Introduction
Gastric cancer (GC) has the fifth highest cancer
incidence and second highest rate of cancer-associated mortalities
among all malignant neoplasms worldwide (1). Although curative resection (R0) with
lymph node dissection and adjuvant chemotherapy has prolonged the
survival of patients with GC, the recurrence rate of R0 cases
remains at ~30% in patients with stage II/III GC (2). Peritoneal recurrence is the most
frequent recurrence pattern in patients with GC following curative
resection, and as such, peritoneal recurrence is the most common
cause of subsequent cancer-associated mortality (3).
Stromal cell-derived factor 1α (SDF1α, also termed
CXC ligand 12) and its receptor C-X-C chemokine receptor type 4
(CXCR4) have been known to serve a critical role in cancer cell
migration and proliferation in solid tumors, including GC (4,5), breast
(6), esophageal (7), prostate tumor (8), pancreatic cancer (9,10),
melanoma (11), colon (12), ovarian (13) and lung cancer (14).
Various types of solid tumors, including GC have a
heterogeneously hypoxic environment which is currently thought to
be associated with aggressive tumor phenotypes (15–19).
Clinical and experimental data on GC also provide evidence of an
association between the hypoxic environment and a poor prognosis
(16,18). Therefore, a hypoxic environment has
been considered to be associated with aggressive tumor phenotypes
of gastric carcinomas (20,21), including the metastatic ability of
cancer cells (22).
Our recent study reported that the progression of GC
may be recognized as the product of evolving crosstalk between the
cancer cells and their surrounding tumor stroma (23,24). The
results of our previous study reported that SDF1 from tumor stromal
cells may stimulate the proliferation of GC cells through the CXCR4
axis in hypoxic microenvironments (4). Certain studies also reported that the
expression of CXCR4 in cancer cells has been upregulated under
hypoxia (25,26). However, the clinical association
between the expression of SDF1α/CXCR4 and hypoxic conditions in GC
has been unclear. The present study investigated the
clinicopathological significance of SDF1α and CXCR4 expression and
a hypoxic environment in GC at stage II and III.
Materials and methods
Clinical materials
Human GC tissues were obtained from a total of 185
patients with stage II or III GC, who had undergone resection of a
primary GC at Osaka City University Hospital. Patients with stage I
or stage IV GC were excluded. None of the patients had undergone
preoperative radiation and/or chemotherapy. The pathological
diagnoses and classifications were made according to classified by
the Japanese Classification of Gastric Carcinoma 3rd English
edition (27) or the Union for
International Cancer Control Tumor-Node-Metastasis classification
of malignant tumors (28). Table I shows the clinicopathological
characteristics of 185 patients with stage II and III GC. The study
protocol conformed to the ethical guidelines of the Declaration of
Helsinki (29). The present study
was conducted with the approval of the Ethical Committee of Osaka
City University (reference number 924). Written informed consent
was obtained from all patients prior to treatment.
 | Table I.Clinicopathological features of 185
patients with stage II or III gastric cancer. |
Table I.
Clinicopathological features of 185
patients with stage II or III gastric cancer.
| Clinicopathological
feature | n (n=185) |
|---|
| Sex |
|
|
Female | 76 |
|
Male | 109 |
| Age, years |
|
|
<70 | 102 |
|
≥70 | 83 |
| Macroscopic
type |
|
| Type
4 | 23 |
|
Other | 162 |
| Histological
type |
|
|
Intestinal | 85 |
|
Diffuse | 100 |
| Infiltration
pattern |
|
|
a/b | 123 |
| c | 58 |
| Lymph node
metastasis |
|
|
Negative | 44 |
|
Positive | 141 |
| Stage | 78 |
| II |
|
|
III | 107 |
| Lymphatic
invasion |
|
|
Negative | 29 |
|
Positive | 155 |
| Venous
invasion |
|
|
Negative | 130 |
|
Positive | 55 |
Immunohistochemical techniques
The GC tissue was preserved by fixing in a solution
of 10% neutral-buffered formalin for ~24 h at room temperature.
Immunohistochemical staining was performed on 4-µm sections of
formalin-fixed paraffin-embedded tissue. The slides were
deparaffinized in xylene and rehydrated in decreasing
concentrations of ethyl alcohol. The sections were heated for 10
min at 105°C by autoclave in Target Retrieval Solution (Dako;
Agilent Technologies, Inc.). The sections were blocked for 10 min
at room temperature with 10% normal goat serum (Histofine Simple
Stain™ MAX-PO; Nichirei Biosciences Inc.) and subsequently
incubated with 3% hydrogen peroxide to block endogenous peroxidase
activity. Immunohistochemistry was performed using the following
antibodies: Anti-CXCR4 (cat. no. ab124824; dilution 1:100; Abcam),
anti-SDF1α (cat. no. MAB350; dilution 1:200; R&D Systems,
Inc.), and a hypoxic marker, carbonic anhydrase 9 (CA9; clone; cat.
no. M75; dilution 1:1,000; Novus Biologicals, LLC). The specimens
were incubated with the antibodies at 4°C overnight, followed by
three washes with PBS. The slides were treated with
streptavidin-peroxidase reagent and were incubated in PBS
diaminobenzidine and 1% hydrogen peroxide vol/vol, followed by
counterstaining with Mayer's hematoxylin for 1 min at room
temperature and analysis of three fields per sample under a light
microscope (magnification, ×100).
