Introduction
With a worldwide annual mortality of >200,000,
prostate cancer has become the second leading cause of
cancer-related mortality in men (1,2).
According to the recommended guidelines and current research,
several biomarkers, such as prostate-specific antigen and prostate
cancer antigen 3, are used as screening indicators to detect
patients at risk of developing prostate cancer or to monitor
postoperative patients (3,4). However, the pathogenesis of prostate
carcinoma has yet to be fully elucidated. Further research on the
development and progression of prostate cancer are key to reducing
recurrence and mortality. Therefore, it is crucial to explore novel
reliable biomarkers that are involved in the pathogenesis of
prostate neoplasms.
Testican-1 (SPOCK1) is a member of the secreted
protein acidic and rich in cysteine family that encodes a
matricellular Ca2+-binding glycoprotein and plays a key
role in cell cycle regulation, apoptosis, DNA repair and metastasis
(5–7). Due to its characteristic N-terminus,
follistatin-like domain and C-terminus, SPOCK1 was found to be
involved in cell proliferation, adhesion and migration (8). Recent studies reported that SPOCK1 is
abnormally expressed in various tumors and is involved in
glioblastoma cell invasion, hepatocellular carcinoma progression
and the regulation of epithelial-to-mesenchymal transition (EMT) in
lung cancer (9–11). In vitro and in vivo
assays revealed that SPOCK1 promotes tumor growth and metastasis in
human prostate cancer via several pathways, such as via PI3K/Akt,
Wnt/catenin, Bcl-2 family and matrix metalloproteinases (MMPs)
(12,13). However, the mechanism underlying
SPOCK1 overexpression remains unclear.
MicroRNAs (miRs/miRNAs), which are evolutionary
conserved small non-coding RNAs, have been identified as regulators
of gene expression and protein translation (14). Recent studies revealed different
roles of miR-155-5p in various types of cancer (15–20).
miR-155-5p was shown to be downregulated in gastric tumors
(15), and overexpression of
miR-155-5p inhibited the proliferation and promoted apoptosis of
gastric cancer cell lines via decreasing mitogen-activated protein
kinase 10, while downregulation of miR-155-5p decreased cisplatin
sensitivity (16). In addition,
miR-155-5p was shown to suppress cell migration and invasion in the
lung adenocarcinoma A549 cell line by targeting Smad2 (17). However, several studies have reported
an opposite effect of miR-155-5p on other carcinomas. In colorectal
carcinoma, miR-155-5p expression was upregulated and promoted the
proliferation, invasion and metastasis of colorectal cancer cells
(18). Increased miR-155-5p
expression may also contribute to the suppression of tumor cell
death in osteosarcoma (19). A
recent study demonstrated that miR-155-5p contributes to
EMT-associated oral squamous cell carcinoma (OSCC) progression and
serves as a biomarker for predicting relapse, particularly for
patients with early-stage OSCC (20).
Based on data obtained from TargetScan and miRBase,
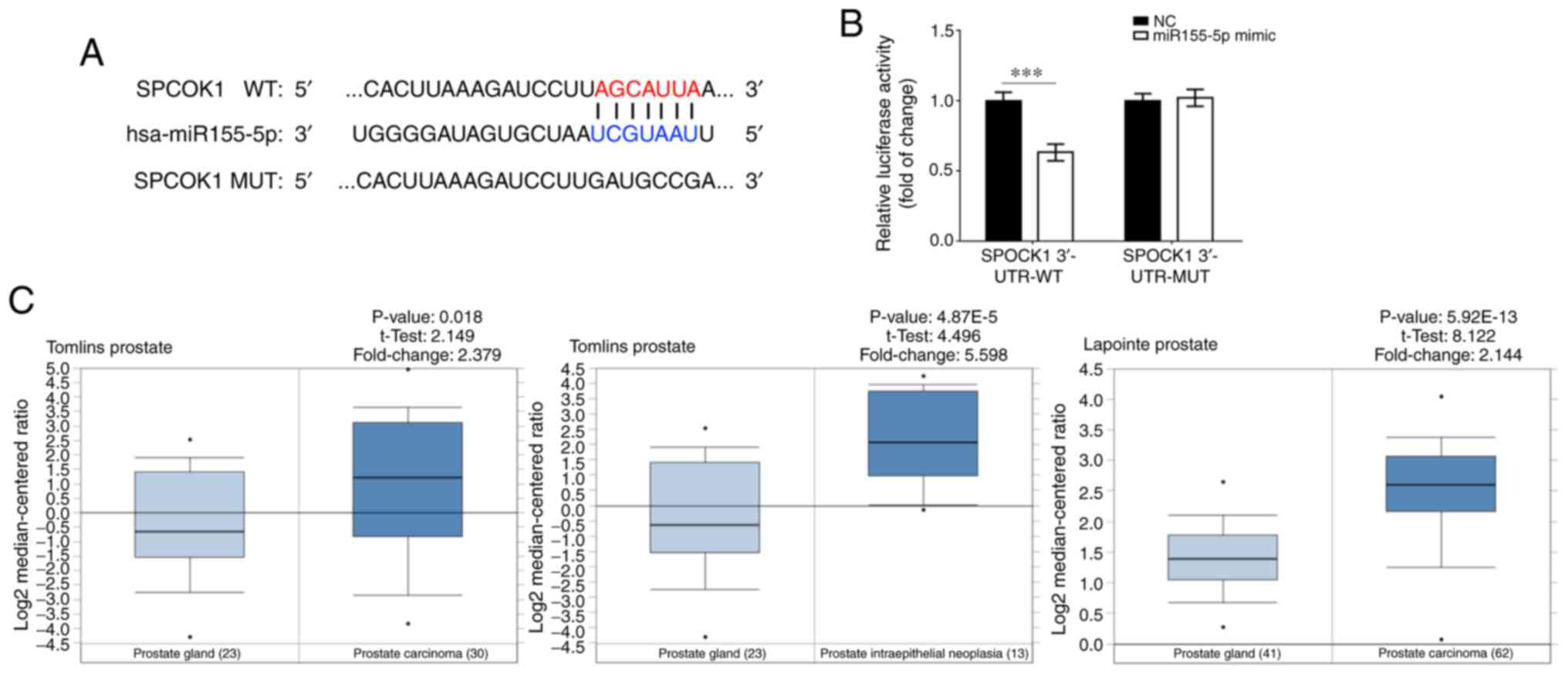
miR-155-5p appears to directly target SPOCK1 (Fig. 1A). Due to the carcinogenic function
of SPOCK1 in prostate cancer, it was hypothesized that miR-155-5p
may inhibit the invasion and migration of prostate cancer cells via
targeting SPOCK1. However, to the best of our knowledge, few
studies to date have investigated whether miR155-5p plays role in
prostate tumorigenesis and cancer progression. Therefore, the aim
of the present study was to investigate the expression of SPOCK1
and miR155-5p in 41 cases of prostate cancer using reverse
transcription-quantitative PCR (RT-qPCR) analysis, evaluate the
association between SPOCK1 and miR-155-5p in prostate cancer, and
determine the role of miR-155-5p in the invasion and migration of
prostate cancer cells in vitro.
