Despite significant progress being made in the
prevention and treatment of gastric cancer (GC) in the past
decades, GC is still one of the most concerning malignancies as the
majority of patients are diagnosed at an advanced stage of disease.
Globally, GC ranked fifth for cancer incidence and third for cancer
deaths, accounting for 1.3 million estimated incident cases and
819,000 estimated deaths in 2015 (1). Geographically, GC is more prevalent in
developing countries, with the majority of cases and deaths
occurring in Eastern Asia, including China, Japan and Korea,
followed by Central Europe, Eastern Europe and South America
(2). Etiologically, GC is a
multifactorial disease attributed to both host and environmental
factors. Proposed risk factors for GC include Helicobacter
pylori (H. pylori) infection, smoking, alcohol, obesity,
salt intake, atrophic gastritis (AG), intestinal metaplasia (IM)
and family history of GC. The current therapy for GC includes
surgery, chemotherapy, radiotherapy, VEGFR (ramucirumab) and
targeted therapy against HER2 (trastuzmab) (3). The overall outcome of GC is largely
associated with the stage of the disease at diagnosis. For early GC
limited to the mucosa and submucosa, the 5-year survival rate is
>90% (4,5). However, due to the lack of
distinguishable symptoms at early stages and effective mass
screening programs worldwide, the majority of GC cases are
typically detected at stage IIIA-IV, with an estimated 5-year
survival rate of <30% and a median survival of 12 months
(4,5).
To date, four GC screening methodologies have been
implemented in clinical settings: H. pylori serology, serum
pepsinogen (PG) testing, indirect upper gastrointestinal series
(UGIS) and endoscopy. Since the 1960s, population-based GC
screening programs in several high-prevalence nations such as Japan
and Korea have achieved significantly improved survival and cure
rates using the above methods. These programs demonstrate the
effectiveness of mass screening for GC (6–8).
However, each of these screening tools has its limitations. For
instance, H. pylori serology is unable to detect
premalignant lesions, such as longstanding AG and IM. Therefore,
H. pylori serology alone is not useful as a screening test
for GC (9). The combination of upper
endoscopy with pathological biopsy examination is the primary
screening technique in the majority of these programs and the gold
standard for confirmation of diagnosis (10). In general, endoscopy is superior to
UGIS in sensitivity and cost-effectiveness for detecting early GC
(11–13). However, endoscopy is an invasive
technique that has infrequent but serious complications, and its
utility depends largely on the skill of the endoscopist (14). Therefore, the use of endoscopy in
mass-screening programs in low-prevalence and low-income countries
is impractical and likely to be associated with low participation
rates. Currently, the only non-invasive test for GC detection in
the clinical setting is the PG assay (15). Changes in serum PG levels reflect the
function of the gastric mucosa. Decreased PGI levels and PGI/PGII
ratio are indicators of atrophic changes in the gastric corpus. PG
tests can detect gastric mucosal atrophy with a sensitivity of
66.7–84.6% and a specificity of 73.5–87.1% (16,17).
However, PG assay's sensitivity for GC detection ranges from 36.8
to 62.3% (18,19), which is too low to be acceptable for
population-based screening. Therefore, new assays with improved
sensitivity, specificity and cost-effectiveness are needed.
Recent advances in genetic testing, such as
next-generation sequencing (NGS) and digital PCR, and
bioinformatics have accelerated the research on liquid biopsy
greatly, which have high potential to change the clinical
management of patients with cancer. Meanwhile, considerable efforts
have been made to identify novel, early-stage GC biomarkers with
potential utility in clinical liquid biopsy testing. Such
biomarkers include cell-free DNA (cfDNA), cell-free RNA (cfRNA),
proteins, autoantibodies, circulating tumor cells (CTCs),
cancer-derived extracellular vesicles (EVs) and metabolites. A
number of comprehensive reviews were recently published on CTCs,
proteomics, cfRNA biomarkers, exosomes and EVs in GC (20,21). The
current review provides an overview of the recent advances in the
early detection of GC using liquid biopsy, with a focus on cfDNA,
and their origin and mechanism of release into the bloodstream, as
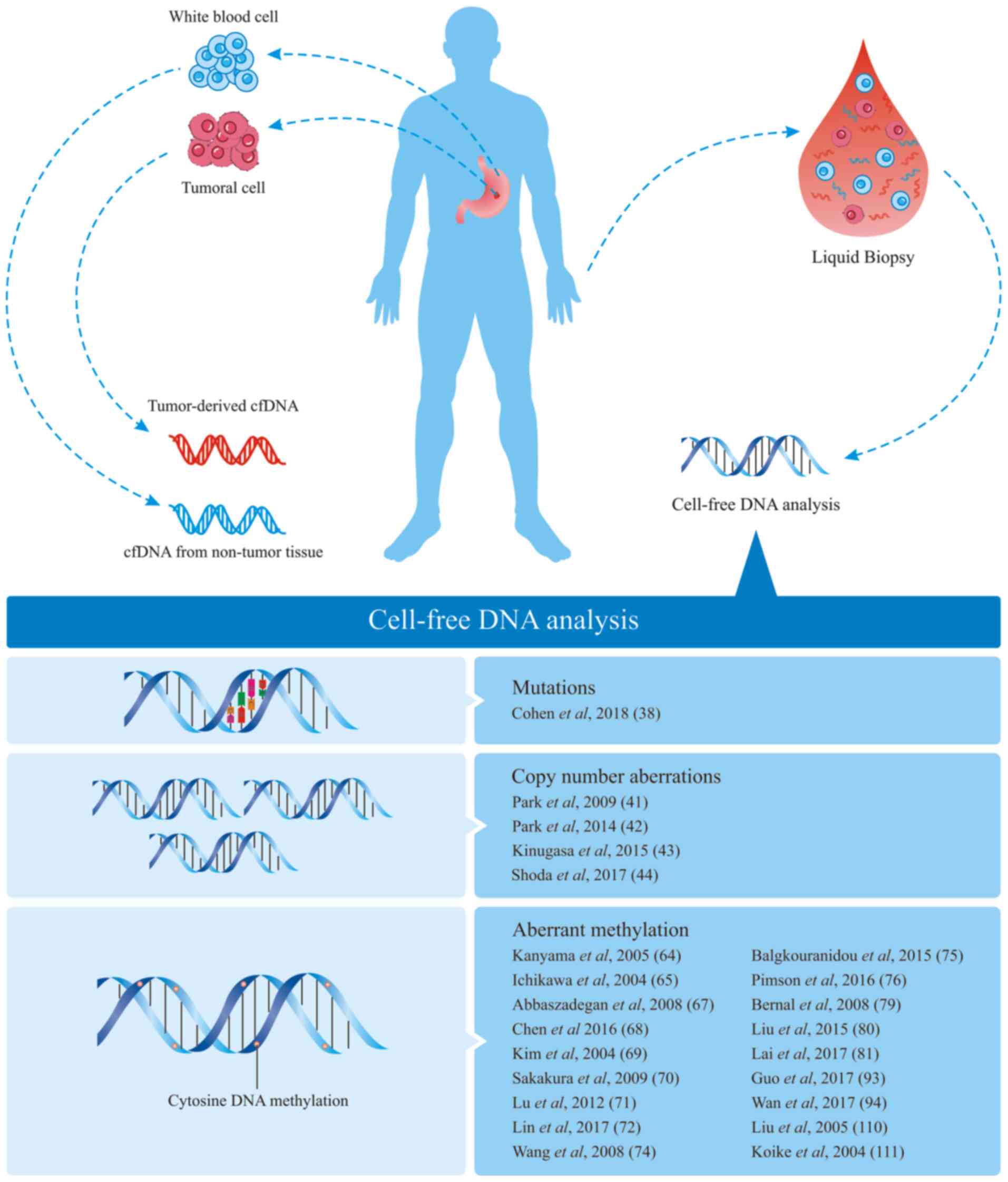
well as their potential utility in clinical practice (Fig. 1).