Immunohistochemical determination of
SDF1α, CXCR4 and CA9
Positive immunostaining was evaluated by two
independent investigators who were blinded to patient outcomes and
clinicopathological features. A numerical scoring system with two
categories was used to assess the intensity and the extent of
immunoreactivity. The proportion score was an estimate of the
proportion of positive cells: 0, no immunoreactive cells; 1,
<20% immunoreactive cells; 2, 20–50% immunoreactive cells; and
3, ≥50% immunoreactive cells. The intensity score estimates the
average staining intensity of positive tumor cells: 0, no staining;
1, weak positive membrane staining; 2, moderate; and 3, strong
staining. The two scores were multiplied together to give a final
numerical score ranging between 0 and 9. The cases were considered
positive if the score was 5 or more.
Statistical analysis
The χ2 test or Fisher's exact test were
used to determine the significance of the difference between the
covariates. Survival curves were constructed using Kaplan-Meier
survival analysis and compared using the log-rank test. The
influence of each prognostic factor on patient survival was
evaluated using Cox regression analysis. All analyses were
performed using SPSS software version 22.0 (IBM Corp.). P<0.05
was considered to indicate a statistically significant
difference.
Results
Association between
clinicopathological features and CA9 expression, CXCR4 expression
in cancer cells, and SDF1α expression in the stromal cells
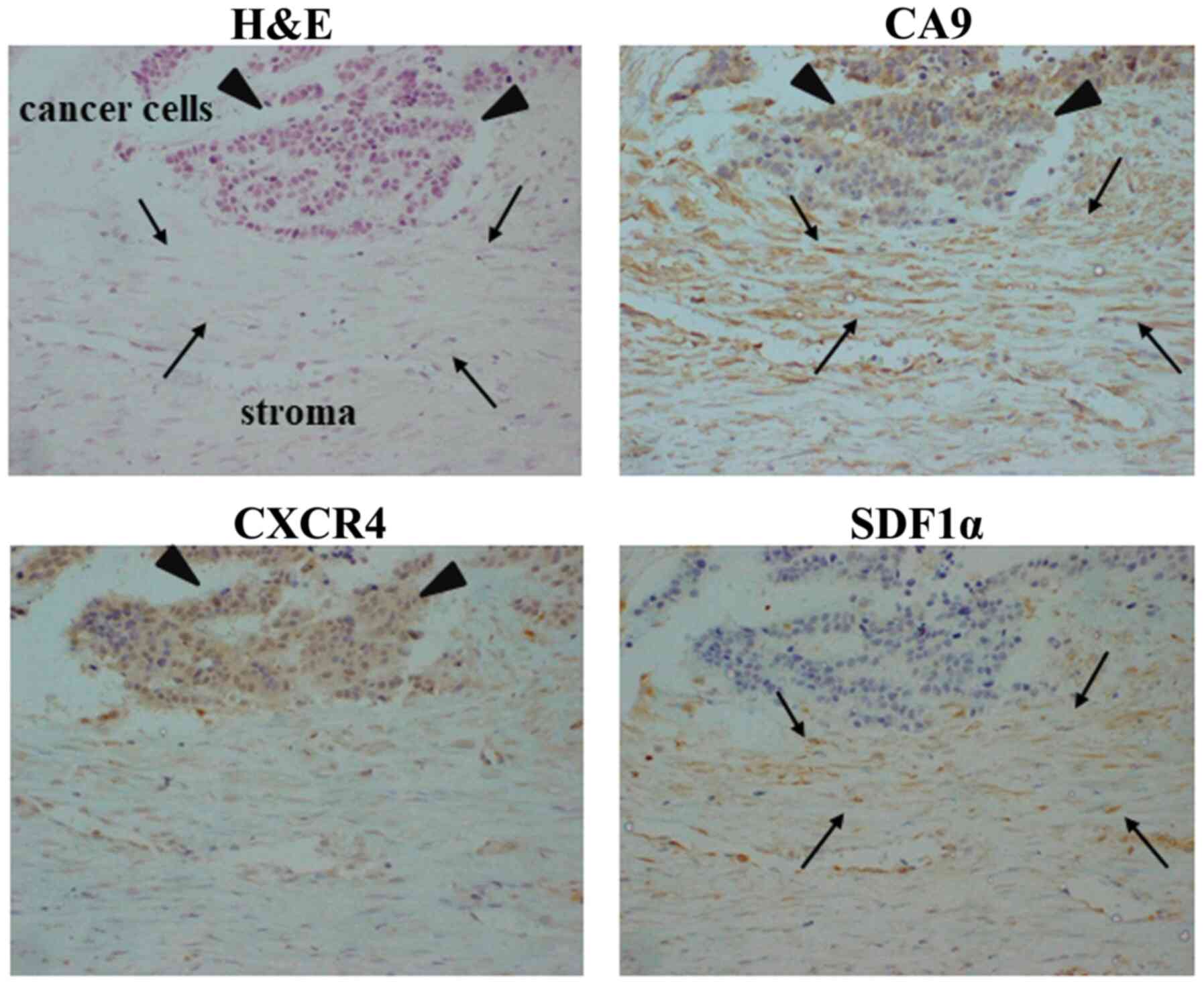
Representative images of CA9, CXCR4 and SDF1α
immunostaining are presented in Fig.
