Introduction
Malignant tumor cells derived from the urinary
epithelium are called urothelial carcinomas (UCs), and these
constitute the fourth most common cancer in the world (1). Bladder cancer (BC) is the most common
type of UC, and accounts for 90–95% of the total UC cases (2). In Western countries, the prevalence of
upper tract urothelial carcinomas (UTUCs) is notably less compared
with that of BC, which accounts for only 5–10% of all UC cases, and
the incidence of UTUC in the renal pelvis is ~2-3-fold more common
than that in the ureter, and the male-to-female ratio is ~2-3:1
(3). However, in Taiwan, UTUCs
account for 30% of all UC cases, and the incidence of UTUC in the
renal pelvis is similar to that in the ureter with a ratio of
~1.1:0.9 (4). In addition, there are
more female patients with UTUC than males (5). The high recurrence rate, high
progression potential and frequent distant metastasis are the main
reasons why UC has poor clinical outcomes even when diagnosed at an
early stage. In a clinical setting, previous studies have shown
that tumor (T) stage, tumor grade, tumor size and lymph node
metastasis are important prognostic predictors for UTUC (6,7). The
present study aimed to identify the biomarkers of advanced UTUC
based on pathological features from human tissue samples.
PTEN is a dual protein/lipid phosphatase that
dephosphorylates phosphatidyl-inositol (3,4,5)-triphosphate (PIP3) into
phosphatidylinositol 4,5-biphosphate (PIP2). Deletion, mutation or
silencing due to high levels of promoter methylation causes loss of
PTEN activity in a number of primary and metastatic cancers
(8,9). Moreover, loss of PTEN is associated
with an aggressive tumor phenotype and poor clinical outcomes in UC
(10). Concordantly, PTEN is also a
negative regulator of the PI3K/Akt/mTOR signaling pathway (11) (Fig.
1). Akt is recruited to the cell membrane by PIP3, and
Phosphoinositide-dependent kinase-1 (PDK1) phosphorylates Akt on
threonine (Thr)308 (12). The
mammalian target of rapamycin complex 2 (mTORC2) complex then
phosphorylates Akt on serine (Ser)473 via a positive feedback loop
(13), leading to full activation of
Akt. Phosphorylated Akt activates mTORC1 to regulate the
phosphorylation of the S6 protein and the initial translational
factor in eukaryotes, eukaryotic initiation factor 4E binding
protein 1 (4E-BP1) (14). Activated
Akt phosphorylates several downstream effectors that regulate a
variety of essential processes such as cell growth, cell
metabolism, cell survival and protein synthesis (15). Although the phosphorylation of both
Thr308 and Ser473 is thought to be mandatory for complete
activation of the Akt pathway, there are still discrepancies about
the association of this with clinical outcomes. Gallay et al
(16) demonstrated that the levels
of phosphorylation on Thr308, instead of on Ser473, were
significantly associated with poor clinical outcomes, including
overall survival, event-free survival and relapse-free survival, in
acute myeloid leukemia. By contrast, Freudlsperger et al
(17) showed that the relative
levels of phosphorylated (p)Akt on Ser473 were associated with
overall survival and progression-free survival in patients with
advanced head and neck squamous cell carcinoma, but the relative
levels of pAkt on Thr308 were not correlated with patient
outcomes.
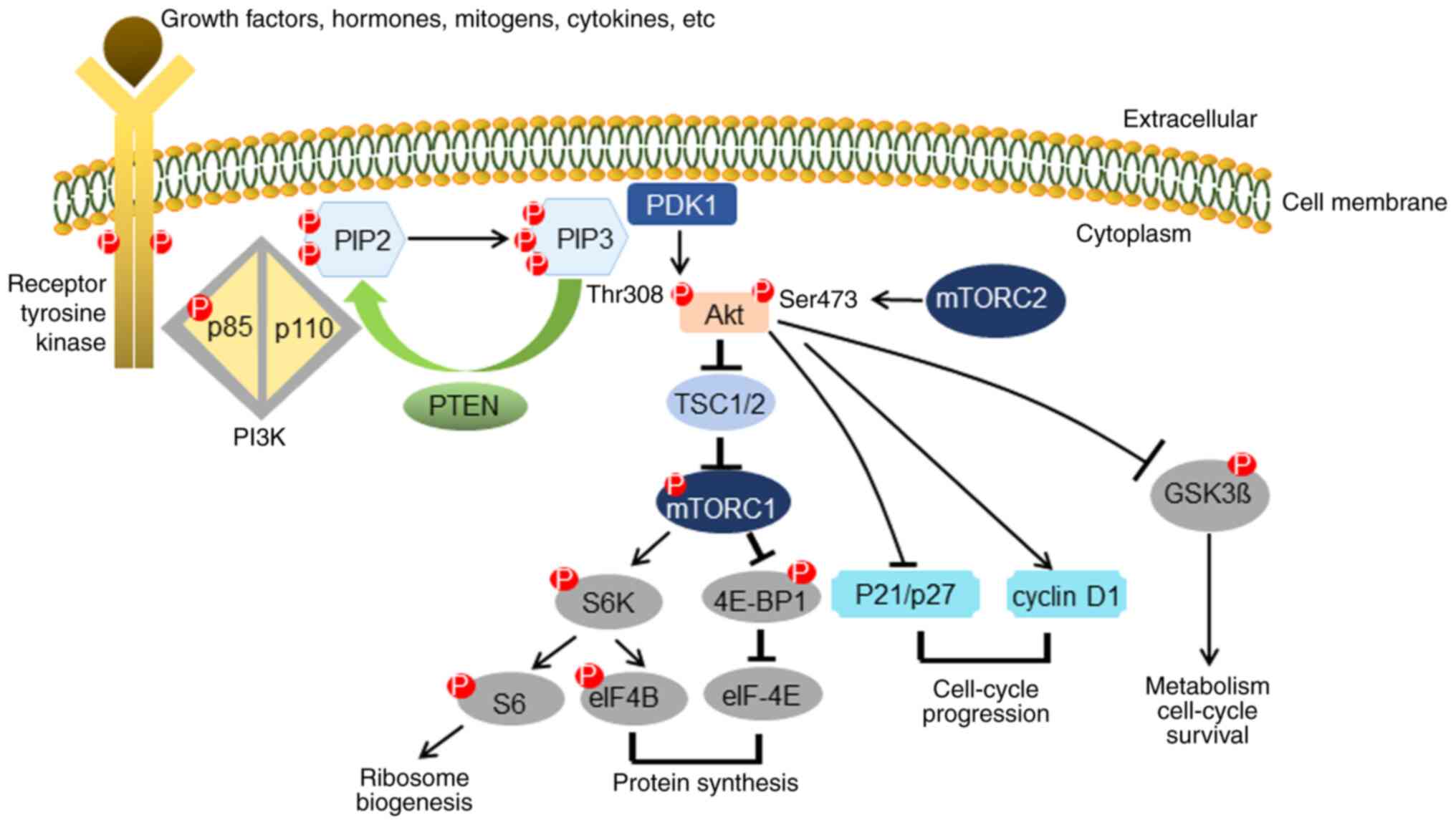 | Figure 1.PTEN in the PI3K/Akt signaling
pathway. PTEN is a negative regulator in the PI3K/Akt signaling
pathway. Akt is recruited to the cell membrane by PIP3, and PDK1
phosphorylates Akt on Thr308. Another cascade is started by mTORC2,
which phosphorylates Akt on Ser473 via a positive feedback loop and
leads to complete activation of Akt. Via activation of mTORC1,
phosphorylation of both the S6K protein and the initial
translational factor in eukaryotes, 4E-BP1, will occur. Activated
Akt phosphorylates several downstream effectors that regulate a
variety of essential processes such as cell growth, cell
metabolism, cell survival and protein synthesis. PIP3,
phosphatidyl-inositol (3,4,5)-triphosphate; Thr, threonine; Ser,
serine; PDK1, phosphoinositide-dependent kinase-1; mTORC1,
mammalian target of rapamycin complex 1; 4E-BP1, eukaryotic
initiation factor 4E binding protein 1; S6K, ribosomal protein S6
kinase. |
Activation of PI3K and Akt is reported to induce
ovarian (18), breast (18,19),
esophageal (20) and pancreatic
cancer (21), among other (9,22). The
PI3K/Akt/mTOR signaling pathway serves an essential role in various
cellular processes, including cell growth, cell motility, cell
survival, angiogenesis and cell metabolism (23–25).
This pathway is also documented to be associated with
carcinogenesis in UC (26), and
plays a central role in resistance to chemotherapy and radiation
therapy in multiple cancer types (27).
In the present study, PTEN gene alterations
were investigated using fluorescence in-situ hybridization
(FISH), and the protein expression levels of PTEN, phosphorylation
of Ser473 in Akt (pAktSer473) and phosphorylation of
Thr308 in Akt (pAktThr308) were analyzed using
immunohistochemistry (IHC) in UTUC tissues from 65 patients.