cfDNA is circulating extracellular DNA existing in
the blood serum or plasma, synovial fluid, cerebrospinal fluid and
other body fluids. Physiological events producing cfDNA include
cellular apoptosis, secretion, micrometastasis and necroptosis
(22). In patients with cancer, the
plasma cfDNA levels are 2–3-fold higher than those in normal
healthy groups (23), suggesting its
potential as a complementary biomarker for cancer detection, as
well as an indicator of prognosis and therapeutic response. As
shown in Table I, a prospective
study quantified the cfDNA levels in serum samples from patients
with benign or malignant gastrointestinal tract disease using a
radioimmunoassay, and it showed that the cfDNA levels in patients
with malignant diseases were significantly higher than those of
patients with benign diseases (24).
Consistently, Sai et al (25)
reported that increased plasma total cfDNA in patients with GC
could be detected compared with the undetectable levels of healthy
controls (Table I). The authors
measured the short and long forms of β-actin in plasma samples from
patients with GC and healthy controls by quantitative PCR (qPCR),
and found that the cfDNA concentration in patients with GC was
significantly higher. These studies suggest the potential use of
serum cfDNA concentration assay to detect GC.
There are various methods for quantitative cfDNA
detection, but their efficiency is limited by sample preparation
and assay procedures. To address this issue, Park et al
(26) developed an Alu-qPCR assay
for measuring cfDNA concentrations, which demonstrated better
sensitivity and reproducibility compares with other technologies,
based on Ultraviolet-visible (UV–Vis) spectrophotometry or the
PicoGreen fluorophore. Applying this assay, the cfDNA levels were
compared between patients with GC and those of age-matched healthy
controls, and found that the mean levels of plasma cfDNA were
higher in the GC group than in the control group (Table I). To understand the dynamics of
cfDNA levels pre- and post-surgery, Kim et al (27) measured the plasma cfDNA levels of
patients with GC and healthy controls by qPCR. The samples of the
patients with GC were collected before and 24 h after surgery. The
results showed that average cfDNA levels were increased in patients
with GC compared with those of healthy controls, and there was a
positive dose-dependent association with more advanced cancer
staging. Meanwhile, the levels of cfDNA in the 24-h-post-surgery
group decreased significantly, thus supporting the utility of using
cfDNA to monitor disease severity and therapeutic efficacy.
Since the measurement of cfDNA levels does not
require any prior knowledge of genetic alterations in tumor tissue,
this test could be highly useful in non-invasive assays for early
GC detection. However, the application of cfDNA quantification
alone for early cancer diagnosis is limited by several obstacles:
i) Circulating cfDNA is unstable and its kinetics has not yet been
well defined, which may affect assay robustness and
standardization; ii) cfDNA levels cannot differentiate cancer type
or tissue of origin; iii) cfDNA testing is relatively nonspecific,
as numerous patients with non-cancer conditions, such as
inflammatory disease, infections and cardiovascular disease, and
even healthy individuals after exercise, show elevated cfDNA levels
(29,30). Therefore, it is expected that the
combined detection of cfDNA levels with other markers may achieve
improved clinical performance.
Genetic alterations such as mutations, rearrangement
and amplification of driver genes result in tumorigenesis. Recent
advancements in NGS techniques have identified various nucleotide
mutations associated with GC (Table
II). The top mutated genes showing higher mutation frequency
were TP53, TTN, MUC16, CDH1, KMT2C and MLH1 (31–33). In
1977, Leon et al (34)
reported that numerous patients with cancer had elevated
circulating cfDNA, and found that this DNA was tumor derived.
Since, scientists focused their attention on the content of ctDNA.
Some of the aberrant DNA shed into the blood by cancer cells, such
as EGFR and KRAS mutations, can be potential
biomarkers. Previous studies have demonstrated the detectability of
mutant DNA released from tumor tissues. In fact, ctDNA analysis has
emerged as an additional diagnostic tool to guide clinical
management of certain cancer types including lung cancer and colon
cancer (35,36). However, the use of ctDNA for early
cancer detection is complicated by the generally low abundance of
ctDNA in early-stage cancer and the technical challenges in its
detection.
Applying the droplet digital PCR (ddPCR) assay,
which is currently the most sensitive method, Bettegowda et
al (37) evaluated ctDNA in 640
plasma samples from patients with various cancer types and stages.
ctDNA was detected in >75% of patients with several advanced
cancer types, including melanoma, and pancreatic, breast,
hepatocellular, ovarian, colorectal, bladder, gastroesophageal, and
head and neck cancer. In patients with localized tumors, ctDNA was
detectable only in a subset of patients with gastroesophageal
cancer (57%), colorectal cancer (CRC) (73%), pancreatic cancer
(48%) and breast adenocarcinoma (50%). When KRAS mutations
were tested for in ctDNA in an additional panel of 206 patients
with metastatic CRC, the sensitivity and specificity of detection
were 87.2 and 99.2%, respectively. These results indicate that the
detectability of ctDNA is affected by cancer type and stage.
DNA modifications such as 5-methylcytosine (5-mC)
and 5-hydroxymethylcytosine (5-hmC) could serve as ideal biomarkers
for cancer diagnosis. The 5-mC remodeling of DNA has been reported
to be involved in cancer initiation, progression and therapeutic
response (45). Except for 5-mC, a
previous study by Li et al (46) showed that 5-hmC from circulating
cfDNA was highly predictive of colorectal and gastric cancer, and
was superior to conventional biomarkers and comparable to 5-hmC
biomarkers from tissue biopsies. Hypermethylation of promoter CpG
islands in tumor suppressor genes plays a crucial role in
carcinogenesis (47–49). Eyvazi et al (50) verified the promoter methylation of
EphA5, HS3ST2 and CDH11 genes in patients with GC using
paraffin-embedded tissue sections. Recently, cfDNA in blood plasma
has become a promising cancer biomarker for early diagnosis
(51). The abnormal methylation of a
large number of genes has been demonstrated to have utility for
non-invasive detection of cancer in plasma or serum samples
(52–54). Among them, Septin 9 gene
methylation, detectable as hypermethylated Septin 9 DNA
fragments in blood plasma, is a front-runner for the clinical
screening of CRC (55). Septin
9, a member of the Septin family, was originally identified in
myeloid neoplasia (56). It
functions as a tumor suppressor gene in multiple cancer types
(57). Consequently, several assay
kits have been developed to detect methylated Septin 9. Epi
proColon, the first commercial methylated Septin 9 assay,
has been approved by the USA Food and Drug Administration (FDA) for
average-risk patients over the age of 50 years, and has also been
approved in Europe and China (58).