1. CA9 was heterogeneously expressed on stromal cells (arrows)
and GC cells (arrowheads). SDF1α expression in stromal cells was
observed primarily in the cytoplasm of fibroblast-like stromal
cells (arrows). CXCR4 expression was observed primarily in cancer
cells (arrowheads). CA9 expression was significantly associated
with CXCR4 expression in the cancer cells and SDF1α expression in
the stromal cells (P=0.001), and was significantly associated with
macroscopic type 4 tumor (P=0.021), and a pattern of tumor
infiltration into the surrounding tissue (P=0.005). CA9 expression,
CXCR4 expression in the cancer cells, and SDF1α expression in the
stromal cells (CA9/CXCR4/SDF1α) were significantly associated with
macroscopic type 4 (P=0.012) and a pattern of tumor infiltration
into the surrounding tissue (P<0.001; Table II).
 | Table II.Association between
clinicopathological features and CA9/CXCR4/SDF1α expression in
stage II and III gastric cancer. |
Table II.
Association between
clinicopathological features and CA9/CXCR4/SDF1α expression in
stage II and III gastric cancer.
|
| CA9 expression |
| CA9/CXCR4/SDF1α
expression |
|
|---|
|
|
|
|
|
|
|---|
| Factors | Positive n=96
(%) | Negative n=89
(%) | P-value | Positive n=20
(%) | Negative n=165
(%) | P-value |
|---|
| Age, years |
|
≥70 | 41 (49.4) | 42 (50.6) | 0.632 | 9 (10.8) | 74 (89.2) | 1.000 |
|
<70 | 54 (52.9) | 48 (47.1) |
| 11 (10.8) | 91 (89.2) |
|
| Sex |
|
Female | 32 (42.1) | 44 (57.9) | 0.036 | 10 (13.2) | 66 (86.8) | 0.391 |
|
Male | 63 (57.8) | 46 (42.2) |
| 10 (9.2) | 99 (90.8) |
|
| Macroscopic
type |
| Type
4 | 17 (73.9) | 6 (26.1) | 0.021 | 6 (26.1) | 17 (73.9) | 0.012 |
|
Other | 78 (48.1) | 84 (51.9) |
| 14 (8.6) | 165 (91.4) |
|
| Tumor size, mm |
|
≥50 | 60 (52.6) | 54 (47.4) | 0.659 | 10 (8.8) | 104 (91.2) | 0.258 |
|
<50 | 35 (49.3) | 36 (50.7) |
| 10 (14.1) | 61 (85.9) |
|
| Histological
type |
|
Diffuse | 58 (58.0) | 42 (42) | 0.050 | 14 (14) | 86 (86) | 0.508 |
|
Intestinal | 37 (43.5) | 48 (56.5) |
| 6 (7.1) | 79 (92.9) |
|
| aInfiltration pattern |
| INF
a/b | 53 (43.1) | 70 (56.9) | 0.005 | 7 (5.7) | 116 (94.3) | <0.001 |