Materials and methods
Patients and tissue samples
A total of 41 Chinese patients with prostate cancer
who were admitted to Kunshan Hospital of Traditional Chinese
Medicine between January, 2015 and December, 2018 were selected.
Tumor tissues from each patient and matched adjacent non-tumor
tissues were obtained with the patient's authorization. The mean
age of the whole patient sample was 73.54±16.02 years and the
characteristics of all the patients are summarized in Table I. Total RNA was extracted to
investigate the expression of SPOCK1 and miR155-5p. The protocol of
the present study was approved by the Chinese Medicine Hospital
Ethics Committee and all the patients provided written informed
consent.
 | Table I.Characteristics of 41 patients with
prostate cancer. |
Table I.
Characteristics of 41 patients with
prostate cancer.
| Characteristics | N |
|---|
| Age at diagnosis,
years |
|
|
<70 | 16 |
| ≥70 | 25 |
| Baseline
prostate-specific antigen level, ng/ml |
|
|
<4 | 0 |
| 4-9 | 18 |
| ≥10 | 23 |
| Clinical stage |
|
| T1c | 14 |
| T2 | 17 |
| T3 | 10 |
| Gleason score |
|
| ≤7 | 19 |
|
>7 | 22 |
Data mining of datasets
Two datasets were selected from Oncomine (https://www.oncomine.org/): Tomlins Prostate (23
samples of normal prostate gland tissue, 13 samples of prostate
intraepithelial neoplasia and 30 samples of prostate carcinoma) and
Lapointe Prostate (41 samples of normal prostate gland tissue and
62 samples of prostate carcinoma). These datasets compared the
expression of SPOCK1 between prostate cancer and normal tissues.
Bioinformatics analysis using TargetScan and miRBase was conducted
and SPOCK1 was identified as a direct target gene of
miR-155-5p.
Cell lines and cell culture
The normal human prostate cell line RWPE-2 and four
prostate cancer cell lines (PC3, 22Rv1, DU145 and LNCaP) were
obtained from FuDan IBS Cell Center (Shanghai, China). The cells
were cultured in corresponding media (RPMI-1640 medium for LNCaP,
DU145 and RWPE-2 cells; F-12 medium for PC3 cells; and DMEM for
22Rv1 cells) supplemented with 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.) and 1% antibiotic/antimycotic solution
(Sigma-Aldrich; Merck KGaA) at 37°C with 5% CO2,
according to the guidelines of the FuDan IBS Cell Center.
Cell transfection and luciferase
reporter assay
PC3 cells were cultured in a 6-well plate and
transfected with miR-155-5p mimic and miR-155-5p negative control
(NC; Shanghai GenePharma Co., Ltd.) when the cells were 70%
confluent. After 48 h, RT-qPCR was performed to evaluate
transfection efficiency. SPOCK1 expression was evaluated using
RT-qPCR and western blot assays to verify whether SPOCK1 is a
direct target gene of miR-155-5p.
For the luciferase reporter assay, PC3 cells were
seeded into 96-well plates at a density of 2×104
cells/well and grown to 70% confluence. Cells were co-transfected
with miR-155-5p mimic or miR155-5p NC for 48 h at 37°C, and
SPOCK1-3′-untranslated region (UTR)-wild-type (WT) or
SPOCK1-3′-UTR-mutant (MUT) plasmids (Shanghai GenePharma Co., Ltd.)
using Lipofectamine™ 2000 (Thermo Fisher Scientific, Inc.). A
luciferase assay kit (BioLux® Gaussia; New England
BioLabs, Inc.) was used to evaluate luciferase activity according
to the manufacturer's protocol.
Western blot analysis
Total protein was extracted using RIPA buffer
supplemented with PMSF (Beyotime Institute of Biotechnology). The
samples were loaded in pre-cast protein electrophoresis gels
(Thermo Fisher Scientific, Inc.) and were transferred to PVDF
membranes (Beyotime Institute of Biotechnology). The following
antibodies were used: Rabbit anti-human SPOCK1 polyclonal antibody
(1:2,000; cat. no. ab229935; Abcam), rabbit anti-human MMP3
monoclonal antibody (1:2,000; cat. no. ab52915; Abcam), rabbit
anti-human MMP9 polyclonal antibody (1:1,000; cat. no. ab38898;
Abcam), mouse anti-human vimentin monoclonal antibody (1:1,000;
cat. no. ab8978; Abcam), rabbit anti-human β-catenin polyclonal
antibody (1:6,000; cat. no. ab32572; Abcam), rabbit anti-human
N-cadherin monoclonal antibody (1:1,000, cat. no. ab202030; Abcam),
rabbit anti-human E-cadherin monoclonal antibody (1:1,000; cat. no.