Meta-analysis of existing clinical data showed that the assay's
sensitivity and specificity were 71 and 92%, respectively,
demonstrating its reliability for CRC detection (59,60). In
2016, a blood test that detects circulating methylated Septin
9 DNA was approved by the FDA to provide an alternative
screening modality (61), thus
paving the way for the development of a new generation of liquid
biopsy tests for early cancer detection.
Of note, there is obvious heterogeneity in gene
methylation frequencies between different studies, which might be
attributable to differences in samples size, technique variations
and geographical differences. To obtain a better understanding of
this variance, Hu et al (82)
recently performed a meta-analysis to evaluate the pooled
sensitivity and specificity of the results of 32 studies, which
included 4,172 patients with GC and 2,098 controls. Collectively,
the overall sensitivity of DNA methylation-based blood test for
detecting GC was 57% (95% CI, 50–63%), while the specificity was
97% (95% CI, 95–98%). The sensitivity and specificity of tests
covering multiple methylated genes were 76% (95% CI, 64–84%) and
85% (95% CI, 65–95%), respectively. These results indicate that
blood-based DNA methylation tests have high specificity but modest
sensitivity for detecting GC. Evaluating multiple methylated genes
or using plasma samples seems to improve diagnostic
sensitivity.
Besides the methylation markers described above,
increased methylation of numerous other genes in cfDNA has also
been reported in GC (83–86) (Table
V). However, due to the limited sample sizes and method
variants, further studies are needed to demonstrate their
analytical and clinical validity.
Although the genetic landscape for GC has been well
researched and a large number of candidate biomarkers have been
detected in blood samples in the past decades, none of these have
yet progressed into clinical assays for GC. A number of challenges
account for this delay.
First and foremost is the lack of biomarker
validation studies demonstrating acceptable sensitivities and
specificities for clinical use. In fact, most of the proposed
biomarkers were identified or validated in retrospective studies
with limited sample sizes. Quantification of plasma cfDNA alone,
for example, is insufficient as a clinical biomarker due to its
lack of specificity. On the other hand, detection of mutations or
rearrangements of ctDNA seems more intriguing due to their
biological relevance for tumor initiation and development. However,
even the most commonly mutated genes, such as TP53 and
KRAS, are typically aberrant in <50% of the cases in any
particular cancer type. On this context, it is assumed that
multigene panel analysis of ctDNA could lead to increased test
sensitivity. However, the mutations of these genes are often
located in different exons, and their abundance in circulation is
generally elevated only in late-stage cancers. This impairs the
detection of DNA-based sequence variations, as well as their
utility in early cancer detection. For instance, in the recent
CancerSEEK study, detecting mutations of 16 genes only achieved 40%
sensitivity for early-stage tumors (38). By contrast, assaying DNA methylation
(which is the epigenetic modification of CpG dinucleotides) is more
robust and consistent than testing genetic alterations. There is
accumulating evidence that cfDNA gene hypermethylation is more
readily detectable than genetic mutations in patients with GC or
pre-cancerous diseases such as intestinal metaplasia and dysplasia.
For example, the hypermethylation of a number of genes, including
RunX3, RPRM and RASSF1A, was significantly elevated
in plasma samples of patients with early-stage GC. Aside from Epi
proColon, the first FDA-approved gene methylation-based GC assay,
Epi proLung, which tests for plasma SHOX2 and PTGER4
gene methylation, recently received a CE-IVD mark in Europe for
lung cancer detection as well (87).
These developments demonstrate the current utility and future
potential of cfDNA methylation assays for cancer detection.
In addition to testing sensitivity and specificity,
tracking tumor location is another challenge for cfDNA-based tests.
For instance, although hypermethylation of Septin 9 is
preferentially detected in patients with CRC, it is also present in
some patients with primary lung and stomach cancer (88,89). In
fact, even CancerSEEK had to rely on conventional protein tumor
biomarkers to track the tissue of origin. However, in patients with
early-stage cancer, conventional protein tumor biomarkers can be
difficult to detect. Recent studies using computer based-analyses
of genome-wide methylation signatures have demonstrated certain
potential for identifying the presence, type and location of tumors
(90–93). For instance, methylated haplotype
load, an analysis of tissue-specific methylation haplotype blocks
using whole-genome bisulfite sequencing (WGBS) data, can help to
identify cancer-associated biomarkers in both tissue and plasma
samples (94). Preliminary data from
this analysis demonstrated its potential in determining tissue of
origin as well as in predicting cancer development and progression
from plasma samples of patients with lung cancer and CRC.
Similarly, Kang et al (92)
developed CancerLocator, a probabilistic approach to WGBS analysis
that is used to predict disease burden and the tissue of origin of
ctDNA based on the genome-wide methylation profile of its cfDNA.
However, further prospective studies with larger sample sizes are
required to validate its utility in clinical settings.
Besides biomarker validation, another challenge for
developing cfDNA-based tests in GC is the lack of a standard
consensus for experimental procedures, including sampling, storage
conditions, cfDNA isolation and enrichment, data analysis and
results interpretation (95).
Currently, the technologies used for cfDNA detection include qPCR,
next-generation sequencing (NGS), dPCR and ultra-sensitive
amplification refractory mutation system (ARMS) PCR. Each of these
methods has its advantages and drawbacks (96).
Recently, liquid biopsy testing by qPCR (Cobas and
Therascreen) was FDA-approved for EGFR exon 19 deletions,
EGFR L858R and EGFR T790M in patients with NSCLC.
However, these kits have only been validated for allele frequencies
of >1%, which is not sufficient when attempting to detect tumors
at early stages (97–99).
By contrast, dPCR has the highest testing
sensitivity and suitability in liquid biopsies (100,101).
Compared with the characteristics of NGS, dPCR is more
cost-effective and has faster turnaround times. However, it has a
lower throughput, and can only detect a limited number of known
mutations at a time (102). NGS, on
the other hand, is theoretically able to detect numerous gene
mutations, amplifications and fusions in parallel with higher
throughput, and has already been approved for tumor-tissue
profiling in the clinic (103,104).
Despite this, conventional NGS has relatively low-detection
sensitivity and a high-error rate, thus limiting its usefulness for
analyzing cfDNA, which occurs in low abundance in plasma samples.
New targeted- and genome-wide-NGS approaches for liquid biopsy
testing have been developed with improved sensitivity and
error-suppression rates (105,106).
However, the complicated process, quality control and
cost-effectiveness of NGS still need to be improved for clinical
applications.
On the other hand, an ultra-sensitive ARMS PCR assay
(Udx-PCR, Super-ARMS) was developed, which can detect mutant ctDNA
at an allele frequency of 0.1–0.02% in the background of 10–50 ng
wild-type DNA (46,107). This is comparable to the detection
sensitivity of dPCR, but with significantly improved robustness,
cost-effectiveness and procedural ease.