| INF
c | 38 (65.5) | 20 (34.5) |
| 12 (20.7) | 46 (79.3) |
|
| Stage |
| II | 35 (44.9) | 43 (55.1) | 0.132 | 8 (13.0) | 70 (87.0) | 0.836 |
|
III | 60 (56.1) | 47 (43.9) |
| 12 (26.8) | 95 (73.2) |
|
| Lymph node
metastasis |
|
Positive | 73 (51.8) | 68 (48.2) | 0.837 | 15 (10.6) | 126 (89.4) | 0.727 |
|
Negative | 22 (50.0) | 22 (50.0) |
| 5 (11.4) | 39 (88.6) |
|
| Lymphatic
invasion |
|
Positive | 76 (49.0) | 79 (51.0) | 0.103 | 15 (9.7) | 140 (90.3) | 0.230 |
|
Negative | 19 (65.5) | 10 (34.5) |
| 5 (17.2) | 24 (82.8) |
|
| Venous
invasion |
|
Positive | 28 (50.9) | 27 (49.1) | 0.938 | 5 (9.1) | 50 (90.9) | 0.624 |
|
Negative | 67 (51.5) | 63 (48.5) |
| 15 (11.5) | 115 (88.5) |
|
| CXCR4/SDF1α
expression |
|
Positive | 20 (83.3) | 4 (16.7) | 0.001 |
|
|
|
|
Negative | 75 (46.6) | 86 (53.4) |
|
|
|
|
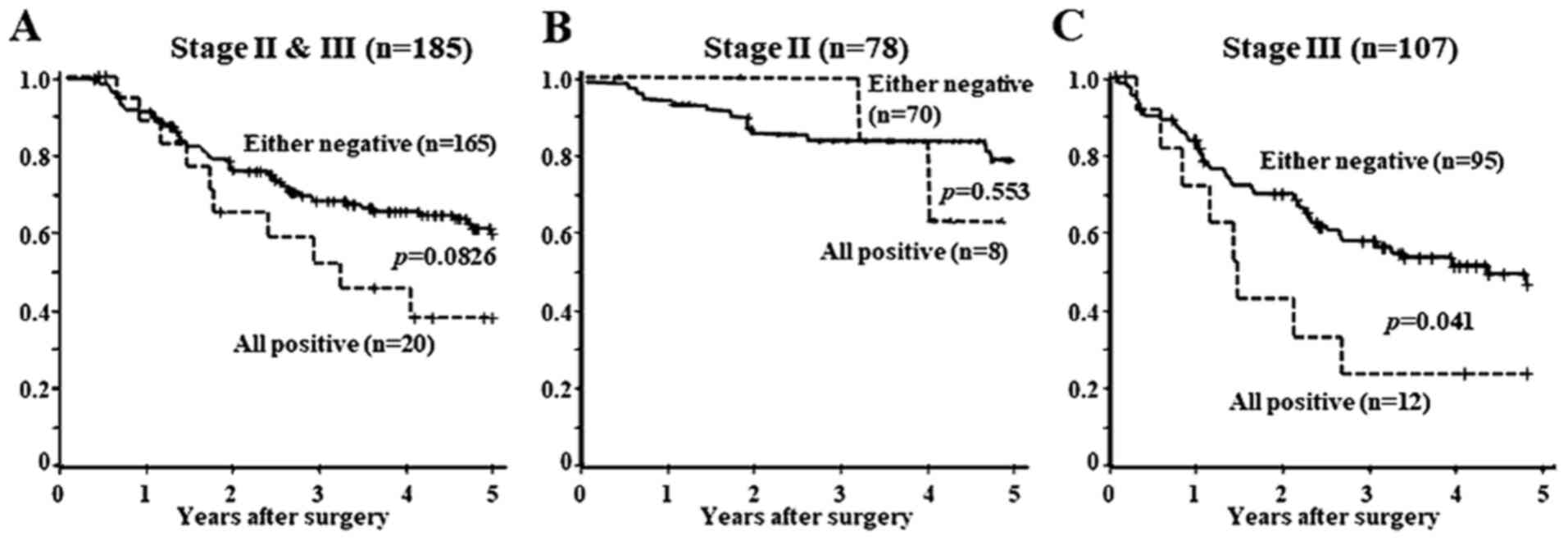
Survival analysis
Fig. 2 shows the
Kaplan-Meier survival curve for all 185 patients according to CXCR4
and SDF1α expression. The patients who were positive for all CA9,
CXCR4, and SDF1α were defined as the CA9/CXCR4/SDF1α-positive
group, whereas the those who were negative for CA9, CXCR4 or SDF1α
were termed CA9/CXCR4/SDF1α-negative. The prognosis of the
CA9/CXCR4/SDF1α-positive group tended to be poorer compared with
that of the CA9/CXCR4/SDF1α-negative patients with stage II or III
GC (Fig. 2A; P=0.0826). The
prognosis of patients with stage II GC was not different between
the all CA9/CXCR4/SDF1α-positive and -negative groups (Fig. 2B). By contrast, the prognosis of the
CA9/CXCR4/SDF1α-positive group was significantly poorer compared
with that of the CA9/CXCR4/SDF1α-negative patients with stage III
GC (Fig. 2C; P=0.041).
As presented in Table
III, the univariate analysis revealed that CA9/CXCR4/SDF1α,
age, macroscopic type and tumor size were each significantly
associated with a poor prognosis. The multivariate analysis
revealed that macroscopic type was independent prognostic factor,
whereas CA9/CXCR4/SDF1α expression was not.
 | Table III.Univariate and multivariate analysis
with respect to overall survival in gastric cancer. |
Table III.
