 is overexpressed in human SOC
tissues
First, bioinformatic analysis was performed to
explore the mRNA expression levels of AK4 in SOC and normal tissues
at using the GEPIA database. AK4 mRNA expression was increased in
426 cancer tissues compared with 88 normal tissues from different
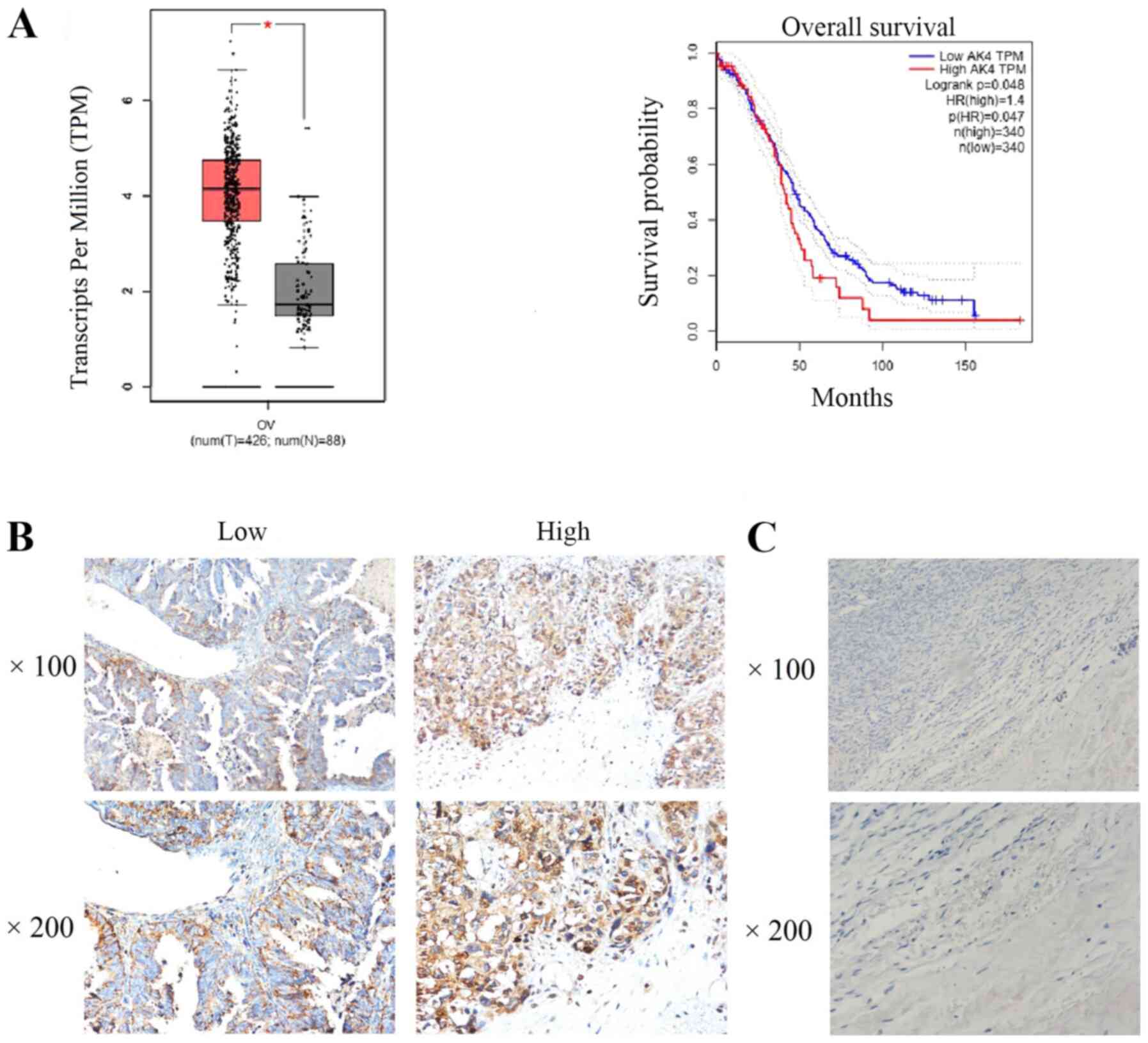
patients (P<0.05; Fig. 1A).