Materials and methods
Patients and tissue samples
Patients with confirmed diagnosis of UTUC after
ureteroscopic biopsy or computed tomography-guided biopsy, who then
underwent nephroureterectomy between January 2007 and October 2014,
were included in the present study. Patients with insufficient
tissue for complete pathologic review, including the diagnosis of
atypical urothelial cells, or without the required clinical data,
including demographic and survival information, were excluded. An
informed consent form regarding the utilization of residual tissues
for medical research was signed at the outpatient visit after
explanation by the physician, and all specimens were sent to the
tissue bank of Linkou Chang Gung Memorial Hospital (Taoyuan,
Taiwan) immediately after the operation at 4°C and then stored at
−80°C. The present study was approved by The Human Subject Research
Ethics Committee/Institutional Review Board (IRB) of Linkou Chang
Gung Memorial Hospital (approval no. 201601555B0). The
clinicopathological details of 65 patients with UTUC were analyzed
in the study. In total, 30 (46%) patients were male and 35 (54%)
were female, with a median age of 77 years (range, 49–98 years).
Additionally, 22 slides of normal kidney (19 slides) and ureter (3
slides) tissues were obtained to use as reference. According to
guidelines (28,29), nephroureterectomy would be the
standard treatment for these patients. As for T3 or T4 lesions,
neoadjuvant chemotherapy would be applied if patients were fit
enough, followed by surgery if resectable. Adjuvant systemic
treatment was initiated if recurrence was found. For all patients,
clinical data, including age, sex, history of smoking, alcohol
consumption, diabetes mellitus, hypertension, tumor location,
histology grade, tumor stage based on the American Joint Committee
on Cancer classification (30),
metastasis, recurrence and urolithiasis, were recorded (Table I). The overall survival was
calculated independently and stratified according to the noteworthy
parameters.
 | Table I.Clinicopathological features of 65
patients with upper tract urothelial carcinoma. |
Table I.
Clinicopathological features of 65
patients with upper tract urothelial carcinoma.
| Variables | Values |
|---|
| Median age (range),
years | 77 (49–98) |
| Sex, n (%) |
|
|
Male | 30 (46) |
|
Female | 35 (54) |
| Tumor localization,
n (%) |
|
| Renal
pelvis | 60 (92) |
|
Ureter | 4 (6) |
| Renal
pelvis and ureter | 1 (2) |
| Histological type,
n (%) |
|
|
IUC | 42 (65) |
|
PUC | 21 (32) |
|
IPUC | 2 (3) |
| pT stage, n
(%) |
|
|
pTa | 16 (25) |
|
pT1 | 12 (18) |
|
pT2 | 11 (17) |
|
pT3 | 20 (31) |
|
pT4 | 6 (9) |
| Tumor grade, n
(%) |
|
|
Low | 11 (17) |
|
High | 54 (83) |
| Metastasis, n
(%) |
|
|
Yes | 16 (25) |
| No | 49 (75) |
| Recurrence, n
(%) |
|
|
Yes | 8 (12) |
| No | 57 (88) |
| History of smoking,
n (%) |
|
|
Yes | 17 (26) |
| No | 48 (74) |
| History of alcohol,
n (%) |
|
|
Yes | 9 (14) |
| No | 56 (86) |
| History of
urolithiasisa, n
(%) |
|
|
Yes | 13 (20) |
| No | 52 (80) |
| History of
diabetes, n (%) |
|
|
Yes | 17 (26) |
| No | 48 (74) |
| History of
hypertension, n (%) |
|
|
Yes | 32 (49) |
| No | 33 (51) |
| Median
follow-up (range), months | 96 (30–159) |
FISH for analysis of the PTEN
gene
Two commercially available dual-color FISH probes
(CytoTest Inc.; cat no. CT-PAC101 and CT-LSP042) were designed to
detect copy number changes in the region of the human PTEN
gene, which is located on chromosome 10q23. The probes hybridized
to chromosome 10 in both metaphase and interphase and the Locus
Specific Probe (LSP), which is around 470 kb in length, exhibited
an orange fluorescent signal under the appropriate filters. The
other probe, the Chromosome 10 Counting Probe (CCP10), exhibiting a
green signal, served as an internal control according to the nature
derived from chromosome 10-specific pericentromeric DNA. First,
4-µm-thick formalin-fixed paraffin-embedded (FFPE) samples were
deparaffinized in 3 washes of xylene for 5 min each, and then
samples were rehydrated using a descending ethanol series (100, 85
and 70%). Samples were washed in 4X saline sodium citrate (SSC) at
room temperature (RT) for 30 min in a rotating shaker, and then
were treated with 1 M sodium thiocyanate (NaSCN) at RT overnight.
Lastly, slides were washed in distilled water for 5 min. The
specimens were digested in 250 µm 10% pepsin in 0.01 M HCl. Probes
and target DNA were then co-denatured at 82°C for 10 min and
hybridized overnight in a 37°C incubator. Post-hybridization washes
were performed in 2X SSC at RT for 5 min and in 0.3% NP40/2X SSC at
73°C for 2 min, followed by a 1-min wash at RT in distilled water.
All images were captured using a Leica DM2500 fluorescence
microscope (Leica Microsystems GmbH) using a magnification of ×63
with an ASI CCD camera (CCD-1300DS; Applied Spectral Imaging), and
were subsequently analyzed with FISHView EXPO version 5.5 software
(Applied Spectral Imaging). To evaluate the PTEN copy
number, signals in 300 non-overlapping nuclei in each sample were
counted using the aforementioned software. DAPI staining of nuclei
(using 1 µg/ml at room temperature for 30 min) was performed after
resuspension of the cell pellet into absolute ethanol at −20°C
(Sigma-Aldrich; Merck KGaA). After staining, the slides were ready
for interpretation in reference to the corresponding hematoxylin
and eosin (H&E)-stained tissue identified in the areas of
carcinoma. H&E staining was performed by the hospital according
to routine protocols and a series of 10 slides for the same patient
was retrieved after IRB approval. Heterozygous deletion of
PTEN was defined as a ratio of PTEN signal/centromere
10 probe signal <0.5. In addition, homozygous deletion of
PTEN was defined as a complete absence of PTEN probe
signal in >60% of tumor nuclei per sample, but there could be
1–2 PTEN signals in adjacent cells.
IHC for PTEN, pAktSer473
and pAktThr308
A total of 65 FFPE UTUC tissue samples were
collected between January 2007 and October 2014. The areas of
carcinoma were identified by three researchers, including one
pathologist, in H&E-stained tissues. IHC staining was performed
in the present study using primary antibodies against PTEN (clone
D4.3 XP; 1:250 dilution; cat. no. 9188), pAktSer473
(clone 736E11; 1:200 dilution; cat. no. 3787) and
pAktThr308 (clone 244F9; 1:100 dilution; cat. no. 4056)
(all Cell Signaling Technology, Inc.). All 4-µm FFPE UTUC tissues
were deparaffinized with xylene and rehydrated in 100, 85 and 70%
ethanol (Sigma-Aldrich; Merck KGaA). The procedure was performed
according to that reported in our previous study with modifications
(31). Antigen retrieval was
performed by heating the slides at 95°C in sodium citrate buffer
(10 mM sodium citrate, 0.05% Tween-20, pH 6.0) for 20 min.
Endogenous peroxidase activity was quenched by incubation with
hydrogen peroxidase (Thermo Fisher Scientific, Inc.) for 10 min at
RT, followed by an ultraviolet block (Thermo Fisher Scientific,
Inc.) for 5 min at RT to prevent non-specific background staining.
Slides were incubated for 16 h at 4°C with the aforementioned
rabbit anti-human PTEN, pAKTSer473 and
pAKTThr308 antibodies for each group. After 16 h of
incubation, the slides were left for 1 h at RT, followed by primary
antibody amplifier Quanto (Thermo Fisher Scientific, Inc.)
treatment for 10 min at RT. After application of the secondary
antibody for 10 min at RT (UltraVision Quanto Detection System;
cat. no. TL-060-QHD; ready to use; Thermo Fisher Scientific, Inc.),
the slides were stained using the chromogen 3,3′-diaminobenzidine
tetrahydrochloride (Dako; Agilent Technologies, Inc.) for 20 sec at
RT. Finally, all slides were counterstained with hematoxylin for 20
sec, dehydrated in 95 and 99% ethanol for 2 min each, and mounted,
all at RT. Negative control slides were incubated with PBS at 4°C
overnight. Slides from normal kidney or ureter were stained as
aforementioned and used as positive controls. All slides were
scanned using a high-resolution brightfield APERIO®
ScanScope (Leica Microsystems, Inc.) at ×40 magnification, and
digital images were used for scoring of the immunoreactivity of
targeted proteins.