Univariate and multivariate analysis
with respect to overall survival in gastric cancer.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variables | Hazard ratio | 95% CI | P-value | Hazard ratio | 95% CI | P-value |
|---|
|
CA9/CXCR4/SDF1α |
| Either
negative vs. all positive | 1.860 | 1.094–3.163 | 0.022 | 1.583 | 0.925–2.710 | 0.094 |
| Age, years |
| >70
vs. <70 | 1.659 | 1.050–2.622 | 0.030 | 1.379 | 0.852–2.233 | 0.191 |
| Sex |
| Female
vs. male | 1.179 | 0.733–1.896 | 0.497 |
|
|
|
| Macroscopic
type |
| Type 4
vs. other types | 3.779 | 2.219–6.434 | <0.001 | 2.685 | 1.475–4.886 | 0.001 |
| Tumor size, mm |
| <50
vs. ≥50 | 2.385 | 1.414–4.024 | <0.001 | 1.593 | 0.894–2.837 | 0.114 |
| Histological
type |
|
Intestinal vs. diffuse | 1.341 | 0.840–2.141 | 0.219 |
|
|
|
| Lymphatic
invasion |
|
Negative vs. positive | 1.779 | 0.816–3.880 | 0.147 |
|
|
|
Discussion
CA9 is upregulated under hypoxic conditions through
the upregulation and stabilization of hypoxia-inducible factor 1α
(HIF-1α), which binds to the hypoxia-responsive element present in
the promoter regions of CA9 (30).
Therefore, CA9 was considered to indicate hypoxic loci, and was
used as a hypoxic marker in the present study.
SDF1α was expressed in GC cells and stromal cells,
as previously reported (31,32). In the GC microenvironment, SDF1α
expression was observed mainly in the cytoplasm of fibroblast-like
stromal cells, particularly frequently in the macroscopic type 4 or
diffuse-type GC with abundant stromal cells. By contrast, the SDF1α
expression on the cancer cells was observed primarily at the cell
membrane. The SDF1α expression on the cancer cells was
significantly associated with SDF1α expression on the stromal
cells. SDF1α was first cloned from bone marrow-derived stromal
cells (33) and was reported to be
expressed on various stromal cells (34,35).
These results suggested that SDF1α on the membrane of cancer cells
may be derived from fibroblast-like stromal cells.
SDF1α was not expressed by any gastric or pancreatic
cancer cell lines (36,37). Therefore, in the present study the
SDF1α expression on stromal cells was investigated. Orimo et
al (38) also demonstrated that
SDF1α released by stromal fibroblasts directed the paracrine
stimulation of tumor cells through CXCR4 expressed on breast cancer
cells. SDF1α signaling may be associated with the malignant
progression of cancer cells. It was also observed that the SDF1α
expression on the tumor stromal cells was associated with the
diffuse type, while that on the cancer cells was associated with
the intestinal type (data not shown). SDF1α signaling may be
different between the histological types of GC.
In the present study, CXCR4 expression on cancer
cells was associated with macroscopic type 4, lymph node metastasis
and peritoneal metastasis. It has been reported that CXCR4
expression was associated with lymph node or liver metastasis in GC
(34,35), and was a prognostic factor in GC
(39–41). In the present study, patients with
CXCR4 and SDF1α expression exhibited significantly poorer
prognoses. The results of the present study suggested that the
SDF1α/CXCR4 axis may serve an important role in the progression of
cancer, and that the expression of these molecules may be a useful
prognostic factor for patients with stage III GC.
Hypoxia is thought to be associated with aggressive
tumor phenotypes of gastric carcinomas (42,43),
including the metastatic ability of cancer cells (44,45).
Clinical and experimental data have also provided evidence of an
association between the hypoxic environment and a poor prognosis
(45,46). In the present study, CA9, which was
used to investigate the hypoxic cells, was demonstrated to be
expressed heterogeneously in a gastric tumor, and it was found that
the CA9 expression was significantly associated with the CXCR4
expression on the cancer cells and the SDF1α expression on the
stromal cells. These results suggested that hypoxia, which was
evaluated by CA9 staining, may induce SDF1a and CXCR4. Recent
studies have demonstrated that SDF1α is upregulated in fibroblasts
to fulfill its role in cell protection against hypoxia (4,32). These
results suggested that the heterogeneous hypoxic environment in
cancer may be one of the reasons for cancer heterogenicity, which
is associated with tumor resistance for various types of therapy
(15,47,48).
SDF1α may serve as a protective factor to promote
cell repair following hypoxic injury via its main receptor, CXCR4
(49). Our previous study
demonstrated that the hypoxic condition affected the expression
level of certain receptors of cancer cells (17,18,50). The
results of our present study suggested that these results indicated
that hypoxia may upregulate SDFα production from stromal cells and
CXCR4 expression in cancer cells. Therefore, the SDF1α/CXCR4 axis
may serve an important role in the progression of GC cells in
hypoxia.
In conclusion, the SDF1α/CXCR4 axis may be involved
in the progression of GC at stage II and III, particularly under
hypoxic conditions.
Acknowledgements
Not applicable.
Funding
The present study was partially founded by the
KAKENHI (Grant-in-Aid for Scientific Research; nos. 18H02883 and
23390329) from the Ministry of Education, Science, Sports, Culture
and Technology of Japan.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MY and HK designed, performed the experiments and
co-wrote the manuscript. GT, TF, YY, TS, ST and AS prepared the
samples. SN, SK, MY and TT accumulated the data. TS, KK, ST and MO
sampled the material and MO reviewed manuscript. All authors read
and approved the final manuscript.