Patients with high AK4 expression had a decreased overall survival
time compared with patients with low AK4 expression (P=0.048;
Fig. 1A). These data suggested that
AK4 may be used to predict a poor prognosis in patients with SOC.
IHC assays were subsequently performed using SOC tissues and their
paired normal tissues collected at the Second Hospital of
Lianyungang to detect the expression level of AK4. The staining
results indicated that AK4 was markedly expressed in SOC tissues
compared with in the adjacent tissues (Fig. 1B and C).
To further investigate the effect of AK4 on SOC
progression, SOC tissue samples were divided into AK4 high or low
expression groups, according to the staining index. The
clinicopathological characteristics, including patient age, tumor
size, preoperative chemotherapy, differentiation, FIGO stage, and
lymph node metastasis, were compared between high and low AK4
expression groups (Table I). No
significant associations were found between AK4 expression and age
(P=0.513), preoperative chemotherapy (P=0.149), differentiation
(P=0.113) and lymph node metastasis (P=0.230). However, AK4
expression was significantly associated with tumor size (P=0.009)
and FIGO stage (P=0.023) (Table I).
These data indicated that AK4 may promote the progression of
SOC.
Knockdown of AK4 suppresses the
proliferation, migration and invasion of SOC cells in vitro
To further investigate the involvement of AK4 in the
progression of SOC, shRNA plasmids targeting AK4 or NC shRNA
plasmids were transfected into two SOC cell lines, CAOV3 and
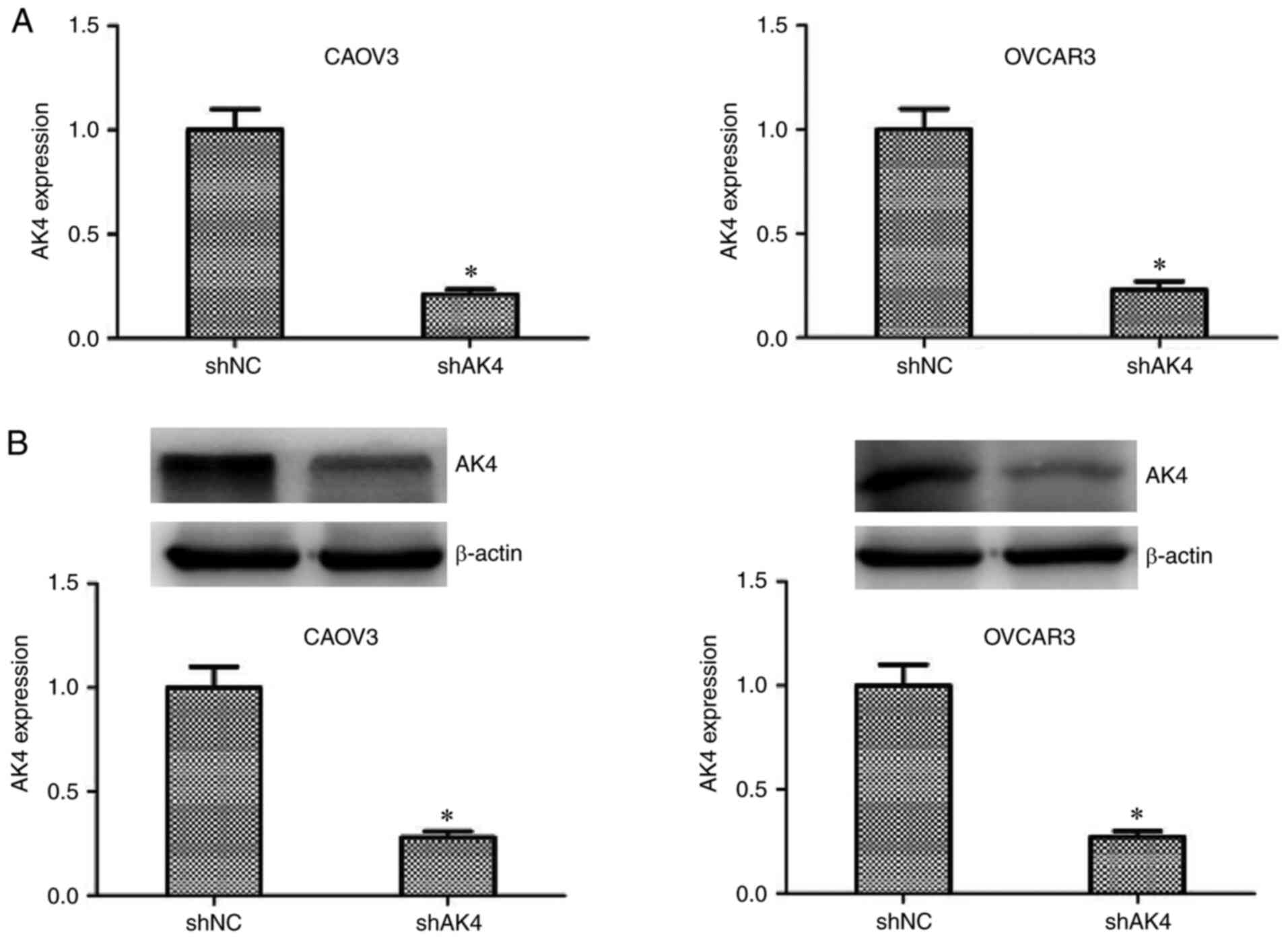
OVCAR3. The results revealed that AK4 mRNA and protein expression
levels were significantly decreased following transfection with AK4
shRNA plasmids in CAOV3 and OVCAR3 cells compared with the
respective NC shRNA groups (P<0.05; Fig. 2).
Subsequently, the possible effects of AK4 on the
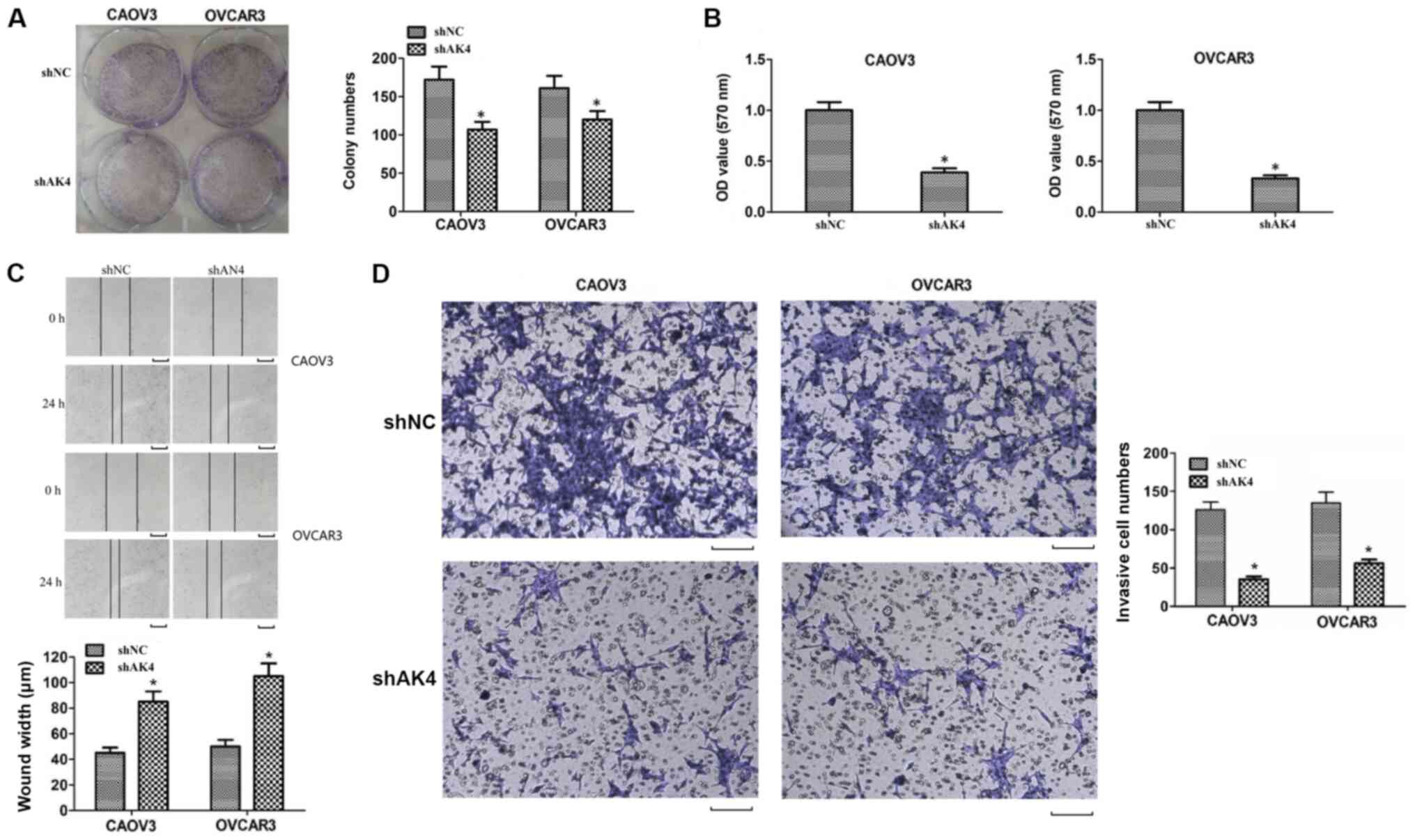
viability of SOC cells were assessed through colony formation and
MTT assays. The number of colonies in the AK4 depletion group was
significantly decreased compared with the NC shRNA group in CAOV3
and OVCAR3 cells (P<0.05; Fig.
3A). Similarly, the results of MTT assays also revealed that
the OD value in the AK4 shRNA group was significantly decreased
compared with the NC shRNA group (P<0.05; Fig. 3B). These results demonstrated that
AK4 may play a role in the regulation of SOC cell viability.
The possible effects of AK4 on the migration and
invasion of SOC cells were evaluated via wound healing and
Transwell assays, respectively. An impaired wound healing capacity
was observed in the AK4 shRNA group compared with the shNC group in
CAOV3 and OVCAR3 cells in vitro (P<0.05; Fig. 3C). Additionally, Transwell assays
