Introduction
Endometrial carcinoma accounts for 4.8% of all
cancer cases in women, and the life-time risk of development is
2–3%. Endometrial carcinoma is the most common type of malignancy
of the female genital tract and the fourth most common type of
cancer in females after breast, colorectal and lung cancer.
Endometrial carcinoma can be either hereditary or sporadic in 10
and 90% of women, respectively (1–8).
Endometrial carcinoma is classified as type I or type II based on
the pathological histology and clinical profile. Type I endometrial
carcinoma usually occurs in pre- and peri-menopausal women,
accounts for 80–90% of endometrial cancer cases, and consists of
endometrioid or mucinous adenocarcinomas. Type I endometrial
carcinoma is an estrogen-dependent neoplasm of a low histological
grade and arises through persistent, unopposed estrogen stimulation
of the endometrium with a strong link to obesity. Typically, type I
endometrial carcinoma neoplasms begin as endometrial hyperplasia
and in particular, complex endometrial hyperplasia with atypia. The
early diagnosis and treatment of endometrial carcinoma type I has
favorable prognosis with a 5-year overall survival rate of 85–90%
(1,5,9–11). Type II endometrial adenocarcinoma
accounts for 10–20% of endometrial cancer cases and consists of
high grade serous papillary and clear cell adenocarcinoma. They are
estrogen-independent neoplasms and frequently begin as endometrial
atrophy. Endometrial carcinoma type II neoplasms are aggressive
with exhibit metastasis at an early stage, and patients have a poor
prognosis and a 5-year survival rate between 30–70% (9,12).
The insulin-like growth factor (IGF) system
includes: i) IGF-1, which is a growth hormone-dependent growth
factor with a molecular weight of 7,650 kDa, also known as
somatomedin C; ii) IGF-2, which is a small peptide that shares ~60%
of amino acid homology with IGF-1 and 40% with pro-insulin; iii)
type 1 IGF receptor (IGF-1R), which is a tyrosine kinase receptor
with >50% of homology with the insulin receptor (IR) and binds
to IGF-1 with the highest affinity or to IGF-2 with low affinity,
and mediates the majority of the somatomedin-like actions of IGF-1
and IGF-2; iv) type 2 IGF receptor (IGF-2R), which is a
transmembrane single-chain glycoprotein known as cation-independent
mannose-6-phosphate receptor and binds to IGF-1 with very low
affinity and to IGF-2 but not to insulin; v) IR, which is a cell
surface tyrosine kinase receptor that activates the
ras-raf-MAPK-ERK, PI3K-AKT and mTOR signaling pathways, and binds
primarily to insulin, and to a lesser degree with IGF-1 and IGF-2;
vi) IR isoform A (IR-A), which is derived from alternative splicing
of IR mRNA with the absence of exon 11, and exhibits a high
affinity for insulin; vii) IR isoform B (IR-B), which is derived
from alternative splicing of IR mRNA with inclusion of exon 11, and
has a lower binding affinity for insulin than IR-A; viii) the
IR/IGR-1R hybrid, which is hypothesized to function predominantly
as an IGF-1R; and ix) at least six insulin-like growth factor
binding proteins (IGFBPs) IGFBP-1-6, which bind to IGF-1 and IGF-2
with high affinity (13–26). The availability and biological
activities of IGFs are controlled and modulated by IGFBPs (27,28). For
example, IGFBP-3 binds to IGF-1 forming a 150 kDa ternary complex,
which protects IGF-1 from proteolytic degradation. Pericellular
proteases found in biological fluids, cleave IGFBP-3, releasing
free IGF-1 at the cell surface, which diffuses into tissues and
binds to IGF-1R to exert its biological functions (20,21,29–36).
IGFBPs compete with IGF-1R and have higher binding affinity to
IGF-1 compared with IGF-1R. Binding of IGF-1 to IGFBPs suppresses
IGF-1 actions (35,37,38).
Additionally, there is a group of cysteine-rich proteins, known as
IGFBP-related proteins that shares important structural
similarities with IGFBPs, but this group of proteins exhibits a low
binding affinity for IGF (30).
In humans, the IGF-1 gene is present in the human
genome as a single copy with 6 exons and 5 introns. The
transcription of the IGF-1 gene is controlled by two promoters (P1
and P2), which are located before exons 1 and 2 respectively
(39–43). It is hypothesized that the P2
promoter encodes the endocrine IGF-1 form, which remains under the
control of growth hormone (42).
Alternative splicing of the exons of the IGF-1 genes produces
multiple heterogeneous IGF-1 mRNA transcripts, including isoforms
of mRNA called IGF-1Ea, IGF-1Eb and IGF-1Ec (42,44–48). The
translation of these mRNA isoforms produces various isoforms of
precursor IGF-1 proteins (45).
IGF-1 activity is mediated by mature IGF-1, but IGF-1 is
synthesized as a precursor protein (49). The precursor protein is termed
prepro-IGF-1, which contains a signal peptide and pro-IGF-1
(50). The signal peptide is removed
in the endoplasmic reticulum (50).
Specific enzymes cleave post-translationally the polypeptidic
pro-IGF-1 into mature IGF-1, and free the carboxyl-terminal
extension E-peptide (30,51–54). The
E-peptide possesses distinct bioactivity compared with the mature
form of IGF-1 (30,55,56).
Exon 3 encodes parts of the signal peptide and the mature peptide,
which is common to all IGF-1 isoforms (47). Exon 4 encodes the rest of the mature
peptide and the proximal part of the E-domain (47). Therefore, the mature IGF-1 molecule
is encoded exclusively by exons 3 and 4 and is composed of 70 amino
acids (47). The mature IGF peptide
is the biologically active peptide, and is responsible for binding
to the receptors (30). Peptide Ec
is encoded by exon 4, and parts of exons 5 and 6 in the IGF-1Ec
isoform and is composed of the last 40 amino acids at the
COO-terminal of the IGF-1Ec isoform (45–47,57–60).
Fig. 1 shows the molecular structure
of the human igf-1 gene, the various IGF-1 mRNA isoforms and
their IGF-1 protein isoforms (IGF-1Ea, IGF-1Eb and IGF-1Ec).
IGF-1Eb and IGF-1Ec are produced in negligible amounts, and the
predominantly expressed isoform under physiological conditions is
IGF-1Ea (60). IGF-1 synthesis in
several tissues exerts autocrine and paracrine effects. IGF-1
production in the liver exhibits endocrine activity primarily
(13,19,21,39,47,61,62).
Various studies have demonstrated the presence of IGF-1 in the
endometrium during a normal menstrual cycle (63). Estrogen increases the expression of
IGF-1 in the uterus and IGF-1 is required to mediate its mitogenic
effect on the endometrium via IGF-1R, and possibly via hybrid
IR/IGF-1R (20,27,34,64–66).
Progesterone has been reported to increase IGF synthesis to
antagonize estrogen-induced cell proliferation (27,28).
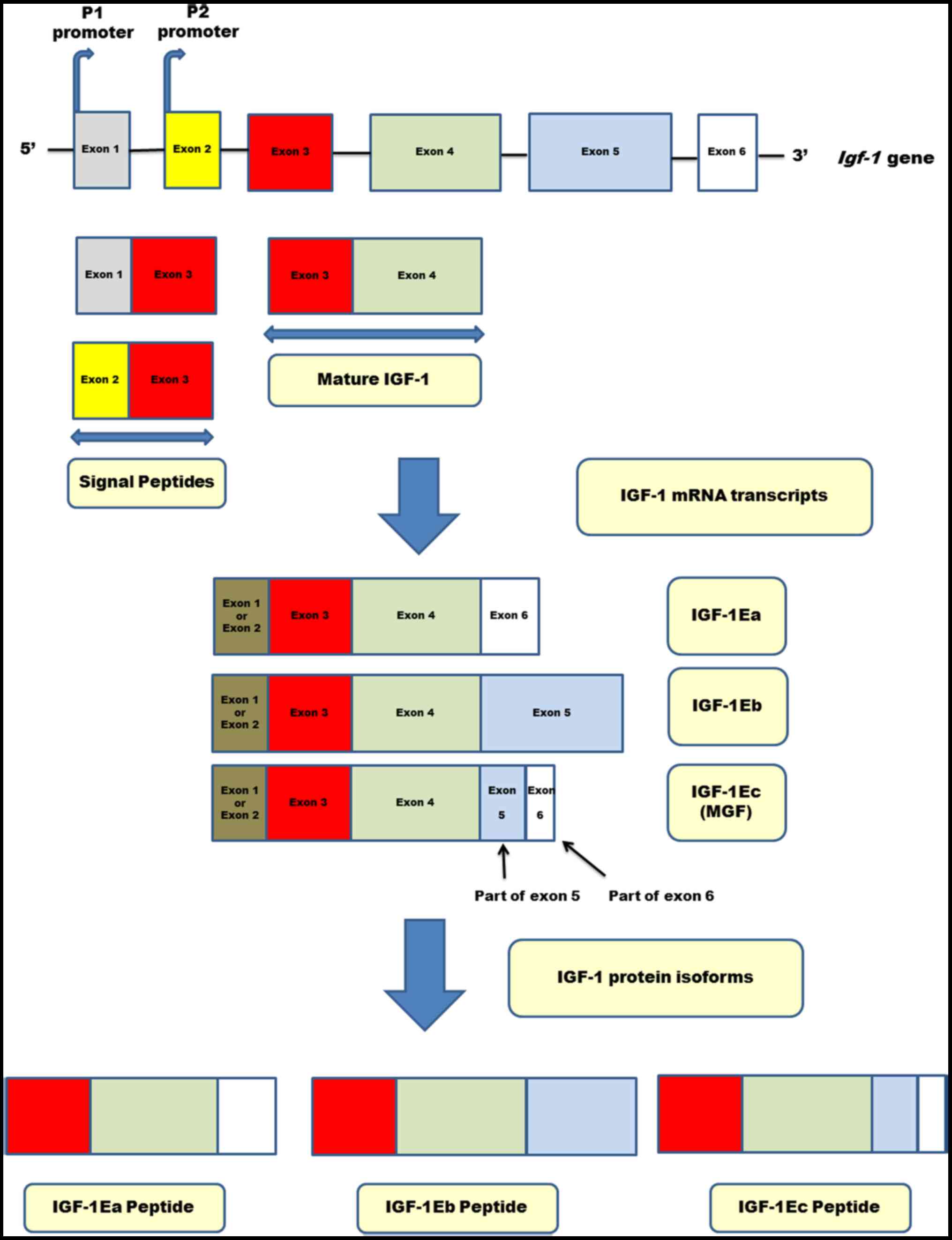 | Figure 1.Molecular structure of the human
igf-1 gene. The human igf-1 gene is composed of six
exons and five introns. Transcription is controlled by one of the
two promoters (P1 and P2) located in exon 1 and 2, respectively.
The P2 promoter encodes the endocrine IGF-1 form, which remains
under the control of growth hormone (GH). Various IGF-1 mRNA
isoforms are generated by alternative splicing. Exons 1 and 2 are
alternatively utilized and comprise class I and II, respectively.
Exons 3 and 4 are common part of all known isoforms. The IGF-1Ea
isoform is encoded by exons 3, 4 and 6; exon 5 is absent in isoform
IGF-1Ea. The IGF-1Eb isoform is encoded by exons 3, 4 and 5. The
IGF-1Ec isoform is encoded by exons 3, 4 and parts of exons 5 and
6. The translation of these mRNA isoforms produces the
corresponding IGF-1 protein isoforms, i.e. the IGF-1Ea, IGF-1Eb and
IGF-1c pro-peptides. The mature peptide of IGF-1 is encoded
exclusively by exons 3 and 4, is the common part of each IGF-1
isoform, responsible for binding to the receptors, and is composed
of 70 amino acids. IGF, insulin-like growth factor. |
There is epidemiological evidence suggestive of a
significantly higher risk of endometrial carcinoma in women with
chronic hyperinsulinemia from insulin resistance (67). In addition, IGF-1 and IGF-2 have been
reported to be involved in endometrial carcinogenesis (68). However, the results of various
studies which evaluated the biological significance of IGF-1 and
IGF-2 in endometrial carcinoma have produced conflicting results,
and are the subject of debate (22).
The biological and clinical role of IGF-1Ec isoform expression in
endometrial carcinogenesis has not been studied so far, to the best
of our knowledge. The aim of the present study was to investigate
the expression of IGF-1Ec isoform immunohistochemically in
endometrial carcinoma in Greek patients, in order to determine its
potential role in endometrial carcinogenesis, and whether it is
associated with well-established clinicopathological
parameters.
Material and methods
Patients
A total of 99 patients with primary endometrial
carcinoma who underwent surgical treatment with total abdominal
hysterectomy and bilateral salpingo-oophorectomy were selected
randomly and studied retrospectively using archived formalin-fixed
paraffin-embedded specimens. The control group with normal
endometrium consisted of 30 cases (including 12 proliferative and 8
secretory cases of endometrium, and 10 cases of atrophic
endometrium); the group with hyperplastic endometrium consisted of
30 cases (including 28 simple, 1 complex and 1 complex with atypia
endometrial hyperplasia). In addition, non-neoplastic endometrial
tissue adjacent to endometrial tumor was studied in 9 cases.
Endometrial biopsies without hysterectomy specimens were excluded.
All patients included in the present study had not received
radiation therapy, hormonal therapy or neoadjuvant chemotherapy
prior to the surgery. Additionally, the patients with normal or
hyperplastic endometrium did not have concomitant ovarian lesions.
In the patients with endometrial carcinoma, the following
histopathological parameters were determined: Histological tumor
type, histological grade, depth of myometrial invasion, presence of
lymph-vascular space invasion, presence of fallopian tube or
ovarian invasion and presence of tumoral necrosis. Pelvic and
para-aortic lymph nodes were not dissected in all of patients.
Endometrial carcinomas were graded according to the World Health
Organization (WHO) system. Clinical staging for all of the patients
was performed using CT scans and MRI. Pathological confirmation of
extension to the cervix was also taken into consideration for the
staging of the disease. Additional details regarding the patients
with endometrial carcinoma used in the present study have been
described previously (69). Patients
with metastases in the pelvic or para-aortic or inguinal lymph
nodes were excluded from the study (FIGO stages IIIc and IVb)
(69). The present study was
approved by the Ethics Committee of the Medical School of the
Kapodistrian University of Athens. All patients provided written
informed consent before the study.
Histological analysis and evaluation
of immunohistochemistry
For histological examination, endometrial carcinomas
were orientated longitudinally, routinely fixed with formalin (4%
final concentration) and embedded in paraffin for subsequent
immunohistochemical analysis. For histological analysis routine
staining with hematoxylin and eosin was used, and a pathologist
confirmed the pathological diagnosis and identified areas of tumor
mass. The phosphatase and tensin homologue deleted on chromosome 10
(PTEN), p53 and survivin immunohistochemical staining was performed
and evaluated as described previously (69,70).
Immunohistochemical staining for IGF-1Ec was performed as follows:
4-µm-thick microtome sections were prepared from the paraffin-fixed
samples, making sure to include a sufficient quantity of neoplasm
mass, which was mounted on a silane-coated glass slides, dried at
37°C overnight, dewaxed in xylene and rehydrated in serial
dilutions of ethanol. Endogenous peroxidase activity was quenched
with 1% hydrogen peroxide in distilled water for 15 min. After two
serial washes with distilled water and PBS buffer, the sections
were incubated with polyclonal anti-IGF-IEc antiserum (1:1,000 in
PBS) overnight at 4°C. After washing with PBS, secondary
biotinylated goat anti-rabbit IgG (Dako Real EnVision) was added
for 25 min at room temperature, followed again by repeated PBS
washes. The immunocomplex was visualized by incubating the sections
in a solution of 3,3-diaminobenzidine (Dako Real EnVision) in PBS
for 10 min. Sections were stained in hematoxylin for 5 min, washed
in distilled water, dehydrated in serial dilutions of ethanol and
xylene and finally mounted in dibutyl phthalate xylene. Tissue
sections were visualized under a light microscope. The
immunohistochemical staining was followed by a series of positive
and negative control reactions. Positive control sections for
specificity included staining of positive controls of prostate
carcinoma that are known to be IGF-1Ec positive. Negative controls
were processed without adding the primary antibody in the
experiment.
All slides were scored by an independent pathologist
who was blinded to the characteristics of the tumors, in 10
randomly selected fields of view, at a magnification of ×400 and
the results are expressed as a percentage of positive staining. The
neoplastic, hyperplastic and normal endometrium were evaluated
separately. The scores of immunohistochemical expression of IGF-1Ec
isoform, PTEN, p53 and survivin were classified into the following
four categories: 0,=<5% immunopositive cells; 1,=5-25%
immunopositive cells; 2,=25-75% immunopositive cells; 3,=>75%
immunopositive cells. Staining intensity was defined as follows: 0,
negative; 1, weakly positive; 2, moderately positive; and 3,
strongly positive. The sum of the stain intensity and positive cell
scores was used as the result for each section, as scored as: 0, -;
1 and 2, +; 3 and 4, ++; and 5 and 6, +++.
Statistical analysis
Categorical variables are presented as absolute (n)
and relative (%) frequencies, whereas continuous variables are
presented as the median range. Associations between categorical
variables were assessed using an exact Pearson's χ2
test. For continuous variables, differences in medians between two
groups were assessed using a Mann-Whitney U test, and differences
between three groups were assessed using a Kruskal-Wallis test with
Dunn test for post hoc analysis. Correlations between continuous
variables were assessed by Spearman's rho (ρ). Multivariate linear
regression models were fitted to assess the effects of covariates
on the intensity of survivin staining, whilst adjusting for
potential confounding variables. P<0.05 was considered to
indicate a statistically significant difference. Data were analyzed
using SPSS version 23.0 (IBM Corp.).
Results
Patient characteristics
The median age of the 99 patients with endometrial
carcinoma was 64 years (range 42,90). The endometrial carcinoma
group included 86.9% (86 cases) endometrioid carcinomas and 13.1%
(13 cases) clear cell and papillary serous endometrial carcinomas.
Using the WHO grading system, the cases were distributed as
follows: Well differentiated adenocarcinomas (grade 1), 20.2% (20
cases); moderately differentiated adenocarcinomas (grade 2), 49.5%
(49 cases); and poorly differentiated adenocarcinomas (grade 3),
30.3% (30 cases). Lymph-vascular space invasion was observed in
14.1% (14 cases), while fallopian tube and/or ovarian invasion was
observed in 19% (19 cases) cases. Presence of tumoral necrosis was
detected in 7.1% (7 cases). The demographic characteristics of the
patients with endometrial carcinoma has been previously published
(69).
Immunohistochemical expression of
IGF-1Ec in endometrial carcinoma
The scores of IGF-1Ec immunohistochemical expression
were not statistically significantly associated with the mean age
of the patients (P=0.402), the clinical stage (P=0.223), the depth
of myometrial invasion (P=0.287), lymph-vascular space invasion
(P=0.121), and fallopian tube and/or ovarian invasion (P=0.523)
(Table I). The findings were
suggestive for the histological differentiation (P=0.056). There
was a statistically significant correlation between histological
types and the scores of immunohistochemical IGF-1Ec expression
(P=0.039). Endometrioid carcinomas included 18 cases (20.9%) with
5–25% IGF-1Ec immunopositive cells, 49 cases (57.0%) with 25–75%
IGF-1Ec immunopositive cells, and 13 cases (15.1%) with >75%
IGF-1Ec immunopositive cells. In the patients with clear cell and
papillary serous endometrial carcinomas, IGF-1Ec was
immunohistochemically expressed in 5–25% of cells in 7 cases
(53.8%) and in 25–75% of cells in 5 cases (38.5%). In particular,
in patients with endometrioid carcinomas, there were a larger
number of cases with low IGF-1Ec expression, and a smaller number
of high IGF-1Ec expression than expected. The opposite was true for
the patients with clear cell and serous papillary adenocarcinomas.
In addition, there was a statistically significant association
between tumoral necrosis and the immunohistochemical scores of
IGF-1Ec expression (P=0.004). In the presence of tumoral necrosis,
immunohistochemical scores for IGF-1 were: 5–25% of cells in 1 case
(14.3%), 25–75% of cells in 0 cases (0.0%), and >75% of cells in
5 cases (71.4%). If necrosis was absent, the corresponding
frequencies for the three categories of IGF-1Ec expression were 7
cases (13.5%), 32 cases (61.5%) and 10 cases (19.2%) respectively.
Therefore, the presence of tumoral necrosis was associated with a
larger number of patients with high IGF-1Ec expression and lower
number of patients with moderate IGF-1Ec expression, than expected.
The opposite was true for the absence of tumoral necrosis (Table II).
 | Table I.Association between
clinicopathological characteristics and scores of
immunohistochemical IGF-1Ec expression. |
Table I.