Ethics statement and consent to
participate
The present study was conducted with the approval of
the Ethical Committee of Osaka City University (reference no. 924).
Written informed consent was obtained from all patients prior to
treatment.
Patient consent for publication
Written informed consent was obtained from all
patients.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Global Burden of Disease Cancer
Collaboration, ; Fitzmaurice C, Dicker D, Pain A, Hamavid H,
Moradi-Lakeh M, MacIntyre MF, Allen C, Hansen G, Woodbrook R, et
al: The global burden of cancer 2013. JAMA Oncol. 1:505–527. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Miki Y, Yashiro M, Ando K, Okuno T,
Kitayama K, Masuda G, Tamura T, Sakurai K, Toyokawa T, Kubo N, et
al: Examination of cancer cells exposed to gastric serosa by
serosal stamp cytology plus RT-PCR is useful for the identification
of gastric cancer patients at high risk of peritoneal recurrence.
Surg Oncol. 26:352–358. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Togano S, Yashiro M, Miki Y, Yamamoto Y,
Sera T, Kushitani Y, Sugimoto A, Kushiyama S, Nishimura S, Kuroda
K, et al: Microscopic distance from tumor invasion front to serosa
might be a useful predictive factor for peritoneal recurrence after
curative resection of T3-gastric cancer. PLoS One. 15:e02259582020.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kinoshita H, Yashiro M, Fukuoka T,
Hasegawa T, Morisaki T, Kasashima H, Masuda G, Noda S and Hirakawa
K: Diffuse-type gastric cancer cells switch their driver pathways
from FGFR2 signaling to SDF1/CXCR4 axis in hypoxic tumor
microenvironments. Carcinogenesis. 36:1511–1520. 2015.PubMed/NCBI
|
|
5
|
Lee HJ and Jo DY: The role of the
CXCR4/CXCL12 axis and its clinical implications in gastric cancer.
Histol Histopathol. 27:1155–1161. 2012.PubMed/NCBI
|
|
6
|
Muller A, Homey B, Soto H, Ge N, Catron D,
Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, et al:
Involvement of chemokine receptors in breast cancer metastasis.
Nature. 410:50–56. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gockel I, Schimanski CC, Heinrich C,
Wehler T, Frerichs K, Drescher D, von Langsdorff C, Domeyer M,
Biesterfeld S, Galle PR, et al: Expression of chemokine receptor
CXCR4 in esophageal squamous cell and adenocarcinoma. BMC Cancer.
6:2902006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Engl T, Relja B, Blumenberg C, Müller I,
Ringel EM, Beecken WD, Jonas D and Blaheta RA: Prostate tumor
CXC-chemokine profile correlates with cell adhesion to endothelium
and extracellular matrix. Life Sci. 78:1784–1793. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu QY, Yang CK, Rong LJ, Li JC and Lei LM:
Investigation of the association between C-X-C motif chemokine
receptor subunits and tumor infiltration levels and prognosis in
patients with early-stage pancreatic ductal adenocarcinoma. Oncol
Lett. 20:162020.PubMed/NCBI
|
|
10
|
Katsumoto K and Kume S: The role of
CXCL12-CXCR4 signaling pathway in pancreatic development.
Theranostics. 3:11–17. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Scala S, Giuliano P, Ascierto PA, Ieranò
C, Franco R, Napolitano M, Ottaiano A, Lombardi ML, Luongo M,
Simeone E, et al: Human melanoma metastases express functional
CXCR4. Clin Cancer Res. 12:2427–2433. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang SS, Han ZP, Jing YY, Tao SF, Li TJ,
Wang H, Wang Y, Li R, Yang Y, Zhao X, et al:
CD133+CXCR4+ colon cancer cells exhibit
metastatic potential and predict poor prognosis of patients. BMC
Med. 10:852012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chiaramonte R, Colombo M, Bulfamante G,
Falleni M, Tosi D, Garavelli S, De Simone D, Vigolo E, Todoerti K,
Neri A and Platonova N: Notch pathway promotes ovarian cancer
growth and migration via CXCR4/SDF1alpha chemokine system. Int J
Biochem Cell Biol. 66:134–140. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cavallaro S: CXCR4/CXCL12 in
non-small-cell lung cancer metastasis to the brain. Int J Mol Sci.
14:1713–1727. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kitayama K, Yashiro M, Morisaki T, Miki Y,
Okuno T, Kinoshita H, Fukuoka T, Kasashima H, Masuda G, Hasegawa T,
et al: Pyruvate kinase isozyme M2 and glutaminase might be
promising molecular targets for the treatment of gastric cancer.