3195; Cell Signaling Technology, Inc.) and rabbit anti-human GAPDH
polyclonal antibody (1:1,000; cat. no. 5174; Cell Signaling
Technology, Inc.). The formula used to measure relative protein
expression was as follows: Relative protein expression = Grey value
of each protein/grey value of GAPDH.
RT-qPCR analysis
Total RNA was extracted from cells or tissues using
TRIzol® reagent (Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. RNA was
reverse-transcribed into cDNA using the miScript II RT kit
(Invitrogen; Thermo Fisher Scientific, Inc.). An iQ5 Real-Time PCR
detection system (Bio-Rad Laboratories, Inc.) with the SYBR Premix
Ex Taq™ kit (Takara Bio, Inc.) were used. The following primer
pairs were used for the qPCR: miR-155-5p forward,
5′-UAAUACCGUCUUAAAACCGU-3′ and reverse, 5′-UUCUGGGAACGUGAAACCT-3′;
U6 forward, 5′-CGCTTCGGCAGCACATATACTAAAATTGGAAC-3′ and reverse,
5′-GCTTCACGAATTTGCGTGTCATCCTTGC-3′; SPOCK1 forward,
5′-AAGGGTCAAGCAGGAGGTCAT-3′ and reverse,
5′-CACGAGGATGCGAACAGAGTC-3′; and GAPDH forward,
5′-GAAGGTGAAGGTCGGAGT-3′ and reverse, 5′-GAAGATGGTGATGGGATTTC-3′.
The following thermocycling conditions were used for the qPCR: 94°C
for 4 min, 40 cycles of 95°C for 1 min, 60°C for 1 min and 72°C for
1 min. Subsequently, 2−ΔΔCq value/fold change was used
to calculate the relative expression [ΔΔCq =
(CtrLV-SPARCL1-PGK-Puro-SAPRCL1-CqrLV-SPARCL1-PGK-Puro-GAPDH)-(CqEmpty-Vector-SPARCL1-CqEmpty-Vector-GAPDH)]
(21).
Wound healing assay
To assess the role of miR-155-5p on the migration of
PC3 cells, transfected cells were seeded in 6-well plates to
complete confluence. The cell monolayer was scratched with a
sterile plastic 200-µl micropipette tip and PBS (Gibco; Thermo
Fisher Scientific, Inc.) was used to remove loose cells.
Subsequently, serum-free Opti-MEM (Gibco; Thermo Fisher Scientific,
Inc.) was added to the wells. Following incubation at 37°C with 5%
CO2 for 24 h, images were captured using an inverted
microscope (magnification ×40, IX73; Olympus Corporation). The
following formula was used: Residual wound area rate (relative
proportion of the wound) = (width of the wound/initial width on day
0) ×100%.
Transwell assay
Transwell culture inserts (pore size, 8 mm;
Guangzhou Jet Bio-Filtration Co., Ltd.) were placed into the wells
of 24-well plates to separate upper and lower chambers. The upper
side of the membrane was precoated with Matrigel and incubated at
37°C for 1 h for gel formation. Subsequently, F-12 medium was added
to the lower chamber, whereas 1×105 cells/well in
Opti-MEM were added to the upper chamber. After 72 h of incubation
at 37°C, the number of invading cells was counted using a counting
chamber under an inverted light microscope (magnification ×100;
IX73; Olympus Corporation).
Statistical analysis
Continuous variables are expressed as the mean ± SD
and analyzed with an unpaired t-test. Pearson's correlation test
was used to analyze the relationship between miR-155-5p and SPOCK1
expression in prostate tumors. The F-test with Tukey's test was
used to compare relative expression levels of miR-155-5p and SPOCK1
mRNA in RWPE-2 and prostate cancer cell lines. All data were
analyzed using SPSS 20.0 (IBM, Corp.) or GraphPad Prism 6.0
(GraphPad Software, Inc.). P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression levels and correlation
analysis of miR-155-5p and SPOCK1 in prostate cancer
Based on TargetScan and miRBase analysis, miR-155-5p
was found to directly target SPOCK1. A WT-SPOCK1 and MUT-SPOCK1
luciferase reporter gene vector containing a 7-bp mutation on the
predicted miR-155-5p binding site was constructed (Fig. 1A). The luciferase reporter assay
revealed that luciferase activity was decreased in the SPOCK1
3′-UTR WT group transfected with miR-155-5p mimic, thus suggesting
that SPOCK1 may be a direct target gene of miR-155-5p (Fig. 1B).