Association between
clinicopathological characteristics and scores of
immunohistochemical IGF-1Ec expression.
|
| IGF-1Ec staining
pattern scores (%) |
|
|---|
|
|
|
|
|---|
| Clinicopathological
parameters | 5-25%
positivity | 25-75%
positivity | >75%
positivity | P-value |
|---|
| Age, years |
|
|
| 0.402 |
|
<60 | 2 (8.7) | 15 (65.2) | 5 (21.7) |
|
|
≥60 | 16 (21.1) | 41 (53.9) | 13 (17.1) |
|
| Histological
type |
|
|
| 0.039 |
|
Endometrioid | 18 (20.9) | 49 (57.0) | 13 (15.1) |
|
| Clear
cell and papillary serous | 0 (0.0) | 7 (53.8) | 5 (38.5) |
|
| Clinical stage |
|
|
| 0.223 |
| I | 12 (17.6) | 42 (61.8) | 10 (14.7) |
|
| II | 4 (26.7) | 6 (40.0) | 4 (26.7) |
|
|
III | 0 (0.0) | 2 (40.0) | 2 (40.0) |
|
| Histological
differentiation |
|
|
| 0.056 |
| G1 | 5 (25.0) | 9 (45.0) | 3 (15.0) |
|
| G2 | 12 (24.5) | 30 (61.2) | 6 (12.2) |
|
| G3 | 1 (3.3) | 17 (56.7) | 9 (30.0) |
|
| Myometrial
invasion |
|
|
| 0.287 |
|
<1/2 | 9 (26.5) | 16 (47.1) | 6 (17.6) |
|
|
≥1/2 | 9 (13.8) | 40 (61.5) | 12 (18.5) |
|
| Lymph-vascular
space invasion |
|
|
| 0.121 |
|
Positive | 0 (0.0) | 8 (57.1) | 6 (42.9) |
|
|
Negative | 8 (15.7) | 29 (56.9) | 10 (19.6) |
|
| Fallopian tube
and/or ovarian invasion |
|
|
| 0.523 |
|
Positive | 4 (21.1) | 8 (42.1) | 6 (31.6) |
|
|
Negative | 3 (11.1) | 15 (55.6) | 6 (22.2) |
|
| Tumoral
necrosis |
|
|
| 0.004 |
|
Yes | 1 (14.3) | 0 (0.0) | 5 (71.4) |
|
| No | 7 (13.5) | 32 (61.5) | 10 (19.2) |
|
 | Table II.Association between
clinicopathological characteristics and intensity of
immunohistochemical IGF-1Ec expression. |
Table II.
Association between
clinicopathological characteristics and intensity of
immunohistochemical IGF-1Ec expression.
|
| IGF-1Ec staining
pattern intensity (%) |
|
|---|
|
|
|
|
|---|
| Clinicopathological
parameters | Moderate | Strong | P-value |
|---|
| Age, years |
|
| 0.802 |
|
<60 | 16 (69.6) | 7 (30.4) |
|
|
≥60 | 46 (60.5) | 25 (32.9) |
|
| Histological
type |
|
| 0.327 |
|
Endometrioid | 56 (65.1) | 26 (30.2) |
|
| Clear
cell and papillary serous | 6 (46.2) | 6 (46.2) |
|
| Clinical stage |
|
| 0.546 |
| I | 41 (60.3) | 24 (35.3) |
|
| II | 11 (73.3) | 3 (20.0) |
|
|
III | 2 (40.0) | 2 (40.0) |
|
| Histological
differentiation |
|
| 0.115 |
| G1 | 12 (60.0) | 7 (35.0) |
|
| G2 | 36 (73.5) | 12 (24.5) |
|
| G3 | 14 (46.7) | 13 (43.3) |
|
| Myometrial
invasion |
|
| 1.000 |
|
<1/2 | 22 (64.7) | 11 (32.4) |
|
|
≥1/2 | 40 (61.5) | 21 (32.3) |
|
| Lymph-vascular
space invasion |
|
| 0.768 |
|
Positive | 9 (64.3) | 5 (35.7) |
|
|
Negative | 29 (56.9) | 20 (39.2) |
|
| Fallopian tube
and/or ovarian invasion |
|
| 0.125 |
|
Positive | 14 (73.7) | 4 (21.1) |
|
|
Negative | 14 (51.9) | 12 (44.4) |
|
| Tumoral
necrosis |
|
| 0.677 |
|
Yes | 3 (42.9) | 3 (42.9) |
|
| No | 31 (59.56) | 20 (38.5) |
|
According to the intensity of IGF-1Ec expression out
of 99 cases, 32 cases (32.3%) exhibited strong expression and 62
cases (62.6%) showed moderate expression. The intensity of IGF-1Ec
was not statistically significantly associated with the age of the
patients (P=0.802), the histological type (P=0.327), clinical stage
(P=0.546), histological differentiation (P=0.115), depth of
myometrial invasion (P=1.000), lymph-vascular space invasion
(P=0.768), fallopian tube and/or ovarian invasion (P=0.125) and the
presence of tumoral necrosis (P=0.677) (Table II).
Table III shows the
sum of staining intensity and scores of IGF-1Ec immunopositive
cells in association with the clinicopathological characteristics.
There was no association between the sum of staining intensity and
scores of IGF-1Ec immunopositive cells with the age of the patients
(P=0.875), histological types (P=0.383), clinical stage (P=0.512),
depth of myometrial invasion (P=0.091), fallopian tube and/or
ovarian invasion (P=0.557), presence of lymph-vascular space
invasion (P=0.724) and tumoral necrosis (P=0.108). However, there
was a statistical significance between the sum of staining
intensity and scores of IGF-1Ec immunopositive cells and the
histological differentiation (P=0.014). Significantly, patients at
Grade 1 had a lower sum of stain intensity and scores of IGF-1Ec
immunopositive cells compared to patients at Grade 3 who had a
higher sum of immunopositivity.
 | Table III.Association between
clinicopathological characteristics and sum of stain intensity and
scores of IGF-1Ec expression. |
Table III.
Association between
clinicopathological characteristics and sum of stain intensity and
scores of IGF-1Ec expression.
|
|
| IHC results of
IGF-1Ec, n (%) |
|
|---|
|
|
|
|
|
|---|
|
Characteristics | Cases, n (%) | 0 | + | ++ | +++ | P-value |
|---|
| Age, years |
|
|
|
|
| 0.875 |
|
<60 | 22 (23.9) | 0 (0.0) | 2 (8.7) | 13 (56.5) | 7 (30.4) |
|
|
≥60 | 70 (76.1) | 0 (0.0) | 7 (9.2) | 36 (47.4) | 27 (35.5) |
|
| Histological
type |
|
|
|
|
| 0.383 |
|
Endometrioid | 80 (87.0) | 0 (0.0) | 9 (10.5) | 43 (50.0) | 28 (32.6) |
|
| Clear
cell and papillary serous | 12 (13.0) | 0 (0.0) | 0 (0.0) | 6 (46.2) | 6 (46.2) |
|
| Clinical stage |
|
|
|
|
| 0.512 |
| I | 64 (78.0) | 0 (0.0) | 7 (10.3) | 33 (48.5) | 24 (35.3) |
|
| II | 14 (17.1) | 0 (0.0) | 1 (6.7) | 9 (60.0) | 4 (26.7) |
|
|
III | 4 (4.9) | 0 (0.0) | 0 (0.0) | 1 (20.0) | 3 (60.0) |
|
| Histological
differentiation |
|
|
|
|
| 0.014 |
| G1 | 17 (18.5) | 0 (0.0) | 4 (20.0) | 6 (30.0) | 7 (35.0) |
|
| G2 | 48 (52.2) | 0 (0.0) | 4 (8.2) | 32 (65.3) | 12 (24.5) |
|
| G3 | 27 (29.3) | 0 (0.0) | 1 (3.3) | 11 (36.7) | 15 (50.0) |
|
| Myometrial
invasion |
|
|
|
|
| 0.091 |
|
<1/2 | 31(33.7) | 0 (0.0) | 6 (17.6) | 14 (41.2) | 11 (32.4) |
|
|
≥1/2 | 61 (66.3) | 0 (0.0) | 3 (4.6) | 35 (53.8) | 23 (35.4) |
|
| Lymph-vascular
space invasion |
|
|
|
|
| 0.724 |
|
Positive | 47 (77.0) | 0 (0.0) | 3 (5.9) | 24 (47.1) | 20 (39.2) |
|
|
Negative | 14 (23.0) | 0 (0.0) | 0 (0.0) | 7 (50.0) | 7 (50.0) |
|
| Fallopian tube
and/or ovarian invasion |
|
|
|
|
| 0.557 |
|
Positive | 18 (42.9) | 0 (0.0) | 1 (5.3) | 11 (57.9) | 6 (31.6) |
|
|
Negative | 24 (57.1) | 0 (0.0) | 2 (7.4) | 10 (37.0) | 12 (44.4) |
|
| Tumoral
necrosis |
|
|
|
|
| 0.108 |
|
Yes | 6 (10.9) | 0 (0.0) | 3 (5.8) | 26 (50.0) | 20 (38.5) |
|
| No | 49 (89.1) | 0 (0.0) | 0 (0) | 1 (14.3) | 5 (71.4) |
|
Correlation analysis between
concomitant expression of IGF-1Ec and PTEN
The correlation between IGF-1Ec and PTEN expression
was assessed using Spearman's correlation coefficient. According to
the proportion of immunopositive cells (scores), there was
concomitant expression of IGF-1Ec and PTEN in 27.0% of cases (17
out of 63) compared with 73.0% of cases without such co-expression,
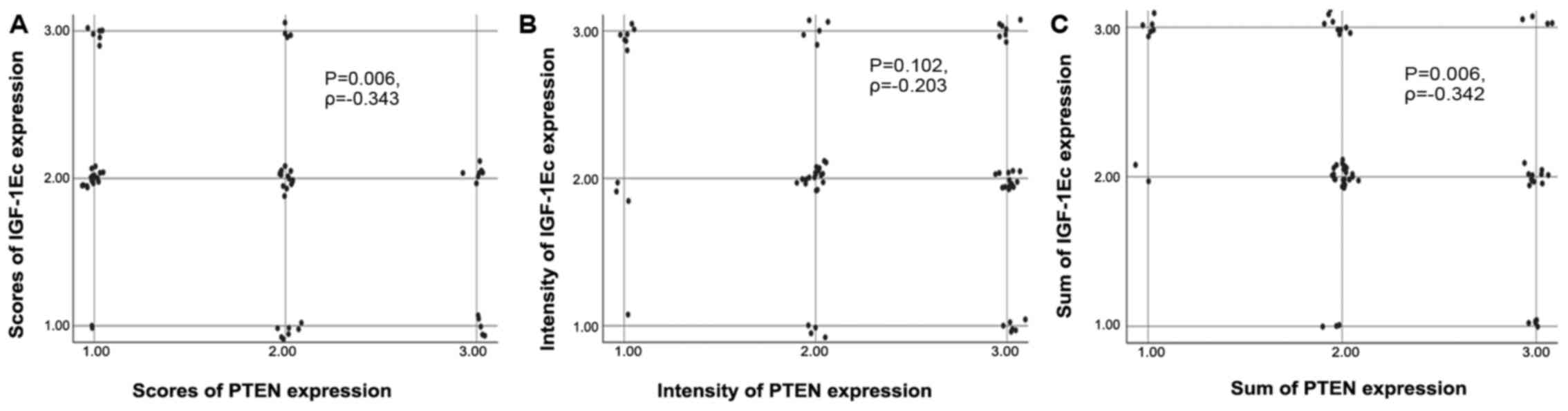
and this was statistically significant (P=0.006, Ρ=−0.343). The
correlation between scores of IGF-1Ec and PTEN immunopositive cells
is shown in the scatterplot (Fig.
2A).
According to the intensity of staining there was
concomitant expression of IGF-1Ec and PTEN in 28.8% of cases (19
out of 66) compared with 71.2% cases without such co-expression,
but this was not significant (P=0.102, Ρ=−0.203). The correlation
between intensity of IGF-1Ec and PTEN immunopositivity is shown in
the scatterplot (Fig. 2B).
Based on the sum of staining (scores and intensity)
there was concomitant expression of IGF-1Ec and PTEN in 42.9 of
cases (27 out of 63) compared with 57.1% without such
co-expression, and the difference was statistically significant
(P=0.006, Ρ=−0.342). The correlation between the sum of IGF-1Ec and
PTEN immunopositivity is shown in the scatterplot (Fig. 2C).
In the case of concomitant expression of IGF-1Ec and
PTEN, there was no correlation between the scores of
immunohistochemical expression and the age of the patients
(P=0.599), histological type (P=1.000), clinical stage (P=0.294),
histological differentiation (P=0.494), depth of myometrial
invasion (P=0.563), lymph-vascular space invasion (P=1.000),
fallopian tube and/or ovarian invasion (P=0.357) and the presence
of tumoral necrosis (P=1.000) (Table
IV).
 | Table IV.Co-expression of IGF-1Ec and PTEN in
endometrial carcinoma according to scores of immunopositive cells
in relation to clinicopathological parameters. |
Table IV.
Co-expression of IGF-1Ec and PTEN in
endometrial carcinoma according to scores of immunopositive cells
in relation to clinicopathological parameters.
|
Characteristics | Patients with
IGF-1Ec and PTEN low scores expression, n (%) | Patients with
either IGF-1Ec or PTEN moderate scores expression, n (%) | Patients with
IGF-1Ec and PTEN high scores expression, n (%) | P-value |
|---|
| Age, years |
|
|
| 0.599 |
|
<60 | 0 (0.0) | 19 (82.6) | 0 (0.0) |
|
|
≥60 | 2 (2.6) | 49 (64.5) | 0 (0.0) |
|
| Histological
type |
|
|
| 1.000 |
|
Endometrioid | 2 (2.3) | 61 (70.9) | 0 (0.0) |
|
| Clear
cell and papillary serous | 0 (0.0) | 7(53.8) | 0 (0.0) |
|
| Clinical stage |
|
|
| 0.294 |
| I | 1 (1.5) | 52 (76.5) | 0 (0.0) |
|
| II | 1 (6.7) | 6 (40.0) | 0 (0.0) |
|
|
III | 0 (0.0) | 3 (60.0) | 0 (0.0) |
|
| Histological
differentiation |
|
|
| 0.494 |
| G1 | 0 (0.0) | 14 (70.0) | 0 (0.0) |
|
| G2 | 2 (4.1) | 35 (71.4) | 0 (0.0) |
|
| G3 | 0 (0.0) | 19 (63.3) | 0 (0.0) |
|
| Myometrial
invasion |
|
|
| 0.563 |
|
<1/2 | 0 (0.0) | 22 (64.7) | 0 (0.0) |
|
|
≥1/2 | 2 (3.1) | 46 (70.8) | 0 (0.0) |
|
| Lymph-vascular
space invasion |
|
|
| 1.000 |
|
Yes | 0 (0.0) | 10 (71.4) | 0 (0.0) |
|
| No | 2 (3.9) | 32 (62.7) | 0 (0.0) |
|
| Fallopian tube
and/or ovarian invasion |
|
|
| 0.357 |
|
Yes | 1 (5.3) | 9 (47.4) | 0 (0.0) |
|
| No | 0 (0.0) | 18 (66.7) | 0 (0.0) |
|
| Tumoral
necrosis |
|
|
| 1.000 |
|
Yes | 0 (0.0) | 1 (14.3) | 0 (0.0) |
|
| No | 2 (3.8) | 35 (67.3) | 0 (0.0) |
|
With the co-expression of IGF-1Ec and PTEN there was
no correlations between the intensity of immunohistochemical
IGF-1Ec expression and the age of the patients (P=1.000),
histological differentiation (P=0.351), depth of myometrial
invasion (P=1.000), presence of fallopian tube and/or ovarian
invasion (P=0.352) and the presence of tumoral necrosis (P=0.637)
(Table V). The findings were
suggestive of an association with clinical stage (P=0.056). There
was a significant association with the histological type (P=0.014).
Specifically, the number of patients with endometrioid carcinomas
and IGF-1Ec and PTEN concomitant moderate expression intensity was
higher than expected, whereas the opposite was true for the group
of patients with clear and serous papillary carcinomas.
Additionally, a statistical significance was found for
lymph-vascular space invasion (P=0.021; Table V). In this case, the number of
patients with no lymph-vascular space invasion and concomitant
IGF-1Ec and PTEN expression of moderate intensity was higher,
whereas the number of patients with no lymph-vascular space
invasion and both IGF-1Ec and PTEN high expression was lower. The
opposite was true for the patients with presence of lymph-vascular
space invasion.
 | Table V.Co-expression of IGF-1Ec and PTEN in
endometrial carcinoma according to stain intensity of
immunopositive cells in relation to clinicopathological
parameters. |
Table V.
Co-expression of IGF-1Ec and PTEN in
endometrial carcinoma according to stain intensity of
immunopositive cells in relation to clinicopathological
parameters.
|
Characteristics | Patients with
IGF-1Ec and PTEN weak positive expression, n (%) | Patients with
either IGF-1Ec or PTEN moderate positive expression, n (%) | Patients with
IGF-1Ec and PTEN strong positive expression, n (%) | P-value |
|---|
| Age, years |
|
|
| 1.000 |
|
<60 | 0 (0.0) | 18 (78.3) | 2 (8.7) |
|
|
≥60 | 1 (1.3) | 41 (53.9) | 5 (6.6) |
|
| Histological
type |
|
|
| 0.014 |
|
Endometrioid | 0 (0.0) | 53 (61.6) | 4 (4.7) |
|
| Clear
cell and papillary serous | 1 (7.7) | 6 (46.2) | 3 (23.1) |
|
| Clinical stage |
|
|
| 0.056 |
| I | 0 (0.0) | 41 (60.3) | 3 (4.4) |
|
| II | 0 (0.0) | 10 (66.7) | 1 (6.7) |
|
|
III | 0 (0.0) | 2 (40.0) | 2 (40.0) |
|
| Histological
differentiation |
|
|
| 0.351 |
| G1 | 0 (0.0) | 9 (45.0) | 1 (5.0) |
|
| G2 | 0 (0.0) | 32 (65.3) | 2 (4.1) |
|
| G3 | 1 (3.3) | 18 (60.0) | 4 (13.3) |
|
| Myometrial
invasion |
|
|
| 1.000 |
|
<1/2 | 0 (0.0) | 19 (55.9) | 2 (5.9) |
|
|
≥1/2 | 1 (1.5) | 40 (61.5) | 5 (7.7) |
|
| Lymph-vascular
space invasion |
|
|
| 0.021 |
|
Yes | 0 (0.0) | 7 (50.0) | 4 (28.6) |
|
| No | 1 (2.0) | 33 (64.7) | 2 (3.9) |
|
| Fallopian tube
and/or ovarian invasion |
|
|
| 0.352 |
|
Yes | 1 (5.3) | 11 (57.9) | 3 (15.8) |
|
| No | 0 (0.0) | 18 (66.7) | 2 (7.4) |
|
| Tumoral
necrosis |
|
|
| 0.637 |
|
Yes | 0 (0.0) | 5 (71.4) | 2 (28.6) |
|
| No | 1 (1.9) | 31 (59.6) | 4 (7.7) |
|
With the concomitant IGF-1Ec and PTEN expression,
the sum of scores and staining intensity of the IGF-1Ec
immunopositive cells was not correlated with the age of the
patients (P=1.000), histological type (P=1.000), clinical stage
(P=0.688), histological differentiation (P=1.000) or the depth of
myometrial invasion (P=1.000) (Table
VI).
 | Table VI.Co-expression of IGF-1Ec and PTEN in
endometrial carcinoma according to sum of stain intensity and
scores of immunopositive cells in relation to clinicopathological
parameters. |
Table VI.
