Lung cancer is the leading cause of cancer mortality
worldwide, and >1 million people die of this disease each year
since 2006 (1). However, the
complicated mechanisms involved in tumor development have not been
completely elucidated, which impedes the development of early lung
cancer diagnosis and therapy (2,3). Hence,
to develop effective clinical therapies against lung cancer, it is
extremely urgent that the potential molecular mechanism of lung
cancer development be further explored. Previous studies have
discovered that a class of small non-coding RNAs, namely microRNAs
(miRNAs), serve as intermediate regulators of cellular biological
functions such as cell development, growth and apoptosis, which has
opened up a whole field of genomics called microRNomics (4,5).
Importantly, numerous studies have shown that miRNAs play critical
roles in the development and metastasis of lung cancer, and miRNAs
have emerged as potential factors in the early diagnosis and
prognosis of lung cancer.
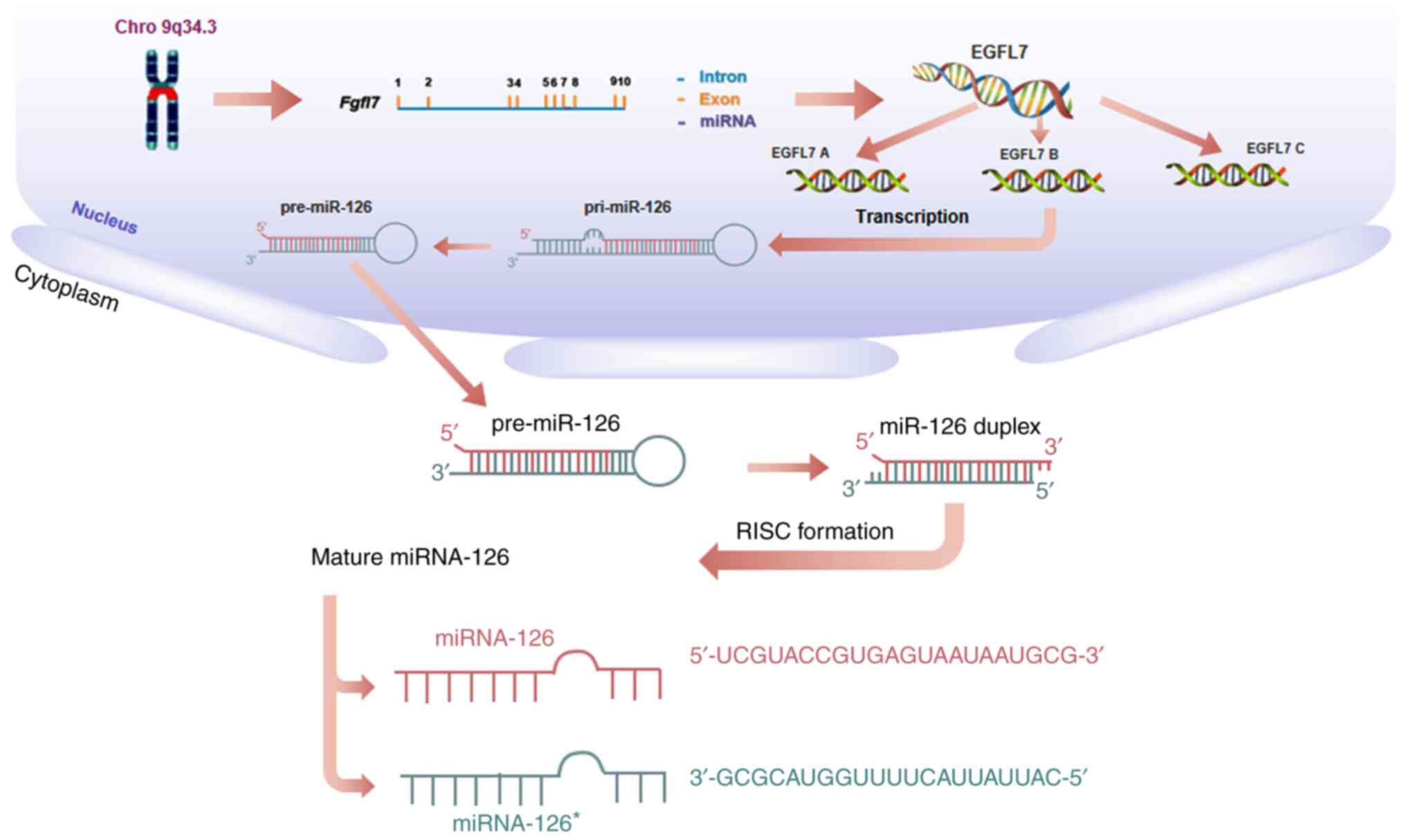
MicroRNA-126 (miRNA-126, miR-126) is an important
member of the miRNA family, encoded by intron 7 of epidermal growth
factor-like domain-containing gene 7 (EGFL7) on human
chromosome 9q34.3 (6,7). Three EGFL7 isoforms (named EGFL7
isoform A, B and C), all of which contain the same open reading
frame but are transcribed from separate promoters, utilize
alternative exons (Fig. 1). At the
transcriptional level, miR-126 may silence genes such as mTOR,
PIK3R2, and indirectly regulate EGFL7 isoform B (8–10).
Currently, miR-126 has been reported to have altered expression in
various cancer tissues, including liver, colorectal cancer and
melanoma as well as lung cancer, and it has been well studied for
its role in lung cancer tumorigenesis as well as in diagnosis and
therapy (2,11–15),
indicating the promising role of miR-126 in lung cancer
treatment.
Numerous studies have documented that miR-126 can
control the growth of lung cancer cells. For instance, miR-126 can
mediate the activation of the signal transducer and activator of
transcription 3 signaling pathway to regulate the malignant
biological behavior of non-small cell lung cancer (NSCLC) cells
including their proliferation, migration, cell cycle entry and
apoptosis susceptibility (16). In
addition, miR-126 can activate the proapoptotic and antimetastatic
effects in lung cancer cells by blocking the vascular endothelial
growth factor A (VEGF-A)/vascular endothelial growth factor
receptor-2 (VEGFR-2)/extracellular protein kinase (ERK) signaling
pathway (17). Recently, Chen et
al (18) demonstrated that
silencing miR-126 could reverse the anticancer effects of naringin
on NSCLC cell growth, in which naringin induces a reduction in the
phosphatidylinositol-3 protein kinase (PI3K)/protein kinase B
(AKT)/mechanistic target of rapamycin and suppresses vascular cell
adhesion molecule 1 protein levels. In addition, miR-126 exhibits a
suppressive effect on NSCLC cell invasion by interacting with three
hub genes: VEGFA, AKT1, and Kirsten rat sarcoma viral oncogene
homologue (19). Additionally, other
studies have demonstrated that miR-126 can suppress the growth,
migration and invasion of NSCLC cells by targeting chemokine (C-C
motif) receptor 1 and solute carrier family 7 (cationic amino acid
transporter, y+ system) member 5 (SLC7A5) or v-crk sarcoma virus
CT10 oncogene homologue (Crk) (20–23),
indicating that miR-126 may control the growth of lung cancer cells
through multiple targets (Table
I).