suggested that the knockdown of AK4 efficiently suppressed invasion
of CAOV3 and OVCAR3 cells. Collectively, these results indicated
that AK4 affected the migration and invasion of SOC cells in
vitro.
AK4 knockdown inhibits tumor growth
and metastasis of SOC cells in vivo
The possibility that AK4 contributes to tumor growth
and metastasis of SOC cells was further assessed using a mouse
xenograft model and a lung metastasis model.
To confirm the aforementioned in vitro
results, CAOV3 cells were first infected with AK4 shRNA
lentiviruses to stably knock down the expression of AK4, and
subcutaneously injected into nude mice. After 14 days, the tumors
began to form and the volume of tumors was measured every 3 days.
After 29 days, all tumors were excised and representative images
were shown (P<0.05; Fig. 4A). The
growth curves of tumors in NC and AK4 shRNA groups were also shown
in Fig. 4A. Tumor volume in the AK4
depletion groups was significantly lower compared with that in the
shNC group on day 29 (Fig. 4A).
Additionally, an increased lung metastasis degree was observed
after 7 weeks of injection compared with the NC group (P<0.05;
Fig. 4B) Lung tissues were isolated
from mice injected with NC and AK4 shRNA cells, images were
captured and individual tissues were analyzed. The tumor volume in
the AK4 shRNA group was significantly decreased compared with that
in the NC shRNA group (P<0.05; Fig.
4B).