Co-expression of IGF-1Ec and PTEN in
endometrial carcinoma according to sum of stain intensity and
scores of immunopositive cells in relation to clinicopathological
parameters.
|
Characteristics | Patients with
IGF-1Ec and PTEN + expression, n (%) | Patients with
either IGF-1Ec or PTEN ++ expression, n (%) | Patients with
IGF-1Ec and PTEN +++ expression, n (%) | P-value |
|---|
| Age, years |
|
|
| 1.000 |
|
<60 | 0 (0.0) | 16 (69.6) | 1 (4.3) |
|
|
≥60 | 0 (0.0) | 46 (60.5) | 3 (3.9) |
|
| Histological
type |
|
|
| 1.000 |
|
Endometrioid | 0 (0.0) | 55
(64.0) | 4 (4.7) |
|
| Clear
cell and papillary serous | 0 (0.0) | 7 (53.8) | 0 (0.0) |
|
| Clinical stage |
|
|
| 0.688 |
| I | 0 (0.0) | 41 (60.3) | 4 (5.9) |
|
| II | 0 (0.0) | 10 (66.7) | 0 (0.0) |
|
|
III | 0 (0.0) | 4 (80.0) | 0 (0.0) |
|
| Histological
differentiation |
|
|
| 1.000 |
| G1 | 0 (0.0) | 10 (50.0) | 1 (5.0) |
|
| G2 | 0 (0.0) | 35 (71.4) | 2 (4.1) |
|
| G3 | 0 (0.0) | 17 (56.7) | 1 (3.3) |
|
| Myometrial
invasion |
|
|
| 1.000 |
|
<1/2 | 0 (0.0) | 18 (52.9) | 1 (2.9) |
|
|
≥1/2 | 0 (0.0) | 44 (67.7) | 3 (4.6) |
|
| Lymph-vascular
space invasion |
|
|
| – |
|
Yes | 0 (0.0) | 11 (78.6) | 0 (0.0) |
|
| No | 0 (0.0) | 30 (58.8) | 0 (0.0) |
|
| Fallopian tube
and/or ovarian invasion |
|
|
| – |
|
Yes | 0 (0.0) | 13 (68.4) | 0 (0.0) |
|
| No | 0 (0.0) | 17 (63.0) | 0 (0.0) |
|
| Tumoral
necrosis |
|
|
| – |
|
Yes | 0 (0.0) | 5 (71.4) | 0 (0.0) |
|
| No | 0 (0.0) | 31 (59.6) | 0 (0.0) |
|
Correlation analysis between
concomitant expression of IGF-1Ec and p53
The correlation between IGF-1Ec and p53 expression
was assessed using Spearman's correlation coefficient. Based on the
proportion of immunopositive cells (scores), concomitant expression
of IGF-1Ec and p53 was observed in 46.8% of cases (36 out of 77)
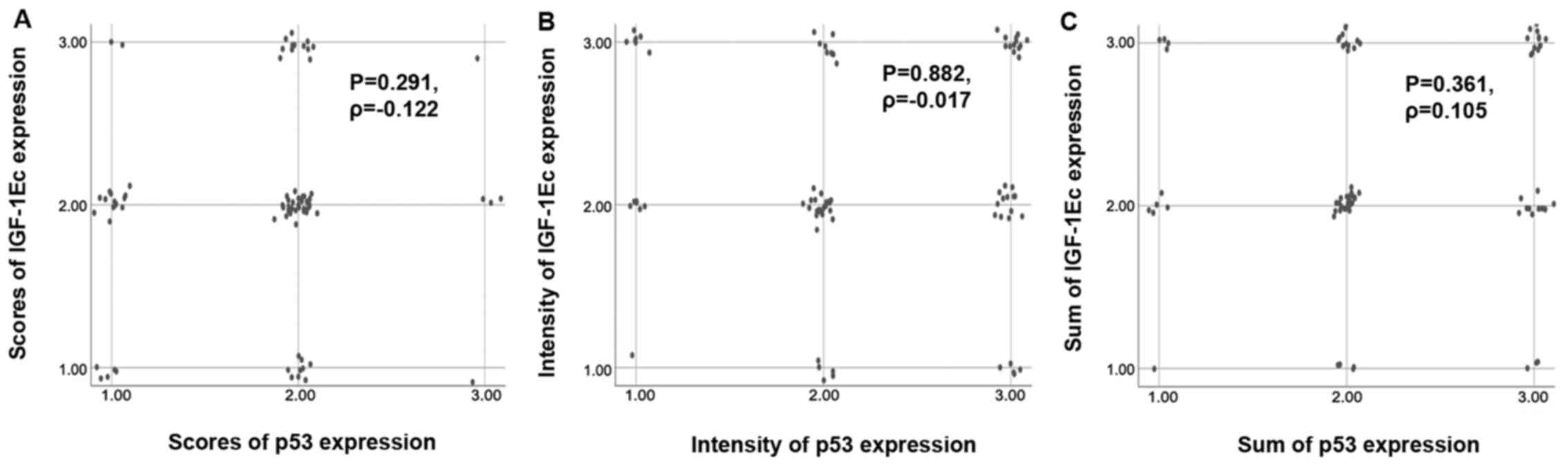
compared with 53.2% of cases without co-expression. The difference
was not significant (P=0.291, Ρ=−0.122). The correlation between
scores of IGF-1Ec and p53 immunopositive cells is shown in the
scatterplot (Fig. 3A).
According to the intensity of staining, there was
concomitant expression of IGF-1Ec and p53 in 44.3% of cases (35 out
of 79) compared with 55.7% without such co-expression; the
difference was not significant (P=0.882, Ρ=−0.017). The correlation
between intensity of IGF-1Ec and p53 immunopositivity is shown in
the scatterplot (Fig. 3B).
According to the sum of staining (scores and
intensity) there was concomitant expression of IGF-1Ec and p53 in
50.6% of cases (39 out of 77) compared with 49.4% of cases without
such co-expression. The findings were not statistically significant
(P=0.361, Ρ=0.105). The correlation between the sum of survivin and
p53 immunopositivity is shown in the scatterplot (Fig. 3C).
In the case of concomitant expression of IGF-1Ec and
p53 there was no significant correlation between the scores of
IGF-1Ec expression and the age of the patients (P=0.490),
histological types (P=0.654), clinical stage (P=0.257),
histological differentiation (P=0.751), depth of myometrial
invasion (P=1.000), presence of lymph-vascular space invasion
(P=0.156), presence of fallopian tube and/or ovarian invasion
(P=0.705) and tumoral necrosis (P=0.125) as well (Table VII).
 | Table VII.Co-expression of IGF-1Ec and p53 in
endometrial carcinoma according to scores of immunopositive cells
in relation to clinicopathological parameters. |
Table VII.
Co-expression of IGF-1Ec and p53 in
endometrial carcinoma according to scores of immunopositive cells
in relation to clinicopathological parameters.
|
Characteristics | Patients with
IGF-1Ec and p53 low scores expression, n (%) | Patients with
either IGF-1Ec or p53 moderate scores expression, n (%) | Patients with
IGF-1Ec and p53 high scores expression, n (%) | P-value |
|---|
| Age, years |
|
|
| 0.490 |
|
<60 | 0 (0.0) | 21 (91.3) | 0 (0.0) |
|
|
≥60 | 5 (6.6) | 60 (78.9) | 1 (1.3) |
|
| Histological
type |
|
|
| 0.654 |
|
Endometrioid | 5 (5.8) | 68 (79.1) | 1 (1.2) |
|
| Clear
cell and papillary serous | 0 (0.0) | 13 (100.0) | 0 (0.0) |
|
| Clinical stage |
|
|
| 0.257 |
| I | 3 (4.4) | 57 (83.8) | 0 (0.0) |
|
| II | 1 (6.7) | 10 (66.7) | 1 (6.7) |
|
|
III | 0 (0.0) | 5 (100.0) | 0 (0.0) |
|
| Histological
differentiation |
|
|
| 0.751 |
| G1 | 1 (5.0) | 15 (75.0) | 0 (0.0) |
|
| G2 | 3 (6.1) | 38 (77.6) | 0 (0.0) |
|
| G3 | 1 (3.3) | 28 (93.3) | 1 (3.3) |
|
| Myometrial
invasion |
|
|
| 1.000 |
|
<1/2 | 2 (5.9) | 27 (79.4) | 0 (0.0) |
|
|
≥1/2 | 3 (4.6) | 54 (83.1) | 1 (1.5) |
|
| Lymph-vascular
space invasion |
|
|
| 0.156 |
|
Yes | 0 (0.0) | 13 (92.9) | 1 (7.1) |
|
| No | 3 (5.9) | 38 (74.5) | 0 (0.0) |
|
| Fallopian tube
and/or ovarian invasion |
|
|
| 0.705 |
|
Yes | 1 (5.3) | 14 (73.7) | 1 (5.3) |
|
| No | 1 (3.7) | 22 (81.5) | 0 (0.0) |
|
| Tumoral
necrosis |
|
|
| 0.125 |
|
Yes | 0 (0.0) | 4 (57.1) | 1 (14.3) |
|
| No | 3 (5.8) | 41 (78.8) | 0 (0.0) |
|
The staining intensity was not correlated with the
age of the patients (P=0.371), clinical stage (P=0.272), depth of
myometrial invasion (P=0.374), the presence of lymph-vascular space
invasion (P=0.674), the presence of fallopian tube and/or ovarian
invasion (P=0.698) and the presence of tumoral necrosis (P=0.581)
(Table VIII). However, a
statistical significance was found between the IGF-1Ec staining
intensity and the histological types (P=0.002). Specifically, in
patients with endometrioid carcinomas, there were a larger number
of patients with concomitant IGF-1Ec and p53 moderate intensity
staining than with both IGF-1Ec and p53 high positive intensity.
The opposite associations were observed in patients with clear and
serous papillary carcinoma. In addition, a statistically
significant correlation was found for the histological
differentiation as well (P=0.007) (Table VIII). Specifically, in patients
with Grade 2 carcinoma, a larger number of patients with
concomitant IGF-1Ec and p53 moderate positive intensity staining
were observed, whereas in the same group fewer patients with both
IGF-1Ec and p53 high positive intensity staining were observed. The
opposite association was observed in patients with Grade 3
carcinoma.
 | Table VIII.Co-expression of IGF-1Ec and p53 in
endometrial carcinoma according to stain intensity of
immunopositive cells in relation to clinicopathological
parameters. |
Table VIII.
Co-expression of IGF-1Ec and p53 in
endometrial carcinoma according to stain intensity of
immunopositive cells in relation to clinicopathological
parameters.
|
Characteristics | Patients with
IGF-1Ec and p53 weak positive expression, n (%) | Patients with
either IGF-1Ec or p53 moderate positive expression, n (%) | Patients with
IGF-1Ec and p53 strong positive expression, n (%) | P-value |
|---|
| Age, years |
|
|
| 0.371 |
|
<60 | 0 (0.0) | 18 (78.3) | 1 (4.3) |
|
|
≥60 | 1 (1.3) | 45 (59.2) | 12 (15.8) |
|
| Histological
type |
|
|
| 0.002 |
|
Endometrioid | 1 (1.2) | 59 (68.6) | 7 (8.1) |
|
| Clear
cell and papillary serous | 0 (0.0) | 4 (30.8) | 6 (46.2) |
|
| Clinical stage |
|
|
| 0.272 |
| I | 1 (1.5) | 44 (64.7) | 6 (8.8) |
|
| II | 0 (0.0) | 9 (60.0) | 3 (20.0) |
|
|
III | 0 (0.0) | 2 (40.0) | 2 (40.0) |
|
| Histological
differentiation |
|
|
| 0.007 |
| G1 | 1 (5.0) | 11 (55.0) | 2 (10.0) |
|
| G2 | 0 (0.0) | 37 (75.5) | 3 (6.1) |
|
| G3 | 0 (0.0) | 15 (50.0) | 8 (26.7) |
|
| Myometrial
invasion |
|
|
| 0.374 |
|
<1/2 | 0 (0.0) | 22 (64.7) | 2 (5.9) |
|
|
≥1/2 | 1 (1.5) | 41 (63.1) | 11 (16.9) |
|
| Lymph-vascular
space invasion |
|
|
| 0.674 |
|
Yes | 0 (0.0) | 10 (71.4) | 3 (21.4) |
|
| No | 0 (0.0) | 33 (64.7%) | 6 (11.8) |
|
| Fallopian tube
and/or ovarian invasion |
|
|
| 0.698 |
|
Yes | 0 (0.0) | 12 (63.2) | 4 (21.1) |
|
| No | 0 (0.0) | 18 (66.7) | 4 (14.8) |
|
| Tumoral
necrosis |
|
|
| 0.581 |
|
Yes | 0 (0.0) | 4 (57.1) | 2 (28.6) |
|
| No | 0 (0.0) | 33 (63.5) | 7 (13.5) |
|
The sum of scores and staining intensity of the
IGF-1Ec immunopositive cells was correlated with histological type
(P=0.049) and histological differentiation (P=0.002). There was no
correlation found with age (P=0.375), clinical stage (P=0.119),
depth of myometrial invasion (P=0.121), the presence of
lymph-vascular space invasion (P=0.229), the presence of fallopian
tube and/or ovarian invasion (P=0.465) and the presence of tumoral
necrosis (P=0.079) (Table IX).
 | Table IX.Co-expression of IGF-1Ec and p53 in
endometrial carcinoma according to sum of stain intensity and
scores of immunopositive cells in relation to clinicopathological
parameters. |
Table IX.
Co-expression of IGF-1Ec and p53 in
endometrial carcinoma according to sum of stain intensity and
scores of immunopositive cells in relation to clinicopathological
parameters.
|
Characteristics | Patients with
IGF-1Ec and p53 + expression, n (%) | Patients with
either IGF-1Ec or p53 ++ expression, n (%) | Patients with
IGF-1Ec and p53 +++ expression, n (%) | P-value |
|---|
| Age, years |
|
|
| 0.375 |
|
<60 | 0 (0.0) | 19 (82.6) | 1 (4.3) |
|
|
≥60 | 1 (1.3) | 47 (61.8) | 12 (15.8) |
|
| Histological
type |
|
|
| 0.049 |
|
Endometrioid | 1 (1.2) | 59 (68.6) | 8 (9.3) |
|
| Clear
cell and papillary serous | 0 (0.0) | 7 (53.8) | 5 (38.5) |
|
| Clinical stage |
|
|
| 0.119 |
| I | 1 (1.5) | 47 (69.1) | 5 (7.4) |
|
| II | 0 (0.0) | 9 (60.0) | 4 (26.7) |
|
|
III | 0 (0.0) | 2 (40.0) | 2 (40.0) |
|
| Histological
differentiation |
|
|
| 0.002 |
| G1 | 1 (5.0) | 10 (50.0) | 2 (10.0) |
|
| G2 | 0 (0.0) | 38 (77.6) | 2 (4.1) |
|
| G3 | 0 (0.0) | 18 (60.0) | 9 (30.0) |
|
| Myometrial
invasion |
|
|
| 0.121 |
|
<1/2 | 0 (0.0) | 23 (67.6) | 1 (2.9) |
|
|
≥1/2 | 1 (1.5) | 43 (66.2) | 12 (18.5) |
|
| Lymph-vascular
space invasion |
|
|
| 0.229 |
|
Yes | 0 (0.0) | 10 (71.4) | 4 (28.6) |
|
| No | 0 (0.0) | 33 (64.7) | 5 (9.8) |
|
| Fallopian tube
and/or ovarian invasion |
|
|
| 0.465 |
|
Yes | 0 (0.0) | 12 (63.2) | 5 (26.3) |
|
| No | 0 (0.0) | 18 (66.7) | 4 (14.8) |
|
| Tumoral
necrosis |
|
|
| 0.079 |
|
Yes | 0 (0.0) | 3 (42.9) | 3 (42.9) |
|
| No | 0 (0.0) | 34 (65.4) | 6 (11.5) |
|
Correlation analysis between
concomitant expression of IGF-1Ec and survivin
The correlation between IGF-1Ec and survivin
expression was assessed using Spearman's correlation coefficient.
According to the proportion of immunopositive cells (scores), there
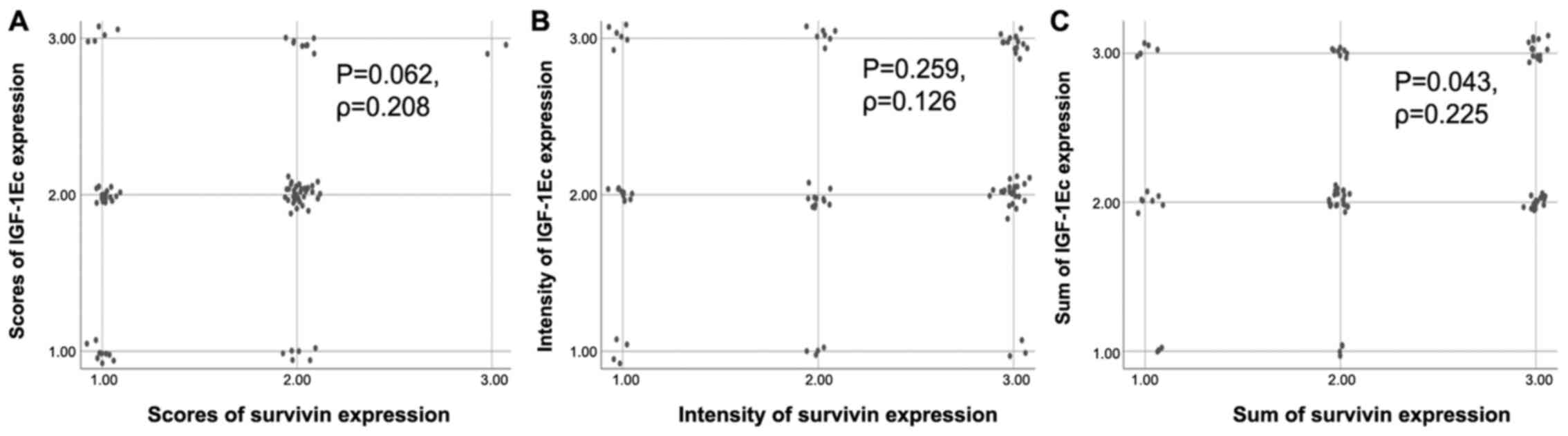
was concomitant expression between IGF-1Ec and survivin in 54.3% of
cases (44 out of 81) compared with 45.7% of cases without such
co-expression; the difference was not significant (P=0.062,
Ρ=0.208). The correlation between scores of IGF-1Ec and survivin
immunopositive cells is shown in the scatterplot (Fig. 4A).
According to the intensity of staining there was
concomitant expression between IGF-1Ec and survivin in 36.6% of
cases (30 out of 82) compared with 63.4% without such
co-expression; the difference was not statistically significant
(P=0.259, Ρ=0.126). The correlation between intensity of IGF-1Ec
and survivin immunopositivity is shown in the scatterplot (Fig. 4B).
According to the sum of staining (scores and
intensity) there was concomitant expression of IGF-1Ec and survivin
in 49.4% of cases (40 out of 81) compared with 50.6% without such
co-expression. The findings were statistically significant
(P=0.043, Ρ=0.225). The correlation between sum of IGF-1Ec and
survivin immunopositivity is shown in the scatterplot (Fig. 4C).
In the case of concomitant expression of IGF-1Ec and
survivin, there was a significant correlation between the scores of
immunohistochemical expression and the clinical stage (P=0.040),
histological differentiation (P=0.024), lymph-vascular space
invasion (P=0.034) and the presence of tumoral necrosis (P=0.008).
There was no correlation found for the age of the patients
(P=0.558), histological types (P=0.508), the depth of the
myometrial invasion (P=0.171) and the presence of fallopian tube
and/or ovarian invasion (P=0.341) (Table
X).
 | Table X.Co-expression of IGF-1Ec and survivin
in endometrial carcinoma according to scores of immunopositive
cells in relation to clinicopathological parameters. |
Table X.
Co-expression of IGF-1Ec and survivin
in endometrial carcinoma according to scores of immunopositive
cells in relation to clinicopathological parameters.
|
Characteristics | Patients with
IGF-1Ec and survivin low scores expression, n (%) | Patients with
either IGF-1Ec or survivin moderate scores expression, n (%) | Patients with
IGF-1Ec and survivin high scores expression, n (%) | P-value |
|---|
| Age, years |
|
|
| 0.558 |
|
<60 | 1 (4.3) | 18 (78.3) | 0 (0.0) |
|
|
≥60 | 8 (10.5) | 54 (71.1) | 2 (2.6) |
|
| Histological
type |
|
|
| 0.508 |
|
Endometrioid | 9 (10.5) | 62 (72.1) | 2 (2.3) |
|
| Clear
cell and papillary serous | 0 (0.0) | 10 (76.9) | 0 (0.0) |
|
| Clinical stage |
|
|
| 0.040 |
| I | 6 (8.8) | 50 (73.5) | 0 (0.0) |
|
| II | 2 (13.3) | 11 (73.3) | 1 (6.7) |
|
|
III | 0 (0.0) | 2 (40.0) | 1 (20.0) |
|
| Histological
differentiation |
|
|
| 0.024 |
| G1 | 4 (20.0) | 11 (55.0) | 0 (0.0) |
|
| G2 | 5 (10.2) | 38 (77.6) | 0 (0.0) |
|
| G3 | 0 (0.0) | 23 (76.7) | 2 (6.7) |
|
| Myometrial
invasion |
|
|
| 0.171 |
|
<1/2 | 5 (14.7) | 20 (58.8) | 0 (0.0) |
|
|
≥1/2 | 4 (6.2) | 52 (80.0) | 2 (3.1) |
|
| Lymph-vascular
space invasion |
|
|
| 0.034 |
|
Yes | 0 (0.0) | 10 (71.4) | 2 (14.3) |
|
| No | 4 (7.8) | 40 (78.4) | 0 (0.0) |
|
| Fallopian tube
and/or ovarian invasion |
|
|
| 0.341 |
|
Yes | 2 (7.4) | 20 (74.1) | 0 (0.0) |
|
| No | 2 (10.5) | 13 (68.4) | 2 (10.5) |
|
| Tumoral
necrosis |
|
|
| 0.008 |
|
Yes | 1 (14.3) | 3 (42.9) | 2 (28.6) |
|
| No | 3 (5.8) | 41 (78.8) | 0 (0.0) |
|
In the case of concomitant expression of IGF-1Ec and
survivin, the IGF-1Ec staining intensity was correlated with
histological differentiation (P=0.030) (Table XI). Specifically, in Grade 2
patients, there were a larger number of patients with concomitant
IGF-1Ec and survivin moderate expression than expected.