It is universally acknowledged that
epithelial-mesenchymal transition (EMT) serves a critical role in
lung cancer metastasis (24–26). Studies have demonstrated that miR-126
is a key regulator that controls EMT signaling in lung cancer. For
instance, in SPC-A1 lung cancer cells, ectopic expression of
miR-126 significantly suppresses the EMT process by directly
targeting PI3K/AKT/Snail signaling, which is considered to be the
initial step of tumor metastasis (27). Consistently, the upregulation of
miR-126 can also inhibit the migratory and invasive abilities of
NSCLC cells by decreasing the expression of the target gene
PIK3R2 and influencing the transduction of the phosphatase
and tensin homologue (PTEN)/PI3K/AKT signaling pathway (10,28).
Further studies have demonstrated that both strands of the miR-126
and the complementary ‘passenger strand’ of the miR-126 duplex
simultaneously target cytokine stromal cell-derived factor-1α to
reduce the recruitment of mesenchymal stem cells and inflammatory
monocytes to primary tumors, thereby inhibiting lung metastasis
(29). This indicates that miR-126
may regulate metastasis by affecting the tumor microenvironment
(29). In addition, miR-126 has been
identified in the miRNA-transcription factor (TF) target regulatory
network in cancer metastasis (30).
Novel differentially expressed genes including glutamate receptor,
metabotropic 8 and dachshund family transcription factor 1 are all
regulated by miR-126, which is implicated in the lymph node
metastasis process of small cell lung cancer (30). However, the exact role of miR-126 in
TF-miR networks, especially the interaction among these different
TFs in lung cancer metastasis remains to be elucidated.
Recent studies have suggested that miR-126 may serve
as an exciting new diagnostic biomarker of lung cancer (Table II). For example, Shang et al
(55) demonstrated that the
specificity and sensitivity of serum miR-126 levels in predicting
NSCLC development were 84.3 and 96.40%, respectively. In addition,
Yang et al (56) demonstrated
that the diagnostic performances of miRNAs such as miR-21, miR-223,
miR-155 and miR-126 in serum and plasma in lung cancers were good
and that serum miRNAs performed better compared with plasma miRNAs,
with a sensitivity of 79%, specificity of 78%, positive likelihood
ratio of 3.7, diagnostic odds ratio of 14 and area under the curve
(AUC) of 85%. Even the diagnostic accuracy of these miRNAs on stage
I/II and an additional stage I group was similar to that of
all-stage lung cancer, which suggests that circulating miRNAs can
correctly distinguish every stage of lung cancer from controls
correctly (56). In addition, the
expression of miR-126 in serum of patients with asbestos-related
NSCLC was comparable to that of patients with asbestos-unrelated
NSCLC, which may indicate the diagnostic value of miR-126 in
asbestos-related NSCLC (57). In
contrast, another recent study mentioned that the levels of both
serum and serum exosomal miR-126 in the early NSCLC group were
significantly lower compared with those in the healthy control
group but not significantly different from those in the benign lung
lesions group (58). Thus far, the
specific value of the role of miR-126 in early NSCLC and benign
lung lesions still needs to be clarified further. Zhu et al
(59) reported that circulating
miRNAs, including miR-126, had a good diagnostic value for lung
cancers, with a sensitivity of 60.7%, a specificity of 92.5%, and
an AUC of 79.3%. It is also worth noting that further logistic
regression model analysis revealed that the combination of miR-126,
miR-182, miR-183 and miR-210 with carcinoembryonic antigen
substantially increased the diagnostic value, with an AUC of 96.5%;
sensitivity, 81.2%; specificity, 100.0%; and accuracy, 90.8%
(59). The aforementioned results
indicated that circulating miR-126, particularly in combination
with multiple other miRNAs, may serve as a promising biomarker for
the diagnosis of lung cancer (56,59,60).
In the past decade, a large number of studies have
documented that miR-126 may also be a meaningful prognostic marker
of lung cancer (19,21,63–65)
(Table II). Recently, Xu et
al (66) found that high plasma
expression level of miR-126 in patients with lung cancer was
associated with shorter disease-free survival [hazard ratios (HRs)
of univariate Cox and multivariate Cox were 1.867 and 1.582,
respectively; 95% confidence intervals (CIs) of univariate Cox and
multivariate Cox were 1.386–2.515 and 1.158–2.161, respectively]
and overall survival (OS) (HRs of univariate Cox and multivariate
Cox were 1.706 and 1.320, respectively; 95% CIs of univariate Cox
and multivariate Cox were 1.218–2.388 and 0.932–1.817,
respectively). In addition, not only was the expression of miR-126
apparently downregulated in tumor tissue compared with benign
control tissue in NSCLCs, but the expression level was also
significantly higher in NSCLCs with a tumor size of ≤3 cm compared
with those with a tumor size of >3 cm, indicating the
association between miR-126 expression and tumor size (67). In addition, Jusufovic et al
(68) reported that low expression
of miR-126 in lung cancer tissue was a negative prognostic factor
for progression-free survival (PFS) (HR, 0.10; 95% CI, 0.04–0.21)
and OS (HR, 0.14; 95% CI,0.06–0.31) in patients with NSCLC, which
may be related to elevated MVD and lung cancer angiogenesis.
Finally, Lønvik et al (69)
discovered that intranuclear miRNA processing enzyme Drosha/miR-126
coexpression had a negative impact on the disease-specific survival
rate. Additionally, lower expression of combined miRNAs (let-7b and
miR-126) was closely associated with lower PFS time (HR,0.05; 95%
CI, 0.02–0.14) and OS time (HR, 0.05; 95% CI, 0.02–0.16) in
patients with NSCLC (68). However,
the possible difference in the prognostic value between single
miR-126 and coexpression of miR-126 with other factors in patients
with NSCLC prognosis needs to be further elucidated. In brief, the
aforementioned results may contribute to proving the prognostic
value of miR-126 in lung cancer.
Currently, some drugs for treating lung cancer
target the VEGF signaling pathway (70–72).
