Introduction
Colorectal cancer (CRC), accounts for ~10% of all
tumor-related deaths, is the third most common type of cancer
worldwide (1,2). Despite advances in diagnostic and
therapeutic strategies, the prognosis of patients with CRC remains
poor with high 5-year mortality (3).
Oxaliplatin in combination with 5-fluorouracil and leucovorin is
the first-line chemotherapeutic strategy for CRC, which functions
as a DNA transcription and replication suppressor by forming
platinum-DNA adducts (4).
Oxaliplatin resistance commonly contributes to the failure of CRC
therapy and worse clinical outcomes for patients with CRC. Thus,
there is an urgent need to identify novel anticancer drugs for
CRC.
Polyphyllin, which is derived from Paris
polyphyllin, has been used as a clinical drug in China
(5). In previous years, polyphyllin
was reported to be a potential anticancer drug in numerous types of
human cancers, including breast cancer, non-small cell lung cancer
and acute myeloid leukemia (6,7).
Recently, polyphyllin VII was found to have anticancer activity and
be involved in inhibiting cancer growth, suppressing cancer cell
metastasis, inducing cell cycle arrest and apoptosis in cancer
cells (8–10). Chen et al (11) found that polyphyllin VII suppressed
cell growth and induced autophagy in nasopharyngeal cancer via AKT
signaling. Zhang et al (12)
reported that polyphyllin VII could affect mitogen-activated
protein kinase (MAPK) signaling and mitochondrial dysfunction to
promote cell apoptosis in liver cancer cells. Although the
antitumor activities of polyphyllin VII has been demonstrated in
other types of cancer, the effects of polyphyllin VII on CRC
progression and its underlying mechanisms remain unclear.
The present study aimed to determine the potential
antitumor effects of polyphyllin VII in CRC. The effects of
polyphyllin VII on CRC proliferation, cell cycle, apoptosis and
migration were evaluated. Microarray analysis was performed to
identify downstream targets of polyphyllin VII in CRC. The present
study showed that polyphyllin VII could be a potential novel
therapeutic drug for CRC.
Materials and methods
Cell culture and treatment
The human CRC HCT116 cell line was obtained from
American Type Culture Collection. Cells were cultured in RPMI-1640
medium with 10% FBS (both Thermo Fisher Scientific, Inc.) and 100
U/ml penicillin-streptomycin at 37°C in a humidified atmosphere
containing 5% CO2.
Cell viability assay
Cell Counting Kit-8 (CCK-8; Dojindo Molecular
Technologies, Inc.) assay was used to detect the effects of
polyphyllin VII (Chengdu Must Bio-Technology Co., Ltd.) on CRC cell
viability, according to the manufacturer's instructions. Briefly,
1×104 HCT116 cells/well were seeded into 96-well plates.
Subsequently, 0.9197 µg/ml polyphyllin VII was added and cells were
incubated at 37°C for 5 days. Cultured HCT116 cells were incubated
for 72 h in the presence of vehicle or polyphyllin VII at the
indicated doses (0.1, 0.2, 0.4, 0.6, 0.8, 1.0, 1.2, 1.4, 2.0 and 5
µg/ml). At each time point (days 1, 2, 3, 4 and 5), 10 µl CCK-8
solution was added to the cells. Following 2 h of incubation, the
absorbance was detected at a wavelength of 450 nm.
Cell cycle assay
Following polyphyllin VII treatment, HCT116 cells
were collected and washed with PBS. Subsequently, cells
(1×106) were treated with 0.03% Triton X-100 for 15 min,
and stained with propidium iodide (PI; 50 ng/ml) and 80 µg/ml
ribonuclease A (RNAse; Gibco; Thermo Fisher Scientific, Inc.) at
room temperature for 15 min. Cell cycle distribution was detected
using a flow cytometer (FACSCalibur; BD Biosciences) and analyzed
using ModFit 5.0 software (Verity Software House, Inc.).
Annexin V/PI double staining
The effects of polyphyllin VII on CRC cell apoptosis
were determined using the Annexin V-FITC/PI apoptosis detection kit
(EMD Millipore), according to the manufacturer's instructions.
Briefly, cells were washed three times with PBS and resuspended in
1X binding buffer provided by the apoptosis kit. Subsequently, 2
µg/ml Annexin V-FITC and 2.5 µg/ml PI were added to the
1×106 cells. Following incubation in the dark for 15
min, the early and late apoptosis statuses were analyzed using a
flow cytometer, using FACSCalibur software (BD Biosciences).
Apoptotic cells were subsequently analyzed via flow cytometry,
using MoFlo XDP (Beckman Coulter, Inc.).
Colony formation assay
The colony formation assay was conducted according
to Chen et al (11). Briefly,
1×104 HCT116 cells/well were seeded into a six-well
plate. At 24 h later, 0.9197 µg/ml of polyphyllin VII were added to
the cells and incubated at 37°C for 2 weeks. After 2 weeks,
colonies (>50 cells) were stained with 0.3% crystal violet
solution.
Wound healing assay
A total of 1×106 HCT116 cells/well were
seeded into a six-well plate. When cell confluency reached ~80%, a
scratch was created in the cell monolayer using a sterile
micropipette tip. The detached cells were then washed with PBS.
Then, the RPMI-1640 medium (Thermo Fisher Scientific, Inc.) was
replaced with 1 ml fresh RPMI 1640 medium without FBS. The extent
of wound healing was observed at 0, 8 and 30 h. Images were
captured in five randomly selected fields using an inverted
microscope. Migration ability was determined by calculating the
change in uncovered area between 0 and 24 h using MetaMorph
software (MetaMorph Inc.).
Transwell assay
The invasion assay was performed using
Matrigel® (BD Biosciences), which was used in 8-mm pore
size Transwell plates (Corning, Inc.). A total of 5×104
HCT116 cells, cultured in RPMI-1640 medium with 2% FBS, were seeded
into the upper chamber. In addition, RPMI-1640 with 10% FBS was
added to the bottom chamber. Following incubation for 72 h at 37°C,
the cells were fixed with a 4% paraformaldehyde solution for 30 min
at room temperature, followed by rinsing with PBS and staining with
a 0.5% crystal violet dye solution for 10 min at room temperature.
Subsequently, the cell counting was performed with a light
microscope at ×200 magnification.
Western blot analysis
Cells were lysed with RIPA lysis buffer (Applygen
Technologies, Inc.). The BCA assay kit (Sangon Technology) was used
to detect the protein concentration. Proteins (35 µg/lane) were
subjected to 10% SDS-PAGE, and then subsequently transferred onto a
PVDF membrane (EMD Millipore). Membranes were then incubated with
5% non-fat milk for 1 h at room temperature, and stained with
primary antibodies against ribonucleoside-diphosphate reductase
subunit M2 (RRM2; 1:1,000; cat. no. ab172476; Abcam), Bax (1:1,000;
cat. no. ab32503; Abcam), Bcl-2 (1:1,000; cat. no. ab59348; Abcam),
structural maintenance of chromosomes protein 4 (SMC4; 1:1,000;
cat. no. HPA029449; Sigma-Aldrich; Merck KGaA), cleaved caspase-3
(1:1,000; cat. no. 9661S; Cell Signaling Technology, Inc.) and
anti-GAPDH (1:1,000; cat. no. sc-32233; Santa Cruz Biotechnology,
Inc.) at 4°C overnight. Subsequently, the membrane was washed three
times in TBS with 5% Tween-20, and then incubated with horseradish
peroxidase-conjugated anti-rabbit (cat. no. IH-0011; 1:5,000
dilution) and anti-mouse (cat. no. IH-0031; 1:5,000 dilution) (both
Beijing Dingguo Changsheng Biotechnology Co., Ltd.) antibodies for
1 h at room temperature. Finally, the membrane was visualized with
Enhanced Chemiluminescence Plus reagents (Thermo Fisher Scientific,
Inc.). Protein band density was normalized to the corresponding
GAPDH density.