Furthermore, the opposite associations were observed in Grade 3
patients. There was no correlation found for the age of the
patients (P=0.155), histological types (P=0.084), clinical stage
(P=1.000), depth of myometrial invasion (P=0.615), the presence of
lymph-vascular space invasion (P=0.360), fallopian tube and/or
ovarian invasion (P=0.338) and tumoral necrosis (P=0.119) (Table XI).
 | Table XI.Co-expression of IGF-1Ec and survivin
in endometrial carcinoma according to stain intensity of
immunopositive cells in relation to clinicopathological
parameters. |
Table XI.
Co-expression of IGF-1Ec and survivin
in endometrial carcinoma according to stain intensity of
immunopositive cells in relation to clinicopathological
parameters.
|
Characteristics | Patients with
IGF-1Ec and survivin weak positive expression, n (%) | Patients with
either IGF-1Ec or survivin moderate positive expression, n (%) | Patients with
IGF-1Ec and survivin strong positive expression, n (%) | P-value |
|---|
| Age, years |
|
|
| 0.155 |
|
<60 | 1 (4.3) | 18 (78.3) | 1 (4.3) |
|
|
≥60 | 3 (3.9) | 43 (566) | 14 (18.4) |
|
| Histological
type |
|
|
| 0.084 |
|
Endometrioid | 4 (4.7) | 57 (66.3) | 11 (12.8) |
|
| Clear
cell and papillary serous | 0 (0.0) | 4 (30.8) | 4 (30.8) |
|
| Clinical stage |
|
|
| 1.000 |
| I | 2 (2.9) | 43 (63.2) | 10 (14.7) |
|
| II | 0 (0.0) | 9 (60.0) | 2 (13.3) |
|
|
III | 0 (0.0) | 2 (40.0) | 1 (20.0) |
|
| Histological
differentiation |
|
|
| 0.030 |
| G1 | 2 (10.0) | 11 (55.0) | 3 (15.0) |
|
| G2 | 2 (4.1) | 37 (75.5) | 4 (8.2) |
|
| G3 | 0 (0.0) | 13 (43.3) | 8 (26.7) |
|
| Myometrial
invasion |
|
|
| 0.615 |
|
<1/2 | 2 (5.9) | 22 (64.7) | 4 (11.8) |
|
|
≥1/2 | 2 (3.1) | 39 (60.0) | 11 (16.9) |
|
| Lymph-vascular
space invasion |
|
|
| 0.360 |
|
Yes | 0 (0.0) | 8 (57.1) | 4 (28.6) |
|
| No | 3 (5.9) | 30 (58.8%) | 7 (13.7) |
|
| Fallopian tube
and/or ovarian invasion |
|
|
| 0.338 |
|
Yes | 0 (0.0) | 12 (63.2) | 2 (10.5) |
|
| No | 3 (11.1) | 15 (55.6) | 5 (18.5) |
|
| Tumoral
necrosis |
|
|
| 0.119 |
|
Yes | 0 (0.0) | 3 (42.9) | 3 (42.9) |
|
| No | 3 (5.8) | 31 (59.6) | 6 (11.5) |
|
In the case of concomitant expression of IGF-1Ec and
survivin, the sum of scores and staining intensity of the IGF-1Ec
immunopositive cells was correlated with histological
differentiation (P=0.008) and the presence of tumoral necrosis
(P=0.030) (Table XII). In terms of
histological differentiation, in Grade 2 patients a larger number
of patients with concomitant IGF-1Ec and survivin sum moderate
expression was observed than expected, whereas in the same group, a
lower number of patients with both high sum score of IGF-1Ec and
survivin expression was observed. The opposite associations were
observed in Grade 3 patients. Additionally, in patients without
tumoral necrosis, a larger number of patients with concomitant
IGF-1Ec and survivin moderate sum expression was observed, and a
lower number of patients with both high IGF-1Ec and survivin sum
expression. The opposite associations were observed in patients
with tumoral necrosis. There was no correlation found for the age
of the patients (P=0.221), histological type (P=0.784), clinical
stage (P=0.235), depth of myometrial invasion (P=0.421), presence
of lymph-vascular space invasion (P=0.297) and presence of
fallopian tube and/or ovarian invasion (0.546) (Table XII).
 | Table XII.Co-expression of IGF-1Ec and survivin
in endometrial carcinoma according to sum of stain intensity and
scores of immunopositive cells in relation to clinicopathological
parameters. |
Table XII.
Co-expression of IGF-1Ec and survivin
in endometrial carcinoma according to sum of stain intensity and
scores of immunopositive cells in relation to clinicopathological
parameters.
|
Characteristics | Patients with
IGF-1Ec and survivin + expression, n (%) | Patients with
either IGF-1Ec or survivin ++ expression, n (%) | Patients with
IGF-1Ec and survivin +++ expression, n (%) | P-value |
|---|
| Age, years |
|
|
| 0.221 |
|
<60 | 1 (4.3) | 17 (73.9) | 1 (4.3) |
|
|
≥60 | 2 (2.6) | 45 (59.2) | 15 (19.7) |
|
| Histological
type |
|
|
| 0.784 |
|
Endometrioid | 3 (3.5) | 55 (64.0) | 13 (15.1) |
|
| Clear
cell and papillary serous | 0 (0.0) | 7 (53.8) | 3 (23.1) |
|
| Clinical stage |
|
|
| 0.235 |
| I | 2 (2.9) | 44 (64.7) | 9 (13.2) |
|
| II | 0 (0.0) | 10 (66.7) | 3 (20.0) |
|
|
III | 0 (0.0) | 1 (20.0) | 2 (40.0) |
|
| Histological
differentiation |
|
|
| 0.008 |
| G1 | 2 (10.0) | 9 (45.0) | 3 (15.0) |
|
| G2 | 1 (2.0) | 39 (79.6) | 4 (8.2) |
|
| G3 | 0 (0.0) | 14 (46.7) | 9 (30.0) |
|
| Myometrial
invasion |
|
|
| 0.421 |
|
<1/2 | 2 (5.9) | 20 (58.8) | 4 (11.8) |
|
|
≥1/2 | 1 (1.5) | 42 (64.6) | 12 (18.5) |
|
| Lymph-vascular
space invasion |
|
|
| 0.297 |
|
Yes | 2 (3.9) | 30 (58.8) | 7 (13.7) |
|
| No | 0 (0.0) | 8 (57.1) | 5 (35.7) |
|
| Fallopian tube
and/or ovarian invasion |
|
|
| 0.546 |
|
Yes | 0 (0.0) | 12 (63.2) | 4 (21.1) |
|
| No | 2 (7.4) | 15 (55.6) | 4 (14.8) |
|
| Tumoral
necrosis |
|
|
| 0.030 |
|
Yes | 0 (0.0) | 2 (28.6) | 4 (57.1) |
|
| No | 2 (3.8) | 32 (61.5) | 6 (11.5) |
|
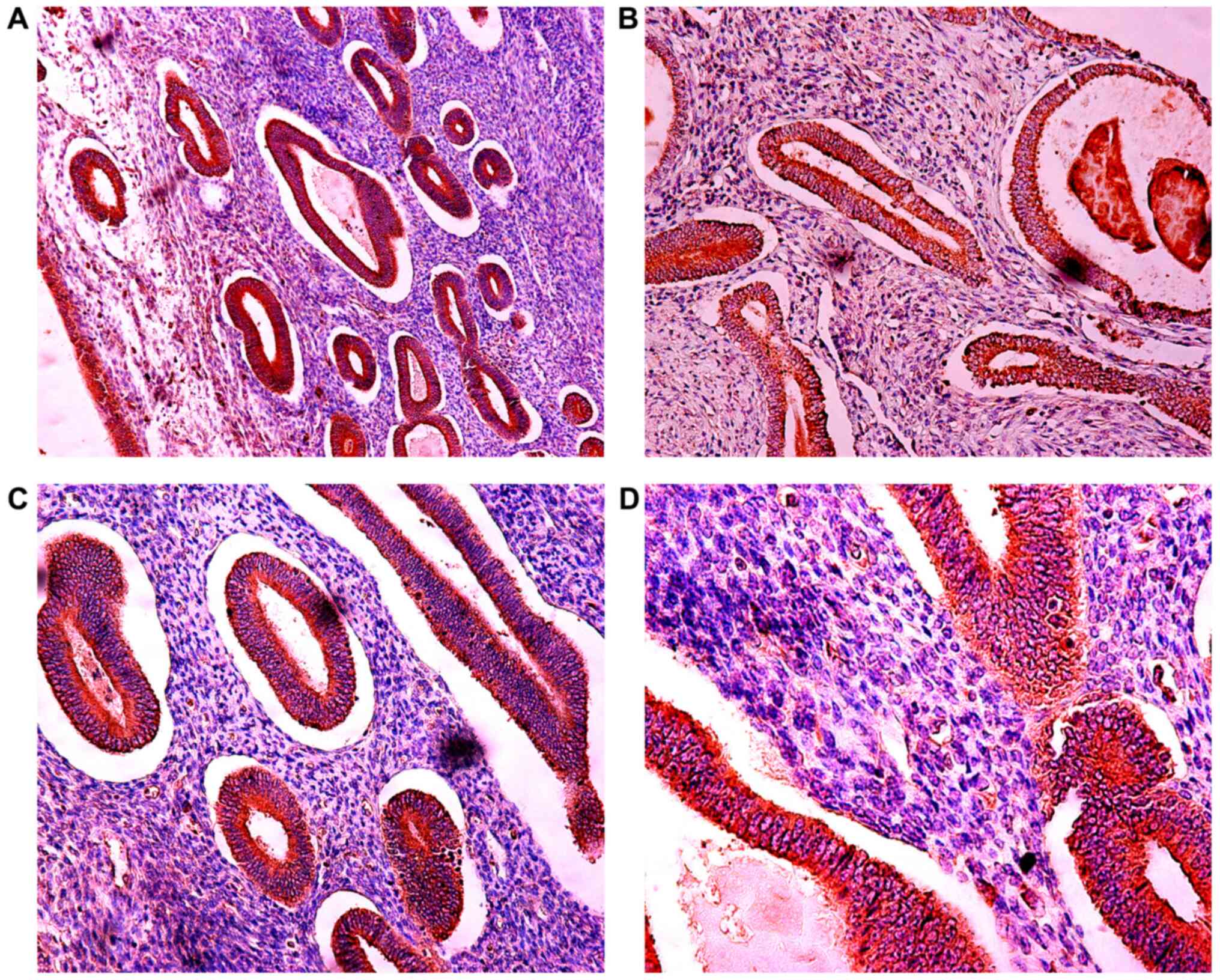
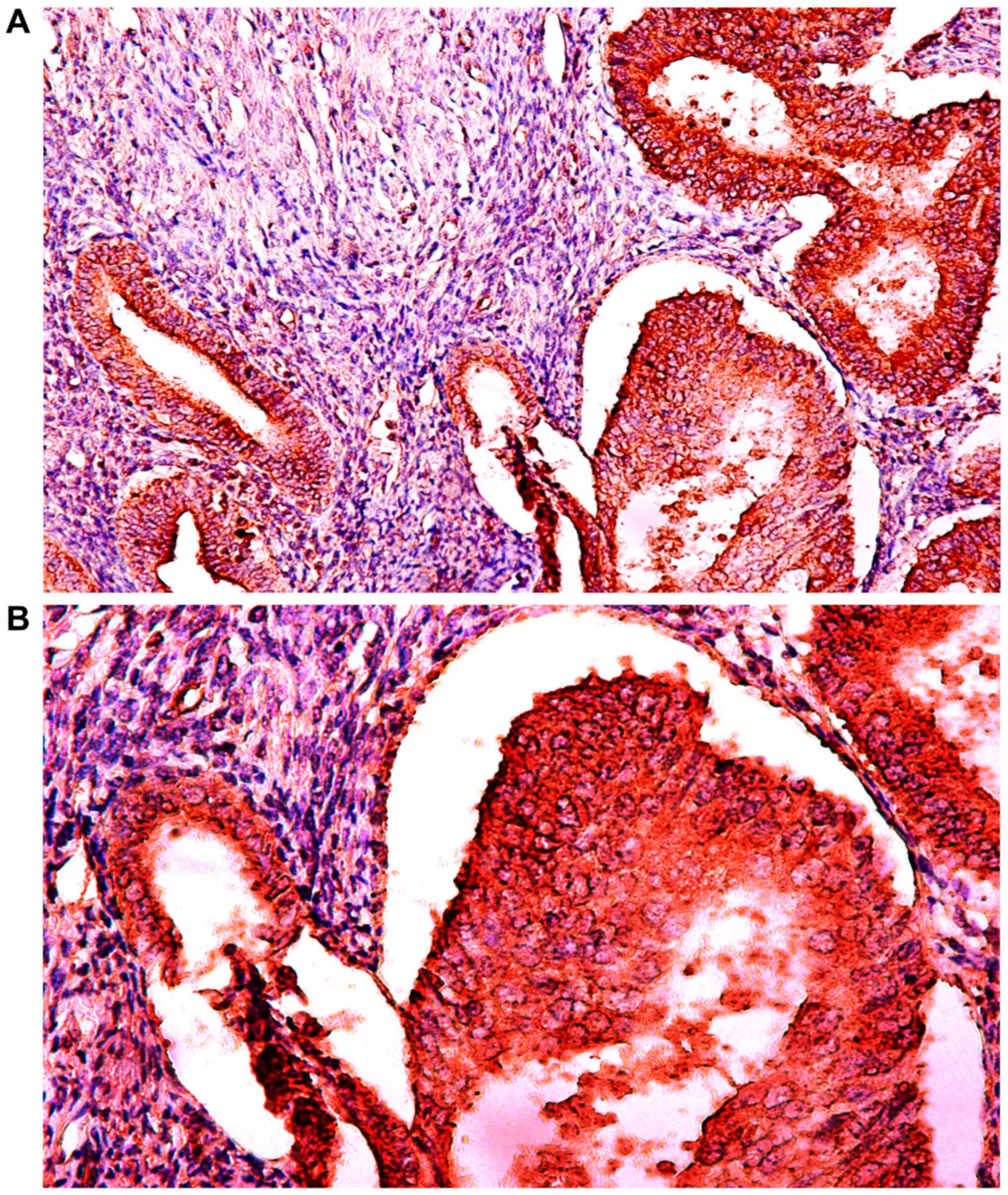
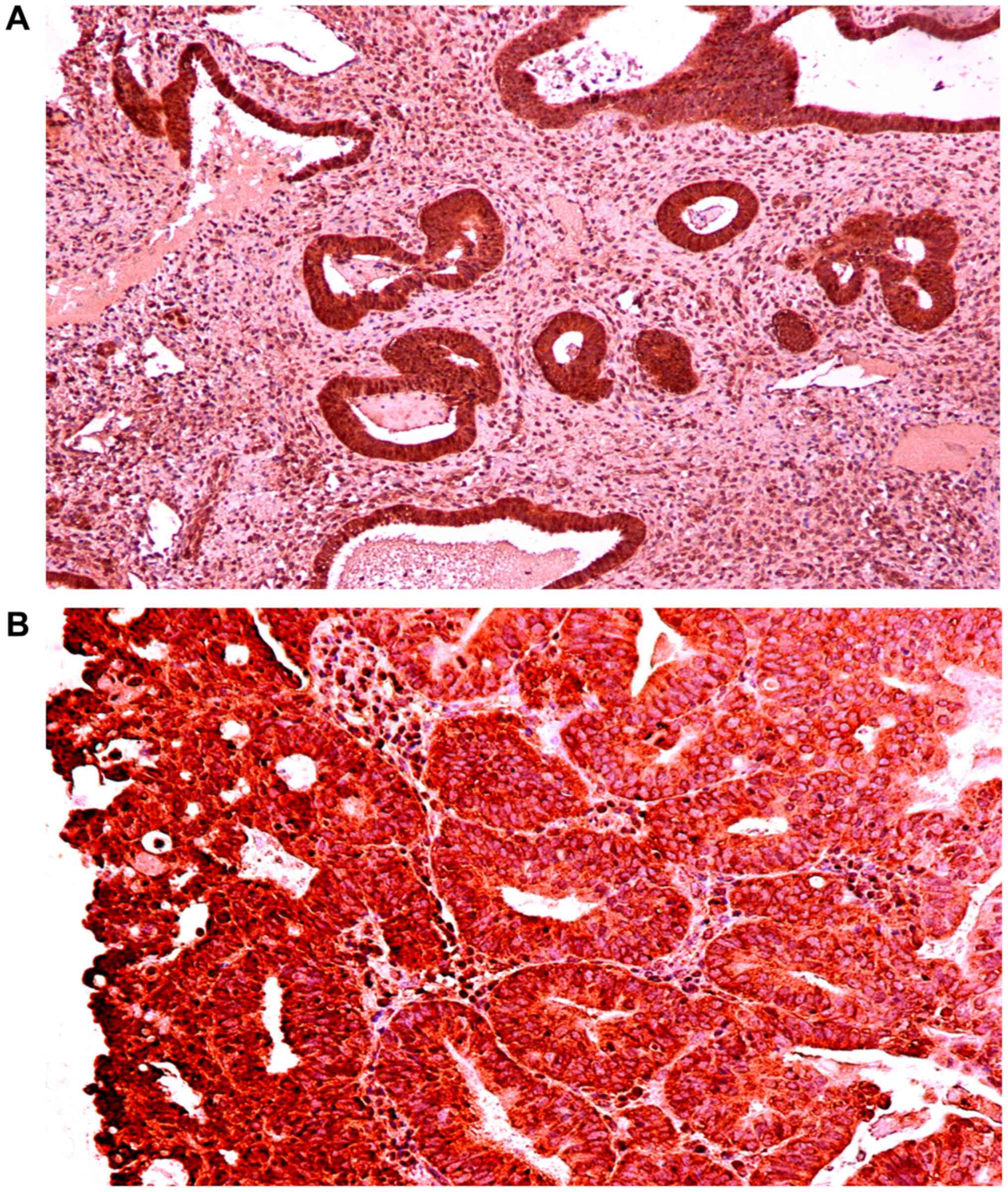
Expression of IGF-1Ec in the normal
endometrium and endometrial hyperplasia, and comparison with
expression in endometrial carcinoma
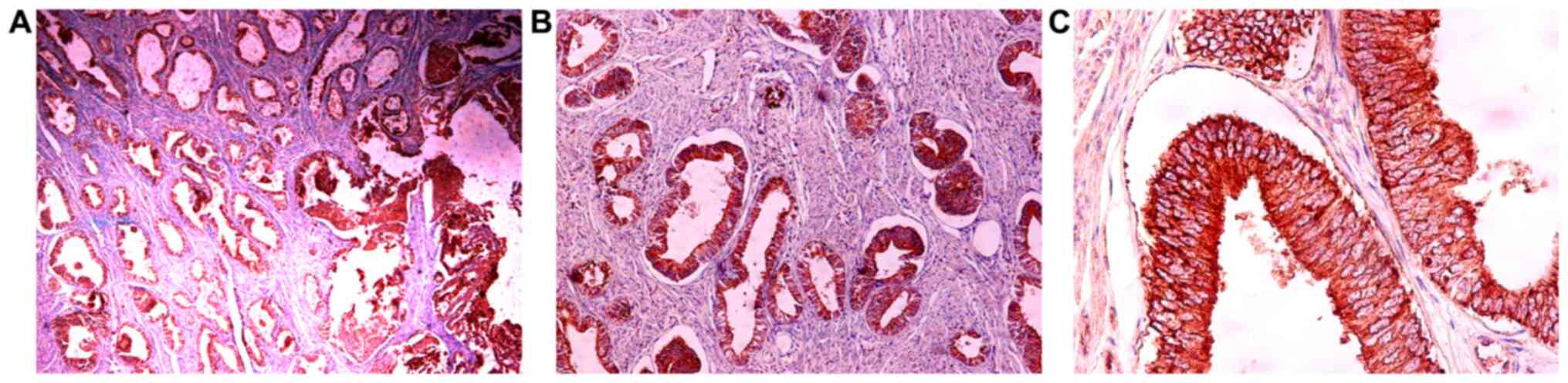
The expression of IGF-1Ec in endometrioid
endometrial adenocarcinoma, normal endometrium and complex
endometrial hyperplasia with atypia are shown in Figs. 5, 6
and 7, respectively. Fig. 8 shows the IGF-1Ec immunoexpression in
simple and complex endometrial hyperplasia. In normal endometrium
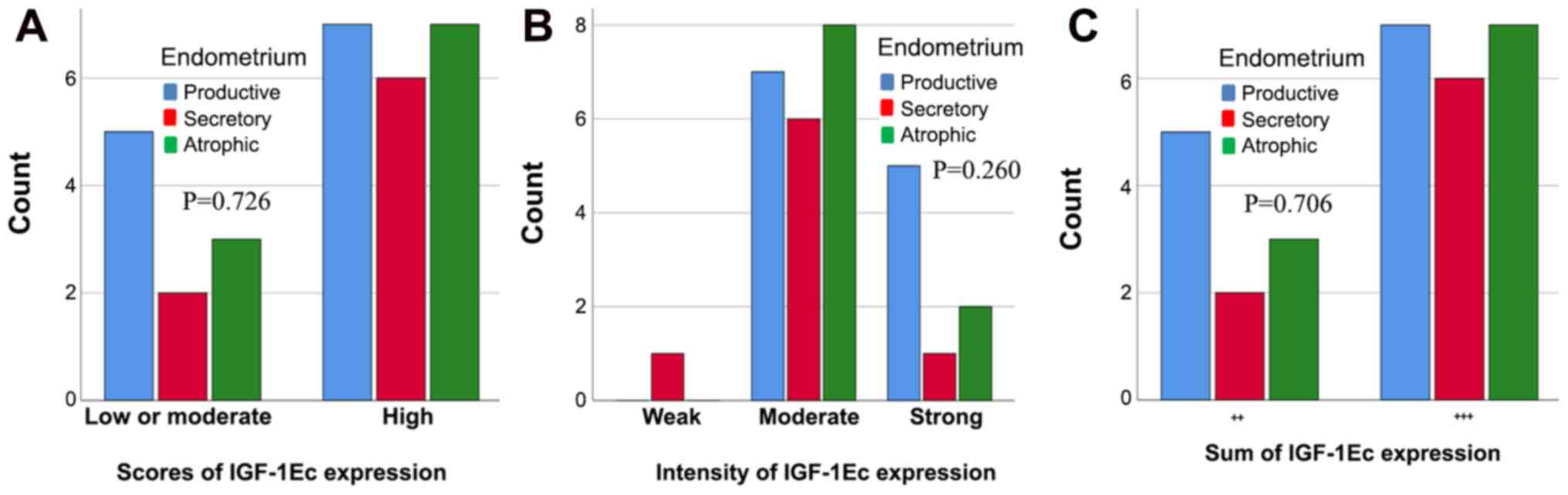
IGF-1Ec, staining was observed in 100% of cases and in 100% of
cases of endometrial hyperplasia. There were no statistically
significant differences in the IGF-1Ec expression between normal
endometrium and the proliferative or secretary phase, or with
atrophic endometrium based on the scores (P=0.726), the intensity
(P=0.260) or the sum of immunopositivity (P=0.706) (Fig. 9). However, there were differences in
the levels of IGF-1Ec expression between normal endometrium,
endometrial hyperplasia and endometrial carcinomas.