VEGF signaling can promote the growth of vascular endothelial cells
and vascular repair through connexin 43 (17,73–78).
miR-126 was reported as one of the most important regulators of
signaling for angiogenic growth factors, such as VEGF, fibroblast
growth factor, insulin like growth factor and endothelial growth
factor that manipulate vascular integrity and angiogenesis,
indicating its potential value in lung cancer treatment (40,53)
(Table III). For instance, Sun
et al (40) first reported
that the expression of EGFL7 increased significantly in
vitro and in vivo after transfection with a miR-126
overexpression plasmid that targets EGFL7. Subsequently, miR-126
restoration led to a reduction in the number of A549 cell
initiating differentiation, accompanied by significant inhibition
of tumor cell proliferation in vitro and tumor formation
in vivo (40). Recently, Jia
et al (27) used a xenograft
Lewis lung carcinoma model in C57BL/6 mice to detect the role of
miR-126 in lung cancer metastasis and found that the stable ectopic
expression of miR-126 significantly suppressed the formation of
lung metastases following the surgical removal of the primary
tumors. Meanwhile, decreased expression of EMT markers was observed
in the miR-126 overexpression group compared with the negative
control group. The results of the aforementioned study indicated
that miR-126 could suppress EMT and metastasis of lung cancer cells
by targeting PI3K/AKT/Snail signaling (27). Enhanced expression of miR-126 also
increased the sensitivity of NSCLC cells to anticancer agents
through the negative regulation of the
VEGF/PI3K/AKT/resistance-associated protein 1 signaling pathway
(3). Notably, some studies also
demonstrated that miR-126 could promote ionizing radiation-induced
apoptosis through the PI3K-AKT pathway in lung cancer (79,80).
In addition to the studies mentioned above, miR-126
was also regarded as a crucial regulator involved in the effect of
other factors in lung cancer (Table
III). For instance, Li et al (81) reported that long non-coding
RNA-PVT1-5 (lncRNA-PVT1-5) may act as a competing endogenous RNA
for miR-126 to promote cell proliferation by regulating the
miR-126/SLC7A5 pathway, suggesting that the
lncRNA-PVT1-5/miR-126/SLC7A5 regulatory network may shed light on
tumorigenesis in lung cancer. Furthermore, exosomes are a delivery
systems with low immunogenicity and toxicity that are not
recognized by the mononuclear phagocyte system (82). These special properties of exosomes
make them appropriate to serve as targeted delivery systems in
cancer therapy (83,84). Recently, Nie et al (85) demonstrated that miR-126 loaded into
231-Exo (miRNA-231-Exo) led to an effective inhibitory effect on
proliferation and migration in A549 lung cancer cells via the
interruption of the PTEN/PI3K/AKT signaling pathway. miR-126-laden
231-Exo, which can effectively escape innate immune cells, also
demonstrated a potent cancer inhibition effect in a lung cancer
metastasis model in mice.
In addition, miR-126 also serves a vital role when
other therapeutic approaches using other drugs are used in lung
cancer. For instance, cryptotanshinone is an important inhibitor of
the development of lung cancer cells (86). The expression levels of miR-126 were
upregulated by treatment with cryptotanshinone in A549 cells
(87). In addition, one new study
demonstrated that tubeimoside-1 (TBMS1) could elevate miR-126