Microarray and gene expression
analysis
Gene expression profiling was analyzed using the
PrimeView™ Human Gene Expression Arrays (Affymetrix; Thermo Fisher
Scientific, Inc.), as described previously (13); the CEL-files of the raw data were
obtained by Affymetrix GeneChip® Command
Console® v1.0 Software (Affymetrix; Thermo Fisher
Scientific, Inc.). Total RNA was extracted from HCT116 cells using
TRIzol reagent 3 days after 0.9197 µg/ml polyphyllin VII and
control treatment (DMSO) at 37°C for 3 days. RNA quantity and
quality were assessed with a NanoDrop™ 2000 spectrophotometer
(Thermo Fisher Scientific, Inc.) and an Agilent Bioanalyzer 2100.
Briefly, reverse transcription, cDNA synthesis, and labeling were
all performed using the GeneChip 3′ IVT Expression kit (Thermo
Fisher Scientific, Inc.). Microarray hybridization, washing and
staining were carried out using the GeneChip Hybridization Wash and
Stain kit (Thermo Fisher Scientific, Inc.).
The limma method in Bioconductor (version 3.38.3;
http://www.bioconductor.org) was used to
identify the genes that were differentially expressed between the
two groups (14). Genes with an
adjusted P<0.05 after FDR correction and a fold-change of >2
or <0.5 were considered as differentially expressed genes
(DEGs).
GO and KEGG pathway analyses
Database for Annotation, Visualization and
Integrated Discovery (DAVID) 6.8 (http://david.abcc.ncifcrf.gov/) is an online platform
that is used for gene annotation, visualization and integrated
discovery (15). Gene Ontology (GO)
(16) and Kyoto Encyclopedia of
Genes and Genomes (KEGG) (17)
pathway enrichment analyses were implemented with the DAVID
database. Using this comprehensive tool, the biological meaning
behind the DEGs can be understood more quickly and effectively.
P<0.05 indicated a statistically significant difference.
Protein-protein interaction (PPI)
network
The Search Tool for the Retrieval of Interacting
Genes (STRING, http://string-db.org) database was
used to construct the PPI network (18). A confidence score ≥0.9 was set as the
cut-off criterion, and disconnected nodes were excluded from the
network. CytoHubba (19) and
Molecular Complex Detection (MCODE) (20) in Cytoscape 3.5.1 (21) were performed to identify hub genes
and significant modules of the PPI network. The filter conditions
were as follows: Degree cut-off ≥2 and nodes with edges
≥2-core.
Gene expression profiling interactive
analysis (GEPIA)
The present study used GEPIA (22) dataset to analyze the expression
levels of DEGs after polyphyllin VII treatment in order to explore
the differential expression and its effect on the prognosis of
patients with rectum adenocarcinoma (READ) and colon adenocarcinoma
(COAD).
Statistical analysis
Each experiment was performed at least three times
in the present study. Statistical analysis was conducted using SPSS
13.0 software (SPSS, Inc.). Statistical significance between two
groups was determined using student's t-test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Polyphyllin VII inhibits CRC cell
proliferation and colony formation
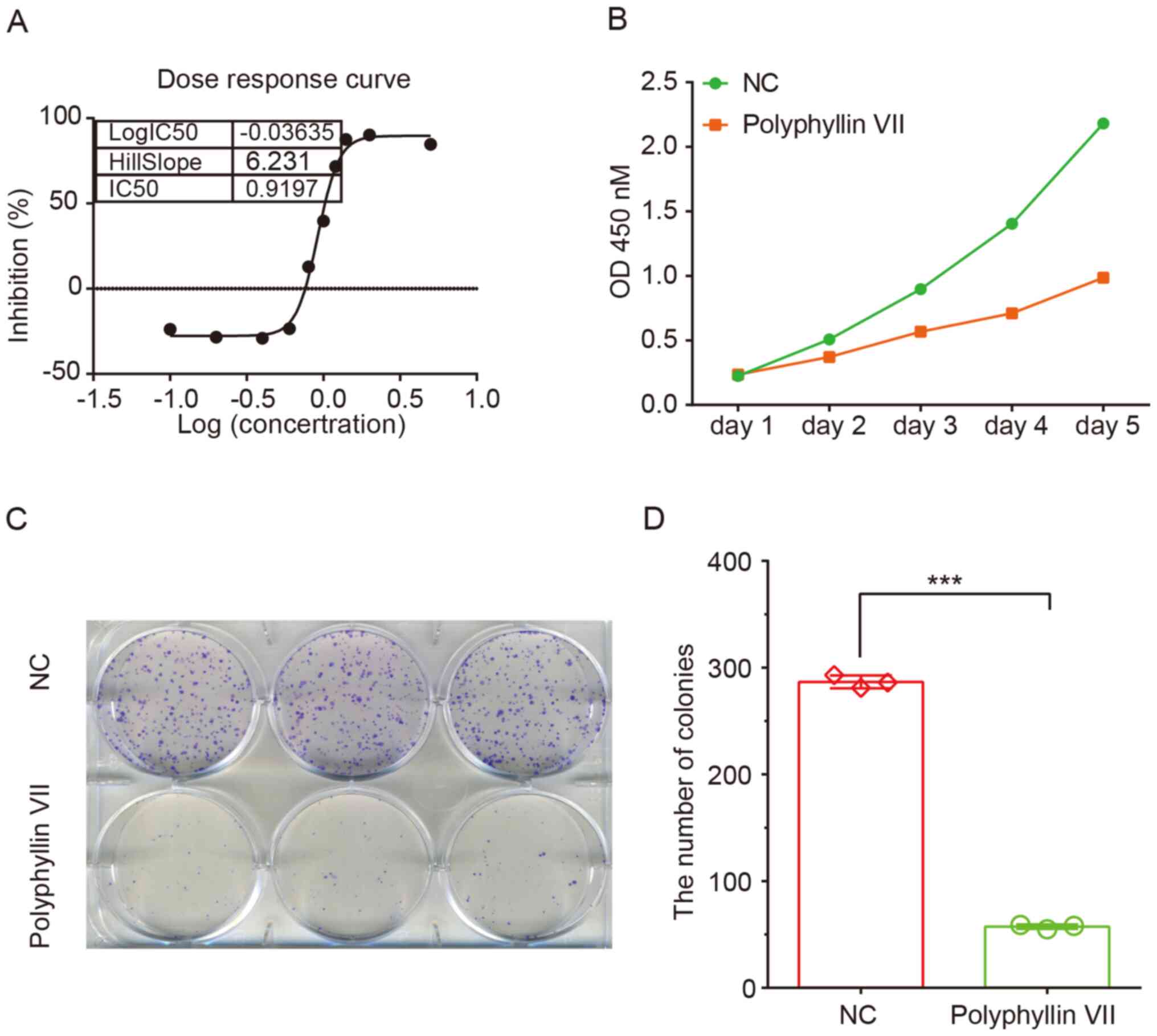
The effects of polyphyllin VII on CRC proliferation
were first examined using a CCK-8 assay. As shown in Fig. 1, compared with the control group,
polyphyllin VII treatment resulted in a significant reduction in
HCT116 cell viability. The IC50 at 72 h was 0.9197
µg/ml, which was the dose used for further experiments (Fig. 1A).
A CCK-8 assay was further used to evaluate the
effect of polyphyllin VII treatment on CRC cell proliferation. The
results showed that polyphyllin VII treatment notably inhibited
HCT116 cell proliferation compared with the control group (Fig. 1B). In addition, polyphyllin VII
treatment significantly suppressed HCT116 cell colony formation.
The relative colony number in the polyphyllin VII treatment group
decreased by 85% in HCT116 cells (Fig.
1C and D).