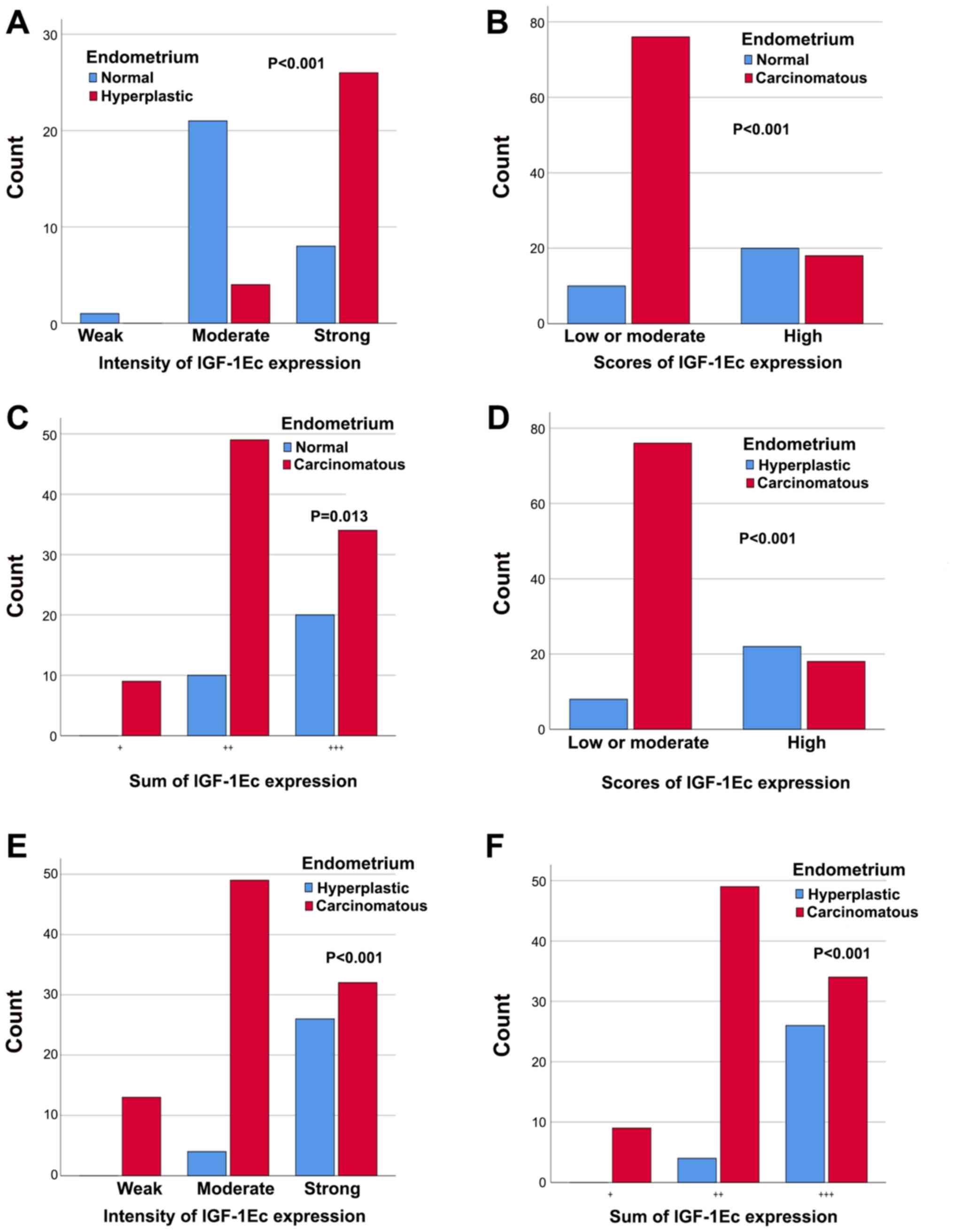
In particular, the intensity of IGF-1Ec
immunostaining was statistically significantly different between
normal and hyperplastic endometrium (P<0.001); in the group of
patients with normal endometrium, there were more cases with
moderate expression and fewer patients with high expression, while
in the endometrial carcinoma group the opposite was observed
(Fig. 10A). There was no
statistically significant correlation between the scores or the sum
of IGF-1Ec expression between normal and hyperplastic endometrium
(P=0.779 and P=0.125 respectively).
In addition, the scores of IGF-1Ec expression was
statistically significantly different between normal endometrium
and endometrial carcinomas (P<0.001); the low or moderate scores
of IGF-1Ec expression were significantly higher in patients with
endometrial carcinoma compared with patients with normal
endometrium (Fig. 10B).
Furthermore, the sum of IGF-1Ec immunoexpression was statistically
significant between normal endometrium and endometrial carcinomas
(P=0.013); the (+) or (++) or (+++) sum of IGF-1Ec expression were
significantly higher in patients with endometrial carcinoma
compared with patients with normal endometrium (Fig. 10C). However, there was no
statistically significant correlation between the intensity of
IGF-1Ec expression between normal and carcinomatous endometrium
(P=0.142).
The scores of IGF-1Ec expression were statistically
significantly different between endometrial hyperplasia cases and
carcinomas (P<0.001); more patients with low or moderate IGF-1Ec
expression were observed in the group with endometrial carcinomas
compared with patients with endometrial hyperplasia (Fig. 10D). Additionally, the intensity of
IGF-1Ec expression was statistically significantly different
between endometrial hyperplasia cases and carcinoma cases
(P<0.001); patients with endometrial carcinoma had higher counts
of weak, moderate and strong IGF-1Ec positive expression, compared
with patients with hyperplastic endometrium (Fig. 10E). The sum of IGF-1Ec expression
was statistically significantly different between endometrial
hyperplasia and carcinoma (P<0.001); the (+) or (++) or (+++)
sum of IGF-1Ec expression was significantly higher in patients with
endometrial carcinoma compared with patients with hyperplastic
endometrium (Fig. 10F).
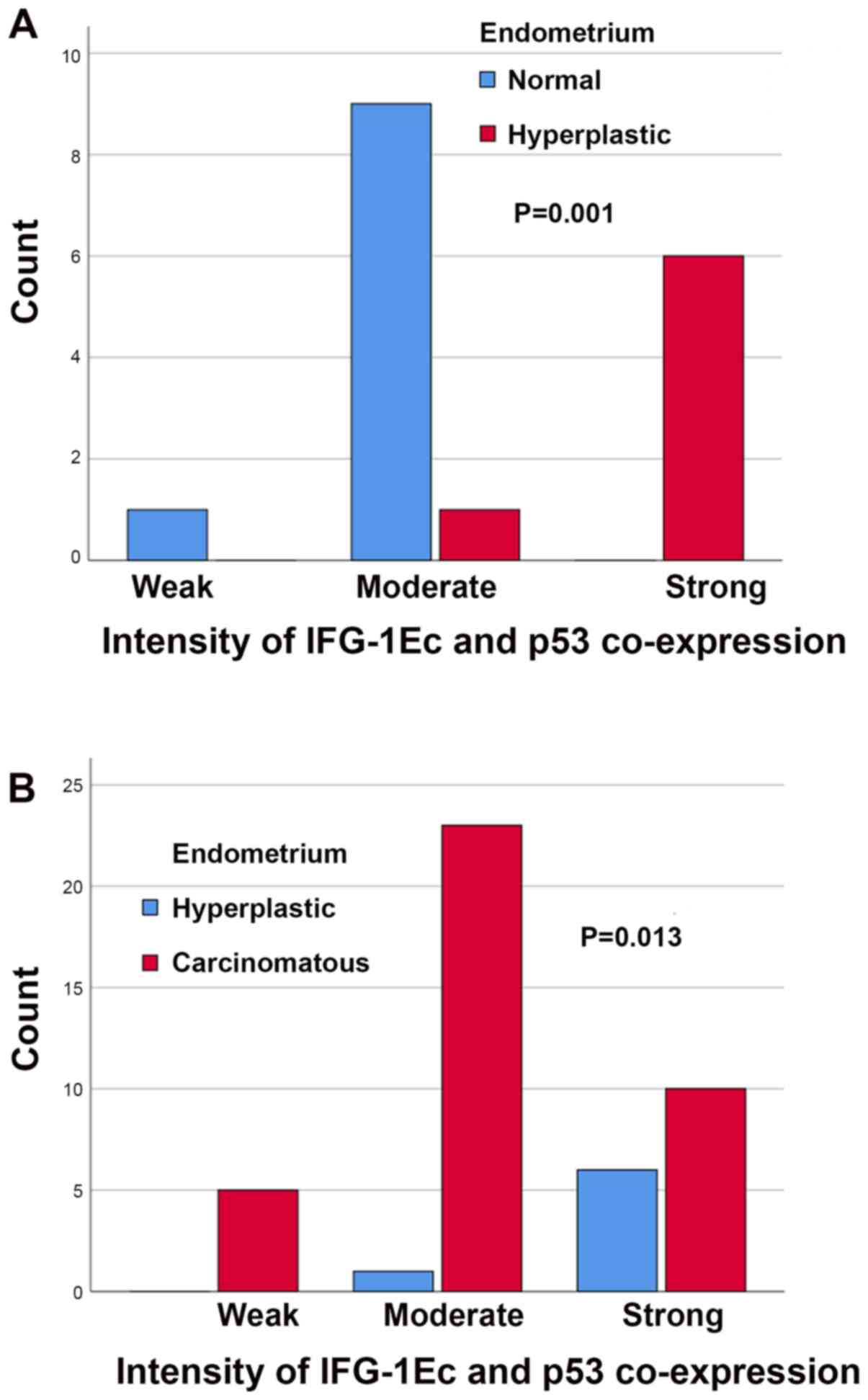
In the case of concomitant IGF-1Ec and p53
expression, there were no statistically significant differences
between normal and hyperplastic endometrium with scores (P=1.000)
or the sum (P=1.000) of immunopositivity. However, there was a
statistically significant difference between the intensity of
concomitant IGF-1Ec and p53 immunostaining (P=0.001); in the group
of patients with endometrial hyperplasia, there were more patients
with strong immunostaining compared with patients with normal
endometrium (Fig. 11A). In the case
of concomitant IGF-1Ec and p53 expression there were no
statistically significant differences between normal and
carcinomatous endometrium in the scores (P=1.000), intensity
(P=0.171) or sum (P=1.000) of immunopositivity. In the case of
concomitant IGF-1Ec and p53 expression there were no statistically
significant differences between hyperplastic and carcinomatous
endometrium with the scores (P=0.673) or sum (P=1.000) of
immunopositivity. However, there was a statistically significant
difference in the intensity of concomitant IGF-1Ec and p53
immunostaining (P=0.013); more patients with moderate intensity
were observed in the group of patients with endometrial carcinomas
compared with patients with endometrial hyperplasia (Fig. 11B).
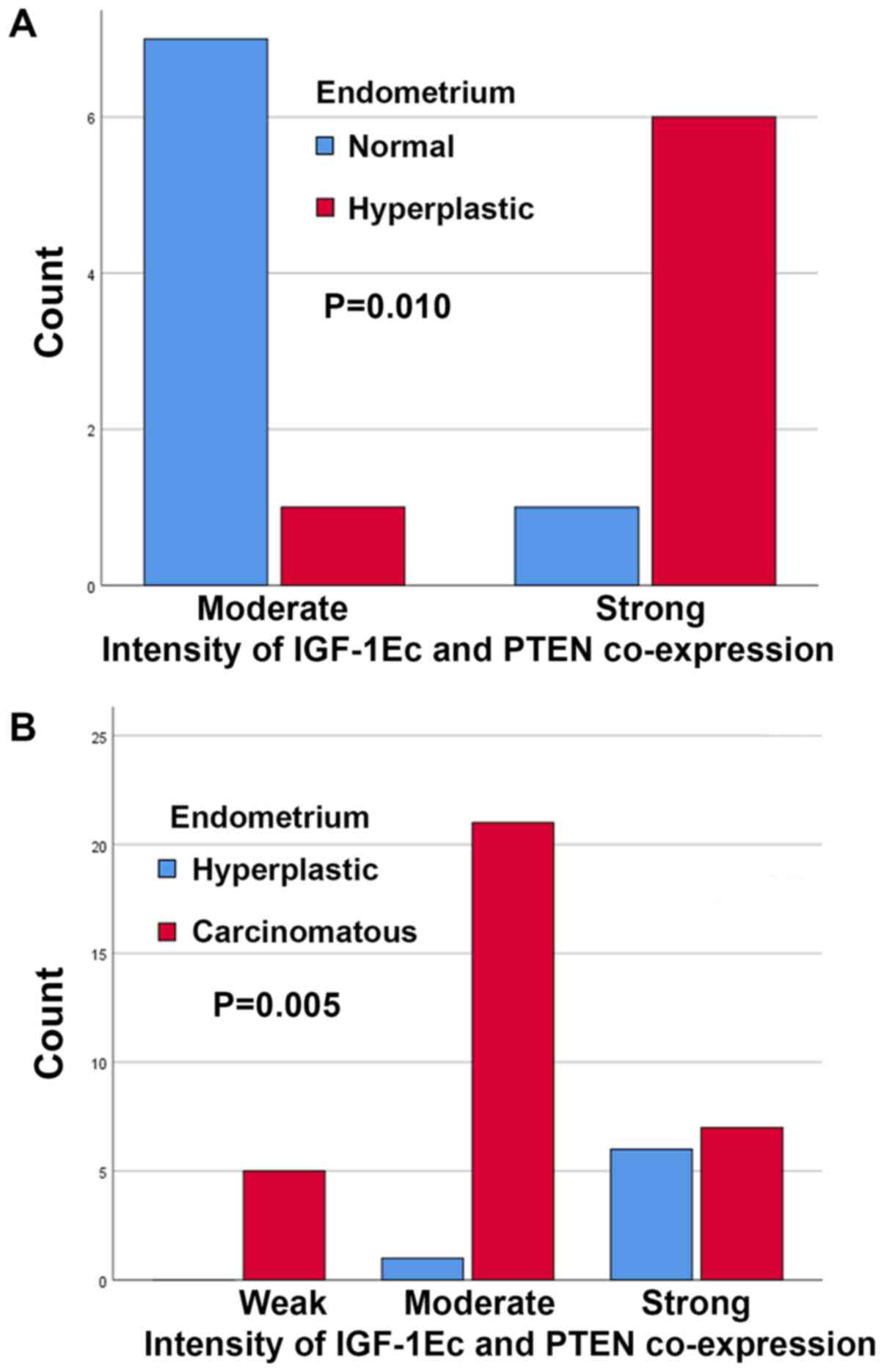
In the case of concomitant IGF-1Ec and PTEN
expression there were no statistically significant differences
between normal and hyperplastic endometrium with scores (P=0.444)
or sum (P=0.282) of immunopositivity. However, there was a
statistically significant difference in the intensity of
concomitant IGF-1Ec and PTEN immunostaining (P=0.010); in the group
of patients with normal endometrium there were fewer patients with
strong immunostaining compared with patients with endometrial
hyperplasia (Fig. 12A). In the case
of concomitant IGF-1Ec and PTEN expression, there were no
statistically significant differences between normal and
carcinomatous endometrium with scores (P=1.000), or intensity
(P=0.446) and sum (P=0.282) of immunopositivity. In the case of
concomitant IGF-1Ec and PTEN expression, there were no
statistically significant differences between hyperplastic and
carcinomatous endometrium with scores (P=0.108) or sum (P=0.330) of
immunopositivity. However, there was a statistically significant
difference in the intensity of concomitant IGF-1Ec and PTEN
immunostaining (P=0.005); stronger expression was observed in
patients with endometrial carcinomas compared with patients with
endometrial hyperplasia (Fig.
12B).