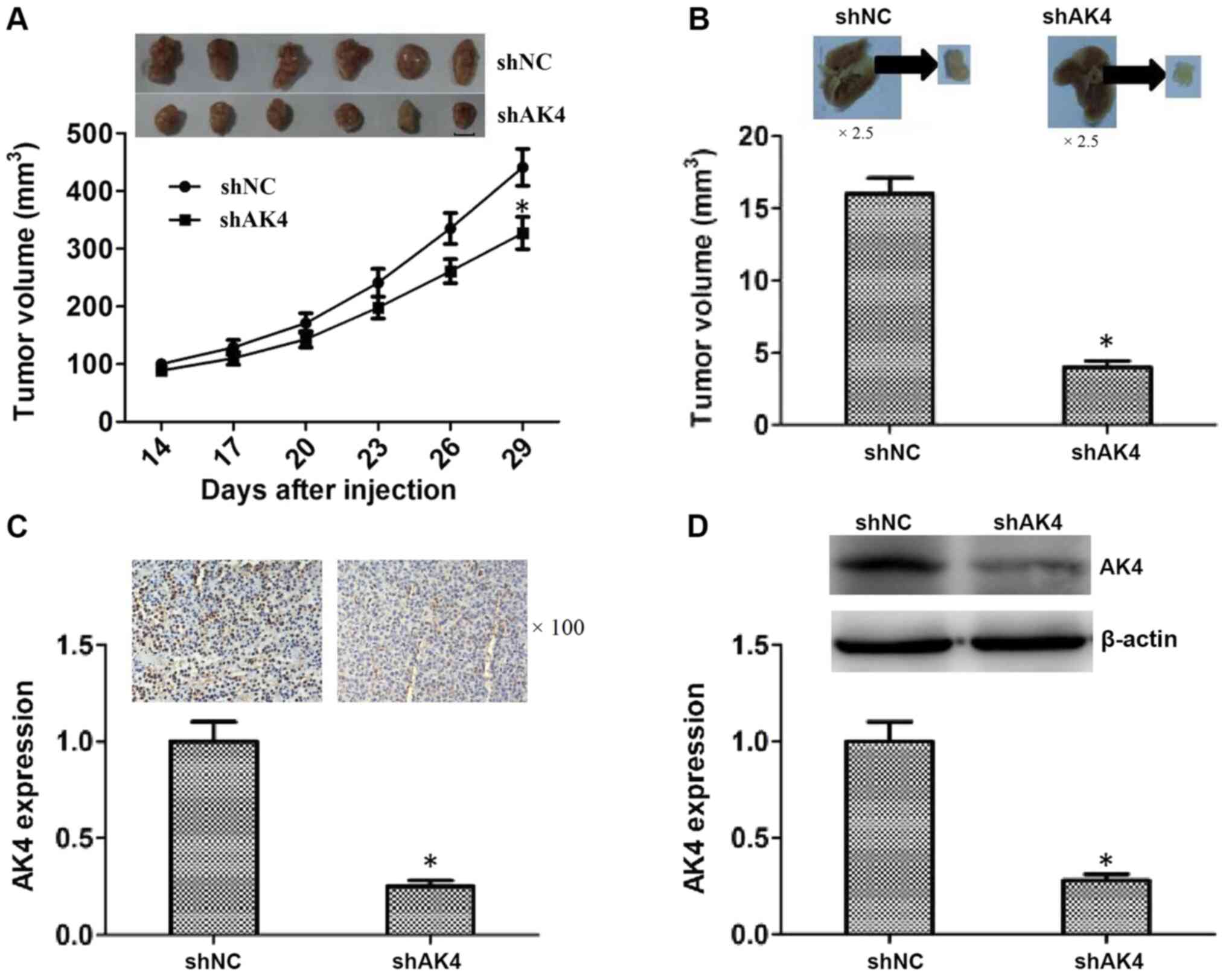 | Figure 4.AK4 induces tumor growth and
metastasis of SOC cells in mice. (A) CAOV3 cells infected with NC
or AK4 shRNA lentiviruses were implanted into nude mice. After 14
days, tumors began to form and the volume of each tumor was
measured every 3 days (n=6 in each group). After 29 days, all
tumors were isolated and the growth curves were analyzed according
to the average volume of 5 tumors in the AK4 and NC shRNA groups.
Scale bar, 5 mm. (B) CAOV3 cells were infected with NC or AK4 shRNA
lentiviruses, implanted into the tail vein of nude mice. Lung
tissues were isolated after 7 weeks, images were captured and the
degree of lung metastasis was calculated. Magnification, ×2.5. (C)
Immunohistochemical assays revealed the expression levels of AK4 in
NC and AK4 stable knockdown primary tumors excised from mice.
Magnification, ×100. (D) Western blotting assays showed the
expression levels of AK4 in NC and AK4 shRNA tumors. The results
are presented as the mean ± standard deviation. *P<0.05 vs.
shNC. NC, negative control; AK4, adenylate kinase 4; sh, short
hairpin RNA. |
Further, IHC assays were conducted to confirm the
effective silencing of AK4 in the primary tumor tissues (Fig. 4C). The expression levels of AK4 were
also determined in the primary tumor tissues using western
blotting, and the results revealed that AK4 protein expression
levels were decreased in the AK4 shRNA group compared with that in
the shNC group (Fig. 4D). Overall,
the results of the in vivo assays indicated the potential
involvement of AK4 in tumor growth and metastasis of SOC cells
in vivo.