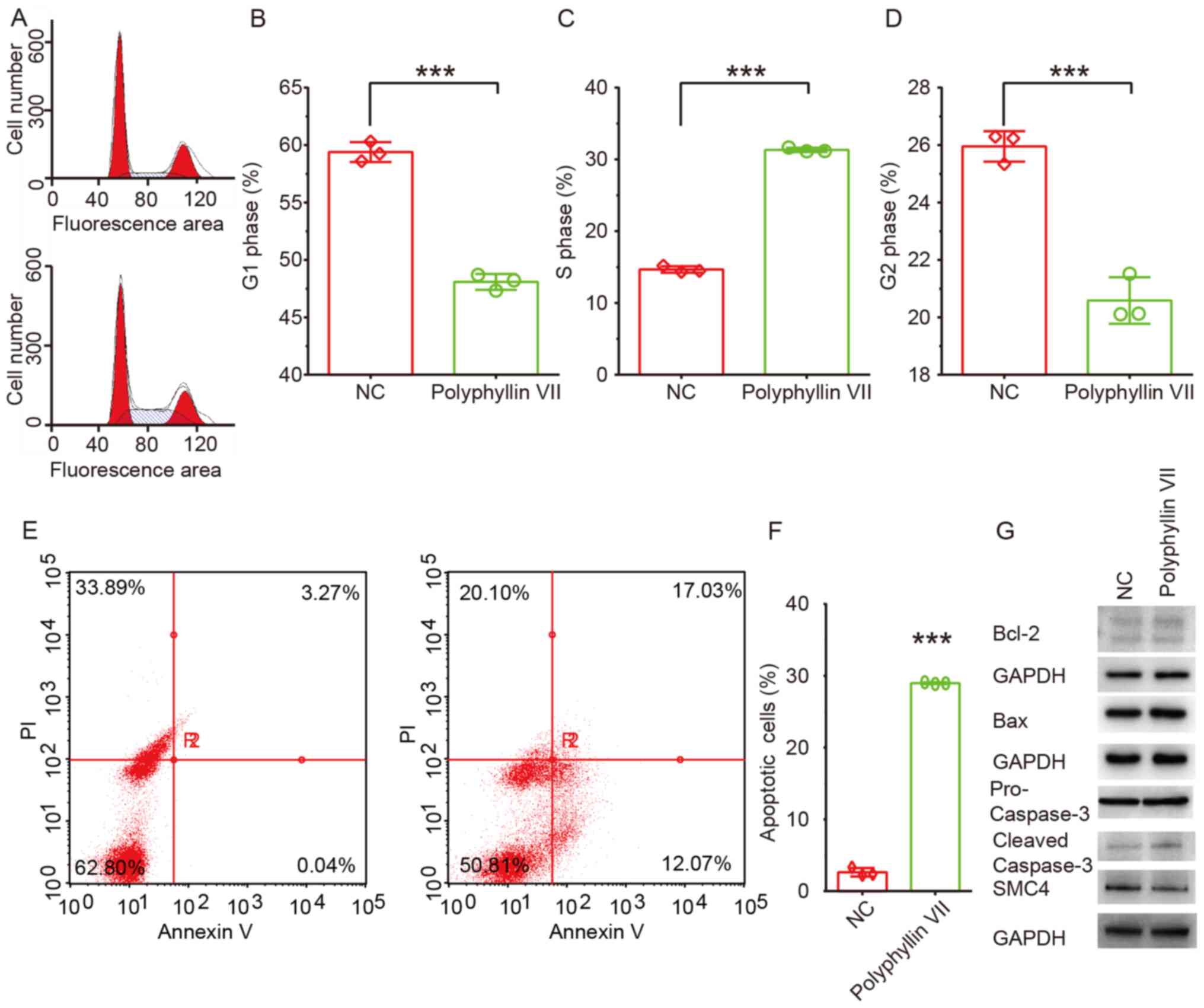
Polyphyllin VII induces cell cycle
arrest and apoptosis in CRC cells
In order to explore how polyphyllin VII suppresses
CRC cell proliferation, the cell cycle of CRC cells after
polyphyllin VII treatment was analyzed using flow cytometry. The
results showed that polyphyllin VII could significantly induce cell
cycle arrest in S phase, which led to a significant increase of
cells in S phase, but a significant decrease of cells in the G1 and
G2 phases (Fig. 2A-D).
The effect of polyphyllin VII on CRC cell apoptosis
was then assessed using flow cytometry. At 3 days post-incubation,
the number of apoptotic HCT116 cells in the polyphyllin VII group
increased by 50% compared with the control group (Fig. 2E and F). Then, the protein expression
levels of pro caspase-3, cleaved caspase-3, Bcl-2 and Bax were
detected in CRC cells following polyphyllin VII treatment. A
notable increase in the expression levels of cleaved caspase-3 and
Bax were observed (Fig. 2G).
However, no significant changes in the expression levels of pro
caspase 3 and Bcl-2 were observed in CRC cells after polyphyllin
VII treatment (Fig. 2G).
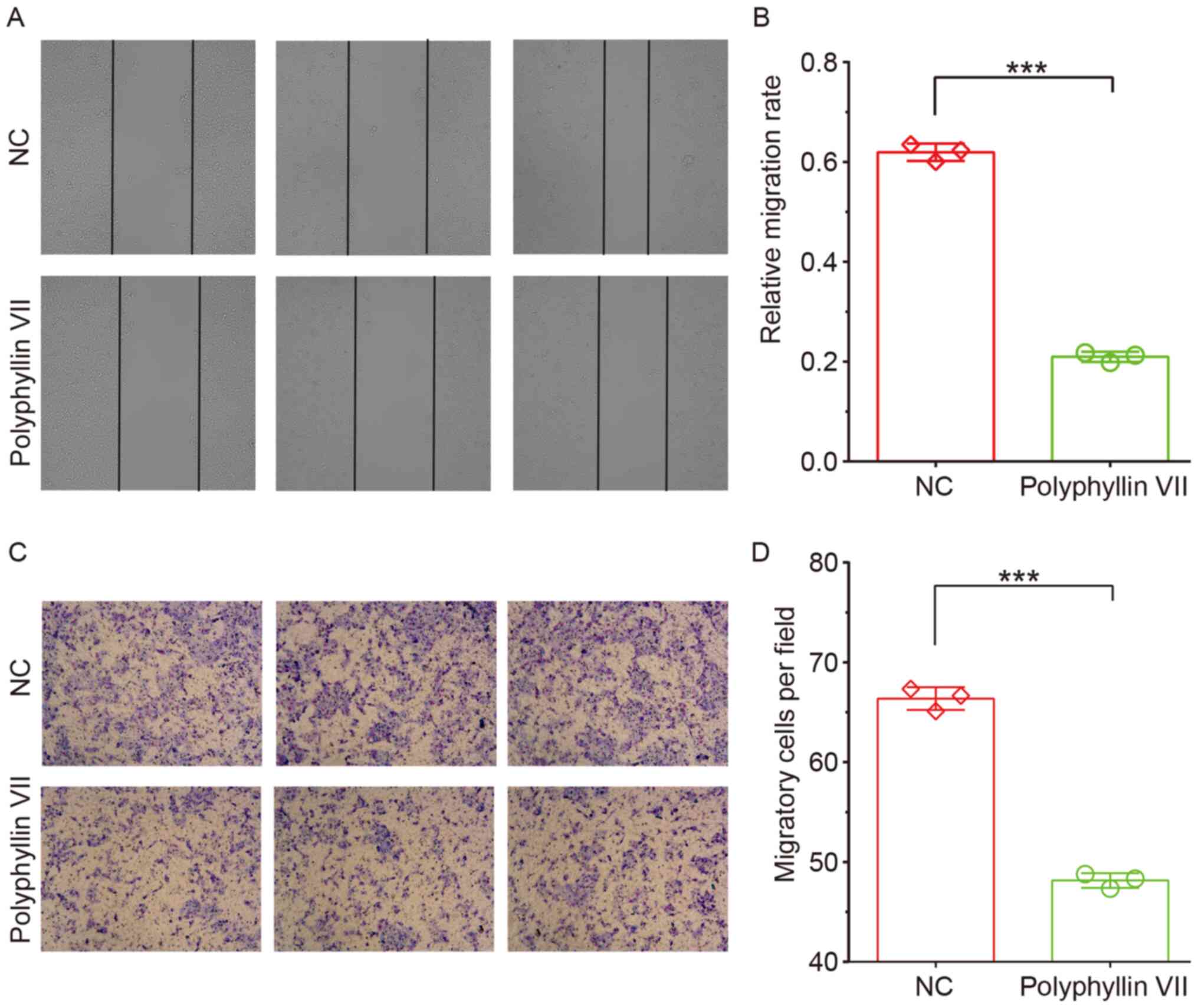
Polyphyllin VII suppresses CRC
invasion
Subsequently, the effects of polyphyllin VII
treatment on CRC cell migration were determined using a wound
healing assay. Cells treated with polyphyllin VII showed lower
wound healing ability (Fig. 3A and
B). A Transwell assay was also performed to validate the
present findings. As shown in Fig.