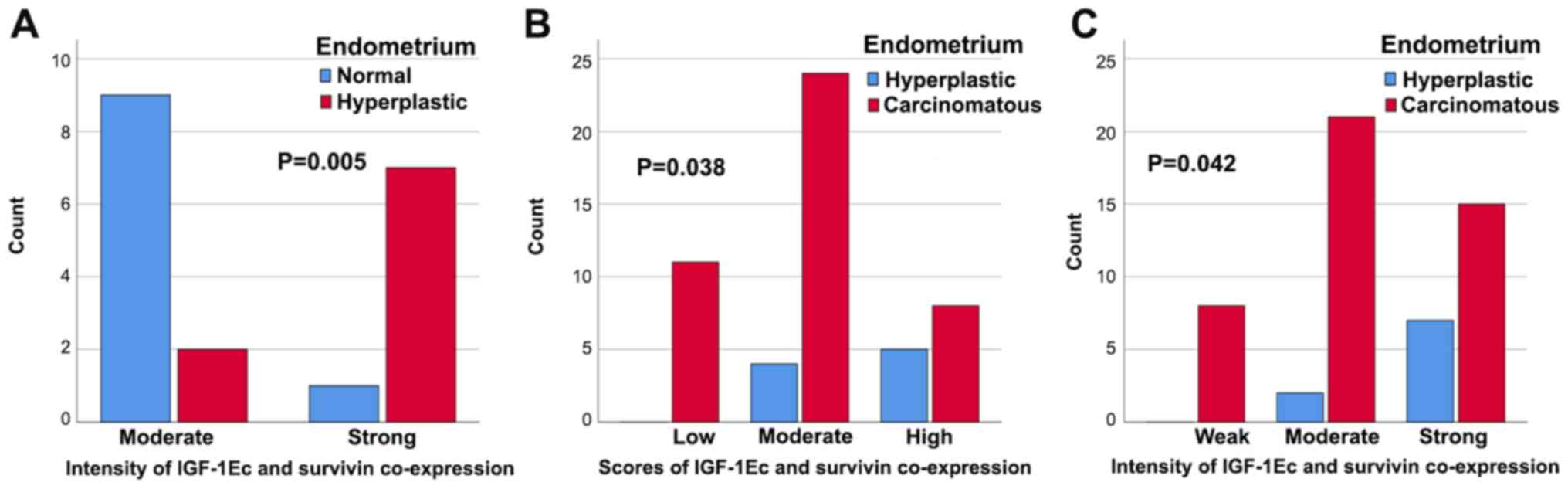
In the case of concomitant IGF-1Ec and survivin
expression there were no statistically significant differences
between normal and hyperplastic endometrium with scores (P=1.000)
or sum (P=0.375) of immunopositivity. However, there was a
statistically significant correlation in the intensity of
concomitant IGF-1Ec and survivin immunostaining (P=0.005); stronger
expression was observed in hyperplastic endometrium compared with
normal endometrium (Fig. 13A). In
the case of concomitant IGF-1Ec and survivin expression, there were
no statistically significant differences between normal and
carcinomatous endometrium with scores (P=0.163), intensity
(P=0.069) or sum (P=1.000) of immunopositivity. In the case of
concomitant IGF-1Ec and survivin expression there were
statistically significant differences between hyperplastic end
carcinomatous endometrium and scores (P=0.038) or intensity
(P=0.042) of immunopositivity. More patients showed higher scores
and stronger concomitant expression of IGF-1Ec and survivin in
patients with endometrial carcinoma compared with patients with
hyperplastic endometrium (Fig. 13B and
C, respectively).
Discussion
For normal endometrial physiology, appropriate
balances between cellular proliferation, metabolism, cell cycle
arrest and apoptosis are of critical importance. The exact
pathophysiology of endometrial carcinogenesis has not been
accurately determined, and may vary between individuals. A wealth
of evidence has shown that activation of oncogenes, inactivation or
deficiency of tumor suppressor genes, or inhibition of apoptosis
results in endometrial carcinogenesis and metastasis. Some of the
possible causes of all these genetic alterations include gene
mutations, amplifications and chromosomal translocations or
rearrangements (71–74). Thus, clinicians should take into
consideration multiple factors which may affect therapeutic options
in patients with endometrial carcinoma, to determine the
appropriate therapeutic regimen, and avoid over- or under-treatment
(75,76). In clinical practice, well-established
clinicopathological, prognostic and neoplastic factors are used,
such as the histological type, histological grade, depth of
myometrial invasion, cervical involvement, lymphovascular space
invasion, or presence of tumoral necrosis. However, some patients
with endometrial carcinoma may benefit from screening for molecular
markers, such as growth or survival factors, oncogenes and tumor
suppressor genes. The purpose of the ongoing research in this field
is to allow for more optimal and personalized therapeutic regimens,
with the possibility gene targeting therapies, and to improve
estimation of the risk of recurrence and therefore accurately
predict prognosis (75,77,78).
Endometrial carcinoma type I is frequently associated with
alterations in phosphatase and tensing homologue deleted on
chromosome 10 (PTEN), K-ras, β-catenin,
phosphatidyl-inositol-3-kinase catalytic subunit (PIK3CA), MutL
homolog (MLH)-1 and MLH-6, and alterations in DNA-mismatch repair
genes and microsatellite instability (79,80).
Endometrial carcinoma type II is frequently associated with
alterations in p53, p16, human epidermal growth factor receptor
type 2 (also known as proto-oncogene neu or receptor
tyrosine-protein kinase, c-erbB−2), E-type Cyclin E1,
c-MYC, fibroblast growth factor receptor 3, SOX17, STK15 and
E-cadherin, including loss of heterozygosity (79–84).
Metabolic syndrome is accompanying by chronic
proinflammatory status and is associated with the development of
cardiovascular disease and diabetic morbidity and mortality
(85,86). Most patients with metabolic syndrome
have hyperinsulinemia caused by insulin resistance (87). Metabolic syndrome is diagnosed by
abdominal obesity defined by waist circumference (>102-cm in men
and >88-cm in women), dyslipidemia accompanying by elevated
triglyceride levels, low high-density lipoprotein-cholesterol
levels, hyperglycemia (fasting blood glucose >110 mg/dl) and
raised blood pressure (blood pressure >130/85 mmHg) (86,87).
Also, metabolic syndrome is a risk for common malignancies
including liver, gastric, esophageal, colorectal, bladder, breast,
hepatic, pancreatic, renal, endometrial, cervical and ovarian
cancer (86–89). It seems that disarrangement by the
adipose tissue in the regulation of hormones and cytokines such as
sex steroids, leptin, adipokines, tumor necrosis factor (TNF)-α and
plasminogen activator inhibitor-1 leads to chronic inflammation and
related carcinogenesis (86,90,91).
Chronic mitogenic endometrial stimulation by the elevated free
estrogen levels is thought to be a risk factor for carcinogenesis
through the activation of estrogen receptor-α. In addition,
estrogens can induce the secretion of vascular endothelial factor
and stimulate angiogenesis (92,93).
Disturbances in the function of the IGF/IGF-1R/IGFBPs system may be
responsible for the induction of carcinogenesis (22). In particular, in colon cancer it has
been shown that IGF-1 is associated with an increase in motility
and migration of malignant colonic cells through reorganization of
integrin receptors, modulation of E-cadherin/catenins complex
function and activation of protein kinases C-γ and C-δ (94,95).
Additionally, there may be a close association between IGF-1 and
regulation of vascular endothelial growth factor (VEGF) expression
in human colon cancer through induction of transcription of the
VEGF gene (96). In a mouse model of
colon cancer, the administration of recombinant human IGF-1 has
been shown to increase tumor mass, growth of cecum tumors, and
increase metastasis to the liver, supporting the hypothesis that
circulating IGF-1 levels may serve an important role in tumor
development and metastasis of these neoplasms (97). In addition, elevated IGF-1 levels in
obesity and hyperinsulinemia promote endometrial neoplastic
transformation (97). Impaired
glucose management, hyperglycemia and expression of insulin
receptor trigger cancer cell proliferation and inhibit cancer cell
apoptosis (92,98). Furthermore, there is a growing
interest in the different expression patterns of IGF-1 splice
transcripts in normal vs. pathological tissues in order to
determine the potential role of IGF-1 isoforms and immature IGF-1
products, and in particular, the role of IGF-1Ec in the development
of several pathological conditions (47,48,60,99,100).
The IGF-1Ec peptide is a cellular and secreted mitogen peptide,
which was originally identified in the liver and serves a critical
role in development and growth of the skeletal muscle (46,101–105).
IGF-1Ec may serve a role in skeletal and cardiac muscle
regeneration and hypertrophy after exercise-induced skeletal muscle
damage or myocardial infarction (106–108).
Milingos et al (20)
suggested that the expression of IGF-1Ec isoform may be associated
with the progression of endometriosis. In addition, it has been
shown that in prostate cancer cells the exogenous administration of
a synthetic 24-amino acid peptide of the COO-terminal of the
IGF-1Ec isoform (parts of exons 5 and 6 of the igf-1 gene)
were associated with the proliferation of neoplasmatic cells
(109). Therefore, there is strong
evidence that IGF-1Ec serve a pivotal role in stimulating somatic
cell proliferation and growth, in regulating differentiation and
migration, and reducing apoptosis during cancer cell growth
(21,22,32,35,58,59,66,110–113).
Furthermore, clinical studies have shown that treatment with
metformin exhibits antiproliferative effects and restrains
endometrial cancer growth (114,115).
In the present study, the expression of IGF-1Ec in
endometrial adenocarcinoma tissues was compared with adjacent
histologically normal tissues, endometrial hyperplasia and normal
endometrial tissues. Positive staining for IGF-1Ec was observed in
the primary endometrial tumors and hyperplastic endometrium, the
first study to show this, to the best of our knowledge. The
intensity or sum of IGF-1Ec expression in normal, hyperplastic and
malignant endometrium were also shown to differ. The continuous
expression of IGF-1Ec supports an autocrine and/or paracrine role
in the endometrial pathophysiology. This, it is hypothesized that
the different levels of expression of IGF-1Ec may be a critical
mediator of transformation from normal endometrium to hyperplastic
and then to cancerous. The upregulated expression of IGF-1Ec in
premalignant biopsy samples may be a marker of progression to
malignancy. Thus, IGF-1Ec upregulation may initiate malignant
endometrial transformation, highlighting the importance of IGF-1Ec
in progression of endometrial carcinoma. Continuous stimulation of
IGF-1Ec with a possible synergistic action with growth or survival
factors, oncogenes, tumor suppressor genes or hormones may result
in uncontrolled cell proliferation resulting in malignant
endometrial transformation. This hypothesis is supported by the
results of the present study, which showed significantly stronger
co-expression of IGF-1Ec with p53, PTEN or survivin in hyperplastic
vs. normal or cancerous vs. hyperplastic endometrium.
Interestingly, there was no statistically significant difference
between normal-adjacent and cancerous endometrium, suggesting that
in the histologically normal adjacent endometrium, the necessary
molecular alterations for its cancerous transformation may have
taken place. In addition, IGF-1Ec expression was significantly
higher in non-endometrioid (serous papillary or clear cell)
compared with endometrioid endometrial adenocarcinomas, emphasizing
the importance of IGF-1Ec expression in the development of
non-estrogen dependent carcinomas. Furthermore, expression of
IGF-1Ec in the presence of tumoral necrosis in endometrial
adenocarcinomas was significantly increased, suggesting an
association between IGF-1Ec expression and more aggressive
endometrial tumors, as it has been shown that tumoral necrosis is
strongly associated with aggressive behavior of endometrial cancer,
and is associated with hypoxia, angiogenesis and inflammation
response (116). Furthermore, there
was a positive correlation between the sum of staining-intensity
and the scores of IGF-1Ec immunopositive cells and the tumor grade
of endometrial carcinomas, suggesting that higher expression of
IGF-1Ec may contribute to a higher degree of differentiation during
endometrial carcinogenesis, and may be associated with the
malignant progression in these patients. The molecular pathway of
endometrial carcinogenesis is mediated by IGF-1Ec in high-grade
uterine endometrioid adenocarcinomas may be similar to that of
non-endometrioid (serous papillary and clear cell) endometrial
adenocarcinomas. This hypothesis is supported by the active
involvement of IGF-1Ec in the carcinogenesis of other types of
cancer. In particular, increased expression of IGF-1Ec has been
reported in prostate cancer, osteosarcomas, neuroendocrine
neoplasms and thyroid carcinomas (25,100,109,117–119).
Armakolas et al (109)
showed significantly higher expression of IGF-1Ec in prostate
tissues and human prostate cancer cell lines compared with normal
prostate tissues, and the normal prostate epithelial cells did not
express IGF-1Ec. Immunohistochemical analysis from prostate cancer
specimens showed that IGF-1Ec expression was significantly
positively associated with prostate cancer stage (117). A synthetic analogue of the Ec
domain, human Ec, has been shown to promote progression in
vitro in human prostate cancer PC-3 cells (118). Philippou et al (100) assessed the expression of IGF-1Ea,
IGF-1b and IGF-1Ec isoforms in human osteoblast-like osteosarcoma
MG-63 cells, and found that these cells expressed only the IGF-1Ea
and IGF-1Ec transcripts. Alexandraki et al (25) investigated IGF-1Ec expression in
neuroendocrine neoplasms and found cytoplasmic staining in 49% (23
out of 47 cases). Furthermore, samples from metastatic
neuroendocrine neoplasms exhibited significantly higher
immunohistochemical expression compared with primary tumors
(25). Karagiannis et al
(119) investigated the expression
of IGF-1Ec in thyroid cancer specimens using immunohistochemistry,
and found that the expression of IGF-1Ec was significantly
associated with advanced cancer stages, and with muscular and
capsule malignant invasion. Together, these findings support the
hypothesis that IGF-1Ec expression is directly involved in
tumorigenesis, although the exact mechanisms remain to be
determined. It has been proposed that Ec domain of the IGF-1Ec
isoform may possibly mediate its mitogenic bioactivity through
autonomous mechanisms, independent of IGF-1R and IR (100,109).
This hypothesis is supported by our findings, showing a strong
connection between high levels of IGF-1Ec expression and
non-endometrioid endometrial carcinomas, which develop
independently from estrogen action.
It has been shown that the IGF-1 pathway serves an
important role in the normal proliferation of epithelium and
stromal cells in normal menstruating or premenopausal endometrium
(63,120,121).
IGF-1 and IGF-2 promote cellular growth and differentiation, and
act as mediators of steroid hormones (122). Estrogen modulates IGF signaling by
increasing the expression of IGF-1 and activation of IGF-1R,
whereas progesterone increases the synthesis of IGFBPs to
antagonize estrogen-induced cell proliferation (20,27,28,34,64–66).
Zhou et al (123) found that
endometrial IGF-1 expression was maximal during the proliferative
phase, and not in the secretory phase of the menstrual cycle.
Results of various studies which evaluated the role of IGF-1 in the
biology of endometrial cancer are ambiguous and conflicting
(22). Rutanen et al
(27) found that the expression of
IGFBP-2, IGFBP-4 and IGFBP-5 in endometrial cancer tissues did not
differ from those in normal endometrium. Laatikainen et al
(124) found that the expression of
IGF-1 was similar in tamoxifen-treated and control patients. Elkas
et al (125) found that
IGF-1 and IGFBP-1 were expressed in normal and neoplastic
endometrium of all patients, regardless of tamoxifen treatment.
Mairano et al (126) showed
that the levels of IGF-1 and IGF-1R proteins measured by
immunohistochemistry were the same between normal and malignant
endometrium. However, O'Toole et al (127) found lowered IGF-1, ERa, ERb and
progesterone expression in endometrial carcinoma tissues compared
with normal premenopausal and postmenopausal endometrial samples.
IGF-1 is also modulated by interactions with growth factors
(128). Soufla et al
(129) showed that IGF-1 and
epidermal growth factor (EGF) down-regulation and simultaneous
fibroblast growth factor-2 up-regulation may be involved in
endometrial carcinogenesis. Furthermore, it has been shown that
upregulation of IGF-1R is correlated with expression of VEGF factor
C and this may be important in lymph node metastasis in patients
with endometrial adenocarcinoma (130).
PTEN is a tumor suppressor gene which modulates
cell proliferation and cell survival, and is frequently mutated or
deleted in several types of cancer. PTEN functions as a lipid
phosphatase and serves a key role in regulation of the PI3
kinase/Akt pathway. The IGF system serves an important role in cell
proliferation and cell survival via the PI3 kinase/AKT and MAP
kinase pathways in several neoplasmatic cells. In human gastric
adenocarcinoma cells, Yi et al (131) suggested that PTEN may inhibit the
anti-apoptotic properties of IGF. PTEN may block IGF-IGFR-induced
Akt activity and may also regulate the expression of members of the
IGF system, in particular through up-regulation of IGFBP-3. The
association between IGF-1R activation status and PTEN expression
status in cases of complex atypical endometrial hyperplasia and
endometrial carcinoma were investigated by McCamplell et al
(132), who suggested that elevated
levels of activated IGF-1R and concomitant PTEN may be associated
with an increased risk of either endometrial carcinoma or
subsequent development of endometrial carcinoma. In endometrial
cancer cells, Dong et al (133) showed that picropodophyllin, an
IGF-1R inhibitor in the growth of endometrial cancer, resulted in
down-regulation of IGF-1R phosphorylation and reduced cell
proliferation via the PI3K/Akt signaling pathway. In the present
study, there was a negative association between the proportion of
immunopositive cells (scores) or the sum of staining (scores and
intensity) and the coexistence of IGF-1Ec and PTEN expression.
Thus, IGF-1Ec and PTEN may work in opposite directions from each
other during endometrial carcinogenesis. These findings suggest
that cases with high IGF-1Ec staining score show decreased scores
of PTEN staining scores and vice versa. The molecular mechanism
underlying this negative association between these two mediators of
apoptosis remains to be determined. There was, a significant
association between the intensity of IGF-1Ec and PTEN positivity
with the histological type or the presence of lymph-vascular space
invasion. Therefore, these findings suggest that the opposite
relationship between IGF-1Ec and PTEN may result in a worse
prognosis during endometrial carcinogenesis.
The nuclear transcript p53 is a tumor suppressor
gene with strong apoptotic effects. There is considerable evidence
showing the interplay between the IGF signaling pathway and the
tumor suppressor gene p53. In prostate cancer cell lines in
vitro, Leung et al (134) suggested IGF-1 was potentially
down-regulated by p53. Tucci (135)
suggested a potential association between p53 and IGF-1 signaling
pathways in dietary caloric restriction-induced life extension; p53
may regulate longevity via TOR/insulin/IGF-1 signaling. Luo et
al (136) showed that prolonged
activation of IGF-1 signaling enhanced ameliorative hepatic
steatosis and fibrogenesis via inhibition of nuclear p53-progerin
interaction. A possible molecular connection between p53 and IGF-1
was also suggested by Tran et al (137) involving IGF-1-SIRT1-p53 signaling
in cellular senescence and aging. Attias-Geva et al
(138) assessed the involvement of
p53 in regulation of IGF-1R gene expression in uterine serous
carcinoma cell lines and found that wild type p53 repressed the
IGF-1R promoter. In the present study, there was no association
between the concomitant expression of IGF-1Ec and p53. However,
there was a significant association between the concomitant IGF-1Ec
and p53 stain intensity or the sum of their scores and staining
intensity with the histological type and/or the histological grade
of endometrial carcinomas; non-endometrioid carcinomas or Grade 3
endometrial adenocarcinomas showed concomitant high IGF-1Ec and p53
immunostaining. These results suggest that the co-expression of
IGF-1Ec and p53 serves an important role in the development of
non-endometrioid or high-grade endometrial adenocarcinoma.
Survivin is a unique member of the inhibitor of
apoptosis family of proteins, and regulates cell proliferation.
Survivin is upregulated in the majority of human tumors through
poorly defined mechanisms (139–144).
Additionally, activation of the Stat3 transcription pathway
determines the radio-sensitivity of cancer cells (145). In hepatocellular carcinoma cell
lines it has been shown that IGF-1 and survivin are able to
co-regulate metastasis; surviving may serve a vital role in the
IGF-1 signaling pathway by mediating epithelial-mesenchymal
transition (EMT) through the up-regulation of the expression of EMT
markers, and the knockdown of surviving expression may suppress
metastasis of cancer cells (78). In
addition, in prostate cancer cells a positive correlation between
IGF and survivin was observed. IGF-1/mTOR signaling may increase
survivin-expression via rapid changes in mRNA translation (146). Furthermore, in rat prostatic
epithelial cells, it has been shown that IGF-1 signaling increased
the expression of survivin via a PI3K/Akt/mTOR1 pathway and
promoted cell growth by suppressing Smad-dependent autocrine
transforming growth factor-β signaling (147). In renal cell lines, Sato et
al (148) suggested that cell
proliferation and survivin expression were induced by IGF-1. In
pancreatic cancer cells, IGF-1 may promote radio-resistance through
activation of the Stat3 pathway (145). Oh et al (149) suggested that treatment with a
farnesyltransferase inhibitor in head and neck squamous cell
carcinoma and non-small cell lung cancer cell lines activated the
IGF-1R/PI3-kinase/Akt signaling pathway, leading to increased
mTOR-mediated protein synthesis of survivin. Morgillo et al
(150) suggested that enhanced
synthesis of survivin protein mediated by the IGF-1R/EGFR
heterodimer counteracts the antitumor action of erlotinib, an EGFR
tyrosine kinase inhibitor in human NSCLC cell lines. In particular,
erlotinib induced heterodimerization of IGF-1R/EGFR, and stimulated
the IGF-1R and PI3K/Akt/mTOR1 pathways resulting in an increase in
EGFR and survivin protein expression; however, assessment of
combined treatment with erlotinib and inhibitors of IGF-1R or mTOR
resulted in suppression of survivin and EGFR expression (150). In the present study, a positive
association was observed between the sum of staining (scores and
intensity) and the coexistence of IGF-1Ec and survivin expression.
Thus, IGF-1Ec and survivin may promote endometrial carcinogenesis
together, regulating in parallel each other's actions, and
therefore may share a common molecular pathway. In addition, in the
present study, there was an association between the scores of
immunohistochemical concomitant expression of IGF-1Ec and survivin
and the clinical stage, histological differentiation,
lymph-vascular space invasion and the presence of tumoral necrosis.