Discussion
Surgical adjuvant chemotherapy is the most common
therapeutic method for advanced SOC; however, it has little effect
on improving patient survival rate (3). For this type of malignant tumor with
high metastasis rates and fast progression, targeted therapy would
be a suitable treatment method (8).
Liposome targeted therapy is an effective strategy for tumors prone
to metastasis (23,24). A number of targeted therapy drugs,
such as bevacizumab, an anti-angiogenic agent, and olaparib, a
poly-ADP ribose polymerase inhibitor, have been tested in clinical
trials for SOC or used in the treatment of patients with high-grade
SOC (25,26). The current study demonstrated that a
member of the adenylate kinase protein family, AK4, could serve as
a promising therapeutic target for the treatment of SOC. Analysis
of TCGA database data and IHC assays revealed a high expression
level of AK4 in human SOC tissues compared with healthy control
samples. The present study suggested that AK4 may be involved in
SOC progression and serve as a potential therapeutic target.
Further research is required to fully elucidate the underlying
molecular mechanisms.
In the current study, the protein expression of AK4
was markedly enhanced in human SOC tissues compared with adjacent
normal tissues, which is consistent with previous investigations.
Jan et al (17) reported that
AK4 was significantly upregulated when glioma cells were exposed to
hypoxia and was associated with hypoxia-inducible
factor-1α-mediated invasion and migration in human glioma cells,
indicating that AK4 is a possible cancer biomarker (27).
Colony formation and MTT assays revealed an effect
of AK4 on SOC cell proliferation. Additionally, wound healing and
Transwell assays further showed that AK4 mediated the migration and
invasion of SOC cells in vitro. Consistent with these data,
the subsequent in vivo results showed that AK4 promoted
tumor growth and lung metastasis of SOC cells in mice. Therefore,
the results of the present study indicated that AK4 may affect the
progression of SOC. Previous studies have also confirmed that AK4
played a role in cancer development. AK4 could modulate anticancer
drug sensitivity in lung cancer via the regulation of mitochondrial
activity (28). AK4 could also
affect multi-chemoresistance and radioresistance in osteosarcoma
and esophageal cancer, and AK4 expression was regulated by
miR-199a-3p (21,29). Another study indicated that AK4 also
affected the proliferation and invasion of bladder cancer cells
in vitro (20). These
studies, together with the findings of the present study, indicate
that AK4 may play a role in cancer progression.
In the in vivo metastasis model used in the
present study, AK4 knockdown could significantly inhibit tumor
growth and lung metastasis degree following intravenous injection
of cancer cells, suggesting that AK4 played a role in promoting
tumor growth and metastasis. In a previous study on lung cancer,
AK4 expression promoted the invasion step of the
invasion-metastasis cascade by repressing ATF3 expression and
resulted in relieving the expression of the downstream effector,
MMP2 (17). In a mouse model of
bladder cancer, AK4 shRNA transfection markedly inhibited tumor
growth and metastasis compared with that in a scramble control
group (20). AK4 is also a potential
oncogene upregulated in metastatic colorectal cancer and exhibiting
in vitro oncogenic properties (30). In addition, withaferin-A was
identified as a potential agent for the treatment of metastatic
lung cancer that could regulate AK4-associated gene expression
(19).
In conclusion, the present study investigated the
possible involvement of AK4 in the progression of SOC. The results
revealed high expression levels of AK4 in human SOC tissues and AK4
was associated with the tumor size and FIGO stage of patients with
SOC. Furthermore, AK4 affected the proliferation, migration, and
invasion of SOC cells in vitro, and contributed to tumor
growth and metastasis of SOC cells in mice. Therefore, AK4 could
serve as a promising therapeutic target for SOC treatment.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MH and XQ were responsible for study conception and
design. MH, XQ, YW and FM performed the experiments. Acquisition of
data was performed by MH, XQ and YW. Analysis and interpretation of
data, writing, review and/or revision of the manuscript, and
administrative and technical support were performed by MH and XQ.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Animal Care and Use Committee of the Second People's Hospital of
Lianyungang (Lianyungang, China) and the Human Ethics Committee of
the Second People's Hospital of Lianyungang (Lianyungang, China).