Recent milestones in cfDNA analysis as a liquid
biopsy for early cancer detection pave the way for its adoption in
clinical practice. Among them, Epi proColon is the first
population-based CRC screening product that detects gene
methylation in plasma samples. Another study led by Chan et
al (108) indicated that
detection of Epstein-Barr virus DNA in plasma is effective for the
screening of early asymptomatic nasopharyngeal carcinoma.
Additionally, CancerSEEK has demonstrated how a single assay can
screen multiple cancer types by combining ctDNA and protein
biomarkers (38). Liquid biopsy draw
the attention of independent libraries and commercial companies. In
a recent research by GRAIL, which focused on applying cfDNA liquid
biopsy to cancer early-stage diagnosis, the sensitivity of stage
I–III was 67.3% (CI, 60.7–73.3%) in a pre-specified set of 12
cancer types (such as anus, bladder, colon/rectum, esophagus, head
and neck, liver/bile-duct, lung, lymphoma, ovary, pancreas, plasma
cell neoplasm and stomach), and 43.9% (CI, 39.4–48.5%) in all
cancer types (54). In other studies
on breast cancer, 358 cancer and 452 normal cases were included.
The results indicated that for three types of breast cancer (triple
negative, HER2-positive/hormone receptor-positive and
HER2-negative), the sensitivity was 58, 40 and 15%, respectively.
This sensitivity shows that cfDNA liquid biopsy is still far from
ready-to-use for clinic diagnosis (109). Nonetheless, since non-invasive and
low-cost cfDNA testing still plays an important role in
consumer-grade cancer diagnosis and cancer treatment management,
such as personalized medicine and cancer prognosis. In those
studies, two available approaches for early cancer detection using
liquid biopsy were presented: i) One assay for one cancer
(one-to-one); and ii) one assay for multiple cancer types
(one-to-many) by utilizing genome-wide profiling or a large genetic
signature panel.
Although numerous potential biomarkers have already
been identified in GC, the development of a new generation of
minimally invasive cfDNA-based tests for GC early detection must
consider their clinical validity and utility. These require
collaborative efforts in two areas: i) Developing new assays with
improved sensitivity, reproducibility, procedural standardization
and cost-effectiveness; and ii) validating emerging biomarkers in
larger prospective clinical studies. Although the ‘one-to-many’
liquid biopsy approach is more attractive in the long term, it
presents greater challenges than the ‘one-to-one’ approach. With
these recent advances in cancer genetics and assay modalities,
particularly the clinical implementation of circulating methylated
DNA-based CRC and lung cancer screening tests, it is expected that
a new, clinically effective, liquid biopsy assay for early
detection of GC will be available in the near future.
Not applicable.
No funding was received.
Not applicable.
ZH performed researched data and wrote this
manuscript, HZ contributed to discussions of content and helped to
draft the manuscript. BY revised the draft of the manuscript. DY
designed the study and revised the manuscript. All authors read and
approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Global Burden of Disease Cancer
Collaboration, ; Fitzmaurice C, Allen C, Barber RM, Barregard L,
Bhutta ZA, Brenner H, Dicker DJ, Chimed-Orchir O, Dandona R, et al:
Global, regional, and national cancer incidence, mortality, years
of life lost, years lived with disability, and disability-adjusted
life-years for 32 cancer groups, 1990 to 2015: A systematic
analysis for the global burden of disease study. JAMA Oncol.
3:524–548. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Karimi P, Islami F, Anandasabapathy S,
Freedman ND and Kamangar F: Gastric cancer: Descriptive
epidemiology, risk factors, screening, and prevention. Cancer
Epidemiol Biomarkers Prev. 23:700–713. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Takahashi T, Saikawa Y and Kitagawa Y:
Gastric cancer: Current status of diagnosis and treatment. Cancers
(Basel). 5:48–63. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Orditura M, Galizia G, Sforza V,
Gambardella V, Fabozzi A, Laterza MM, Andreozzi F, Ventriglia J,
Savastano B, Mabilia A, et al: Treatment of gastric cancer. World J
Gastroenterol. 20:1635–1649. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rosati G, Ferrara D and Manzione L: New
perspectives in the treatment of advanced or metastatic gastric
cancer. World J Gastroenterol. 15:2689–2692. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hamashima C, Shibuya D, Yamazaki H, Inoue
K, Fukao A, Saito H and Sobue T: The Japanese guidelines for
gastric cancer screening. Jpn J Clin Oncol. 38:259–267. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Choi KS, Jun JK, Lee HY, Park S, Jung KW,
Han MA, Choi IJ and Park EC: Performance of gastric cancer
screening by endoscopy testing through the national cancer
screening program of Korea. Cancer Sci. 102:1559–1564. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Leung WK, Wu MS, Kakugawa Y, Kim JJ, Yeoh
KG, Goh KL, Wu KC, Wu DC, Sollano J, Kachintorn U, et al: Screening
for gastric cancer in Asia: Current evidence and practice. Lancet
Oncol. 9:279–287. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ley C, Mohar A, Guarner J,
Herrera-Goepfert R, Figueroa LS, Halperin D and Parsonnet J:
Screening markers for chronic atrophic gastritis in Chiapas,
Mexico. Cancer Epidemiol Biomarkers Prev. 10:107–112.
2001.PubMed/NCBI
|
|
10
|
Katai H and Sano T: Early gastric cancer:
Concepts, diagnosis, and management. Int J Clin Oncol. 10:375–383.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tashiro A, Sano M, Kinameri K, Fujita K
and Takeuchi Y: Comparing mass screening techniques for gastric
cancer in Japan. World J Gastroenterol. 12:4873–4874.
2006.PubMed/NCBI
|
|
12
|
Tonouchi H, Mohri Y, Kobayashi M, Tanaka
K, Ohi M and Kusunoki M: Laparoscopy-assisted distal gastrectomy
with laparoscopic sentinel lymph node biopsy after endoscopic
mucosal resection for early gastric cancer. Surg Endosc.
21:1289–1293. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kusano C, Iwasaki M, Kaltenbach T, Conlin
A, Oda I and Gotoda T: Should elderly patients undergo additional
surgery after non-curative endoscopic resection for early gastric
cancer? Long-term comparative outcomes. Am J Gastroenterol.
106:1064–1069. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
ASGE Standards of Practice Committee, ;
Ben-Menachem T, Decker GA, Early DS, Evans J, Fanelli RD, Fisher
DA, Fisher L, Fukami N, Hwang JH, et al: Adverse events of upper GI
endoscopy. Gastrointest Endosc. 76:707–718. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mukoubayashi C, Yanaoka K, Ohata H, Arii
K, Tamai H, Oka M and Ichinose M: Serum Pepsinogen and Gastric
Cancer Screening. Intern Med. 46:261–266. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Miki K, Ichinose M, Shimizu A, Huang SC,
Oka H, Furihata C, Matsushima T and Takahashi K: Serum pepsinogens
as a screening test of extensive chronic gastritis. Gastroenterol
Jpn. 22:133–141. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nasrollahzadeh D, Aghcheli K, Sotoudeh M,
Shakeri R, Persson EC, Islami F, Kamangar F, Abnet CC, Boffetta P,
Engstrand L, et al: Accuracy and cut-off values of pepsinogens I,
II and gastrin 17 for diagnosis of gastric fundic atrophy:
Influence of gastritis. PLoS One. 6:e269572011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Miki K and Urita Y: Using serum
pepsinogens wisely in a clinical practice. J Dig Dis. 8:8–14. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang YK, Yu JC, Kang WM, Ma ZQ, Ye X,
Tian SB and Yan C: Significance of serum pepsinogens as a biomarker
for gastric cancer and atrophic gastritis screening: A systematic
review and meta-analysis. PLoS One. 10:e01420802015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhou J, Ma X, Bi F and Liu M: Clinical
significance of circulating tumor cells in gastric cancer patients.