3, the number of polyphyllin VII-treated HCT116 cells that
passed through the Transwell membrane significantly decreased
compared with the control group (Fig. 3C
and D). The results indicated that polyphyllin VII could
suppress CRC metastasis.
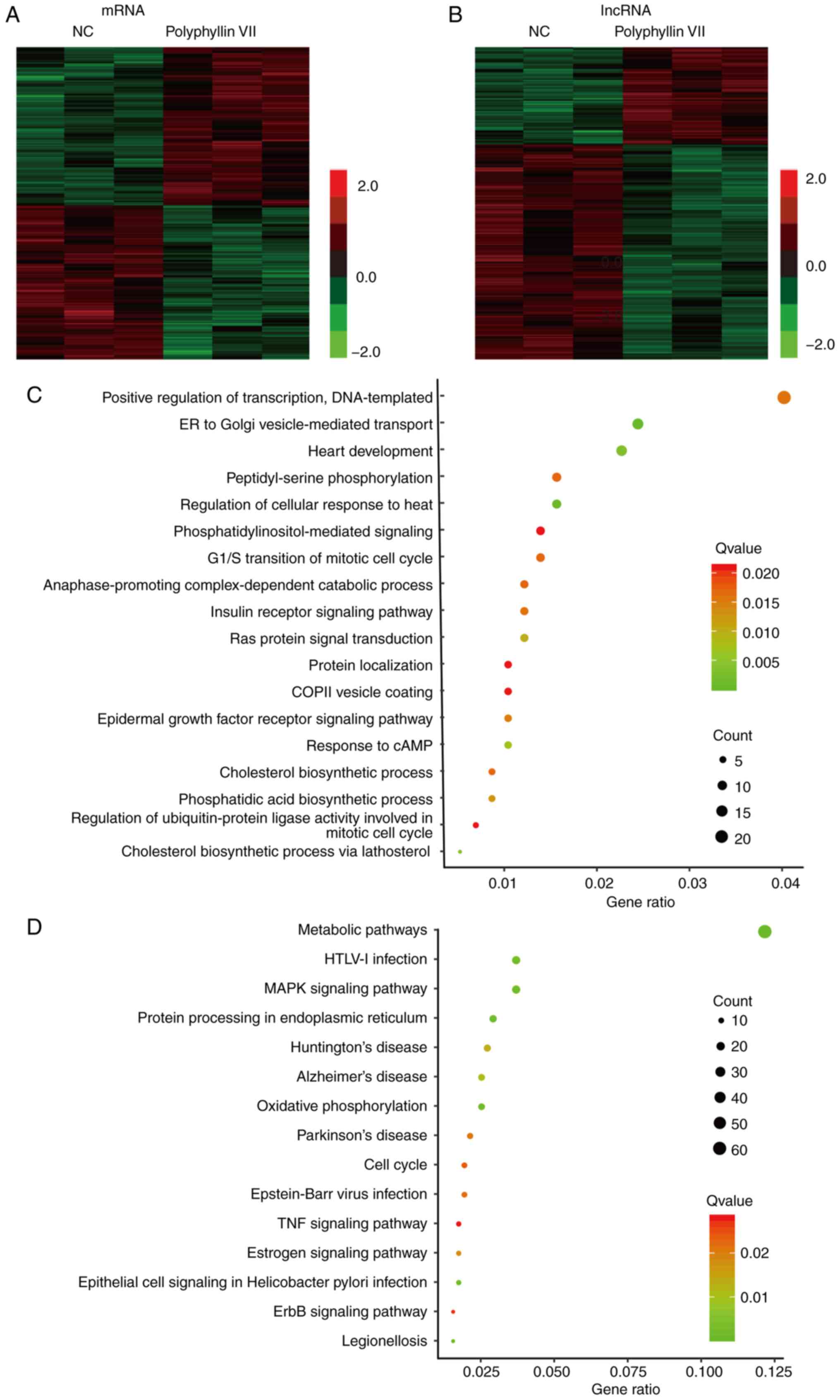
Identification of polyphyllin
VII-regulated DEGs in CRC
The present study screened differentially expressed
mRNAs and long non-coding RNAs (lncRNAs) following polyphyllin VII
treatment in CRC using microarray analysis. Genes with a
fold-change in expression of ≥2.0 and P<0.05 between control and
polyphyllin VII-treated samples were identified to be
differentially expressed.
As shown in Fig. 4,
469 mRNAs were detected to be differentially regulated by a
fold-change ≥2.0, among which 411 mRNAs were upregulated, while 511
mRNAs were downregulated (Fig. 4A).
The top 10 differentially expressed mRNAs are listed in Table I. A total of 347 upregulated lncRNAs
and 1,765 downregulated lncRNAs were observed after polyphyllin VII
treatment in CRC samples (Fig. 4B).
Several well-known lncRNAs, such as urothelial cancer associated 1
(UCA1), nuclear enriched abundant transcript 1 (NEAT1), metastasis
associated lung adenocarcinoma transcript 1 (MALAT1) and ZNFX1
antisense RNA 1 were identified to be polyphyllin VII targets. The
top 10 differentially expressed lncRNAs are listed in Table II. Hierarchical clustering showed
differentially expressed mRNAs and lncRNAs in CRC.
 | Table I.Top 10 differentially expressed mRNAs
following treatment with polyphyllin VII. |
Table I.
Top 10 differentially expressed mRNAs
following treatment with polyphyllin VII.
| Gene symbol | Polyphyllin
VII | Control | Fold-change | ANOVA P-value |
|---|
| HSPA1B | 11.14 | 12.98 | −3.57 |
1.86×10−3 |
| ADM | 7.87 | 9.69 | −3.54 |
3.94×10−4 |
| HSPA1A | 9.52 | 11.17 | −3.14 |
1.11×10−3 |
| HSPH1 | 11.32 | 12.74 | −2.69 |
1.64×10−4 |
| SLC38A1 | 12.71 | 14.09 | −2.60 |
6.41×10−5 |
| KLF7 | 12.35 | 11.16 | 2.28 |
1.90×10−2 |
| MSL1 | 9.60 | 8.39 | 2.31 |
1.67×10−2 |
| KRTAP2-2 | 12.94 | 11.63 | 2.47 |
3.96×10−3 |
| KBTBD3 | 5.48 | 4.14 | 2.54 |
1.62×10−2 |
| TAS2R30 | 7.83 | 6.27 | 2.95 |
3.10×10−2 |
 | Table II.Top 10 differentially expressed long
non-coding RNAs following treatment with polyphyllin VII. |
Table II.