expression, whereas overexpressing miR-126 inactivated the
VEGF-A/VEGFR-2/ERK signaling pathway, which promoted proapoptotic
and antimetastatic effects in NCI-H1299 cells (17). The aforementioned study may offer a
theoretical foundation for miR-126 serving a critical role in the
treatment of TBMS1 in lung cancer (17). Similarly, other researchers reported
that computed tomography-guided percutaneous radio frequency
ablation was an effective treatment for primary NSCLCs and
secondary lung tumors in hepatocellular carcinoma; the efficacy of
ablation may be related to its ability to normalize deregulated
expression of miR-126 (88).
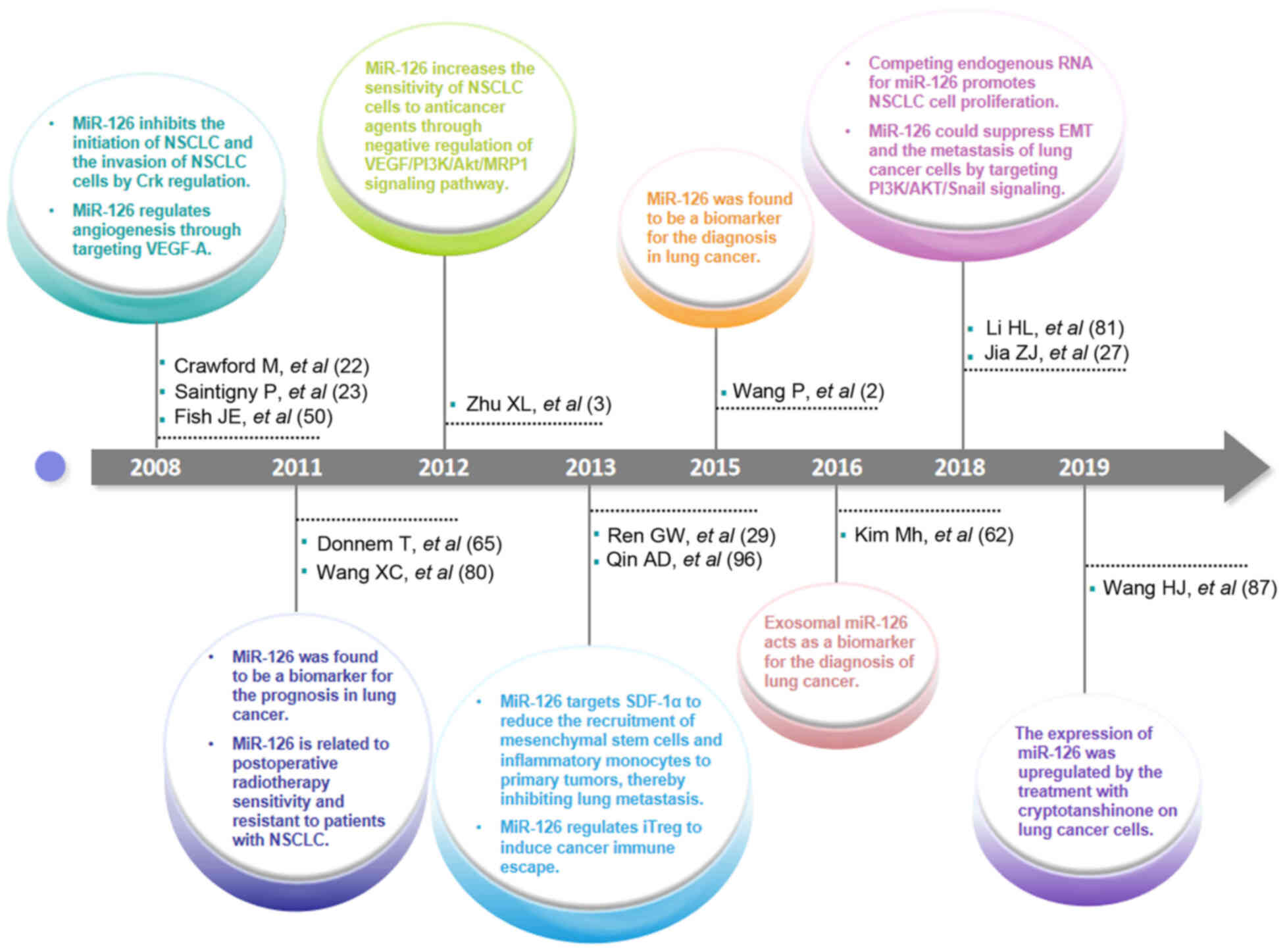
To date, important progress in the research on the
role of miR-126 in the tumorigenesis, diagnosis, prognosis and
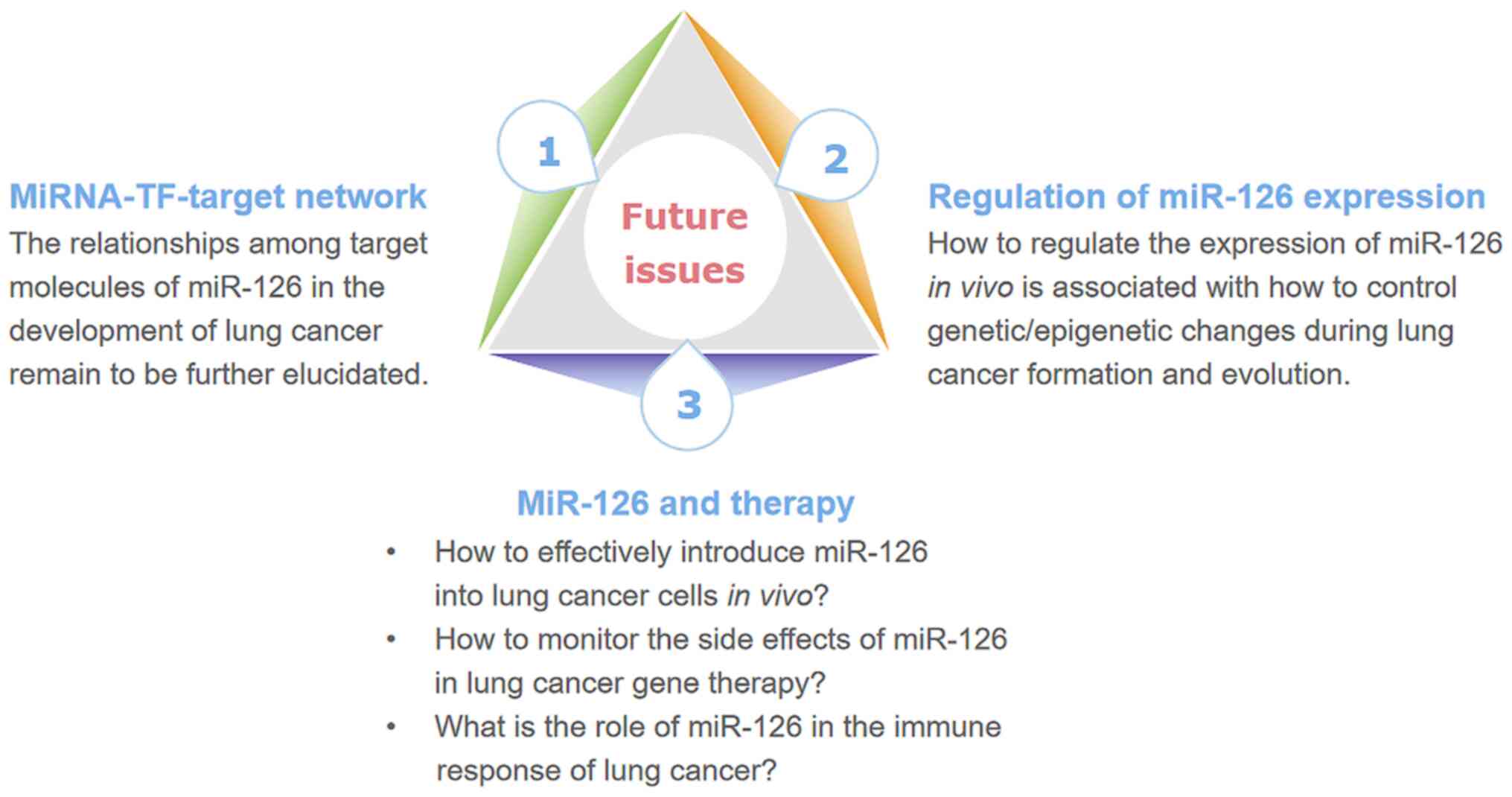
therapy of lung cancer has been achieved (Fig. 2). However, some scientific issues
remain to be further elucidated (Fig.
3). Firstly, the relationships among different target molecules
of miR-126 in the development of lung cancer have not yet been
clearly elucidated. Notably, Barshack et al (89) demonstrated that miR-126 was
significantly upregulated in liver cancer lung metastasis,
indicating that miR-126 may play different roles through distinct
targets in some special types of cancer (90,91). For
instance, in lung tumor tissues, Tafsiri et al (90) reported that miR-126 was downregulated
in 15 out of 18 adenocarcinoma samples, but there was no
significant correlation between squamous cell carcinoma and
expression of miR-126. Chen et al (91) demonstrated the result of pathway
enrichment analysis of the target genes that the target genes of
miR-126 in NSCLC [FOXO3, phosphoinositide 3-kinase catalytic
subunit δ (PIK3CD) and PIK3R2] were different from those in
colorectal cancer (Bcl-2, PIK3CD, PIK3R2). The underlying molecular
mechanisms may be related to the involvement of miR-126 in an
miRNA-TF-target network that contains PIK3CD, PIK3R2, forkhead box
protein O3, plexin B2 and tuberous sclerosis 1 (91). Secondly, some challenges need to be
further addressed. For example, it is unclear how to effectively
introduce miR-126 into lung cancer cells in vivo and how to
monitor the side effects of miR-126 in lung cancer gene therapy. In
particular, some evidences have shown that miR-126 also plays a
vital role in the function of immune cells including effector T
cells and regulatory T cells (Tregs) (92–96).
Additionally, miR-126 is also likely to be involved in
CD4+ T cell function (97). Hence, further investigation of the
role of miR-126 in lung cancer, including in the immune response,
may be valuable for the progression of miRNA-based immunotherapy
against lung cancer.
No applicable.
The present study was supported by the Program for
High level Innovative Talents in the Guizhou Province (grant no.
QKH-RC-2016-4031), the National Natural Science Foundation of China
(grant no. 31760258), the Program for New Century Excellent Talents
in University, Ministry of Education of China (grant no.
NCET-12-0661), the Program for Excellent Young Talents of Zunyi
Medical University (grant no. 15ZY-001) and the Project of Guizhou
Provincial Department of Science and Technology (grant no.
2009C491).
Data sharing is not applicable to this article, as
no datasets were generated or analyzed during the current
study.