Scoring of PTEN, pAktSer473
and pAktThr308 protein expression levels
The IHC score in each case was independently
evaluated by one pathologist, one doctor and one researcher. Tumors
stained by each marker were evaluated for the location of staining
(nuclear or cytoplasmic), extent of staining (percentage of
positive cells, 0–100%) and intensity of staining (0, negative; 1,
weak; 2, medium; and 3, intense), and a cut-off value was based on
the median H-score in tumor (32).
Adjacent normal tissues were defined as areas surrounding the tumor
in each section with confirmation by pathologist in case of
uncertainty. To determine the percentage of positive cells and the
staining intensity, the H-score was calculated by the sum of the
products of the intensity and extent of expression scores,
obtaining a value from 0 to 300. For statistical analysis, the
final H-score was divided into two scoring categories. First, cases
with H-scores ≥ the median were considered to have high expression;
by contrast, cases with H-scores < the median were considered to
have low expression (Table II).
Second, the results for the nuclear to cytoplasmic expression ratio
were stratified into four groups: Nuclear high/cytoplasmic high,
nuclear high/cytoplasmic low, nuclear low/cytoplasmic high and
nuclear low/cytoplasmic low.
 | Table II.Immunohistochemistry distribution in
adjacent normal vs. tumor tissues, and association between
cytoplasmic and nuclear expression levels. |
Table II.
Immunohistochemistry distribution in
adjacent normal vs. tumor tissues, and association between
cytoplasmic and nuclear expression levels.
| A, PTEN
expression |
|---|
|
|---|
| Location | Normal tissues, n
(%) | Tumor tissues, n
(%) | P-value | χ2a |
|---|
| Nuclear |
|
| 0.572 | 0.319 |
|
Low | 11 (50) | 37 (57) |
|
|
|
High | 11 (50) | 28 (43) |
|
|
| Cytoplasmic |
|
| 0.420 | 0.651 |
|
Low | 10 (45) | 36 (55) |
|
|
|
High | 12 (55) | 29 (45) |
|
|
|
| B,
pAktSer473 expression |
|
|
Location | Normal tissues,
n (%) | Tumor tissues, n
(%) | P-value | χ2a |
|
| Nuclear |
|
| 0.072 | 5.241 |
|
Low | 10 (45) | 43 (66) |
|
|
|
High | 11 (50) | 22 (34) |
|
|
| Absent
data | 1 (5) | 0 (0) |
|
|
| Cytoplasmic |
|
| 0.003 | 11.340 |
|
Low | 9
(41) | 50 (77) |
|
|
|
High | 12 (55) | 15 (23) |
|
|
| Absent
data | 1 (4) | 0 (0) |
|
|
|
| C,
pAktThr308 expression |
|
|
Location | Normal tissues,
n (%) | Tumor tissues, n
(%) | P-value | χ2a |
|
| Nuclear |
|
| 0.113 | 2.510 |
|
Low | 10 (45) | 42 (65) |
|
|
|
High | 12 (55) | 23 (35) |
|
|
| Cytoplasmic |
|
| 0.147 | 2.104 |
|
Low | 10 (45) | 41 (63) |
|
|
|
High | 12 (55) | 24 (37) |
|
|
Statistical analysis
The expression levels of protein in adjacent normal
tissues and UTUCs were compared using non-parametric statistics.
The association between PTEN gene alternation, protein
expression levels and various clinical characteristics, including
that between protein expression in UTUCs and pT stage status (low
vs. high), were evaluated using χ2 test or Fisher's
exact test (Table III). Two-way
ANOVA with Bonferroni's correction was used to compare the
differences between normal and tumor cells in the cytoplasm and
nucleus, respectively. Patients who were lost to follow-up were
censored on the date of the last visit. All significant parameters
from the univariate analyses were included in the multivariate
analyses. The probabilities of overall survival were calculated
using Kaplan-Meier analysis, and log-rank tests were used to
compare overall survival between patient groups. P<0.05 was
considered to indicate a statistically significant difference. All
statistical analyses were performed using SPSS software version
20.0 (IBM Corp.) or GraphPad Prism version 7.00 (GraphPad Software,
Inc.).
 | Table III.Association between
clinicopathological features and target protein expression. |
Table III.
Association between
clinicopathological features and target protein expression.
|
| pT stage | Tumor grade |
|---|
|
|
|
|
|---|
| Variables | pTa-1, n (%) | pT2-4, n (%) | P-value | Low, n (%) | High, n (%) | P-value |
|---|
| Low cytoplasmic
PTEN protein expression | 11 (17) | 25 (38) | 0.023 | 4 (6) | 32 (49) | – |
| High nuclear
pAktThr308 protein expression | 17 (26) | 25 (38) | – | 3 (5) | 39 (60) | 0.026 |
| High cytoplasmic
pAktThr308 protein expression | 18 (28) | 23 (35) | – | 3 (5) | 38 (58) | 0.031 |
Results
PTEN alterations identified using
FISH
In total, 60 (92%) tumors were located in the renal
pelvis and 4 (6%) tumors were located in the ureter, with 1 (2%)
tumor located in both the renal pelvis and the ureter (Table I). A total of 16 (25%) patients
presented with Ta, 12 (18%) patients presented with pT1, 11 (17%)
patients presented with pT2, 20 (31%) patients presented with pT3
and 6 (9%) patients presented with pT4. In total, 11 (17%) patients
had low-grade tumors, while the remaining 54 (83%) patients had
high-grade tumors. A total of 16 (25%) patients had metastasis, and
the median follow-up time was 96 months (range, 30–159 months;
Table I). PTEN gene deletions
were found in 33.8% (22/65) of all UTCUs (Table SI). Representative images of the
FISH results are illustrated in Fig.
S1. Among the specimens with PTEN deletion, 18 (81.8%)
samples showed heterozygous deletion and 1 sample had a homozygous
deletion. Monosomy of chromosome 10 was detected in 3 samples (data
not shown). PTEN gene translocation was found in 1 of the
heterozygous deletion samples. However, there was no significant
association between PTEN gene alteration, protein expression
(Table SI) or clinicopathological
parameters (data not shown).
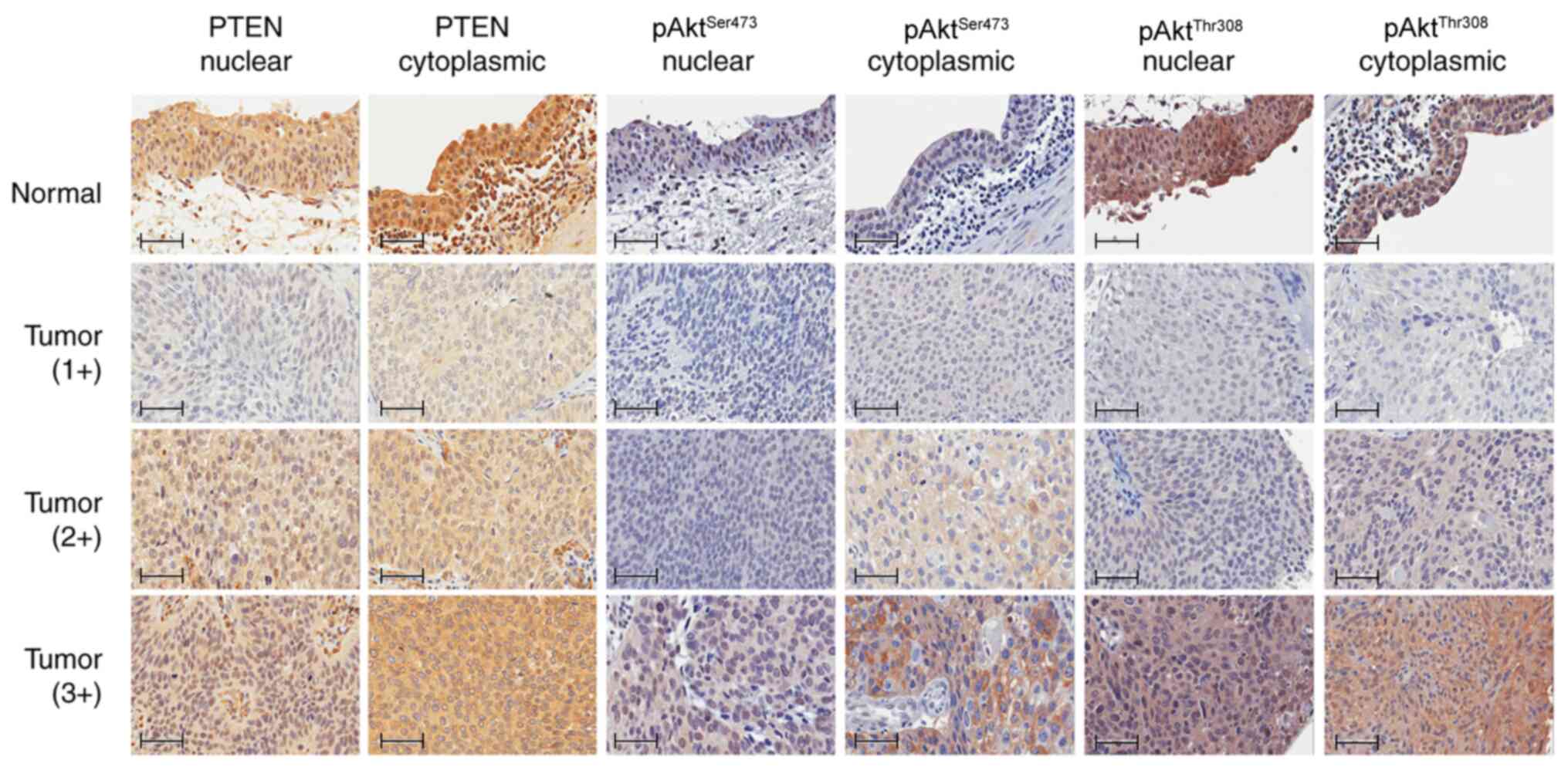
Protein expression levels identified
using IHC
Representative patterns of protein expression levels
detected by IHC are depicted in Fig.