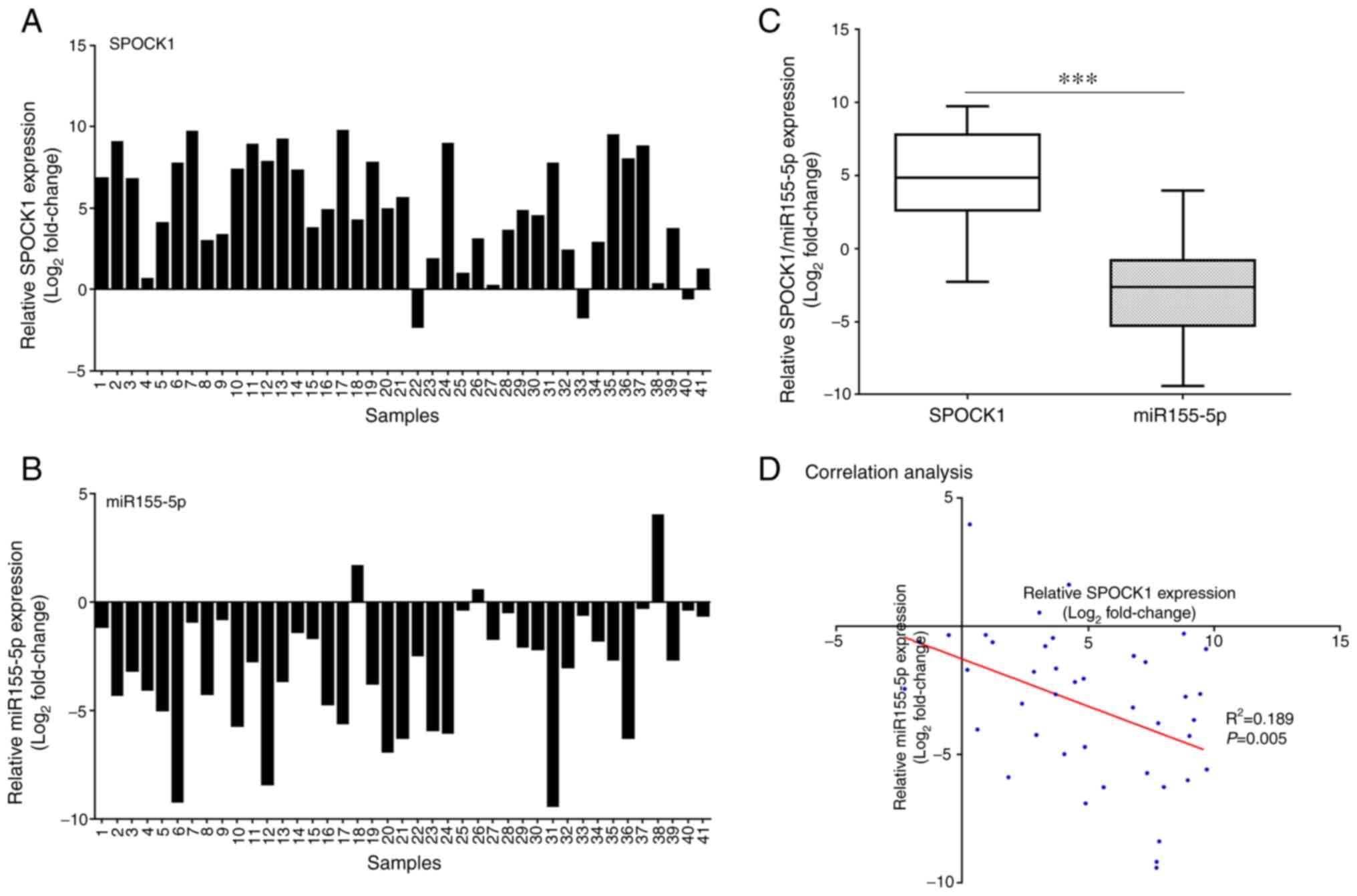
SPOCK1 gene expression was found to be significantly
higher in prostate carcinoma compared with that in corresponding
non-tumor tissues (Figs. 1C and
2A). Furthermore, miR-155-5p
expression in prostate tumor tissues was lower compared with that
in normal tissues and significantly lower compared with SPOCK1
expression in prostate tumor tissues (Fig. 2B and C). In addition, there was a
negative correlation between the expressions of SPOCK1 and
miR-155-5p in prostate tumors (Fig.
2D).
Overexpression of miR-155-5p inhibits
SPOCK1 expression in PC3 cells
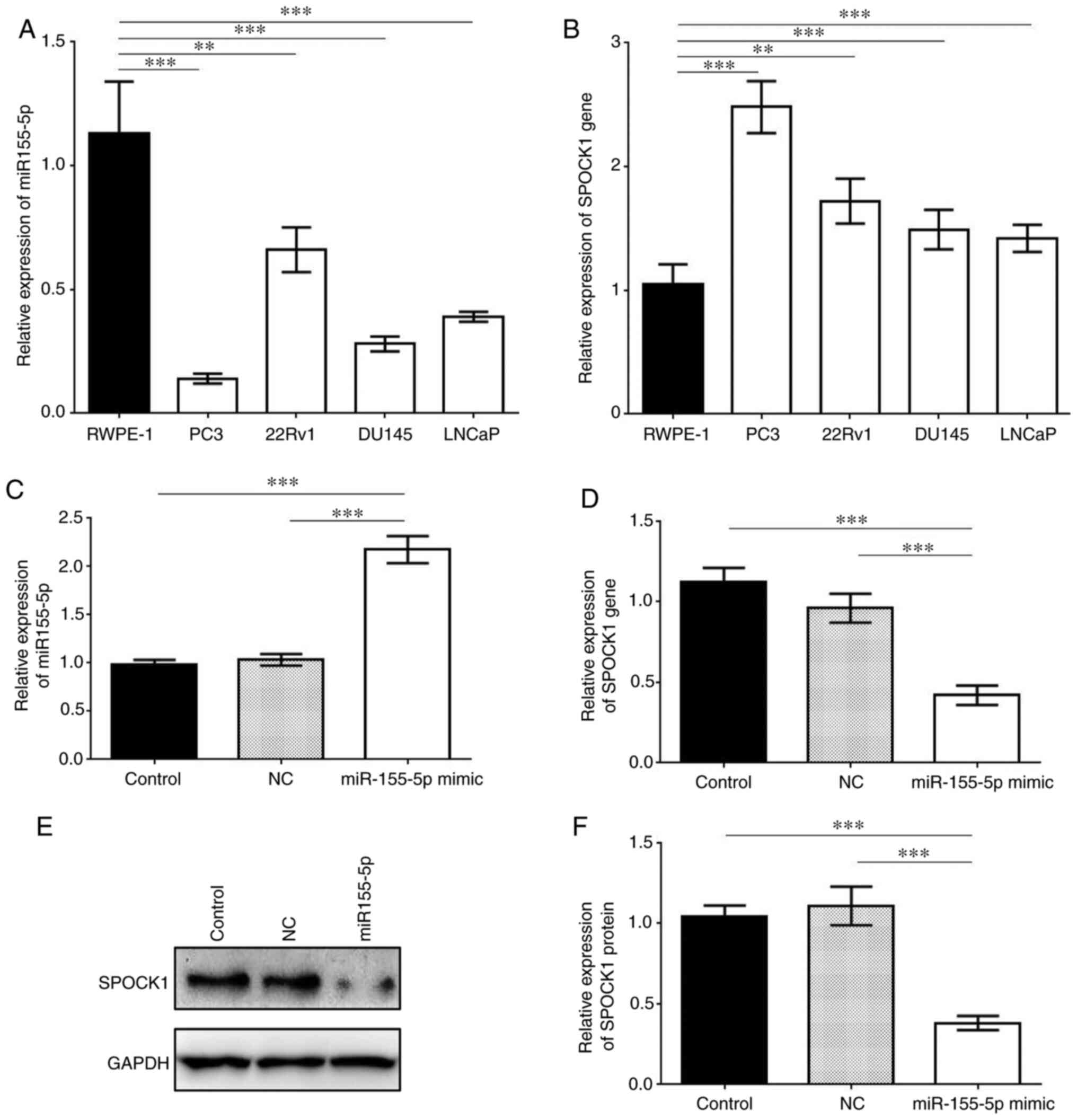
Compared with RWPE-2 (normal prostate cell line),
the four prostate cancer cell lines (PC3, 22Rv1, DU145 and LNCaP)
exhibited significantly lower expression of miR-155-5p, while the
expression levels of SPOCK1 in these cancer cell lines were
significantly higher (P<0.01 or P<0.001; Fig. 3A and B). PC3 cells exhibited the