All patients signed informed consent forms prior to surgery.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AK4
|
adenylate kinase 4
|
|
SOC
|
serous ovarian cancer
|
|
IHC
|
immunohistochemical
|
|
OS
|
overall survival
|
References
|
1
|
Gutiérrez-Castañeda LD, Tovar-Parra D,
Quintero G, Amezquita L, Guerrero C and Sanabria D: Isolation and
phenotypic characterization of tumor cells of patients with a
diagnosis of ovarian cancer. J Cell Physiol. 235:3320–3328. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Antar A, AlJawad H, AlMuslim A and
El-Majzoub N: Isolated late recurrence of epithelial ovarian cancer
in cervical lymph nodes. Eur J Gynaecological Oncol. 40:1067–1069.
2019.
|
|
3
|
Bouberhan S, Shea M and Cannistra SA:
Advanced epithelial ovarian cancer: Do more options mean greater
benefits? J Clin Oncol. 37:1359–1364. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zuberi M, Mir R, Khan I, Javid J, Guru SA,
Bhat M, Sumi MP, Ahmad I, Masroor M, Yadav P, et al: The promising
signatures of circulating microRNA-145 in epithelial ovarian cancer
patients. MicroRNA. 9:49–57. 2019. View Article : Google Scholar
|
|
5
|
Lalremmawia H and Tiwary BK:
Identification of molecular biomarkers for ovarian cancer using
computational approaches. Carcinogenesis. 40:742–748. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ida T, Fujiwara H, Kiriu T, Taniguchi Y
and Kohyama A: Relationship between the precursors of high grade
serous ovarian cancer and patient characteristics: Decreased
incidence of the p53 signature in pregnant women. J Gynecol Oncol.
30:e962019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kreuzinger C, von der Decken I, Wolf A,
Gamperl M, Koller J, Karacs J, Pfaffinger S, Bartl T, Reinthaller
A, Grimm C, et al: Patient-derived cell line models revealed
therapeutic targets and molecular mechanisms underlying disease
progression of high grade serous ovarian cancer. Cancer Lett.
459:1–12. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Clifford C, Vitkin N, Nersesian S,
Reid-Schachter G, Francis JA and Koti M: Multi-omics in high-grade
serous ovarian cancer: Biomarkers from genome to the immunome. Am J
Reprod Immunol. 80:e129752018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gadducci A, Guarneri V, Peccatori FA,
Ronzino G, Scandurra G, Zamagni C, Zola P and Salutari V: Current
strategies for the targeted treatment of high-grade serous
epithelial ovarian cancer and relevance of BRCA mutational status.
J Ovarian Res. 12:92019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ivy SP, Kunos CA, Arnaldez FI and Kohn EC:
Defining and targeting wild-type BRCA high-grade serous ovarian
cancer: DNA repair and cell cycle checkpoints. Expert Opin Investig
Drugs. 28:771–785. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Song K, Wang Y, Li Y, Ding C, Cai R, Tao
G, Zhao P, Xia Q and He H: A convenient, rapid, sensitive, and
reliable spectrophotometric assay for adenylate kinase activity.
Molecules. 24:6632019. View Article : Google Scholar
|
|
12
|
Liu R, Ström AL, Zhai J, Gal J, Bao S,
Gong W and Zhu H: Enzymatically inactive adenylate kinase 4
interacts with mitochondrial ADP/ATP translocase. Int J Biochem
Cell Biol. 41:1371–1380. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yoneda T, Sato M, Maeda M and Takagi H:
Identification of a novel adenylate kinase system in the brain:
Cloning of the fourth adenylate kinase. Brain Res Mol Brain Res.
62:187–195. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Panayiotou C, Solaroli N, Johansson M and
Karlsson A: Evidence of an intact N-terminal translocation sequence
of human mitochondrial adenylate kinase 4. Int J Biochem Cell Biol.