2, and the results with further subgroup categorization based
on the median H score are summarized in Table II. The distribution of protein
expression levels between tumor and normal tissues was not
significantly different according to the classification of
expression levels above or below the median H score, with the
exception of cytoplasmic spAktSer473 protein expression
levels, which were lower in tumor tissues compared with those in
normal tissues (P=0.003). As for PTEN expression in the nucleus,
57% of tumors and 50% of normal tissues had low expression levels
(χ2=0.319; P=0.572). Similarly, for PTEN expression in
the cytoplasm, 55% of tumors vs. 45% of normal tissues had low
expression levels (χ2=0.651; P=0.420). As for
pAktSer473 expression in the nucleus, 66% of tumors and
45% of normal tissues had low expression levels
(χ2=5.241; P=0.072), while a significant difference was
found in the cytoplasm with 77% of tumors vs. 41% of normal tissues
having low expression (χ2=11.343; P=0.003). As for
pAktThr308 expression in the nucleus, 65% of tumors and
45% of normal tissues had low expression levels
(χ2=2.510; P=0.113), and a similar trend was observed in
the cytoplasm with 63% of tumors vs. 45% of normal tissues having
low expression (χ2=2.104; P=0.147). On the other hand,
statistically significant differences were observed in the protein
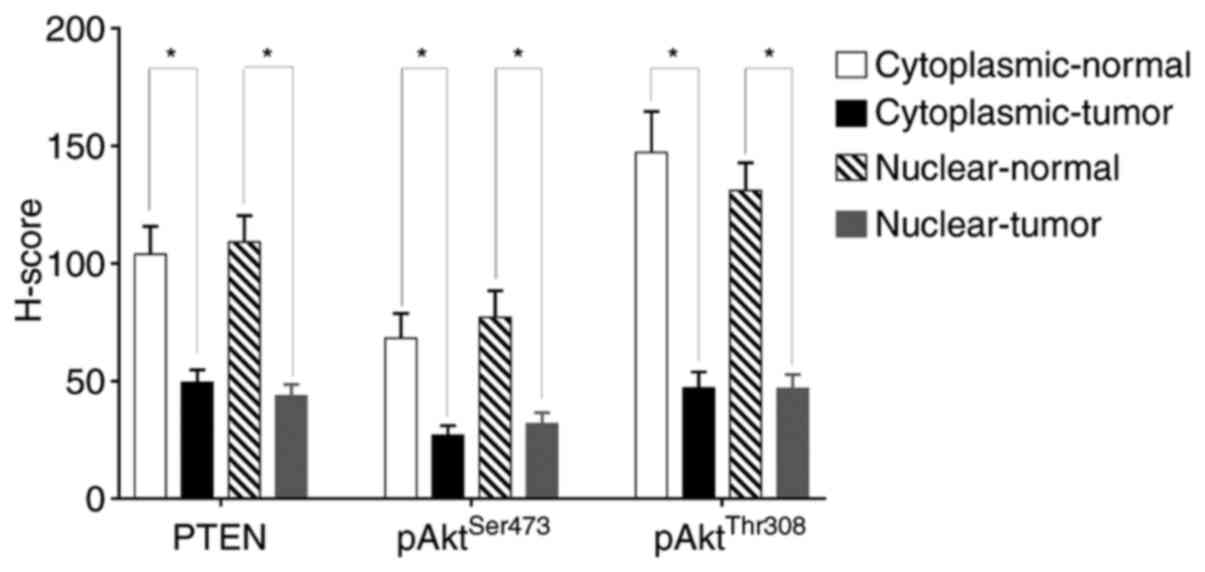
expression levels between tumor and adjacent normal tissues
(Fig. 3). PTEN,
pAktSer473 and pAktThr308 levels were all
significantly lower in UTUC tissues compared with those in adjacent
normal tissues in terms of H scores. The mean PTEN expression in
the cytoplasm between normal and tumor tissues was 104 and 49.53,
while that in the nucleus was 109.2 and 43.86, respectively; mean
pAktSer473 expression in the cytoplasm between normal
and tumor tissues was 68.2 and 26.9, while that in the nucleus was
77.12 and 31.86, respectively; mean pAktThr308
expression in the cytoplasm between normal and tumor tissues was
147.3 and 47.03, while that in the nucleus was 131.1 and 46.95,
respectively.
Association of PTEN results identified
using FISH and IHC
A total of 11/18 samples with heterozygous
PTEN gene deletion showed lower nuclear and cytoplasmic PTEN
protein expression compared with the expression levels of the other
samples, and 7 samples had high expression levels. Overall, 2 out
of 3 samples with monosomic PTEN gene deletion showed lower
nuclear and cytoplasmic PTEN protein expression compared with that
in the other sample, which had high expression levels. One sample
with homozygous PTEN gene deletion showed no PTEN protein
expression in neither the nucleus or the cytoplasm. There was no
significant association between PTEN gene alteration and
nuclear PTEN protein expression (P=0.434), cytoplasmic PTEN protein
expression (P=0.338), nuclear pAktSer473 protein
expression (P=0.423), cytoplasmic pAktSer473 protein
expression (P=0.566), nuclear pAktThr308 protein
expression (P=0.906) or cytoplasmic pAktThr308 protein
expression (P=0.634) (Table
SI).
Association of patient characteristics
with protein expression levels
Table III
summarizes the association of protein expression levels with
clinicopathological parameters. Lower cytoplasmic PTEN protein
expression levels were significantly associated with advanced T
stage (T2-pT4 vs. pTa and pT1, 38 vs. 17%, P=0.023), and expression
of nuclear and cytoplasmic pAktThr308 protein was
associated with higher tumor grade (high grade vs. low grade, 60
vs. 5%, P=0.026 and 58 vs. 5%, P=0.031, respectively). However,
pAktSer473 protein expression was not associated with T
stage or tumor grade.
Univariate and multivariate analyses
of clinical characteristics and protein expression levels
In the univariate analysis, high T stage [P=0.01;
hazard ratio (HR)=3.40; 95% CI, 1.34–8.60), metastatic status
(P=0.003; HR=3.55; 95% CI, 1.55–8.11), low cytoplasmic PTEN protein
expression (P=0.016; HR=3.14, 95% CI, 1.24–7.95) and high
cytoplasmic pAktSer473 protein expression (P=0.019;
HR=2.71; 95% CI, 1.18–6.21) were predictive of poor overall
survival. However, in the multivariate analysis, only metastatic
status (P=0.031, HR=2.73, 95% CI, 1.10–6.78), low cytoplasmic PTEN
protein expression (P=0.017; HR=3.29; 95% CI, 1.24–8.73) and high
cytoplasmic pAktSer473 protein expression (P=0.027;
HR=2.64; 95% CI, 1.12–6.23) were significantly associated with poor
prognosis (Table IV).
 | Table IV.Univariate and multivariate analyses
for predictors of overall survival. |
Table IV.