Oncotarget. 8:25713–25720. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu Y, Ling Y, Qi Q, Lan F, Zhu M, Zhang
Y, Bao Y and Zhang C: Prognostic value of circulating tumor cells
in advanced gastric cancer patients receiving chemotherapy. Mol
Clin Oncol. 6:235–242. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schwarzenbach H, Hoon DS and Pantel K:
Cell-free nucleic acids as biomarkers in cancer patients. Nat Rev
Cancer. 11:426–437. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
De Mattos-Arruda L, Olmos D and Tabernero
J: Prognostic and predictive roles for circulating biomarkers in
gastrointestinal cancer. Future Oncol. 7:1385–1397. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shapiro B, Chakrabarty M, Cohn EM and Leon
SA: Determination of circulating DNA levels in patients with benign
or malignant gastrointestinal disease. Cancer. 51:2116–2120. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sai S, Ichikawa D, Tomita H, Ikoma D, Tani
N, Ikoma H, Kikuchi S, Fujiwara H, Ueda Y and Otsuji E:
Quantification of plasma cell-free DNA in patients with gastric
cancer. Anticancer Res. 27:2747–2751. 2007.PubMed/NCBI
|
|
26
|
Park JL, Kim HJ, Choi BY, Lee HC, Jang HR,
Song KS, Noh SM, Kim SY, Han DS and Kim YS: Quantitative analysis
of cell-free DNA in the plasma of gastric cancer patients. Oncol
Lett. 3:921–926. 2012.PubMed/NCBI
|
|
27
|
Kim K, Shin DG, Park MK, Baik SH, Kim TH,
Kim S and Lee S: Circulating cell-free DNA as a promising biomarker
in patients with gastric cancer: Diagnostic validity and
significant reduction of cfDNA after surgical resection. Ann Surg
Treat Res. 86:136–142. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Qian C, Ju S, Qi J, Zhao J, Shen X, Jing
R, Yu J, Li L, Shi Y, Zhang L, et al: Alu-based cell-free DNA: A
novel biomarker for screening of gastric cancer. Oncotarget.
8:54037–54045. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kolesnikova EV, Tamkovich SN, Bryzgunova
OE, Shelestyuk PI, Permyakova VI, Vlassov VV, Tuzikov AS, Laktionov
PP and Rykova EY: Circulating DNA in the blood of gastric cancer
patients. Ann N Y Acad Sci. 1137:226–231. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Coimbra S, Catarino C, Costa E, Oliveira
H, Figueiredo A, Rocha-Pereira P and Santos-Silva A: Circulating
cell-free DNA levels in Portuguese patients with psoriasis vulgaris
according to severity and therapy. Br J Dermatol. 170:939–942.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen C, Shi C, Huang X, Zheng J, Zhu Z, Li
Q, Qiu S, Huang Z, Zhuang Z, Wu R, et al: Molecular profiles and
metastasis markers in Chinese patients with gastric carcinoma. Sci
Rep. 9:139952019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wu R, Li Q, Wu F, Shi C and Chen Q:
Comprehensive analysis of CDC27 related to peritoneal metastasis by
whole exome sequencing in gastric cancer. Onco Targets Ther.
13:3335–3346. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang R, Song S, Harada K, Ghazanfari
Amlashi F, Badgwell B, Pizzi MP, Xu Y, Zhao W, Dong X, Jin J, et
al: Multiplex profiling of peritoneal metastases from gastric
adenocarcinoma identified novel targets and molecular subtypes that
predict treatment response. Gut. 69:18–31. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Leon SA, Shapiro B, Sklaroff DM and Yaros
MJ: Free DNA in the serum of cancer patients and the effect of
therapy. Cancer Res. 37:646–650. 1977.PubMed/NCBI
|
|
35
|
Chen K, Zhao H, Yang F, Hui B, Wang T,
Wang LT, Shi Y and Wang J: Dynamic changes of circulating tumour
DNA in surgical lung cancer patients: Protocol for a prospective
observational study. BMJ Open. 8:e0190122018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bachet JB, Bouché O, Taieb J, Dubreuil O,
Garcia ML, Meurisse A, Normand C, Gornet JM, Artru P, Louafi S, et
al: RAS mutation analysis in circulating tumor DNA from patients
with metastatic colorectal cancer: The AGEO RASANC prospective
multicenter study. Ann Oncol. 29:1211–1219. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bettegowda C, Sausen M, Leary RJ, Kinde I,
Wang Y, Agrawal N, Bartlett BR, Wang H, Luber B, Alani RM, et al:
Detection of circulating tumor DNA in early- and late-stage human
malignancies. Sci Transl Med. 6:224ra242014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cohen JD, Li L, Wang Y, Thoburn C, Afsari
B, Danilova L, Douville C, Javed AA, Wong F, Mattox A, et al:
Detection and localization of surgically resectable cancers with a
multi-analyte blood test. Science. 359:926–930. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Deng N, Goh LK, Wang H, Das K, Tao J, Tan
IB, Zhang S, Lee M, Wu J, Lim KH, et al: A comprehensive survey of
genomic alterations in gastric cancer reveals systematic patterns
of molecular exclusivity and co-occurrence among distinct
therapeutic targets. Gut. 61:673–684. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Qian Z, Zhu G, Tang L, Wang M, Zhang L, Fu
J, Huang C, Fan S, Sun Y, Lv J, et al: Whole genome gene copy
number profiling of gastric cancer identifies PAK1 and KRAS gene
amplification as therapy targets. Genes Chromosomes Cancer.
53:883–894. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Park KU, Lee HE, Park DJ, Jung EJ, Song J,
Kim HH, Choe G, Kim WH and Lee HS: MYC quantitation in cell-free
plasma DNA by real-time PCR for gastric cancer diagnosis. Clin Chem
Lab Med. 47:530–536. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Park KU, Lee HE, Nam SK, Nam KH, Park DJ,
Kim HH, Kim WH and Lee HS: The quantification of HER2 and MYC gene
fragments in cell-free plasma as putative biomarkers for gastric
cancer diagnosis. Clin Chem Lab Med. 52:1033–1040. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kinugasa H, Nouso K, Tanaka T, Miyahara K,
Morimoto Y, Dohi C, Matsubara T, Okada H and Yamamoto K: Droplet
digital PCR measurement of HER2 in patients with gastric cancer. Br
J Cancer. 112:1652–1655. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Shoda K, Ichikawa D, Fujita Y, Masuda K,
Hiramoto H, Hamada J, Arita T, Konishi H, Komatsu S, Shiozaki A, et
al: Monitoring the HER2 copy number status in circulating tumor DNA
by droplet digital PCR in patients with gastric cancer. Gastric
Cancer. 20:126–135. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Taby R and Issa JP: Cancer epigenetics. CA
Cancer J Clin. 60:376–392. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Li Y, Xu H, Su S, Ye J, Chen J, Jin X, Lin
Q, Zhang D, Ye C and Chen C: Clinical validation of a highly
sensitive assay to detect EGFR mutations in plasma cell-free DNA
from patients with advanced lung adenocarcinoma. PLoS One.