Top 10 differentially expressed long
non-coding RNAs following treatment with polyphyllin VII.
| Gene symbol | Polyphyllin
VII | Control | Fold-change | ANOVA P-value |
|---|
| RNU6-175P | 6.10 | 7.88 | −3.44 |
2.72×10−3 |
| LDHAP5 | 7.20 | 8.82 | −3.07 |
9.78×10−3 |
| UCA1 | 13.06 | 14.56 | −2.82 |
2.70×10−5 |
| RPS27P18 | 3.84 | 5.20 | −2.57 |
2.63×10−3 |
| RP11-84C10.3 | 5.24 | 6.59 | −2.55 |
1.03×10−2 |
| AC114498.1 | 11.13 | 9.62 | 2.85 |
2.46×10−2 |
| ADAM20P1 | 7.04 | 5.52 | 2.88 |
6.81×10−3 |
| AC069063.1 | 7.32 | 5.768 | 2.95 |
2.54×10−2 |
| RP11-255C15.3 | 5.10 | 3.53 | 2.96 |
9.51×10−3 |
| RP11-53O19.3 | 7.97 | 5.93 | 4.12 |
4.45×10−3 |
GO and KEGG analysis of polyphyllin
VII-regulated genes in CRC
Furthermore, GO and KEGG analyses were performed to
identify polyphyllin VII targets. GO analysis showed that
polyphyllin VII targets were significantly associated with multiple
biological processes, including ‘ER to Golgi vesicle-mediated
transport’, ‘heart development’, ‘regulation of cellular response
to heat’, ‘cholesterol biosynthetic process via lathosterol’,
‘response to cAMP’, ‘Ras protein signal transduction’,
‘phosphatidic acid biosynthetic process’, ‘epidermal growth factor
receptor signaling pathway’, ‘insulin receptor signaling pathway’,
‘positive regulation of transcription, DNA-templated’,
‘peptidyl-serine phosphorylation’, ‘cholesterol biosynthetic
process’, ‘anaphase-promoting complex-dependent catabolic process’,
‘G1/S transition of mitotic cell cycle’,
‘phosphatidylinositol-mediated signaling’, ‘regulation of
ubiquitin-protein ligase activity involved in mitotic cell cycle’,
‘COPII vesicle coating’ and ‘protein localization’ (Fig. 4C).
KEGG pathway analysis revealed that polyphyllin VII
target genes were enriched in ‘metabolic pathways’, ‘protein
processing in endoplasmic reticulum’, ‘oxidative phosphorylation’,
‘epithelial cell signaling in Helicobacter pylori
infection’, ‘legionellosis’, ‘MAPK signaling pathway’, ‘HTLV–I
infection’, ‘Alzheimer's disease’, ‘Huntington's disease’,
‘estrogen signaling pathway’, ‘Parkinson's disease’, ‘Epstein-Barr
virus infection’, ‘cell cycle’, ‘ErbB signaling pathway’ and ‘TNF
signaling pathway’ (Fig. 4D).
PPI network analysis of polyphyllin
VII-regulated genes in CRC
Based on the STRING database (18), PPI networks were constructed for
polyphyllin VII-induced and reduced genes in CRC. Following the
construction of the PPI network, hub PPI networks were identified
using the MCODE plugin (degree cut-off ≥2 and nodes with edges
≥2-core). The top polyphyllin VII-induced hub modules (module A)
and top polyphyllin VII-reduced hub modules (module B) are shown in
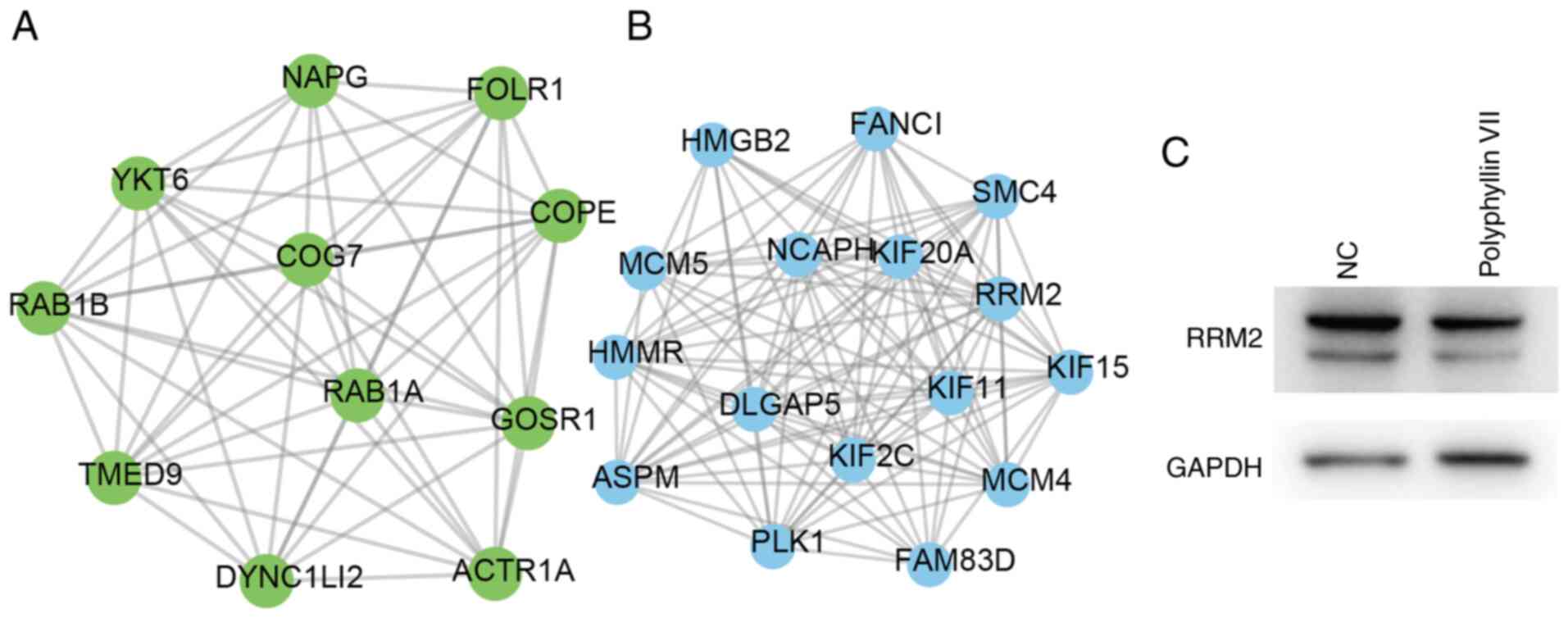
Fig. 5. Module A contained 11 nodes
and 53 edges, including RAB1B, RAB1A, TMED9, YKT6, COG7, GOSR1,
NAPG, FOLR1, COPE, DYNC1LI2 and ACTR1A (Fig. 5A). Module B contained 16 nodes and
106 edges, including ASPM, PLK1, HMGB2, KIF2C, KIF15, FAM83D,
KIF11, KIF20A, FANCI, HMMR, MCM5, MCM4, NCAPH, SMC4, RRM2 and
DLGAP5 (Fig. 5B). In order to
confirm the effects of polyphyllin VII on these proteins, the
protein expression levels of SMC4 and RRM2 were detected in HCT116
cells following polyphyllin VII treatment. The results showed that
Polyphyllin VII treatment led to a notable decrease of SMC4
(Fig. 2G) and RRM2 (Fig. 5C).
Key polyphyllin VII-regulated genes
are dysregulated in CRC
To explore the clinical implication of key
polyphyllin VII-regulated genes in CRC, their expression levels in
CRC samples were analyzed using the GEPIA database. As shown in
Fig. 6, the results showed that
ACTR1A (Fig. 6A), DYNC1LI2 (Fig. 6B) and COG7 (Fig. 6C) were significantly downregulated in
both COAD and READ samples compared with normal tissues. Whereas,
HMGB2 (Fig. 6D), PLK1 (Fig. 6E), DLGAP5 (Fig. 6F), RRM2 (Fig. 6G), SMC4 (Fig. 6H), NCAPH (Fig. 6I), MCM4 (Fig. 6J), HMMR (Fig. 6K), FANCI (Fig. 6L), KIF20A (Fig. 6M), KIF11 (Fig. 6N), KIF15 (Fig. 6O), KIF2C (Fig. 6P) and MCM5 (Fig. 6Q) were significantly upregulated in
COAD and READ samples compared with normal tissues.