Univariate and multivariate analyses
for predictors of overall survival.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Variables | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| pT stage |
| 0.01 |
| – |
|
pTa-pT1 | 1 |
|
|
|
|
pT2-pT4 | 3.40
(1.34–8.60) |
|
|
|
| Metastasis |
| 0.003 |
| 0.031 |
| No | 1 |
| 1 |
|
|
Yes | 3.55
(1.55–8.11) |
| 2.73
(1.10–6.78) |
|
| Cytoplasmic PTEN
protein expression |
| 0.016 |
| 0.017 |
|
High | 1 |
| 1 |
|
|
Low | 3.14
(1.24–7.95) |
| 3.29
(1.24–8.73) |
|
| Cytoplasmic
pAktSer473 protein expression |
| 0.019 |
| 0.027 |
|
Low | 1 |
| 1 |
|
|
High | 2.71
(1.18–6.21) |
| 2.64
(1.12–6.23) |
|
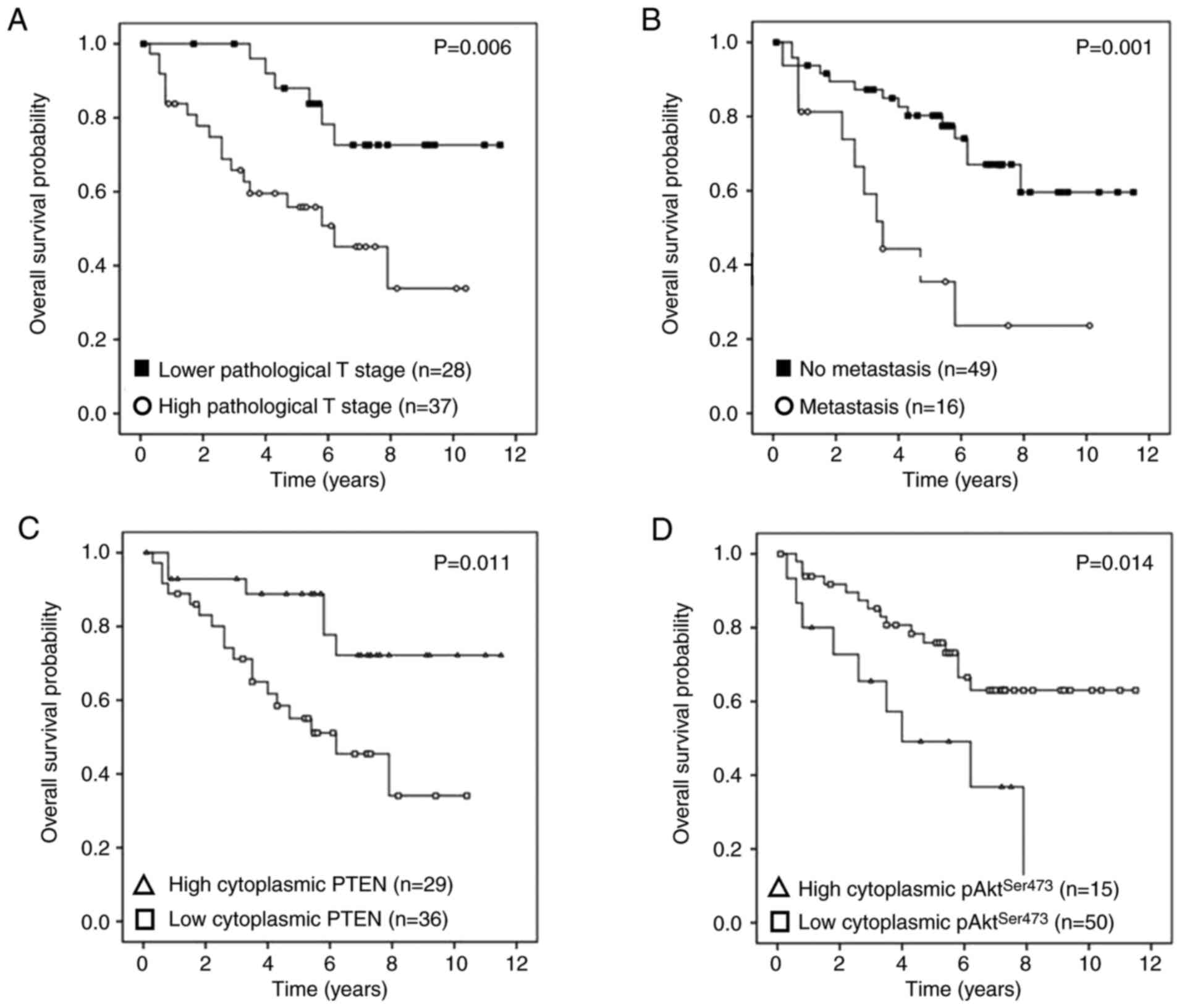
Stratification of survival differences
according to protein expression subgroups
The results of Kaplan-Meier analysis and log-rank
test for overall survival according to clinicopathological
parameters and protein expression are presented in Figs. 4 and 5. Survival was poor in patients with high T
stage (P=0.006) (Fig. 4A),
metastatic disease (P=0.001) (Fig.
4B), low cytoplasmic PTEN protein expression (P=0.011)
(Fig. 4C) and high cytoplasmic
pAktSer473 protein expression (P=0.014) (Fig. 4D). The co-expression phenotypes with
low cytoplasmic PTEN and high cytoplasmic pAktSer473
(PTENlow/pAktSer473/high) (P<0.001)
(Fig. 5A), low nuclear and/or
cytoplasmic PTEN and high pAktThr308
(PTENlow/pAktThr308/high) (P=0.024) (Fig. 5B), low nuclear and/or cytoplasmic
PTEN and high pAktSer473
(PTENlow/pAktSer473/high) (P=0.016) (Fig. 5C) and low cytoplasmic PTEN with high
pAktSer473 and pAktThr308
(PTENlow/pAktSer473/high/pAktThr308/high)
(P=0.001) (Fig. 5D) were
demonstrated to have unfavorable impacts on overall survival.
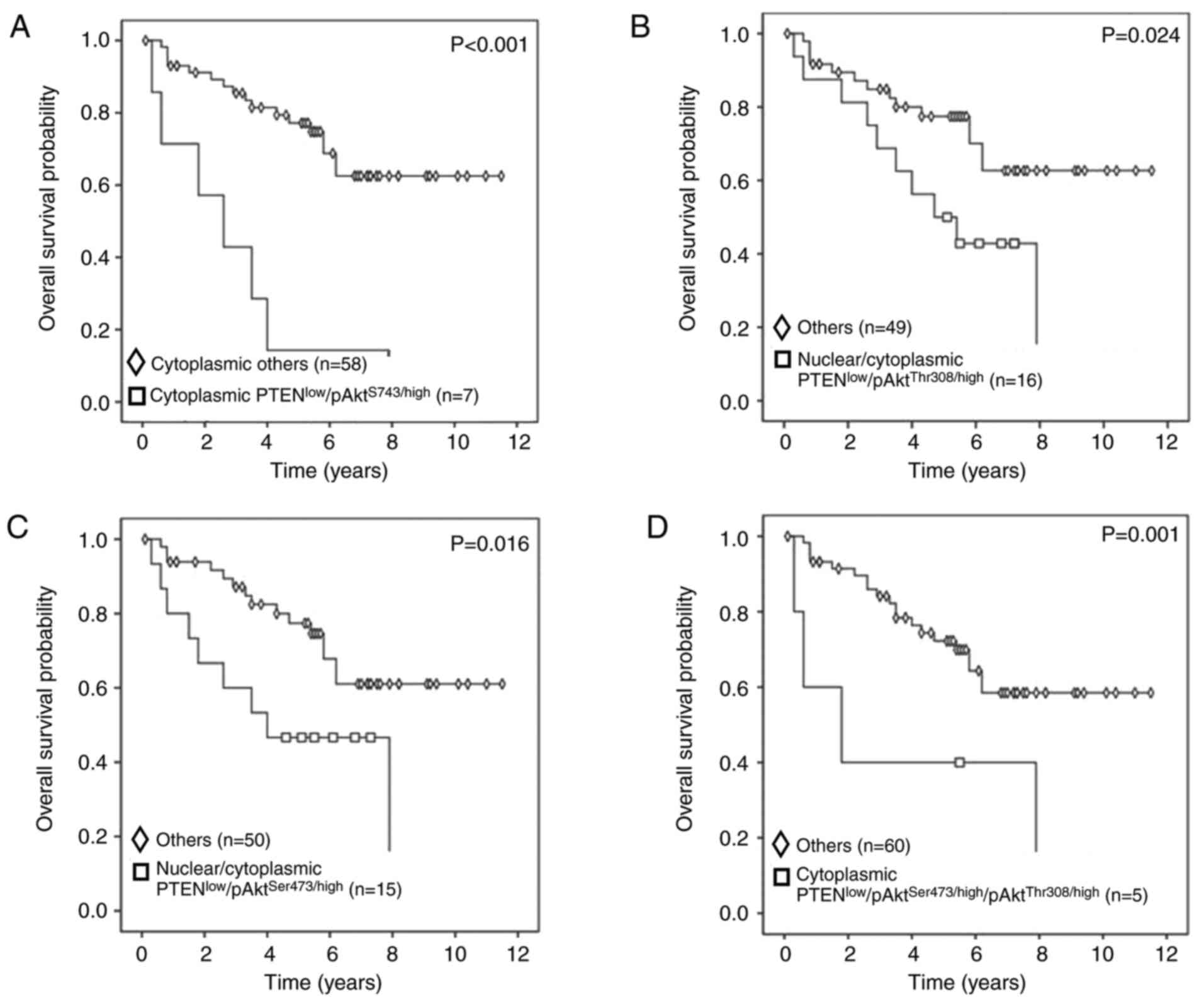 | Figure 5.Kaplan-Meier curves and log-rank
tests for overall survival according to protein co-expression. (A)
Patients in the cytoplasmic PTENlow and
pAktSer473/high group had significantly shorter survival
compared with those in other groups, including cytoplasmic
PTENlow/pAktSer473/low, cytoplasmic
PTENhigh/pAktSer473/low and cytoplasmic
PTENhigh/pAktSer473/high (P<0.001). (B)
Patients in the nuclear and/or cytoplasmic PTENlow and
pAktThr308/high group had significantly shorter survival
compared with those in other groups, including nuclear/cytoplasmic
PTENhigh/pAktThr308/high, nuclear/cytoplasmic
PTENlow/pAktThr308/low and
nuclear/cytoplasmic PTENhigh/pAktThr308/low
(P=0.024). (C) Patients in the nuclear and/or cytoplasmic
PTENlow and pAktSer473/high group showed
significantly shorter survival than thos in other groups, including
PTENhigh/pAktSer473/high, cytoplasmic
PTENlow/pAktSer473/low and cytoplasmic
PTENhigh/pAktSer473/low (P=0.016). (D)
Patients in the cytoplasmic PTENlow and combined
pAktSer473/high and pAktThr308/high group had
significantly shorter survival than those in other groups,
including
PTENlow/pAktSer473/low/pAktThr308/high,
PTENlow/pAktSer473/high/pAktThr308/low,
PTENlow/pAktSer473/low/pAktThr308/low,
PTENhigh/pAktSer473/low/pAktThr308/high,
PTENhigh/pAktSer473/high/pAktThr308/low,
PTENhigh/pAktSer473/low/pAktThr308/low
and
PTENhigh/pAktSer473/high/pAktThr308/high
(P=0.001). p, phosphorylated; Ser, serine; Thr, threonine; T,
tumor. |
Discussion
In the present study, lower protein expression
levels of PTEN, pAktSer473 and pAktThr308 in
either the cytoplasm or nucleus were observed in UTUC compared with
those in adjacent normal tissues (P<0.001; Fig. 3). In addition, high T stage,
metastatic disease, low cytoplasmic PTEN protein expression levels,
high cytoplasmic pAktSer473 protein expression levels,
co-expression of low cytoplasmic/nuclear PTEN and high cytoplasmic
pAktThr308 and high pAktSer473 had
unfavorable impacts on overall survival (Figs. 4 and 5).