Furthermore, in the case of concomitant expression of IGF-1Ec and
survivin, the sum of scores and staining intensity of
immunopositive cells was associated with the histological
differentiation, or the presence of tumoral necrosis. These results
suggest the importance of further studying the coexistence of
IGF-1Ec and survivin in the development of more aggressive
endometrial carcinomas.
The development of endometrial adenocarcinoma is
complicated and is based on multiple steps. The mapping of IGF-1Ec
in normal, hyperplastic and malignant endometrium allows for a
better understanding of endometrial carcinogenesis. Continuous
endometrial stimulation of IGF-1Ec with concomitant expression of
PTEN, p53 and survivin may initiate uncontrolled cell proliferation
and result in malignant transformation. The results of the present
study highlight the importance of IGF-1Ec expression in the
progression of endometrial carcinoma, which progresses from normal
endometrium to hyperplasia and then on to carcinoma. In addition,
the different levels of IGF-1Ec expression in normal, hyperplastic
and malignant endometrium, and the synergistic action of IGF-1Ec
with p53, PTEN or survivin observed in the present study support
the autocrine and/or paracrine role with growth factors, survival
factors, oncogenes and/or tumor suppressor genes during the
endometrial pathophysiology.
The upregulated expression of IGF-1Ec in
non-endometrioid (serous papillary or clear cell) compared with
endometrioid adenocarcinomas emphasize the importance of IGF-1Ec
expression in the development of non-estrogen dependent carcinomas.
Furthermore, the upregulated expression of IGF-1Ec in the presence
of tumoral necrosis in endometrial adenocarcinomas suggest an
association between IGF-1Ec expression and more aggressive
endometrial tumors. The present study showed that upregulation of
IGF-1Ec is associated with a high histological grade in endometrial
carcinoma, and supports the hypothesis that IGF-1Ec serves an
important role in differentiation and progression of endometrial
carcinogenesis. Additionally, the present study is the first to
show that the molecular pathway of endometrial carcinogenesis is
similar between high grade and non-endometrioid (serous papillary
and clear cell) uterine endometrial adenocarcinomas; and this
pathway may be activated by the IGF-1Ec upregulation. These results
suggest that higher levels of IGF-1Ec expression are associated
with more aggressive behavior in endometrial adenocarcinomas. These
findings reflect the biological behavior and spreading patterns of
endometrial carcinomas which may in turn allow for more accurate
prognostic prediction. Although our study shows for the first time
a potential role of high scores of IGF-1Ec in non-endometrioid
(serous papillary or clear cell) compared to endometrioid
endometrial carcinomas, it also has a number of limitations
including the relative low number of studied cases of clear cell
and serous papillary compared to endometrioid adenocarcinomas (13
vs. 86 cases, respectively). In addition, the experimental design
of our study did not allow fresh frozen endometrial tissue
collection for analysis of the IGF-1Ec mRNA expression.
Furthermore, the research design of the present study did not
consider the examination of hormone receptor expression in
endometrial tumors for confirmation of our statement on
hormone-insensitivity.
In conclusion, the concomitant expression between
IGF-1Ec and PTEN, p53 and survivin highlights the molecular
mechanisms which may be involved in endometrial carcinogenesis.
IGF-1Ec and PTEN may act together opposing direction from each
other during endometrial carcinogenesis. The inverse association
between the intensity of IGF-1Ec and PTEN positivity, and the
histological type and the presence of lymph-vascular space invasion
may suggest a regulatory role of IGF-1Ec in modulating PTEN
expression, resulting in a worse prognosis during endometrial
carcinogenesis. In contrast, the positive correlation between
IGF-1Ec and survivin suggests that these molecules may share a
common molecular pathway, and may act in parallel as potential
mediators during endometrial carcinogenesis. A combination of high
positive levels of IGF-1Ec expression and survivin expression was
associated with a high clinical stage, high grade of histological
differentiation, presence of lymph-vascular space invasion and
presence of tumoral necrosis. These findings suggest that IGF-1Ec
and survivin work cooperatively resulting in the development of
more aggressive endometrial carcinomas. Finally, the association
between the concomitant IGF-1Ec and p53 staining intensity or the
sum of their scores and staining intensity, and the histological
types or the histological grade was shown. It is hypothesized that
the concomitant upregulation of IGF-1Ec and p53 serves an important
role in the development of non-endometrioid endometrial carcinomas
and in the progression to high grade endometrial tumors.
Acknowledgements
The present study is part of a thesis for a Doctor
of Philosophy (PhD) in Obstetrics and Gynecology, Medical School,
Kapodistrian University of Athens, Greece for Mr. Aggelis
Stavropoulos
Funding
Not applicable.
Availability of data and materials
The datasets used and/or analyzed during this study
are available from the corresponding author on reasonable
request.
Authors' contributions
TV and AT were responsible for the provision of the
study materials. TV, AT, VKV and FNV were responsible for the
collection and assembly of the data. AS, MV, AP, TV, VKV, FNV, AT,
ACL and MK performed the data analysis and interpretation. AS, MV,
AP, TV, VKV, FNV, AT, ACL and MK contributed to the writing,
drafting, revising, editing, reviewing and the conception and
design of the study. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Medical School of Kapodistrian University of Athens, Greece. All
patients provided written informed consent before the study for
participation.
Patient consent for publication
All patients included in this study at the time of
data collection provided consent for their data to be used in this
publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Creasman WT, Odicine F, Maisonneuve P,
Quinn MA, Beller U, Benedet JL, Heintz AP, Ngan HY and Pecorelli S:
Carcinoma of the corpus uteri. FIGO 26th annual report on the
results of treatment in gynecological cancer. Int J Gynaecol
Obstet. 95 (Suppl 1):S105–S143. 2006. View Article : Google Scholar
|
|
2
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Doll A, Abal M, Rigau M, Monge M, Gonzalez
M, Demajo S, Colás E, Llauradó M, Alazzouzi H, Planagumá J, et al:
Novel molecular profiles of endometrial cancer-new light through
old windows. J Steroid Biochem Mol Biol. 108:221–229. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bray F, Dos Santos Silva I, Moller H and
Weiderpass E: Endometrial cancer incidence trends in Europe:
Underlying determinants and prospects for prevention. Cancer
Epidemiol Biomarkers Prev. 14:1132–1142. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kourea HP, Nikolaou M, Tzelepi V, Adonakis
G, Kardamakis D, Tsapanos V, Scopa CD, Kalofonos C and Decavalas G:
Expression of phosphorylated Akt, mTOR and MAPK in type I
endometrial carcinoma: Clinical significance. Anticancer Res.
35:2321–2331. 2015.PubMed/NCBI
|
|
6
|
Shevra CR, Ghosh A and Kumar M: Cyclin D1
and Ki-67 expression in normal, hyperplastic and neoplastic
endometrium. J Postgrad Med. 61:15–20. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Morice P, Leary A, Creutzberg C,
Abu-Rustum N and Darai E: Endometrial cancer. Lancet.
387:1094–1108. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Takeshita S, Yamashita Y, Shiomi K, Suzuki
N, Yoshida J, Naiki-Ito A, Suzuki S, Akatsuka S, Toyokuni S,
Takahashi T, et al: Expression of P-REX2a is associated with poor
prognosis in endometrial malignancies. Oncotarget. 9:24778–24786.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bokhman JV: Two pathogenetic types of
endometrial carcinoma. Gynecol Oncol. 15:10–17. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Parazzini F, La Vecchia C, Bocciolone L
and Franceschi S: The epidemiology of endometrial cancer. Gynecol
Oncol. 41:1–16. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Steiner E, Eicher O, Sagemuller J, Schmidt
M, Pitch H, Tanner B, Hengstler JG, Hofmann M and Knapstein PG:
Multivariate independent prognostic factors in endometrial
carcinoma: A clinicopathological study in 181 patients: 10
experience at the department of obstetrics and gynecology of the
mainz university. Int J Gynecol Cancer. 13:197–203. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Prat J: Prognostic parameters of
endometrial carcinoma. Hum Pathol. 35:649–662. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Daughaday WH and Rotwein P: Insulin-like
factors I and II. Peptide, messenger ribonucleic acid and gene
structures, serum and tissue concentrations. Endocr Rev. 10:68–91.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
De Meyts P and Whittaker J: Structural
biology of insulin and IGF1 receptors: Implications for drug
design. Nat Rev Drug Discov. 1:769–783. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Federici M, Porzio O, Zucaro L, Giovannone
B, Borboni P, Marini MA, Lauro D and Sesti G: Increased abundance
of insulin/IGF-I hybrid receptors in adipose tissue from NIDDM
patients. Mol Cell Endocrinol. 135:41–47. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nakae J, Kido Y and Accili D: Distinct and
overlapping functions of insulin and IGF-I receptors. Endocr Rev.
22:818–835. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Baserga R, Peruzzi F and Reiss K: The
IGF-1 receptor in cancer biology. Int J Cancer. 107:873–877. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Taguchi A and White MF: Insulin-like
signaling, nutrient homeostasis, and life span. Annu Rev Physiol.
70:191–212. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Samani AA, Yakar S, LeRoith D and Brodt P:
The role of the IGF system in cancer growth and metastasis:
Overview and recent insights. Endocr Rev. 28:20–47. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Milingos DS, Philippou A, Armakolas A,
Papageorgiou E, Sourla A, Protopapas A, Liapi A, Antsaklis A,
Mastrominas M and Koutsilieris M: Insulin-like growth factor-1Ec
(MGF) expression in eutropic and ectopic endometrium:
Characterization of the MGF E-peptide actions in vitro. Mol Med.
17:21–28. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jones JI and Clemmons DR: Insulin-like
growth factors and their binding proteins: Biological actions.
Endocr Rev. 13:3–34. 1995. View Article : Google Scholar
|
|
22
|
Majchrzak-Baczmańska D and Malinowski A:
Does IGF-1 play a role in the biology of endometrial cancer?
Ginekol Pol. 87:598–604. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Flannery CA, Saleh FL, Choe GH, Selen DJ,
Kodaman PH, Kliman HJ, Wood TL and Taylor HS: Differential
expression of IR-A, IR-B and IGF-1R in endometrial physiology and
distinct signature in adenocarcinoma. J Clin Endocrinol Metab.
101:2883–2891. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dai C, Li N, Song G, Yang Y and Ning X:
Insulin-like growth factor 1 regulates growth of endometrial
carcinoma through PI3k signaling pathway in insulin-resistant type
2 diabetes. Am J Transl Res. 8:3329–3336. 2016.PubMed/NCBI
|
|
25
|
Alexandraki KI, Philippou A, Boutzios G,
Theohari I, Koutsilieris M, Delladetsima IK and Kaltsas GA: IGF-IEc
expression is increased in secondary compared to primary foci in
neuroendocrine neoplasms. Oncotarget. 8:79003–79011. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mazerbourg S and Monget P: Insulin-like
growth factor binding proteins and IGFBP proteases: A dynamic
system regulating the ovarian folliculogenesis. Front Endocrinol
(Lausanne). 9:1342018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rutanen EM, Nyman T, Lehtovirta P, Ammala
M and Pekonen F: Suppressed expression of insulin-like growth
factor binding protein-1 mRNA in the endometrium: A molecular
mechanism associating endometrial cancer with its risk factors. Int
J Cancer. 59:307–312. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Suvanto-Luukkonen E, Sundostrom H,
Penttinen J, Kauppila A and Rutane EM: Insulin-like growth
factor-binding protein-1: A biochemical marker of endometrial
response to progestin during hormone replacement therapy.
Maturitas. 22:255–262. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Vassilakos G, Philippou A, Tsakiroglou P
and Koutsilieris M: Biological activity of the e domain of the
IGF-1Ec as addressed by synthetic peptides. Hormones (Athens).
13:182–196. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Philippou A, Maridaki M, Pneumaticos S and
Koutsilieris M: The complexity of the IGF1 gene splicing,
posttranslational modification and bioactivity. Mol Med.
20:202–214. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kornfeld S: Structure and function of the
mannose-6-phosphate/insulinlike growth factor II receptors. Annu
Rev Biochem. 61:307–330. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
LeRoith D and Roberts CT Jr: The
insulin-like growth factor system and cancer. Cancer Lett.
195:127–137. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Baxter RC and Martin JL: Binding proteins
for the insulin-like growth factors: Structure, regulation and
function. Prog Growth Factor Res. 1:49–68. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Murphy LJ and Ghahary A: Uterine
insulin-like growth factor-1: Regulation of expression and its role
in estrogen-induced uterine proliferation. Endocr Rev. 11:443–453.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yu H and Rohan T: Role of the insulin-like
growth factor family in cancer development and progression. J Natl
Cancer Inst. 92:1472–1489. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mourmouras N, Philippou A, Christopoulos
P, Kostoglou K, Grivaki C, Konstantinidis C, Serafetinides E,
Delakas D and Koutsilieris M: Different expression of IGF-I
transcripts in bladder cancer. Anticancer Res. 38:3453–3459. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kelley KM, Oh Y, Gargosky SE, Gucev Z,
Matsumoto T, Hwa V, Ng L, Simpson DM and Rosenfeld RG: Insulin-like
growth factor-binding proteins (IBFBPs) and their regulatory
dynamics. Int J Biochem Cell Biol. 28:619–637. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Clemmons DR: Insulin-like growth factor
binding proteins and their role in controlling IGF actions.
Cytokine Growth Factor Rev. 8:45–62. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Burroughs KD, Donn SE, Barrett JC and
Taylor JA: Insulin-like growth factor-I: A key regulator of human
cancer risk? J Natl Cancer Inst. 91:579–581. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Adamo ML, Ben-Hur H, LeReith D and Roberts
CT Jr: Transcription initiation in the two leader exons of the rat
IGF-1 gene occurs from disperse versus localized sites. Biochem
Biophys Res Commun. 176:887–893. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Adamo ML, Neuenschwander S, LeRoith D and
Roberts CT Jr: Structure, expression, and regulation of the IGF-I
gene. Adv Exp Med Biol. 343:1–11. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Adamo ML: Regulation of insulin-like
growth factor I gene expression. Implications for normal and
pathological growth. Diabetes Rev. 3:2–27. 1995.
|
|
43
|
Simmons JG, Van Wyk JJ, Hoyt EC and Lund
PK: Multiple transcription start sites in the rat insulin-like
growth factor-I gene give rise to IGF-I mRNAs that encode different
IGF-I precursors and are processed differently in vitro. Growth
Factors. 9:205–221. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Bell GI, Stempien MM, Fong NM and Rall LB:
Sequences of liver cDNAs encoding two different mouse insulin-like
growth factor 1 precursors. Nucleic Acids Res. 14:7873–7882. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Temmerman L, Slonimsky E and Rosenthal N:
Class 2 IGF-1 isoforms are dispensabile for viability, growth and
maintenance of IGF-1 serum levels. Growth Horm IGF Res. 20:255–263.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Matheny RW Jr, Nindl BC and Adamo ML:
Minireview: Mechano-growth factor: A putative product of IGF-I gene
expression involved in tissue repair and regeneration.
Endocrinology. 151:865–875. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kasprzak A, Szaflarski W, Szmeja J,
Andrzejewska M, Przybyszewska W, Kaczmarek E, Koczorowska M,
Kościński T, Zabel M and Drews M: Differential expression of IGF-1
mRNA isoforms in colorectal carcinoma and normal colon tissue. Int
J Oncol. 42:305–316. 2013.PubMed/NCBI
|
|
48
|
Philippou A, Armakolas A and Koutsilieris
M: Evidence for the possible biological significance of the igf-1
gene alternative splicing in prostate cancer. Front Endocrinol
(Lausanne). 4:312013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Jansen M, van Schaik FM, Richer AT,
Bullock B, Woods DE, Gabbay KH, Nauubaum AL, Sussenbach JS and Van
den Brande JL: Sequence of cDNA encoding human insulin-like growth
factor I precursor. Nature. 306:609–611. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Brisson BK, Spinazzola J, Park S and
Barton ER: Viral expression of insulin-like growth factor I
E-peptide increases skeletal muscle mass but at the expense of
strength. Am J Physiol Endocrinol Metab. 306:E965–E974. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Rotwein P, Pollock KM, Didier DK and Krivi
GG: Organization and sequence of the human insulin-like growth
factor I gene. Alternative RNA processing produces two insulin-like
growth factor I precursor peptides. J Biol Chem. 261:4828–4832.
1986.PubMed/NCBI
|
|
52
|
Duguay SJ: Post-translational processing
of insulin-like growth factors. Horm Metab Res. 31:43–49. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Barton ER: The ABCs of IGF-I isoforms:
Impact on muscle hypertrophy and implications for repair. Appl
Physiol Nutr Metab. 31:791–797. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wallis M: New insulin-like growth factor
(IGF)-precursor sequences from mammalian genomes: The molecular
evolution of IGFs and associated peptides in primates. Growth Horm
IGF Res. 19:12–23. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Siegfriend JM, Kasprzyk PG, Treston AM,
Mulshine JL, Quinn KA and Cuttitta F: A mitogenic peptide amide
encoded within the E peptide domain of the insulin-like growth
factor IB prohormone. Proc Natl Acad Sci USA. 89:8107–8111. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Kuo YH and Chen TT: Novel activities of
pro-IGF-I E peptides: Regulation of morphological differentiation
and anchorage-independent growth in human neuroblastoma cells. Exp
Cell Res. 280:75–89. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Chew SL, Lavender P, Clark AJ and Ross RJ:
An alternatively spliced human insulin-like growth factor-1
transcript with hepatic tissue expression that diverts away from
mitogenic IBE1 peptide. Endocrinology. 136:1939–1944. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Tian XC, Chen MJ, Pantschenko AG, Yang TJ
and Chen TT: Recombinant E-peptides of pro-IGF-I have mitogenic
activity. Endocrinology. 140:3387–3390. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Chen MJ, Chiou PP, Lin P, Lin CM, Siri S,
Peck K and Chen TT: Suppression of growth and cancer-induced
angiogenesis of aggressive human breast cancer cells (MDA-MB-231)
on the chorionallantoic membrane of developing chicken embryos by
E-peptide of pro-IGF-I. J Cell Biochem. 101:1316–1327. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Armakolas A, Dimakakos A, Loucogiannaki C,
Armakolas N, Antonopoulos A, Florou C, Tsioli P, Papageorgiou E,
Alexandrou TP, Stathaki M, et al: IL-6 is associated to IGF-1Ec
upregulation and Ec peptide secretion, from prostate tumors. Mol
Med. 24:62018. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Garrouste F, Remacle-Bonnet M, Fauriat C,
Marvaldi J, Luis J and Pommier G: Prevention of cytokine-induced
apoptosis by insulin-like growth factor-I is independent of cell
adhesion molecules ijn HT29-D4 colon carcinoma cells-evidence for a
NF-kappaB-dependent survival mechanism. Cell Death Differ.
9:768–779. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Alexia C, Fallot G, Lasfer M,
Schweizer-Groyer G and Groyer A: An evaluation of the role of
insulin-like growth factors (IGF) and of type-I IGF receptor
signaling in hepatocarcinogenesis and in the resistance of
hepatocarcinoma cells against drug-induced apoptosis. Biochem
Pharmacol. 86:1003–1015. 2004. View Article : Google Scholar
|
|
63
|
Wang HS and Chard T: IGFs and IGF-binding
proteins in the regulation of human ovarian and endometrial
function. J Endocrinol. 161:1–13. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Bogdanos J, Karamanolakis D, Tenta R,
Tsintavis A, Milathianakis C, Mitsiades C and Koutsilieris M:
Endocrine/paracrine/autocrine survival factor activity of bone
microenviroment participates in the development of androgen
ablation and chemotherpay refractoriness of prostate cancer
metastasis in skeleton. Endocr Relat Cancer. 10:279–289. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Koutsilieris M: Pathophysiology of uterine
leiomyomas. Biochem Cell Biol. 70:273–278. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Koutsilieris M, Mitsiades C and Sourla A:
Insulin-like growth factor I and urokinase-type plasminogen
activator bioregulation system as a survival mechanism of prostate
cancer cells in osteoblastic metastases: Development of
anti-survival factor therapy for hormone-reflactory prostate
cancer. Mol Med. 6:251–267. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Hernandez AV, Pasupuleti V, Benites-Zapata
VA, Thota P, Deshpande A and Perez-Lopez FR: Insulin resistance and
endometrial cancer risk: A systemic review and meta-analysis. Eur J
Cancer. 51:2747–2758. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Pavelic J, Radakovic B and Pavelic K:
Insulin-like growth factor 2 and its receptors (IGF 1R and IGF
2R/mannose 6-phospate) in endometrial adenocarcinoma. Gynecol
Oncol. 105:727–735. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Stavropoulos A, Varras M, Vasilakaki T,
Varra VK, Tsavari A, Varra FN, Nonni A, Kavantzas N and Lazaris AC:
Expression of p53 and PTEN in human primary endometrial carcinomas:
Clinicopathological and immunohistochemical analysis and study of
their concomitant expression. Oncol Lett. 17:4575–4589.