42:62–69. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Miyoshi K, Akazawa Y, Horiguchi T and Noma
T: Localization of adenylate kinase 4 in mouse tissues. Acta
Histochem Cytochem. 42:55–64. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kong F, Binas B, Moon JH, Kang SS and Kim
HJ: Differential expression of adenylate kinase 4 in the context of
disparate stress response strategies of HEK293 and HepG2 cells.
Arch Biochem Biophys. 533:11–17. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jan YH, Tsai HY, Yang CJ, Huang MS, Yang
YF, Lai TC, Lee CH, Jeng YM, Huang CY, Su JL, et al: Adenylate
kinase-4 is a marker of poor clinical outcomes that promotes
metastasis of lung cancer by downregulating the transcription
factor ATF3. Cancer Res. 72:5119–5129. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jan YH, Lai TC, Yang CJ, Huang MS and
Hsiao M: A co-expressed gene status of adenylate kinase 1/4 reveals
prognostic gene signature associated with prognosis and sensitivity
to EGFR targeted therapy in lung adenocarcinoma. Sci Rep.
9:123292019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jan YH, Lai TC, Yang CJ, Lin YF, Huang MS
and Hsiao M: Adenylate kinase 4 modulates oxidative stress and
stabilizes HIF-1α to drive lung adenocarcinoma metastasis. J
Hematol Oncol. 12:122019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xin F, Yao DW, Fan L, Liu JH and Liu XD:
Adenylate kinase 4 promotes bladder cancer cell proliferation and
invasion. Clin Exp Med. 19:525–534. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zang C, Zhao F, Hua L and Pu Y: The
miR-199a-3p regulates the radioresistance of esophageal cancer
cells via targeting the AK4 gene. Cancer Cell Int. 18:1862018.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang Y, Zhao Z, Xie C and Zhao Y:
Dual-targeting liposome modified by glutamic hexapeptide and folic
acid for bone metastatic breast cancer. Chem Phys Lipids.
228:1048822020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhao Z, Zhao Y, Xie C, Chen C, Lin D, Wang
S, Lin D, Cui X, Guo Z and Zhou J: Dual-active targeting liposomes
drug delivery system for bone metastatic breast cancer: Synthesis
and biological evaluation. Chem Phys Lipids. 223:1047852019.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yamamoto TM, McMellen A, Watson ZL,
Aguilera J, Ferguson R, Nurmemmedov E, Thakar T, Moldovan GL, Kim
H, Cittelly DM, et al: Activation of Wnt signaling promotes
olaparib resistant ovarian cancer. Mol Carcinog. 58:1770–1782.
2019. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Secord AA, McCollum M, Davidson BA,
Broadwater G, Squatrito R, Havrilesky LJ, Gabel AC, Starr MD, Brady
JC, Nixon AB and Duska LR: Phase II trial of nintedanib in patients
with bevacizumab-resistant recurrent epithelial ovarian, tubal, and
peritoneal cancer. Gynecol Oncol. 153:555–561. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lichtenfels R, Dressler SP, Zobawa M,
Recktenwald CV, Ackermann A, Atkins D, Kersten M, Hesse A,
Puttkammer M, Lottspeich F and Seliger B: Systematic comparative
protein expression profiling of clear cell renal cell carcinoma: A
pilot study based on the separation of tissue specimens by
two-dimensional gel electrophoresis. Mol Cell Proteomics.
8:2827–2842. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fujisawa K, Terai S, Takami T, Yamamoto N,
Yamasaki T, Matsumoto T, Yamaguchi K, Owada Y, Nishina H, Noma T
and Sakaida I: Modulation of anti-cancer drug sensitivity through
the regulation of mitochondrial activity by adenylate kinase 4. J
Exp Clin Cancer Res. 35:482016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lei W, Yan C, Ya J, Yong D, Yujun B and
Kai L: MiR-199a-3p affects the multi-chemoresistance of
osteosarcoma through targeting AK4. BMC Cancer. 18:6312018.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ji Y, Yang C, Tang Z, Yang Y, Tian Y, Yao
H, Zhu X, Zhang Z, Ji J and Zheng X: Adenylate kinase hCINAP
determines self-renewal of colorectal cancer stem cells by
facilitating LDHA phosphorylation. Nat Commun. 8:153082017.
View Article : Google Scholar : PubMed/NCBI
|