Based on the results from the paired samples
analyzed using IHC, the PTEN protein expression levels in either
the cytoplasm or nucleus were significantly higher in adjacent
normal tissues compared with those in tumor tissues (all
P<0.001; Fig. 3). Therefore,
alterations in gene structure were further investigated, and the
majority of cases presented PTEN gene heterozygous
deletions, with only one case showing a homozygous deletion, which
resulted in the absence of PTEN protein expression.
Nevertheless, a previous study demonstrated that
PTEN deletion in bladder cancer was significantly associated
with recurrence in the Ta stage of disease and with progression in
the T1 stage of disease (10). The
present study focused on UTUC, and a high proportion of PTEN
gene deletions (22/65; 33.8%) were observed in UTUC tissues. A
previous report by Rieken et al (33) revealed that loss of PTEN protein
expression was rare, and was associated with an aggressive
phenotype, high tumor grade, high tumor stage and metastasis in
UTUC. Further association with poor overall mortality was also
documented. Although the lower number of cases showing loss of PTEN
protein expression in Western countries contrasts with the higher
rate in Taiwan (34), the results
regarding patient outcomes were similar in the present study.
In addition, the correlation between the location of
proteins in tumor cells and patient survival rate was further
analyzed. Notably, it was found that patients with low cytoplasmic
PTEN and high pAktSer473 expression levels (the
PTENlow/pAktSer473/high group) had
significantly shorter overall survival compared with that of other
groups (P<0.001). Similarly, patients with low nuclear and/or
cytoplasmic PTEN and high pAktThr308 expression levels
(the PTENlow/pAktThr308/high group) had
significantly shorter overall survival compared with that of other
groups (P=0.024). Moreover, patients with low nuclear and/or
cytoplasmic PTEN and high pAktSer473 expression levels
(the PTENlow/pAktSer473/high group) also
showed significantly shorter overall survival compared with that of
other groups (P=0.016). Patients with low cytoplasmic PTEN and high
pAktSer473 and pAktThr308 expression levels
(the
PTENlow/pAktSer473/high/pAktThr308/high
group) had significantly shorter survival compared with that of
other groups (P=0.001) (Fig. 5).
When patients lost PTEN expression, regardless of location in the
cytoplasm or nucleus, this was significantly associated with less
favorable survival outcomes. Additionally, to the best of our
knowledge, homozygous deletion of PTEN is uncommon in urothelial
cancer (10), and the present study
is the first to report this.
Contrary to expectations, the protein expression
levels of pAktSer473 and pAktThr308 were
lower in tumor samples compared with those in normal tissue samples
(Fig. 3). These results coincide
with those of a study by Munari et al (35), in which 99 archival FFPE tissues were
evaluated for the expression status and prognostic significance of
members of the mTOR signaling pathway in UTUC. Significantly higher
expression levels of PTEN and pAkt were found in benign urothelium
tissues compared with those in paired tumor samples. A possible
reason for this could be that the prevalence of PTEN loss in
tumors varies between 8 and 36% of cases (33), and activation of Akt phosphorylation
sites is regulated by different downstream pathways.
PTEN is a tumor suppressor gene, and
dephosphorylation of PIP3 into PIP2 can prevent hyperactivation of
Akt (11). According to the findings
of Makboul et al (36),
decreased PTEN protein expression levels were significantly
associated with high-grade tumors in UC and with poorly
differentiated squamous cell carcinomas. A number of studies have
indicated that PTEN deletion was more prevalent in the
nucleus compared with that in the cytoplasm (37,38).
Loss of cytoplasmic PTEN expression was primarily observed in the
pT2-pT4 stages of UC, which was documented in 77% (10/13) of UC
cases (39). In addition, several
studies demonstrated that the active Akt protein is an important
regulatory factor in cancer cells, giving rise to uncontrolled
proliferation without apoptosis (26,40). For
example, the activation of Akt is primarily driven by the
phosphorylation of two residues, Thr308 and Ser473, which are
located in the activation loop and in the C-terminal hydrophobic
motif of the protein, respectively (41). The site of Ser473 phosphorylation is
known to be associated with tumor formation, and its
phosphorylation may be triggered by mTORC2 activation (17,42).
However, protein activation mediated by phosphorylated Thr308,
which in turn is regulated by PDK1, is considered necessary and
sufficient to stimulate Akt signaling in cells; therefore,
phosphomimetics are commonly used to study the biology of Akt
signaling, although they may be insufficient in clarifying the
mechanism of the whole Akt signaling pathway. Gallay et al
indicated that pAktrThr308 could be a diagnostic marker
of Akt activity (16). Moreover, Akt
phosphorylation at Thr308 is associated with human non-small cell
lung cancer (43) (Fig. 1).
In the present study, both cytoplasmic and nuclear
PTEN levels in tumors were compared with those in adjacent normal
cells (P<0.001; Fig. 3), and
lower expression levels of cytoplasmic PTEN were found in muscle
invasive disease (high stage, pT2-4) compared with those in
non-muscle invasive disease (low stage, pTa-1; P=0.023; Table II). Furthermore, high expression
levels of cytoplasmic and nuclear pAktThr308 were
associated with high tumor grade (P=0.031 and P=0.026,
respectively). Similar results from stage I lung adenocarcinoma
also revealed that a PTEN(−)/pAkt(+)/pmTOR(+) phenotype was
associated with poor overall survival (44). In agreement with these data, the
present study showed that low cytoplasmic PTEN protein expression
levels were observed in high T stage tissues, and high nuclear or
cytoplasmic pAktThr308 protein expression levels were
observed in high tumor grade tissues. Furthermore, it was revealed
that high T stage, metastasis, low cytoplasmic PTEN protein
expression and high cytoplasmic pAktSer473 protein
expression levels were strong predictors of poor survival in
univariate analysis (Table IV). In
multivariate analysis, metastasis, low cytoplasmic PTEN protein
expression levels and high cytoplasmic pAktSer473
protein expression levels remained strong predictors. Table II showed the association between
clinical features and target protein levels by IHC, which showed no
specific association between pAktSer473,
pAktThr308 and metastasis. However, patients with high
cytoplasmic/nuclear pAktThr308 possessed high tumor
grades in the present cohort. Therefore, it was hypothesized that
pAktSer473 and pAktThr308 are not directly
associated with metastasis. It is also noteworthy that high T stage
in the present cohort was not a significant predictor in the
multivariate analysis. A possible reason for this phenomenon may be
that cisplatin-based chemotherapy works well for patients who
progress to locally advanced or metastatic disease as standard
first-line systemic treatment. Therefore, in the present cohort
with the majority of patients at the Ta-T2 (60%) stages, T stage is
not a significant predictor for overall survival in the
multivariate analysis. Cha et al, Margulis et al and
Ehdaie et al (45–47) reported that UTUC with lymph node
metastasis was associated with worse cancer-specific and overall
survival compared with UTUC without lymph node metastasis. In
addition, two other studies have shown that nuclear pAkt expression
levels and pathological stage were associated with poor prognosis
in UTUC (48,49), supporting the present findings and
suggesting that the PTEN/PI3K/pAkt signaling pathway is important
in the carcinogenesis of UTUC.