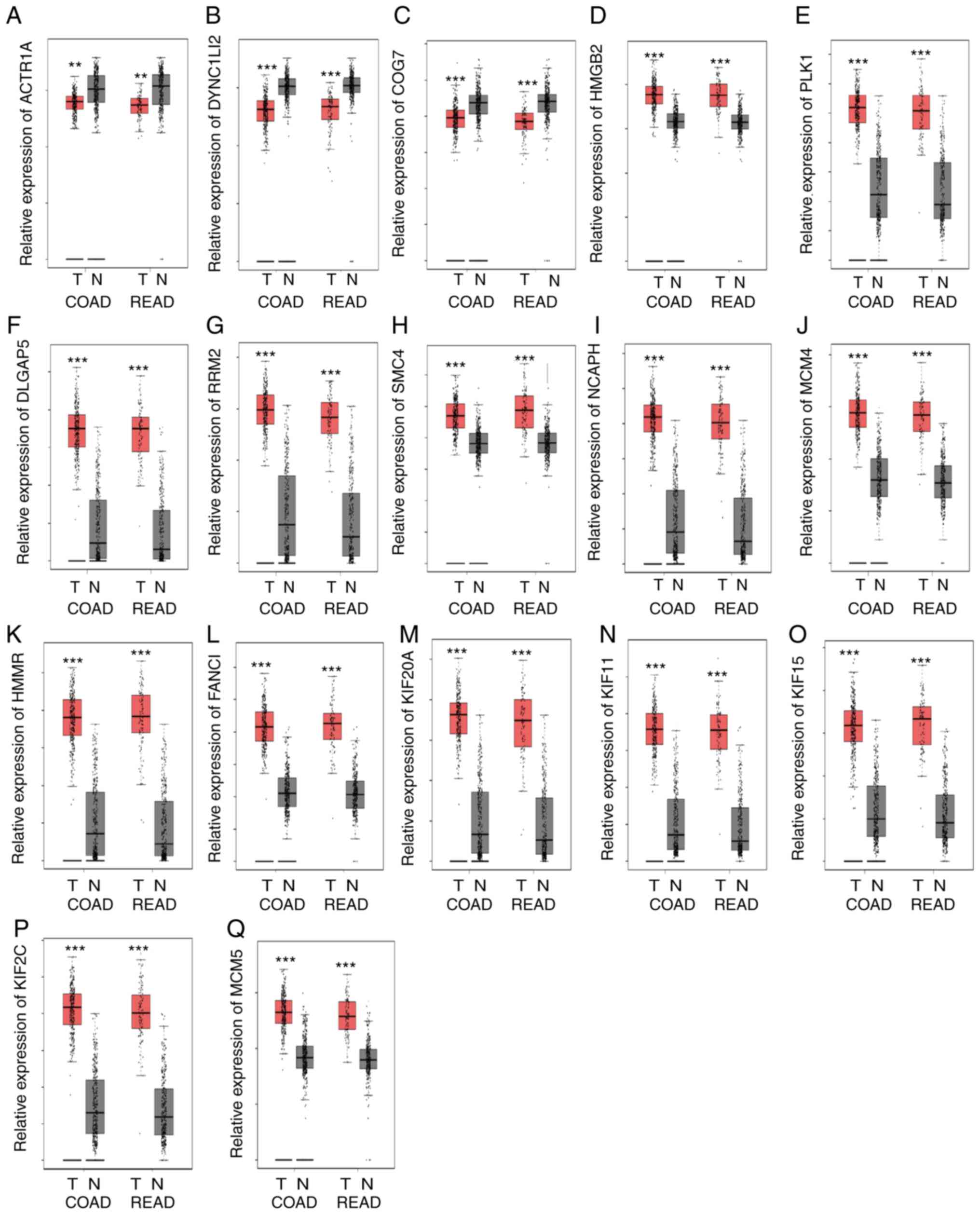 | Figure 6.Polyphyllin VII target genes are
dysregulated in CRC samples. (A) ACTR1A, (B) DYNC1LI2 and (C) COG7
were downregulated in COAD and READ samples compared with normal
tissues. (D) HMGB2, (E) PLK1, (F) DLGAP5, (G) RRM2, (H) SMC4, (I)
NCAPH, (J) MCM4, (K) HMMR, (L) FANCI, (M) KIF20A, (N) KIF11, (O)
KIF15, (P) KIF2C and (Q) MCM5 were upregulated in COAD and READ
samples compared with normal tissues. **P<0.01 and ***P<0.001
vs. normal tissue. N, normal; T, tumor; COAD, colon adenocarcinoma;
READ, rectum adenocarcinoma. |
Discussion
There is an urgent need to identify novel anticancer
drugs for CRC. In the past decades, multiple studies demonstrated
that natural herbal products could be promising novel
chemotherapeutics for the treatment of human cancers. Various
herbal products were found to exhibit anticancer activity by
suppressing cell proliferation and metastasis and inducing cell
apoptosis and autophagy (23–25).
Previous studies reported that polyphyllin could suppress the
progression of multiple human cancers, including lung (26), gastric (27), breast (28) and prostate (29) cancer. Polyphyllin exerts its roles in
cancers by inducing tumor cell apoptosis (30), suppressing tumor cell proliferation
(31), angiogenesis (32) and reversing multidrug resistance of
tumor cells (33,34). Exploring the antitumor effect of
polyphyllin could provide novel drugs for CRC treatment.
The anticancer effects of polyphyllin VII has been
demonstrated in a number of cancers. The results showed that
polyphyllin VII could induce cancer cell apoptosis via regulating
caspase protein activation in oral cancer (30). Several recent studies demonstrated
that polyphyllin VII could induce the autophagy process in tumor
cells (11,30,35).
Distant metastasis is the primary cause of cancer-related deaths.
Wang et al (36) reported
that polyphyllin VI could suppress breast cancer invasion,
suggesting the anti-metastatic roles of polyphyllin. The present
study revealed that polyphyllin VII could significantly inhibit CRC
proliferation, and induce cell cycle arrest and CRC cell apoptosis.
A notable increase of cleaved caspase-3 and Bax expression was also
observed. These results were consistent with previous reports
(11,12,30).
Moreover, the anti-metastatic effects of polyphyllin VII in CRC
cells were validated. These results suggested that polyphyllin VII
could be a potential antitumor drug for CRC.
Recently, emerging studies have demonstrated that
lncRNAs play crucial roles in human cancer progression (37,38). In
CRC, it has been reported that lncRNAs are involved in regulating
tumor proliferation, metastasis and therapeutic resistance. The
lncRNA CALIC was found to promote CRC metastasis via upregulating
AXL receptor tyrosine kinase (39).
SNHG6 was revealed to suppress CRC cell proliferation and
metastasis by targeting ETS proto-oncogene 1 transcription factor
(40). To the best of our knowledge,
the present study, for the first time, identified 347 upregulated
lncRNAs and 1,765 downregulated lncRNAs following polyphyllin VII
treatment in CRC cells. Several well-known lncRNAs, such as UCA1
(41), NEAT1 (42), and MALAT1 (43) were identified to be polyphyllin VII
targets. UCA1 was previously found to be overexpressed in both CRC
tissues and plasma samples, and its expression was correlated with
shorter survival times, which suggested that UCA1 could be a
potential biomarker for CRC (41).
Knockdown of UCA1 suppressed CRC migration and
epithelial-mesenchymal transition, and enhanced the
radiosensitivity of CRC cells (44).
Cui et al (41) found that
UCA1 regulated CRC progression via sponging microRNA (miR)-28-5p.
NEAT1 promoted CRC progression via activating cell division protein
kinase 6 (42). MALAT1 was
upregulated in CRC samples. A previous study reported that MALAT1
served as an oncogene in CRC by promoting invasion and metastasis
(45). These results suggested that
lncRNAs may also play a crucial role in regulating the antitumor
effects of polyphyllin VII in CRC.