12:e01833312017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Baylin SB, Herman JG, Graff JR, Vertino PM
and Issa JP: Alterations in DNA methylation: A fundamental aspect
of neoplasia. Adv Cancer Res. 72:141–196. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Jones PA and Laird PW: Cancer-epigenetics
comes of age. Nat Genet. 21:163–167. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ebrahimi V, Soleimanian A, Ebrahimi T,
Azargun R, Yazdani P, Eyvazi S and Tarhriz V: Epigenetic
modifications in gastric cancer: Focus on DNA methylation. Gene.
742:1445772020. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Eyvazi S, Khamaneh AM, Tarhriz V,
Bandehpour M, Hejazi MS, Sadat ATE and Sepehri B: CpG islands
methylation analysis of CDH11, EphA5, and HS3ST2 genes in gastric
adenocarcinoma patients. J Gastrointest Cancer. 51:579–583. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Donaldson J and Park BH: Circulating tumor
DNA: Measurement and clinical utility. Annu Rev Med. 69:223–234.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Chen X, Gole J, Gore A, He Q, Lu M, Min J,
Yuan Z, Yang X, Jiang Y, Zhang T, et al: Non-invasive early
detection of cancer four years before conventional diagnosis using
a blood test. Nat Commun. 11:34752020. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Jiang P, Chan KCA and Lo YMD:
Liver-derived cell-free nucleic acids in plasma: Biology and
applications in liquid biopsies. J Hepatol. 71:409–421. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Liu MC, Oxnard GR, Klein EA, Swanton C,
Seiden MV, Liu MC, Oxnard GR, Klein EA, Smith D, Richards D, et al:
Sensitive and specific multi-cancer detection and localization
using methylation signatures in cell-free DNA. Ann Oncol.
31:745–759. 2020. View Article : Google Scholar
|
|
55
|
U.S. Food and Drug Administration (FDA), .
Premarket Approval (PMA) for Epi ProColon. FDA; Silver Spring, MD:
2016
|
|
56
|
Bennett KL, Karpenko M, Lin MT, Claus R,
Arab K, Dyckhoff G, Plinkert P, Herpel E, Smiraglia D and Plass C:
Frequently methylated tumor suppressor genes in head and neck
squamous cell carcinoma. Cancer Res. 68:4494–4499. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Kreuziger LM, Porcher JC, Ketterling RP
and Steensma DP: An MLL-SEPT9 fusion and t(11;17)(q23;q25)
associated with de novo myelodysplastic syndrome. Leuk Res.
31:1145–1148. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Shirley M: Epi proColon® for
colorectal cancer screening: A profile of its use in the USA. Mol
Diagn Ther. 24:497–503. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Church TR, Wandell M, Lofton-Day C, Mongin
SJ, Burger M, Payne SR, Castaños-Vélez E, Blumenstein BA, Rösch T,
Osborn N, et al: Prospective evaluation of methylated SEPT9 in
plasma for detection of asymptomatic colorectal cancer. Gut.
63:317–325. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Nian J, Sun X, Ming S, Yan C, Ma Y, Feng
Y, Yang L, Yu M, Zhang G and Wang X: Diagnostic accuracy of
methylated SEPT9 for blood-based colorectal cancer detection: A
systematic review and meta-analysis. Clin Transl Gastroenterol.
8:e2162017. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Cai L, Hood S, Kallam E, Overman D, Barker
K, Rutledge D, Riojas J, Best C, Eisenberg M and Kam-Morgan L: Epi
proColon®: Use of a non-invasive SEPT9 gene methylation
blood test for colorectal cancer screening: A national laboratory
experience. J Clin Epigenet. 4:72018. View Article : Google Scholar
|
|
62
|
Sherr CJ: The pezcoller lecture: Cancer
cell cycles revisited. Cancer Res. 60:3689–3695. 2000.PubMed/NCBI
|
|
63
|
Weisenberger DJ: Characterizing DNA
methylation alterations from the cancer genome atlas. J Clin
Invest. 124:17–23. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Kanyama Y, Hibi K, Nakayama H, Kodera Y,
Ito K, Akiyama S and Nakao A: Detection of p16 promoter
hypermethylation in serum of gastric cancer patients. Cancer Sci.
94:418–420. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Ichikawa D, Koike H, Ikoma H, Ikoma D,
Tani N, Otsuji E, Kitamura K and Yamagishi H: Detection of aberrant
methylation as a tumor marker in serum of patients with gastric
cancer. Anticancer Res. 24:2477–2481. 2004.PubMed/NCBI
|
|
66
|
Guo L, Huang C and Ji QJ: Aberrant
promoter hypermethylation of p16, survivin, and retinoblastoma in
gastric cancer. Bratisl Lek Listy. 118:164–168. 2017.PubMed/NCBI
|
|
67
|
Abbaszadegan MR, Moaven O, Sima HR,
Ghafarzadegan K, A'Rabi A, Forghani MN, Raziee HR, Mashhadinejad A,
Jafarzadeh M, Esmaili-Shandiz E and Dadkhah E: p16 promoter
hypermethylation: A useful serum marker for early detection of
gastric cancer. World J Gastroenterol. 14:2055–2060. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Chen F, Liu X, Bai J, Pei D and Zheng J:
The emerging role of RUNX3 in cancer metastasis (Review). Oncol
Rep. 35:1227–1236. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Kim TY, Lee HJ, Hwang KS, Lee M, Kim JW,
Bang YJ and Kang GH: Methylation of RUNX3 in various types of human
cancers and premalignant stages of gastric carcinoma. Lab Invest.
84:479–484. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Sakakura C, Hamada T, Miyagawa K, Nishio
M, Miyashita A, Nagata H, Ida H, Yazumi S, Otsuji E, Chiba T, et
al: Quantitative analysis of tumor-derived methylated RUNX3
sequences in the serum of gastric cancer patients. Anticancer Res.
29:2619–2625. 2009.PubMed/NCBI
|
|
71
|
Lu XX, Yu JL, Ying LS, Han J, Wang S, Yu
QM, Wang XB, Fang XH and Ling ZQ: Stepwise cumulation of RUNX3
methylation mediated by Helicobacter pylori infection
contributes to gastric carcinoma progression. Cancer.