QC designed and wrote the study. SC and YZ designed
the the study. JZ wrote the manuscript. LX conceived, designed and
wrote the manuscript. All authors have read and approved this
manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Lam S, Chari R, Lockwood W, Lam W,
MacAulay C, Sin D, Gazdar A, Khoury J, Yao RS and You M: Progress
in lung cancer chemoprevention. Cancer Epidem Biomar. 4:46–52.
2006.
|
|
2
|
Wang P, Yang D, Zhang H, Wei X, Ma T,
Cheng Z, Hong Q, Hu J, Zhou H, Song Y, et al: Early detection of
lung cancer in serum by a panel of microRNA biomarkers. Clin Lung
Cancer. 16:313–319.e1. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhu X, Li H, Long L, Hui L, Chen H, Wang
X, Shen H and Xu W: MiR-126 enhances the sensitivity of non-small
cell lung cancer cells to anticancer agents by targeting vascular
endothelial growth factor A. Acta Biochim Biophys Sin (Shanghai).
44:519–526. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Latronico MV, Catalucci D and Condorelli
G: Emerging role of microRNAs in cardiovascular biology. Circ Res.
101:1225–1236. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang C: MicroRNomics: A newly emerging
approach for disease biology. Physiol Genomics. 33:139–147. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Peng Y, Chao FF, Cai YP, Teng W and Qiu
CG: MiR-126 inhibits the proliferation of myocardial fibroblasts by
regulating EGFL7-mediated EGFR signal pathway. Int J Clin Exp Med.
10:6158–6166. 2017.
|
|
7
|
Liu F, Zhang H, Lu S, Wu Z, Zhou L, Cheng
Z, Bai Y, Zhao J, Zhang Q and Mao H: Quantitative assessment of
gene promoter methylation in non-small cell lung cancer using
methylation-sensitive high-resolution melting. Oncol Lett.
15:7639–7648. 2018.PubMed/NCBI
|
|
8
|
Saito Y, Friedman JM, Chihara Y, Egger G,
Chuang JC and Liang G: Epigenetic therapy upregulates the tumor
suppressor microRNA-126 and its host gene EGFL7 in human cancer
cells. Biochem Biophys Res Commun. 379:726–731. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wei L, Chen Z, Cheng N, Li X, Chen J, Wu
D, Dong M and Wu X: MicroRNA-126 inhibit viability of colorectal
cancer cell by repressing mTOR induced apoptosis and autophagy.
Onco Targets Ther. 13:2459–2468. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Song L, Li D, Gu Y, Wen ZM, Jie J, Zhao D
and Peng LP: MicroRNA-126 targeting PIK3R2 inhibits NSCLC A549 cell
proliferation, migration, and invasion by regulation of
PTEN/PI3K/AKT pathway. Clin Lung Cancer. 17:e65–e75. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Świtlik WZ, Karbownik MS, Suwalski M,
Kozak J and Szemraj J: Serum miR-210-3p as a potential noninvasive
biomarker of lung adenocarcinoma: A preliminary study. Genet Test
Mol Biomarkers. 23:353–358. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Świtlik W, Karbownik MS, Suwalski M, Kozak
J and Szemraj J: MiR-30a-5p together with miR-210-3p as a promising
biomarker for non-small cell lung cancer: A preliminary study.
Cancer Biomark. 21:479–488. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zheng W, Zhou Y, Lu J, Xu H, Lei L, Chen
C, Zhao J and Xu L: The prognostic value of miR-126 expression in
non-small-cell lung cancer: A meta-analysis. Cancer Cell Int.
17:712017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim JE, Eom JS, Kim WY, Jo EJ, Mok J, Lee
K, Kim KU, Park HK, Lee MK and Kim MH: Diagnostic value of
microRNAs derived from exosomes in bronchoalveolar lavage fluid of
early-stage lung adenocarcinoma: A pilot study. Thorac Cancer.
9:911–915. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen P, Gu YY, Ma FC, He RQ, Li ZY, Zhai
GQ, Lin X, Hu XH, Pan LJ and Chen G: Expression levels and
co-targets of miRNA-126-3p and miRNA-126-5p in lung adenocarcinoma
tissues: An exploration with RT-qPCR, microarray and bioinformatic
analyses. Oncol Rep. 41:939–953. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang Z, Wang J, Cheng J and Yu X: Effects
of miR-126 on the STAT3 signaling pathway and the regulation of
malignant behavior in lung cancer cells. Oncol Lett. 15:8412–8416.
2018.PubMed/NCBI
|
|
17
|
Shi H, Bi H, Sun X, Dong H, Jiang Y, Mu H,
Liu G, Kong W, Gao R and Su J: Antitumor effects of Tubeimoside-1
in NCI-H1299 cells are mediated by microRNA-126-5p-induced
inactivation of VEGF-A/VEGFR-2/ERK signaling pathway. Mol Med Rep.
17:4327–4336. 2018.PubMed/NCBI
|
|
18
|
Chen M, Peng W, Hu S and Deng J:
MiR-126/VCAM-1 regulation by naringin suppresses cell growth of
human non-small cell lung cancer. Oncol Lett. 16:4754–4760.
2018.PubMed/NCBI
|
|
19
|
Song F, Xuan Z, Yang X, Ye X, Pan Z and
Fang Q: Identification of key microRNAs and hub genes in
non-small-cell lung cancer using integrative bioinformatics and
functional analyses. J Cell Biochem. 121:2690–2703. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu R, Zhang YS, Zhang S, Cheng ZM, Yu JL,
Zhou S and Song J: MiR-126-3p suppresses the growth, migration and
invasion of NSCLC via targeting CCR1. Eur Rev Med Pharmacol Sci.
23:679–689. 2019.PubMed/NCBI
|
|
21
|
Wang J, Ding M, Zhu H, Cao Y and Zhao W:
Up-regulation of long noncoding RNA MINCR promotes non-small cell
of lung cancer growth by negatively regulating miR-126/SLC7A5 axis.
Biochem Biophys Res Commun. 508:780–784. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Crawford M, Brawner E, Batte K, Yu L,
Hunter MG, Otterson GA, Nuovo G, Marsh CB and Nana-Sinkam SP:
MicroRNA-126 inhibits invasion in non-small cell lung carcinoma
cell lines. Biochem Biophys Res Commun. 373:607–612. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Saintigny P, Ren H, Zou XC and Mao L:
MicroRNA (miRNA) species differentially expressed between
immortalized normal bronchial epithelial cells and non-small cell
lung. Cancer Res. 373:607–612. 2008.
|
|
24
|
Song L, Li XX, Liu XY, Wang Z, Yu Y, Shi
M, Jiang B and He XP: EFEMP2 suppresses the invasion of lung cancer
cells by inhibiting epithelial-mesenchymal transition (EMT) and
down-regulating MMPs. Onco Targets Ther. 13:1375–1396. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen J, Tong W, Liao M and Chen D:
Inhibition of arachidonate lipoxygenase12 targets lung cancer
through inhibiting EMT and suppressing RhoA and NF-κB activity.