lowest miR-155-5p and highest SPOCK1 expression levels. When
miR-155-5p was upregulated by miR-155-5p mimic, the relative
expression of SPOCK1 markedly decreased compared with that in the
control groups, as shown by both RT-qPCR and western blot assays
(Fig. 3). Therefore, SPOCK1 was
suppressed by miR-155-5p in PC3 cells due to their specific binding
site.
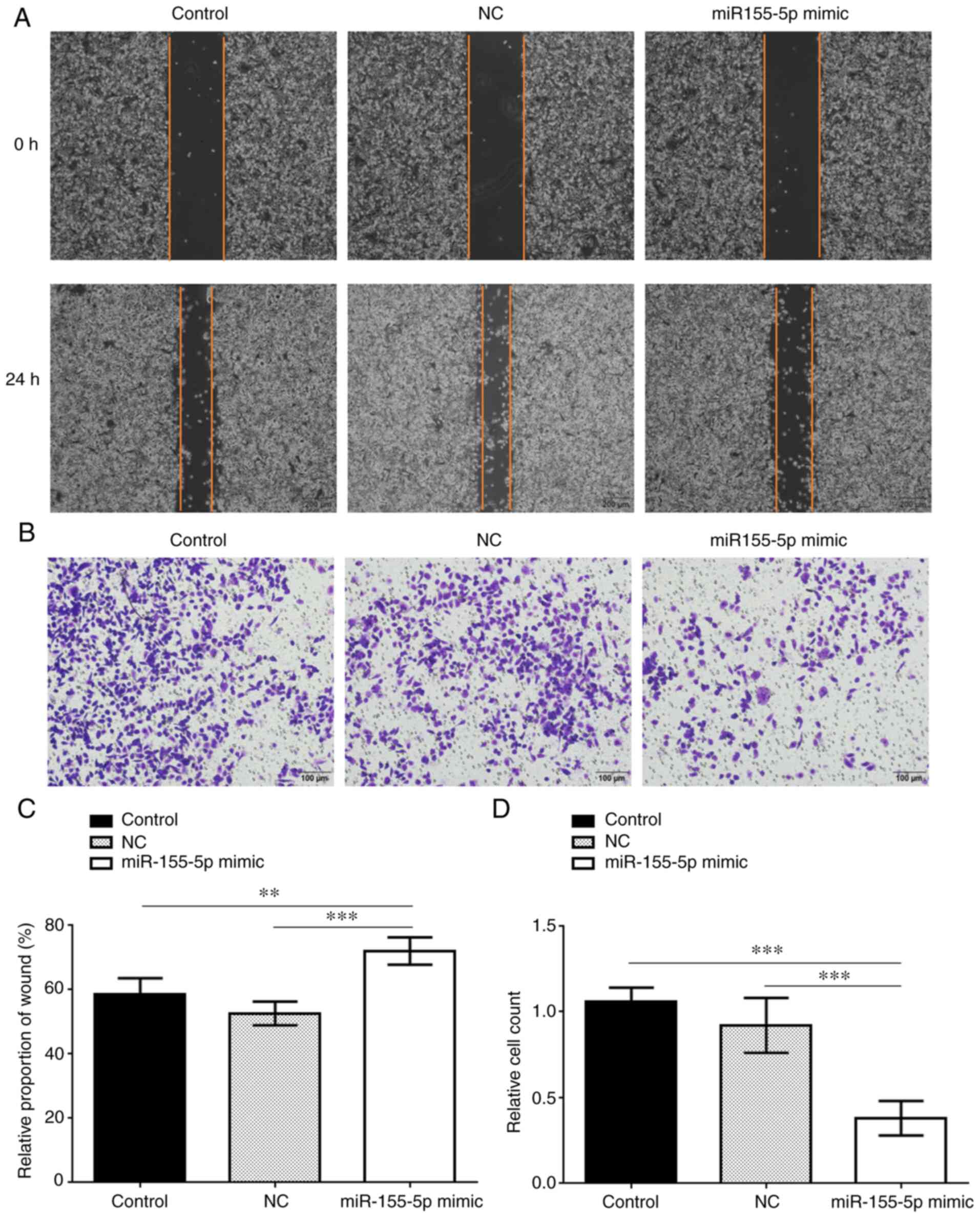
Overexpression of miR-155-5p
suppresses invasion and migration of PC3 cells
Cell migration and invasion were measured by scratch
and Transwell assays, respectively, and were both found to be
significantly inhibited in the miR-155-5p mimic group compared with
the control groups (Fig. 4). In
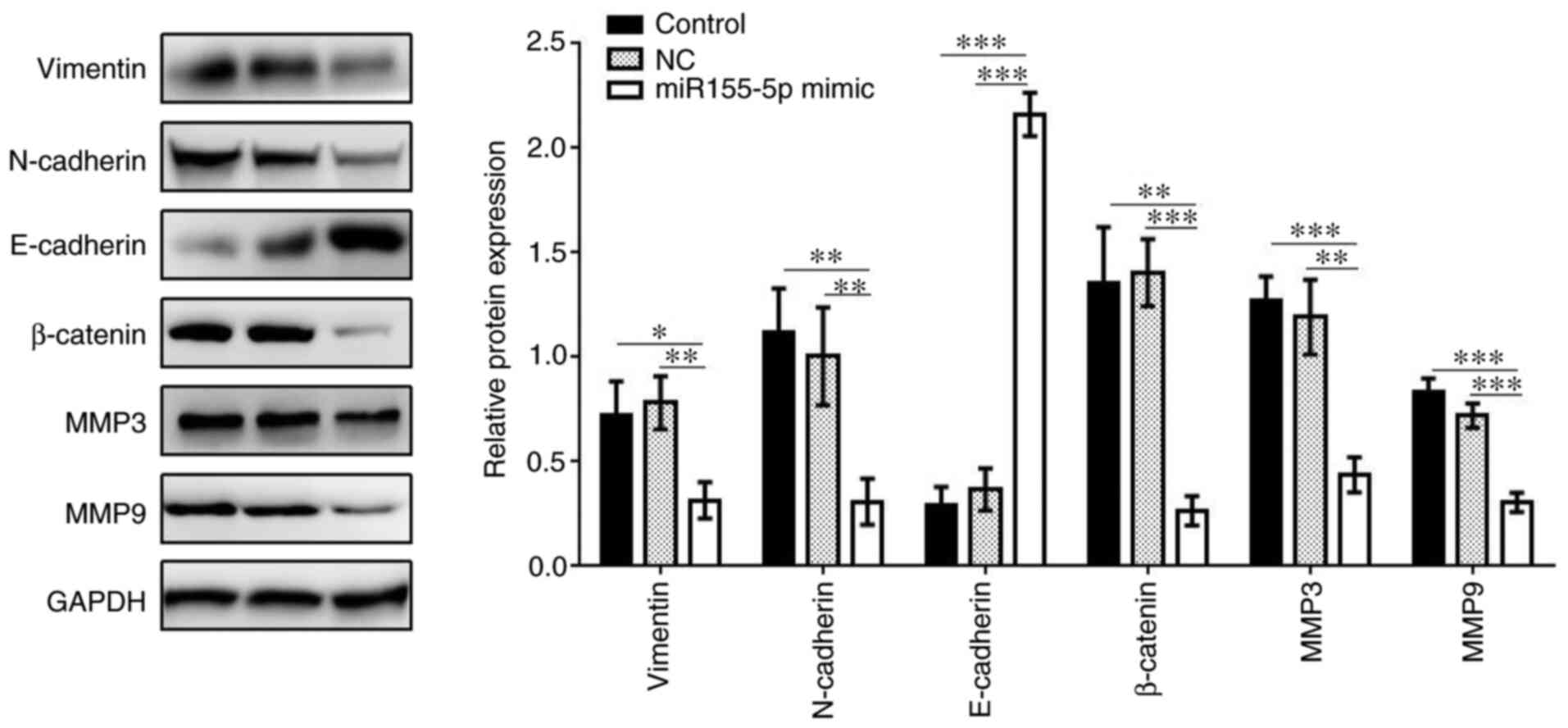
addition, the expression levels of vimentin, N-cadherin, β-catenin,
MMP3 and MMP9 were all markedly decreased in the miR-155-5p mimic
group compared with those in the control groups, while the
expression of E-cadherin was upregulated in the miR-155-5p mimic
group (Fig. 5). Therefore,
miR-155-5p may inhibit the invasion and migration ability of PC3
cells via downregulating the expression of SPOCK1 and its
downstream regulators.
Discussion
SPOCK1 is a matricellular Ca2+-binding
glycoprotein that has a characteristic N-terminus, follistatin-like
domain and C-terminus, which plays a key role in cancer metastasis.
SPOCK1 expression was found to be significantly higher in prostate
carcinoma compared with that in the normal prostate gland by both
Oncomine data and the results of the present study. In previous
studies, SPOCK1 was identified as a novel oncogene with critical
effects on the PI3K/Akt and Wnt/β-catenin pathways, the Bcl-2
family and MMPs (12,13,22).
Since SPOCK1 plays a role in the tumorigenesis and
progression of prostate cancer, the present study investigated the
inhibitory effect of an upstream regulator, miR-155-5p, on the
invasion and migration of prostate cancer cells in vitro.