Moreover, the present study found that polyphyllin
VII affected a series of cancer-related pathways, including ‘ER to
Golgi vesicle-mediated transport’, ‘regulation of cellular response
to heat’, ‘response to cAMP’, ‘Ras protein signal transduction’,
‘metabolic pathways’, ‘MAPK signaling pathway’, ‘cell cycle’, ‘ErbB
signaling pathway’ and ‘TNF signaling pathway’. These pathways are
associated with the tumorigenesis and development of CRC. For
example, the p38α/MAPK pathway promotes cell metabolism, invasion,
autophagy, inflammation and angiogenesis in CRC and has been
validated as a key factor in CRC therapy and chemoresistance
(46). The abnormal regulation of
the cell cycle plays a crucial role in promoting tumor growth.
Moreover, in the present study, a PPI network was constructed to
identify key polyphyllin VII-regulated genes in CRC. Two hub
networks regulated by polyphyllin VII were revealed in this study.
A series of genes, such as RAB1B, RAB1A, TMED9, YKT6, COG7, GOSR1,
NAPG, FOLR1, COPE, DYNC1LI2, ACTR1A, ASPM, PLK1, HMGB2, KIF2C,
KIF15, FAM83D, KIF11, KIF20A, FANCI, HMMR, MCM5, MCM4, NCAPH, SMC4,
RRM2 and DLGAP5, were regarded as key regulators. Moreover,
polyphyllin VII-regulated genes were significantly overexpressed in
CRC samples compared with normal tissues. Among these genes, RRM2,
SMC4 and MCM4 have been reported to play a regulatory role in CRC
proliferation and migration. In the present study, polyphyllin VII
treatment led to a significant decrease of RRM2 and SMC4
expression. Overexpression of RRM2 is correlated with poor survival
in patients with CRC (47). SMC4
could predict survival and serve as a therapeutic target for CRC
(48).
In summary, the present demonstrated that
polyphyllin VII suppressed CRC proliferation and migration, but
induced apoptosis and cell cycle arrest. Microarray analysis
identified that polyphyllin VII could affect multiple
protein-coding genes and non-coding RNAs. Bioinformatics analysis
revealed that polyphyllin VII regulated multiple pathways in CRC,
including ‘ER to Golgi vesicle-mediated transport’, ‘response to
cAMP’, ‘Ras protein signal transduction’, ‘metabolic pathways’,
‘MAPK signaling pathway’ and ‘cell cycle’. PPI networks analysis
identified RRM2, SMC4 and MCM4 as the key targets of polyphyllin
VII in CRC. Finally, the results demonstrated that these key
polyphyllin VII-regulated genes were dysregulated in CRC. Taken
together, these results indicated that polyphyllin VII could be a
novel antitumor drug for the treatment of CRC.
Acknowledgements
Not applicable.
Funding
This work was supported by the Natural Science
Foundation of Hunan Province (grant no. 2018JJ3312), Hunan Health
Committee for Scientific Research Projects (grant no. C2019067),
Academic Topics of Hunan Academy of Traditional Chinese Medicine
(grant no. 201701), the Changsha Science and Technology Bureau
(grant no. kq1907124) and the Department of Education of Hunan
Province (grant no. 19b432).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CS and WT designed the study. CS, BP and XY
performed the experiments. CS wrote the paper. CS and BP conducted
statistical analysis and interpreted the data. CS, BP, XY and WT
reviewed and edited the manuscript. All authors read and approved
the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chakradhar S: Colorectal cancer: 5 big
questions. Nature. 521:S162015. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brody H: Colorectal cancer. Nature.
521:S12015. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rawla P, Sunkara T and Barsouk A:
Epidemiology of colorectal cancer: Incidence, mortality, survival,
and risk factors. Prz Gastroenterol. 14:89–103. 2019.PubMed/NCBI
|
|
4
|
Andre T, Boni C, Mounedji-Boudiaf L,
Navarro M, Tabernero J, Hickish T, Topham C, Zaninelli M, Clingan
P, Bridgewater J, et al: Oxaliplatin, fluorouracil, and leucovorin
as adjuvant treatment for colon cancer. N Engl J Med.
350:2343–2351. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Teng WJ, Chen P, Zhu FY, Di K, Zhou C,
Zhuang J, Cao XJ, Yang J, Deng LJ and Sun CG: Effect of Rhizoma
paridis total saponins on apoptosis of colorectal cancer cells and
imbalance of the JAK/STAT3 molecular pathway induced by IL-6
suppression. Genet Mol Res. 14:5793–5803. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Attar R, Tabassum S, Fayyaz S, Ahmad MS,
Nogueira DR, Yaylim I, Timirci-Kahraman O, Kucukhuseyin O, Cacina
C, Farooqi AA and Ismail M: Natural products are the future of
anticancer therapy: Preclinical and clinical advancements of viscum
album phytometabolites. Cell Mol Biol (Noisy-le-Grand). 61:62–68.
2015.PubMed/NCBI
|
|
7
|
da Rocha AB, Lopes RM and Schwartsmann G:
Natural products in anticancer therapy. Curr Opin Pharmacol.
1:364–369. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang C, Li C, Jia X, Wang K, Tu Y, Wang
R, Liu K, Lu T and He C: In vitro and in vivo anti-inflammatory
effects of polyphyllin VII through downregulating MAPK and NF-kB
pathways. Molecules. 24:8752019. View Article : Google Scholar
|
|
9
|
Wu Z and Zhang J, Xu F, Wang Y and Zhang
J: Rapid and simple determination of polyphyllin I, II, VI, and VII
in different harvest times of cultivated Paris polyphylla Smith
var. yunnanensis (Franch.) Hand.-Mazz by UPLC-MS/MS and FT-IR. J
Nat Med. 71:139–147. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lin Z, Liu Y, Li F, Wu J, Zhang G, Wang Y,
Lu L and Liu Z: Anti-lung cancer effects of polyphyllin VI and VII
potentially correlate with apoptosis in vitro and in vivo.
Phytother Res. 29:1568–1576. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen JC, Hsieh MJ, Chen CJ, Lin JT, Lo YS,
Chuang YC, Chien SY and Chen MK: Polyphyllin G induce apoptosis and
autophagy in human nasopharyngeal cancer cells by modulation of AKT
and mitogen-activated protein kinase pathways in vitro and in vivo.
Oncotarget. 7:70276–70289. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang C, Jia X, Bao J, Chen S, Wang K,
Zhang Y, Li P, Wan JB, Su H, Wang Y, et al: Polyphyllin VII induces
apoptosis in HepG2 cells through ROS-mediated mitochondrial
dysfunction and MAPK pathways. BMC Complement Altern Med.
16:582016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang F, Liu H, Zhao J, Ma X and Qi W:
POLR1B is upregulated and promotes cell proliferation in non-small
cell lung cancer. Oncol Lett. 19:671–680. 2020.PubMed/NCBI
|
|
14
|
Ritchie ME, Phipson B, Wu D, Hu Y, Law CW,
Shi W and Smyth GK: Limma powers differential expression analyses
for RNA-sequencing and microarray studies. Nucleic Acids Res.
43:e472015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dennis G Jr, Sherman BT, Hosack DA, Yang
J, Gao W, Lane HC and Lempicki RA: DAVID: Database for annotation,
visualization, and integrated discovery. Genome Biol. 4:P32003.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ashburner M, Ball CA, Blake JA, Botstein
D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT,
et al: Gene ontology: Tool for the unification of biology. The gene
ontology consortium. Nat Genet. 25:25–29. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kanehisa M and Goto S: KEGG: Kyoto
encyclopedia of genes and genomes. Nucleic Acids Res. 28:27–30.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Szklarczyk D, Franceschini A, Wyder S,
Forslund K, Heller D, Huerta-Cepas J, Simonovic M, Roth A, Santos
A, Tsafou KP, et al: STRING v10: Protein-protein interaction
networks, integrated over the tree of life. Nucleic Acids Res.