118:5507–5517. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Lin Z, Luo M, Chen X, He X, Qian Y, Lai S,
Si J and Chen S: Combined detection of plasma ZIC1, HOXD10 and
RUNX3 methylation is a promising strategy for early detection of
gastric cancer and precancerous lesions. J Cancer. 8:1038–1044.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Shi DT, Han M, Gao N, Tian W and Chen W:
Association of RASSF1A promoter methylation with gastric cancer
risk: A meta-analysis. Tumour Biol. 35:943–948. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Wang YC, Yu ZH, Liu C, Xu LZ, Yu W, Lu J,
Zhu RM, Li GL, Xia XY, Wei XW, et al: Detection of RASSF1A promoter
hypermethylation in serum from gastric and colorectal
adenocarcinoma patients. World J Gastroenterol. 14:3074–3080. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Balgkouranidou I, Matthaios D,
Karayiannakis A, Bolanaki H, Michailidis P, Xenidis N, Amarantidis
K, Chelis L, Trypsianis G, Chatzaki E, et al: Prognostic role of
APC and RASSF1A promoter methylation status in cell free
circulating DNA of operable gastric cancer patients. Mutat Res.
778:46–51. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Pimson C, Ekalaksananan T, Pientong C,
Promthet S, Putthanachote N, Suwanrungruang K and Wiangnon S:
Aberrant methylation of PCDH10 and RASSF1A genes in blood samples
for non-invasive diagnosis and prognostic assessment of gastric
cancer. PeerJ. 4:e21122016. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Ohki R, Nemoto J, Murasawa H, Oda E,
Inazawa J, Tanaka N and Taniguchi T: Reprimo, a new candidate
mediator of the p53-mediated cell cycle arrest at the G2 phase. J
Biol Chem. 275:22627–22630. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Ooki A, Yamashita K, Yamaguchi K, Mondal
A, Nishimiya H and Watanabe M: DNA damage-inducible gene, Reprimo
functions as a tumor-suppressor and is suppressed by promoter
methylation in gastric cancer. Mol Cancer Res. 11:1362–1374. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Bernal C, Aguayo F, Villarroel C, Vargas
M, Díaz I, Ossandon FJ, Santibáñez E, Palma M, Aravena E,
Barrientos C and Corvalan AH: Reprimo as a potential biomarker for
early detection in gastric cancer. Clin Cancer Res. 14:6264–6269.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Liu L and Yang X: Implication of Reprimo
and hMLH1 gene methylation in early diagnosis of gastric carcinoma.
Int J Clin Exp Pathol. 8:14977–14982. 2015.PubMed/NCBI
|
|
81
|
Lai J, Wang H, Luo Q, Huang S, Lin S,
Zheng Y and Chen Q: The relationship between DNA methylation and
Reprimo gene expression in gastric cancer cells. Oncotarget.
8:108610–108623. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Hu W, Zheng W, Liu Q, Chu H, Chen S, Kim
JJ, Wu J and Si J: Diagnostic accuracy of DNA methylation in
detection of gastric cancer: A meta-analysis. Oncotarget.
8:113142–113152. 2015. View Article : Google Scholar
|
|
83
|
Chen X, Lin Z, Xue M, Si J and Chen S:
Zic1 promoter hypermethylation in plasma DNA is a potential
biomarker for gastric cancer and intraepithelial neoplasia. PLoS
One. 10:e01339062015. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Xue WJ, Feng Y, Wang F, Li P, Liu YF, Guo
YB, Wang ZW and Mao QS: The value of serum RASSF10 hypermethylation
as a diagnostic and prognostic tool for gastric cancer. Tumour
Biol. 37:11249–11257. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Cheung KF, Lam CN, Wu K, Ng EK, Chong WW,
Cheng AS, To KF, Fan D, Sung JJ and Yu J: Characterization of the
gene structure, functional significance, and clinical application
of RNF180, a novel gene in gastric cancer. Cancer. 118:947–959.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Liu C, Li N, Lu H, Wang Z, Chen C, Wu L,
Liu J, Lu Y and Wang F: Circulating SFRP1 promoter methylation
status in gastric adenocarcinoma and esophageal square cell
carcinoma. Biomed Rep. 3:123–127. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
epigenomics: Epigenomics AG gets CE-IVD
Mark for Lung Cancer Test Epi proLung(R). 2017.
|
|
88
|
Powrózek T, Krawczyk P, Kucharczyk T and
Milanowski J: Septin 9 promoter region methylation in free
circulating DNA-potential role in noninvasive diagnosis of lung
cancer: Preliminary report. Med Oncol. 31:9172014. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Lee HS, Hwang SM, Kim TS, Kim DW, Park DJ,
Kang SB, Kim HH and Park KU: Circulating methylated septin 9
nucleic Acid in the plasma of patients with gastrointestinal cancer
in the stomach and colon. Transl Oncol. 6:290–296. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Lehmann-Werman R, Neiman D, Zemmour H,
Moss J, Magenheim J, Vaknin-Dembinsky A, Rubertsson S, Nellgård B,
Blennow K, Zetterberg H, et al: Identification of tissue-specific
cell death using methylation patterns of circulating DNA. Proc Natl
Acad Sci USA. 113:E1826–E1834. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Snyder MW, Kircher M, Hill AJ, Daza RM and
Shendure J: Cell-free DNA comprises an in vivo nucleosome footprint
that informs its tissues-of-origin. Cell. 164:57–68. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Kang S, Li Q, Chen Q, Zhou Y, Park S, Lee
G, Grimes B, Krysan K, Yu M, Wang W, et al: CancerLocator:
Non-invasive cancer diagnosis and tissue-of-origin prediction using
methylation profiles of cell-free DNA. Genome Biol. 18:532017.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Guo S, Diep D, Plongthongkum N, Fung HL
and Zhang K and Zhang K: Identification of methylation haplotype
blocks aids in deconvolution of heterogeneous tissue samples and
tumor tissue-of-origin mapping from plasma DNA. Nat Genet.
49:635–642. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Wan JCM, Massie C, Garcia-Corbacho J,
Mouliere F, Brenton JD, Caldas C, Pacey S, Baird R and Rosenfeld N:
Liquid biopsies come of age: Towards implementation of circulating
tumour DNA. Nat Rev Cancer. 17:223–238. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Heitzer E, Haque IS, Roberts CES and
Speicher MR: Current and future perspectives of liquid biopsies in
genomics-driven oncology. Nat Rev Genet. 20:71–88. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Leung F, Kulasingam V, Diamandis EP, Hoon
DS, Kinzler K, Pantel K and Alix-Panabières C: Circulating tumor
DNA as a cancer biomarker: Fact or fiction? Clin Chem.