Biochem Biophys Res Commun. 524:803–809. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tao L, Shu-Ling W, Jing-Bo H, Ying Z, Rong
H, Xiang-Qun L, Wen-Jie C and Lin-Fu Z: MiR-451a attenuates
doxorubicin resistance in lung cancer via suppressing epithelial
mesenchymal transition (EMT) through targeting c-Myc. Biomed
Pharmacother. 125:1099622020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jia Z, Zhang Y, Xu Q, Guo W and Guo A:
MiR-126 suppresses epithelial-to-mesenchymal transition and
metastasis by targeting PI3K/AKT/Snail signaling of lung cancer
cells. Oncol Lett. 15:7369–7375. 2018.PubMed/NCBI
|
|
28
|
Yang X, Chen BB, Zhang MH and Wang XR:
MicroRNA-126 inhibits the proliferation of lung cancer cell line
A549. Asian Pac J Trop Med. 8:239–242. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ren G and Kang Y: A one-two punch of
miR-126/126* against metastasis. Nat Cell Biol. 15:231–233. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang Z, Lu B, Sun L, Yan X and Xu J:
Identification of candidate genes or microRNAs associated with the
lymph node metastasis of SCLC. Cancer Cell Int. 18:1612018.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang J, Chen J, Guo Y, Wang B and Chu H:
Strategies targeting angiogenesis in advanced non-small cell lung
cancer. Oncotarget. 8:53854–53872. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li FJ, Huang J, Ji D, Meng Q, Wang C, Chen
S, Wang X, Zhu Z, Jiang C, Shi Y, et al: Azithromycin effectively
inhibits tumor angiogenesis by suppressing vascular endothelial
growth factor receptor 2-mediated signaling pathways in lung
cancer. Oncol Lett. 14:89–96. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lauridant G, Kotecki N, Pannier D and
Dansin E: The role of angiogenesis inhibitors in the treatment of
lung cancer. Oncologie. 18:409–418. 2016. View Article : Google Scholar
|
|
34
|
Tian RH, Wu X, Liu X, Yang JW, Ji HL and
Yan YJ: The role of angiogenesis inhibitors in the treatment of
elderly patients with advanced non-small-cell lung cancer: A
meta-analysis of eleven randomized controlled trials. J Cancer Res
Ther. 12:571–575. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang S, Aurora AB, Johnson BA, Qi X,
McAnally J, Hill JA, Richardson JA, Bassel-Duby R and Olson EN: The
endothelial-specific microRNA miR-126 governs vascular integrity
and angiogenesis. Dev Cell. 15:261–271. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hong G, Kuek V, Shi J, Zhou L, Han X, He
W, Tickner J, Qiu H, Wei Q and Xu J: EGFL7: Master regulator of
cancer pathogenesis, angiogenesis and an emerging mediator of bone
homeostasis. J Cell Physiol. 233:8526–8537. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Johnson L, Huseni M, Smyczek T, Lima A,
Yeung S, Cheng JH, Molina R, Kan D, De Mazière A, Klumperman J, et
al: Anti-EGFL7 antibodies enhance stress-induced endothelial cell
death and anti-VEGF efficacy. J Clin Invest. 123:3997–4009. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Usuba R, Pauty J, Soncin F and Matsunaga
YT: EGFL7 regulates sprouting angiogenesis and endothelial
integrity in a human blood vessel model. Biomaterials. 197:305–316.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Monaco F, Gaetani S, Alessandrini F,
Tagliabracci A, Bracci M, Valentino M, Neuzil J, Amati M, Bovenzi
M, Tomasetti M and Santarelli L: Exosomal transfer of miR-126
promotes the anti-tumour response in malignant mesothelioma: Role
of miR-126 in cancer-stroma communication. Cancer Lett. 463:27–36.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sun Y, Bai Y, Zhang F, Wang Y, Guo Y and
Guo L: MiR-126 inhibits non-small cell lung cancer cells
proliferation by targeting EGFL7. Biochem Biophys Res Commun.
391:1483–1489. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Shen X, Zhi Q, Wang Y, Li Z, Zhou J and
Huang J: Hypoxia induces multidrug resistance via enhancement of
epidermal growth Factor-like domain 7 expression in non-small lung
cancer cells. Chemotherapy. 62:172–180. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Caporali S, Amaro A, Levati L, Alvino E,
Lacal PM, Mastroeni S, Ruffini F, Bonmassar L, Antonini Cappellini
GC, Felli N, et al: MiR-126-3p down-regulation contributes to
dabrafenib acquired resistance in melanoma by up-regulating ADAM9
and VEGF-A. J Exp Clin Cancer Res. 38:2722019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Di Martino S, Acierno C and Licito A:
Experimental study on the prevention of liver cancer angiogenesis
via miR-126. Promising results for targeted therapy. Eur Rev Med
Pharmacol Sci. 22:853–855. 2018.PubMed/NCBI
|
|
44
|
Pishavar E and Behravan J: MiR-126 as a
therapeutic agent for diabetes mellitus. Curr Pharm Des.
23:3309–3314. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhou F, Jia X, Yang Y, Yang Q, Gao C, Hu
S, Zhao Y, Fan Y and Yuan X: Nanofiber-mediated microRNA-126
delivery to vascular endothelial cells for blood vessel
regeneration. Acta Biomater. 43:303–313. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Dong G, Lin XH, Liu HH, Gao DM, Cui JF,
Ren ZG and Chen RX: Intermittent hypoxia alleviates increased VEGF
and Pro-angiogenic potential in liver cancer cells. Oncol Lett.
18:1831–1839. 2019.PubMed/NCBI
|
|
47
|
Gu C, Zou S, He C, Zhou J, Qu R, Wang Q,
Qi J, Zhou M, Yan S and Ye Z: Long non-coding RNA CCAT1 promotes
colorectal cancer cell migration, invasiveness and viability by
upregulating VEGF via negative modulation of microRNA-218. Exp Ther
Med. 19:2543–2550. 2020.PubMed/NCBI
|
|
48
|
Yoon NA, Jung SJ, Choi SH, Ryu JH, Mani M,
Lee UH, Vo MT, Jeon DY, Chung SW, Ju Lee B, et al: DRG2 supports
the growth of primary tumors and metastases of melanoma by
enhancing VEGF-A expression. FEBS J. 287:2070–2086. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Boudria A, Abou Faycal C, Jia T, Gout S,
Keramidas M, Didier C, Lemaître N, Manet S, Coll JL, Toffart AC, et
al: VEGF165b, a splice variant of VEGF-A, promotes lung
tumor progression and escape from anti-angiogenic therapies through
a β1 integrin/VEGFR autocrine loop. Oncogene. 38:1050–1066. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Fish JE, Santoro MM, Morton SU, Yu S, Yeh
RF, Wythe JD, Ivey KN, Bruneau BG, Stainier DY and Srivastava D:
MiR-126 regulates angiogenic signaling and vascular integrity. Dev
Cell. 15:272–284. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Qu Y, Wu J, Deng JX, Zhang YP, Liang WY,
Jiang ZL, Yu QH and Li J: MicroRNA-126 affects rheumatoid arthritis
synovial fibroblast proliferation and apoptosis by targeting PIK3R2
and regulating PI3K-AKT signal pathway. Oncotarget. 7:74217–74226.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ye L, Peng Y, Mo J and Yao Y: MiR-126
enhances VEGF expression in induced pluripotent stem cell-derived
retinal neural stem cells by targeting spred-1. Int J Clin Exp
Pathol. 11:1023–1030. 2018.PubMed/NCBI
|
|
53
|
Yücel EI and Sahin M: Fenretinide reduces
angiogenesis by downregulating CDH5, FOXM1 and eNOS genes and
suppressing microRNA-10b. Mol Biol Rep. 47:1649–1658. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Liu X, Tufman A, Behr J, Kiefl R, Goldmann
T and Huber RM: Role of the erythropoietin receptor in lung cancer
cells: Erythropoietin exhibits angiogenic potential. J Cancer.