43:D447–D452. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chin CH, Chen SH, Wu HH, Ho CW, Ko MT and
Lin CY: CytoHubba: Identifying hub objects and sub-networks from
complex interactome. BMC Syst Biol. 8 (Suppl 4):S112014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Minguez P, Letunic I, Parca L and Bork P:
PTMcode: A database of known and predicted functional associations
between post-translational modifications in proteins. Nucleic Acids
Res. 41:D306–D311. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shannon P, Markiel A, Ozier O, Baliga NS,
Wang JT, Ramage D, Amin N, Schwikowski B and Ideker T: Cytoscape: A
software environment for integrated models of biomolecular
interaction networks. Genome Res. 13:2498–2504. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tang Z, Li C, Kang B, Gao G, Li C and
Zhang Z: GEPIA: A web server for cancer and normal gene expression
profiling and interactive analyses. Nucleic Acids Res. 45:W98–W102.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tsai YT, Lai JN, Lo PC, Chen CN and Lin
JG: Prescription of Chinese herbal products is associated with a
decreased risk of invasive breast cancer. Medicine (Baltimore).
96:e79182017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sparber A, Jonas W, White J, Derenzo E,
Johnson E and Bergerson S: Cancer clinical trials and subject use
of natural herbal products. Cancer Invest. 18:436–439. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Spaulding-Albright N: A review of some
herbal and related products commonly used in cancer patients. J Am
Diet Assoc. 97 (Suppl 10):S208–S215. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu Y, Si Y, Xiang Y, Zhou T, Liu X, Wu M,
Li W, Zhang T, Xiang K, Zhang L, et al: Polyphyllin I activates
AMPK to suppress the growth of non-small-cell lung cancer via
induction of autophagy. Arch Biochem Biophys. 687:1082852020.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dong R, Guo J, Zhang Z, Zhou Y and Hua Y:
Polyphyllin I inhibits gastric cancer cell proliferation by
downregulating the expression of fibroblast activation protein
alpha (FAP) and hepatocyte growth factor (HGF) in cancer-associated
fibroblasts. Biochem Biophys Res Commun. 497:1129–1134. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
He DX, Li GH, Gu XT, Zhang L, Mao AQ, Wei
J, Liu DQ, Shi GY and Ma X: A new agent developed by
biotransformation of polyphyllin VII inhibits chemoresistance in
breast cancer. Oncotarget. 7:31814–31824. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang D, Liu S, Liu Z, Ma C, Jiang Y, Sun
C, Li K, Cao G, Lin Z, Wang P, et al: Polyphyllin I induces cell
cycle arrest in prostate cancer cells via the upregulation of IL6
and P21 expression. Medicine (Baltimore). 98:e177432019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hsieh MJ, Chien SY, Lin JT, Yang SF and
Chen MK: Polyphyllin G induces apoptosis and autophagy cell death
in human oral cancer cells. Phytomedicine. 23:1545–1554. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hong F, Gu W, Jiang J, Liu X and Jiang H:
Anticancer activity of polyphyllin I in nasopharyngeal carcinoma by
modulation of lncRNA ROR and P53 signalling. J Drug Target.
27:806–811. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang M, Zou J, Zhu H, Liu S, Wang H, Bai P
and Xiao X: Paris saponin II inhibits human ovarian cancer
cell-induced angiogenesis by modulating NF-kB signaling. Oncol Rep.
33:2190–2198. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lou W, Chen Y, Zhu KY, Deng H, Wu T and
Wang J: Polyphyllin I overcomes EMT-associated resistance to
erlotinib in lung cancer cells via IL-6/STAT3 pathway inhibition.
Biol Pharm Bull. 40:1306–1313. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zheng R, Jiang H, Li J, Liu X and Xu H:
Polyphyllin II restores sensitization of the resistance of PC-9/ZD
cells to gefitinib by a negative regulation of the PI3K/Akt/mTOR
signaling pathway. Curr Cancer Drug Targets. 17:376–385. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cui J, Man S, Cui N, Yang L, Guo Q, Ma L
and Gao W: The synergistic anticancer effect of formosanin C and
polyphyllin VII based on caspase-mediated cleavage of Beclin1
inhibiting autophagy and promoting apoptosis. Cell Prolif.
52:e125202019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang P, Yang Q, Du X, Chen Y and Zhang T:
Targeted regulation of Rell2 by microRNA-18a is implicated in the
anti-metastatic effect of polyphyllin VI in breast cancer cells.
Eur J Pharmacol. 851:161–173. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Joung J, Engreitz JM, Konermann S,
Abudayyeh OO, Verdine VK, Aguet F, Gootenberg JS, Sanjana NE,
Wright JB, Fulco CP, et al: Genome-scale activation screen
identifies a lncRNA locus regulating a gene neighbourhood. Nature.
548:343–346. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Parikshak NN, Swarup V, Belgard TG, Irimia
M, Ramaswami G, Gandal MJ, Hartl C, Leppa V, Ubieta LT, Huang J, et
al: Genome-wide changes in lncRNA, splicing, and regional gene
expression patterns in autism. Nature. 540:423–427. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kawasaki Y, Miyamoto M, Oda T, Matsumura
K, Negishi L, Nakato R, Suda S, Yokota N, Shirahige K and Akiyama
T: The novel lncRNA CALIC upregulates AXL to promote colon cancer
metastasis. EMBO Rep. 20:e470522019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Meng S, Jian Z, Yan X, Li J and Zhang R:
LncRNA SNHG6 inhibits cell proliferation and metastasis by
targeting ETS1 via the PI3K/AKT/mTOR pathway in colorectal cancer.
Mol Med Rep. 20:2541–2548. 2019.PubMed/NCBI
|
|
41
|
Cui M, Chen M, Shen Z, Wang R, Fang X and
Song B: LncRNA-UCA1 modulates progression of colon cancer through
regulating the miR-28-5p/HOXB3 axis. J Cell Biochem. Jan
16–2019.(Epub ahead of print). View Article : Google Scholar
|
|
42
|
He Z, Dang J, Song A, Cui X, Ma Z and
Zhang Z: NEAT1 promotes colon cancer progression through sponging
miR-495-3p and activating CDK6 in vitro and in vivo. J Cell
Physiol. 234:19582–19591. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wu Q, Meng WY, Jie Y and Zhao H: LncRNA
MALAT1 induces colon cancer development by regulating
miR-129-5p/HMGB1 axis. J Cell Physiol. 233:6750–6757. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yang X, Liu W, Xu X, Zhu J, Wu Y, Zhao K,
He S, Li M, Wu Y, Zhang S, et al: Downregulation of long noncoding
RNA UCA1 enhances the radiosensitivity and inhibits migration via
suppression of epithelialmesenchymal transition in colorectal
cancer cells. Oncol Rep. 40:1554–1564. 2018.PubMed/NCBI
|
|
45
|
Ji Q, Cai G, Liu X, Zhang Y, Wang Y, Zhou
L, Sui H and Li Q: MALAT1 regulates the transcriptional and
translational levels of proto-oncogene RUNX2 in colorectal cancer
metastasis. Cell Death Dis. 10:3782019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Braicu C, Buse M, Busuioc C, Drula R,
Gulei D, Raduly L, Rusu A, Irimie A, Atanasov AG, Slaby O, et al: A
comprehensive review on MAPK: A promising therapeutic target in
cancer. Cancers (Basel). 11:16182019. View Article : Google Scholar
|
|
47
|
Yoshida Y, Tsunoda T, Doi K, Tanaka Y,
Fujimoto T, Machida T, Ota T, Koyanagi M, Takashima Y, Sasazuki T,
et al: KRAS-mediated up-regulation of RRM2 expression is essential
for the proliferation of colorectal cancer cell lines. Anticancer
Res. 31:2535–2539. 2011.PubMed/NCBI
|
|
48
|
Li X, Chen W, Jia J, You Z, Hu C, Zhuang
Y, Lin Z, Liu Y, Yang C and Xu R: The long non-coding RNA-RoR
promotes the tumorigenesis of human colorectal cancer by targeting
miR-6833-3p through SMC4. Onco Targets Ther. 13:2573–2581. 2020.
View Article : Google Scholar : PubMed/NCBI
|