The incidence of UTUC accounts for 5–10% of that of
all UCs in Western countries, while the incidence is as high as 30%
in Taiwan, and UTUC is associated with consumption of Chinese
herbal medicines containing aristolochic acid (AA) (50). In the present study of 65 patients
with UTUC, high T stage, metastasis, low cytoplasmic PTEN protein
expression levels, high cytoplasmic pAktSer473 protein
expression levels and co-expression of low cytoplasmic PTEN and
high pAktSer473 were strong predictors of poor overall
survival. Munari et al (35)
and Izquierdo et al (48)
also reported that high T stage, lymph node metastasis and
lymphovascular invasion were significant predictors of tumor
progression and increased cancer-specific mortality in UTUC.
Moreover, Shin et al (44)
demonstrated that a PTEN(−)/pAkt(+)/pmTOR(+) result from IHC
analysis was associated with poor prognosis in stage I non-small
cell lung carcinoma. Although these aforementioned factors are
highly associated with cancer prognosis when considered together,
they are not synergistic and do not have any effect on their own.
Koletsas et al (39) found
that PTEN expression loss was not associated with Akt activation,
suggesting that more complicated pathways could be involved through
crosstalk or synergistic effects rather than direct activation
(38). By contrast, a study
evaluating the mTOR pathway members in patients with UTUC showed
that none of the existing biomarkers were useful for predicting
tumor progression or cancer-specific mortality (35). These findings implied that
differences in ethnicity or environmental factors, such as AA, may
make the clinical presentation of the disease variable and
unpredictable.
There are some limitations in the present study.
First, a couple of clinical factors were also associated with
oncological outcomes, for example, lymph node status. However,
lymph node dissection is not the routine procedure during
nephroureterectomy, especially when the pre-operative image study
is negative for lymph node disease in patients with UTUC. Although
there were 6 patients (9%) with T4 disease, representing the
high-risk group for nodal disease, the present study only
incorporated distant metastasis as a predictor of long-term
oncological outcome instead of addressing the association with
lymph node status. Future studies should include lymph node
dissection in the prospective setting. Second, FISH and IHC were
performed to quantify the expression levels of targeted proteins;
despite meticulous quality control and standardization of every
procedure, experimental errors may still have occurred in these
tests. Alternative analyses should be performed in future work,
including western blotting and ELISA for determination of protein
expression levels, in order to investigate associations with other
notable targets. If further studies showed a significant
association with the mechanism of invasion or proliferation, the
levels of target proteins before and after systemic treatment could
also be explored. The present study showed the preliminary results
of the hypothesis, indicating that low PTEN expression with
upregulation of phosphorylated AktSer473 and
AktThr308 may be associated with poor overall survival
in UTUC and may be applied as predictors for outcome stratification
before surgery. However, in real world practice, it is hard to
obtain a large enough sample size of patients with UTUC before
surgery for tissue sampling. If technical advancements reach a
level of improved accuracy in regard to small tissue volumes, it
may be helpful to predict the outcome of surgery or to enable more
personalized planning prior to surgery for patients with UTUC with
positive markers.
To the best of our knowledge, the present study is
the first to evaluate the association of PTEN gene
alteration and the co-expression of PTEN, pAktSer473 and
pAktThr308 with clinicopathological parameters and
outcomes in UTUC. The present results suggest that patients with
negative protein expression patterns should receive further
personalized follow-up and adjuvant treatment.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Ms. Shu-Ting Gan,
the analyst at the Center for Big Data Analytics and Statistics
(CLRPG3D0046) at the Linkou Chang Gung Memorial Hospital (Taoyuan,
Taiwan) for the statistical consultation and manuscript
revision.
Funding
The present study was supported by Linkou Chang
Gung Memorial Hospital (grant nos. CORPG3G0081 and
NTUT-CGMH-106-08).
Availability of data and materials
The datasets used and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
WHW, LCL and STP conceived and designed the study.
KJY, LCL, YJP, CKC and YTC analyzed and interpreted the data. WHW,
KJY and LCL drafted the initial manuscript, and YJP, STP and CKC
critically revised the manuscript for important intellectual
content. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by The Human Subject
Research Ethics Committee/Institutional Review Board (approval no.
201601555B0) of Linkou Chang Gung Memorial Hospital (Taoyuan,
Taiwan). All patients provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
UCs
|
urothelial carcinomas
|
|
UTUC
|
upper tract urothelial carcinoma
|
|
BC
|
bladder cancer
|
|
PIP3
|
phosphatidyl-inositol (3,4,5)-triphosphate
|
|
PIP2
|
phosphatidylinositol
4,5-biphosphate
|
|
FFPE
|
formalin-fixed, paraffin-embedded
|
|
FISH
|
fluorescence in situ
hybridization
|
References
|
1
|
Ploeg M, Aben KK and Kiemeney LA: The
present and future burden of urinary bladder cancer in the world.
World J Urol. 27:289–293. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Verhoest G, Shariat SF, Chromecki TF,
Raman JD, Margulis V, Novara G, Seitz C, Remzi M, Rouprêt M, Scherr
DS and Bensalah K: Predictive factors of recurrence and survival of
upper tract urothelial carcinomas. World J Urol. 29:495–501. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lai MN, Wang SM, Chen PC, Chen YY and Wang
JD: Population-based case-control study of Chinese herbal products
containing aristolochic acid and urinary tract cancer risk. J Natl
Cancer Inst. 102:179–186. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Taiwan Health Promotion Administration
Ministry of Health and Welfare Taiwan, . Cancer Registry Annual
Report, 2014 Taiwan. 1212016.
|
|
6
|
Rouprêt M, Babjuk M, Compérat E, Zigeuner
R, Sylvester RJ, Burger M, Cowan NC, Böhle A, Van Rhijn BW,
Kaasinen E, et al: European association of urology guidelines on
upper urinary tract urothelial cell carcinoma: 2015 Update. Eur
Urol. 68:868–879. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kikuchi E, Margulis V, Karakiewicz PI,
Roscigno M, Mikami S, Lotan Y, Remzi M, Bolenz C, Langner C, Weizer
A, et al: Lymphovascular invasion predicts clinical outcomes in
patients with node-negative upper tract urothelial carcinoma. J
Clin Oncol. 27:612–618. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vivanco I and Sawyers CL: The
phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev
Cancer. 2:489–501. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Parsons DW, Wang TL, Samuels Y, Bardelli
A, Cummins JM, DeLong L, Silliman N, Ptak J, Szabo S, Willson JK,
et al: Colorectal cancer: Mutations in a signalling pathway.
Nature. 436:7922005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cordes I, Kluth M, Zygis D, Rink M, Chun
F, Eichelberg C, Dahlem R, Fisch M, Höppner W, Wagner W, et al:
PTEN deletions are related to disease progression and unfavourable
prognosis in early bladder cancer. Histopathology. 63:670–677.
2013.PubMed/NCBI
|
|
11
|
Chalhoub N and Baker SJ: PTEN and the
PI3-kinase pathway in cancer. Annu Rev Pathol. 4:127–150. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Stokoe D, Stephens LR, Copeland T, Gaffney
PR, Reese CB, Painter GF, Holmes AB, McCormick F and Hawkins PT:
Dual role of phosphatidylinositol-3,4,5-trisphosphate in the
activation of protein kinase B. Science. 277:567–570. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sarbassov DD, Guertin DA, Ali SM and
Sabatini DM: Phosphorylation and regulation of Akt/PKB by the
rictor-mTOR complex. Science. 307:1098–1101. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sabatini DM: mTOR and cancer: Insights
into a complex relationship. Nat Rev Cancer. 6:729–734. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hay N: The Akt-mTOR tango and its
relevance to cancer. Cancer Cell. 8:179–183. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gallay N, Dos Santos C, Cuzin L, Bousquet
M, Simmonet Gouy V, Chaussade C, Attal M, Payrastre B, Demur C and
Récher C: The level of AKT phosphorylation on threonine 308 but not
on serine 473 is associated with high-risk cytogenetics and
predicts poor overall survival in acute myeloid leukaemia.
Leukemia. 23:1029–1038. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Freudlsperger C, Horn D, Weißfuß S,
Weichert W, Weber KJ, Saure D, Sharma S, Dyckhoff G, Grabe N,
Plinkert P, et al: Phosphorylation of AKT(Ser473) serves as an
independent prognostic marker for radiosensitivity in advanced head
and neck squamous cell carcinoma. Int J Cancer. 136:2775–2785.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Campbell IG, Russell SE, Choong DY,
Montgomery KG, Ciavarella ML, Hooi CS, Cristiano BE, Pearson RB and
Phillips WA: Mutation of the PIK3CA gene in ovarian and breast
cancer. Cancer Res. 64:7678–7681. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kirkegaard T, Witton CJ, McGlynn LM, Tovey
SM, Dunne B, Lyon A and Bartlett JM: AKT activation predicts
outcome in breast cancer patients treated with tamoxifen. J Pathol.