11:6090–6100. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Shang AQ, Xie YN, Wang J, Sun L, Wei J, Lu
WY, Lan JY, Wang WW, Wang L and Wang LL: Predicative values of
serum microRNA-22 and microRNA-126 levels for non-small cell lung
cancer development and metastasis: A case-control study. Neoplasma.
64:453–459. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Yang Y, Hu Z, Zhou Y, Zhao G, Lei Y, Li G,
Chen S, Chen K, Shen Z, Chen X, et al: The clinical use of
circulating microRNAs as non-invasive diagnostic biomarkers for
lung cancers. Oncotarget. 8:90197–90214. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Santarelli L, Gaetani S, Monaco F, Bracci
M, Valentino M, Amati M, Rubini C, Sabbatini A, Pasquini E, Zanotta
N, et al: Four-miRNA signature to identify asbestos-related lung
malignancies. Cancer Epidemiol Biomarkers Prev. 28:119–126. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Wu Q, Yu L, Lin X, Zheng Q, Zhang S, Chen
D, Pan X and Huang Y: Combination of serum miRNAs with serum
exosomal miRNAs in early diagnosis for non-small-cell lung cancer.
Cancer Manag Res. 12:485–495. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Zhu W, Zhou K, Zha Y, Chen D, He J, Ma H,
Liu X, Le H and Zhang Y: Diagnostic value of serum miR-182,
miR-183, miR-210, and miR-126 levels in patients with early-stage
non-small cell lung cancer. PLoS One. 11:e01530462016. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Wang W, Ding M, Duan X, Feng X, Wang P,
Jiang Q, Cheng Z, Zhang W, Yu S, Yao W, et al: Diagnostic value of
plasma microRNAs for lung cancer using support vector machine
model. J Cancer. 10:5090–5098. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Bagheri A, Khorshid HRK, Tavallaie M,
Mowla SJ, Sherafatian M, Rashidi M, Zargari M, Boroujeni ME and
Hosseini SM: A panel of noncoding RNAs in non-small-cell lung
cancer. J Cell Biochem. Nov;28.2018.doi: 10.1002/jcb.28111 (Epub
ahead of print).
|
|
62
|
Kim MH, Jo EJ, Eom JS, Mok JH, Ki KL, Kim
U, Park HK and Lee MK: Diagnostic value of microRNAs derived
exosomes from bronchoalveolar lavage fluid in early stage lung
adenocarcinoma. Chest. 150:703A2016. View Article : Google Scholar
|
|
63
|
Ulivi P, Petracci E, Marisi G, Baglivo S,
Chiari R, Billi M, Canale M, Pasini L, Racanicchi S, Vagheggini A,
et al: Prognostic role of circulating miRNAs in early-stage
non-small cell lung cancer. J Clin Med. 8:1312019. View Article : Google Scholar
|
|
64
|
Chen SW, Wang TB, Tian YH and Zheng YG:
Down-regulation of microRNA-126 and microRNA-133b acts as novel
predictor biomarkers in progression and metastasis of non small
cell lung cancer. Int J Clin Exp Pathol. 8:14983–14988.
2015.PubMed/NCBI
|
|
65
|
Donnem T, Lonvik K, Eklo K, Berg T, Sorbye
SW, Al-Shibli K, Al-Saad S, Andersen S, Stenvold H, Bremnes RM and
Busund LT: Independent and tissue-specific prognostic impact of
miR-126 in nonsmall cell lung cancer: Coexpression with vascular
endothelial growth factor-A predict poor survival. Cancer.
117:3193–3200. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Xu X, Zhu S, Tao Z and Ye S: High
circulating miR-18a, miR-20a, and miR-92a expression correlates
with poor prognosis in patients with Non-small cell lung cancer.
Cancer Med. 7:21–31. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Kim MK, Jung SB, Kim JS, Roh MS, Lee JH,
Lee EH and Lee HW: Expression of microRNA miR-126 and miR-200c is
associated with prognosis in patients with non-small cell lung
cancer. Virchows Arch. 465:463–471. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Jusufovic E, Rijavec M, Keser D, Korošec
P, Sodja E, Iljazović E, Radojević Z and Košnik M: Let-7b and
miR-126 are down-regulated in tumor tissue and correlate with
microvessel density and survival outcomes in non-small-cell lung
cancer. PLoS One. 7:e455772012. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Lønvik K, Sørbye SW, Nilsen MN and
Paulssen RH: Prognostic value of the MicroRNA regulators Dicer and
Drosha in non-small-cell lung cancer: Co-expression of Drosha and
miR-126 predicts poor survival. BMC Clin Pathol. 14:452014.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Shi H, Bi H, Sun X, Dong H, Jiang Y, Mu H,
Li W, Liu G, Gao R and Su J: Tubeimoside-1 inhibits the
proliferation and metastasis by promoting miR-126-5p expression in
non-small cell lung cancer cells. Oncol Lett. 16:3126–3134.
2018.PubMed/NCBI
|
|
71
|
Rai MK, Goyal R, Bhutani MK, Kaneria J,
Mahendru K and Sharma N: Efficacy and safety profile of combined
targeted therapy against Egfr and Vegf in patients with previously
treated advanced non-small-cell lung cancer: A systematic review
and meta-analysis. Value Health. 18:A4302015. View Article : Google Scholar
|
|
72
|
Yin W, Zhu J, Gonzalez-Rivas D, Okumura M,
Rocco G, Pass H, Jiang G and Yang Y: Construction of a novel
bispecific antibody to enhance antitumor activity against lung
cancer. Adv Mater. 30:e18054372018. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Leung DW, Cachianes G, Kuang WJ, Goeddel
DV and Ferrara N: Vascular endothelial growth factor is a secreted
angiogenic mitogen. Science. 246:1306–1309. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Sheikh AM, Yano S, Mitaki S, Haque MA,
Yamaguchi S and Nagai A: A Mesenchymal stem cell line (B10)
increases angiogenesis in a rat MCAO model. Exp Neurol.