Although previous research reported the carcinogenic role of
miR-155-5p in colorectal carcinoma, osteosarcoma and oral squamous
cell carcinoma (18–20), recent studies demonstrated that
miR-155-5p inhibited cell proliferation and promoted apoptosis in
gastric cancer cell lines via decreasing mitogen-activated protein
kinase 10, while downregulation of miR-155-5p decreased cisplatin
sensitivity (15,16). In addition, miR-155-5p suppressed the
migration and invasion of the lung adenocarcinoma A549 cell line by
targeting Smad2 (17). In a previous
study, promoter-associated CpG islands of miR-155 were frequently
hypermethylated in prostate cancer, but not in benign prostatic
hyperplasia samples (23). Due to
methylation, highly significant downregulation of miR-155-5p was
detected in prostate cancer compared with benign samples (23). In the present study, RT-qPCR analysis
demonstrated that the expression of miR-155-5p in prostate tumor
tissues was also higher compared with that in normal prostate
tissues. Furthermore, another study focused on chronic
non-bacterial prostatitis (CNP) and revealed that rno-miR-155-5p
levels in rat prostate samples of the carrageenan injection group
were significantly upregulated compared with the blank control
(without any interference) or normal saline injection groups, which
may prove to be of value for identifying novel mechanisms of action
of miRNAs in immune regulation and effective target-specific
theragnosis of CNP (24). Therefore,
the present study demonstrated that decreased levels of miR-155-5p
were involved in prostate tumorigenesis and cancer progression.
In the present study, SPOCK1 was identified as a
target gene of miR-155-5p in PC3 cells via bioinformatics analysis,
luciferase reporter assays, RT-qPCR analysis and western blotting.
RT-qPCR also revealed the presence of a negative association
between SPOCK1 and miR-155-5p in prostate tumor tissues and cell
lines. This indicated that miR-155-5p downregulation may play a
role in SPOCK1-mediated prostate cancer progression.
EMT plays a key role in cancer metastasis,
particularly in prostate neoplasms (25). Recent studies demonstrated that
SPOCK1 regulates the EMT process during cancer metastasis (12,13,26).
MMP3 and MMP9 are two mesenchymal markers that promote EMT and
distant metastasis (27). In a
previous study, SPOCK1 knockdown in PC3 cells significantly
inhibited cell invasion and migration via downregulation of MMP3
and MMP9 (12). In addition, EMT and
metastasis of prostate cancer were also regulated by the
Wnt/β-catenin signaling pathway, which was found to be aberrantly
activated in prostate cancer (28).
Therefore, the present study investigated the expression of MMP3,
MMP9 and key proteins of the Wnt/β-catenin signaling pathway to
elucidate the potential molecular mechanisms underlying the effect
of miR-155-5p on the PC3 cell line. Upon miR-155-5p mimic
transfection, the protein levels of vimentin, β-catenin,
N-cadherin, MMP3 and MMP9 were decreased, while the protein levels
of E-cadherin were increased. These results indicated that
miR-155-5p could exert its tumor-suppressor role by suppressing
MMP3 and MMP9 expression and modulating the Wnt/β-catenin signaling
pathway.
In conclusion, the present study demonstrated that
the oncogene SPOCK1 is a target gene of miR-155-5p in prostate
cancer. SPOCK1 expression was found to be negatively correlated
with that of miR-155-5p in both prostate tumor tissues and cell
lines. In addition, miR-155-5p suppressed the invasion and
migration of PC3 cells by decreasing the expression of MMPs and
regulating the Wnt/β-catenin signaling pathway. The findings of the
present study suggest that miR-155-5p acts as a tumor suppressor
gene in prostate carcinoma, inhibits SPOCK1-mediated prostate
cancer progression, and may be a valuable candidate for targeted
therapy.
Acknowledgements
Not applicable.
Funding
This study was supported by the Kunshan Science and
Technology Program of Social Development (grant no. KS1834).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LYY and JOY conceived and planned the study. LYY,
JM, XMZ and JOY performed the experiments. LYY and JM analyzed the
data. All authors read and approved the manuscript and agreed to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
The protocol of the present study was approved by
the Chinese Medicine Hospital Ethics Committee and all the patients
provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
All the authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cao L, Lee CH, Ning J, Handy BC, Wagar EA
and Meng QH: Combination of prostate cancer antigen 3 and
prostate-specific antigen improves diagnostic accuracy in men at
risk of prostate cancer. Arch Pathol Lab Med. 14:1106–1112. 2018.
View Article : Google Scholar
|
|
3
|
Nordström T, Akre O, Aly M, Grönberg H and
Eklund M: Prostate-Specific antigen (PSA) density in the diagnostic
algorithm of prostate cancer. Prostate Cancer Prostatic Dis.
21:57–63. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chunhua L, Zhao H, Zhao H, Lu Y, Wu J, Gao
Z, Li G, Zhang Y and Wang K: Clinical significance of peripheral
blood PCA3 gene expression in early diagnosis of prostate cancer.
Transl Oncol. 11:628–632. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bradshaw AD and Sage EH: SPARC, a
matricellular protein that functions in cellular differentiation
and tissue response to injury. J Clin Invest. 107:1049–1054. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ma LJ, Wu WJ, Wang YH, Wu TF, Liang PI,
Chang IW, He HL and Li CF: SPOCK1 overexpression confers a poor
prognosis in urothelial carcinoma. J Cancer. 7:467–476. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dhamija R, Graham JM Jr, Smaoui N,
Thorland E and Kirmani S: Novel de novo SPOCK1 mutation in a
proband with developmental delay, microcephaly and agenesis of
corpus callosum. Eur J Med Genet. 57:181–184. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Song X, Han P, Liu J, Wang Y, Li D, He J,
Gong J, Li M, Tu W, Yan W, et al: Up-Regulation of SPOCK1 induces
epithelial-mesenchymal transition and promotes migration and
invasion in esophageal squamous cell carcinoma. J Mol Histol.