2019.PubMed/NCBI
|
|
70
|
Stavropoulos A, Varras M, Vasilakaki T,
Varra VK, Varra FN, Tsavari A, Nonni A, Kavantzas N and Lazaris AC:
Expression of anti-apoptotic protein survivin in human endometrial
carcinoma: Clinical and pathological correlations as a separate
factor and in combination with concomitant PTEN and p53 expression.
Oncol Lett. 20:1033–1054. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Galimi F, Torti D, Sassi F, Isella C, Corà
D, Gastaldi S, Ribero D, Muratore A, Massucco P, Siatis D, et al:
Genetic and expression analysis of MET, MACC1, and HGF in
metastatic colorectal cancer: Response to met inhibition in patient
xenografts and pathologic correlations. Clin Cancer Res.
17:3146–3156. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Li M, Xin X, Wu T, Hua T and Wang H and
Wang H: Stromal cells of endometrial carcinoma promotes
proliferation of epithelial cells through the HGF/c-Met/Akt
signaling pathway. Tumour Biol. 36:6239–6248. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Harpaz N, Taboada S, Ko HM, Yu J, Yang Q,
Xu H and Cao W: Expression of MACC1 and MET in inflammatory bowel
disease-associated colonic neoplasia. Inflamm Bowel Dis.
20:703–711. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Zhang Q, Xu P, Lu Y and Dou H: Correlation
of MACC1/c-Myc expression in endometrial carcinoma with
clinical/pathlogical features or prognosis. Med Sci Monit.
24:4738–4744. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Yang E and Yan H: Expression of ELF5 in
endometrial carcinoma tissues and its clinical significance. Oncol
Lett. 16:3473–3480. 2018.PubMed/NCBI
|
|
76
|
Singh N, Hirschowitz L, Zaino R,
Alvarado-Cabrero I, Duggan MA, Ali-Fehmi R, Euscher E, Hecht JL,
Horn LC, Ioffe O, et al: Pathologic prognostic factors in
endometrial carcinoma (other than tumor type and grade). Int J
Gynecol Pathol. 38 (Suppl 1):S93–S113. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Sadlecki P, Bodnar M, Grabiec M, Marszalek
A, Walentowicz P, Sokup A, Zegarska J and Walentowicz-Sadlecka M:
The role of Hypoxia-inducible factor-1α, Glucose thransporter-1,
(GLUT-1) and carbon anhydrase IX in endometrial cancer patients.
Biomed Res Int. 2014:6168502014. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Liu F, Sun Y, Liu B, Lu J, Li H, Zhu H,
Gao H, Zhou X and Chang H: Insulin-like growth factor-1 induces
epithelial-mesenchymanl transition in hepatocellular carcinoma by
activating survivin. Oncol Rep. 40:952–958. 2018.PubMed/NCBI
|
|
79
|
Bansal N, Yendluri V and Wenham RM: The
molecular biology of endometrial cancers and the implications for
pathogenesis, classification, and targeted therapies. Cancer
Control. 16:8–13. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Llauradó M, Ruiz A, Majem B, Ertekin T,
Colás E, Pedrola N, Devis L, Rigau M, Sequeiros T, Montes M, et al:
Molecular bases of endometrial cancer: New roles for new actors in
the diagnosis and the therapy of the disease. Mol Cell Endocrinol.
358:244–255. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Meng X, Dizon DS, Yang S, Wang X, Zhu D,
Thiel KW and Leslie KK: Strategies for molecularly enhanced
chemotherpay to achieve synthetic lethality in endometrial tumors
with mutant p53. Obstet Gynecol Int. 2013:8281652013. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Sugihara T: Loss of adherens junction
protein E-cadherin is a biomarker of high grade histology and poor
prognosis in endometrial cancer. Ann Clin Lab Res. 4:12016.
View Article : Google Scholar
|
|
83
|
Gómez-Macías GS, Garza-Rodríguez ML,
Garza-Guajardo R, Monsiváis-Ovalle D, Ancer-Rodríguez J,
Barrera-Saldaña HA and Barboza-Quintana O: Overexpression of the
matrix metalloproteinase 11 gene is a potential biomarker for type
1 endometrial cancer. Oncol Lett. 16:1073–1078. 2018.PubMed/NCBI
|
|
84
|
Buchynska LG, Brieiva OV and Iurchenko NP:
Assessment of HER-2/neu, c-MYC and CCNE1 gene copy number
variations and protein expression in endometrial carcinomas. Exp
Oncol. 41:138–143. 2019.PubMed/NCBI
|
|
85
|
Reaven GM: Banting lecture 1988. Role of
insulin resistance in human disease. Diabetes. 37:1595–1607. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Lee DY and Lee TS: Associations between
metabolic syndrome and gynecologic cancer. Obstet Gynecol Sci.
63:215–224. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Esposito K, Capuano A and Giugliano D:
Metabolic syndrome and cancer: Holistic or reductionist? Endocrine.
45:362–364. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Esposito K, Chiodini P, Colao A, Lenzi A
and Giugliano D: Metabolic syndrome and risk of cancer: A
systematic review and meta-analysis. Diabetes Care. 35:2402–2411.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Trabert B, Wentzensen N, Felix AS, Yang
HP, Sherman ME and Brinton LA: Metabolic syndrome and risk of
endometrial cancer in the united states: A study in the
SEER-medicare linked database. Cancer Epidemiol Biomarkers Prev.
24:261–267. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Jee SH, Kim HJ and Lee J: Obesity, insulin
resistance and cancer risk. Yonsei Med J. 46:449–455. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
van Kruijsdijk RC, van der Wall E and
Visseren FL: Obesity and cancer: The role of dysfunctional adipose
tissue. Cancer Epidemiol Biomarkers Prev. 18:2569–2578. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Iyengar NM, Hudis CA and Dannenberg AJ:
Obesity and cancer: Local and systemic mechanisms. Annu Rev Med.
66:297–309. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Yagen JD and Davidson NE: Estrogen
carcinogenesis in breast cancer. N Engl J Med. 354:270–282. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
André F, Rigot V, Remacle-Bonnet M, Luis
J, Pommier G and Marvaldi J: Protein kinases C-gamma and -delta are
involved in insulin-like growth factor I-induced migration of
colonic epithelial cells. Gastroenterology. 116:64–77. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
André F, Rigot V, Thimonier J, Montixi C,
Parat F, Pommier G, Marvaldi J and Luis J: Integrins and E-cadherin
cooperate with IGF-I to induce migration of epithelial colonic
cells. Int J Cancer. 83:497–505. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Akagi Y, Liu W, Zebrowski B, Xie K and
Ellis LM: Regulation of vascular endothelial growth factor
expression in human colon cancer by insulin-like growth factor-I.
Cancer Res. 58:4008–4014. 1998.PubMed/NCBI
|
|
97
|
Wu Y, Yakar S, Zhao L, Hemminghausen L and
LeRoith D: Circulating insulin-like growth factor-I levels regulate
colon cancer growth and metastasis. Cancer Res. 62:1030–1035.
2002.PubMed/NCBI
|
|
98
|
Fader AN, Arriba LN, Frasure HE and von
Gruenigen VE: Endometrial cancer and obesity: Epidiomiology,
biomarkers, prevention and survivorship. Gynecol Oncol.
114:121–127. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Koczorowska MM, Kwasniewska A and
Gozdzicka-Jozefiak A: IGF1 mRNA isoform expression in the cervix of
HPV-positive woman with pre-cancerous and cancerous lesions. Exp
Ther Med. 2:149–156. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Philippou A, Armakolas A, Pantleakou Z,
Pissimissis N, Nezos A, Theos A, Kaparelou M, Armakolas N,
Pneumaticos SG and Koutsilieris M: IGFF1Ec expression in MG-63
human osteoblast-like osteosarcoma cells. Anticancer Res.
31:4259–4265. 2011.PubMed/NCBI
|
|
101
|
McKoy G, Ashley W, Mander J, Yang SY,
Williams N, Russell B and Goldspink G: Expression of insulin-like
growth factor-1 splice variants and structural genes in rabbit
skeletal muscle induced by stretch and stimulation. J Physiol.
516:583–592. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Hameed M, Orrell RW, Cobbold M, Goldspink
G and Harridge SD: Expression of IGF-I splice variants in young and
old human skeletal muscle after high resistance exercise. J
Physiol. 547:247–254. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Hameed M, Lange KH, Andersen JL,
Schjerling P, Kjaer M, Harrigde SD and Goldspink G: The effect of
recombinant human growth hormone and resistance training of IGF-I
mRNA expression in the muscles of elderly men. J Physiol.
555:231–240. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Bickel CS, Slade J, Mahoney E, Haddad F,
Dudley GA and Adams GR: Time course of molecular responses of human
skeletal muscle to acute bouts or resistance exercise. J Appl
Physiol (1985). 98:482–488. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Kim JS, Cross JM and Bamman MM: Impact of
resistance loading on myostatin expression and cell cycle
regulation in young and older men and women. Am J Physiol
Endocrinol Metab. 288:E1110–E1119. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Philippou A, Papageorgiou E, Bogdanis G,
Halapas A, Sourla A, Maridaki M, Pissimissis N and Koutsilieris M:
Expression of IGF-1 isoforms after exercise-induced muscle damage
in humans: Characterization of the MGF E peptide actions in vitro.
In Vivo. 23:567–575. 2009.PubMed/NCBI
|
|
107
|
Carpenter V, Matthews K, Devlin G, Stuart
S, Jensen J, Conaglen J, Jeanplong F, Goldspink P, Yang SY,
Goldspink G, et al: Mechano-growth factor reduces loss of cardiac
function in acute myocardial infarction. Heart Lung Circ. 17:33–39.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Stavropoulou A, Halapas A, Sourla A,
Philippou A, Papageorgiou E, Papalois A and Koutsilieris M: IGF-1
expression in infarcted myocardium and MGF E peptide actions in rat
cardiomyocytes in vitro. Mol Med. 15:127–135. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Armakolas A, Philippou A, Panteleakou Z,
Nezos A, Sourla A, Petraki C and Koutsilieris M: Preferential
expression of IGF-IEc (MGF) transcript in cancerous tissues of
human prostate: Evidence for a novel and autonomous growth factor
activity of MGF E peptide in human prostate cancer cells. Prostate.
70:1233–1242. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Liu JP, Baker J, Perkins AS, Robertson EJ
and Efstratiadis A: Mice carrying null mutations of the genes
encoding insulin-like growth factor I (Igf-1) and type 1 IGF
receptor (Igf1r). Cell. 75:59–72. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Siddle K, Ursø B, Niesler CA, Cope DL,
Molina L, Surinya KH and Soos MA: Specificity in ligand binding and
intracellular signaling by insulin and insulin-like growth factor
receptors. Biochem Soc Trans. 29:513–525. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Laviola L, Natalicchio A and Giorgiono F:
The IGF-I signaling pathway. Curr Pharm Des. 13:663–669. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Barton ER, Park S, James JK, Makarewich
CA, Philippou A, Eletto D, Lei H, Brisson B, Ostrovsky O, Li Z and
Argon Y: Deletion of muscle GRP94 impairs both muscle and body
growth by inhibiting local IGF production. FASEB J. 26:3691–3702.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Wu JW, Boudreau DM, Park Y, Simonds NI and
Freedman AN: Commonly used diabetes and cardiovascular medications
and cancer recurrence and cancer-specific mortality: A review of
the literature. Expert Opin Drug Saf. 13:1071–1099. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Zhang ZJ and Li S: The prognostic value of
metformin for cancer patients with concurrent diabetes: A
systematic review and meta-analysis. Diabetes Obes Metab.
16:707–710. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Bredholt G, Mannelqvist M, Stefansson IM,
Birkeland E, Bø TH, Øyan AM, Trovik J, Kalland KH, Jonassen I,
Salvesen HB, et al: Tumor necrosis is an important hallmark of
aggressive endometrial cancer and associates with hypoxia,
angiogenesis and inflammation responses. Oncotarget. 6:39676–39691.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Armakolas A, Kararelou M, Dimakakos A,
Papageorgiou E, Armakolas N, Antonopoulos A, Petraki C, Lekarakou
M, Lelovas P, Stathaki M, et al: Oncogenic role of the Ec peptide
of the IGF-1Ec isoform in prostate cancer. Mol Med. 21:167–179.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Papageorgiou E, Philippou A, Armakolas A,
Christopoulos PF, Dimakakos A and Koutsilieris M: The human Ec
peptide: The active core of a progression growth factor with
species-specific mode of action. Hormones (Athens). 15:423–434.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Karagiannis AK, Philippou A,
Tseleni-Balafouta S, Zevolis E, Nakouti T, Tsopanomichalou-Gklotsou
M, Psarras V and Koutsilieris M: IGF-IEc expression is associeted
with advanced differentied thyroid cancer. Anticancer Res.
39:2811–2819. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Irwin JC, de las Fuentes L, Dsupin BA and
Giudice LC: Insulin-like growth factor regulation of human
endometrial stromal cell function: Coordinate effects on
insulin-like growth factor binding protein-1, cell proliferation
and prolactin secretion. Regul Pept. 48:165–177. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Tang XM, Rossi MJ, Mastrerson BJ and
Chegini N: Insulin-like growth factor I (IGF-I), IGF-I receptors,
and IGF binding proteins 1–4 in human uterine tissue: Tissue
localization and IGF-I action in endometrial stromal and myometrial
smooth muscle cells in vitro. Biol Reprod. 50:1113–1125. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Giudice LC, Dsupin BA, Jin IH, Vu TH and
Hoffman AF: Differential expression of messenger ribonucleic acids
encoding insulin-like growth factors and their receptors in human
uterine endometrium and decidua. J Clin Endocrinol Metab.
76:1115–1122. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Zhou J, Dsupin BA, Giudice LC and Bondy
CA: Insulin-like growth factor system gene expression in human
endometrium during menstrual cycle. J Clin Endocrinol Metab.
79:1723–1734. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Laatikainen TJ, Toma EI and Voutilainen
RJ: The expression of insulin-like growth factor and its binding
protein RNA in the endometrium of postmenopausal patients with
breast cancer receiving tamoxifen. Cancer. 76:1406–1410. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Elkas J, Gray K, Howard L, Petit N, Pohl J
and Armstrong A: The effects of tamoxifen on endometrial
insulin-like growth factor-1 expression. Obstet Gynecol. 91:45–50.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Mairano E, Loverro G, Viale G, Giannini T,
Napoli A and Perlino E: Insulin-like growth factor-I expression in
normal and diseased endometrium. Int J Cancer. 80:188–193. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
127
|
O'Toole SA, Dunn E, Sheppard BL, Sheils O,
O'Leary JJ, Wuttke W and Seidlova-Wuttke D: Oestrogen regulated
gene expression in normal and malignant endometrial tissue.
Maturitas. 51:187–198. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Bruchim I, Sarfstein R and Werner H: The
IGF hormonal network in endometrial cancer: Functions, regulation,
and targeting approaches. Front Endocrinol (Lausanne). 5:762014.
View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Soufla G, Sifakis S and Spandidos DA: FGF2
transcript levels are positively correlated with EGF and IGF-1 in
the malignant endometrium. Cancer Lett. 259:146–155. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Pengchong H and Tao H: Expression of
IGF-1R, VEGF-C and D2-40 and their correlation with lymph node
metastasis in endometrial adenocarcinoma. Eur J Gynecol Oncol.
32:660–664. 2011.
|
|
131
|
Yi HK, Kim SY, Hwang PH, Kim CY, Yang DH,
Oh Y and Lee DY: Impact of PTEN on the expression of insulin-like
growth factors (IGFs) and IGF-binding proteins in human gastric
adenocarcinoma cells. Biochem Biophys Res Commun. 330:760–767.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
McCamplell AS, Broaddus RR, Loose DS and
Davies PJ: Overexpression of the insulin-like growth factor I
receptor and activation of the AKT pathway in hyperplastic
endometrium. Clin Cancer Res. 12:6373–6378. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Dong L, Du M and Lv Q: Picropodophyllin
inhibits type I endometrial cancer cell proliferation via
disruption of the PI3K/Akt pathway. Acta Biochim Biophys Sin
(Shanghai). 51:753–760. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Leung PS, Aronson WJ, Ngo TH, Golding LA
and Barnard RJ: Exercise alters the IGF axis in vivo and increases
p53 protein in prostate tumor cells in vitro. J Appl Physiol
(1985). 96:450–454. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Tucci P: Caloric restriciton: Is mammalian
life exptension linked to p53? Aging (Albany NY). 4:525–534. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Luo X, Jiang X, Li J, Bai Y, Li Z, Wei P,
Sun S, Liang Y, Han S, Li X and Zhang B: Insulin-like growth
factor-1 attenuates oxidative stress-induced hepatocyte premature
senescence in liver fibrogenesis via regulating nuclear
p53-progerin interaction. Cell Death Dis. 10:4512019. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Tran D, Bergholz J, Zhang H, He H, Wang Y,
Zhang Y, Li Q, Kirkland JL and Xiao ZX: Insulin-like growth
factor-1 regulates the SIRT1-p53 pathway in cellular senescence.
Aging Cell. 13:669–678. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Attias-Geva Z, Bentov I, Kidrom D, Amichay
K, Sarfstein R, Fishman A, Bruchim I and Werner H: p53 regulates
insulin-like growth factor-I receptor gene expression in uterine
serous carcinoma and predicts responsiveness to an insulin-like
growth factor-I receptor-directed targeted therapy. Eur J Cancer.
48:1570–1580. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Altieri DC: Survivin, cancer networks and
pathway-directed drug discovery. Nat Rev Cancer. 8:61–70. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Takai N, Miyazaki T, Nishida M, Nasu K and
Miyakawa I: Survivin expression correlates with clinical stage,
histological grade, invasive behavior and survival rate in
endometrial carcinoma. Cancer Lett. 184:105–116. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Tran J, Master Z, Yu JL, Rak J, Dumont DJ
and Kerbel RS: A role for survivin in chemoresistance of
endothelial cells mediated by VEGF. Proc Natl Acad Sci USA.
99:4349–4354. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Altieri DC: Survivin, versatile modulation
of cell division and apoptosis in cancer. Oncogene. 22:8581–8589.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Altieri DC: Survivin and apoptosis
control. Adv Cancer Res. 88:31–35. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Mita AC, Mita MM, Nawrocki ST and Giles
FJ: Survivin: Key regular of mitosis and apoptosis and novel target
for cancer therapeutics. Clin Cancer Res. 14:5000–5005. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Liu X, Chen H, Hou Y, Ma X, Ye M, Huang R,
Hu B, Cao H, Xu L, Liu M, et al: Adaptive EGF expression sensitizes
pancreatic cancer cells to ionizing radiation through activation of
the cyclin D1/P53/PARP pathway. Int J Oncol. 54:1466–1480.
2019.PubMed/NCBI
|
|
146
|
Vaira V, Lee CW, Goel HL, Bosari S,
Languino LR and Altieri DC: Regulation of surivivn expression by
IGF-I/mTOR signaling. Oncogene. 26:2678–2684. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Song K, Shankar E, Yang J, Bane KL,
Wahdan-Alaswad R and Danielpour D: Critical role of a
survivin/TGF-β/mTORC1 axis in IGF-I-mediated growth of prostate
epithelial cells. PLoS One. 8:e618962013. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Sato A, Oya M, Ito K, Mizuno R, Horiguchi
Y, Umezawa K, Hayakawa M and Murai M: Survivin associates with cell
proliferation in renal cancer cells: Regulation of survivin
expression by insulin-like growth factor-1, interferon-gamma and a
novel NK-kappaB inhibitor. Int J Oncol. 28:841–846. 2006.PubMed/NCBI
|
|
149
|
Oh SH, Jin Q, Kim ES, Khuri FR and Lee HY:
Insulin-like growth factor-I receptor signaling pathway induces
resistance to the apoptotic activitis of SCH66336 (lonafarnib)
through Akt/mammalian target of rapamycin-mediated increases in
survivin expression. Clin Cancer Res. 14:1581–1589. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Morgillo F, Woo JK, Kim ES, Hong WK and
Lee HY: Heterodimerization of insulin-like growth factor
receptor/epidermal growth factor receptor and induction of survivin
expression counteract the antitumor action of erlotinid. Cancer
Res. 66:10100–10111. 2006. View Article : Google Scholar : PubMed/NCBI
|