Cancer Sci. 108:2462–2469. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kato Y, Yashiro M, Noda S, Kashiwagi S,
Matsuoka J, Fuyuhiro Y, Doi Y and Hirakawa K: Expression of a
hypoxia-associated protein, carbonic anhydrase-9, correlates with
malignant phenotypes of gastric carcinoma. Digestion. 82:246–251.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Noda S, Yashiro M, Nshii T and Hirakawa K:
Hypoxia upregulates adhesion ability to peritoneum through a
transforming growth factor-beta-dependent mechanism in diffuse-type
gastric cancer cells. Eur J Cancer. 46:995–1005. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Matsuoka J, Yashiro M, Doi Y, Fuyuhiro Y,
Kato Y, Shinto O, Noda S, Kashiwagi S, Aomatsu N, Hirakawa T, et
al: Hypoxia stimulates the EMT of gastric cancer cells through
autocrine TGFβ signaling. PLoS One. 8:e623102013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hirakawa T, Yashiro M, Doi Y, Kinoshita H,
Morisaki T, Fukuoka T, Hasegawa T, Kimura K, Amano R and Hirakawa
K: Pancreatic fibroblasts stimulate the motility of pancreatic
cancer cells through IGF1/IGF1R signaling under hypoxia. PLoS One.
11:e01599122016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kasashima H, Yashiro M, Kinoshita H,
Fukuoka T, Morisaki T, Masuda G, Sakurai K, Kubo N, Ohira M and
Hirakawa K: Lysyl oxidase is associated with the
epithelial-mesenchymal transition of gastric cancer cells in
hypoxia. Gastric Cancer. 19:431–442. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kasashima H, Yashiro M, Nakamae H,
Kitayama K, Masuda G, Kinoshita H, Fukuoka T, Hasegawa T, Nakane T,
Hino M, et al: CXCL1-Chemokine (C-X-C Motif) receptor 2 signaling
stimulates the recruitment of bone marrow-derived mesenchymal cells
into diffuse-type gastric cancer stroma. Am J Pathol.
186:3028–3039. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kasashima H, Yashiro M, Nakamae H, Masuda
G, Kinoshita H, Morisaki T, Fukuoka T, Hasegawa T, Sakurai K,
Toyokawa T, et al: Bone marrow-derived stromal cells are associated
with gastric cancer progression. Br J Cancer. 113:443–452. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yashiro M, Matsuoka T and Ohira M: The
significance of scirrhous gastric cancer cell lines: The molecular
characterization using cell lines and mouse models. Hum Cell.
31:271–281. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Okuno T, Yashiro M, Masuda G, Togano S,
Kuroda K, Miki Y, Hirakawa K, Ohsawa M, Wanibuchi H and Ohira M:
Establishment of a new scirrhous gastric cancer cell line with
FGFR2 overexpression, OCUM-14. Ann Surg Oncol. 26:1093–1102. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Oh YS, Kim HY, Song IC, Yun HJ, Jo DY, Kim
S and Lee HY: Hypoxia induces CXCR4 expression and biological
activity in gastric cancer cells through activation of
hypoxia-inducible factor-1α. Oncol Rep. 28:2239–2246. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Romain B, Hachet-Haas M, Rohr S, Brigand
C, Galzi JL, Gaub MP, Pencreach E and Guenot D: Hypoxia
differentially regulated CXCR4 and CXCR7 signaling in colon cancer.
Mol Cancer. 13:582014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Japanese Gastric Cancer Association:
Japanese classification of gastric carcinoma: 3rd English edition.
Gastric Cancer. 14:101–112. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Greene FL and Sobin LH: A worldwide
approach to the TNM staging system: Collaborative efforts of the
AJCC and UICC. J Surg Oncol. 99:269–272. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shephard DA: The 1975 declaration of
helsinki and consent. Can Med Assoc J. 115:1191–1192.
1976.PubMed/NCBI
|
|
30
|
Eckert AW, Horter S, Bethmann D, Kotrba J,
Kaune T, Rot S, Bache M, Bilkenroth U, Reich W, Greither T, et al:
Investigation of the prognostic role of carbonic anhydrase 9 (CAIX)
of the cellular mRNA/protein level or soluble CAIX protein in
patients with oral squamous cell carcinoma. Int J Mol Sci.
20:3752019. View Article : Google Scholar
|
|
31
|
Hitchon C, Wong K, Ma G, Reed J, Lyttle D
and El-Gabalawy H: Hypoxia-induced production of stromal
cell-derived factor 1 (CXCL12) and vascular endothelial growth
factor by synovial fibroblasts. Arthritis Rheum. 46:2587–2597.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu H, Liu S, Li Y, Wang X, Xue W, Ge G
and Luo X: The role of SDF-1-CXCR4/CXCR7 axis in the therapeutic
effects of hypoxia-preconditioned mesenchymal stem cells for renal
ischemia/reperfusion injury. PLoS One. 7:e346082012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tashiro K, Tada H, Heilker R, Shirozu M,
Nakano T and Honjo T: Signal sequence trap: A cloning strategy for
secreted proteins and type I membrane proteins. Science.
261:600–603. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Iwasa S, Yanagawa T, Fan J and Katoh R:
Expression of CXCR4 and its ligand SDF-1 in intestinal-type gastric
cancer is associated with lymph node and liver metastasis.