207:139–146. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Okano J, Snyder L and Rustgi AK: Genetic
alterations in esophageal cancer. Methods Mol Biol. 222:131–145.
2003.PubMed/NCBI
|
|
21
|
Asano T, Yao Y, Zhu J, Li D, Abbruzzese JL
and Reddy SA: The PI 3-kinase/Akt signaling pathway is activated
due to aberrant Pten expression and targets transcription factors
NF-kappaB and c-Myc in pancreatic cancer cells. Oncogene.
23:8571–8580. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Broderick DK, Di C, Parrett TJ, Samuels
YR, Cummins JM, McLendon RE, Fults DW, Velculescu VE, Bigner DD and
Yan H: Mutations of PIK3CA in anaplastic oligodendrogliomas,
high-grade astrocytomas, and medulloblastomas. Cancer Res.
64:5048–5050. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shaw RJ and Cantley LC: Ras, PI(3)K and
mTOR signalling controls tumour cell growth. Nature. 441:424–430.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Knowles MA, Platt FM, Ross RL and Hurst
CD: Phosphatidylinositol 3-kinase (PI3K) pathway activation in
bladder cancer. Cancer Metastasis Rev. 28:305–316. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Courtney KD, Corcoran RB and Engelman JA:
The PI3K pathway as drug target in human cancer. J Clin Oncol.
28:1075–1083. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Askham JM, Platt F, Chambers PA, Snowden
H, Taylor CF and Knowles MA: AKT1 mutations in bladder cancer:
Identification of a novel oncogenic mutation that can co-operate
with E17K. Oncogene. 29:150–155. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Brognard J, Clark AS, Ni Y and Dennis PA:
Akt/protein kinase B is constitutively active in non-small cell
lung cancer cells and promotes cellular survival and resistance to
chemotherapy and radiation. Cancer Res. 61:3986–3997.
2001.PubMed/NCBI
|
|
28
|
Clark PE, Agarwal N, Biagioli MC,
Eisenberger MA, Greenberg RE, Herr HW, Inman BA, Kuban DA, Kuzel
TM, Lele SM, et al: Bladder cancer. J Natl Compr Canc Netw.
11:446–475. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rouprêt M, Babjuk M, Compérat E, Zigeuner
R, Sylvester R, Burger M, Cowan N, Böhle A, Van Rhijn BW, Kaasinen
E, et al: European guidelines on upper tract urothelial carcinomas:
2013 Update. Eur Urol. 63:1059–1071. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Edge SB and Compton CC: The American joint
committee on cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Weng WH, Ahlén J, Aström K, Lui WO and
Larsson C: Prognostic impact of immunohistochemical expression of
ezrin in highly malignant soft tissue sarcomas. Clin Cancer Res.
11:6198–6204. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gonzalez-Roibon ND, Chaux A, Al-Hussain T,
Osunkoya AO, Bezerra SM, Hicks J, Epstein JI and Netto GJ:
Dysregulation of mammalian target of rapamycin pathway in
plasmacytoid variant of urothelial carcinoma of the urinary
bladder. Hum Pathol. 44:612–622. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rieken M, Shariat SF, Karam JA, Foerster
B, Khani F, Gust K, Abufaraj M, Wood CG, Weizer AZ, Raman JD, et
al: Frequency and prognostic value of PTEN loss in patients with
upper tract urothelial carcinoma treated with radical
nephroureterectomy. J Urol. 198:1269–1277. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Qian CN, Furge KA, Knol J, Huang D, Chen
J, Dykema KJ, Kort EJ, Massie A, Khoo SK, Vanden Beldt K, et al:
Activation of the PI3K/AKT pathway induces urothelial carcinoma of
the renal pelvis: Identification in human tumors and confirmation
in animal models. Cancer Res. 69:8256–8264. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Munari E, Fujita K, Faraj S, Chaux A,
Gonzalez-Roibon N, Hicks J, Meeker A, Nonomura N and Netto GJ:
Dysregulation of mammalian target of rapamycin pathway in upper
tract urothelial carcinoma. Hum Pathol. 44:2668–2676. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Makboul R, Refaiy A, Abdelkawi IF, Hameed
DA, Elderwy AA, Shalaby MM, Merseburger AS and Hussein MR:
Alterations of mTOR and PTEN protein expression in schistosomal
squamous cell carcinoma and urothelial carcinoma. Pathol Res Pract.
212:385–392. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tsuruta H, Kishimoto H, Sasaki T, Horie Y,
Natsui M, Shibata Y, Hamada K, Yajima N, Kawahara K, Sasaki M, et
al: Hyperplasia and carcinomas in Pten-deficient mice and reduced
PTEN protein in human bladder cancer patients. Cancer Res.
66:8389–8396. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Platt FM, Hurst CD, Taylor CF, Gregory WM,
Harnden P and Knowles MA: Spectrum of phosphatidylinositol 3-kinase
pathway gene alterations in bladder cancer. Clin Cancer Res.
15:6008–6017. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Koletsas N, Koletsa T, Choidas S,
Anagnostopoulos K, Touloupidis S, Zaramboukas T, Raptou G,
Papadopoulos N and Lambropoulou M: Immunohistochemical
investigation of HER/AKT/mTOR pathway and cellular adhesion
molecules in urothelial carcinomas. Patholog Res Int.
2017:67941502017.PubMed/NCBI
|
|
40
|
Brugge J, Hung MC and Mills GB: A new
mutational AKTivation in the PI3K pathway. Cancer Cell. 12:104–107.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Franke TF, Kaplan DR, Cantley LC and Toker
A: Direct regulation of the Akt proto-oncogene product by
phosphatidylinositol-3,4-bisphosphate. Science. 275:665–668. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kreisberg JI, Malik SN, Prihoda TJ,
Bedolla RG, Troyer DA, Kreisberg S and Ghosh PM: Phosphorylation of
Akt (Ser473) is an excellent predictor of poor clinical outcome in
prostate cancer. Cancer Res. 64:5232–5236. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Vincent EE, Elder DJ, Thomas EC, Phillips
L, Morgan C, Pawade J, Sohail M, May MT, Hetzel MR and Tavaré JM:
Akt phosphorylation on Thr308 but not on Ser473 correlates with Akt
protein kinase activity in human non-small cell lung cancer. Br J
Cancer. 104:1755–1761. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Shin E, Choi CM, Kim HR, Jang SJ and Park
YS: Immunohistochemical characterization of the mTOR pathway in
stage-I non-small-cell lung carcinoma. Lung Cancer. 89:13–18. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Cha EK, Shariat SF, Kormaksson M, Novara
G, Chromecki TF, Scherr DS, Lotan Y, Raman JD, Kassouf W, Zigeuner
R, et al: Predicting clinical outcomes after radical
nephroureterectomy for upper tract urothelial carcinoma. Eur Urol.
61:818–825. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Margulis V, Shariat SF, Matin SF, Kamat
AM, Zigeuner R, Kikuchi E, Lotan Y, Weizer A, Raman JD and Wood CG;
Upper Tract Urothelial Carcinoma CollaborationThe Upper Tract
Urothelial Carcin, : Outcomes of radical nephroureterectomy: A
series from the upper tract urothelial carcinoma collaboration.
Cancer. 115:1224–1233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ehdaie B, Chromecki TF, Lee RK, Lotan Y,
Margulis V, Karakiewicz PI, Novara G, Raman JD, Ng C, Lowrance WT,
et al: Obesity adversely impacts disease specific outcomes in
patients with upper tract urothelial carcinoma. J Urol. 186:66–72.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Izquierdo L, Truan D, Mengual L, Mallofré
C and Alcaraz A: HER-2/AKT expression in upper urinary tract
urothelial carcinoma: Prognostic implications. Anticancer Res.
30:2439–2445. 2010.PubMed/NCBI
|
|
49
|
Wheat JC, Weizer AZ, Wolf JS Jr, Lotan Y,
Remzi M, Margulis V, Wood CG, Montorsi F, Roscigno M, Kikuchi E, et
al: Concomitant carcinoma in situ is a feature of aggressive
disease in patients with organ confined urothelial carcinoma
following radical nephroureterectomy. Urol Oncol. 30:252–258. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Poon SL, Pang ST, McPherson JR, Yu W,
Huang KK, Guan P, Weng WH, Siew EY, Liu Y, Heng HL, et al:
Genome-wide mutational signatures of aristolochic acid and its
application as a screening tool. Sci Transl Med. 5:197ra1012013.
View Article : Google Scholar : PubMed/NCBI
|