311:182–193. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Kong Z, Hong Y, Zhu J, Cheng X and Liu Y:
Endothelial progenitor cells improve functional recovery in focal
cerebral ischemia of rat by promoting angiogenesis via VEGF. J Clin
Neurosci. 55:116–121. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Ruan W, Zhao F, Zhao S, Zhang L, Shi L and
Pang T: Knockdown of long noncoding RNA MEG3 impairs
VEGF-stimulated endothelial sprouting angiogenesis via modulating
VEGFR2 expression in human umbilical vein endothelial cells. Gene.
649:32–39. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Melincovici CS, Boşca AB, Şuşman S,
Mărginean M, Mihu C, Istrate M, Moldovan IM, Roman AL and Mihu CM:
Vascular endothelial growth factor (VEGF)-key factor in normal and
pathological angiogenesis. Rom J Morphol Embryol. 59:455–467.
2018.PubMed/NCBI
|
|
78
|
Li L, Liu H, Xu C, Deng M, Song M, Yu X,
Xu S and Zhao X: VEGF promotes endothelial progenitor cell
differentiation and vascular repair through connexin 43. Stem Cell
Res Ther. 8:2372017. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Long L, Zhang X, Bai J, Li Y, Wang X and
Zhou Y: Tissue-specific and exosomal miRNAs in lung cancer
radiotherapy: From regulatory mechanisms to clinical implications.
Cancer Manag Res. 11:4413–4424. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Wang XC, Du LQ, Tian LL, Wu HL, Jiang XY,
Zhang H, Li DG, Wang YY, Wu HY, She Y, et al: Expression and
function of miRNA in postoperative radiotherapy sensitive and
resistant patients of non-small cell lung cancer. Lung Cancer.
72:92–99. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Li H, Chen S, Liu J, Guo X, Xiang X, Dong
T, Ran P, Li Q, Zhu B, Zhang X, et al: Long non-coding RNA PVT1-5
promotes cell proliferation by regulating miR-126/SLC7A5 axis in
lung cancer. Biochem Biophys Res Commun. 495:2350–2355. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Fortunato O, Gasparini P, Boeri M and
Sozzi G: Exo-miRNAs as a new tool for liquid biopsy in lung cancer.
Cancers (Basel). 11:8882019. View Article : Google Scholar
|
|
83
|
Kibria G, Ramos EK, Wan Y, Gius DR and Liu
H: Exosomes as a drug delivery system in cancer therapy: Potential
and challenges. Mol Pharm. 15:3625–3633. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Kobayashi M, Sawada K, Miyamoto M, Shimizu
A, Yamamoto M, Kinose Y, Nakamura K, Kawano M, Kodama M, Hashimoto
K and Kimura T: Exploring the potential of engineered exosomes as
delivery systems for tumor-suppressor microRNA replacement therapy
in ovarian cancer. Biochem Biophys Res Commun. 527:153–161. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Nie H, Xie X, Zhang D, Zhou Y, Li B, Li F,
Li F, Cheng Y, Mei H, Meng H and Jia L: Use of lung-specific
exosomes for miRNA-126 delivery in non-small cell lung cancer.
Nanoscale. 12:877–887. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Qi P, Li Y, Liu X, Jafari FA, Zhang X, Sun
Q and Ma Z: Cryptotanshinone suppresses non-Small cell lung cancer
via microRNA-146a-5p/EGFR Axis. Int J Biol Sci. 15:1072–1079. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Wang H, Zhang Y, Zhang Y, Liu W and Wang
J: Cryptotanshinone inhibits lung cancer invasion via
microRNA-133a/matrix metalloproteinase 14 regulation. Oncol Lett.
18:2554–2559. 2019.PubMed/NCBI
|
|
88
|
Hu X, Zhang F, Liu XR, Wu YT and Ni YM:
Efficacy and potential MicroRNA mechanism for computed
tomography-guided percutaneous radiofrequency ablation of primary
lung cancer and lung metastasis from liver cancer. Cell Physiol
Biochem. 33:1261–1271. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Barshack I, Meiri E, Rosenwald S, Lebanony
D, Bronfeld M, Aviel-Ronen S, Rosenblatt K, Polak-Charcon S,
Leizerman I, Ezagouri M, et al: Differential diagnosis of
hepatocellular carcinoma from metastatic tumors in the liver using
microRNA expression. Int J Biochem Cell Bio. 42:1355–1362. 2010.
View Article : Google Scholar
|
|
90
|
Tafsiri E, Darbouy M, Shadmehr MB,
Zagryazhskaya A, Alizadeh J and Karimipoor M: Expression of miRNAs
in non-small-cell lung carcinomas and their association with
clinicopathological features. Tumour Biol. 36:1603–1612. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Chen Q, Hu H, Jiao D, Yan J, Xu W, Tang X,
Chen J and Wang J: MiR-126-3p and miR-451a correlate with
clinicopathological features of lung adenocarcinoma: The underlying
molecular mechanisms. Oncol Rep. 36:909–917. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Kontarakis Z, Rossi A, Ramas S, Dellinger
MT and Stainier DYR: Mir-126 is a conserved modulator of lymphatic
development. Dev Biol. 437:120–130. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Agudo J, Ruzo A, Tung N, Salmon H, Leboeuf
M, Hashimoto D, Becker C, Garrett-Sinha LA, Baccarini A, Merad M
and Brown BD: The miR-126-VEGFR2 axis controls the innate response
to pathogen-associated nucleic acids. Nat Immunol. 15:54–62. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Ferretti C and La Cava A: MiR-126, a new
modulator of innate immunity. Cell Mol Immunol. 11:215–217. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Peng J, Yu Z, Xue L, Wang JB, Li J, Liu D,
Yang Q and Lin Y: The effect of foxp3-overexpressing Treg cells on
non-small cell lung cancer cells. Mol Med Rep. 17:5860–5868.
2018.PubMed/NCBI
|
|
96
|
Qin A, Wen Z, Zhou Y, Li Y, Li Y, Luo J,
Ren T and Xu L: MicroRNA-126 regulates the induction and function
of CD4(+) Foxp3(+) regulatory T cells through PI3K/AKT pathway. J
Cell Mol Med. 17:252–264. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Chu F, Hu Y, Zhou Y, Guo M, Lu J, Zheng W,
Xu H, Zhao J and Xu L: MicroRNA-126 deficiency enhanced the
activation and function of CD4+T cells by elevating
IRS-1 pathway. Clin Exp Immunol. 191:166–179. 2018. View Article : Google Scholar : PubMed/NCBI
|