Anticancer Res. 29:4751–4758. 2009.PubMed/NCBI
|
|
35
|
Zhao BC, Wang ZJ, Mao WZ, Ma HC, Han JG,
Zhao B and Xu HM: CXCR4/SDF-1 axis is involved in lymph node
metastasis of gastric carcinoma. World J Gastroenterol.
17:2389–2396. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yasumoto K, Koizumi K, Kawashima A, Saitoh
Y, Arita Y, Shinohara K, Minami T, Nakayama T, Sakurai H, Takahashi
Y, et al: Role of the CXCL12/CXCR4 axis in peritoneal
carcinomatosis of gastric cancer. Cancer Res. 66:2181–2187. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Koshiba T, Hosotani R, Miyamoto Y, Ida J,
Tsuji S, Nakajima S, Kawaguchi M, Kobayashi H, Doi R, Hori T, et
al: Expression of stromal cell-derived factor 1 and CXCR4 ligand
receptor system in pancreatic cancer: A possible role for tumor
progression. Clin Cancer Res. 6:3530–3535. 2000.PubMed/NCBI
|
|
38
|
Orimo A, Gupta PB, Sgroi DC,
Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL
and Weinberg RA: Stromal fibroblasts present in invasive human
breast carcinomas promote tumor growth and angiogenesis through
elevated SDF-1/CXCL12 secretion. Cell. 121:335–348. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jiang Q, Sun Y and Liu X: CXCR4 as a
prognostic biomarker in gastrointestinal cancer: A meta-analysis.
Biomarkers. 24:510–516. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yu C and Zhang Y: Characterization of the
prognostic values of CXCR family in gastric cancer. Cytokine.
123:1547852019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ishigami S, Natsugoe S, Okumura H,
Matsumoto M, Nakajo A, Uenosono Y, Arigami T, Uchikado Y, Setoyama
T, Arima H, et al: Clinical implication of CXCL12 expression in
gastric cancer. Ann Surg Oncol. 14:3154–3158. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Nayak A, Roy AD, Rout N, Singh SP,
Bhattacharyya A and Roychowdhury A: HIF1α-dependent upregulation of
ATAD2 promotes proliferation and migration of stomach cancer cells
in response to hypoxia. Biochem Biophys Res Commun. 523:916–923.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
de Barros Moreira Beltrão H, de Paula
Cerroni M, de Freitas DR, das Neves Pinto AY, da Costa Valente V,
Valente SA, de Góes Costa E and Sobel J: Investigation of two
outbreaks of suspected oral transmission of acute chagas disease in
the amazon region, para state, Brazil, in 2007. Trop Doct.
39:231–232. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Li Q, Zhu CC, Ni B, Zhang ZZ, Jiang SH, Hu
LP, Wang X, Zhang XX, Huang PQ, Yang Q, et al: Lysyl oxidase
promotes liver metastasis of gastric cancer via facilitating the
reciprocal interactions between tumor cells and cancer associated
fibroblasts. EBioMedicine. 49:157–171. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bubnovskaya L and Osinsky D: Tumor
microenvironment and metabolic factors: Contribution to gastric
cancer. Exp Oncol. 42:2–10. 2020.PubMed/NCBI
|
|
46
|
Zhang WJ, Chen C, Zhou ZH, Gao ST, Tee TJ,
Yang LQ, Xu YX, Pang TH, Xu XY, Sun Q, et al: Hypoxia-inducible
factor-1 alpha correlates with tumor-associated macrophages
infiltration, influences survival of gastric cancer patients. J
Cancer. 8:1818–1825. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kato Y, Yashiro M, Fuyuhiro Y, Kashiwagi
S, Matsuoka J, Hirakawa T, Noda S, Aomatsu N, Hasegawa T, Matsuzak
T, et al: Effects of acute and chronic hypoxia on the
radiosensitivity of gastric and esophageal cancer cells. Anticancer
Res. 31:3369–3375. 2011.PubMed/NCBI
|
|
48
|
Al-Juboori SI, Vadakekolathu J, Idri S,
Wagner S, Zafeiris D, Rd Pearson J, Almshayakhchi R, Caraglia M,
Desiderio V, Miles AK, et al: PYK2 promotes HER2-positive breast
cancer invasion. J Exp Clin Cancer Res. 38:2102019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Liu S, Jia X, Li C, Han X, Yan W and Xing
Y: CXCR7 silencing attenuates cell adaptive response to stromal
cell derived factor 1alpha after hypoxia. PLoS One. 8:e552902013.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kato Y, Yashiro M, Noda S, Tendo M,
Kashiwagi S, Doi Y, Nishii T, Matsuoka J, Fuyuhiro Y, Shinto O, et
al: Establishment and characterization of a new hypoxia-resistant
cancer cell line, OCUM-12/hypo, derived from a scirrhous gastric
carcinoma. Br J Cancer. 102:898–907. 2010. View Article : Google Scholar : PubMed/NCBI
|