46:347–356. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li Y, Chen L, Chan TH, Liu M, Kong KL, Qiu
JL, Li Y, Yuan YF and Guan XY: SPOCK1 is regulated by CHD1L and
blocks apoptosis and promotes HCC cell invasiveness and metastasis
in mice. Gastroenterology. 144:179–191. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Colin C, Baeza N, Bartoli C, Fina F, Eudes
N, Nanni I, Martin PM, Ouafik L and Figarella-Branger D:
Identification of genes differentially expressed in glioblastoma
versus pilocytic astrocytoma using suppression subtractive
hybridization. Oncogene. 25:2818–2826. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Miao L, Wang Y, Xia H, Yao C, Cai H and
Song Y: SPOCK1 is a novel transforming growth factor-beta target
gene that regulates lung cancer cell epithelial-mesenchymal
transition. Biochem Biophys Res Commun. 440:792–797. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen Q, Yao YT, Xu H, Chen YB, Gu M, Cai
ZK and Wang Z: SPOCK1 promotes tumor growth and metastasis in human
prostate cancer. Drug Des Devel Ther. 10:2311–2321. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang C, Fischer-Kešo R, Schlechter T,
Ströbel P, Marx A and Hofmann I: Plakophilin 1-deficient cells
upregulate SPOCK1: Implications for prostate cancer progression.
Tumour Biol. 36:9567–9577. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Torrini C, Cubero RJ, Dirkx E, Braga L,
Ali H, Prosdocimo G, Gutierrez MI, Collesi C, Licastro D, Zentilin
L, et al: Common regulatory pathways mediate activity of MicroRNAs
inducing cardiomyocyte proliferation. Cell Rep. 27:2759–2771. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li H, Xie S, Liu M, Chen Z, Liu X, Wang L,
Li D and Zhou Y: The clinical significance of downregulation of
mir-124-3p, mir-146a-5p, mir-155-5p and mir-335-5p in gastric
cancer tumorigenesis. Int J Oncol. 45:197–208. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li S, Zhang T, Zhou X, Du Z, Chen F, Luo J
and Liu Q: The tumor suppressor role of miR-155-5p in gastric
cancer: The tumor suppressor role of miR-155-5p in gastric cancer.
Oncol Lett. 16:2709–2714. 2018.PubMed/NCBI
|
|
17
|
Lin J, Chen Y, Liu L, Shen A and Zheng W:
MicroRNA-155-5p suppresses the migration and invasion of lung
adenocarcinoma A549 cells by targeting smad2. Oncol Lett.
16:2444–2452. 2018.PubMed/NCBI
|
|
18
|
Qu YL, Wang HF, Sun ZQ, Tang Y, Han XN, Yu
XB and Liu K: Up-Regulated miR-155-5p promotes cell proliferation,
invasion and metastasis in colorectal carcinoma. Int J Clin Exp
Pathol. 8:6988–6994. 2015.PubMed/NCBI
|
|
19
|
Bhattacharya S, Chalk AM, Ng AJ, Martin
TJ, Zannettino AC, Purton LE, Lu J, Baker EK and Walkley CR:
Increased miR-155-5p and reduced miR-148a-3p contribute to the
suppression of osteosarcoma cell death. Oncogene. 35:5282–5294.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim H, Yang JM, Ahn SH, Jeong WJ, Chung JH
and Paik JH: Potential oncogenic role and prognostic implication of
microRNA-155-5p in oral squamous cell carcinoma. Anticancer Res.
38:5193–5200. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu YZ, Guo YF, Wang L, Tan LJ, Liu XG,
Pei YF, Yan H, Xiong DH, Deng FY, Yu N, et al: Genome-Wide
association analyses identify SPOCK as a key novel gene underlying
age at menarche. PLoS Genet. 5:e10004202009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Daniunaite K, Dubikaityte M, Gibas P,
Bakavicius A, Lazutka JR, Ulys A, Jankevicius F and Jarmalaite S:
Clinical significance of miRNA host gene promoter methylation in
prostate cancer. Hum Mol Genet. 26:2451–2461. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang L, Liu Y, Chen XG, Zhang Y, Chen J,
Hao ZY, Fan S, Zhang LG, Du HX and Liang CZ: MicroRNA expression
profile in chronic nonbacterial prostatitis revealed by
next-generation small RNA sequencing. Asian J Androl. 21:351–359.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen WY, Tsai YC, Yeh HL, Suau F, Jiang
KC, Shao AN, Huang J and Liu YN: Loss of SPDEF and gain of TGFBI
activity after androgen deprivation therapy promote EMT and bone
metastasis of prostate cancer. Sci Signal. 10:4922017. View Article : Google Scholar
|
|
26
|
Huber MA, Azoitei N, Baumann B, Grünert S,
Sommer A, Pehamberger H, Kraut N, Beug H and Wirth T: NF-kappaB is
essential for epithelial-mesenchymal transition and metastasis in a
model of breast cancer progression. J Clin Invest. 114:569–581.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang H, Liang J, Zhou J, Mi J, Ma K, Fan
Y, Ning J, Wang C, Wei X and Li E: Knockdown of RHOC by shRNA
suppresses invasion and migration of cholangiocellular carcinoma
cells via inhibition of MMP2, MMP3, MMP9 and epithelial-mesenchymal
transition. Mol Med Rep. 13:5255–5261. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen L, Mai W, Chen M, Hu J, Zhuo Z, Lei
X, Deng L, Liu J, Yao N, Huang M, et al: Arenobufagin inhibits
prostate cancer epithelial-mesenchymal transition and metastasis by
down-regulating β-catenin. Pharmacol Res. 123:130–142. 2017.
View Article : Google Scholar : PubMed/NCBI
|