62:1054–1060. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Bartels S, Persing S, Hasemeier B,
Schipper E, Kreipe H and Lehmann U: Molecular analysis of
circulating cell-free DNA from lung cancer patients in routine
laboratory practice: A cross-platform comparison of three different
molecular methods for mutation detection. J Mol Diagn. 19:722–732.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Wan R, Wang Z, Lee JJ, Wang S, Li Q, Tang
F, Wang J, Sun Y, Bai H, Wang D, et al: Comprehensive analysis of
the discordance of EGFR mutation status between tumor tissues and
matched circulating tumor DNA in advanced non-small cell lung
cancer. J Thorac Oncol. 12:1376–1387. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Guttery DS, Page K, Hills A, Woodley L,
Marchese SD, Rghebi B, Hastings RK, Luo J, Pringle JH, Stebbing J,
et al: Noninvasive detection of activating estrogen receptor 1
(ESR1) mutations in estrogen receptor-positive metastatic breast
cancer. Clin Chem. 61:974–982. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Busser B, Lupo J, Sancey L, Mouret S,
Faure P, Plumas J, Chaperot L, Leccia MT, Coll JL, Hurbin A, et al:
Plasma circulating tumor DNA levels for the monitoring of melanoma
patients: Landscape of available technologies and clinical
applications. Biomed Res Int. 2017:59861292017. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Rachiglio AM, Esposito Abate R, Sacco A,
Pasquale R, Fenizia F, Lambiase M, Morabito A, Montanino A, Rocco
G, Romano C, et al: Limits and potential of targeted sequencing
analysis of liquid biopsy in patients with lung and colon
carcinoma. Oncotarget. 7:66595–66605. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Xu S, Lou F, Wu Y, Sun DQ, Zhang JB, Chen
W, Ye H, Liu JH, Wei S, Zhao MY, et al: Circulating tumor DNA
identified by targeted sequencing in advanced-stage non-small cell
lung cancer patients. Cancer Lett. 370:324–331. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Newman AM, Lovejoy AF, Klass DM, Kurtz DM,
Chabon JJ, Scherer F, Stehr H, Liu CL, Bratman SV, Say C, et al:
Integrated digital error suppression for improved detection of
circulating tumor DNA. Nat Biotechnol. 34:547–555. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Phallen J, Sausen M, Adleff V, Leal A,
Hruban C, White J, Anagnostou V, Fiksel J, Cristiano S, Papp E, et
al: Direct detection of early-stage cancers using circulating tumor
DNA. Sci Transl Med. 9:eaan24152017. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Belic J, Koch M, Ulz P, Auer M, Gerhalter
T, Mohan S, Fischereder K, Petru E, Bauernhofer T, Geigl JB, et al:
Rapid identification of plasma DNA samples with increased ctDNA
levels by a modified FAST-SeqS approach. Clin Chem. 61:838–849.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Kinde I, Wu J, Papadopoulos N, Kinzler KW
and Vogelstein B: Detection and quantification of rare mutations
with massively parallel sequencing. Proc Natl Acad Sci USA.
108:9530–9535. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Zhai J, Wu Y, Luo X, Li X and Yu DH:
Abstract 643: An ultra-sensitive multiplex allele-specific
real-time PCR (Udx-PCR) assay for detection of KRAS BRAF NRAS
mutations in colorectal cancer. Cancer Res. 78:6432018.
|
|
108
|
Chan KCA, Woo JKS, King A, Zee BCY, Lam
WKJ, Chan SL, Chu SWI, Mak C, Tse IOL, Leung SYM, et al: Analysis
of plasma epstein-barr virus DNA to screen for nasopharyngeal
cancer. N Engl J Med. 377:513–522. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Fiala C and Diamandis EP: Can Grail find
the trail to early cancer detection? Clin Chem Lab Med. 57:403–406.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Liu YH, Zhang LH, Ren H, Zhang GG, Qin F,
Kong GZ, Deng GR and Ji JF: Promoter hypermethylation of p16 gene
in pre- and post-operative plasma of patients with gastric
adenocarcinoma. Beijing Da Xue Xue Bao Yi Xue Ban. 37:257–260.
2005.(In Chinese). PubMed/NCBI
|
|
111
|
Koike H, Ichikawa D, Ikoma H, Otsuji E,
Kitamura K and Yamagishi H: Comparison of methylation-specific
polymerase chain reaction (MSP) with reverse
transcriptase-polymerase chain reaction (RT-PCR) in peripheral
blood of gastric cancer patients. J Surg Oncol. 87:182–186. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Guo X, Liu W, Pan Y, Ni P, Ji J, Guo L,
Zhang J, Wu J, Jiang J, Chen X, et al: Homeobox gene IRX1 is a
tumor suppressor gene in gastric carcinoma. Oncogene. 29:3908–3920.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Zheng Y, Chen L, Li J, Yu B, Su L, Chen X,
Yu Y, Yan M, Liu B and Zhu Z: Hypermethylated DNA as potential
biomarkers for gastric cancer diagnosis. Clin Biochem.
44:1405–1411. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Ng EK, Leung CP, Shin VY, Wong CL, Ma ES,
Jin HC, Chu KM and Kwong A: Quantitative analysis and diagnostic
significance of methylated SLC19A3 DNA in the plasma of breast and
gastric cancer patients. PLoS One. 6:e222332011. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Chen L, Su L, Li J, Zheng Y, Yu B, Yu Y,
Yan M, Gu Q, Zhu Z and Liu B: Hypermethylated FAM5C and MYLK in
serum as diagnosis and pre-warning markers for gastric cancer. Dis
Markers. 32:195–202. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Raja UM, Gopal G and Rajkumar T:
Intragenic DNA methylation concomitant with repression of ATP4B and
ATP4A gene expression in gastric cancer is a potential serum
biomarker. Asian Pac J Cancer Prev. 13:5563–5568. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Ling ZQ, Lv P, Lu XX, Yu JL, Han J, Ying
LS, Zhu X, Zhu WY, Fang XH, Wang S and Wu YC: Circulating
methylated XAF1 DNA indicates poor prognosis for gastric cancer.
PLoS One. 8:e671952013. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Balgkouranidou I, Karayiannakis A,
Matthaios D, Bolanaki H, Tripsianis G, Tentes AA, Lianidou E,
Chatzaki E, Fiska A, Lambropoulou M, et al: Assessment of SOX17 DNA
methylation in cell free DNA from patients with operable gastric
cancer. Association with prognostic variables and survival. Clin
Chem Lab Med. 51:1505–1510. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Zhang H, Song Y, Xia P, Cheng Y, Guo Q,
Diao D, Wang W, Wu X, Liu D and Dang C: Detection of aberrant
hypermethylated spastic paraplegia-20 as a potential biomarker and
prognostic factor in gastric cancer. Med Oncol. 31:8302014.
View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Wang G, Zhang W, Zhou B, Jin C, Wang Z,
Yang Y, Wang Z, Chen Y and Feng X: The diagnosis value of promoter
methylation of UCHL1 in the serum for progression of gastric
cancer. Biomed Res Int. 2015:7410302015. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Li WH, Zhou ZJ, Huang TH, Guo K, Chen W,
Wang Y, Zhang H, Song YC and Chang DM: Detection of OSR2, VAV3, and
PPFIA3 methylation in the serum of patients with gastric cancer.
Dis Markers. 2016:57805382016. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Hu H, Chen X, Wang C, Jiang Y, Li J, Ying
X, Yang Y, Li B, Zhou C, Zhong J, et al: The role of TFPI2
hypermethylation in the detection of gastric and colorectal cancer.
Oncotarget. 8:84054–84065. 2017. View Article : Google Scholar : PubMed/NCBI
|